Abstract
Donkey-hide gelatine (DHG) is a well-known, animal-derived traditional Chinese medicine material called Colla corii asini (known in Chinese as “E’jiao”). Because DHG is claimed to have properties that are beneficial to health, its consumption has increased, but its production has decreased. Thus, the incidence of DHG adulteration has become increasingly serious. In this study, a loop-mediated isothermal amplification (LAMP) assay was developed for the authentication of DHG. Identification of donkey DNA from DHG was performed specifically and rapidly within one hour by LAMP primers. Moreover, the sensitivity of LAMP in authenticating DHG was 10−3 ng, which revealed a 105-fold higher sensitivity than that of conventional PCR. The relative detection limit was 0.1% DHG in the adulterants, including gelatines of horse, cow, pork, goat, sheep or chicken origins. When genomic DNAs extracted from heat-treated DHG samples, including boiling or autoclaving for 40 min, were used as templates, DHG detection by LAMP was unchanged and reproducible. In conclusion, the LAMP assay established herein could potentially be applied for the authentication of DHG and DHG-related products in herbal or food markets.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04319-3) contains supplementary material, which is available to authorized users.
Keywords: Donkey-hide gelatines (DHG), Loop-mediated isothermal amplification (LAMP), Authentication
Introduction
Donkey-hide gelatine (DHG) is a well-known, animal-derived traditional Chinese medicine material (CMM) called Colla corii asini (known in Chinese as “E’jiao”) that has been used in China for a long time. DHG has not only been used in medical applications but is also a precious and expensive food or cosmetic ingredient for health-care product processing (Wang et al. 2017). Recently, because of its decreased production, high rate of consumption and claimed functional benefits, the price of DHG has increased. Previous studies of DHG have documented that it has been used for tonifying liver blood and kidney yin fluid and to stop bleeding (Cho 1996). DHG is rich in collagen and polysaccharides and has been known to promote haematopoiesis, increase the haemoglobin concentration, upregulate the number of peripheral erythrocytes, and even improve anaemia in pregnant women with thalassemia (Wang et al. 2017; Li et al. 2016). Other health benefits, such as anti-tumour, antiaging and bone-repairing activities, have also been reported in the medical application of DHG (Liu et al. 2005; Wang et al. 2012; Gao et al. 2004). Since numerous scientific studies have demonstrated the biological characteristics or activity of DHG, the quality of DHG, especially its authenticity and adulteration, has gradually become of importance. For example, fake DHGs, such as those produced from horses, pigs or cattle, have frequently appeared in the CMM market. Consequently, to ensure the authenticity of DHG in food, cosmetic or medical products and to reduce consumers' anxiety, DHG authentication has become significant.
Currently, numerous well-developed detection methods for DHG authentication have been reported in previous studies, including conventional observation of the appearance and structural characteristics of DHG or chromatographic identification of amino acids, peptides or specific proteins in DHG using liquid chromatography-tandem mass spectrometry (LC–MS/MS) (China Medical Science Press 2015; Yang et al. 2015; Li et al. 2017). However, physical examinations only provide preliminary information on the quality of DHG rather than its authenticity. Additionally, the accuracy of confirmation relies on the technical experience of the examiner. For the identification of specific digested proteins or peptides in the DHG, the integrity of the utilized mass spectral protein database is usually a bottleneck for DHG authentication. Moreover, time-consuming pre-treatment protocols and the need for well-equipped mass spectrometry laboratories in order to conduct chromatographic analyses becomes inconvenient during practical authentication. Using DNA molecular techniques for DHG authentication has also been established in previous reports because of the higher stability of DNA during harsh extraction conditions (Zhao et al. 2005; Zuo et al. 2017).
A nucleic acid amplification technique termed loop-mediated isothermal amplification (LAMP) has been reported for biomaterial diagnosis. Because of its high specificity, sensitivity and effectiveness, LAMP has been widely applied for microorganism, animal and plant identification (Woźniakowski and Tarasiuk 2015; Lee et al. 2017; Abdulmawjood et al. 2014). Theoretically, under isothermal conditions, at least four specific primers can be used to anneal six distinct DNA sequences of the target gene simultaneously for nucleic acid amplification when the LAMP reaction is performed. Moreover, Bst DNA polymerase also has strand-displacement activity; there is no need to denature DNA at high temperatures by using a thermal cycler during DNA amplification via LAMP. Therefore, LAMP has high potential to be rapidly applied for the sensitive and specific point-of-care detection of sample DNA.
In this study, a rapid, sensitive and specific LAMP assay for the authentication of DHG was established. The sequence of 12S ribosomal DNA (12S rDNA) was used as the target for the designation of LAMP primers to validate the primer specificity. The effect of the DNA integrity on DHG authentication was evaluated with DHG and various commercial processed DHG-related products purchased from a market to determine the feasibility of the assay. To the best of our knowledge, this work is the first report of a LAMP assay used for the determination of DHG authenticity.
Materials and methods
Samples
Two donkey-hide gelatine (DHG) samples, Zhengjiao donkey glue (ZDG) and Shandong donkey glue (SDG), were purchased from folk pharmacies (Pingtung, Taiwan) in January 2017 and were authenticated by professor Wen-Te Chang of China Medical University, Department of Chinese Pharmaceutical Science and Chinese Medicine Resources (Taichung, Taiwan). Five commercial food products containing DHG, including amber donkey glues date, crystal glue dates, sesame donkey glue dates, golden donkey glue dates, and donkey glue snacks manufactured in Hong Kong and Taiwan, were collected from an Internet store and a local supermarket (Pingtung, Taiwan) in March 2017. Additionally, one DHG-containing product of concentrated Chinese medicine granules (CCMGs), coptis-donkey glue powder, was purchased from a pharmaceutical company (Taoyuan, Taiwan). Well-authenticated donkey genomic DNA was collected from Dr. Xue-Mei Qin of the Modern Research Center for Traditional Chinese Medicine of Shanxi University in China for usage as the standard DNA. Samples of these DHG and DHG-related products were deposited at the Department of Food Science of the National Pingtung University of Science and Technology.
DNA extraction
Genomic DNA from the DHG samples was extracted as described by Kumeta et al. (2014) with modification. Briefly, DHG samples were frozen using liquid nitrogen and then ground in a ceramic mortar and pestle; the resultant samples were stored at − 80 °C for further experiments. One gram of DHG powder was suspended in 1 mL of digestion buffer (10 mM Tris-HCl, 25 mM EDTA, 100 mM NaCl, 0.5% SDS, pH 8.0) and then subjected to a water bath at 56 °C for 1 h until completely dissolved. Then, protease K (20 mg/mL) was added for sample digestion during incubation for 1 h at 56 °C. After centrifugation (12,000×g, 4 °C) for 10 min, the supernatant was collected and subjected to genomic DNA extraction using a QIAqick® nucleotide eemoval kit (QIAGEN) according to the manufacturer’s instructions.
LAMP primer design
The LAMP primers (DHG-F3, DHG-B3, DHG-FIP and DHG-BIP) (Table 1) for DHG authentication were designed by using the commercial software Primer Explorer V4 (https://loopamp.eiken.co.jp/e/index.html; Eiken Chemical Co., Ltd., Tokyo, Japan) based on the consensus sequence of 12S ribosomal DNA (rDNA) obtained from GenBank (https://www.ncbi.nlm.nih.gov). The accession numbers of the 12S ribosomal DNA sequences of Equus asinus in GenBank were FM 16411, KT829587 and AF221593 and were used for the alignment of consensus sequences. The primer positions are depicted in the alignment of the consensus sequence of 12S rDNA, as illustrated in supplementary Fig. 1.
Table 1.
LAMP primers for donkey-hide glue (DHG) authentication used in this study.
| Primer name | Sequence (5′–3′) |
|---|---|
| DHG-F3 | 5′-TGGAGAGAAATGGGCTACA-3′ |
| DHG-B3 | 5′-CATGGTTTTGTGTAATATTGTGA-3′ |
| DHG-FIP(F1c-F2) | 5′-TCCCATGGGCTACACCTTGA-AAACCCTAAACAAGGTACCAA-3′ |
| DHG-BIP(B1c-B2) | 5′-ACTCTAAGAACAAGAACTCAACCC-AATCCTCCTTCGGTCTCT-3′ |
Fig. 1.
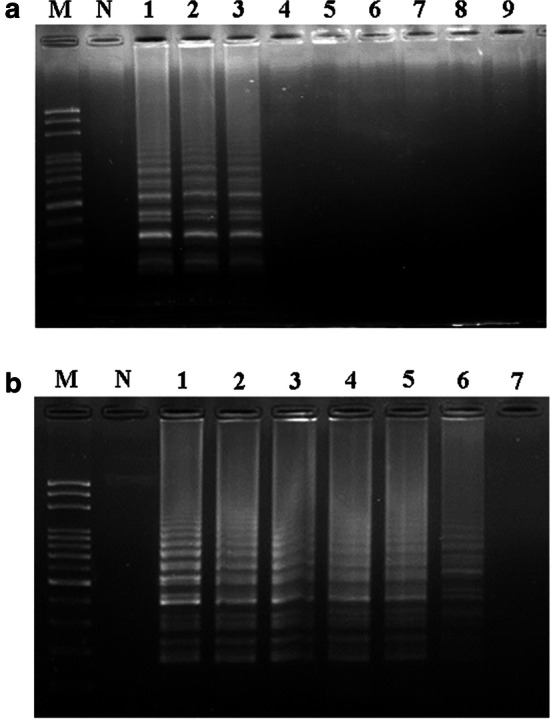
Validation of the specificity of the LAMP primers and the detection limit for DHG identification. The specificity of LAMP for DHG identification was validated by using 12S rDNA-based LAMP primers (a). Purified donkey DNA (10−1 μg) and genomic DNA samples from two DHGs, Shandong donkey glue (SDG) and Zhengjiao donkey glue (ZDG), were used as templates for the LAMP reactions. Equal amounts of genomic DNA from adulterants, including those of horse, cow, pork, goat, sheep or chicken origin, were also used. Lanes M and N, respectively, represent the 100 bp DNA ladder and the negative control. Lanes 1–9 represent various DNAs: 1, donkey; 2, Shandong donkey glue (SDG); 3, Zhengjiao donkey glue (ZDG) 4, horse; 5, cow; 6, pork; 7, goat; 8, sheep; and 9, chicken. The detection limit of the LAMP primers for DHG identification was determined (b). Samples containing donkey DNA mixed with various percentages of DNA from DHG adulterant LAMP primers were used for donkey DNA detection. Lanes M and N represent the 100 bp DNA ladder and the negative control, respectively. Lane 1: donkey DNA; Lanes 2–7: 50%, 10%, 5%, 1%, 0.1% and 0.01% donkey DNA mixed with different DNAs from the three DHG adulterants, respectively
DHG authentication via the LAMP reaction
The LAMP reaction was performed as described by Lee et al. (2017). Briefly, various quantities of genomic DNA of DHG were applied for each LAMP reaction. Eight U of Bst DNA polymerase (New England Biolabs, Frankfurt, Germany) was added to the LAMP reaction mixture containing 1 × Bst DNA polymerization buffer, 200 μM dNTPs, 0.5 μM DHG-F3 and DHG-B3 primers, and 4 μM DHG-FIP and DHG-BIP primers. The mixtures were isothermally incubated at 61 °C for 60 min using a PCR Express thermal cycler (TP600, Takara, Japan), and the reaction was terminated by heating at 80 °C for 5 min. The resultant mixture was stored at 4 °C until further analysis.
Analysis of LAMP products
LAMP products were detected by DNA electrophoresis using a 2% agarose gel. After electrophoresis, the gel was stained with ethidium bromide for observation of the DNA banding under UV excitation.
PCR
Two primers (DHG-F3 and DHG-B3), as shown in Table 1, were used as forward and reverse primers for PCR. PCR was carried out in a final volume of 25 µL containing various amounts of extracted DHG genomic DNA, 1 U Taq DNA polymerase, 200 μM dNTPs and 0.4 µM primers (DHG-F3 and DHG-B3). The reaction was started with denaturation (95 °C, 6 min), followed by 40 cycles of denaturation (94 °C, 30 s), annealing (60 °C, 30 s) and extension (72 °C, 30 s), and a final extension step (72 °C, 7 min) to complete the reaction in the PCR Express thermal cycler (TP600, Takara, Japan).
Authentication of boiled and steamed DHG by LAMP
Ten grams of each DHG sample was treated by boiling and steaming. DHG samples were directly immersed in boil water for 20, 40 and 60 min, followed by cooling in an ice bath. For the steaming process, DHG samples were autoclaved for 20, 40 or 60 min at 121 °C under a pressure of 15 psi. Then, the steamed DHG was cooled in an ice bath. The resultant DHG samples from boiling and steaming were subjected to genomic DNA extraction by following the previously described procedure. The purified DNA obtained from the boiled or steamed DHG was subjected to LAMP and PCR analysis.
Specificity of the LAMP assay
Six common genomic DNA samples of DHG adulterants, including those originating from horse, cow, pork, goat, sheep and chicken products, were purchased from BIOFOOD as a mixed kit (BIOTOOLS B&M Labs, Madrid, Spain). After quantification, 100 ng of DNA of each DHG adulterant was used for the LAMP reaction as the template DNA for the validation of primer specificity.
Sensitivity of the LAMP assay
Different amounts of genomic DHG DNA (102 ng, 101 ng, 100 ng, 10−1 ng, 10−2 ng, 10−3 ng and 10−4 ng, prepared by serial dilution) were used as template DNA to determine the sensitivity of the LAMP primer.
Authentication of DHG mixed with adulterants in different ratios
Genomic DNA of DHG adulterants of horse, cow and pork origin were placed in equal amounts in a test tube and vortexed to mix completely. The adulterant DNA mixture was added to donkey DNA at different ratios (50%, 10%, 5%, 1%, 0.1% and 0.01% by serial dilution). The resultant mixtures of DNA were used as template DNA and then subjected to LAMP.
Results
Establishment of a LAMP for the authentication of DHG
To establish a LAMP assay to detect donkey DNA for DHG authentication, specific LAMP primers were designed based on the consensus sequence of 12S ribosomal DNA of Equus asinus (Table 1). When LAMP primers were used to perform DNA amplification, the LAMP products were produced with a typical pattern of ladder-like DNA fragments on the agarose gel that was similar to that of the reference donkey DNA and the extracted DNAs from the DHG samples (Fig. 1a, lanes 1–3). This result demonstrated that the LAMP primers developed herein enabled the targeting of DNA templates derived from donkey origins to initiate DNA amplification. In contrast, no LAMP products were revealed when non-donkey genomic DNAs, such as those from the DHG adulterants of horse, cow, pork, goat, sheep or chicken origins, were used in the reactions (Fig. 1a, lanes 4–9). Moreover, the LAMP assay did not exhibit interferences with the mixing of donkey DNA and adulterant genomic DNA. At least 0.1% of the total donkey genomic DNA within the sample was required to achieve a specific detection of donkey DNA (Fig. 1b, lanes 1–6). These results demonstrated that the LAMP primers established herein for DHG authentication were specific.
Sensitivity of LAMP for DHG detection
To investigate the sensitivity of the LAMP assay, various quantities of DHG genomic DNA extracted from Zhengjiao donkey glue (ZDG) were subjected to the LAMP reaction. As demonstrated in Fig. 2a, the minimal amount of DHG genomic DNA required was 10−3 ng for detection by LAMP. A strong LAMP pattern was revealed on the gel except for when 10−3 ng of template DNA from DHG was used in the reaction (Fig. 2a, lane 7). Using the current primers DHG-F3 and DHG-B3, 102 ng genomic DNA from DHG was needed for PCR for DHG authentication (Fig. 2b). Strong and specific DNA band of nearly 200 bp was observed on the gel only when genomic DNA from DHG was employed for PCR (Fig. 2b, lane 2). Taken together, these results indicate that the LAMP assay applied herein demonstrated 105-fold higher sensitivity than that of the PCR assay for DHG authentication.
Fig. 2.
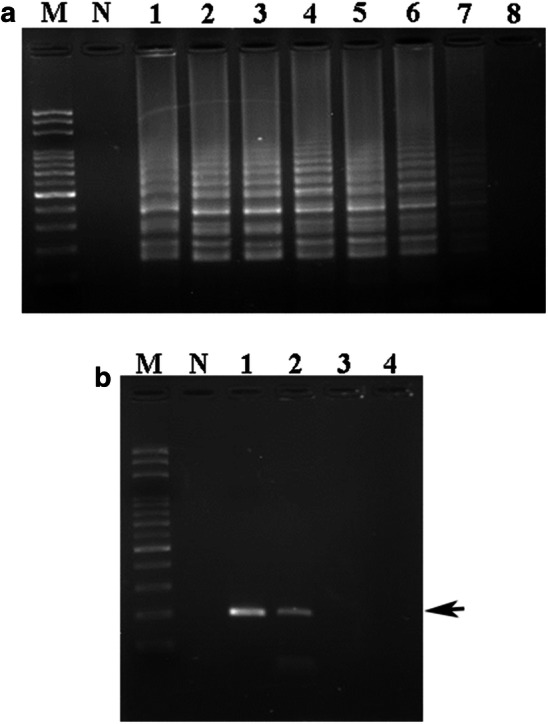
Sensitivity of specific LAMP and PCR primers used for the detection of donkey DNA in DHG products. Various amounts of donkey DNA obtained from DHG samples were prepared for LAMP and PCR assays. a For the LAMP assay, lanes M and N represent the 100 bp DNA ladder and the negative control, respectively. Lane 1: positive control. Lanes 2–8 represent the different amounts of DHG DNA added to the LAMP reaction: 2, 102 ng; 3, 101 ng; 4, 100 ng; 5, 10−1 ng; 6, 10−2 ng; 7, 10−3 ng; and 8, 10−4 ng. b For PCR, lanes M and N represent the 100 bp DNA ladder and the negative control, respectively. Lane 1 represents the positive control. Lanes 2–4 represent the different amounts of DHG DNA added to the reaction: 2, 102 ng; 3, 101 ng; and 4, 100 ng. The black arrow represents the specific amplified PCR product in the agarose gel
Effect of heat processing on DHG authentication by the LAMP assay
Various processes, including boiling and steaming, in food production might cause severe DNA damage that could interfere with DNA authentication. Thus, to examine the effect of heat treatment on DHG authentication by the LAMP assay, boiled and steamed DHG samples were prepared for further authentication tests. Boiled DHG samples with boiling times of 20 and 40 min did not exhibit different LAMP reaction results for the detection of genomic DNA from DHG; only the LAMP products of the DHG sample boiled for 60 min were not amplified (Fig. 3a, lanes 2–4). For the steamed DHG samples, those that underwent autoclaving for 20 and 40 min did not exhibit changed LAMP products from DHG DNA (Fig. 3b, lanes 2–4). In contrast, PCR was not able to identify any donkey DNA from any DHG samples that had been boiled or autoclaved DHG (Fig. 3c, d). Taken together, these results demonstrated that short-term boiling or steaming did not influence the outcome of the LAMP assay but completely inhibited DHG authentication by PCR (Fig. 3a–d).
Fig. 3.
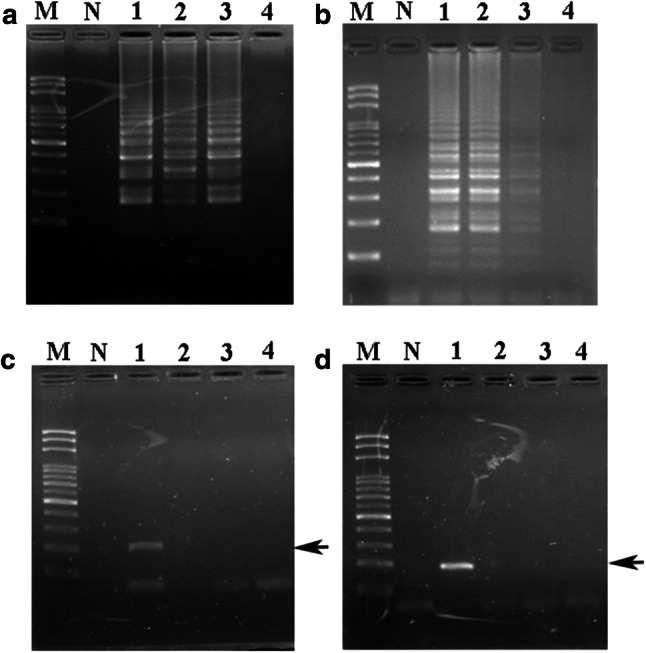
Effect of boiling and autoclave-steaming procedures on DHG authentication by LAMP (a, b) and PCR (c, d). When LAMP and PCR primers were used, specific DNA amplification was examined using DHG genomic DNA as a template obtained from samples treated with various heat processing conditions. Lane M: 100 bp DNA ladder, Lane N: negative control. For the boiling treatment (a, c), lane 1 represents positive control and lane 2-4 indicates that the DHG DNA was boiled for 20, 40 and 60 min, respectively. For the autoclave-steaming procedure (b, d), lane 1 respresnts positive control and lane 2-4 indicates that the DHG DNA was steamed for 20, 40 and 60 min, respectively. The black arrow represents the specific amplified PCR product in the agarose gel
Application of LAMP to DHG authentication in commercial DHG products
To evaluate the applicability of the LAMP assay in practical DHG authentication, six commercial food products containing DHG were purchased for the detection of donkey DNA. When LAMP was performed, four of the six commercial DHG products had detectable donkey DNA, including the sesame donkey glue dates, golden donkey glue dates, donkey glue snacks and coptis-donkey glue powder (Fig. 4a, lanes 4–7). The results of DHG authentication by PCR were not in accordance with the LAMP results (Fig. 4b). No DHG products were identified as having detectable donkey DNA by PCR (Fig. 4b, lanes 2–7). These results suggest that DHG-containing products can be authenticated rapidly and sensitively by the developed LAMP assay. However, the manufacturer-labelled formulations of all six commercial DHG foods or products did not agree with our LAMP results. After establishing the specificity and validation of the LAMP primers in this work, this LAMP assay displays practical applications for DHG authentication in the market.
Fig. 4.
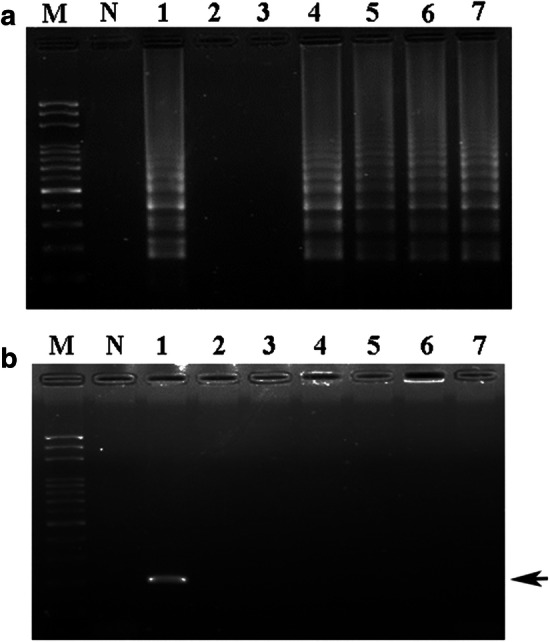
Analysis of LAMP (a) and PCR (b) assays on DGH authentication in commercial DHG products. LAMP and PCR assays were performed to detect donkey DNA extracted from six purchased commercial DHG products. Total genomic DNA extracted from each DHG sample as a template was used for the LAMP and PCR assays. LAMP (a) and PCR (b) products were detected by electrophoresis. Lane M represents the 100 bp DNA ladder; lane N represents the negative control. Lanes 1 to 7 represent the sample DNA of donkey, amber donkey glue dates, crystal glue dates, sesame donkey glue dates, golden donkey glue dates, donkey glue snacks and coptis-donkey glue powder, respectively. The black arrow indicates the specific amplified PCR product in the agarose gel (b)
Discussion
DHG is a very popular, expensive and precious animal-derived CMM in herbal markets. DHG is produced from the donkey hide of Equus asinus. DHG is not only a functional ingredient in food products but also a prescribed medicine in traditional Chinese medicine practices. Conventionally, some methods, including examination of its structural appearance and spectrophotometry, have been applied to authenticate DHG in previous studies (China Medical Science Press 2015; Yang et al. 2015; Li et al. 2017). The currently available assays do not provide intact information on authenticity during the discrimination of DHG from its adulterants. Moreover, these methods rely heavily on the examiner’s experience or equipment acquisition. Thus, other alternative methods with increased convenience, effectiveness and precision still need to be developed.
Several advantages of DNA-based authentication methods have been shown, including reliability, effectiveness and sensitivity, compared to those of other methods. Recently, an increasing number of isothermal DNA amplification techniques, such as loop-mediated isothermal amplification (LAMP), have been applied as detection methods to rapidly authenticate microorganisms, animals and medicinal plants (Woźniakowski et al. 2015; Lee et al. 2017; Abdulmawjood et al. 2014). LAMP is quite sensitive, specific and easy to perform for the on-site detection of biomaterials. The Bst DNA polymerase used in the LAMP reaction has DNA amplifying activity combined with DNA displacement. Thus, DNA can be amplified under isothermal incubation, such as simple water bath heating at a constant temperature; that is, a thermal cycler is not needed. In this study, specific LAMP primers were developed and used for the authentication of DHG. This LAMP primer set yielded an assay with specificity, sensitivity and efficiency for detecting donkey DNA. The LAMP assay displayed 105-fold higher sensitivity than that of PCR for DHG authentication. In addition to sensitivity improvement, detection was achieved within 1 h, which is also an advantage of this donkey DNA LAMP assay. In contrast, performing PCR requires at least 2 h for thermal cycling. Thus, LAMP is a competitive and alternative method for application in various situations, such as in on-site detection and analysis of samples containing few amplicons or low-integrity DNA when DHG authentication is performed.
In general, DHG is frequently used in commercial foods or related products, which presents a challenge for DHG authentication. Different heating processes, such as boiling and steaming, might destroy the amplicons that influence the initiation of DNA amplification. The LAMP assay established herein was performed successfully to authenticate donkey DNA, and 0.1% DHG was the relative detection limit when DNA of typical DHG adulterants was presented. Moreover, the performance of LAMP did not significantly change for different types of processed DHG (boiled or steamed) in this work. Conventional PCR for DHG detection did not display results parallel to those of the LAMP assay (Fig. 3c, d). This indicated that LAMP exhibited better sensitivity than that of PCR for the identification of donkey DNA in DHG (Fig. 3). Thus, this LAMP assay may be more applicable and effective for the detection of donkey DNA in commercial DHG products than PCR. In practical applications of the authentication of commercial DHG products by LAMP, as described in Fig. 4, this assay could also be applied for internal quality control for preventing adulteration or in routine inspections by government authorities for product labelling (Lee et al. 2017). To the best of our knowledge, this is the first report authenticating donkey DNA in DHG with rapid, specific and sensitive detection by a LAMP assay.
Conclusion
An isothermal DNA amplification method was developed and was shown to be fast, specific and sensitive for the authentication of DHG in this work. This assay can not only authenticate DHG but can also verify donkey DNA to expose adulteration of commercial DHG-related products in the market.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This research was supported by the grant from the Ministry of Science and Technology (MOST 105-2221-E-020-031-MY2), Taiwan.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
This article does not contain any studies with human or animal subjects.
Informed consent.
Not applicable.
Informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdulmawjood A, Grabowski N, Fohler S, Kittler S, Nagengast H, Klein G. Development of loop-mediated isothermal amplification (LAMP) assay for rapid and sensitive identification of ostrich meat. PLoS ONE. 2014;9:e100717. doi: 10.1371/journal.pone.0100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China Medical Science Press (2015) Pharmacopoeia of the People's Republic of China. In: 2015th ed. vol I. China Medical Science Press, The Pharmacopoeia Commission of PRC; Beijing, pp 189–90
- Cho HY. Oriental medicine: a modern interpretation. Compton: Yuin University Press; 1996. [Google Scholar]
- Gao Y, Dong FH, Zheng J. Influence of E Jiao on related genes expression during bone repair. Chin J Orthop Trauma. 2004;17:520–523. [Google Scholar]
- Kumeta Y, Maruyama T, Asama H, Yamamoto Y, Hakamatsuka T, Goda Y. Species identification of Asini Corii Collas (donkey glue) by PCR. J Nat Med. 2014;68:181–185. doi: 10.1007/s11418-013-0790-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Su TY, Lien YY, Sheu SS. The development of loop-mediated isothermal amplification (LAMP) ssays for the rapid authentication of five forbidden vegetables in strict vegetarian diets. Sci Rep. 2017;7:44238. doi: 10.1038/srep44238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Shi F, Gong L, Hang B, Li D, Chi L. Species-specific identification of collagen components in Colla corii asini using nano-liquid chromatography tandem mass spectrometry proteomic approach. Int J Nanomed. 2017;12:4443–4454. doi: 10.2147/IJN.S136819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, He H, Yang L, Li X, Li D, Luo S. Therapeutic effect of Colla corri asini on improving anemia and haemoglobin composition in pregnant women with thalassemia. Int J Hematol. 2016;104:559–565. doi: 10.1007/s12185-016-2069-0. [DOI] [PubMed] [Google Scholar]
- Liu PM, You JH, Tian SS, Xie XJ, Xie FS. Animal experiment of the medicine containing blood serum of Ejiao induced lung cancer PG cell apoptosis. Pract J Med Pharm. 2005;22:426–427. [Google Scholar]
- Wang DL, Liu MX, Cao JC, Cheng YN, Zhuo C, Xu HY, Tian SS, Zhang Y, Zhang J, Wang FS. Effect of Colla corii asini (E'jiao) on d-galactose induced aging mice. Biol Pharm Bull. 2012;35:2128–2132. doi: 10.1248/bpb.b12-00238. [DOI] [PubMed] [Google Scholar]
- Wang D, Ru W, Xu Y, Zhang J, He X, Fan G, Mao B, Zhou X, Qin Y. Chemical constituents and bioactivities of Colla corri asini. Drug Discov Ther. 2017;8:201–207. doi: 10.5582/ddt.2014.01038. [DOI] [PubMed] [Google Scholar]
- Woźniakowski G, Tarasiuk K. Visual detection of goose haemorrhagic polyomavirus in geese and ducks by loop-mediated isothermal amplification. Avian Pathol. 2015;44:311–318. doi: 10.1080/03079457.2015.1049585. [DOI] [PubMed] [Google Scholar]
- Yang H, Shen Y, Xu Y, Maqueda AS, Zheng J, Wu Q, Tam JP. A novel strategy for the discrimination of gelatinous Chinese medicines based on enzymatic digestion followed by nano-flow liquid chromatography in tandem with orbitrap mass spectrum detection. Int J Nanomed. 2015;10:4947–4955. doi: 10.2147/IJN.S82291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CJ, Han GC, Qin YH, Wu C. Differentiating among horse (Equus caballus), donkey (Equus asinus) and their hybrids with combined analysis of nuclear and mitochondrial gene polymorphism. J Anim Breed Genet. 2005;122:285–288. doi: 10.1111/j.1439-0388.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- Zuo HL, Zhao J, Wang YT, Xia ZN, Hu YJ, Yang FQ. Identification of the adulterated Asini corii colla with cytochrome c oxidase subunit I gene-based polymerase chain reaction. Pharmacogn Res. 2017;9:313–318. doi: 10.4103/pr.pr_33_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


