Rhizobia improve legume N nutrition, and modulate other aspects of host nutrition and development. Symbiont impact on host resource allocation and root distribution, important for soil resource acquisition, is discussed.
Keywords: Legumes, nutrition, rhizobia, roots, root system architecture, symbiosis
Abstract
Legumes form symbioses with rhizobia to fix N2 in root nodules to supplement their nitrogen (N) requirements. Many studies have shown how symbioses affect the shoot, but far less is understood about how they modify root development and root system architecture (RSA). RSA is the distribution of roots in space and over time. RSA reflects host resource allocation into below-ground organs and patterns of host resource foraging underpinning its resource acquisition capacity. Recent studies have revealed a more comprehensive relationship between hosts and symbionts: the latter can affect host resource acquisition for phosphate and iron, and the symbiont’s production of plant growth regulators can enhance host resource flux and abundance. We review the current understanding of the effects of rhizobia–legume symbioses on legume root systems. We focus on resource acquisition and allocation within the host to conceptualize the effect of symbioses on RSA, and highlight opportunities for new directions of research.
Introduction: Importance of root system architecture for soil resource acquisition
The term ‘root system’ typically refers to the entire root network of a plant, and hence its root system architecture (RSA) is the distribution of this network in space which determines the plant’s capacity to absorb soil resources (de Dorlodot et al., 2007; Tian and Doerner, 2013). Hence, root distribution in the pedosphere is a critical determinant of a plant’s capacity to efficiently, and in competition with other plants, capture below-ground resources. Most resources in soils are heterogeneously distributed and some, such as phosphorus (P) or iron (Fe), are also immobile and therefore roots must be in close proximity to deposits for acquisition (Vance, 2001). Other, more mobile resources, such as nitrate and water, are soluble and percolate into deeper layers. It follows that distinct RSAs are optimal for acquisition of different resources (e.g. deep rooted for water uptake and shallow rooted for uptake of phosphate) (Ho et al., 2005; Uga et al., 2013). Under combined stresses (e.g. low P and drought), a dimorphic root system performs best (Ho et al., 2005). Plants must therefore make architectural trade-offs when roots need to acquire multiple resources with distinct distribution patterns in the soil. This architectural plasticity is underpinned by changes to the growth behaviour of distinct roots and is essential to optimize plant resource acquisition. RSA is therefore not only a reflection of an individual plant’s (external) resource acquisition strategy in its specific environment, but also a history of the (internal) resource allocation (investment) to optimally capture below-ground resources.
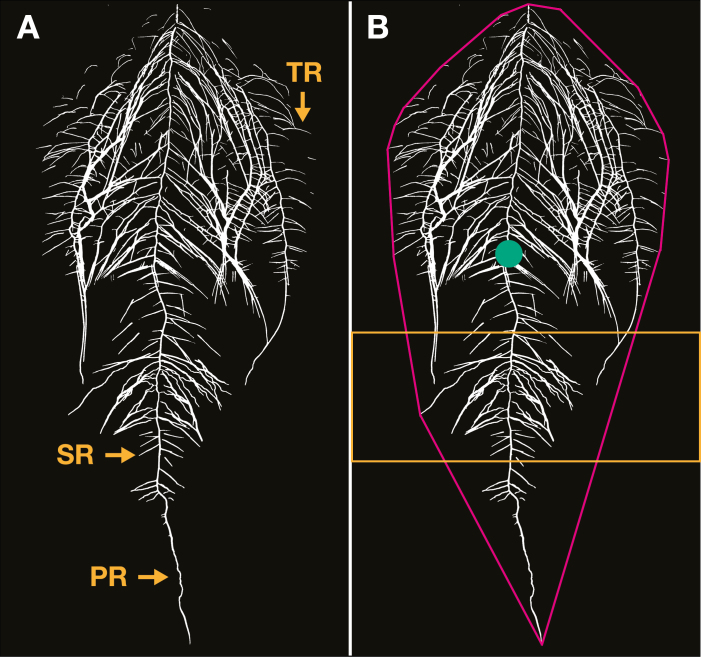
The characterization of root systems based on their architecture, using parameters which describe and quantify its shape, extent, and density as well as changes over time, which can only be measured in situ in the pedosphere, can inform on the plant’s resource capture and use strategies (Fig. 1) (Burridge et al., 2016, 2017; de Dorlodot et al., 2007; Tian and Doerner, 2013). However, such RSA-centric approaches are still not widely employed, probably because many methods used to acquire data, such as X-ray tomography, are costly and inaccessible to many (Kumi, 2015; Metzner et al., 2015). Other methods, which do not consider the positional information of roots in the soil, are more common, since these data are easier to obtain (Fig. 1). For example, most studies analyse the root system in terms of parameters such as length or mass, or as different root classes [e.g. number and length of primary roots (PRs) and lateral roots (LRs)] (Lynch, 2007; Lynch and Wojciechowski, 2015).
Fig. 1.
Comparison of traditional and architectural root system analysis. (A) Traditional root system analyses prioritize hierarchical and quantitative relationships between root classes without consideration of their spatial distribution. Thus, the primary root (PR) gives rise to secondary roots (SRs), which in turn give rise to tertiary roots (TRs), and so forth. All roots apart from the PR are collectively termed lateral roots. Measurements usually include number, average, and total length for each root class, and sometimes parameters that refer to the whole root system such as total root length and area. (B) In contrast, architectural analysis does not emphasize root rank but prioritizes their distribution in space to determine their functional contribution to the acquisition of soil resources. Major RSA parameters that describe said shape are the convex hull (the area of the smallest polygon covering the whole root system when projected in 2D, which represents the area the roots are exploring) (magenta); the centroid (the centre of mass of the root system bounded by the convex hull) (green dot); vertical root density (length or area of roots in a given area or volume, which indicates the intensity of soil exploitation in that space) (an example of such a segment is shown between the yellow lines); and other parameters such as total length, area, and depth of the root system. The image corresponds to a chickpea root system captured by the system of Bontpart et al. (2019, Preprint).
Importance of biotic interactions for root system architecture
Since RSA is directly tied to the capacity of the plant to acquire soil resources, biotic interactions where microorganisms improve plant resource acquisition might have an impact on it. For example, the application of P-solubilizing microorganisms under P-limited conditions might alter RSA due to the reduced need of the plant to exploit heterogeneously distributed P-rich patches. Symbioses between plant roots and bacteria and/or fungi, which entail a direct exchange of nutrients and host-derived resources, for example carbon (C)-rich compounds such as sugars and amino acids, are likely to have a bigger impact on RSA. Therefore, studying how these interactions modify RSA, as well as nutrient acquisition, shoot and root development, plant biomass, and C fluxes will result in a more comprehensive understanding of the different mechanisms and regulatory pathways through which RSA is regulated. In this review, we will focus on the legume–rhizobia symbiosis as a model for how microorganisms modify RSA.
The legume–rhizobia symbiosis improves N nutrition under limiting conditions
Legume–rhizobia symbiosis
Legumes are the second most important group of crops after cereals, accounting for 26% of global crop production, making them an important source of food and income for many (Medeot et al., 2010). Legumes are also a key part of the nitrogen cycle in both agricultural and natural environments since they form symbioses with rhizobia, soil endosymbiotic α- and β-proteobacteria able to fix N2 inside modified roots called root nodules (Fig. 2) (Allito et al., 2015; Poole et al., 2018). We use the term rhizobia generically here to include the following genera: Bradyrhizobium, Mesorhizobium, Rhizobium, and Sinorhizobium.
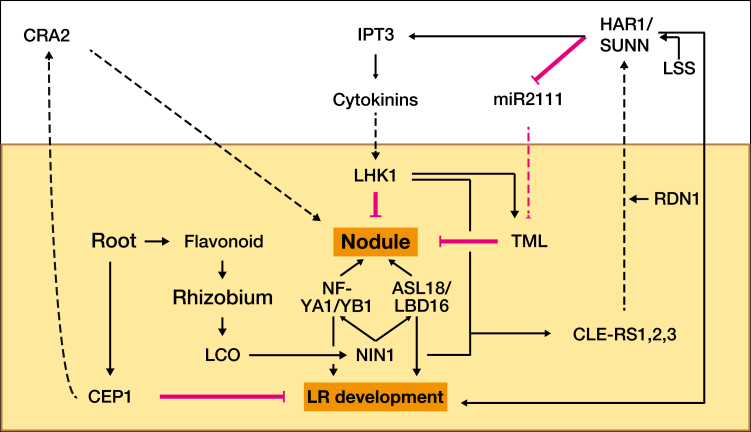
Fig. 2.
Autoregulation of nodulation (AON). Low N levels induce roots to produce flavonoids which stimulate rhizosphere-associated rhizobia to produce LCO. In L. japonicus, these regulate the major transcription factor NIN which activates both NF subunits YA1/YB1 as well as ASL18/LBD16, whose interaction initiates nodulation and nodule development. NIN also induces the expression of genes coding for nodulation-suppressing CLE peptides [mainly CLE ROOT SIGNAL 1 and 2 (CLE-RS1/2)], which are transported to the shoot and perceived by their receptor HAR1. This results in decreased accumulation and shoot–root mobilization of miR2111, which negatively regulates the nodulation suppressor TML. CLE–HAR1 interaction also leads to higher CK synthesis and signalling due to increased expression of IPTG3 and CK translocation to the roots to suppress further nodulation events through their receptor LHK1. This receptor also induces the expression of CLE-RS3, inducing a positive feedback loop on its own regulation, and is required for TML effect in the root cortex. In M. truncatula, low N conditions also stimulate roots to synthetize CEP1, which is mobilized to the shoot where its candidate receptor CRA2 positively regulates nodulation. RDN1 arabinosylates rhizobia-induced CLE12, similar to how CLE-RS2 is arabinosylated in L. japonicus to interact with HAR1, while LSS (LIKE SUNN SUPERNODULATOR) also further regulates the expression of the HAR1 orthologue SUNN. Mutations in these genes affect LR development since they also participate in the plant response to external N. Finally, NF-YA1/YB1 and ASL18/LBD16 have positive roles in LR development. Together, these genetic interactions show how several of the genes implicated in nodule development and the AON pathway overlap with those that regulate LR development. Black lines with arrowheads signify a positive effect while blunt-ended magenta lines indicate a negative effect. Solid lines indicate regulation while dashed lines signal mobilization to a different plant organ. Nodule and LR development are highlighted and in bold. Light brown indicates below-ground.
When soil N levels, and hence plant N resources, are low, legume roots exude flavonoids that attract compatible rhizobia to roots, where these produce diffusible lipo-chitooligosacharides (LCOs) (Fig. 2). When the plant perceives a compatible LCO, a signalling cascade is triggered that results in the expression of symbiotic genes such as NODULE INCEPTION PROTEIN (NIN). NIN is a transcription factor that increases the expression of both nuclear factor-Y (NF-Y) subunit genes NF-YA1 and NF-YB1, and ASYMMETRIC LEAVES LIKE 18/LATERAL ORGAN BOUNDARIES DOMAIN 16 (ASL18/LBD16). NF-Y and ASL18 proteins interact with each other to relay the rhizobia-mediated signal which ultimately initiates development of the nodule primordia through activation of cell division in the cortex layer (reviewed in Liu et al., 2018; Soyano et al., 2019).
Most rhizobia enter the root through an infection thread, a host-produced structure that guides it from the root hair to the nodule primordium, while others enter by intercellular penetration (crack entry), for example in peanut (Arachis hypogaea L.). Endodermis and pericycle cells also form part of the developing nodule; however, these are not infected by rhizobia (Xiao et al., 2014). In the nodule, rhizobia differentiate into a bacteroid and fix atmospheric N2 into ammonia, which is protonated into ammonium and captured in organic forms such as glutamine to be used by the plant for growth and development (reviewed in Poole et al., 2018; Ferguson et al., 2019). Nodules can be indeterminate, having a persistent meristem like those of Medicago species and pea, or determinate, which do not have an active meristem, such as in Lotus japonicus and soybean (Kohlen et al., 2018).
Similarities between nodule and lateral root development
In model legumes, LRs are predominantly derived from pericycle cells in both indeterminate (Herrbach et al., 2014) and determinate nodule-forming species (Held et al., 2014); however, endodermal and cortical divisions can also be observed (Xiao et al., 2014). In contrast, nodule primordia in Medicago truncatula are predominantly founded by the inner cortical cell layers (Xiao et al., 2014). Both organs initiate from their founder cells in response to localized auxin accumulation, and auxin-responsive genes such as the meristem identity genes WUSCHEL RELATED HOMEOBOX 5 (WOX5) and PLETHORA are up-regulated at the initiation site of both LRs and nodules. Furthermore, higher expression of auxin response factors and auxin biosynthesis genes such as YUCCA are also common to both processes (reviewed in Bishopp and Bennett, 2019). However, while LRs emerge from pre-defined founder cells, nodules are formed in response to LCO perception, which initiates cytokinin (CK)-induced gene expression (reviewed in Schiessl et al., 2019). In M. truncatula, CKs, via CYTOKININ RESPONSE 1, promote auxin accumulation in the cortex by increasing the expression of NIN, and are also antagonistic to LR development (Schiessl et al., 2019).
Root nodules are modified LRs, and several of the regulatory steps for LR development and nodule organogenesis are shared (Herrbach et al., 2014; Xiao et al., 2014), with 75% overlap in the gene expression changes induced in LRs and nodules (Schiessl et al., 2019). That explains why ectopic expression of NIN or mutations in its targets affect both nodule and LR development (Soyano et al., 2013, 2019).
ASL18/LBD16 has recently been found to be a key link of nodule evolution from LRs (Fig. 2). It is involved in LR development, and in L. japonicus it has intronic NIN-binding sequences which are conserved in most leguminous ASL18/LBD16 genes but not in non-leguminous orthologues. These sequences are sufficient for NIN-induced ASL18/LBD16 expression in the nodule primordia (Soyano et al., 2019). Like many LR regulatory genes, ASL18/LBD16 is induced by auxin, whereas neither NIN nor NF-YA1/2 is. Thus, LRs and nodules share many mechanistic similarities during their early development, indicating that a major part of the LR regulatory programme has been recruited for nodule development during legume evolution. A major unanswered question is how this developmental machinery is harnessed by and responsive to the hosts’ resource acquisition and allocation strategies, and if and how this coupling has changed in the context of nodulation.
Rhizobia–legume symbioses stimulate growth and enhance plant development
In the presence of compatible rhizobia and in N-limited conditions, entering into symbiosis is usually the most efficient way for legumes to acquire more N. Compared with non-nodulated plants, symbiosis provides a competitive advantage by increasing N levels by up to 5-fold (Solaiman et al., 2011; Wang et al., 2011; Regus et al., 2015; Goh et al., 2016). Rhizobia-dependent N fixation itself is essential for increased plant N levels in N deficit conditions: when two Lotus species were inoculated with rhizobial strains with a reduced capacity to fix N2, this resulted in a lower increase in plant biomass; rhizobia null mutants unable to fix N2 failed to increase plant biomass (Regus et al., 2015; Quides et al., 2017).
Symbiosis requires the host to allocate C to the symbiont, which in some legumes can comprise up to 14% of their total photosynthates (Kaschuk et al., 2009, 2010a), but in low N conditions this allocation is an investment and not a cost: first, under conditions of nutrient, including N, limitation, growth is rapidly uncoupled from photosynthetic C fixation. Declining growth leads to a reduction in sink strength which in turn diminishes the amount of C that growing organs are able to utilize (Fig. 3). Such C sink limitation is caused by the sink organ’s inability to utilize C in adverse environmental conditions (e.g. high temperature or drought) or mineral nutrient deficiencies (e.g. N or P) (Körner, 2015). Secondly, C sink limitation also leads to an accumulation of (unutilized) soluble carbohydrates and starch in source tissues which also negatively impacts photosynthetic C fixation (Paul and Foyer, 2001)
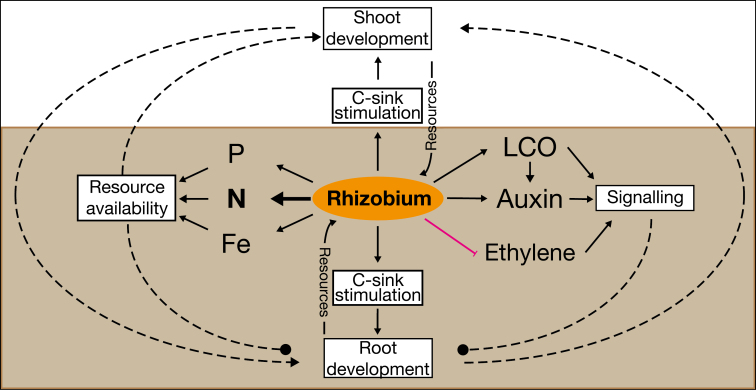
Fig. 3.
Overview of mechanisms by which rhizobia affects root development. Rhizobia modulate root development through several pathways. The major one is by increasing available N through the fixation of atmospheric N2, but it can also increase P and Fe availability by increasing rhizosphere acidity and secreting compounds that mobilize or chelate both compounds, such as siderophores for Fe. In this way, rhizobia contribute to plant resource acquisition, which has a positive effect on shoot development, and its effect on roots will depend on the new nutritional status of the plant to stimulate or inhibit the development of certain roots in certain parts of the root system. Furthermore, rhizobia can synthetize both auxin and LCOs, which further increase root IAA levels, and reduce ethylene concentration by modulating its biosynthesis. The resulting readjustment of the root’s hormonal status alters root signalling which activates/inhibits the initiation and development of specific roots in response to these altered hormone levels. Finally, rhizobia, and the developing nodules, consume C which increases root C sink strength and removes C sink limitation of roots and leaves, thus contributing to increased photosynthesis and enhanced development of both organs. Solid lines indicate a direct positive effect on a specific process, while blunt-ended magenta lines indicate a negative effect. Rhizobia improvement of plant N nutrition is shown in bold to highlight its major contribution to plant development. Text in boxes indicates key processes. Dashed lines with an arrowhead indicate a positive, indirect impact on organ development, while dashed lines with a circle indicate an impact (either positive or negative) on root development. Brown indicates below-ground.
Symbiosis and N2 fixation therefore stimulate growth by improving N nutrition and simultaneously reducing or removing the host’s C sink limitation. As a consequence, active symbioses result in higher photosynthesis and C fixation by feed-forward stimulation as reported for soybean (Fig. 3) (Harris et al., 1985; Zhou et al., 2006; Kaschuk et al., 2010a), Lotus (Regus et al., 2015), and Vicia faba (Kucey and Paul, 1982). This probably explains the somewhat paradoxical observation that even in cases where leaf N levels are lower than those of control N-fertilized plants, rhizobia-nodulated plants have higher C fixation. For example, Kaschuk et al. (2012) report that symbiosis in soybean increased photosynthesis by up to 31% due to increased sink stimulation and decreased starch and soluble sugar accumulation, which removes carbohydrate inhibition of photosynthesis in source tissues (Azcón-Bieto, 1983; Kaschuk et al., 2009, 2010a). Higher yields in nodulated compared with N-fertilized control plants have also been reported in other legumes (Kaschuk et al., 2010b). Increased C consumption in nodules for N2 fixation in alfalfa and M. truncatula (Larrainzar et al., 2014; Gebril et al., 2015; Kaur et al., 2019), and export of fixed-N metabolites to the host in soybean (Collier and Tegeder, 2012; Carter and Tegeder, 2016) enhanced plant biomass, which stimulated N fixation in turn.
Increased C consumption by nodules should only have a positive effect on photosynthesis when adequate N2 levels are fixed to support the higher metabolic demands of the host. Indeed, nodulated common bean, pea, and soybean have higher photosynthetic rates compared with uninoculated plants only at high N2 fixation rates (Bethlenfalvay et al., 1978; Zhou et al., 2006; Bambara and Ndakidemi, 2009). Similar findings are reported in pea plants treated with LCOs compared with untreated controls when grown in soil with native rhizobia, which showed a higher photosynthetic rate and N content due to more abundant nodulation and N2 fixation activity (Podleśny et al., 2014; Siczek et al., 2014).
Interestingly, nodules that fix higher amounts of N2 are also allocated more resources compared with those that fix low quantities, which are penalized by the host in terms of C allocation (Simms et al., 2006; Regus et al., 2015; Westhoek et al., 2017). The most productive symbioses have high sink strength nodules which also allocate high amounts of organic N to the host, thereby creating a strong, positive feedback on plant growth under N-limiting conditions (Kaschuk et al., 2010a; Quides et al., 2017). This explains why in many symbioses, nodule biomass is proportional to the N2-fixing capacity of the rhizobia (Pampana et al., 2016; Quides et al., 2017).
N availability per se impacts resource allocation to roots
Due to the interdependency of N and photosynthesis, plant N levels are a major factor regulating resource partitioning between shoots and roots in many species (Schortemeyer et al., 1999; Goh et al., 2019). When shoot N availability is limiting, a higher proportion of the plant’s total C will be invested in the root system to underpin foraging and N acquisition to satisfy the shoot’s requirements (Fig. 3). Conversely, when shoot N levels are high, C flux to the roots is proportionally, but not absolutely, decreased. Such modifications to root N allocation are reported in many legumes in response to nodulation such as in several Medicago species (Goh et al., 2016), Acacia melanoxylon (Schortemeyer et al., 1999), and soybean (Wang et al., 2011), but also in non-legumes such as tobacco (Scheible et al., 1997) and Arabidopsis (Yan et al., 2019) due to higher available N.
Taken together, this is strong evidence that active symbioses can stimulate growth and photosynthesis by multiple mechanisms (Fig. 3): symbioses not only directly enhance host N availability, but also stimulate C fixation via relief from feed-back restrictions on photosynthesis. In rhizobia–legume symbiosis, relief from N and C limitation is co-dependent.
We posit that actively N-fixing symbioses are likely to modify host root systems by modulation of sink strength and resource fluxes, which affects shoot–root resource allocation. Such changes are likely also to affect host plant growth regulator (e.g. auxin) signalling, because enhanced sucrose transport to sink organs via phloem augments the amount of auxin transported by this route (Petrášek and Friml, 2009). A major and largely unanswered question is whether this modification of host root systems is global or, alternatively, leads to changes in its distribution in the pedosphere; that is, changes to RSA.
Effects of rhizobia symbiosis on legume root system architecture
Active symbioses provide a continuous N supply, decreasing host requirements for N foraging. Consequently, if N is no longer the most limiting resource, host resource partitioning and acquisition are predicted to be correspondingly altered to prioritize the assimilation of other, now more limiting, soil resources such as P. To maximize the host’s return on its overall below-ground investment, it would need to modify its foraging strategy, and hence root development, to efficiently use root-allocated resources to absorb these more limiting resources (reviewed in Kaschuk et al., 2010a; Goh et al., 2016; Ferguson et al., 2019). Consistent with this, rhizobial symbiosis increases root depth in common bean (Sofi et al., 2017), which reflects a higher demand for water, and increases growth angle on field-grown soybean, indicating a higher exploitation of the topsoil (Yang et al., 2017), a common RSA response to increase immobile P absorption (Williamson et al., 2001; Péret et al., 2011).
Unfortunately, our current state of understanding of how rhizobia modify legume RSA lacks breadth and detail due to several historical, technical, and conceptual limitations. First, in many experiments, the root system is not studied in detail or not at all (Mishra et al., 2011; Ndakidemi et al., 2011; Quides et al., 2017). Secondly, many experimental growth systems are not explicitly designed to study RSA: for example, root systems in soil-grown plants cultivated in pots or long tubes lose their in situ RSA once they are removed for imaging or analysis (Wang et al., 2011; Ravikumar, 2012; Yang et al., 2017). Thirdly, there is a lack of awareness or use of RSA-specific parameters, which describe and quantify the root system within the soil for analyses (Burridge et al., 2016, 2017). Since few data of possible RSA changes in active rhizobia–legume symbioses are available, we will review the effect of symbioses on host nutrition, growth, and nutrient signalling, C acquisition and flux, and root development to then consider their possible impact on root architecture.
Impacts of rhizobia–legume symbioses on host root development
Modification of legume root traits by rhizobia
Multiple studies have shown that many rhizobia–legume symbioses modify root traits, irrespective of host or symbiont species (Table 1). The general conclusion from these studies is that rhizobia–legume symbioses positively regulate various aspects of root development. The key question becomes: are these changes to root development more likely to be isometric (i.e. a linear, proportional increase of RSA trait values) or allometric (i.e. changes to scale and relative proportions of RSA parameters)? Although measurements of RSA parameters are generally missing, it is likely that these vary specifically in the course of symbioses. Due to the changing identity of the most limiting nutrient resulting from the provision of fixed N, and the resultant changes to C and N resource allocation, changes to RSA parameters will reflect altered priorities in resource acquisition and therefore are likely to be allometric rather than isometric.
Table 1.
Effect of different rhizobia on shoot and root system modifications, and N content in different legumes
| Host | Symbiont | Growth medium | Shoot trait | Root trait | N changes | Comment | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Species | Varieties | Species | Strains | ||||||
| Phaseolus vulgaris | 4 | R. leguminosarum | 2 | Inert | Weight | Weight | Yes | Higher root weight in 1–2 varieties with 1–2 strains. In one variety both strains increased shoot and root mass | Franzini et al. (2010) |
| 1 | Rhizobium spp. and R. tropici | 7 | Soil mix | Weight | Weight, length | Yes | Karaca and Uyanöz (2012) | ||
| 6 | R. phaseoli | 1 | Soil mix | Weight | Weight, depth | NR | Higher weight/depth in 5–6 varieties. Higher shoot and root biomass in 4 varieties | Sofi et al. (2017) | |
| 1 | R. phaseoli | 1 | Soil | Weight | Weight, length | Yes | No changes in root length | Stajković et al. (2011) | |
| Glycine max | 2 | Bradyrhizobium spp. | 1 | Soil | Weight | Weight, length | Yes | Small increases in root traits. Higher shoot and root mass in one variety, lower shoot/root ratio with nodulation | Wang et al. (2011) |
| >10 | Rhizobium spp. and R. tropici | 1 | Soil | Weight | Weight, length, area | NR | Field experiment | Yang et al. (2017) | |
| 1 | B. japonicum | 1 | Hydroponic | No | Length, area | No | Egamberdieva et al. (2017) | ||
| 1 | Bradyrhizobium spp. | 1 | Hydroponic | NR | Length | NR | Inferred higher root length | Li et al. (2015) | |
| Vigna unguiculata | 1 | Rhizobium spp. and R. tropici | 1 | Soil mix | Weight | Weight, length | NR | Arumugam et al. (2010) | |
| Vigna mungo | 1 | Rhizobium spp. and R. tropici | 1 | Soil | Height | Length | Yes | Badar and Qureshi (2012) | |
| 1 | R. japonicum | 1 | Soil | Height, leaf and branch number | Number | NR | Ravikumar (2012) | ||
| Vigna radiata | 1 | R. japonicum | 1 | Soil | Height, leaf and branch number | Number | NR | Ravikumar (2012) | |
| Cicer ariеtinum | 1 | Rhizobium spp. and R. tropici | 10 | Soil | Weight | Weight, length | NR | Higher root development with 3 strains in greenhouse, no changes in field with those 3 | Khaitov et al. (2016) |
| 1 | M. ciceri | 1 | Soil | Weight | Weight, length | Yes | Moradi et al. (2013) | ||
| 1 | Rhizobium spp. and R. tropici | 4 | Soil | Weight | Weight, length | Yes | Higher length with 3 strains, weight with 2 strains. Higher shoot weight and root length in 2 strains | Solaiman et al. (2011) | |
| Lens culinaris | 1 | R. leguminosarum | 1 | Soil | Weight | Weight, length | Yes | Mishra et al. (2011) | |
| Arachis hypogaea | 1 | Rhizobium spp. and R. tropici | 6 | Soil | Height | Length | NR | No changes in field | Sharma et al. (2011) |
| Vicia faba | 1 | Rhizobium spp. and R. tropici | 9 | Two soils | Weight | Length | NR | Higher root length in 3 strains, and shoot weight in 2 of them in one soil | Argaw (2012) |
| Medicago truncatula | 3 | S. meliloti and S. medicae | 2 | Hydroponic | Weight | Weight, length | Yes | Higher weight/length in 1–2 varieties with 1–2 strains. Higher shoot and root weight in one variety with one strain | Kallala et al. (2018) |
| Pisum sativum | >10 | R. leguminosarum bv. viciae | 1 | Hydroponic /inert | NR | NR | NR | Positive relationship between nodule establishment and root system growth | Bourion et al. (2010) |
| Lotus japonicus | 1 | M. loti | 3 | Inert | Weight | NR | NR | Rhizobia increases shoot mass by 6- and 17-fold in growth chambers and greenhouse, respectively. Changes in root traits inferred | Quides et al. 2017 |
| Lotus strigosus | 1 | Bradyhizobium spp. | 4 | Inert | Weight | NR | Yes | 3 strains increased shoot mass by 2- to 6-fold, and N by up to 5-fold. Changes in root traits inferred | Regus et al. (2015) |
| Medicago truncatula, | 1 | S. meliloti | 1 | Inert | Weight | Weight, length | Yes | Stimulated root growth is both observed and inferred. | Goh et al. (2016) |
| Medicago sativa | 1 | S. meliloti | 1 | Inert | Weight | Weight | Yes | Stimulated root growth is both observed and inferred. | Goh et al. (2016) |
| Trifolium subterraneum | 1 | R. leguminosarum bv. trifolii | 1 | Inert | Weight | Weight, length | Yes | Stimulated root growth is both observed and inferred. | Goh et al. (2016) |
| Vicia faba | 1 | R. leguminosarum | 1 | Field | Height, branch number | Weight, length | Yes | Higher branch number, but decreased height. No changes in root length | Desta et al. (2015) |
NR, not reported.
Rhizobia-modified legume root development is conditional on the host and environment
It is important to note that not every study of rhizobia–legume symbiosis revealed modified root traits compared with uninoculated plants. In peanut (A. hypogaea L.), treatment with several rhizobial strains failed to increase root length (Singh et al., 2011), while, in other cases, modified root traits were genotype dependent or specific to certain soils (Franzini et al., 2010; Argaw, 2012; Kallala et al., 2018). In an experiment with shallow- and deep-rooted soybean genotypes, rhizobia inoculation increased root dry mass only for the deep-rooted variety under two contrasting P scenarios, but not in the shallow-rooting variety under both P conditions (Wang et al., 2011). Khaitov et al. (2016) analysed several rhizobia–chickpea symbioses grown in saline soil in greenhouse conditions: three strains resulted in higher root mass and length but, when tested in the field, no differences in root length were found. These reports exemplify that rhizobia–legume symbioses, while generally positively impacting, for example, root mass, length, and/or area, also depend on the specific symbiosis (plant variety and rhizobial strain) and soil environment.
Rhizobia-mediated higher plant N levels impact resource partitioning
In most nodulated legumes with active symbioses, shoot biomass is higher resulting from the co-dependent stimulation of photosynthesis and N fixation (Harris et al., 1985; Kaschuk et al., 2012). Numerous examples suggest that the larger shoot stimulates root development to allow for exploitation of more soil resources to satisfy its higher requirements. Changes in root mass, length, and area would thus reflect a more intensive exploration/exploitation of the soil to provide said resources for increased shoot growth enabled by relief from sink limitation and higher C fixation (Table 1).
The impact of symbioses on source–sink relationships and on shoot and root growth is contingent on the symbiosis delivering substantially improved N nutrition to the host (Table 1). Goh et al. (2016) report for Medicago sativa, M. truncatula, and Trifolium subterraneum that higher N levels are significantly and positively correlated with higher total root length and total first-order LR length. For nodulated chickpea, only strains that resulted in the largest increase of shoot N levels led to increases in root length and mass (Solaiman et al., 2011). As a corollary, in adequate soil N levels or with rhizobial strains that fix low amounts of N2, symbiosis would contribute little or nothing to host nutrition, hence not leading to increases of shoot biomass or to changes to root traits (Franzini et al., 2010; Singh et al., 2011; Argaw, 2012; Kallala et al., 2018).
Interestingly, Goh et al. (2016) also report that reductions to C flux to the roots were observed independent of the rhizobium strain’s ability to fix N2; possibly explained by changes to host plant growth regulator homeostasis. For the T. subterraneum–Rhizobium leguminosarum bv. trifolii interaction, altered plant growth regulator distribution or levels may explain how symbiosis modified the proportion of root-allocated resources targeted to PR or LR development, respectively (Goh et al., 2016). However, more evidence is needed to be confident that this is a widespread mechanism, but this report suggests that rhizobia can potentially also sculpt source–sink relationships by altering host growth regulator distribution.
Rhizobia-dependent N activates plant N utilization and signalling pathways
In non-legume species such as rice (Oryza sativa) and Arabidopsis, nitrate and ammonium activate N utilization and signalling pathways, for example by expression of nitrate-responsive genes (reviewed in Fukushima and Kusano, 2014; Krapp et al., 2014; Medici and Krouk, 2014). A key observation from molecular studies of nitrate-responsive gene expression is the co-regulation of N-, C-, and hormone-responsive pathways, leading to an overall higher steady-state level of metabolism, which also affects root development (reviewed in Hu et al., 2019; Medici et al., 2019; Hu and Chu, 2020). In rice, glutamine is the signal required for nitrate- and ammonium-dependent activation of cytokinin-dependent shoot growth (Kamada-Nobusada et al., 2013). These observations suggest that N fixation products in legumes, possibly glutamine, probably modify N, C, and growth regulator signalling pathways, leading to changes in root development.
Taken together, active symbioses lead to enhanced levels of plant metabolism and growth by a combination of several mechanisms: N2 fixation relieves sink and source tissue-level limits on photosynthesis and growth, resulting in co-stimulation of N and C metabolism. Augmented steady-state metabolism increases shoot and root growth capacity. However, growth stimulation of roots and shoots is unlikely to be uniform and proportional: in most circumstances, other resources (e.g. water, P, or Fe) will rapidly limit growth. Consequently, root foraging for limiting resources is predicted to alter root growth patterns and timing (RSA) by modifying resource partitioning, including by changing plant growth regulator homeostasis (Fig. 3). While much evidence has reported that shoot and root mass and/or length are affected by active symbioses, it remains an open question how active symbioses change RSA.
Rhizobia broadly benefit host non-N resource acquisition and metabolism
Rhizobial symbiosis impacts multiple aspects of legume nutrition and development
Successful symbioses stimulate both host and symbiont metabolism and signalling, resulting in elevated demands on metabolic capacity to underpin enhanced growth. Therefore, when focusing on their metabolism and resource allocation, it is useful to consider both organisms together, as an assemblage of organisms or holobiont; for example, N fixation biochemistry requires elevated levels of Fe and P, and the enhanced metabolism enabled by provision of reduced N to the holobiont increases Fe and P requirements. Therefore, it would be selectively advantageous for rhizobia if they did not fix only N, but would also directly contribute to non-N nutrient acquisition. It is not surprising then that there are many examples where rhizobia are involved in non-N nutrient acquisition for the host (Table 2); and that many also produce plant growth regulators, which can contribute to modifying resource allocation (Abril et al., 2007; Vargas et al., 2009; Qin et al., 2011). Taken together, this multitude of mechanisms involved in the legume–rhizobia symbiosis indicate a much greater contribution by rhizobia to host resource acquisition and partitioning than often recognized (Kaschuk et al., 2010a; Ndakidemi et al., 2011; Goh et al., 2016). The multiple benefits to the plant by the rhizobia has led to the suggestion that the temporary rhizobia–host symbiosis is evolving towards a novel N-fixing organelle (Coba de la Pena et al., 2017).
Table 2.
Effect of rhizobia on P and Fe content in different legumes
| Host | Symbiont | Growth medium | Higher P | Higher Fe | Comment | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Species | Varieties | Species | Strains | |||||
| Phaseolus vulgaris | 1 | R. leguminosarum | 1 | Inert | Yes | NR | Higher P content compared with reference strain | Abril et al. (2007) |
| Phaseolus vulgaris | 4 | R. leguminosarum | 2 | Inert | Yes | NR | One rhizobia strain increased P content in one bean variety | Franzini et al. (2010) |
| Glycine max | 1 | B. elkanii | 1 | Inert | Yes | NR | Higher P content under two N scenarios when fed different insoluble forms of P | Qin et al. (2011) |
| Cicer aritenium | 1 | Rhizobium spp. | 29 | Soil | Yes | NR | 23 rhizobial strains increased P content. Additional experiments achieved similar results. | Imen et al. (2015) |
| Cicer aritenium | 1 | M. mediterraneum, M. tianshanense, and M. ciceri | 4 | Inert | Yes | NR | Higher P content with one Mesorhizobium strain | Rivas et al. (2006) |
| Cicer aritenium | 1 | M. mediterraneum | 1 | Soil | Yes | NR | Higher P content at two different levels of P | Peix et al. (2001) |
| Phaseolus vulgaris | 6 | Rhizobium spp. | 47 | Inert | Yes | Yes | Many rhizobial strains that increase shoot mass in several bean varieties can solubilize P and produce siderophores | Abbaszadeh-dahaji et al. (2012) |
| Hedysarum coronarium | 1 | Rhizobium spp. | 1 | Soil | Yes | Yes | Soumaya et al. (2016) | |
| Glycine max | 2 | Bradyrhizobium spp. | 1 | Soil | Yes | NR | Increase in P content in a P-inefficient variety under several nutritional scenarios | Wang et al. (2011) |
| Vigna mungo | 1 | Rhizobium spp. and R. tropici | 1 | Soil | Yes | NR | Badar and Qureshi (2012) | |
| Phaseolus vulgaris L. | 1 | R. leguminosarum | 1 | Soil/field | NR | Yes | Higher Fe levels under both field and glasshouse conditions | Ndakidemi et al. (2011) |
| Vigna unguiculata | 1 | B. japonicum | 1 | Soil/field | NR | Yes | Higher Fe levels under both field and glasshouse conditions | Nyoki and Ndakidemi (2014) |
| Cajanus cajan | 1 | Rhizobium spp. and Bradyrhizobium spp. | 20 | Inert | NR | Yes | Rhizobia capacity to produce siderophore shows a high correlation with plant Fe content | Duhan et al. (1998) |
| Cajanus cajan | 1 | Rhizobium spp. | 25 | Hydroponic | NR | Yes | High correlation between shoot Fe content and rhizobia siderophore production | Duhan (2013) |
| Lens culinaris | 1 | R. leguminosarum | 1 | Soil | NR | Yes | High correlation between shoot Fe content and rhizobia siderophore production | Mishra et al. (2011) |
| Phaseolus vulgaris | 1 | R. leguminosarum | 1 | Soil/field | Yes | NR | Higher shoot P content under field and glasshouse growth conditions, higher root P under greenhouse growth conditions | Makoi et al. (2013) |
NR, not reported.
However, while little is understood about how the legume–rhizobia symbiosis affects host roots, even less is understood about how the various distinct contributions of rhizobia to host metabolism, signalling, and nutrient acquisition sculpt host RSA, which remains an important open question.
Rhizobia can benefit their host by enhancing assimilation of P and Fe
Many rhizobia can acidify the rhizosphere to stimulate P and Fe solubilization, and/or produce high-affinity siderophores for Fe3+ (Table 2) (Duhan et al., 1998; reviewed in Qin et al., 2011; Jin et al., 2014). As these nutrients become more accessible to roots, they contribute to improved host growth (Orozco-Mosqueda et al., 2013; Geetha and Joshi, 2013; Imen et al., 2015). Thus, rhizobia with these traits have a greater impact on legume growth than those lacking them (Table 2) (Abril et al., 2007; Franzini et al., 2010). The advantages to the host in such symbioses were multiplied if amendments were provided to the crop (Table 2) (Peix et al., 2001; Qin et al., 2011). The beneficial effects of rhizobia on P and Fe acquisition and assimilation may be particularly pronounced in calcareous soils with high pH (Table 2) (Abbaszadeh-dahaji et al., 2012; Soumaya et al., 2016). In an interesting report, rhizobia increased P content in a soybean variety with a root system inefficient for P uptake (deep rooted) under low P conditions, but no changes were observed in a P-efficient system (shallow rooted) (Wang et al., 2011), showing that effects on RSA can be conditional on host genotype. Hence, specific rhizobial strains can significantly contribute to P nutrition in many species or varieties in different environmental conditions, especially when their root systems are not optimal for P acquisition.
Furthermore, several rhizobial strains, including some that increase P content, can also increase the uptake of Fe in hosts such as common bean, chickpea, and cowpea under different environmental conditions (Table 2) (Peix et al., 2001; Ndakidemi et al., 2011; Nyoki and Ndakidemi, 2014). The capacity to enhance Fe uptake is tightly correlated with the level and type of siderophore produced by the rhizobia (Table 2) (Duhan et al., 1998; Duhan, 2013). The beneficial effect of enhanced Fe uptake mediated by microbes associated with the host is not restricted to legumes: in non-legume species such as Zea mays L., Pseudomonas strains able to produce siderophores can also increase Fe content and remove signs of chlorosis (Sharma and Johri, 2003; Singh et al., 2017).
N2 fixation itself benefits from rhizobia-mediated stimulation of P and Fe uptake; for example, Fe is required in high quantities for the N-fixing enzyme nitrogenase and other symbiotic proteins (reviewed in Burton et al., 1998; O’Hara, 2001). Therefore, under Fe or P deficit, rhizobia that increase Fe and/or P levels fix more N2 than strains that do not, as reported for pigeon pea (Duhan et al., 1998; Duhan, 2013) and chickpea (Singh et al., 2014). Nodulation per se also increases Fe absorption: both N2-fixing and non-fixing rhizobia, as well as their siderophores, stimulate the uptake and transport of Fe to the shoot, with nodulation also enhancing root Fe-reductase activity (Derylo and Skorupska, 1992; reviewed in Jin et al., 2014). Thus, nodulation by some rhizobia strains contributes to plant P and Fe acquisition in a host-dependent manner, and is likely to have a major impact on host growth and metabolic capacity in conditions where these nutrients are limiting.
Rhizobia-enhanced N metabolism can modify host nutrient signalling pathways
Higher N availability due to a successful symbiosis is likely also to alter host signalling pathways (Fig. 3): in several non-legumes, NO3– induces the degradation of the host phosphate- and nitrate-responsive signalling repressor SPX4 (reviewed in Hu et al., 2019; Medici et al., 2019; Hu and Chu, 2020). SPX4 co-ordinates utilization of both macronutrients and plant growth, and hence any imbalance in the host will lead to enhanced acquisition of the limiting nutrient. In legumes, high NO3– availability may directly stimulate P acquisition through a similar mechanism to that seen in rice (Kamada-Nobusada et al., 2013). This function of SPX4 could plausibly explain the higher P levels observed in many nodulated legumes (e.g. resulting from increased P solubilization activity by the roots in N-sufficient plants) (Qin et al., 2011). Stimulation of metabolism and growth based on enhanced resource availability provides the basis for modified root system development (Kan et al., 2015), for example to promote root growth in the relatively P-rich topsoil layer, in addition to the effect of rhizobia on soil P solubilization.
Enhanced availability of limiting P and Fe (Table 2) enables elevated host metabolism, photosynthetic activity, and plant growth, as has been reported for alfalfa and Lotus for active symbioses (Li et al., 2013; Regus et al., 2015; Quides et al., 2017). However, these contributions by rhizobia that overcome limiting non N-nutrient levels in the host are likely to be limited to symbioses that fix high amounts of N2 (Belane et al., 2014).
Enhanced assimilation of non-N nutrients mediated by rhizobia affects root traits
Higher non-N nutrient absorption during interactions with rhizobia has been related to changes in root traits: in chickpea inoculated with a strain that solubilizes phosphate and produces siderophores, a higher root length was observed, and this effect was more pronounced when supplied with either insoluble or soluble phosphate (Singh et al., 2014). In Phaseolus vulgaris grown in low P soils, treatment with several P-solubilizing rhizobia increased root dry weight (Korir et al., 2017). In two alfalfa varieties grown with insoluble Ca3PO4, rhizobia enhance root length compared with plants treated with a nutrient solution, with or without N and P (Li et al., 2013). In pigeon pea, rhizobia that synthesize high levels of siderophores have increased root weight compared with strains that produce low levels and with non-inoculated plants (Duhan et al., 1998; Duhan, 2013). Finally, inoculation of peanut and pigeon pea with rhizobia expressing siderophore receptor genes in autoclaved and non-autoclaved soil resulted in increased root mass compared with their non-transformed parental lines in both soils (Arif et al., 2012). These findings suggest that higher non-N nutrient absorption due to rhizobia could have a much more important impact on overall host resource acquisition and partitioning than previously considered, particularly since most studies do not report root traits in detail.
In conclusion, many rhizobia increase P and Fe levels in legume species. A positive impact on plant nutrition and growth, and, in some instances, modification of root traits associated with high P and Fe content, have been observed. We propose that these modifications to the root systems will result from either a reduced need to forage for these nutrients or from more intensive exploration/exploitation if they result from higher host resource demands due to increased metabolism, which may lead to a different RSA. For example, if P is limiting, a rhizobium strain with low capacity to solubilize it will result in an increased exploitation of the topsoil, as seen in the non-legume Arabidopsis (Williamson et al., 2001; Péret et al., 2011), while a strain that solubilizes P will not lead to this change in RSA, since it already makes far more P available to the roots.
Rhizobia effects on plant growth regulator homeostasis modulate root development and resource acquisition
Rhizobia modulate plant growth regulator homeostasis
The symbiont and the host wrestle over their share of the resources available to the holobiont. As part of their arsenal, rhizobia have also evolved mechanisms to modulate host growth regulator homeostasis and signalling, thereby affecting its growth, development, and resource allocation. The best studied of these mechanisms are auxin and LCO biosynthesis, and ethylene signalling. Auxin is implicated in many aspects of plants, and specifically root development, nodulation and nodule development, N-mediated control of RSA, and nutrient acquisition (Pacios-Bras et al., 2003; Liu et al., 2018; Lagunas et al., 2019; reviewed in Sun et al., 2017). Ethylene negatively affects root development and nodulation (reviewed in Okazaki et al., 2004; Saleem et al., 2007). LCOs modulate auxin levels which can stimulate LR formation in legumes (Pacios-Bras et al., 2003), and in the non-legume Brachypodium distachyon (Buendia et al., 2019) (Fig. 1).
Importance of auxin-synthesizing rhizobia for root development
Many rhizobia produce the common auxin indole-3-acetic acid (IAA); in some cases, >90% of the strains that nodulate single host species produce it (Antoun et al., 1998; Vargas et al., 2009; Abbaszadeh-dahaji et al., 2012). In Vigna mungo and Melilotus alba, mature nodules have much higher IAA levels and decreased amounts of its catabolic enzymes than bulk roots; it has been suggested that this IAA might be transported to other tissues to modulate their functions and therefore impact C partitioning within the plant (Datta and Basu, 1998; Ghosh and Basu, 2006) (Fig. 1).
Inoculation of several mung bean (Vigna radiata) varieties with symbionts that produce high IAA levels in vitro increases root length and mass (Anjum et al., 2011). In M. truncatula and alfalfa, a high IAA-producing strain increased the length of the PR and resulted in higher LR development compared with the control, which itself positively correlated with increased nodule number (Pii et al., 2007). Inoculation with an IAA-overproducing strain leads to a higher production of LRs and a more developed M. truncatula and chickpea root system (Bianco and Defez, 2010; Bianco et al., 2014; Singh et al., 2014). The highest increase in shoot dry mass in nodulated P. vulgaris varieties is observed after inoculation with IAA-producing rhizobia; they might also stimulate bulk root development (Abbaszadeh-dahaji et al., 2012). Finally, in soybean, both auxin and nodulation increase the expression of miR167c, which positively regulates both nodulation and LR number and length (Wang et al., 2015).
However, rhizobia may also directly affect host auxin homeostasis: in non-legume species, root colonization (not nodulation) by rhizobia modifies root auxin signalling which also results in changes to RSA. In Arabidopsis, colonization by rhizobia leads to inhibition of PR growth and a 2-fold enhancement in the number of LRs, primarily through altering auxin signalling (Zhao et al., 2017). The same rhizobial strain has also been shown to increase IAA levels in rice roots, but their root system was not further analysed (Biswas et al., 2000).
Auxin effects on nutrient acquisition
Auxin is strongly associated with control of host metabolism and growth; therefore, it is not surprising that rhizobia-derived IAA is correlated with higher nutrient acquisition in symbioses. A high IAA-producing rhizobial strain, and roots of M. truncatula plants nodulated with it, secrete higher amounts of organic acids compared with its low IAA-producing progenitor strain, resulting in higher P solubilization and therefore absorption (Bianco and Defez, 2010). This strain, and other IAA-overproducing strains, also have increased nitrogenase expression (Imperlini et al., 2009; Bianco et al., 2014), which was highly correlated with increased shoot weight (Bianco et al., 2014). In Vicia hirsuta, inoculation with a strain that produces high IAA levels in nodules results in a 2-fold increase in N2 fixation, probably also due to higher nodule mass (Camerini et al., 2008). Similar findings were reported by Kaneshiro and Kwolek (1985) where inoculation of soybean plants with a high IAA-producing mutant resulted in enhanced N2 fixation compared with its parent strain. Finally, auxin also regulates plant responses to Fe deficiency, and microbial auxins enhance its absorption in legumes under low Fe conditions (reviewed in Jin et al., 2014).
Rhizobia can interfere with host ethylene synthesis and signalling to modulate nodule development
In many legume species, ethylene levels quickly increase in response to compatible LCO detection and repress nodule development; mutations in ethylene signalling pathways result in hyperinfected and hypernodulating plants (reviewed in Buhian and Bensmihen, 2018; Reid et al., 2018).
Rhizobia can decrease ethylene levels through two mechanisms: first, the production of the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase which metabolizes the ethylene precursor ACC (reviewed in Ahemad and Kibret, 2014), and, secondly, the synthesis of rhizobitoxine, which inhibits two enzymes required for ethylene biosynthesis upstream of ACC (Duodu et al., 1999; Yasuta et al., 1999; Yuhashi et al., 2000). Loss-of-function mutations of symbiont ACC deaminase or its decreased expression reduce nodulation, nodule development, and shoot biomass (Ma et al., 2003; Uchiumi et al., 2004). Furthermore, loss-of-function mutants in rhizobitoxine synthesis also have more aborted and fewer mature nodules (Duodu et al., 1999; Yasuta et al., 1999; Yuhashi et al., 2000). The addition of an ACC deaminase gene to rhizobial species that do not have it or have low activity of this enzyme greatly enhances their ACC deaminase activity (Ma et al., 2004; Tittabutr et al., 2008). These strains result in higher nodule number and shoot dry mass in alfalfa, and have improved competitiveness compared with their wild-type progenitors (Ma et al., 2004), as well as nodule number and size and root mass in Leucaena leucocephala (Tittabutr et al., 2008).
Thus, the competition between host and symbiont in controlling ethylene homeostasis at an early stage of the interaction is essential for an optimal symbiosis, and therefore for adequate resource allocation (Ma et al., 2003).
The observation of ACC deaminase activity only in differentiated rhizobia and not in free-living ones further supports this notion (Uchiumi et al., 2004). Furthermore, rhizobia that can modulate ethylene production can decrease its levels in roots of older plants when compared with controls (Yuhashi et al., 2000), raising the possibility that this is another mechanism by which rhizobia could modulate the root system. However, there are also reports where the loss of ACC deaminase does not affect nodulation or nodule development (Murset et al., 2012), suggesting that the sensitivity of the symbiosis to ethylene depends on the plant species.
Although regulation of host ethylene synthesis or signalling by rhizobia is essential for an optimal symbiosis, there is only limited evidence of how this can impact root development: only Tittabutr et al. (2008) report increased root mass in L. leucocephala when inoculated with rhizobia with high ACC deaminase activity. In contrast, most studies that report legume root traits use co-inoculation of rhizobia with other bacteria that can decrease ethylene production. In lentils, co-inoculation with R. leguminosarum and any of two ACC deaminase-producing Pseudomonas strains increased both root length and biomass in two different nutritional conditions (Iqbal et al., 2012). Also in this species, inoculation with either a putative Bacillus or Pseudomonas strain with high ACC deaminase activity increased root weight in seedlings (Saini and Khanna, 2013). Co-inoculating common bean with R. tropici and with a transformed endophyte, Serratia grimesii, expressing high levels of ACC deaminase, increases both root and shoot weight as well as nodule number, when compared with co-inoculation with a wild-type S. grimesii, which does not encode an endogenous ACC deaminase (Tavares et al., 2018).
Thus, rhizobia that possess such mechanisms to decrease ethylene synthesis can enhance the strength of the symbiosis, which is likely to stimulate its impact on legume root development and RSA (reviewed in Okazaki et al., 2004; Tittabutr et al., 2008).
Regulation of auxin and root development by LCOs
Through production of LCOs, rhizobia can enhance localized IAA content that stimulates LR formation in legumes (Olah et al., 2005; Herrbach et al., 2017) and non-legumes (Buendia et al., 2019). In soybean, LCO application also results in an increase in total root length and LR formation (Souleimanov et al., 2002). A higher allocation of shoot resources to the roots has been linked in M. truncatula to nodule initiation, which depends mostly on LCO detection (Goh et al., 2016). This may also be due to the high sink strength of the nodules, which in M. truncatula is higher than that of both leaves and roots (Jeudy et al., 2010). Finally, higher root biomass is reported at several developmental stages in pea after seeds were treated with LCOs, though this may also be due to increased shoot N and photosynthesis from more intense nodulation (Podleśny et al., 2014).
Rhizobia modulate holobiont metabolism, growth, and resource acquisition
The capacity of rhizobia to modulate host growth signalling pathways and nutrient acquisition by interfering with, for example, ethylene biosynthesis, changing IAA levels, and producing LCO, raises the possibility that a significant fraction of the effects of active symbioses on the host root system may be caused by these mechanisms rather than by N2 fixation itself. Such mechanisms would also result in strong reinforcement of the resource-based effects of symbioses due to the stimulation of host metabolism, which itself enhances nutrient acquisition. Thus, rhizobia probably influence plant C partitioning by increasing C allocation to roots through modifications in auxin and ethylene signalling, resulting in a modified RSA.
Molecular regulators of rhizobia-mediated modified legume RSA
There is significant conservation of resource-cued signalling mechanisms in LRs and nodule formation (Goh et al., 2016; Lagunas et al., 2019; Schiessl et al., 2019): plant growth regulators, metabolites that function both in signalling and metabolism, and mobile peptide signals and their cognate receptors are involved in both processes (Bensmihen, 2015). In this section, we discuss the role of these signalling mechanisms in nodulation and host root development, with a view to highlighting potential targets of host resource partitioning mechanisms.
Autoregulation of nodulation
In legumes, two shoot-expressed receptors independently and antagonistically regulate nodulation systemically by perceiving mobile signalling peptides produced in the root contingent on soil N availability and infection status (reviewed in Ferguson et al., 2010; Laffont et al., 2019; Nowak et al., 2019). In the autoregulation of nodulation (AON) pathway, SUPER NUMERIC NODULES (SUNN) in M. truncatula or its L. japonicus orthologue HYPERNODULATION ABERRANT ROOT FORMATION1 (HAR1) inhibit nodulation by detecting CLAVATA3/EMBRYO SURROUNDING REGION (CLE) peptides, while the likely receptor of C-TERMINALLY ENCODED PEPTIDE (CEP) peptides, the leucine-rich repeat receptor-like kinase (LRR-RLK) COMPACT ROOT ARCHITECTURE2 (CRA2), stimulates nodulation. Furthermore, in L. japonicus, HAR1 modulates nodulation by inhibiting shoot–root mobilization of miR2111 that represses the nodulation suppressor TOO MUCH LOVE (TML), and by enhancing CK synthesis through activation of ISOPENTENYL TRANSFERASE 3 (IPT3) and translocation to roots to suppress further nodulation events through their receptor LOTUS HISTIDINE KINASE1 (LHK1) (Tsikou et al., 2018). LHK1 also mediates TML responses in the root cortex (Miri et al., 2019). These pathways allow the plant to regulate resource investment into nodule production, and hence are also likely targets of resource partitioning mechanisms (Ito et al., 2007; Murray et al., 2017; Goh et al., 2019). A conceptual model that combines the known regulatory pathways of two legume models (M. truncatula and L. japonicus) is shown in Fig. 2.
AON components participate in N response and root development
CLE and CEP peptides have additional roles in regulating LR development, with different local and systemic effects depending on local soil N conditions (Fig. 2) (Araya et al., 2016; Sun et al., 2017; Taleski et al., 2018). A functional AON pathway is also required for roots to perceive, take up, and mobilize N as well as for normal root development (Schnabel et al., 2005; Lagunas et al., 2019). Goh et al. (2019) show that M. truncatula mutants with loss-of-function alleles of genes involved in regulation of nodulation, such as sunn, ROOT DETERMINED NODULATION 1 (rdn1), and LIKE SUNN SUPERNODULATOR (lss), also have altered biomass allocation, LR length, and density, similar to what Schnabel et al. (2005) have shown for sunn mutants. This is observed independently of nodulation, which exacerbates these differences due to increased resource competition between roots and nodules. Finally, in L. japonicus, the ROOT DETERMINED NODULATION 1 (RDN1) orthologue PLENTY and HAR1 regulate PR length, LR number, and development under both nodulated and uninoculated conditions (Yoro et al., 2019). This reveals a role for the CLE- and CEP-dependent pathway and the AON pathways in the control of C allocation in underground organs (roots and nodules) to acquire nutrients, primarily N (Fig. 2).
Some of these genes evolved in legumes from those required for N status-cued growth regulation, which would explain why they were co-opted into processes related to N acquisition (root development and nodulation). A further example is NIN, whose paralogues are NIN-like proteins that mediate nitrate responses in many plant species (Konishi and Yanagisawa, 2013; Suzuki et al., 2013). Another example is the CEP Receptor 1, which directly binds the AtCEP1 peptide to regulate N demand signalling, and is the LRR-RLK most closely related to CRA2 in Arabidopsis (Laffont et al., 2019). Finally, in Arabidopsis, CLE/CEP peptides and their receptors and downstream components have been characterized as important for LR development in response to N (reviewed in Sun et al., 2017; Liu et al., 2020), which suggests that in legumes some of these genes might regulate root development in response to nodulation. Thus, these genes participate in both root system development and nodulation, making it likely that rhizobia will impact root development, to some degree, through these mechanisms.
Rhizobia-mediated higher shoot nitrogen affects hormone translocation to roots
The effects of rhizobia on root development can also be attributed to higher shoot N levels in nodulated plants, leading to increased shoot–root auxin transport which intensifies LR development (van Noorden et al., 2006; Jin et al., 2012). In M. truncatula, this transport is essential to balance C allocation between shoot and roots in response to variable N availability: balancing C allocation for shoot and roots maintains growth homeostasis and depends on the AON gene SUNN (Jin et al., 2012; Goh et al., 2016). Higher leaf sucrose levels, both from increased photosynthesis and from elevated C sink strength from the nodules, also have the potential to alter RSA by stimulating auxin synthesis and transport to the roots (Sairanen et al., 2012; Liu et al., 2015).
Nitrate-starved shoots transport CKs to the root where they positively regulate LR development to acquire N (Ruffel et al., 2011). Since in many nodulated plants the shoot has higher levels of N than in non-nodulated plants (Regus et al., 2015; Goh et al., 2016), this may results in reduced levels of CK transported to the roots, leading to decreased LR development. Moreover, glutamine relays the nitrate-dependent induction of several genes that increase CK biosynthesis in shoots (Kamada-Nobusada et al., 2013), and also modulates both root growth and nodulation (reviewed in Mohd-Radzman et al., 2013). Thus, high glutamine levels from N2 fixation can have an impact on root growth and nodulation.
Finally, nodulation and LR development share an extensive overlap in their organogenesis and regulatory genes, and both processes share an auxin maximum in the developing organ (Schiessl et al., 2019). This further suggests how nodules evolved as modified roots specialized to acquire N, and how nodulation could alter the expression of genes and hormone levels that impact LR development via changes to resource homeostasis.
Measuring legume RSA in nodulated plants
A need to better understand changes in RSA and its dynamics due to rhizobia
As has been shown in this review, many studies report that rhizobia affect several aspects of legume root development in a species- and environment-dependent way (Franzini et al., 2010; Solaiman et al., 2011; Wang et al., 2011), but it is not fully understood how the symbiosis affects RSA due to technical and conceptual limitations. For example, symbioses with different rhizobial strains but similar root mass, length, or area may actually have very different RSA since each strain has a different impact on plant nutrition and C availability and partitioning. Furthermore, the lack of information regarding root growth dynamics may result in changes being masked because roots were not analysed over time (Khaitov et al., 2016; Kallala et al., 2018). Alternatively, it could also mean that some symbioses have a small impact on root development, or they do not result in a modified RSA (Wang et al., 2011; Argaw, 2012; Kallala et al., 2018).
The studies discussed in this review point to the involvement of a combination of altered fluxes of metabolites and signalling molecules that are responsible for changes in legume root development, and therefore possibly changes in RSA. To understand these processes with a view to make the host more resource-capture and utilization efficient, it is evident that detailed studies using systems that allow the study of legume RSA over a long period of time are required.
Systems to study RSA
Several systems are available to study root development without removing the roots from the soil, allowing study of their in situ RSA and growth dynamics. Agar plates are useful to study small root systems in controlled conditions (Laffont et al., 2019; Schiessl et al., 2019), while semi-hydroponic systems, where roots grow attached to a material such as cloth oriented vertically, exist of variable dimensions (15–120 cm) and have been used in many species (Chen et al., 2011, 2017; Lagunas et al., 2019). However, they lack the interaction of roots in soil that more faithfully reflects the natural abiotic and biotic environment in which they evolved (Morris et al., 2018).
The two most common systems used to image roots in soil and overcome its opacity are: (i) X-ray tomography or MRI; and (ii) rhizoboxes. The former systems scan pots up to 80×15 cm and reconstruct a 3D image of the roots, both thick and fine, but are very costly (Kumi, 2015; Metzner et al., 2015). In the latter, roots grow in thin layers of soil (2–40 mm) bordered by a transparent surface so that root development is easily captured with visible wavelength camera(s), and the system is usually inclined up to 45° to maximize visible roots (Nagel et al., 2012) (Fig. 4). However, it can also be placed at 0° if both sides are to be imaged (Rellan-Alvarez et al., 2015). They can be of considerable dimensions (up to 145×45 cm) (Bontpart et al., 2019, Preprint), allowing the study of legumes with large root systems and for long periods of time such as after flowering.
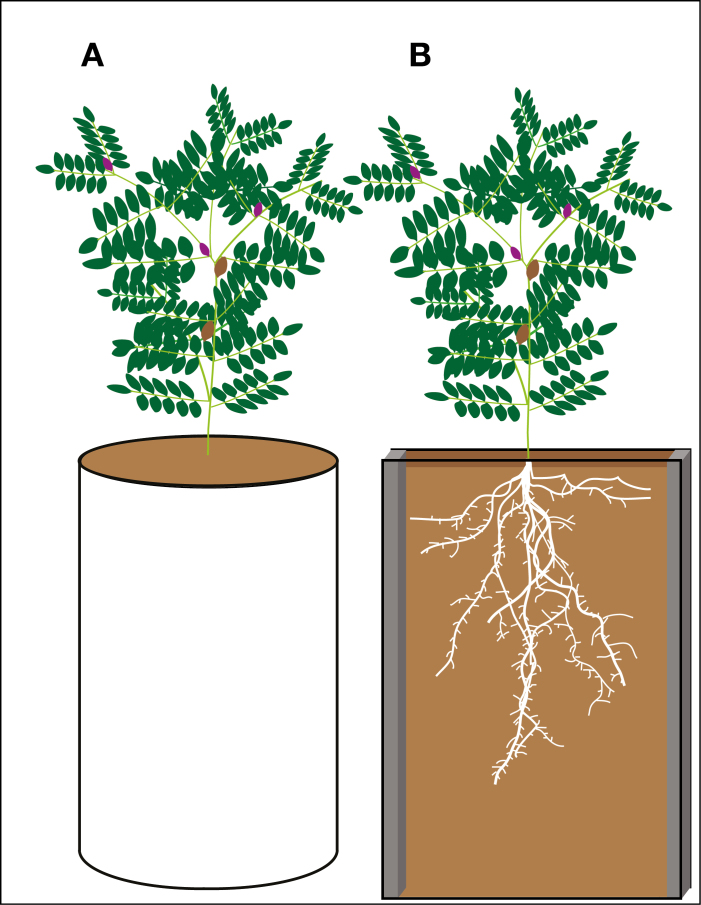
Fig. 4.
Pot and rhizobox growing systems. (A) Pots and long tubes have been extensively used to analyse roots of rhizobia-treated legumes since they are cheap and easy to use. Depending on size, roots develop as they would in a field, with some horizontal constraints. Roots need to be removed from the soil and washed for imaging, which leads to losses, alters their in situ distribution, and precludes repeated measurements on a single plant. (B) Rhizoboxes overcome these limitations and gather high-quality data to continuously analyse root system development and RSA, thereby allowing the study of its dynamics. They have varied dimensions, with at least one transparent side (usually of glass or plastic) that allows capture of root system distribution, either manually or with a camera, without removing them from the growth system. To increase root visibility, rhizoboxes are usually grown at an angle (up to 45°) and their thickness is limited so roots grow in a 2D-like manner. They are also more expensive and have different handling requirements compared with pots and tubes.
The use of these systems to study RSA in a variety of conditions in many symbioses will provide a better understanding of the role of rhizobia in altering legume RSA. For example, systems of larger dimensions allow for longer periods of unrestricted root growth where more evident changes in RSA could be observed. This would also include those that appear only late in the life cycle, specifically after flowering, when nodulation decreases leaf senescence and enhances C assimilation and allocation to roots (Kaschuk et al., 2010a; Li et al., 2016; da Costa Neto et al., 2017). It is not known whether free-living rhizobia in the rhizosphere also contribute to P and Fe acquisition and auxin biosynthesis; it is likely that they do, since they have these effects in gnotobiotic cultures, thus indicating that they are independent of their host (Duhan et al., 1998; Anjum et al., 2011; Qin et al., 2011). Therefore, these systems could determine if and how rhizobia associated with roots contribute to RSA in a significant way.
Time-lapse study of rhizobia–legume symbiosis RSA
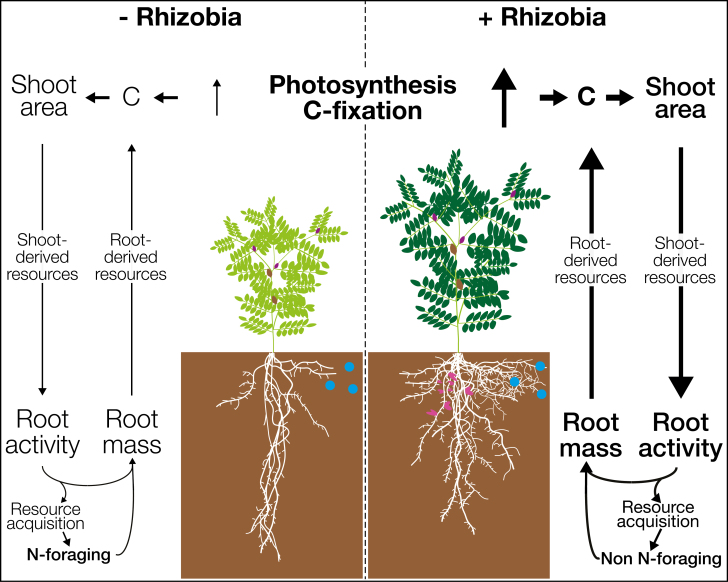
Information regarding how rhizobia modify legume RSA is limited; therefore, we hypothesize here how symbiosis might modify it. Assuming low soil N levels, so the symbiosis is highly beneficial, rhizobia symbiosis will increase plant N levels, photosynthesis, and shoot biomass (Harris et al., 1985; Quides et al., 2017). This will lead to an increase in root mass, length, and area, indicative of higher soil exploitation for resources to satisfy the demands of a larger shoot (Solaiman et al., 2011; Goh et al., 2016). Changes in lateral extension of the root system, and more intense exploitation of the explored soil (root area in the volume/area explored) may be observed due to the higher requirements for immobile resources such as P and reduced need to extensively explore the soil for diffusible N. Depending on water availability, changes in root depth may also be observed, along with higher exploitation of the top layers, a clear indication of a higher P demand. A modified RSA would reveal changes in C partitioning within the root system, indicating where shoot C is being invested underground to forage for resources. A model summarizing how rhizobia modify resource allocation and crosstalk between shoot and roots, and how these changes could affect root development and RSA is shown in Fig. 5.
Fig. 5.
Modification of RSA in legumes due to rhizobia. In low N soil conditions and with no compatible rhizobia (left), legumes need to forage for N themselves; consequently, roots only send relatively low quantities of root-derived resources (specifically N) to the shoot. This leads to low rates of photosynthesis and therefore low levels of C fixation, resulting in slow shoot growth. The shoot will in turn only send low amounts of shoot-derived resources and signals to the roots (e.g. C and auxin along with high levels of CKs). Here, these resources will preferentially be allocated to forage for more N since it limits photosynthesis. When compatible rhizobia are present (right), legumes will enter into symbiosis and produce nodules. These consume C to fix N2, leading to higher amounts of root-derived resources transported to the shoot (e.g. N, along with increased levels of P and Fe in some cases). As a result, photosynthesis, and therefore C fixation, will be considerably higher, leading to a larger shoot area. This will result in increased amounts of C and auxin but lower levels of CKs transported to below-ground organs (shoot-derived resources). Hence, the root system will invest proportionally fewer resources to forage for N and more to obtain water and non-N nutrients to satisfy the demand of the larger shoot. As a consequence, changes to root length/area, vertical distribution, and/or exploration/exploitation of different soil layers can be observed in nodulated legumes. Changes in shoot size, and hypothesized changes in RSA, are shown for the nodulated plant. Note how the nodulated plant exploits P deposits more intensively since its N demands (more critical than P demand) are satisfied to a greater extent. Lines and text in bold indicate greater intensity of a specific process. Nodules are indicated in pink, and P deposits are shown as blue circles.
These modifications will depend on the specific symbiosis, and different strains could result in distinct RSA responses in the same legume variety due to differences in many of their traits (e.g. N2 fixation dynamics, P solubilization, IAA production, or C consumption). For example, high auxin-producing strains could increase root C allocation, as well as length and area of the root system compared with strains that produce low IAA levels (Anjum et al., 2011). High IAA-producing strains will also enhance N and P nutrition which would further increase demand for soil resources and therefore root development (Bianco and Defez, 2010; Bianco et al., 2014). Furthermore, such strains could have an impact even when N levels are not low since this mechanism is independent of providing N to the plant. Similarly, strains that strongly acidify the soil would increase P and Fe nutrition (Abril et al., 2007), specifically useful in soil with high pH such as those reported by Soumaya et al. (2016). These symbioses should have a lower percentage of roots in top layers than those with strains with limited capacity to acidify the soil since they have less need to intensively exploit the soil for these immobile resources. Thus, they could have a higher lateral extension and depth due to the greater need for resources such as water to support growth of the increased shoot resulting from improved C and N fixation. On the other hand, strains that have high C consumption but fix low N levels, or do not fix at all, may possibly result in root systems with few and long PR and LRs since the plant needs to intensively forage the soil for N, and thus its RSA should be similar to that of non-nodulated plants.
Summary and conclusions
Legumes are important crops due to their ability to establish symbioses with rhizobia that allow them to fix N2. However, little is known about how they modify plant resource partitioning and root foraging strategies, and hence RSA. By positive feedback, highly active symbioses stimulate photo-assimilation due to globally enhanced host metabolic capacity. Modification of root traits (such as weight, length, and area) upon symbiosis depends on both partners as well as environmental conditions. Rhizobial symbioses can enhance not only host N but also macro- and micronutrient availability, specifically P and Fe, and some have the ability to additionally sculpt host growth and resource flux and partitioning by producing plant growth regulators such as auxins. Therefore, effective symbioses result in extensive and complex changes that permit the host to modify resource allocation patterns to roots such that its RSA optimizes acquisition of limiting soil resources. Hence, interactions with other microorganisms, such as mycorrhiza and free-living soil bacteria, might also impact RSA depending on which mechanisms and regulatory pathways are stimulated and to what degree. Therefore, RSA embodies host resource partitioning decision making, as well as rhizobia-modified legume nutrition, C flux, and hormonal signalling. This highlights the great utility of time-series experimental studies of RSA in nodulated and non-nodulated hosts to inform on resource partitioning mechanisms, required to develop more resilient and resource-efficient legume and non-legume crops.
Acknowledgements
PD thanks the Biotechnology and Biological Sciences Research Council (BBSRC) for funding (BB/P023487/1). CC thanks CONICYT PFCHA/DOCTORADO BECAS CHILE/2016-72170128 for a PhD scholarship. The authors thank two anonymous reviewers, whose comments resulted in an improved manuscript.
Glossary
Abbreviations
- ACC
1-aminocyclopropane-1-carboxylate
- AON
autoregulation of nodulation
- ASL18/LBD16
ASYMMETRIC LEAVES LIKE 18/LATERAL ORGAN BOUNDARIES DOMAIN 16
- CEP
C-TERMINALLY ENCODED PEPTIDE
- CK
cytokinin
- CLE
CLAVATA3/EMBRYO SURROUNDING REGION
- CRA2
COMPACT ROOT ARCHITECTURE2
- HAR1
HYPERNODULATION ABERRANT ROOT FORMATION1
- IAA
indole-3-acetic acid
- IPT3
ISOPENTENYL TRANSFERASE 3
- LCO
lipo-chitooligosacharide
- LHK1
LOTUS HISTIDINE KINASE1
- LR
lateral root
- LRR-RLK
leucine-rich repeat receptor-like kinase
- NF-Y
nuclear factor-Y
- NIN
NODULE INCEPTION PROTEIN
- PR
primary root
- RDN1
ROOT DETERMINED NODULATION 1
- RSA
root system architecture
- SUNN
SUPER NUMERIC NODULES
- TML
TOO MUCH LOVE
The authors declare no conflicts of interest.
References
- Abbaszadeh-dahaji P, Savaghebi GR, Asadi-rahmani H, Rejali F, Farahbakhsh M, Moteshareh-zadeh B, Omidvari M, Lindstrom K. 2012. Symbiotic effectiveness and plant growh promoting traits in some Rhizobium strains isolated from Phaseolus vulgaris L. Plant Growth Regulation 68, 361–370. [Google Scholar]
- Abril A, Zurdo-Piñeiro JL, Peix A, Rivas R, Velázquez E. 2007. Solubilization of phosphate by a strain of Rhizobium leguminosarum bv. trifolii isolated from Phaseolus vulgaris in El Chaco Arido soil (Argentina). In: Velázquez E, Rodríguez-Barrueco C, eds. First International Meeting on Microbial Phosphate Solubilization. Dordrecht: Springer Netherlands, 135–138. [Google Scholar]
- Ahemad M, Kibret M. 2014. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. Journal of King Saud University - Science 26, 1–20. [Google Scholar]
- Allito BB, Ewusi-Mensah N, Alemneh AA. 2015. Rhizobia strain and host–legume interaction effects on nitrogen fixation and yield of grain legume: a review. Molecular Soil Biology 6, 1–12. [Google Scholar]
- Anjum MA, Zahir Z, Arshad M, Ashraf M. 2011. Isolation and screening of rhizobia for auxin biosynthesis and growth promotion of mung bean (Vigna radiata L.) seedlings under axenic conditions. Soil and Environment 30, 18–26. [Google Scholar]
- Antoun H, Beauchamp CJ, Goussard N, Chabot R, Lalande R. 1998. Potential of Rhizobium and Bradyrhizobium species as plant growth promoting rhizobacteria on non-legumes: effect on radishes (Raphanus sativus L.). Plant and Soil 204, 57–67. [Google Scholar]
- Araya T, von Wirén N, Takahashi H. 2016. CLE peptide signaling and nitrogen interactions in plant root development. Plant Molecular Biology 91, 607–615. [DOI] [PubMed] [Google Scholar]
- Argaw A. 2012. Characterization of symbiotic effectiveness of rhizobia nodulating Faba bean (Vicia faba L.) isolated from central Ethiopia. Research Journal of Microbiology 7, 280–296. [Google Scholar]
- Arif K, Archana G, Anjana JD. 2012. Engineering heterologous iron siderophore complex utilization in rhizobia: effect on growth of peanut and pigeon pea plants. Applied Soil Ecology 53, 65–73. [Google Scholar]
- Arumugam R, Rajasekaran S, Nagarajan SM. 2010. Response of arbuscular mycorrhizal fungi and Rhizobium inoculation on growth and chlorophyll content of Vigna unguiculata (L) Walp Var. Pusa 151. Journal of Applied Science and Environmental Management 14, 113–115. [Google Scholar]
- Azcón-Bieto J. 1983. Inhibition of photosynthesis by carbohydrates in wheat leaves. Plant Physiology 73, 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badar R, Qureshi S. 2012. Comparative effect of Trichoderma hamatum and host-specific Rhizobium species on growth of Vigna mungo. Journal of Applied Pharmaceutical Science 2, 128–132. [Google Scholar]
- Bambara S, Ndakidemi P. 2009. Effects of Rhizobium inoculation, lime and molybdenum on photosynthesis and chlorophyll content of Phaseolus vulgaris L. African Journal of Microbiology Research 3, 791–798. [Google Scholar]
- Belane AK, Pule-Meulenberg F, Makhubedu TI, Dakora FD. 2014. Nitrogen fixation and symbiosis-induced accumulation of mineral nutrients by cowpea (Vigna unguiculata L. Walp.). Crop and Pasture Science 65, 250. [Google Scholar]
- Bensmihen S. 2015. Hormonal control of lateral root and nodule development in legumes. Plants 4, 523–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlenfalvay GJ, Abu-Shakra SS, Phillips DA. 1978. Interdependence of nitrogen nutrition and photosynthesis in Pisum sativum L: I. Effect of combined nitrogen on symbiotic nitrogen fixation and photosynthesis. Plant Physiology 62, 127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C, Defez R. 2010. Improvement of phosphate solubilization and Medicago plant yield by an indole-3-acetic acid-overproducing strain of Sinorhizobium meliloti. Applied and Environmental Microbiology 76, 4626–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C, Senatore B, Arbucci S, Pieraccini G, Defez R. 2014. Modulation of endogenous indole-3-acetic acid biosynthesis in bacteroids within Medicago sativa nodules. Applied and Environmental Microbiology 80, 4286–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopp A, Bennett MJ. 2019. Turning lateral roots into nodules. Science 366, 953–954. [DOI] [PubMed] [Google Scholar]
- Biswas J, Ladha J, Dazzo F. 2000. Rhizobia inoculation improves nutrient uptake and growth of lowland rice. Soil Science Society of America Journal 64, 1644–1650. [Google Scholar]
- Bontpart T, Concha C, Giuffrida V, et al. . 2019. Affordable and robust phenotyping framework to analyse root system architecture of soil-grown plants. bioRxiv, 573139. [Preprint]. [DOI] [PubMed] [Google Scholar]
- Bourion V, Rizvi SM, Fournier S, de Larambergue H, Galmiche F, Marget P, Duc G, Burstin J. 2010. Genetic dissection of nitrogen nutrition in pea through a QTL approach of root, nodule, and shoot variability. Theoretical and Applied Genetics 121, 71–86. [DOI] [PubMed] [Google Scholar]
- Buendia L, Maillet F, O’Connor D, van de-Kerkhove Q, Danoun S, Gough C, Lefebvre B, Bensmihen S. 2019. Lipo-chitooligosaccharides promote lateral root formation and modify auxin homeostasis in Brachypodium distachyon. New Phytologist 221, 2190–2202. [DOI] [PubMed] [Google Scholar]
- Buhian WP, Bensmihen S. 2018. Mini-review: nod factor regulation of phytohormone signaling and homeostasis during rhizobia–legume symbiosis. Frontiers in Plant Science 9, 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge J, Jochua CN, Bucksch A, Lynch JP. 2016. Legume shovelomics: high-throughput phenotyping of common bean (Phaseolus vulgaris L.) and cowpea (Vigna unguiculata subsp. unguiculata) root architecture in the field. Field Crops Research 192, 21–32. [Google Scholar]
- Burridge JD, Schneider HM, Huynh BL, Roberts PA, Bucksch A, Lynch JP. 2017. Genome-wide association mapping and agronomic impact of cowpea root architecture. Theoretical and Applied Genetics 130, 419–431. [DOI] [PubMed] [Google Scholar]
- Burton JW, Harlow C, Theil EC. 1998. Evidence for reutilization of nodule iron in soybean seed development. Journal of Plant Nutrition 21, 913–927. [Google Scholar]
- Camerini S, Senatore B, Lonardo E, Imperlini E, Bianco C, Moschetti G, Rotino GL, Campion B, Defez R. 2008. Introduction of a novel pathway for IAA biosynthesis to rhizobia alters vetch root nodule development. Archives of Microbiology 190, 67–77. [DOI] [PubMed] [Google Scholar]
- Carter AM, Tegeder M. 2016. Increasing nitrogen fixation and seed development in soybean requires complex adjustments of nodule nitrogen metabolism and partitioning processes. Current Biology 26, 2044–2051. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ghanem ME, Siddique KH. 2017. Characterising root trait variability in chickpea (Cicer arietinum L.) germplasm. Journal of Experimental Botany 68, 1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Dunbabin VM, Diggle AJ, Siddique KHM, Rengel Z. 2011. Development of a novel semi-hydroponic phenotyping system for studying root architecture. Functional Plant Biology 38, 355–363. [DOI] [PubMed] [Google Scholar]
- Coba de la Pena T, Fedorova E, Pueyo JJ, Lucas MM. 2017. The symbiosome: legume and rhizobia co-evolution toward a nitrogen-fixing organelle? Frontiers in Plant Science 8, 2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R, Tegeder M. 2012. Soybean ureide transporters play a critical role in nodule development, function and nitrogen export. The Plant Journal 72, 355–367. [DOI] [PubMed] [Google Scholar]
- da Costa Neto VP, Mendes JBS, de Araújo ASF, de Alcântara Neto F, Bonifacio A, Rodrigues AC. 2017. Symbiotic performance, nitrogen flux and growth of lima bean (Phaseolus lunatus L.) varieties inoculated with different indigenous strains of rhizobia. Symbiosis 73, 117–124. [Google Scholar]
- Datta C, Basu PS. 1998. Content of indoleacetic acid and its metabolism in root nodules of Melilotus alba. Folia Microbiologica 43, 427–430. [Google Scholar]
- de Dorlodot S, Forster B, Pagès L, Price A, Tuberosa R, Draye X. 2007. Root system architecture: opportunities and constraints for genetic improvement of crops. Trends in Plant Science 12, 474–481. [DOI] [PubMed] [Google Scholar]
- Derylo M, Skorupska A. 1992. Rhizobial siderophore as an iron source for clover. Physiologia Plantarum 85, 549–553. [Google Scholar]
- Desta Y, Kiros H, Yirga W. 2015. Inoculation, phosphorous and zinc fertilization effects on nodulation, yield and nutrient uptake of Faba bean (Vicia faba L.) grown on calcaric cambisol of semiarid Ethiopia. Journal of Soil Science and Environmental Management 6, 9–15. [Google Scholar]
- Duhan JS. 2013. Tn5 siderophore producing mutants of Rhizobium and its role in nitrogen fixation and iron uptake in pigeonpea. African Journal of Microbiology Research 7, 1459–1464. [Google Scholar]
- Duhan J, Dudeja SS, Khurana AL. 1998. Siderophore production in relation to N2 fixation and iron uptake in pigeon pea–rhizobium symbiosis. Folia Microbiologica 43, 421–426. [Google Scholar]
- Duodu S, Bhuvaneswari TV, Stokkermans TJW, Peters NK. 1999. A positive role for rhizobitoxine in Rhizobium–legume symbiosis. Molecular Plant-Microbe Interactions 12, 1082–1089. [Google Scholar]
- Egamberdieva D, Wirth S, Jabborova D, Rasanen LA, Liao H. 2017. Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. Journal of Plant Interactions 12, 100–107. [Google Scholar]
- Ferguson BJ, Indrasumunar A, Hayashi S, Lin MH, Lin YH, Reid DE, Gresshoff PM. 2010. Molecular analysis of legume nodule development and autoregulation. Journal of Integrative Plant Biology 52, 61–76. [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Mens C, Hastwell AH, Zhang M, Su H, Jones CH, Chu X, Gresshoff PM. 2019. Legume nodulation: the host controls the party. Plant, Cell & Environment 42, 41–51. [DOI] [PubMed] [Google Scholar]
- Franzini VI, Azcón R, Mendes FL, Aroca R. 2010. Interactions between Glomus species and Rhizobium strains affect the nutritional physiology of drought-stressed legume hosts. Journal of Plant Physiology 167, 614–619. [DOI] [PubMed] [Google Scholar]
- Fukushima A, Kusano M. 2014. A network perspective on nitrogen metabolism from model to crop plants using integrated ‘omics’ approaches. Journal of Experimental Botany 65, 5619–5630. [DOI] [PubMed] [Google Scholar]
- Gebril S, Seger M, Villanueva FM, Ortega JL, Bagga S, Sengupta-Gopalan C. 2015. Transgenic alfalfa (Medicago sativa) with increased sucrose phosphate synthase activity shows enhanced growth when grown under N2-fixing conditions. Planta 242, 1009–1024. [DOI] [PubMed] [Google Scholar]
- Geetha SJ, Joshi SJ. 2013. Engineering rhizobial bioinoculants: a strategy to improve iron nutrition. TheScientificWorldJournal 2013, 315890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Basu PS. 2006. Production and metabolism of indole acetic acid in roots and root nodules of Phaseolus mungo. Microbiological Research 161, 362–366. [DOI] [PubMed] [Google Scholar]
- Goh CH, Nicotra AB, Mathesius U. 2016. The presence of nodules on legume root systems can alter phenotypic plasticity in response to internal nitrogen independent of nitrogen fixation. Plant, Cell & Environment 39, 883–896. [DOI] [PubMed] [Google Scholar]
- Goh CH, Nicotra AB, Mathesius U. 2019. Genes controlling legume nodule numbers affect phenotypic plasticity responses to nitrogen in the presence and absence of rhizobia. Plant, Cell & Environment 42, 1747–1757. [DOI] [PubMed] [Google Scholar]
- Harris D, Pacovsky RS, Paul EA. 1985. Carbon economy of soybean–Rhizobium–Glomus associations. New Phytologist 101, 427–440. [DOI] [PubMed] [Google Scholar]
- Held M, Hou H, Miri M, et al. . 2014. Lotus japonicus cytokinin receptors work partially redundantly to mediate nodule formation. The Plant Cell 26, 678–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrbach V, Chirinos X, Rengel D, et al. . 2017. Nod factors potentiate auxin signaling for transcriptional regulation and lateral root formation in Medicago truncatula. Journal of Experimental Botany 68, 569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrbach V, Remblière C, Gough C, Bensmihen S. 2014. Lateral root formation and patterning in Medicago truncatula. Journal of Plant Physiology 171, 301–310. [DOI] [PubMed] [Google Scholar]
- Ho MD, Rosas JC, Brown KM, Lynch JP. 2005. Root architectural tradeoffs for water and phosphorus acquisition. Functional Plant Biology 32, 737– 748. [DOI] [PubMed] [Google Scholar]
- Hu B, Chu C. 2020. Nitrogen–phosphorus interplay: old story with molecular tale. New Phytologist 225, 1455–1460. [DOI] [PubMed] [Google Scholar]
- Hu B, Jiang Z, Wang W, et al. . 2019. Nitrate–NRT1.1B–SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nature Plants 5, 401–413. [DOI] [PubMed] [Google Scholar]
- Imen H, Neila A, Adnane B, Manel B, Mabrouk Y, Saidi M, Bouaziz S. 2015. Inoculation with phosphate solubilizing Mesorhizobium strains improves the performance of chickpea (Cicer arietinum L.) under phosphorus deficiency. Journal of Plant Nutrition 38, 1656– 1671. [Google Scholar]
- Imperlini E, Bianco C, Lonardo E, Camerini S, Cermola M, Moschetti G, Defez R. 2009. Effects of indole-3-acetic acid on Sinorhizobium meliloti survival and on symbiotic nitrogen fixation and stem dry weight production. Applied Microbiology and Biotechnology 83, 727–738. [DOI] [PubMed] [Google Scholar]
- Iqbal MA, Khalid M, Shahzad SM, Ahmad M, Soleman N, Akhtar N. 2012. Integrated use of Rhizobium leguminosarum, plant growth promoting rhizobacteria and enriched compost for improving growth, nodulation and yield of lentil (Lens culinaris Medik.). Chilean Journal of Agricultural Research 72, 104–110. [Google Scholar]
- Ito S, Norikuni O, Sueyoshi K, Ohyama T. 2007. Characteristics of initial growth of hypernodulation soybean mutants, NOD1-3, NOD2-4 and NOD3-7, affected by inoculation of bradyrhizobia and nitrate supply. Soil Science and Plant Nutrition 53, 66–71. [Google Scholar]
- Jeudy C, Ruffel S, Freixes S, et al. . 2010. Adaptation of Medicago truncatula to nitrogen limitation is modulated via local and systemic nodule developmental responses. New Phytologist 185, 817–828. [DOI] [PubMed] [Google Scholar]
- Jin CW, Ye YQ, Zheng SJ. 2014. An underground tale: contribution of microbial activity to plant iron acquisition via ecological processes. Annals of Botany 113, 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Watt M, Mathesius U. 2012. The autoregulation gene SUNN mediates changes in root organ formation in response to nitrogen through alteration of shoot-to-root auxin transport. Plant Physiology 159, 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallala N, M’sehli W, Jelali K, Kais Z, Mhadhbi H. 2018. Inoculation with efficient nitrogen fixing and indoleacetic acid producing bacterial microsymbiont enhance tolerance of the model legume Medicago truncatula to iron deficiency. BioMed Research International 2018, 9134716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada-Nobusada T, Makita N, Kojima M, Sakakibara H. 2013. Nitrogen-dependent regulation of de novo cytokinin biosynthesis in rice: the role of glutamine metabolism as an additional signal. Plant & Cell Physiology 54, 1881–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan CC, Chung TY, Juo YA, Hsieh MH. 2015. Glutamine rapidly induces the expression of key transcription factor genes involved in nitrogen and stress responses in rice roots. BMC Genomics 16, 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshiro T, Kwolek WF. 1985. Stimulated nodulation of soybeans by Rhizobium japonicum mutant (B-14075) that catabolizes the conversion of tryptophan to indol-3yl-acetic acid. Plant Science 42, 141–146. [Google Scholar]
- Karaca U, Uyanöz R. 2012. Effectiveness of native Rhizobium on nodulation and growth properties of dry bean (Phaseolus vulgaris L.). African Journal of Biotechnology 11, 8986–8991. [Google Scholar]
- Kaschuk G, Hungria M, Leffelaar PA, Giller KE, Kuyper TW. 2010a Differences in photosynthetic behaviour and leaf senescence of soybean (Glycine max [L.] Merrill) dependent on N2 fixation or nitrate supply. Plant Biology 12, 60–69. [DOI] [PubMed] [Google Scholar]
- Kaschuk G, Kuyper T, Leffelaar P, Hungria M, Giller K. 2009. Are rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biology and Biochemistry 41, 1233–1244. [Google Scholar]
- Kaschuk G, Leffelaar PA, Giller KE, Alberton O, Hungria M, Kuyper TW. 2010b Responses of legumes to rhizobia and arbuscular mycorrhizal fungi: a meta-analysis of potential photosynthate limitation of symbioses. Soil Biology & Biochemistry 42, 125–127. [Google Scholar]
- Kaschuk G, Yin X, Hungria M, Leffelaar PA, Giller KE, Kuyper TW. 2012. Photosynthetic adaptation of soybean due to varying effectiveness of N2 fixation by two distinct Bradyrhizobium japonicum strains. Environmental and Experimental Botany 76, 1–6. [Google Scholar]
- Kaur H, Peel A, Acosta K, Gebril S, Ortega JL, Sengupta-Gopalan C. 2019. Comparison of alfalfa plants overexpressing glutamine synthetase with those overexpressing sucrose phosphate synthase demonstrates a signaling mechanism integrating carbon and nitrogen metabolism between the leaves and nodules. Plant Direct 3, e00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaitov B, Kurbonov A, Abdiev A, Adilov M. 2016. Effect of chickpea in association with Rhizobium to crop productivity and soil fertility. Eurasian Journal of Soil Science 5, 105. [Google Scholar]
- Kohlen W, Ng JLP, Deinum EE, Mathesius U. 2018. Auxin transport, metabolism, and signalling during nodule initiation: indeterminate and determinate nodules. Journal of Experimental Botany 69, 229–244. [DOI] [PubMed] [Google Scholar]
- Konishi M, Yanagisawa S. 2013. Arabidopsis NIN-like transcription factors have a central role in nitrate signalling. Nature Communications 4, 1617. [DOI] [PubMed] [Google Scholar]
- Korir H, Mungai NW, Thuita M, Hamba Y, Masso C. 2017. Co-inoculation effect of rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Frontiers in Plant Science 8, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. 2015. Paradigm shift in plant growth control. Current Opinion in Plant Biology 25, 107–114. [DOI] [PubMed] [Google Scholar]
- Krapp A, David LC, Chardin C, Girin T, Marmagne A, Leprince AS, Chaillou S, Ferrario-Méry S, Meyer C, Daniel-Vedele F. 2014. Nitrate transport and signalling in Arabidopsis. Journal of Experimental Botany 65, 789–798. [DOI] [PubMed] [Google Scholar]
- Kucey RMN, Paul EA. 1982. Carbon flow, photosynthesis, and N2 fixation in mycorrhizal and nodulated faba beans (Vicia faba L.). Soil Biology and Biochemistry 14, 407–412. [Google Scholar]
- Kumi F. 2015. Review of applying X-ray computed tomography for imaging soil–root physical and biological processes. International Journal of Agricultural and Biological Engineering 8, 1–14. [Google Scholar]
- Laffont C, Huault E, Gautrat P, Endre G, Kalo P, Bourion V, Duc G, Frugier F. 2019. Independent regulation of symbiotic nodulation by the SUNN negative and CRA2 positive systemic pathways. Plant Physiology 180, 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas B, Achom M, Bonyadi-Pour R, et al. . 2019. Regulation of resource partitioning coordinates nitrogen and rhizobia responses and autoregulation of nodulation in Medicago truncatula. Molecular Plant 12, 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrainzar E, Gil-Quintana E, Seminario A, Arrese-Igor C, González EM. 2014. Nodule carbohydrate catabolism is enhanced in the Medicago truncatula A17–Sinorhizobium medicae WSM419 symbiosis. Frontiers in Microbiology 5, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JF, Zhang SQ, Huo P, Shi SL, Miao YY. 2013. Effect of phosphate solubilizing rhizobium and nitrogen fixing bacteria on growth of alfalfa seedlings under P and N deficient conditions. Pakistan Journal of Botany 45, 1557–1562. [Google Scholar]
- Li X, Zhao J, Tan Z, Zeng R, Liao H. 2015. GmEXPB2, a cell wall beta-expansin, affects soybean nodulation through modifying root architecture and promoting nodule formation and development. Plant Physiology 169, 2640–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YS, Liu XB, Wang GH, Yu ZH, Mathesius U, Liu JD, Herbert SJ, Jin J. 2016. Shift in origin of plant nitrogen alters carbon and nitrogen assimilation during reproductive stages of soybean grown in a Mollisol. Crop & Pasture Science 67, 872–880. [Google Scholar]
- Liu B, Wu J, Yang S, Schiefelbein J, Gan Y. 2020. Nitrate regulation of lateral root and root hair development in plants. Journal of Experimental Botany 71 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang C, Yang J, Yu N, Wang E. 2018. Hormone modulation of legume–rhizobial symbiosis. Journal of Integrative Plant Biology 60, 632–648. [DOI] [PubMed] [Google Scholar]
- Liu W, Han X, Zhan G, Zhao Z, Feng Y, Wu C. 2015. A novel sucrose-regulatory MADS-box transcription factor GmNMHC5 promotes root development and nodulation in soybean (Glycine max [L.] Merr.). International Journal of Molecular Sciences 16, 20657–20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2007. Roots of the second green revolution. Australian Journal of Botany 55, 493–512. [Google Scholar]
- Lynch JP, Wojciechowski T. 2015. Opportunities and challenges in the subsoil: pathways to deeper rooted crops. Journal of Experimental Botany 66, 2199–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Charles TC, Glick BR. 2004. Expression of an exogenous 1-aminocyclopropane-1-carboxylate deaminase gene in Sinorhizobium meliloti increases its ability to nodulate alfalfa. Applied and Environmental Microbiology 70, 5891–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Guinel FC, Glick BR. 2003. Rhizobium leguminosarum biovar viciae 1-aminocyclopropane-1-carboxylate deaminase promotes nodulation of pea plants. Applied and Environmental Microbiology 69, 4396–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makoi J, Bambara S, Ndakidemi PA. 2013. Rhizobium inoculation and the supply of molybdenum and lime affect the uptake of macroelements in common bean (‘P. vulgaris L.’) plants. Australian Journal of Crop Science 7, 784–793. [Google Scholar]
- Medeot DB, Paulucci NS, Albornoz AI, Fumero MV, Bueno MA, Garcia MB, Woelke MR, Okon Y, Dardanelli MS. 2010. Plant growth promoting rhizobacteria improving the legume–rhizobia symbiosis. In: Khan MS, Musarrat J, Zaidi A, eds. Microbes for legume improvement. Vienna: Springer Vienna, 473–494. [Google Scholar]
- Medici A, Krouk G. 2014. The primary nitrate response: a multifaceted signalling pathway. Journal of Experimental Botany 65, 5567–5576. [DOI] [PubMed] [Google Scholar]
- Medici A, Szponarski W, Dangeville P, et al. . 2019. Identification of molecular integrators shows that nitrogen actively controls the phosphate starvation response in plants. The Plant Cell 31, 1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzner R, Eggert A, van Dusschoten D, Pflugfelder D, Gerth S, Schurr U, Uhlmann N, Jahnke S. 2015. Direct comparison of MRI and X-ray CT technologies for 3D imaging of root systems in soil: potential and challenges for root trait quantification. Plant Methods 11, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miri M, Janakirama P, Huebert T, Ross L, McDowell T, Orosz K, Markmann K, Szczyglowski K. 2019. Inside out: root cortex-localized LHK1 cytokinin receptor limits epidermal infection of Lotus japonicus roots by Mesorhizobium loti. The New phytologist 222, 1523–1537. [DOI] [PubMed] [Google Scholar]
- Mishra PK, Bisht SC, Ruwari P, Joshi GK, Singh G, Bisht JK, Bhatt JC. 2011. Bioassociative effect of cold tolerant Pseudomonas spp. and Rhizobium leguminosarum-PR1 on iron acquisition, nutrient uptake and growth of lentil (Lens culinaris L.). European Journal of Soil Biology 47, 35–43. [Google Scholar]
- Mohd-Radzman NA, Djordjevic MA, Imin N. 2013. Nitrogen modulation of legume root architecture signaling pathways involves phytohormones and small regulatory molecules. Frontiers in Plant Science 4, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi S, Sheikhi J, Zarei M. 2013. Effects of arbuscular mycorrhizal fungi and Rhizobium on shoot and root growth of chickpea in a calcareous soil. International Journal of Agriculture: Research and Review 3, 381–385. [Google Scholar]
- Morris JL, Puttick MN, Clark JW, Edwards D, Kenrick P, Pressel S, Wellman CH, Yang Z, Schneider H, Donoghue PCJ. 2018. The timescale of early land plant evolution. Proceedings of the National Academy of Sciences, USA 115, E2274–E2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JD, Liu CW, Chen Y, Miller AJ. 2017. Nitrogen sensing in legumes. Journal of Experimental Botany 68, 1919–1926. [DOI] [PubMed] [Google Scholar]
- Murset V, Hennecke H, Pessi G. 2012. Disparate role of rhizobial ACC deaminase in root-nodule symbioses. Symbiosis 57, 43–50. [Google Scholar]
- Nagel KA, Putz A, Gilmer F, et al. . 2012. GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Functional Plant Biology 39, 891–904. [DOI] [PubMed] [Google Scholar]
- Ndakidemi P, Bambara S, Makoi J. 2011. Micronutrient uptake in common bean (‘Phaseolus vulgaris’ L.) as affected by rhizobium inoculation, and the supply of molybdenum and lime. Plant Omics Journal 4, 40–52. [Google Scholar]
- Nowak S, Schnabel E, Frugoli J. 2019. The Medicago truncatula CLAVATA3-LIKE CLE12/13 signaling peptides regulate nodule number depending on the CORYNE but not the COMPACT ROOT ARCHITECTURE2 receptor. Plant Signaling & Behavior 14, 1598730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyoki D, Ndakidemi PA. 2014. Influence of Bradyrhizobium japonicum and phosphorus on micronutrient uptake in cowpea. A case study of zinc (Zn), iron (Fe), copper (Cu) and manganese (Mn). American Journal of Plant Sciences 5, 427–435. [Google Scholar]
- O’Hara G. 2001. Nutritional constraints on root nodule bacteria affecting symbiotic nitrogen fixation: a review. Animal Production Science 41, 417–433. [Google Scholar]
- Okazaki S, Nukui N, Sugawara M, Minamisawa K. 2004. Rhizobial strategies to enhance symbiotic interactions: rhizobitoxine and 1-aminocyclopropane-1-carboxylate deaminase. Microbes and Environments 19, 99–111. [Google Scholar]
- Oláh B, Brière C, Bécard G, Dénarié J, Gough C. 2005. Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. The Plant Journal 44, 195–207. [DOI] [PubMed] [Google Scholar]
- Orozco-Mosqueda Mdel C, Macías-Rodríguez LI, Santoyo G, Farías-Rodríguez R, Valencia-Cantero E. 2013. Medicago truncatula increases its iron-uptake mechanisms in response to volatile organic compounds produced by Sinorhizobium meliloti. Folia Microbiologica 58, 579–585. [DOI] [PubMed] [Google Scholar]
- Pacios-Bras C, Schlaman HR, Boot K, Admiraal P, Langerak JM, Stougaard J, Spaink HP. 2003. Auxin distribution in Lotus japonicus during root nodule development. Plant Molecular Biology 52, 1169–1180. [DOI] [PubMed] [Google Scholar]
- Pampana S, Masoni A, Mariotti M, Ercoli L, Arduini I. 2016. Nitrogen fixation of grain legumes differs in response to nitrogen fertilisation. Experimental Agriculture 54, 66–82. [Google Scholar]
- Paul MJ, Foyer CH. 2001. Sink regulation of photosynthesis. Journal of Experimental Botany 52, 1383–1400. [DOI] [PubMed] [Google Scholar]
- Peix A, Rivas-Boyero AA, Mateos PF, Rodriguez-Barrueco C, Martinez-Molina E, Velazquez E. 2001. Growth promotion of chickpea and barley by a phosphate solubilizing strain of Mesorhizobium mediterraneum under growth chamber conditions. Soil Biology & Biochemistry 33, 103–110. [Google Scholar]
- Péret B, Clément M, Nussaume L, Desnos T. 2011. Root developmental adaptation to phosphate starvation: better safe than sorry. Trends in Plant Science 16, 442–450. [DOI] [PubMed] [Google Scholar]
- Petrásek J, Friml J. 2009. Auxin transport routes in plant development. Development 136, 2675–2688. [DOI] [PubMed] [Google Scholar]
- Pii Y, Crimi M, Cremonese G, Spena A, Pandolfini T. 2007. Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biology 7, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podleśny J, Wielbo J, Podleśna A, Kidaj D. 2014. The pleiotropic effects of extract containing rhizobial Nod factors on pea growth and yield. Central European Journal of Biology 9, 396–409. [Google Scholar]
- Poole P, Ramachandran V, Terpolilli J. 2018. Rhizobia: from saprophytes to endosymbionts. Nature reviews. Microbiology 16, 291–303. [DOI] [PubMed] [Google Scholar]
- Qin L, Jiang H, Tian J, Zhao J, Liao H. 2011. Rhizobia enhance acquisition of phosphorus from different sources by soybean plants. Plant and Soil 349, 25–36. [Google Scholar]
- Quides KW, Stomackin GM, Lee HH, Chang JH, Sachs JL. 2017. Lotus japonicus alters in planta fitness of Mesorhizobium loti dependent on symbiotic nitrogen fixation. PLos One 12, e0185568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar R. 2012. Growth effects of Rhizobium inoculation in some legume plants. International Journal of Current Science, 1–6. [Google Scholar]
- Regus JU, Gano KA, Hollowell AC, Sofish V, Sachs JL. 2015. Lotus hosts delimit the mutualism–parasitism continuum of Bradyrhizobium. Journal of Evolutionary Biology 28, 447–456. [DOI] [PubMed] [Google Scholar]
- Reid D, Liu H, Kelly S, Kawaharada Y, Mun T, Andersen SU, Desbrosses G, Stougaard J. 2018. Dynamics of ethylene production in response to compatible nod factor. Plant Physiology 176, 1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellan-Alvarez R, Lobet G, Lindner H, et al. . 2015. GLO-Roots: an imaging platform enabling multidimensional characterization of soil-grown root systems. eLife 4, e07597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas R, Peix A, Mateos PF, Trujillo ME, Martinez-Molina E, Velazquez E. 2006. Biodiversity of populations of phosphate solubilizing rhizobia that nodulate chickpea in different Spanish soils. Plant and Soil 287, 23–33. [Google Scholar]
- Ruffel S, Krouk G, Ristova D, Shasha D, Birnbaum KD, Coruzzi GM. 2011. Nitrogen economics of root foraging: transitive closure of the nitrate–cytokinin relay and distinct systemic signaling for N supply vs. demand. Proceedings of the National Academy of Sciences, USA 108, 18524–18529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini P, Khanna V. 2013. Preliminary screening for ACC-deaminase production by plant growth promoting rhizobacteria. Journal of Pure and Applied Microbiology 7, 573–576. [Google Scholar]
- Sairanen I, Novák O, Pěnčík A, Ikeda Y, Jones B, Sandberg G, Ljung K. 2012. Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. The Plant Cell 24, 4907–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M, Arshad M, Hussain S, Bhatti AS. 2007. Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. Journal of Industrial Microbiology & Biotechnology 34, 635–648. [DOI] [PubMed] [Google Scholar]
- Scheible W-R, Lauerer M, Schulze E-D, Caboche M, Stitt M. 1997. Accumulation of nitrate in the shoot acts as a signal to regulate shoot–root allocation in tobacco. The Plant Journal 11, 671–691. [Google Scholar]
- Schiessl K, Lilley JLS, Lee T, et al. . 2019. NODULE INCEPTION recruits the lateral root developmental program for symbiotic nodule organogenesis in Medicago truncatula. Current Biology 29, 3657–3668.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel E, Journet EP, de Carvalho-Niebel F, Duc G, Frugoli J. 2005. The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Molecular Biology 58, 809–822. [DOI] [PubMed] [Google Scholar]
- Schortemeyer M, Atkin O, McFarlane N, Evans J. 1999. The impact of elevated atmospheric CO2 and nitrate supply on growth, biomass allocation, nitrogen partitioning and N2 fixation of Acacia melanoxylon. Functional Plant Biology 26, 737–747. [Google Scholar]
- Sharma A, Johri BN. 2003. Growth promoting influence of siderophore-producing Pseudomonas strains GRP3A and PRS9 in maize (Zea mays L.) under iron limiting conditions. Microbiological Research 158, 243–248. [DOI] [PubMed] [Google Scholar]
- Sharma P, Sardana V, Kandola S. 2011. Response of groundnut (Arachis hypogaea L.) to Rhizobium inoculation. Libyan Agriculture Research Center Journal International 2, 101–104. [Google Scholar]
- Siczek A, Lipiec J, Wielbo J, Kidaj D, Szarlip P. 2014. Symbiotic activity of pea (Pisum sativum) after application of Nod factors under field conditions. International Journal of Molecular Sciences 15, 7344–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms EL, Taylor DL, Povich J, Shefferson RP, Sachs JL, Urbina M, Tausczik Y. 2006. An empirical test of partner choice mechanisms in a wild legume–rhizobium interaction. Proceedings of the Royal Society B: Biological Sciences 273, 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GP, Singh PL, Panwar AS. 2011. Response of groundnut (Arachis hypogaea) to biofertilizer, organic and inorganic sources of nutrient in north east India. Legume Research 34, 196–201. [Google Scholar]
- Singh R, Sah S, Singh N. 2017. Iron acquisition in maize (Zea mays L.) using Pseudomonas siderophore. 3Biotech 7121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Gupta G, Khare E, Behal KK, Arora N. 2014. Phosphate solubilizing rhizobia promote the growth of chickpea under buffering conditions. International Journal of Pure and Applied Mathematics 2, 97–106. [Google Scholar]
- Sofi P, Saba I, Amin Z. 2017. Root architecture and rhizobial inoculation in relation to drought stress response in common bean (Phaseolus vulgaris L.). Journal of Applied and Natural Science 9, 502–507. [Google Scholar]
- Solaiman A, Talukder MS, Rabbani MM. 2011. Influence of some Rhizobium strains on chickpea: nodulation, dry matter yield and nitrogen uptake. Bangladesh Journal of Microbiology 27, 61–64. [Google Scholar]
- Souleimanov A, Prithiviraj B, Smith DL. 2002. The major Nod factor of Bradyrhizobium japonicum promotes early growth of soybean and corn. Journal of Experimental Botany 53, 1929–1934. [DOI] [PubMed] [Google Scholar]
- Soumaya TH, Sana DF, Faysal BJ, Imran H. 2016. Effect of Rhizobium inoculation on growth and nutrient uptake of sulla (Hedysarum coronarium L.) grown in calcareous soil of northern Tunisia. Romanian Biotechnological Letters 21, 11779–11786. [Google Scholar]
- Soyano T, Kouchi H, Hirota A, Hayashi M. 2013. Nodule inception directly targets NF-Y subunit genes to regulate essential processes of root nodule development in Lotus japonicus. PLos Genetics 9, e1003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T, Shimoda Y, Kawaguchi M, Hayashi M. 2019. A shared gene drives lateral root development and root nodule symbiosis pathways in Lotus. Science 366, 1021–1023. [DOI] [PubMed] [Google Scholar]
- Stajković O, Delić D, Josic D, Kuzmanović Đ, Rasulić N, Knežević-Vukčević J. 2011. Improvement of common bean growth by co-inoculation with Rhizobium and plant growth-promoting bacteria. Romanian Biotechnological Letters 16, 5919–5926. [Google Scholar]
- Sun CH, Yu JQ, Hu DG. 2017. Nitrate: a crucial signal during lateral roots development. Frontiers in Plant Science 8, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki W, Konishi M, Yanagisawa S. 2013. The evolutionary events necessary for the emergence of symbiotic nitrogen fixation in legumes may involve a loss of nitrate responsiveness of the NIN transcription factor. Plant Signaling & Behavior 8, e25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleski M, Imin N, Djordjevic MA. 2018. CEP peptide hormones: key players in orchestrating nitrogen-demand signalling, root nodulation, and lateral root development. Journal of Experimental Botany 69, 1829–1836. [DOI] [PubMed] [Google Scholar]
- Tavares MJ, Nascimento FX, Glick BR, Rossi MJ. 2018. The expression of an exogenous ACC deaminase by the endophyte Serratia grimesii BXF1 promotes the early nodulation and growth of common bean. Letters in Applied Microbiology 66, 252–259. [DOI] [PubMed] [Google Scholar]
- Tian X, Doerner P. 2013. Root resource foraging: does it matter? Frontiers in Plant Science 4, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittabutr P, Awaya JD, Li QX, Borthakur D. 2008. The cloned 1-aminocyclopropane-1-carboxylate (ACC) deaminase gene from Sinorhizobium sp. strain BL3 in Rhizobium sp. strain TAL1145 promotes nodulation and growth of Leucaena leucocephala. Systematic and Applied Microbiology 31, 141–150. [DOI] [PubMed] [Google Scholar]
- Tsikou D, Yan Z, Holt DB, Abel NB, Reid DE, Madsen LH, Bhasin H, Sexauer M, Stougaard J, Markmann K. 2018. Systemic control of legume susceptibility to rhizobial infection by a mobile microRNA. Science 362, 233–236. [DOI] [PubMed] [Google Scholar]
- Uchiumi T, Ohwada T, Itakura M, et al. . 2004. Expression islands clustered on the symbiosis island of the Mesorhizobium loti genome. Journal of Bacteriology 186, 2439–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, et al. . 2013. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature Genetics 45, 1097–1102. [DOI] [PubMed] [Google Scholar]
- Vance CP. 2001. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiology 127, 390–397. [PMC free article] [PubMed] [Google Scholar]
- van Noorden GE, Ross JJ, Reid JB, Rolfe BG, Mathesius U. 2006. Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant. Plant Physiology 140, 1494–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas LK, Lisboa BB, Schlindwein G, Granada CE, Giongo A, Beneduzi A, Passaglia LMP. 2009. Occurrence of plant growth-promoting traits in clover-nodulating rhizobia strains isolated from different soils in Rio Grande Do Sul state. Revista Brasileira de Ciência do Solo 33, 1227–1235. [Google Scholar]
- Wang X, Pan Q, Chen F, Yan X, Liao H. 2011. Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on soybean growth as related to root architecture and availability of N and P. Mycorrhiza 21, 173–181. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li K, Chen L, et al. . 2015. MicroRNA167-directed regulation of the auxin response factors GmARF8a and GmARF8b is required for soybean nodulation and lateral root development. Plant Physiology 168, 984–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoek A, Field E, Rehling F, Mulley G, Webb I, Poole PS, Turnbull LA. 2017. Policing the legume–Rhizobium symbiosis: a critical test of partner choice. Scientific Reports 7, 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LC, Ribrioux SP, Fitter AH, Leyser HM. 2001. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiology 126, 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao TT, Schilderink S, Moling S, Deinum EE, Kondorosi E, Franssen H, Kulikova O, Niebel A, Bisseling T. 2014. Fate map of Medicago truncatula root nodules. Development 141, 3517–3528. [DOI] [PubMed] [Google Scholar]
- Yan Z, Eziz A, Tian D, Li X, Hou X, Peng H, Han W, Guo Y, Fang J. 2019. Biomass allocation in response to nitrogen and phosphorus availability: insight from experimental manipulations of Arabidopsis thaliana. Frontiers in Plant Science 10, 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhao Q, Li X, Ai W, Liu D, Qi W, Zhang M, Yang C, Liao H. 2017. Characterization of genetic basis on synergistic interactions between root architecture and biological nitrogen fixation in soybean. Frontiers in Plant Science 8, 1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuta T, Satoh S, Minamisawa K. 1999. New assay for rhizobitoxine based on inhibition of 1-aminocyclopropane-1-carboxylate synthase. Applied and Environmental Microbiology 65, 849–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoro E, Nishida H, Ogawa-Ohnishi M, Yoshida C, Suzaki T, Matsubayashi Y, Kawaguchi M. 2019. PLENTY, a hydroxyproline O-arabinosyltransferase, negatively regulates root nodule symbiosis in Lotus japonicus. Journal of Experimental Botany 70, 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhashi K, Ichikawa N, Ezura H, Akao S, Minakawa Y, Nukui N, Yasuta T, Minamisawa K. 2000. Rhizobitoxine production by Bradyrhizobium elkanii enhances nodulation and competitiveness on Macroptilium atropurpureum. Applied and Environmental Microbiology 66, 2658–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CZ, Huang J, Gyaneshwar P, Zhao D. 2017. Rhizobium sp. IRBG74 alters Arabidopsis root development by affecting auxin signaling. Frontiers in Microbiology 8, 2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X-J, Liang Y, Chen H, Shen S-H, Jing Y-X. 2006. Effects of rhizobia inoculation and nitrogen fertilization on photosynthetic physiology of soybean. Photosynthetica 44, 530–535. [Google Scholar]