Light exposure tunes circadian rhythms, which modulate the immune response and affect susceptibility to infection in plants and animals. Though molecular responses to light are defined for model plant and animal hosts, analogous pathways that function in bacterial pathogens are understudied. We examined the response to light exposure in biofilms (matrix-encased multicellular assemblages) of the nonphotosynthetic bacterium Pseudomonas aeruginosa. We found that light at intensities that are not harmful to human cells inhibited biofilm maturation via effects on cellular signals. Because biofilm formation is a critical factor in many types of P. aeruginosa infections, including burn wound infections that may be exposed to light, these effects could be relevant for pathogenicity.
KEYWORDS: PAS domain, Pseudomonas aeruginosa, biofilms, cyclic di-GMP, light, phenazines, photosensing, pyocyanin
ABSTRACT
Light is known to trigger regulatory responses in diverse organisms, including slime molds, animals, plants, and phototrophic bacteria. However, light-dependent processes in nonphototrophic bacteria, and those of pathogens in particular, have received comparatively little research attention. In this study, we examined the impact of light on multicellular development in Pseudomonas aeruginosa, a leading cause of biofilm-based bacterial infections. We grew P. aeruginosa strain PA14 in a colony morphology assay and found that growth under prolonged exposure to low-intensity blue light inhibited biofilm matrix production and thereby the formation of vertical biofilm structures (i.e., “wrinkles”). Light-dependent inhibition of biofilm wrinkling was correlated with low levels of cyclic di-GMP (c-di-GMP), consistent with the role of this signal in stimulating matrix production. A screen of enzymes with the potential to catalyze c-di-GMP synthesis or degradation identified c-di-GMP phosphodiesterases that contribute to light-dependent inhibition of biofilm wrinkling. One of these, RmcA, was previously characterized by our group for its role in mediating the effect of redox-active P. aeruginosa metabolites called phenazines on biofilm wrinkle formation. Our results suggest that an RmcA sensory domain that is predicted to bind a flavin cofactor is involved in light-dependent inhibition of wrinkling. Together, these findings indicate that P. aeruginosa integrates information about light exposure and redox state in its regulation of biofilm development.
IMPORTANCE Light exposure tunes circadian rhythms, which modulate the immune response and affect susceptibility to infection in plants and animals. Though molecular responses to light are defined for model plant and animal hosts, analogous pathways that function in bacterial pathogens are understudied. We examined the response to light exposure in biofilms (matrix-encased multicellular assemblages) of the nonphotosynthetic bacterium Pseudomonas aeruginosa. We found that light at intensities that are not harmful to human cells inhibited biofilm maturation via effects on cellular signals. Because biofilm formation is a critical factor in many types of P. aeruginosa infections, including burn wound infections that may be exposed to light, these effects could be relevant for pathogenicity.
INTRODUCTION
Most organisms experience some degree of light exposure, which is habitat specific and temporally affected by Earth’s rotation. Light quality and intensity directly influence the growth of phototrophic bacteria, which are known to regulate gene expression in response to these parameters. However, light can also affect regulation in chemotrophic bacteria, serving as a proxy for correlating conditions such as those found inside animal hosts or at specific depths in freshwater environments (1). While light-dependent regulation has been described in detail for model phototrophs (1), similar mechanisms that may function in bacteria that do not use light as a source of energy are understudied (2–5).
Some investigation of photosensitivity in chemotrophs has been prompted by the relatively recent identification of light-sensing proteins in diverse nonphototrophic bacteria. In these photosensory proteins, light alters the chemical properties of cofactors, such as flavin or bilin derivatives, and changes in cofactor domains influence interactions with effector domains or other proteins with regulatory functions (6). Such interactions can induce global changes in gene expression and modulate bacterial development, sociality, and behavior. In the plant pathogen Xanthomonas campestris, for example, red light inhibits exopolysaccharide excretion, biofilm development, and virulence via a bacteriophytochrome that binds a bilin cofactor (7). Similar effects have been observed in the hospital-acquired pathogen Pseudomonas aeruginosa, which contains a bacteriophytochrome that participates in a phosphorelay linking light sensing to pathways regulated by quorum sensing (8). In Caulobacter crescentus, Bacillus subtilis, and Brucella abortus, photosensory proteins link the effects of blue light on FMN cofactors to regulation of adhesion, the general stress response, and pathogenicity (9–11). Furthermore, in both chemotrophs and phototrophs, photosensory domains are often found in proteins that are predicted to bind, synthesize, or degrade the signaling molecule cyclic di-GMP (c-di-GMP), underscoring the potential for light to affect multicellularity and motility (12–16).
The formation of biofilms can contribute to virulence and the persistence of bacterial and fungal infections. Exposure to light has been investigated as a form of therapy that negatively affects bacterial survival and has been shown to disrupt the integrity of biofilms formed by diverse nosocomial species, including P. aeruginosa, Staphylococcus aureus, and Acinetobacter baumannii (17–21). Exposure to blue light in particular has also been shown to decrease survival of P. aeruginosa in liquid batch cultures (17, 22). However, these studies focused on the detrimental effects of light on chemotrophic bacteria when it was applied at relatively strong intensities, i.e., on the order of 150 to 600 W/m2. In contrast, most of the studies examining light-dependent regulation of gene expression and behavior in phototrophs, as well as only a limited number of studies examining these processes in chemotrophs (1), have demonstrated responses at light intensities of less than 10 W/m2 (8, 23) or less than 100 μmol photons m−2 s−1 (2–5).
A major goal of research in our laboratory is to define the physiological responses of P. aeruginosa biofilms to different growth conditions and the traits that are relevant for host colonization. These studies have led us to examine the metabolism of phenazines, compounds excreted by P. aeruginosa that shuttle electrons from cells in hypoxic biofilm subzones to oxygen available closer to the biofilm periphery (24). Phenazine production balances the intracellular redox state and influences biofilm morphogenesis by inhibiting matrix production (25–27). We have also shown that biofilm-specific phenazine synthesis is required for full virulence in a mouse lung infection model (28). In the study described here, we investigated the effects of another environmental cue—low light exposure—on biofilm morphogenesis in P. aeruginosa and found that, at low intensities comparable to those controlling processes in phototrophs, specific wavelengths of light inhibited biofilm structure development. Our results indicate that light, like phenazines, modulates biofilm formation via c-di-GMP-dependent mechanisms. We propose a model for the integrated roles of cellular redox state and c-di-GMP in the response of P. aeruginosa biofilms to light.
RESULTS AND DISCUSSION
Light delays wrinkling in P. aeruginosa PA14 colony biofilms.
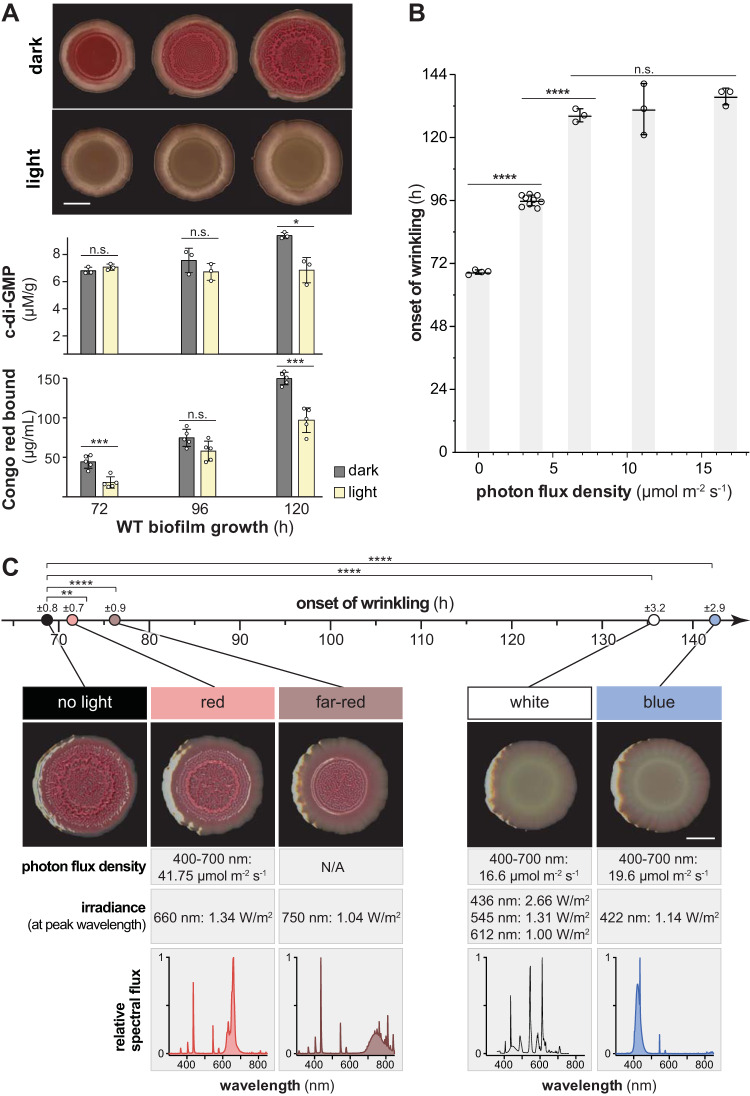
We use a standardized colony morphology assay to study the physiology of biofilm development in P. aeruginosa PA14 (27, 29). In this assay, liquid-culture aliquots are spotted onto an agar-solidified medium containing tryptone and the dyes Congo red and Coomassie blue, followed by incubation under controlled conditions in the dark. On the third day of growth, i.e., at ∼66 to 72 h, wild-type (WT) PA14 biofilms form a circular ridge at the boundary of the original culture droplet. Over the next 24 to 36 h, a whorled wrinkle pattern forms inside this ridge, and short spoke-like wrinkles form that emanate from the ridge toward the edge of the colony (Fig. 1A, top; see also Movies S1 and S2 in the supplemental material) (27). To test the effect of light on PA14 biofilm development, we grew wild-type biofilms under white broad-spectrum light at ∼16 μmol photons m−2 s−1, an intensity that is roughly 10-fold lower than that of sunlight on a winter day in northern Europe (30). This light intensity is lower, by at least an order of magnitude, than the intensities used in the majority of prior studies on light treatment of bacterial biofilms (19–21, 31). We found that biofilm wrinkling was delayed by ∼60 h under these conditions (Fig. 1A, top; see Movies S3 and S4).
FIG 1.
Blue light delays the onset of vertical structure (wrinkle) formation in P. aeruginosa PA14 colony biofilms. Biofilms were grown on colony morphology assay medium in various light conditions. (A, top) WT biofilms grown in the dark or under white fluorescent light at ∼16.5 μmol photons m−2 s−1. Representative biofilms for at least three independent experiments are shown. Scale bar, 5 mm. (Middle) c-di-GMP extracted from biofilms grown in the dark or light and normalized to dry biomass. Each bar represents the mean from three biological replicates (circles), and error bars show the standard deviations. (Bottom) Congo red bound by biofilms grown in the dark or light and harvested at the indicated time points. Each bar represents the mean of five biological replicates (circles), and error bars show the standard deviations. (B) Onset of wrinkling for WT biofilms grown in white fluorescent light at different intensities. Light intensity was measured as the photon flux density between 400 and 700 nm. Each bar shows the mean from at least three biological replicates (circles), and error bars indicate the standard deviations. (C) Onset of wrinkling for WT biofilms grown in the dark or under the light sources indicated (relative spectral flux shown in bottom row). Images are representative of the 120-h time point, and the scale bar is 5 mm. Means of at least three biological replicates per light source are represented by circles (timeline, top). The standard deviation is given above each circle. P values were determined using unpaired two-tailed t tests (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001; n.s., not significant).
P. aeruginosa PA14 biofilm wrinkling is a consequence of matrix secretion, which is stimulated by the cellular signal c-di-GMP (32, 33). Because the dye Congo red binds biofilm matrix (29), the pale coloration of light-exposed biofilms compared to those grown in the dark suggested that the delay in wrinkling we observed resulted from lower levels of matrix production. We confirmed this by performing a quantitative Congo red binding assay with biofilms grown on a dye-free medium (Fig. 1A, bottom). To examine whether the effect of light on matrix production and biofilm structure formation might be mediated by c-di-GMP, we measured c-di-GMP levels in biofilms grown in the presence or absence of broad-spectrum white light (Fig. 1A, middle; see Fig. S1A, left panels). c-di-GMP measurements were normalized to colony biofilm dry weights. Quantifications of colony biofilm dry weight and total protein content (Fig. S1, left panels) both showed that the effects of light on c-di-GMP levels were not reflected in effects on growth. Biofilms grown under light showed lower c-di-GMP levels than those grown in the dark, suggesting that the effect of light exposure on biofilm development is mediated by pathways that control c-di-GMP levels.
Next, we tested whether the intensity or quality of light affected the onset of biofilm wrinkling by subjecting biofilms to a range of intensities of white broad-spectrum light, or growing them under distinct light spectra, and then carrying out time-lapse imaging. The onset of wrinkling was identified using an automated computational algorithm and also subjected to unbiased assessment through scoring by two independent researchers. Biofilms grown at a white broad-spectrum light flux density of ∼3.5 μmol photons m−2 s−1 showed a delayed, intermediate onset of wrinkling, while most of those grown at intensities of ∼7 μmol photons m−2 s−1 and higher did not wrinkle until the end of the experimental time frame at ∼130 h (Fig. 1B). Biofilms grown under blue light showed a pronounced inhibition of wrinkling, while those grown under red or far-red light showed minor delays in the onset of wrinkling (Fig. 1C). Together, these results support a model in which blue light promotes c-di-GMP degradation and/or inhibits c-di-GMP synthesis, thereby inhibiting matrix production and promoting biofilm smoothness.
The phenazine pyocyanin is required for pronounced inhibition of biofilm wrinkling in the presence of light.
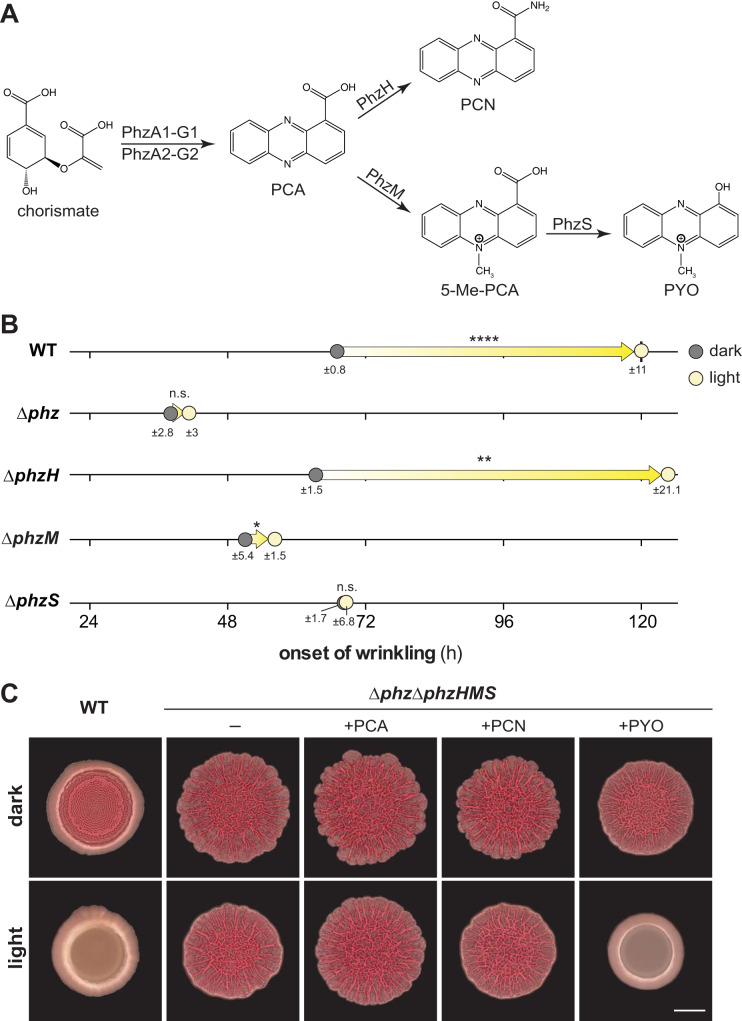
Phenazine production is a key factor that determines the morphogenetic development of PA14 colony biofilms (34). In P. aeruginosa, the phenazine biosynthetic pathway yields phenazine-1-carboxylic acid (PCA), phenazine-1-carboxamide (PCN), and pyocyanin (PYO) (Fig. 2A). 5-Methylphenazine-1-carboxylic acid (5-Me-PCA) is an unstable intermediate between PCA and PYO that can be nonenzymatically converted to other methylated derivatives, such as aeruginosins, under distinct conditions (34–40). When grown in the dark, phenazine-null (Δphz) biofilms showed a much earlier onset of wrinkling, at ∼40 h of incubation, than those formed by the wild type (Fig. 2B; see Movies S1 and S2) (27, 41). We have previously shown that the natural phenazines 5-Me-PCA and PYO and the synthetic phenazine PMS are all sufficient to inhibit PA14 biofilm wrinkle formation in the absence of light (34, 41). Therefore, it is specifically methylated phenazines that inhibit wrinkling in the dark.
FIG 2.
Pyocyanin (PYO) is required for pronounced inhibition of colony biofilm wrinkling in the light. (A) Biosynthetic pathway for phenazine production in P. aeruginosa. PCA, phenazine-1-carboxylic acid; PCN, phenazine-1-carboxamide; 5-Me-PCA, 5-methyl-phenazine-1-carboxylic acid; PYO, pyocyanin. (B) Onset of wrinkling for colony biofilms of PA14 WT and the indicated mutants when grown on colony morphology assay medium. Circles represent averages in the onset of wrinkling for at least three biological replicates, and the standard deviation is listed below each circle. Arrows designate difference in onset of wrinkling in the light compared to dark. P values were determined using unpaired two-tailed t tests and are listed atop each arrow (*, P ≤ 0.05; **, P ≤ 0.01; ****, P ≤ 0.0001; n.s., not significant). (C) Biofilms of PA14 WT or the Δphz ΔphzHMS mutant grown on colony morphology assay medium amended with 200 μM concentrations of the phenazines PCA, PCN, and PYO as indicated. Images shown were taken at the 96-h time point. Scale bar, 5 mm.
Because specific phenazines critically affect the morphogenesis of PA14 biofilms grown in the absence of light, we sought to test the hypothesis that they contribute to the light-dependent effects on biofilm development. We grew phenazine biosynthetic mutants in the presence or absence of light and monitored the onset of biofilm wrinkling using time-lapse imaging. We observed that pronounced light-dependent inhibition of wrinkling—in the standardized colony morphology assay—required PYO production (Fig. 2B; see Movies S3 and S4). The ΔphzS mutant showed no effect of light on the onset of wrinkling, which indicates that 5-Me-PCA and its derivatives other than PYO are not sufficient to mediate the light-dependent delay in wrinkling seen in the wild type when biofilms are grown in this assay.
To test whether PYO alone is sufficient to mediate light-dependent inhibition of biofilm wrinkling, we grew a Δphz ΔphzHMS mutant, which is not able to produce or modify phenazines, on colony morphology assay medium containing one of the exogenously provided phenazines PCA, PCN, and PYO. We found that PYO is sufficient to inhibit wrinkling in the presence of light (Fig. 2C). Δphz ΔphzHMS biofilms grown under all other conditions, including those grown in the absence of phenazines, showed minor yet reproducible effects of light on colony morphology; most notably, light appeared to cause a “smoothing” of the outer rim of the biofilm. However, none of these effects were as dramatic as that of PYO. These results suggest that, although 5-Me-PCA production is sufficient to delay the onset of wrinkling in biofilms grown in the dark, the delay caused by light is specifically mediated by PYO. In addition to their differences in stability and redox potential, 5-Me-PCA and PYO differ in charge and hydrophobicity, properties that may affect their reactivity with bound cofactors such as flavins and that may account for their differential roles in dark- and light-grown biofilms (34).
Growth in the light does not affect PYO production or redox state.
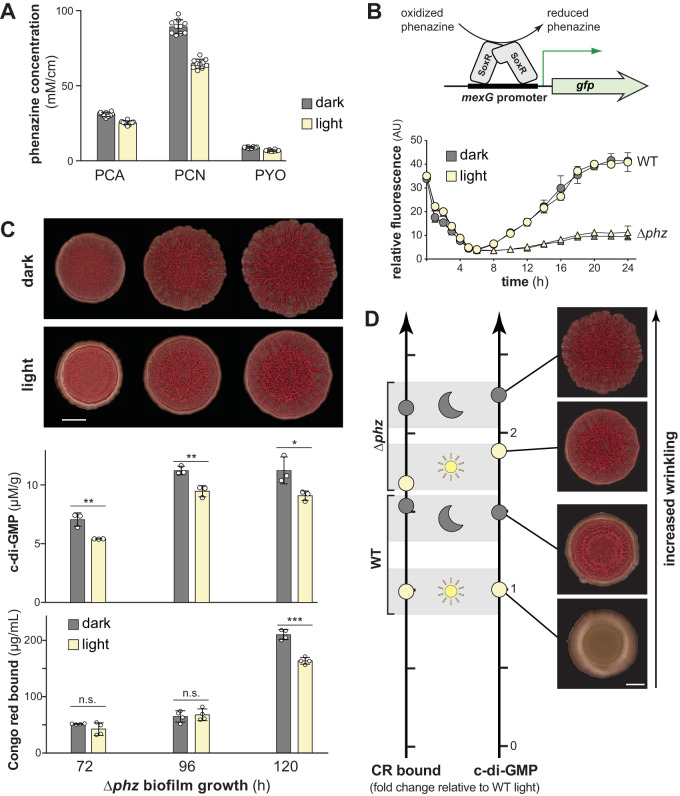
Prior work from our group has shown that, in the absence of light, oxidized phenazines and/or their effects on the cellular redox state promote c-di-GMP degradation and thereby inhibit biofilm wrinkling (25). We therefore wondered whether an increased abundance or a more oxidized redox state of phenazines could be responsible for the effect of light on PA14 biofilm morphogenesis. We measured phenazine production by wild-type PA14 biofilms grown in the presence or absence of light and found that light exposure did not enhance phenazine production (Fig. 3A); thus, the effect of light on PA14 colony development cannot be attributed to an increase in phenazine concentration.
FIG 3.
Light does not increase phenazine production or oxidize the phenazine pool but does decrease c-di-GMP levels in the absence of phenazines. (A) Production of PCA, PCN, and PYO by colony biofilms grown in the dark or light for 96 h. Phenazines were extracted from both the biofilm and the underlying agar for each sample. Phenazine concentration was normalized to colony area. Bars represent the means of 10 biological replicates (circles), and error bars show the standard deviations. (B, top) Schematic showing the mechanism by which SoxR drives expression from the mexG promoter. Phenazines oxidize SoxR and trigger a conformational change that is transduced to the DNA and promotes transcription. (Bottom) Phenazine- and light-dependent expression of the SoxR-regulated mexG reporter in PA14 WT and Δphz strains. The expression of attB::PmexG-gfp in WT and Δphz was determined by measuring GFP fluorescence (excitation, 480 nm; emission, 510 nm) and normalized to the corresponding OD500. Data points represent averages of three biological replicates, and error bars represent the standard deviations. (C, top) PA14 Δphz biofilms grown in the dark or under white fluorescent light at ∼16.5 μmol photons m−2 s−1. The experiment was repeated at least three times. Scale bar, 5 mm. (Middle) c-di-GMP from biofilms grown on colony morphology assay medium and normalized to dry biomass. Bars indicate the averages from three biological replicates (circles), and error bars show the standard deviations. (Bottom) Congo red bound by biofilms grown in the dark or light and harvested at the indicated time points. Each bar represents the mean from four biological replicates (circles), and error bars show standard deviations. The P values were determined using unpaired two-tailed t tests (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; n.s., not significant). (D, left) Relative levels of Congo red (CR) bound by WT and Δphz biofilms grown in dark and light at the 120-h time point, normalized to levels for WT grown in light. (Right) Relative c-di-GMP levels of WT and Δphz biofilms grown in dark and light at the 120-h time point with representative colony images, normalized to levels for WT grown in light. Scale bar, 5 mm.
To test whether light exposure affects the redox state of phenazines in PA14 biofilms, we used reporter strains in which gfp expression is controlled by the promoter found upstream of mexG. The mexGHI-opmD operon is driven by SoxR, a redox-sensitive transcription factor that is directly activated by oxidized phenazines (42–44). When we grew PmexG-gfp reporter strains in liquid cultures, we found that growth in the presence of light had no effect on fluorescence (Fig. 3B). The Δphz control strains showed substantially lower levels of fluorescence, an observation consistent with the role of phenazines in activating SoxR and mexGHI-opmD expression. We conclude that light exposure does not affect the redox state of phenazines in PA14.
PA14 biofilms show PYO-independent effects of light on matrix production and c-di-GMP levels.
In PA14 dark-grown biofilms, phenazine production is correlated with lower c-di-GMP levels and a delayed onset of wrinkling (25). Our observations that matrix and c-di-GMP levels are lower in light-exposed PA14 biofilms and that PYO contributes to light-dependent inhibition of wrinkling raised the question of whether PYO is required for the effects of light on matrix and c-di-GMP levels. We performed the Congo red binding assay on Δphz biofilms grown in the dark and the light and found that, though the effects of light on biofilm morphogenesis are relatively subtle in the Δphz background (Fig. 3C, top; see Movies S1 to S4), this strain showed significantly lower levels of matrix production when biofilms were grown in the light compared to growth in the dark (Fig. 3C, bottom). Similarly, the Δphz strain showed lower c-di-GMP levels for light-exposed compared to dark-grown biofilms, indicating a phenazine-independent mechanism that either inhibits c-di-GMP synthesis or stimulates c-di-GMP degradation in response to light (Fig. 3C, middle; see also Fig. S1A, right panels). We note that although we observed in Δphz biofilms a significant light-dependent decrease in matrix and c-di-GMP, their levels were still higher than for wild-type (i.e., phenazine-producing) biofilms grown in the light or dark (Fig. 3D; see also Fig. S1). We infer that light- and phenazine-sensing are integrated, within the P. aeruginosa regulatory network, at the point of c-di-GMP synthesis or degradation activities and thereby regulate biofilm formation.
PDEs contribute to the light-dependent delay in biofilm structure formation.
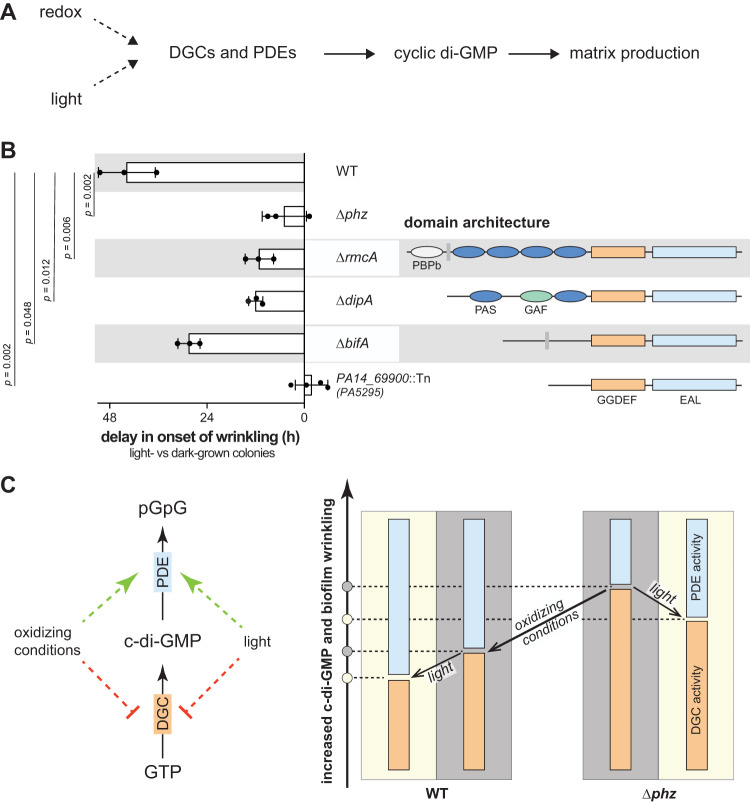
Cellular c-di-GMP levels are modulated by diguanylate cyclases (DGCs), which synthesize c-di-GMP, and phosphodiesterases (PDEs), which degrade it (45). The P. aeruginosa genome encodes 40 proteins with domains implicated in DGC or PDE activity (25, 46, 47). To identify proteins that could link light sensing to modulation of c-di-GMP levels and therefore biofilm matrix production (Fig. 4A), we screened mutants representing each of these proteins for light-dependent inhibition of biofilm wrinkling in the standardized colony morphology assay. We found four mutants that showed attenuated responses to light compared to the wild type (Fig. 4B; see also Movies S5 to S8). All four of the proteins represented by these mutants—PA14_69900, BifA, DipA, and RmcA—contain tandem GGDEF-EAL domains (Fig. 4B). GGDEF domains have the potential for DGC activity, while EAL domains have the potential for PDE activity. Results from prior studies have indicated that each of these proteins functions as a PDE (25, 48–50) and, accordingly, the corresponding mutants were found to show an earlier onset of colony wrinkling in the dark than wild-type PA14 (25) (see Table S1 in the supplemental material).
FIG 4.
c-di-GMP levels are altered in dark versus light through dedicated sensing mechanisms. (A) Schematic for the proposed influence of two external inputs, redox and light, on c-di-GMP levels and matrix production via enzymes with diguanylate cyclase (DGC) or phosphodiesterase (PDE) activities. (B, left) Delay in onset of wrinkling in the light compared to onset of wrinkling in the dark for colony biofilms of PA14 WT and the indicated mutants grown on colony morphology assay medium. Each bar represents the mean from at least three biological replicates (circles), and error bars show the standard deviations. P values were determined using unpaired two-tailed t tests and are listed on the left. (Right) Domain architectures of c-di-GMP-modulating enzymes represented by each mutant. (C, left) Schematic of how external factors, i.e., oxidizing conditions or light, might influence c-di-GMP modulating enzymes, specifically by affecting DGC or PDE activity. (Right) Model showing how c-di-GMP levels decrease with oxidizing conditions, i.e., absence of phenazines, and under light exposure in both PA14 WT and Δphz colony biofilms.
The proteins encoded by screen hits bifA and PA14_69900 are not known to contain sensory domains (Fig. 4B) but may interact with other photosensory proteins that modulate their activities. However, of the four mutations examined, ΔbifA showed the smallest effect on the light-dependent delay in the onset of biofilm wrinkling. Further, we note that although the PA14_69900::Tn mutation abolished the effect of light on biofilm wrinkling, its overall morphotype was most different from the wild type—even in the dark—as exemplified by a distinct pattern of wrinkling and less spreading than biofilms formed by the other three mutants (see Movies S6 and S8). We therefore cannot rule out that the PA14_69900 mutation obscures potential effects of light-dependent c-di-GMP regulation.
Although the ΔbifA, ΔdipA, and ΔrmcA mutations show similar onsets of wrinkling in the dark (Table S1), the light-dependent delay in wrinkling is significantly shortened in ΔdipA and ΔrmcA compared to ΔbifA (Fig. 4B). In this context, it is interesting to note domains in RmcA and DipA with the potential to play sensory roles: RmcA contains an N-terminal, periplasmic arginine-sensing domain (51), followed by four cytoplasmic Per-Arnt-Sim (PAS) domains, while DipA has an N-terminal PAS-GAF-PAS domain architecture. PAS and GAF domains are broadly distributed throughout the tree of life and are frequently involved in sensing stimuli (47, 52–54). Some of the best-known examples of PAS domain-containing proteins mediate behavioral responses to changes in light exposure, oxygen availability, or the cellular redox state (1, 13, 55–58).
We have previously investigated the role of RmcA in sensing phenazines and inhibiting biofilm wrinkling in the dark (25). Based on sequence analysis and modeling, we have suggested that RmcA’s four PAS domains, in order from the N terminus, could bind (i) phenazine, (ii) lipid, (iii) heme, and (iv) FAD, respectively (25, 47). A biochemical characterization of purified RmcA provided support for the notion that this protein may bind phenazine and FAD (25). The capacity for FAD binding is of interest because flavins are critical elements of photosensing in diverse systems (6, 59). Flavin-binding proteins that sense blue light can be categorized into three classes: BLUF proteins (sensors of blue light using FAD), photolyases/cryptochromes (PL/CRY proteins), and light-, oxygen-, and voltage-sensing proteins (LOV proteins) (60–62). LOV proteins are PAS-domain proteins that bind flavins (FMN or FAD) and contain a highly conserved cysteine, which forms a covalent bond with the flavin moiety upon light excitation. Intriguingly, at least some LOV proteins retain their light-dependent signaling abilities when this cysteine is exchanged for another residue (59). Although RmcA does not contain a LOV domain, its putative FAD-binding domain PASd shares significant similarities with NifL from Azotobacter vinelandii, which bears resemblance to LOV domains (Fig. S2B) (25, 47, 59, 63). As an initial assessment of the potential involvement of RmcA’s PASd domain in light-dependent effects on biofilm development, we grew a ΔrmcA mutant and mutants with deletions in the RmcA PASb (“ΔPASb”) and PASd (“ΔPASd”) domains in the colony morphology assay and monitored onset of wrinkling in the presence or absence of light. In the dark, the onset of wrinkling occurred at comparable time points for ΔPASb (55.7 ± 1.6 h), ΔPASd (50.2 ± 2.6 h), and ΔrmcA (51.7 ± 1.9 h) mutants and ahead of the wild type (73.3 ± 2.4 h) (consistent with results reported previously [25]). However, importantly, we observed a significant light-dependent delay in onset of wrinkling only for the ΔPASb mutant and not for the ΔPASd mutant when these mutants were compared to the ΔrmcA mutant (Fig. S2 and Table S1). While PASb contributes to RmcA activity predominantly in the dark, our results indicate that the light-dependent effects of RmcA on biofilm morphogenesis might be mediated through PASd. Together, our findings suggest that some of the major PDEs that attenuate c-di-GMP levels in PA14 dark-grown biofilms contribute to c-di-GMP modulation in response to light and thereby to the effect of light on biofilm development.
Concluding remarks.
The results of this study suggest that integrated information regarding redox and light conditions modulates wrinkle development in PA14 biofilms. We observed that exposure to phenazines, which oxidizes the cellular redox state, and blue light exposure lead to lower levels of c-di-GMP and matrix (Fig. 4C, left panel) and that their cumulative effect profoundly delays the onset of biofilm structure formation (Fig. 1A and 4C, right panel). The lower levels of c-di-GMP could arise from enhanced PDE activity, attenuated DGC activity, or both. In flavoproteins that respond to blue light, high photosensitivity requires that the cofactor be in its oxidized state (64, 65). We speculate that the chemical properties of PYO specifically allow it to modulate the redox states of cellular flavoproteins such that the majority are present in their oxidized forms and that those with the capacity for light sensing, therefore, show enhanced activity. Electron transfer to PYO has been demonstrated for the flavoprotein dihydrolipoamide dehydrogenase (66). In addition, synthetic phenazines were employed and necessary in experiments demonstrating that the cytoplasmic redox potential can shift the photosensitivity of the light-sensing kinase LovK from C. crescentus (67, 68). We note that PYO was unique among the P. aeruginosa phenazines in its capacity to potentiate light-dependent effects on biofilm morphogenesis (Fig. 2C). This finding, in combination with the facts that mutants lacking RmcA or the putative FAD-binding domain of RmcA showed marked attenuations in these effects (Fig. 4B; see also Movies S5 and S7) and that PYO showed the highest affinity for RmcA in previous binding studies (25), suggests that RmcA may sense light in a PYO-dependent manner. This is particularly intriguing given that FAD-binding PAS domains have been shown to act as light sensors (59, 64). We speculate that RmcA integrates phenazine-dependent light and redox signals and transduces this information into regulation of biofilm development in PA14. The biofilms of mutants lacking DipA, which contains an N-terminal PAS-GAF-PAS domain arrangement, showed similar attenuation of the light-induced effects. Whether any of the proteins that we identified sense light directly and how they collectively contribute to mediating light-dependent regulation of biofilm development remain to be elucidated.
Our observations suggest that light-dependent inhibition of PA14 biofilm wrinkling is mediated via the cellular signal c-di-GMP. Figure 4C (right-hand panel) illustrates the relationships between light, redox conditions, and c-di-GMP levels we observed for WT and Δphz biofilms in this study. Oxidation of the cellular redox state, a consequence of phenazine production (26, 27), accounts for a larger relative decrease in c-di-GMP levels than light exposure. Nevertheless, the combined effects of phenazine production and light exposure yield a remarkably low level of c-di-GMP that is manifested as an extreme delay in biofilm structure formation. These results show that P. aeruginosa integrates distinct environmental and physiological cues to regulate multicellular behavior. We note that our c-di-GMP measurements were performed on extracts from whole biofilms, meaning that differences in c-di-GMP levels between subpopulations within biofilms could not be discerned. This may explain why changes in c-di-GMP levels were most consistent with effects on morphology in mature biofilms but less so in early biofilms (i.e., compare Fig. 1A and 3C).
Recently, studies of photodynamic therapy have revealed light, simultaneously applied with antibiotics, as a potentiator of biofilm infection eradication (18, 69, 70). Our finding that PYO, also classified as an antibiotic (71), in conjunction with light diminishes biofilm integrity is consistent with the notion that an oxidizing environment, which is often created as a result of antibiotic treatment, and light together enhance remediation of biofilm-based infections. From the perspective of bacterial physiology, changes in light intensity can accompany changes in other environmental parameters, such as nutrient availability, and chemotrophs could therefore benefit from using light as a proxy signal for conditions that directly affect their own survival. Furthermore, because eukaryotic host organisms show physiological fluctuations that are tuned to light/dark cycling, it makes sense for pathogenic bacteria to monitor light cues that correlate with these changes, for example, variation in immune responses depending on the time of day (72). Finally, a change in light intensity has been speculated to serve as a proxy signal for entry into the host, allowing pathogens to trigger mechanisms of colonization and virulence appropriately (3, 8, 11, 73). For these reasons, as well as because biofilm formation and maintenance are critical components of many types of infections caused by P. aeruginosa, the identification of proteins and small molecules that confer photosensitivity in P. aeruginosa biofilms as described here constitutes an initiative toward better understanding and treating host colonization by this bacterium.
MATERIALS AND METHODS
Bacterial culture and colony morphology assay.
Bacterial strains used in this study are listed in Table S2 in the supplemental material. For colony morphology assays, 1% tryptone plus 1% agar was autoclaved, cooled to 60°C, and 40 μg/ml Congo red (Alfa Aesar) and 20 μg/ml Coomassie blue (OmniPur; MilliporeSigma) dyes were added to the medium. The mixture was poured into plates (100 mm by 100 mm by 15 mm; LDP) at 60 ml per plate and left to cool and solidify overnight (14 to 24 h) at room temperature (25°C). Overnight liquid cultures were grown in lysogeny broth (LB) at 37°C with shaking at 250 rpm for 12 to 16 h. Overnight cultures were diluted 1:100 in fresh LB, and subcultures were grown to midexponential phase (0.4 to 0.6 arbitrary unit [AU] at an optical density at 500 nm [OD500]). Colony biofilms were seeded by spotting 10 μl of this bacterial subculture onto colony morphology medium. Spots were dried and incubated in a Percival CU-22LC9 incubator at 25°C and with 90 to 100% humidity in the dark or under different light conditions (see below). Biofilm development was monitored by taking images at 24-h intervals using a Keyence VHX-1000 microscope or an Epson Expression 11000-XL Photo-Scanner or by taking time-lapse movies in a custom-built movie recording chamber. Time-lapse images were acquired at 15-min intervals by webcam (HD C920 and C930; Logitech) under a 10-s white-light panel (Porta-Trace; Gagne, Inc.) illumination using a customized LabView (National Instruments) integration system.
Onset of wrinkling measurement.
Assessment of onset of wrinkling was aided by the algorithmic prediction and manually curated. Onset of wrinkling was assessed using an unbiased computer vision script, which identifies changes in the grayscale value per pixel (i.e., wrinkles) for a given region of interest (biofilm area) using OpenCV’s Laplacian operators (74). This operation was applied for each image in the time-lapse biofilm movies. The reported times were subjected to unbiased assessment by two independent researchers.
Colony morphology assay under light exposure.
Colony morphology assay was prepared as described above and colony biofilms were incubated in a Percival CU-22LC9 incubator with a built-in lighting system. For all experiments, except for the light titration experiment (Fig. 1B) and light source experiment (Fig. 1C), white-light exposure (bulbs, F17T8/TL841/ALTO; Philips) was calibrated to 16.5 μmol photons m−2 s−1 (7.35 μmol photons m−2 s−1 in the movie chamber). The light titration points (Fig. 1B) were 3.56, 6.84, 11.1, and 16.6 μmol photons m−2 s−1, respectively. Light intensities were measured as photon flux density between 400 and 700 nm using an AMOUR-SL-125-PAR meter (Biospherical Instruments) for all light bulbs except the far-red light bulbs. For the light source experiment (Fig. 1C), the white-light photon flux density was set to 16.6 μmol m−2 s−1, the blue-light photon flux density (bulbs, Actinic Blue, F17T8; Coralife) was set to 19.6 μmol m−2 s−1, and the red-light photon flux density (bulbs, ELA-086, F17T8/IR-660; Percival Scientific) was set to 41.75 μmol m−2 s−1. Light intensities for peak wavelengths of all light bulbs were measured using an S130C photodiode power sensor (Thorlabs) with a PM100A power meter console (Thorlabs). For comparison between light bulbs, the white-light flux densities (analyzed at 436, 545, and 612 nm) were measured as 2.66, 1.31, and 1.0 W/m2, respectively. The blue-light flux density (analyzed at 422 nm) was measured as 1.14 W/m2, the red-light flux density (analyzed at 660 nm) was measured as 1.34 W/m2, and the far-red-light flux density (analyzed at 750 nm; bulbs, ELA-087, F17T8/IR-750; Percival Scientific) was measured as 1.04 W/m2.
Cyclic di-GMP extraction and quantification from biofilms.
Colony biofilms were spotted and grown as described above. Biofilms were scraped off the colony morphology agar plate at 24, 48, 72, 96, and 120 h. Scraped biofilms were transferred to a bead beater tube (19-649; Omni International) containing 1 ml of phosphate-buffered saline (PBS [pH 7.4]) and 0.545 g of 1.4-mm zirconium oxide ceramic beads (19-645; Omni International). Biofilms were homogenized in a Bead Ruptor 12 homogenizer (19-050; Omni International) for 99 s at high speed (5 m/s) at 4°C. Portions (800 μl) of homogenized mixture were transferred to a cold microcentrifuge tube and centrifuged at 16,873× g for 60 s in a benchtop centrifuge (model 5418; Eppendorf) at 4°C. Cell pellets were resuspended in 250 μl of extraction buffer (methanol-acetonitrile-water [40:40:20] with 0.1 N formic acid) and incubated at −20°C for 60 min; the cell debris was then pelleted by centrifugation at 21,130× g for 5 min. Then, 200-μl portions of supernatant were transferred to tubes containing 8 μl of neutralization solution (15% NH4HCO3) and dried in a Vacufuge concentrator (Eppendorf) for 30 to 45 min. Samples were resuspended in mobile phase A (10 mM tributylamine plus 15 mM acetic acid in water-methanol [97:3]) and shipped on dry ice to the mass spectrometry core facility at Michigan State University. c-di-GMP concentrations of samples were quantified with a Quattro Premier XE LC/MS/MS using electrospray ionization analysis. The c-di-GMP concentration of each biofilm was normalized to the dry biomass of the respective sample, which was obtained by drying cell pellets in a Vacufuge concentrator (Eppendorf) and measuring the biomass on an analytical scale (XS64; Mettler Toledo). Experiments were performed in biological triplicates.
Colony morphology assay with exogenous phenazines.
Colony morphology assay medium was prepared as described above and amended with 200 μM phenazine-1-carboxylic acid (PCA; Apexmol), phenazine-1-carboxamide (PCN; Apexmol), or pyocyanin (PYO; Cayman Chemicals). The resulting medium was poured into plates (100 mm by 100 mm by 15 mm; LDP) at 60 ml per plate and left to cool and solidify overnight (14 to 24 h) at room temperature in the dark.
Quantification of phenazine production.
Colony morphology agar was prepared as described above and 4 ml of the mixture were poured into round petri dishes (35 mm by 10 mm; 25373-041; VWR) and left to cool and solidify overnight (14 to 24 h) at 25°C. Then, 10-μl portions of subcultures of WT PA14, grown as described above, were spotted onto a petri dish containing colony morphology medium and grown for 96 h under either white light or dark conditions. Phenazines were harvested from each biofilm and the agar, upon which it was grown by methanol extraction; the agar and biofilm were disrupted with a spatula and transferred to 5 ml of 100% methanol in a 15-ml polypropylene conical tube, and phenazines were extracted overnight in the dark, with constant agitation on a nutator. Portions (300 μl) of extracted phenazine mixture were applied to a Spin-X column with a 0.22-μm filter (VWR 29442-754) and centrifuged at 16,873× g for 5 min, and 200 μl of flowthrough was transferred to a sample vial. The concentrations of PCA, PCN, and PYO were determined by high-performance liquid chromatography (1100 HPLC system; Agilent) as described previously (24, 34). Sample peaks were compared to peaks of pure phenazine standards for PCA, PCN, and PYO, and the area under each peak was used to evaluate the concentration of each phenazine. For each condition, n = 10.
Construction of PmexG-gfp reporter strains.
Transcriptional reporters of SoxR-driven mexG expression were constructed by transforming Escherichia coli S17-1 with plasmid pLD2726 (75). The resulting strain was then used to transfer the plasmid into PA14 WT and Δphz by conjugation, where it was genomically integrated (76). The plasmid backbone was resolved out using FLP recombinase, introduced on plasmid pFLP2, and counterselected on sucrose plates as described previously (76).
Growth curve assay with phenazine oxidation state reporters.
Overnight precultures of reporters (WT attB::PmexG-gfp and Δphz attB::PmexG-gfp) were grown in 1% tryptone medium at 37°C, with shaking at 250 rpm, for 14 h. Overnight cultures were diluted 1:100 in fresh 1% tryptone medium and grown to midexponential phase (0.4 AU at OD500). Two identical assay plates were prepared simultaneously: cultures for the assay were started by inoculating 2 μl of subculture (in technical triplicate) into 200 μl of 1% tryptone per well in a black, flat-bottomed 96-well plate (655097; Greiner Bio-One). Each strain was inoculated in biological triplicates. Both 96-well plates were placed in a 37°C incubator with shaking at 300 rpm; one plate was placed under a dark box, while the other plate was exposed to white LED lights (22.8 μmol photons m−2 s−1). The cell density (OD500) and reporter expression (fluorescence at 480-nm excitation and 510-nm emission) were measured in a BioTek Synergy H1 plate reader every hour for the first 6 h and every 2 h for the next 18 h. Analysis was performed by normalizing each fluorescence value to the corresponding growth value, averaging the technical replicates, followed by averaging the biological replicates.
Preparation of biofilms for protein and matrix quantification.
Liquid subcultures were grown and spotted on 1% tryptone–1% agar plates without dyes, as described above, and colony biofilms were incubated under a constant dark or a constant white-light condition (16.6 μmol photons m−2 s−1). At 72, 96, or 120 h, colony biofilms were scraped from the agar plate using a pipette tip and harvested. Scraped biofilms were transferred to a 2-ml screw cap tube (19-649; Omni International) containing 1 ml of sterile PBS (pH 7.4) and 0.545 g of 1.4-mm zirconium oxide ceramic beads (19-645; Omni International) and homogenized in a Bead Ruptor 12 homogenizer (19-050; Omni International) for 99 s at high speed (5 m/s) at 4°C. The homogenized mixture was split into aliquots of 300 μl each. Two aliquots were frozen at −80°C; one was used for the BCA assay, and one was stored at 4°C to be used for the quantification of matrix (Congo red binding assay).
Protein quantification using Pierce BCA assay.
Homogenized biofilms (harvested as described above) were removed from −80°C, thawed, and diluted 1:10 in sterile PBS. Sodium dodecyl sulfate was added to a final concentration of ∼0.25%, and biofilm cells were sonicated in a water sonicator (G112SP1T_B; Avanti Polar Lipids) for 10 min. The protein concentration of each sample was determined according to the manufacturer’s instructions using a Pierce BCA protein kit assay. Briefly, 25 μl of sonicated biofilm cells was added, in biological triplicates, to a clear, flat-bottomed 96-well plate (655001; Greiner Bio-One). Dilutions of the provided standard were prepared in PBS and used to generate a standard curve. Negative-control wells (n = 2) contained 25 μl of PBS. Then, 200 μl of reagent mix was added to each well, the mixture was incubated at 37°C for 1 h and equilibrated to room temperature for 10 min, and the absorbance at 562 nm (A562) was measured in a BioTek Synergy H1 plate reader. The A562 value of each well was normalized by subtracting the average of the A562 values of the negative-control wells. A standard curve was generated to determine the linear relationship between protein concentration and A562 values and used to quantify the protein concentration for each sample. For each condition tested, the data points represent at least four biological replicates. Each biological replicate value is the average value of three technical replicates.
Matrix quantification by Congo red binding assay.
Homogenized biofilms were removed from 4˚C and cells were pelleted by centrifugation at 16, 873 × g for 2 min in a benchtop centrifuge (model 5418; Eppendorf). Supernatants were removed, and 1 ml of PBS plus 40 μg/ml Congo red was added to each tube. PBS (1 ml) plus 40 μg/ml Congo red (PBS+CR) was used as the negative control. Samples were incubated for 1 h at 37°C with shaking at 250 rpm. Cells were then pelleted by centrifugation at 16, 873 × g for 2 min, and 200 μl of each supernatant was added to a clear, flat-bottomed 96-well plate (655001; Greiner Bio-One). The A490 was determined by using a BioTek Synergy 4 plate reader. The 120-h samples were processed twice, as described above. To determine the amount of bound Congo red, the following equation was used: bound Congo red (μg) = 40(1 – A490 sample/A490 PBS+CR). Data points represent at least four biological replicates, each of which represents the mean from three technical replicates.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yasuhiko Irie for discussion and experimental feedback. Kelly Eckartt and Jeanyoung Jo provided unbiased assessment for the manual determination of biofilm onset of wrinkling. Yihan Zhang provided the algorithm for automated determination of biofilm onset-of-wrinkling. Andreas Hartel, William Stoy, and Joaquim Goes provided technical assistance with the light measurements. Andreas Hartel provided technical assistance with the protein quantification assay. Kelly Eckartt and Andreas Hartel provided feedback on the manuscript. We thank Lijun Chen at the Michigan State University RTSF Mass Spectrometry Core Facility for help with the c-di-GMP analysis.
This study was supported by NIH/NIAID grant R01AI103369 and an NSF CAREER award to L.E.P.D.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Purcell EB, Crosson S. 2008. Photoregulation in prokaryotes. Curr Opin Microbiol 11:168–178. doi: 10.1016/j.mib.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Maresca JA, Keffer JL, Hempel PP, Polson SW, Shevchenko O, Bhavsar J, Powell D, Miller KJ, Singh A, Hahn MW. 2019. Light modulates the physiology of nonphototrophic Actinobacteria. J Bacteriol 201:e00740-18. doi: 10.1128/JB.00740-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santamaría-Hernando S, Rodríguez-Herva JJ, Martínez-García PM, Río-Álvarez I, González-Melendi P, Zamorano J, Tapia C, Rodríguez-Palenzuela P, López-Solanilla E. 2018. Pseudomonas syringae pv. tomato exploits light signals to optimize virulence and colonization of leaves. Environ Microbiol 20:4261–4280. doi: 10.1111/1462-2920.14331. [DOI] [PubMed] [Google Scholar]
- 4.Müller GL, Tuttobene M, Altilio M, Martínez Amezaga M, Nguyen M, Cribb P, Cybulski LE, Ramírez MS, Altabe S, Mussi MA. 2017. Light modulates metabolic pathways and other novel physiological traits in the human pathogen Acinetobacter baumannii. J Bacteriol 199:e00011-17. doi: 10.1128/JB.00011-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu L, McGrane RS, Beattie GA. 2013. Light regulation of swarming motility in Pseudomonas syringae integrates signaling pathways mediated by a bacteriophytochrome and a LOV protein. mBio 4:e00334-13. doi: 10.1128/mBio.00334-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomelsky M, Hoff WD. 2011. Light helps bacteria make important lifestyle decisions. Trends Microbiol 19:441–448. doi: 10.1016/j.tim.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Bonomi HR, Toum L, Sycz G, Sieira R, Toscani AM, Gudesblat GE, Leskow FC, Goldbaum FA, Vojnov AA, Malamud F. 2016. Xanthomonas campestris attenuates virulence by sensing light through a bacteriophytochrome photoreceptor. EMBO Rep 17:1565–1577. doi: 10.15252/embr.201541691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee S, Jemielita M, Stergioula V, Tikhonov M, Bassler BL. 2019. Photosensing and quorum sensing are integrated to control Pseudomonas aeruginosa collective behaviors. PLoS Biol 17:e3000579. doi: 10.1371/journal.pbio.3000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purcell EB, Siegal-Gaskins D, Rawling DC, Fiebig A, Crosson S. 2007. A photosensory two-component system regulates bacterial cell attachment. Proc Natl Acad Sci U S A 104:18241–18246. doi: 10.1073/pnas.0705887104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pané-Farré J, Quin MB, Lewis RJ, Marles-Wright J. 2017. Structure and function of the stressosome signalling hub. Subcell Biochem 83:1–41. doi: 10.1007/978-3-319-46503-6_1. [DOI] [PubMed] [Google Scholar]
- 11.Swartz TE, Tseng T-S, Frederickson MA, Paris G, Comerci DJ, Rajashekara G, Kim J-G, Mudgett MB, Splitter GA, Ugalde RA, Goldbaum FA, Briggs WR, Bogomolni RA. 2007. Blue-light-activated histidine kinases: two-component sensors in bacteria. Science 317:1090–1093. doi: 10.1126/science.1144306. [DOI] [PubMed] [Google Scholar]
- 12.Agostoni M, Koestler BJ, Waters CM, Williams BL, Montgomery BL. 2013. Occurrence of cyclic di-GMP-modulating output domains in cyanobacteria: an illuminating perspective. mBio 4:e00451-13. doi: 10.1128/mBio.00451-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrou J, Crosson S. 2011. Function, structure, and mechanism of bacterial photosensory LOV proteins. Nat Rev Microbiol 9:713–723. doi: 10.1038/nrmicro2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarutina M, Ryjenkov DA, Gomelsky M. 2006. An unorthodox bacteriophytochrome from Rhodobacter sphaeroides involved in turnover of the second messenger c-di-GMP. J Biol Chem 281:34751–34758. doi: 10.1074/jbc.M604819200. [DOI] [PubMed] [Google Scholar]
- 15.Barends TRM, Hartmann E, Griese JJ, Beitlich T, Kirienko NV, Ryjenkov DA, Reinstein J, Shoeman RL, Gomelsky M, Schlichting I. 2009. Structure and mechanism of a bacterial light-regulated cyclic nucleotide phosphodiesterase. Nature 459:1015–1018. doi: 10.1038/nature07966. [DOI] [PubMed] [Google Scholar]
- 16.Tschowri N, Busse S, Hengge R. 2009. The BLUF-EAL protein YcgF acts as a direct anti-repressor in a blue-light response of Escherichia coli. Genes Dev 23:522–534. doi: 10.1101/gad.499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halstead FD, Thwaite JE, Burt R, Laws TR, Raguse M, Moeller R, Webber MA, Oppenheim BA. 2016. Antibacterial activity of blue light against nosocomial wound pathogens growing planktonically and as mature biofilms. Appl Environ Microbiol 82:4006–4016. doi: 10.1128/AEM.00756-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katayama B, Ozawa T, Morimoto K, Awazu K, Ito N, Honda N, Oiso N, Tsuruta D. 2018. Enhanced sterilization and healing of cutaneous pseudomonas infection using 5-aminolevulinic acid as a photosensitizer with 410-nm LED light. J Dermatol Sci 90:323–331. doi: 10.1016/j.jdermsci.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Halstead FD, Hadis MA, Marley N, Brock K, Milward MR, Cooper PR, Oppenheim B, Palin WM. 2019. Violet-blue light arrays at 405 nanometers exert enhanced antimicrobial activity for photodisinfection of monomicrobial nosocomial biofilms. Appl Environ Microbiol 85:e01346-19. doi: 10.1128/AEM.01346-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrer-Espada R, Wang Y, Goh XS, Dai T. 2019. Antimicrobial blue light inactivation of microbial isolates in biofilms. Lasers Surg Med doi: 10.1002/lsm.23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rupel K, Zupin L, Ottaviani G, Bertani I, Martinelli V, Porrelli D, Vodret S, Vuerich R, Passos da Silva D, Bussani R, Crovella S, Parsek M, Venturi V, Di Lenarda R, Biasotto M, Zacchigna S. 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. NPJ Biofilms Microbiomes 5:29. doi: 10.1038/s41522-019-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fila G, Kawiak A, Grinholc MS. 2017. Blue light treatment of Pseudomonas aeruginosa: strong bactericidal activity, synergism with antibiotics and inactivation of virulence factors. Virulence 8:938–958. doi: 10.1080/21505594.2016.1250995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blee JA, Roberts IS, Waigh TA. 2020. Membrane potentials, oxidative stress and the dispersal response of bacterial biofilms to 405 nm light. Phys Biol 17:036001. doi: 10.1088/1478-3975/ab759a. [DOI] [PubMed] [Google Scholar]
- 24.Jo J, Cortez KL, Cornell WC, Price-Whelan A, Dietrich LE. 2017. An orphan cbb3-type cytochrome oxidase subunit supports Pseudomonas aeruginosa biofilm growth and virulence. Elife 6:e30205. doi: 10.7554/eLife.30205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okegbe C, Fields BL, Cole SJ, Beierschmitt C, Morgan CJ, Price-Whelan A, Stewart RC, Lee VT, Dietrich L. 2017. Electron-shuttling antibiotics structure bacterial communities by modulating cellular levels of c-di-GMP. Proc Natl Acad Sci U S A 114:E5236–E5245. doi: 10.1073/pnas.1700264114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price-Whelan A, Dietrich LEP, Newman DK. 2007. Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J Bacteriol 189:6372–6381. doi: 10.1128/JB.00505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietrich LEP, Okegbe C, Price-Whelan A, Sakhtah H, Hunter RC, Newman DK. 2013. Bacterial community morphogenesis is intimately linked to the intracellular redox state. J Bacteriol 195:1371–1380. doi: 10.1128/JB.02273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recinos DA, Sekedat MD, Hernandez A, Cohen TS, Sakhtah H, Prince AS, Price-Whelan A, Dietrich L. 2012. Redundant phenazine operons in Pseudomonas aeruginosa exhibit environment-dependent expression and differential roles in pathogenicity. Proc Natl Acad Sci U S A 109:19420–19425. doi: 10.1073/pnas.1213901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman L, Kolter R. 2004. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol 51:675–690. doi: 10.1046/j.1365-2958.2003.03877.x. [DOI] [PubMed] [Google Scholar]
- 30.Pfeifroth U, Sanchez-Lorenzo A, Manara V, Trentmann J, Hollmann R. 2018. Trends and variability of surface solar radiation in Europe based on surface-and satellite-based data records. J Geophys Res Atmos 123:1735–1754. doi: 10.1002/2017JD027418. [DOI] [Google Scholar]
- 31.Fila G, Krychowiak M, Rychlowski M, Bielawski KP, Grinholc M. 2018. Antimicrobial blue light photoinactivation of Pseudomonas aeruginosa: quorum sensing signaling molecules, biofilm formation and pathogenicity. J Biophotonics 11:e201800079. doi: 10.1002/jbio.201800079. [DOI] [PubMed] [Google Scholar]
- 32.Lee VT, Matewish JM, Kessler JL, Hyodo M, Hayakawa Y, Lory S. 2007. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol Microbiol 65:1474–1484. doi: 10.1111/j.1365-2958.2007.05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hickman JW, Harwood CS. 2008. Identification of FleQ from Pseudomonas aeruginosa as a c‐di-GMP-responsive transcription factor. Mol Microbiol 69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakhtah H, Koyama L, Zhang Y, Morales DK, Fields BL, Price-Whelan A, Hogan DA, Shepard K, Dietrich L. 2016. The Pseudomonas aeruginosa efflux pump MexGHI-OpmD transports a natural phenazine that controls gene expression and biofilm development. Proc Natl Acad Sci U S A 113:E3538–E3547. doi: 10.1073/pnas.1600424113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flood ME, Herbert RB, Holliman FG. 1972. Pigments of Pseudomonas species. V. Biosynthesis of pyocyanin and the pigments of Ps. aureofaciens. J Chem Soc Perkin Trans 1:622–626. doi: 10.1039/p19720000622. [DOI] [PubMed] [Google Scholar]
- 36.Hansford GS, Holliman FG, Herbert RB. 1972. Pigments of Pseudomonas species. Part IV. in vitro and in vivo conversion of 5-methylphenazinium-1-carboxylate into aeruginosin A. J Chem Soc Perkin 1:103–105. doi: 10.1039/p19720000103. [DOI] [PubMed] [Google Scholar]
- 37.Abu EA, Su S, Sallans L, Boissy RE, Greatens A, Heineman WR, Hassett DJ. 2013. Cyclic voltammetric, fluorescence and biological analysis of purified aeruginosin A, a secreted red pigment of Pseudomonas aeruginosa PAO1. Microbiology 159:1736–1747. doi: 10.1099/mic.0.065235-0. [DOI] [PubMed] [Google Scholar]
- 38.Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS. 2001. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol 183:6454–6465. doi: 10.1128/JB.183.21.6454-6465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsons JF, Greenhagen BT, Shi K, Calabrese K, Robinson H, Ladner JE. 2007. Structural and functional analysis of the pyocyanin biosynthetic protein PhzM from Pseudomonas aeruginosa. Biochemistry 46:1821–1828. doi: 10.1021/bi6024403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenhagen BT, Shi K, Robinson H, Gamage S, Bera AK, Ladner JE, Parsons JF. 2008. Crystal structure of the pyocyanin biosynthetic protein PhzS. Biochemistry 47:5281–5289. doi: 10.1021/bi702480t. [DOI] [PubMed] [Google Scholar]
- 41.Dietrich LEP, Teal TK, Price-Whelan A, Newman DK. 2008. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science 321:1203–1206. doi: 10.1126/science.1160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietrich LEP, Price-Whelan A, Petersen A, Whiteley M, Newman DK. 2006. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol 61:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 43.Gu M, Imlay JA. 2011. The SoxRS response of Escherichia coli is directly activated by redox-cycling drugs rather than by superoxide. Mol Microbiol 79:1136–1150. doi: 10.1111/j.1365-2958.2010.07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheplock R, Recinos DA, Mackow N, Dietrich LEP, Chander M. 2013. Species-specific residues calibrate SoxR sensitivity to redox-active molecules. Mol Microbiol 87:368–381. doi: 10.1111/mmi.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Römling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ha D-G, O’Toole GA. 2015. c-di-GMP and its effects on biofilm formation and dispersion: a Pseudomonas aeruginosa review. Microbiol Spectr 3:MB-0003-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dayton H, Smiley MK, Forouhar F, Harrison JJ, Price-Whelan A, Dietrich L. 2020. Sensory domains that control cyclic di-GMP-modulating proteins: a critical frontier in bacterial signal transduction, p 137–158. In Chou S-H, Guiliani N, Lee VT, Römling U (ed), Microbial cyclic di-nucleotide signaling. Springer International Publishing, Cham, Switzerland. [Google Scholar]
- 48.Kuchma SL, Brothers KM, Merritt JH, Liberati NT, Ausubel FM, O’Toole GA. 2007. BifA, a cyclic-Di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol 189:8165–8178. doi: 10.1128/JB.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skotnicka D, Petters T, Heering J, Hoppert M, Kaever V, Søgaard-Andersen L. 2016. Cyclic di-GMP regulates type IV pilus-dependent motility in Myxococcus xanthus. J Bacteriol 198:77–90. doi: 10.1128/JB.00281-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roy AB, Petrova OE, Sauer K. 2012. The phosphodiesterase DipA (PA5017) is essential for Pseudomonas aeruginosa biofilm dispersion. J Bacteriol 194:2904–2915. doi: 10.1128/JB.05346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paiardini A, Mantoni F, Giardina G, Paone A, Janson G, Leoni L, Rampioni G, Cutruzzolà F, Rinaldo S. 2018. A novel bacterial l-arginine sensor controlling c-di-GMP levels in Pseudomonas aeruginosa. Proteins: Struct Funct Bioinf 86:1088–1096. doi: 10.1002/prot.25587. [DOI] [PubMed] [Google Scholar]
- 52.Henry JT, Crosson S. 2011. Ligand-binding PAS domains in a genomic, cellular, and structural context. Annu Rev Microbiol 65:261–286. doi: 10.1146/annurev-micro-121809-151631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aravind L, Ponting CP. 1997. The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem Sci 22:458–459. doi: 10.1016/s0968-0004(97)01148-1. [DOI] [PubMed] [Google Scholar]
- 54.Ho YS, Burden LM, Hurley JH. 2000. Structure of the GAF domain, a ubiquitous signaling motif and a new class of cyclic GMP receptor. EMBO J 19:5288–5299. doi: 10.1093/emboj/19.20.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia D, Watts KJ, Johnson MS, Taylor BL. 2016. Delineating PAS-HAMP interaction surfaces and signalling-associated changes in the aerotaxis receptor Aer. Mol Microbiol 100:156–172. doi: 10.1111/mmi.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gong W, Hao B, Mansy SS, Gonzalez G, Gilles-Gonzalez MA, Chan MK. 1998. Structure of a biological oxygen sensor: a new mechanism for heme-driven signal transduction. Proc Natl Acad Sci U S A 95:15177–15182. doi: 10.1073/pnas.95.26.15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor BL, Zhulin IB. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev 63:479–506. doi: 10.1128/MMBR.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor BL, Rebbapragada A, Johnson MS. 2001. The FAD-PAS domain as a sensor for behavioral responses in Escherichia coli. Antioxid Redox Signal 3:867–879. doi: 10.1089/15230860152665037. [DOI] [PubMed] [Google Scholar]
- 59.Yee EF, Diensthuber RP, Vaidya AT, Borbat PP, Engelhard C, Freed JH, Bittl R, Möglich A, Crane BR. 2015. Signal transduction in light-oxygen-voltage receptors lacking the adduct-forming cysteine residue. Nat Commun 6:10079. doi: 10.1038/ncomms10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Horst MA, Key J, Hellingwerf KJ. 2007. Photosensing in chemotrophic, non-phototrophic bacteria: let there be light sensing too. Trends Microbiol 15:554–562. doi: 10.1016/j.tim.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Zoltowski BD, Gardner KH. 2011. Tripping the light fantastic: blue-light photoreceptors as examples of environmentally modulated protein-protein interactions. Biochemistry 50:4–16. doi: 10.1021/bi101665s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Losi A, Gärtner W. 2012. The evolution of flavin-binding photoreceptors: an ancient chromophore serving trendy blue-light sensors. Annu Rev Plant Biol 63:49–72. doi: 10.1146/annurev-arplant-042811-105538. [DOI] [PubMed] [Google Scholar]
- 63.Key J, Hefti M, Purcell EB, Moffat K. 2007. Structure of the redox sensor domain of Azotobacter vinelandii NifL at atomic resolution: signaling, dimerization, and mechanism. Biochemistry 46:3614–3623. doi: 10.1021/bi0620407. [DOI] [PubMed] [Google Scholar]
- 64.Kopka B, Magerl K, Savitsky A, Davari MD, Röllen K, Bocola M, Dick B, Schwaneberg U, Jaeger K-E, Krauss U. 2017. Electron transfer pathways in a light, oxygen, voltage (LOV) protein devoid of the photoactive cysteine. Sci Rep 7:13346. doi: 10.1038/s41598-017-13420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Losi A, Gärtner W. 2011. Old chromophores, new photoactivation paradigms, trendy applications: flavins in blue light-sensing photoreceptors. Photochem Photobiol 87:491–510. doi: 10.1111/j.1751-1097.2011.00913.x. [DOI] [PubMed] [Google Scholar]
- 66.Glasser NR, Wang BX, Hoy JA, Newman DK. 2017. The pyruvate and α-ketoglutarate dehydrogenase complexes of Pseudomonas aeruginosa catalyze pyocyanin and phenazine-1-carboxylic acid reduction via the subunit dihydrolipoamide dehydrogenase. J Biol Chem 292:5593–5607. doi: 10.1074/jbc.M116.772848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bury A, Hellingwerf KJ. 2014. On the in vivo redox state of flavin-containing photosensory receptor proteins. Methods Mol Biol 1146:177–190. doi: 10.1007/978-1-4939-0452-5_9. [DOI] [PubMed] [Google Scholar]
- 68.Purcell EB, McDonald CA, Palfey BA, Crosson S. 2010. An analysis of the solution structure and signaling mechanism of LovK, a sensor histidine kinase integrating light and redox signals. Biochemistry doi: 10.1021/bi1006404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leanse LG, Dong P-T, Goh XS, Lu M, Cheng J-X, Hooper DC, Dai T. 2019. Quinine enhances photo-inactivation of gram-negative bacteria. J Infect Dis 221:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hendiani S, Rybtke ML, Tolker-Nielsen T, Kashef N. 2019. Sub-lethal antimicrobial photodynamic inactivation affects Pseudomonas aeruginosa PAO1 quorum sensing and cyclic di-GMP regulatory systems. Photodiagnosis Photodyn Ther 27:467–473. doi: 10.1016/j.pdpdt.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 71.Baron SS, Rowe JJ. 1981. Antibiotic action of pyocyanin. Antimicrob Agents Chemother 20:814–820. doi: 10.1128/aac.20.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LAJ. 2014. Circadian clock proteins and immunity. Immunity 40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 73.Idnurm A, Crosson S. 2009. The photobiology of microbial pathogenesis. PLoS Pathog 5:e1000470. doi: 10.1371/journal.ppat.1000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bradski G, Kaehler A. 2008. Learning OpenCV: computer vision with the OpenCV library, 1st ed. O’Reilly Media, Inc., Sebastopol, CA. [Google Scholar]
- 75.Sporer AJ, Beierschmitt C, Bendebury A, Zink KE, Price-Whelan A, Buzzeo MC, Sanchez LM, Dietrich L. 2018. Pseudomonas aeruginosa PumA acts on an endogenous phenazine to promote self-resistance. Microbiology 164:790–800. doi: 10.1099/mic.0.000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin Y-C, Sekedat MD, Cornell WC, Silva GM, Okegbe C, Price-Whelan A, Vogel C, Dietrich L. 2018. Phenazines regulate Nap-dependent denitrification in Pseudomonas aeruginosa biofilms. J Bacteriol 200:e00031-18. doi: 10.1128/JB.00031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.