Abstract
Neurofibromatosis type 1 (NF1) is a life-long neurocutaneous disorder characterized by a predisposition to tumor development, including cutaneous neurofibroma (cNF), the hallmark of the disease. cNF is a histologically benign, multicellular tumor formed in virtually most individuals with NF1. It is considered the most burdensome feature of the disorder due to their physical discomfort, cosmetically disfiguring appearance, and psychosocial burden. Management of cNF remains a challenge in the medical field. Effective medicinal treatment for cNF does not exist at this time. Trials aimed at targeting individual components of the neoplasm such as mast cells with Ketotifen have not shown much success. Physical removal or destruction has been the mainstay of therapy. Surgical removal gives excellent cosmetic results, but risk in general anesthesia may require trained specialists. Destructive laser such as CO2 laser is effective in treating hundreds of tumors at one time but has high risk of scarring hypopigmentation or hyperpigmentation that alter cosmetic outcomes. A robust, low-risk surgical technique has been developed, which may be performed in clinic using traditional biopsy tools that may be more accessible to NF1 patients worldwide than contemporary techniques including Er:YAG or Nd:YAG laser. In this review, specific recommendations for management of cNFs are made based on symptoms, clinical expertise, and available resources. Additionally, antiproliferative agents aimed at stimulating cellular quiescence are explored.
Keywords: current therapy, cutaneous neurofibroma, management of cutaneous neurofibroma, neurofibromatosis type 1, NF1
Key Points.
NF1 patients often identify cNF as their greatest burden within this complex syndrome.
Medical therapies for cNF have been unsuccessful or are undergoing trials.
Surgical removal remains the best treatment approach for cNF.
Neurofibromatosis type I (NF1) is a neurocutaneous disorder characterized by the loss of NF1 (neurofibromin) tumor suppressor gene due to a de novo mutation or through autosomal dominant inheritance.1 The genetic alteration leads to a diverse spectrum of manifestations that can be clinically diagnosed by at least two or more of these features of (1) six or more café-au-lait macules, (2) two or more neurofibromas or one plexiform neurofibroma, (3) freckling in the axillary or inguinal region, (4) Lisch nodules (iris hamartomas), (5) optic gliomas, and (6) osseous lesions.2
Neurofibromas, both cutaneous (dermal) neurofibroma and plexiform neurofibroma, arise from the biallelic loss of NF1 in Schwann cells lineage.1,3,4 The cutaneous neurofibroma (cNF) is a neoplasm of peripheral nerve Schwann cells that presents as a soft nodule in the dermis of the skin at virtually any location in the body.5 The plexiform neurofibroma occurs in more than 30% of those with the NF1 but confers risk to transformation into malignant peripheral nerve sheath tumor that portends a poor 5-year survival prognosis.6 On the other hand, the cNF is present in more than 95% of those with the disease as 2 mm–3 cm, soft, skin-colored nodules covering the skin to the order of tens to thousands.7 They are histology benign and are made up of many cell types without risk of malignant transformation.8
Despite their benign nature, people with NF1 consider cNF to be the most burdensome feature of the disease. Neurological symptoms include irritation, pain, and itching.7 Improper drying after wetting may lead to other complications including maceration, skin breakdown, and superficial infections. Physical disfigurement occurs due to the hundreds to thousands of the cNF that can be present upon one individual.9 Evidence links cNF to lower quality of life due to feelings of embarrassment, interference with daily activities including shopping, trouble with affection toward partners, sexual difficulties, and adverse social implications. People with NF1 may suffer from lower socioeconomic status as a result of their lower self-esteem and risk aversion, and half of those with NF1 suffer from major depressive disorder likely contributed by their cNF burden.10
The biology of cNF is complex that composed of multiple cellular components in a disorganized interaction with extracellular matrix.5,11,12 A nerve is a necessary component for proliferation, development, and maintenance of NF1-deficient Schwann cells through the perineural microenvironment that releases factors such as Neuregulin 1 (NRG1).11 Immune cells are essential constituents of cNF development. Specifically, mast cells are histological hallmarks of cNF and are recruited into the cNF through kit-receptor activation leading to its migration.13 Mast cell degranulation (through trauma or other mechanisms) releases histamine, serotonin, transforming growth factor beta (TGF-B), and other neurotransmitters may be important to cNF development and maintenance.14 Macrophages, the phagocytic leukocytic immune cells, are also present in cNF, but their function in propagation of pathology is currently unknown. Fibroblasts are present in abundance in the cNF and react to TGF-B from mast cells with the deposition of excessive, disorganized collagen and continual reorganization.15 Importantly, these neurofibroma-associated fibroblasts contain separate properties to their fibroblast counterparts in keloids or scar tissues by lacking classical markers such as smooth-muscle actin.16 Other cell types including keratinocytes, melanocytes, and adipocytes are present around cNF but not found to be necessary for driving their development.5 Although the mechanism of pathogenesis is not completely understood, the primary theory is maladaptive response to molecular or physical trauma through hyperactive immune response and excessive fibrosis in the setting of NF1 tumor suppression inactivation in the neoplastic Schwann cells.
Anatomical classification of cNF is ordered by stage according to appearance.17,18 During their nascent stage, cNF cannot be seen by the visible eye, but ultrasound or other forms of imaging can detect the dermal mass.12 The cNF can be classified as flat when their appearance on the skin shows hyperpigmentation or mild epidermal thinning. The sessile stage of the cNF occurs when a visible papule is located on the skin. Subsequently, it moves to the globular stage, which is a larger nodule with a 20–30 mm height and comparable base. The final stage is the pedunculated stage signified by the extrusion of dermal cNF contents into a mass above the skin attached by a stalk.
Currently, no gold-standard treatment exists for cNF. Physical removal remains the most effective method for treating cNF. Physical removal may encompass modalities such as surgical excision with primary closure and modified biopsy removal methodology (Figure 1) or destruction by CO2 laser, electrodessication, and ablation.18–22 Challenges facing removal include tumor regrowth from incomplete excision, significant scarring, and cost burden. Cost remains high because cNF is still classified as an elective, cosmetic treatment by most insurance companies. Additionally, physical removal has no preventive effect on cNF development, which can be improved upon by medicinal therapies.
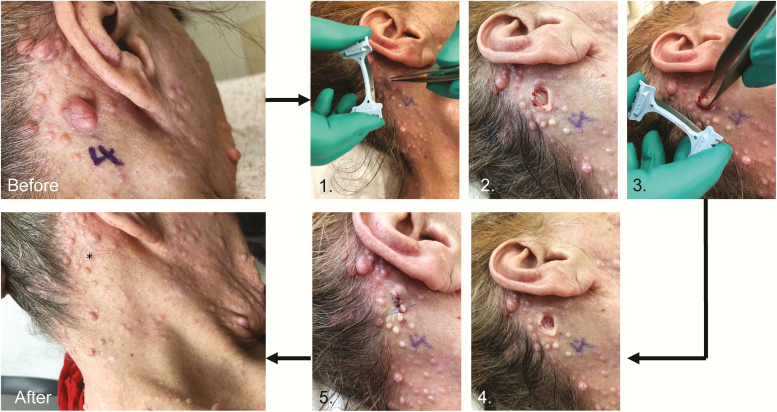
Figure 1.
Modified biopsy removal of cutaneous neurofibroma with 5-month follow-up.18 Before: 2-cm, globular cutaneous neurofibroma before biopsy removal. (1) Dermablade or razor blade shave biopsy of cutaneous neurofibroma above the skin. (2) Soft, pale, dermal component of tumor present. (3) Forceps grasping dermal component to extrude its contents. (4) Empty hole after removal of dermal neurofibroma. (5) Suture to close the skin. After: cutaneous neurofibroma was removed with minimal scar at five-month follow-up. (* = site of tumor removal).
Current medicinal therapies are still under investigation and none is fully effective nor reliable. The past and present therapeutic options are targeting key components to cNF or signaling pathways involved in tumor formation and maintenance, including mTOR, c-Kit, MAPK/MEK, mast cell biochemistry, and cellular proliferative properties.23 Medicinal therapies applied topically will limit systemic exposure to medication.24 Unfortunately, individuals with NF1 may have an extensive tumor burden covering over most of their body surface area making the application of a topical medicine unreasonable. The skin barrier may also prevent dermal penetration of the medicine in the collagenous mass. Systemic therapy would be ideal given the holistic treatment of potentially all cells affected by biallelic loss of NF1 mutation in those with NF1 but risks exposure to agents that may alter normal biology.
Herein, we review the current treatments, both physical and medicinal, for cNF and guide-specific recommendations for cNF treatment based upon this outline. We will also comment on future directions of treatment based on cellular quiescence and genomic editing.
Treatments of Cutaneous Neurofibromas: Physical Removal
To date, physical removal is the most assured method for cNF treatment. Surgery through excision and primary closure was the first technique developed for treatment. Since then, several more modalities have been developed each with their pros and cons (Table 1).
Table 1.
List of studies pertaining to physical removal of cutaneous neurofibromas
| Physical removal | Use, features, efficacy | Limitations and side effects | Sources |
|---|---|---|---|
| Surgery | Large tumors >4 cm; Cosmetically sensitive areas; Histology is available | May require general anesthesia; Require highly trained specialists; Require suture removal; More expensive | 18,25,26 |
| CO2 laser | Small tumors up to 2 cm; Can remove > 100 cNF at once; Rapid surgery | High risk for scarring; Expensive equipment; Require highly trained specialists; Histology is unavailable | 20,27–32 |
| Modified biopsy removal | Small/medium tumors up to 2 cm; Accessible equipment; Can performed by MD, PA, NP; Increased quality of life; Cosmetically sensitive areas; Histology is available; Can remove > 10 tumors per visit | Suture removal is required | 18 |
| Photocoagulation | Minimal discomfort; Local anesthesia; Small/medium tumors <1 cm; Low scar risk; Cosmetically sensitive areas; Healing by secondary intention | Expensive Equipment; Require highly trained specialist; Histology is unavailable | 21,33,34 |
| Electrodessication | Removal > 100 cNF at once; Very small tumors < 5 mm; Accessible equipment; Can perform by MD, PA, NP; Healing by secondary intention | High risk for scarring; Histology is unavailable | 22,35 |
| Radiofrequency ablation/diathermy loop | Rapid surgery; Healing by secondary intention | High risk for scarring; Histology is unavailable | 36 |
cNF, cutaneous neurofibroma. MD, physician. PA, physician assistant. NP, nurse practitioner.
The first recorded publication for cNF removal was from Bromley et al, who utilized surgical excision to remove cNF on 32 patients.19 The technique involves an elliptical excision with removal of the overlaying epidermis and dermal tumor and suturing or healing by primary intention on multiple cNF in each session. Surgical excision has been utilized in a “mega-session” manner where a number of cNFs are removed in one operation with healing by primary or secondary intention depending on the size.25 The “mega-session” technique requires general anesthesia, prior IV antibiotics or topical antibiotics, sterile surgical field, and postoperative pain management. Surgical excision yields favorable postoperative results with minimal scarring and consistently high patient satisfaction.19,26 Additionally, surgical excision can remove giant cNF or cNF in sensitive areas including the eyelids, nipples, genitals, or near neurovascular structures over other physically destructive methods.26 Excision requires highly trained medical specialists including dermatologists, general surgeons, and plastic surgeons who are familiar with the anatomy. Clinics and operational sites should be prepared for hemostasis with aluminum chloride, hyfrecator, or deeper arterial suturing.37 Costs are highly dependent on the method used for excision; thus, access to this technique may be difficult for all patients with NF1. Excision of a few lesions using local anesthesia (1:1000 epinephrine with lidocaine) would cost significantly less than a multi-hour operation requiring general anesthesia in the operating room.38 Operations with elliptical excision may take significantly more time due to the longer excision and accounting the time for suturing.
In 1985, the CO2 laser was introduced to treat a variety of dermatologic skin manifestations.27 The laser employs a 9.4–10.6 μm wavelength laser capable of destroying tissue by rapidly heating and vaporizing intracellular water.39 Since its release, the CO2 has been tested for cNF treatment by a variety of groups.20,21,27–32 In most cases, the CO2 was aimed at the tumor to destroy both the superficial and dermal components leaving a charred center to which healing by secondary intention would occur. The CO2 laser simultaneously seals small nerve endings, rather than leaving frayed endings as occurs with steel scalpel surgery, potentially resulting in less postoperative pain.32 Small lymphatics are also sealed resulting in less postoperative edema. Because this technique achieves hemostasis without sutures, hundreds to thousands of cNF can be treated in one sitting. In all cases, majority of lesions were replaced with a flat, dyspigmented, or depigmented scar that corresponded to the size of the cNF.27,31 Patient satisfaction, despite the resulting obvious adverse coloration or aberrant scarring, was high.21,28,29,31 Recurrence or tumor regrowth was rare at 3–10%.27,28 This method is primarily employed to treat sessile, globular, and pedunculated cNF under 2 cm and should not be used for excessively large cNF with significant dermal mass. Problems include high cost of the machine, expertise or training required for equipment handling, and overall patient access may be limited in certain body areas. Skin-pad burns of uninvolved skin locations may occur when using this equipment, and a 25–50 μm area surrounding the cNF is expected to have thermal necrosis as a byproduct of normal operation.40 Destructive modalities also make challenges for examination by histopathology.
A recent study by Chamseddin et al developed a robust surgical technique for surgical removal of cNF that differs by targeting the distinct anatomy of cNF.18 The cNF first begins as a nascent tumor in the dermis that eventually grows to become visible on the patient’s skin surface. A superficial shave or biopsy will miss a sizeable portion of the tumor in the dermis, which may lead to more significant scarring, regrowth, and will not relieve the pain or itch. The technique comprises a shave biopsy of the soft mass above the skin with a dermablade or razer then using forceps to grasp the dermal component of the tumor, extruding its contents for more visibility, and removing its entirety with the same blade. The end product will be an empty hole, the same size or smaller than the cNF base excised. It can be closed using sutures, surgical glue, or staples dependent on the location of the excision (Figure 1). This technique has excellent postoperative cosmetic results featuring minimal scar size that is less than one required for a complete elliptical excision. In 84 tumors excised, one (1.2%) lesion in an African American male developed hypertrophic scarring and post-inflammatory hyperpigmentation in 12% of cases which improved significantly after 5-month follow-up.18 The Dermatology Life Quality Index (DLQI) showed statistically significant increase in quality of life.18,41 The procedure also does not require antibiotics or sterile gloves due to the superficial nature of the procedure and low risk for infection. The technique relies on local anesthesia and has benefits of worldwide accessibility due to its low-risk setting and reduced costs. Implementation of suturing prevents the removal of hundreds or thousands of cNF in one sitting. Patients can be advised to return for multiple rounds for removal of tens of cNF until acceptable results are obtained.
Contemporary technology has placed focus on cNF treatment with photocoagulation using erbium-doped yttrium aluminum garnet laser (Er:YAG) or neodymium-doped yttrium aluminum garnet laser (Nd:YAG) utilized for cNF treatment in many studies to date.21,33,34 Photocoagulation occurs with Nd:YAG lasers by emitting light at 1064 nm at both pulse and continuous modes for laser-induced thermotherapy to produce tissue destruction by thermal necrosis.42 Er:YAG lasers use an analogous mechanism of light emission but, due to its absorption by water molecules, it may have different outcomes on the heterogenous, extracellular cNF tumors.43 Kriechbaumer et al prospectively compared Er:YAG lasers and CO2 lasers in 21 patients with 15,580 tumors showing Er:YAG lasers had improved the postoperative pain, shorter time to reepithelialization, decreased the duration of postoperative erythema, less thermal necrosis area, and a subjectively improved cosmetic outcome.21 The photocoagulation laser in the large study had no instances of hypertrophic scaring, tumor recurrence at 3.1%, and dyspigmentation in 9% of cases. One subject had a severed subcutaneous bleed requiring deep suturing for homeostasis.21 Another study examined Nd:YAG laser in 12 subjects on 253 cNF which revealed that 40% of cNF treated regressed by at least 75% but around 15% of cNF did not decrease significantly in size.33 A case report where combination of a superficial shave biopsy of the cNF with added laser photocoagulation of the dermal component showed another option for cNF treatment but also risks of hypertrophic scarring and dyspigmentation.34 The photocoagulation lasers may prove to be a superior method for cNF treatment due to its continued high patient satisfaction and cosmetic results.21 This procedure can be performed in an outpatient setting, but the laser is highly expensive and likely only found in well-funded practices and large academic hospital settings, making it inaccessible to most NF1 patients in the world.
Physical destruction of cNF may also be performed by thermal necrosis through other forms including electrodessication and diathermy loop excision. Monopolar diathermy loop uses a heated, metal loop to simultaneously remove and necrotize the cNF tissue and provide cauterization for healing by secondary intention.44 This technique rapidly treats hundreds of tumors in a “mega-session” technique with reported high patient satisfaction.36 This technique is not recommended in cosmetically sensitive areas due to inevitable depigmented scarring at each site of removal.36 Additionally, diathermy loops are not found in every clinical site and thus may be inaccessible to most patients with NF1. Electrodessication, a form of radiofrequency ablation, uses needle-tip cautery at alternating electrical currents to illicit minimal thermal damage to tissue with instant hemostasis. This technique has been used to treat >500 cNF at one sitting but will require general anesthesia under these circumstances.35 Electrodessication should not be employed as the primary mode of cNF removal in patients with limited cNF burden due to epidermal and dermal damage, which will result in dyspigmented scarring as well as in patients with larger cNF.22,35 It is also important to note that patients with cardiac pacemakers should not undergo electrodessication on the trunk, back, or neck.
Treatments of Cutaneous Neurofibromas: Medicinal Topical and Systemic Therapy
To date, there is no topical or systemic medical treatment recommended for cNF. This report highlights successes and failures of past trials for cNF in addition to highlighting current progress for treatment (Table 2). It is important to stress the similarities and differences between plexiform neurofibromas and cNFs, given that many therapies intended for pNF may have consequences for cNF.
Table 2.
List of studies pertaining to medicinal therapy for cutaneous neurofibromas
| Medicinal therapy | Target | Benefits/outcomes | Limitations and side effects | Sources |
|---|---|---|---|---|
| Ketotifen | Mast Cell H1 histamine receptor | Decreased symptoms of pain, itch; Prophylaxis for 30 year case showed anecdotal decreased in tumor burden | Drowsiness | 45–47 |
| Imiquimod | TLR 7/8 | Minimal changes in cNF by calipers | Erythema, irritation | 48 |
| NSAIDS | COX1/COX2 | Local injection of diclofenac leads to 48% of tumors with partial or complete response while others had tumor growth | Erythema, irritation | 49 |
| Photodynamic Therapy | Photosensitizer | Results not yet available | Erythema, irritation | 50, 51 |
| Imatinib | c-KIT | No change in cNF tumor burden | Pancytopenia, Cardiovascular Risks, Gastrointestinal upset, Pulmonary complications | 52, 53 |
| Rapamycin Sirolimus, Everolimus | mTOR | cNF were not reported to change or alter under treatments. A single-arm trial examining everolimus for cNF found no reduction in size nor change in growth under the intervention. | Relatively safe | 54–56 |
| Selumitinib | MEK (MAPK kinase) | cNF was not measured | Elevated creatinine kinase, urticaria, acneiform rash, and in one case decreased left ventricular ejection fraction | 57, 58 |
| Ranibizumab | VEGF | Variable responses, minimal efficacy | Vision changes | 59 |
| Sorefenib | VEGF | cNF was not measured | Vision changes | 60, 71 |
| Everolimus and bevacizumab | mTor, VEGF | Minimal changes in cNF by calipers | 62 | |
| High-dose progesterone | Progesterone Receptor | Increased in tumor burden; No changes in cNF | High Blood Pressure; Mood changes; Drowsiness | 63–65 |
cNF, cutaneous neurofibroma. TLR, toll-like receptor. COX1/COX2, cyclo-oxygenase.
Upon degranulation by trauma or other triggers, mast cells present in the dermis release a host of cellular signals critical to cNF development. Transforming growth factor beta for collagen production from cNF fibroblasts, histamine, vascular endothelial growth factor (VEGF), platelet-derived growth factor, and fibroblast growth factor all contribute to cNF maintenance.23,66 Ketotifen, noncompetitive H1 antihistamine antagonist and mast cell stabilizer, has seen off-label utility by blocking degranulation of mast cells.45 For symptoms from cNF, ketotifen fumarate was shown to have an unequivocal decrease in symptoms of pain and itching of cNF in a study of 10 NF1 patients, likely due to its antihistamine properties.46 Growth rate of cNF slowed over 3 years of treatment, but results were not consistent. One long-term, prospective case report proactively gave an infant with NF1 ketotifen daily for 30 years and reported a paucity of cNFs and the distinctive monotonous uniformity of those present, which were small and flat or barely sessile.47 No double-blinded controlled trials have been performed for ketotifen for cNF size treatment. Based on our current understanding, ketotifen is not useful for mature cNF treatment but could theoretically prohibit the initial growing event. Side effects of the medication are mild, with the most common being mild and transient drowsiness.
Stem cell factor receptor kit (c-KIT) is found on mast cells and has been extensively correlated to NF1-deficient tumor growth in animal studies.13 Imatinib, a small molecule inhibitor of c-KIT, has shown a response to decrease pNF size in 6 of 36 (17%) participants during a phase 2 clinical trial, but the sizes of cNF were not measured.52 A case of an NF1 individual with cutaneous vasculopathy that was treated with Imatinib had no change or reduction in burden of cNF.53 The trial was stopped due to adverse effects that for Imatinib may include gastrointestinal upset, hematologic cytopenia, cardiovascular effects, and pulmonary complications.53,67 Despite these trials, there have not been controlled study investigation on cNF volume or growth rate in the setting of Imatinib treatment, thus no conclusions can be made regarding c-kit inhibition for cNF treatment.
Imiquimod is an immune-response modifier that acts as a toll-like receptor 7 (TLR-7) agonist to modify the innate immune responses.68 A topical application of 5% imiquimod was performed with a primary objective to assess tumor volume by calipers and secondary objective to evaluate the degree of infiltrating inflammatory cells around the region of application.48 After 4 months, cNF showed a 15% reduction in tumor volume, while the control group showed a 10% reduction. Skin inflammation after prolonged treatment was low (5–10%), suggesting that targeting immunogenic response with imiquimod may not be effective.48 This study likely discredits the use of TLR-7 for cNF therapy.
Cells of inflammation including leukocytes and macrophages are present within cNF, yet their function is unknown. Nonsteroidal anti-inflammatory agents (NSAIDS) target the proinflammatory enzymes, COX-1 and/or COX-2, to prevent the release of PGE and prostaglandins.69 Decreased inflammation through NSAIDS was hypothesized as possible mechanisms for treatment. A controlled local injection study with diclofenac showed that 48% of tumors had partial or complete response, while others had tumor growth on treatment.49 In one open, controlled, prospective, proof-of-concept study, 25 mg/ml diclofenac is applied topically twice daily on cNF after microporation with a laser device.70 Results on seven patients have currently not been published. The primary objective is to identify inflammatory process with the presence of tissue necrosis while observing adverse events associated with the study drug. In a related study, researchers injected doxycycline to achieve an 89% total response.
Neoplastic Schwann cell biology is also a primary target for cNF medicinal therapies. Naturally, the tumor Schwann cell utilizes growth factor-initiated RAS signaling cascade to upregulate a PI3K-mTOR survival pathway and the RAF-MEK-ERK transcription and proliferation pathways.71 The NF1 protein, which is absent in tumor cells, inhibits excessive RAS activation and thus preventing activation of these two pathways.72 Drug therapies aimed at downregulating these two pathways at the level of tumor Schwann cell were developed to prevent and treat cNF.
The mTOR pathway is a master regulator of cell growth and metabolism and is important in Schwann cell survival.73 Rapamycin, also known as Sirolimus, is a macrolide compound that inhibits mTOR. Sirolimus and everolimus, other mTOR inhibitors, have been examined in the setting of clinical trials for treatment of pNF and malignant peripheral nerve sheath tumor (MPNST), respectively.54,57 Although they were not the primary outcome, cNF was not reported to change or alter under the treatments. A single-arm trial examining everolimus for cNF found that it did not reduce size nor change growth under the intervention.55 Although the study lacked a control arm, tumor growth was likely not observed due to the quiescence of the matured cNF in the study or the inhibition of mTOR by everolimus. The benefit to rapamycin in a topical regimen applied daily for 6 months did not have significant systemic absorption, and side effects such as pancytopenia were not observed.56 Despite the necessity of mTOR in Schwann cell survival, trials with mTOR antagonists did not have a significant impact on cNF.
The RAF-MEK-ERK pathway is an important regulator of transcription and cell growth and is tightly linked to pathology involved in cNF development through upstream activation by unregulated RAS. Selumitinib is an oral selective inhibitor MAPK kinase (MEK) that has shown activity against several adult cancers.74,75 The drug is undergoing a phase II clinical trial of cNF specifically.58 An earlier study investigated 24 NF1 children who had inoperable pNF with administered Selumitinib twice daily at a dose of 20–30 mg/m2 of body surface area every month.57 Complications included elevated creatinine kinase, urticaria, acneiform rash, and in one case decreased left ventricular ejection fraction. cNF sizes were not measured, but plexiform neurofibromas did have partial responses (>20% decrease volume) in 70% of children, thus signifying growth of pNF relies on MEK.57
VEGF inhibitors have also been trialed stemming from data that show VEGF, the angiogenic signaling molecule, is expressed highly in NF1-deficient tumors.23,76 Ranibizumab, a VEGF antibody, was injected into cNF.59 Uninjected tumors served as internal controls, and primary outcomes were cNF volume changes and interstitial pressure. Reports of outcomes are still expected to be released. Another VEGF inhibitor, Sorefenib, had significant effects on lowering pNF volume by MRI, although cNF response was not measured.60,61 A trial targeting both mTOR inhibition with everolimus and VEGF with bevacizumab in order to examine the pNF and MPNST growth also revealed minimal changes to cNF development or growth.62 One possible reason for this unresponsive nature of cNF to antiproliferative molecules may be the quiescent nature of mature cNF on the skin which do not rely on these signaling pathways after development or whether the trial outcome measure was not sensitive enough to quantify the changes in cNF.
Hormones play a significant role in cNF development. For example, women with NF1 have been reported to have rapid growth in cNF size and numbers during puberty and again, during pregnancy.77 Both progesterone receptors and estrogen receptors have been found in varying degrees within cNF.63,78 In fact, neurofibroma-derived Schwann cells respond by increased proliferation to hormonal (progesterone and estrogen) treatment in vitro, and studies in vivo also support this observation.79,80 Interestingly, two patients with NF1 who took high-dose progesterone had an increased tumor size burden.64 However, a study of 59 women with NF1 who took hormonal contraceptives (progesterone–estrogen combination or progesterone alone) did not reveal an association with cNF growth.64 Thus, the link between hormones and cNF is highly evident in some studies but not in others, and further research to characterize this relationship will be beneficial.63,65 This research should encompass single-sample gene-set enrichment analysis of hormonal pathways in cNF that can reveal hormone impact on individual types of cells within a cNF. To date, growth hormone hypersecretion has been noted in some NF1 patients, but other studies have revealed growth hormone under secretion.81–84 Therefore, the exact role of hormone in NF1 remains undetermined.
Photodynamic therapy (PDT) has been used to treat a variety of dermatologic, hyperproliferative disease including actinic keratosis, basal cell carcinomas, and cutaneous T-cell lymphoma.85 PDT also can kill bacteria and fungi, and destroy viruses that cause warts or molluscum contagiosum.86 A photosensitizer agent, 5-aminiolevulinic acid (ALA), is a precursor to human body’s endogenous photosensitizer Protoporphyrin IX. When illuminated with broadband red light source 570–670 nm, cells that uptake the photosensitizer succumb to death secondary to reaction causing chemical tissue destruction, recruitment of inflammatory cells, and vascular compromise.85 In vitro studies with MPNST cells show a cytotoxic affect.87 However, a case study examining PDT with ALA for pNF specifically did not note any changes to this mass.50 Two clinical trials NCT01682811 (recruiting) and NCT02728388 (not yet recruiting) are examining the impact of ALA-PDT on cNF.51,88
Recommendations for cNF Treatment
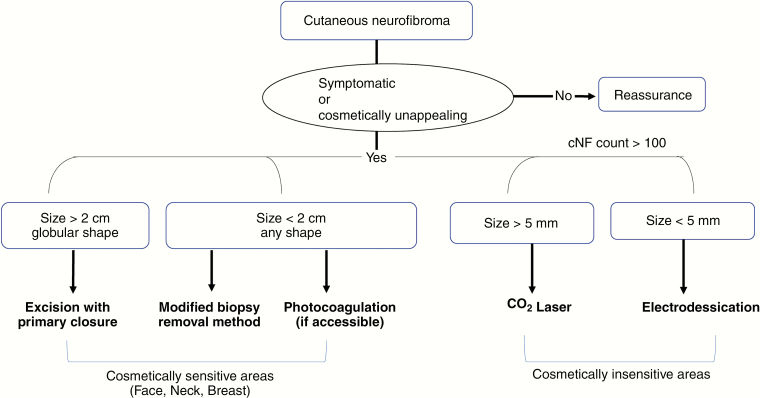
Specific recommendations for treatment of cNF rely on several factors that include equipment availability, time, tumor burden, tumor size, location, and desired cosmetic outcomes (Figure 2).
Figure 2.
Recommendations for physical removal of cutaneous neurofibromas.
Due to its benign nature, cNF ultimately do not contribute to differences in mortality for NF1 patients. Thus, asymptomatic lesions without cosmetic concern should be managed by reassurance alone. Symptomatic lesions, such as itching or pain, can be removed physically. Lesions that trouble the patient due to cosmetic disfigurement should be removed given the strong link between cNF burden and lower quality of life.89 For larger (over 2 cm) cNF with globular morphology, elliptical excision with primary suture closure should be reserved to reduce infection and support faster skin healing. If cosmetically unappealing or in a sensitive area including the face, neck, and breast, the modified biopsy removal method18 or primary excision may be employed to reduce scar size given patients are low risk for hypertrophic or keloidal scarring. Photocoagulation could replace the modified biopsy removal method if equipment and trained specialists are available—although reliability for complete removal of the lesion remains unclear. Given an extensively high cNF burden in the abdomen, chest, or back, more rapid, “mega-session” and cosmetically insensitive techniques can be utilized including CO2 laser for tumors >5 mm and electrodessication for tumors <5 mm without the need for suturing and healing by secondary intention. Risks and benefits for each available procedure should be discussed with the patients.
Future Directions
Surgical and destructive removal is the mainstay and golden standard of therapy for cNFs. Destructive modalities including CO2 lasers, electrodessications, and photocoagulation are effective in the treatment of tackling hundreds of cNF at one sitting. At this time, future research and controlled clinical trials are necessary to target cNF in early stages of development prior to requiring overt treatment. The ideal cNF therapy for patients with NF1 would prevent tumor development from the very beginning. This could come in the form of genetic therapy with genomic editing techniques. The application of CRISPR in theory could be used to correct the initial mutation.90 However, the technique is still incomplete, not yet fully developed and controversial. The advances provided by understanding the biology of cNF derived from recent animal models may afford new opportunity for specific target therapies.23 Lessons learned from the molecular interactions between the neoplastic Schwann cells and their tumor microenvironment within the cNF will provide us new approaches to develop novel therapies to delay and to prevent neurofibroma development in NF1 patients. In this arena, cellular quiescence, halting of the cell-cycle, is at the cornerstone of cNF evolution and should be a prime target for prevention of cNF.91 It has been known that cNF rapidly proliferates in size at the early stage but eventually becomes quiescent in mature stage17 as it shuts down proliferation of the mass when it reaches a certain size and remains unchanged for years. The mechanisms behind quiescence are unknown. Additional studies should be invested to characterize cNF quiescence. Clear targets for this endeavor are examining the cells of origin in early stage and neoplastic cells as well as the tumor microenvironment in the quiescent stage.23 Additionally, prevention of growth and reducing tumor size by minimizing the microenvironment collagen will contribute to overall cNF mass.92
Conclusion
Within the report lies discussion involving current therapy guidelines for cNF management through physical removal and examination of medicinal clinical research which targets cNF biology. Importantly, future directions for research in understanding cellular quiescence in cNF as well as interaction between the neoplastic Schwann cells and its tumor microenvironment in initiating and maintaining cNF will be essential to develop specific and effective therapy for the most common tumor in NF1.
Acknowledgments
LQL holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund, the Thomas L. Shields, M.D. Professorship in Dermatology, and supported by fundings from the Giorgio Foundation, the Neurofibromatosis Therapeutic Acceleration Program, the NF1 Research Consortium Fund, the National Cancer Institute of the National Institutes of Health (grant number R01 CA166593), and the US Department of Defense.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Authorship statement
Designing Research Study: Lu Q. Le. Literature Review: Bahir H. Chamseddin, Lu Q. Le.
Analyzing Data: Bahir H. Chamseddin, Lu Q. Le. Figure Creation: Bahir H. Chamseddin, Lu Q. Le. Drafting Manuscript: Bahir H. Chamseddin, Lu Q. Le. Final Review of the Manuscript: Lu Q. Le.
References
- 1. Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61(1):1–14; quiz 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44(2):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muir D, Neubauer D, Lim IT, et al. Tumorigenic properties of neurofibromin-deficient neurofibroma Schwann cells. Am J Pathol. 2001;158(2):501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu M, Wallace MR, Muir D. Tumorigenic properties of neurofibromin-deficient Schwann cells in culture and as syngrafts in Nf1 knockout mice. J Neurosci Res. 2005;82(3):357–367. [DOI] [PubMed] [Google Scholar]
- 5. Jouhilahti EM, Peltonen S, Callens T, et al. The development of cutaneous neurofibromas. Am J Pathol. 2011;178(2):500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evans DG, Baser ME, McGaughran J, et al. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39(5):311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams VC, Lucas J, Babcock MA, et al. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123(1):124–133. [DOI] [PubMed] [Google Scholar]
- 8. Ortonne N, Wolkenstein P, Blakeley JO, et al. Cutaneous neurofibromas: current clinical and pathologic issues. Neurology. 2018;91(2 Suppl 1):S5–S13. [DOI] [PubMed] [Google Scholar]
- 9. Cannon A, Chen MJ, Li P, et al. Cutaneous neurofibromas in neurofibromatosis type I: a quantitative natural history study. Orphanet J Rare Dis. 2018;13(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen JS, Levy HP, Sloan J, et al. Depression among adults with neurofibromatosis type 1: prevalence and impact on quality of life. Clin Genet. 2015;88(5):425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le LQ, Shipman T, Burns DK, et al. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell Stem Cell. 2009;4(5):453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brosseau JP, Pichard DC, Legius EH, et al. The biology of cutaneous neurofibromas: consensus recommendations for setting research priorities. Neurology. 2018;91(2 Suppl 1):S14–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang FC, Ingram DA, Chen S, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/– and c-kit-dependent bone marrow. Cell. 2008;135(3):437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Payne V, Kam PC. Mast cell tryptase: a review of its physiology and clinical significance. Anaesthesia. 2004;59(7):695–703. [DOI] [PubMed] [Google Scholar]
- 15. Le LQ, Kesterson RA, Guttmann DH. Defining the research landscape for dermal neurofbromas. Oncology Times. 2016;38(18):14–1 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dundr P, Povýsil C, Tvrdík D. Actin expression in neural crest cell-derived tumors including schwannomas, malignant peripheral nerve sheath tumors, neurofibromas and melanocytic tumors. Pathol Int. 2009;59(2):86–90. [DOI] [PubMed] [Google Scholar]
- 17. Riccardi VM. Translational genetics and genomics: the fundamental nature of NF1 neurofibromas. Transl Genet Genom. 2007;1:3–14. [Google Scholar]
- 18. Chamseddin BH, Hernandez L, Solorzano D, et al. Robust surgical approach for cutaneous neurofibroma in neurofibromatosis type 1. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bromley GS, Sherman JE, Goulian D Jr. Neurofibromatosis—distribution of lesions and surgical treatment. Ann Plast Surg. 1982;8:272–276. [DOI] [PubMed] [Google Scholar]
- 20. Becker DW., Jr Use of the carbon dioxide laser in treating multiple cutaneous neurofibromas. Ann Plast Surg. 1991;26(6):582–586. [DOI] [PubMed] [Google Scholar]
- 21. Kriechbaumer LK, Susani M, Kircher SG, et al. Comparative study of CO2- and Er:YAG laser ablation of multiple cutaneous neurofibromas in von Recklinghausen’s disease. Lasers Med Sci. 2014;29(3):1083–1091. [DOI] [PubMed] [Google Scholar]
- 22. Levine SM, Levine E, Taub PJ, et al. Electrosurgical excision technique for the treatment of multiple cutaneous lesions in neurofibromatosis type I. J Plast Reconstr Aesthet Surg. 2008;61(8):958–962. [DOI] [PubMed] [Google Scholar]
- 23. Allaway RJ, Gosline SJC, La Rosa S, et al. Cutaneous neurofibromas in the genomics era: current understanding and open questions. Br J Cancer. 2018;118(12):1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verma SK, Riccardi VM, Plotkin SR, et al. Considerations for development of therapies for cutaneous neurofibroma. Neurology. 2018;91(2 Suppl 1):S21–S30. [DOI] [PubMed] [Google Scholar]
- 25. Onesti MG, Carella S, Spinelli G, et al. The megasession technique for excision of multiple neurofibromas. Dermatol Surg. 2010;36(9):1488–1490. [DOI] [PubMed] [Google Scholar]
- 26. Yuan SM, Cui L, Guo Y, et al. Surgical management of giant neurofibroma in soft tissue: a single-center retrospective analysis. Int J Clin Exp Med. 2015;8(4):5245–5253. [PMC free article] [PubMed] [Google Scholar]
- 27. Roenigk RK, Ratz JL. CO2 laser treatment of cutaneous neurofibromas. J Dermatol Surg Oncol. 1987;13(2):187–190. [DOI] [PubMed] [Google Scholar]
- 28. Chiang YZ, Al-Niaimi F, Ferguson J, et al. Carbon dioxide laser treatment of cutaneous neurofibromas. Dermatol Ther (Heidelb). 2012;2(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Méni C, Sbidian E, Moreno JC, et al. Treatment of neurofibromas with a carbon dioxide laser: a retrospective cross-sectional study of 106 patients. Dermatology. 2015;230(3):263–268. [DOI] [PubMed] [Google Scholar]
- 30. Moreno JC, Mathoret C, Lantieri L, et al. Carbon dioxide laser for removal of multiple cutaneous neurofibromas. Br J Dermatol. 2001;144(5):1096–1098. [DOI] [PubMed] [Google Scholar]
- 31. Ostertag JU, Theunissen CC, Neumann HA. Hypertrophic scars after therapy with CO2 laser for treatment of multiple cutaneous neurofibromas. Dermatol Surg. 2002;28(3):296–298. [DOI] [PubMed] [Google Scholar]
- 32. Gloster HM Jr., Roenigk RK. Carbon dioxide laser for the treatment of cutaneous lesions. Clin Dermatol. 1995;13:25–33. [DOI] [PubMed] [Google Scholar]
- 33. Elwakil TF, Samy NA, Elbasiouny MS. Non-excision treatment of multiple cutaneous neurofibromas by laser photocoagulation. Lasers Med Sci. 2008;23(3):301–306. [DOI] [PubMed] [Google Scholar]
- 34. Kim HJ, Lee KG, Yi SM, et al. Successful treatment of multiple cutaneous neurofibromas using a combination of shave excision and laser photothermocoagulation with a 1,444-nm neodymium-doped yttrium aluminum garnet laser. Dermatol Surg. 2012;38(6):960–963. [DOI] [PubMed] [Google Scholar]
- 35. Lutterodt CG, Mohan A, Kirkpatrick N. The use of electrodessication in the treatment of cutaneous neurofibromatosis: a retrospective patient satisfaction outcome assessment. J Plast Reconstr Aesthet Surg. 2016;69(6):765–769. [DOI] [PubMed] [Google Scholar]
- 36. Roberts AH, Crockett DJ. An operation for the treatment of cutaneous neurofibromatosis. Br J Plast Surg. 1985;38(2):292–293. [DOI] [PubMed] [Google Scholar]
- 37. Chen DL, Carlson EO, Fathi R, et al. Undermining and hemostasis. Dermatol Surg. 2015;41(Suppl 10):S201–S215. [DOI] [PubMed] [Google Scholar]
- 38. Bordianu A, Bobirca F. Facial skin cancer surgery under local anesthesia. J Med Life. 2018;11(3):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brauner GJ. Various laser modalities in the treatment of cutaneous lesions. Clin Podiatr Med Surg. 1992;9(3):687–697. [PubMed] [Google Scholar]
- 40. El-Hoshy K, Abdel-Halim MRE, Dorgham D, et al. Efficacy of fractional carbon dioxide laser in the treatment of mature burn scars: a clinical, histopathological, and histochemical study. J Clin Aesthet Dermatol. 2017;10(12):36–43. [PMC free article] [PubMed] [Google Scholar]
- 41. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. [DOI] [PubMed] [Google Scholar]
- 42. El-Domyati M, El-Ammawi TS, Medhat W, et al. Effects of the Nd:YAG 1320-nm laser on skin rejuvenation: clinical and histological correlations. J Cosmet Laser Ther. 2011;13(3):98–106. [DOI] [PubMed] [Google Scholar]
- 43. Trelles MA, Vélez M, Mordon S. Correlation of histological findings of single session Er:YAG skin fractional resurfacing with various passes and energies and the possible clinical implications. Lasers Surg Med. 2008;40(3):171–177. [DOI] [PubMed] [Google Scholar]
- 44. Storm FK, Harrison WH, Elliott RS, et al. Thermal distribution of magnetic-loop induction hyperthermia in phantoms and animals: effect of the living state and velocity of heating. Int J Radiat Oncol Biol Phys. 1982;8(5):865–871. [DOI] [PubMed] [Google Scholar]
- 45. Riccardi VM. Mast-cell stabilization to decrease neurofibroma growth. Preliminary experience with ketotifen. Arch Dermatol. 1987;123(8):1011–1016. [PubMed] [Google Scholar]
- 46. Riccardi VM. A controlled multiphase trial of ketotifen to minimize neurofibroma-associated pain and itching. Arch Dermatol. 1993;129:577–581. [PubMed] [Google Scholar]
- 47. Riccardi VM. Ketotifen suppression of NF1 neurofibroma growth over 30 years. Am J Med Genet A. 2015;167(7):1570–1577. [DOI] [PubMed] [Google Scholar]
- 48. Massachusetts General Hospital. [NCT00865644] Topical imiquimod 5% cream for treatment of cutaneous neurofibromas in adults with neurofibromatosis 1 no title. Clinicaltrials.gov. 2009. [Google Scholar]
- 49. Fundação Educacional Serra dos Órgãos. [NCT03090971] Use of topical liquid diclofenac following laser microporation of cutaneous neurofibromas in patients with NF1. Clinicaltrials.gov. 2017. [Google Scholar]
- 50. Hamdoon Z, Jerjes W, Al-Delayme R, et al. Solitary giant neurofibroma of the neck subjected to photodynamic therapy: case study. Head Neck Oncol. 2012;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Whelan HT. [NCT02728388] Photodynamic therapy for benign dermal neurofibromas. Clinicaltrials.gov. 2016. [Google Scholar]
- 52. Robertson KA, Nalepa G, Yang FC, et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial. Lancet Oncol. 2012;13(12):1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Khelifa I, Saurat JH, Prins C. Use of imatinib in a patient with cutaneous vasculopathy in the context of von Recklinghausen disease/neurofibromatosis. Br J Dermatol. 2015;172(1):253–256. [DOI] [PubMed] [Google Scholar]
- 54. Assistance Publique - Hôpitaux de Paris. [NCT01412892] Use of RAD001 as monotherapy in the treatment of neurofibromatosis 1 related internal plexiform neurofibromas (NFitor). Clinicaltrials.gov. 2011. [Google Scholar]
- 55. The University of Texas Health Science Center - Houston. [NCT02332902] Everolimus for treatment of disfiguring cutaneous lesions in neurofibromatosis1 CRAD001CUS232T (DCLNF1). Clinicaltrials.gov. 2014. [Google Scholar]
- 56. The University of Texas Health Science Center - Houston. [NCT01031901] Topical rapamycin therapy to alleviate cutaneous manifestations of Tuberous Sclerosis Complex (TSC) and Neurofibromatosis I (NF1). Clinicaltrials.gov. 2009. [Google Scholar]
- 57. Dombi E, Baldwin A, Marcus LJ, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. National Cancer Institute (NCI). [NCT02839720] Selumetinib in treating patients with neurofibromatosis type 1 and dermal neurofibroma. Clinicaltrials.gov. 2016. [Google Scholar]
- 59. Massachusetts General Hospital. [NCT00657202] Ranibizumab for neurofibromas associated with NF1. Clinicaltrials.gov. 2008. [Google Scholar]
- 60. Wu J, Dombi E, Jousma E, et al. Preclincial testing of sorafenib and RAD001 in the Nf(flox/flox);DhhCre mouse model of plexiform neurofibroma using magnetic resonance imaging. Pediatr Blood Cancer. 2012;58(2):173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim A, Dombi E, Tepas K, et al. Phase I trial and pharmacokinetic study of sorafenib in children with neurofibromatosis type I and plexiform neurofibromas. Pediatr Blood Cancer. 2013;60(3):396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Widemann BC, Lu Y, Reinke D, et al. Targeting sporadic and neurofibromatosis type 1 (NF1) related refractory malignant peripheral nerve sheath tumors (MPNST) in a phase II study of everolimus in combination with bevacizumab (SARC016). Sarcoma. 2019;2019:7656747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McLaughlin ME, Jacks T. Progesterone receptor expression in neurofibromas. Cancer Res. 2003;63(4):752–755. [PubMed] [Google Scholar]
- 64. Lammert M, Mautner VF, Kluwe L. Do hormonal contraceptives stimulate growth of neurofibromas? A survey on 59 NF1 patients. BMC Cancer. 2005;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sbidian E, Duong TA, Valeyrie-Allanore L, et al. Neurofibromatosis type 1: neurofibromas and sex. Br J Dermatol. 2016;174(2):402–404. [DOI] [PubMed] [Google Scholar]
- 66. Yang FC, Chen S, Clegg T, et al. Nf1+/– mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Hum Mol Genet. 2006;15(16):2421–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Azizi G, Mirshafiey A. Imatinib mesylate: an innovation in treatment of autoimmune diseases. Recent Pat Inflamm Allergy Drug Discov. 2013;7(3):259–267. [DOI] [PubMed] [Google Scholar]
- 68. Navi D, Huntley A. Imiquimod 5 percent cream and the treatment of cutaneous malignancy. Dermatol Online J. 2004;10(1):4. [PubMed] [Google Scholar]
- 69. Zhang X, Morham SG, Langenbach R, et al. Malignant transformation and antineoplastic actions of nonsteroidal antiinflammatory drugs (NSAIDs) on cyclooxygenase-null embryo fibroblasts. J Exp Med. 1999;190(4):451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Geller M, Filho AB, Oliveira L, et al. A proof-of-concept assessment of the safety and efficacy of intralesional diclofenac in the treatment of cutaneous neurofibromas. Int. J. Clin. Med. 2015;6:975–983. [Google Scholar]
- 71. Ratner N, Miller SJ. A RASopathy gene commonly mutated in cancer: the neurofibromatosis type 1 tumour suppressor. Nat Rev Cancer. 2015;15(5):290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22. [DOI] [PubMed] [Google Scholar]
- 73. Johannessen CM, Reczek EE, James MF, et al. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102(24):8573–8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Melosky B, Bradbury P, Tu D, et al. Selumetinib in patients receiving standard pemetrexed and platinum-based chemotherapy for advanced or metastatic KRAS wildtype or unknown non-squamous non-small cell lung cancer: a randomized, multicenter, phase II study. Canadian Cancer Trials Group (CCTG) IND.219. Lung Cancer. 2019;133:48–55. [DOI] [PubMed] [Google Scholar]
- 75. Ahsan S, Ge Y, Tainsky MA. Combinatorial therapeutic targeting of BMP2 and MEK-ERK pathways in NF1-associated malignant peripheral nerve sheath tumors. Oncotarget. 2016;7(35):57171–57185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kawachi Y, Xu X, Ichikawa E, et al. Expression of angiogenic factors in neurofibromas. Exp Dermatol. 2003;12(4):412–417. [DOI] [PubMed] [Google Scholar]
- 77. Roth TM, Petty EM, Barald KF. The role of steroid hormones in the NF1 phenotype: focus on pregnancy. Am J Med Genet A. 2008;146A(12):1624–1633. [DOI] [PubMed] [Google Scholar]
- 78. Geller M, Mezitis SG, Nunes FP, et al. Progesterone and estrogen receptors in neurofibromas of patients with NF1. Clin Med Pathol. 2008;1:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Overdiek A, Winner U, Mayatepek E, et al. Schwann cells from human neurofibromas show increased proliferation rates under the influence of progesterone. Pediatr Res. 2008;64(1):40–43. [DOI] [PubMed] [Google Scholar]
- 80. Fishbein L, Zhang X, Fisher LB, et al. In vitro studies of steroid hormones in neurofibromatosis 1 tumors and Schwann cells. Mol Carcinog. 2007;46(7):512–523. [DOI] [PubMed] [Google Scholar]
- 81. Cunha KS, Barboza EP, Fonseca EC. Identification of growth hormone receptor in plexiform neurofibromas of patients with neurofibromatosis type 1. Clinics (Sao Paulo). 2008;63(1):39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bizzarri C, Bottaro G. Endocrine implications of neurofibromatosis 1 in childhood. Horm Res Paediatr. 2015;83(4):232–241. [DOI] [PubMed] [Google Scholar]
- 83. Howell SJ, Wilton P, Lindberg A, et al. Growth hormone replacement and the risk of malignancy in children with neurofibromatosis. J Pediatr. 1998;133(2):201–205. [DOI] [PubMed] [Google Scholar]
- 84. Vassilopoulou-Sellin R. Precocious puberty, growth hormone deficiency, and neurofibromatosis. J Pediatr. 1996;128(1):166. [DOI] [PubMed] [Google Scholar]
- 85. Rkein AM, Ozog DM. Photodynamic therapy. Dermatol Clin. 2014;32(3):415–425, x. [DOI] [PubMed] [Google Scholar]
- 86. Rossi R, Bruscino N, Ricceri F, et al. Photodynamic treatment for viral infections of the skin. G Ital Dermatol Venereol. 2009;144(1):79–83. [PubMed] [Google Scholar]
- 87. Lee MJ, Hung SH, Huang MC, et al. Doxycycline potentiates antitumor effect of 5-aminolevulinic acid-mediated photodynamic therapy in malignant peripheral nerve sheath tumor cells. Plos One. 2017;12(5):e0178493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Whelan HT. [NCT01682811] Photodynamic therapy (PDT) for benign dermal neurofibromas (NF1). Clinicaltrials.gov. 2012. [Google Scholar]
- 89. Vranceanu AM, Merker VL, Park E, et al. Quality of life among adult patients with neurofibromatosis 1, neurofibromatosis 2 and schwannomatosis: a systematic review of the literature. J Neurooncol. 2013;114(3):257–262. [DOI] [PubMed] [Google Scholar]
- 90. Bradford J, Perrin D. A benchmark of computational CRISPR-Cas9 guide design methods. PLoS Comput Biol. 2019;15(8):e1007274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. So WK, Cheung TH. Molecular regulation of cellular quiescence: a perspective from adult stem cells and its niches. Methods Mol Biol. 2018;1686:1–25. [DOI] [PubMed] [Google Scholar]
- 92. Mitra M, Ho LD, Coller HA. An in vitro model of cellular quiescence in primary human dermal fibroblasts. Methods Mol Biol. 2018;1686:27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]