Abstract
Plexiform neurofibromas (PNF) are peripheral nerve tumors caused by bi-allelic loss of NF1 in the Schwann cell (SC) lineage. PNF are common in individuals with Neurofibromatosis type I (NF1) and can cause significant patient morbidity, spurring research into potential therapies. Immune cells are rare in peripheral nerve, whereas in PNF 30% of the cells are monocytes/macrophages. Mast cells, T cells, and dendritic cells (DCs) are also present. NF1 mutant neurofibroma SCs with elevated Ras-GTP signaling resemble injury-induced repair SCs, in producing growth factors and cytokines not normally present in SCs. This provides a cytokine-rich environment facilitating PNF immune cell recruitment and fibrosis. We propose a model based on genetic and pharmacologic evidence in which, after loss of Nf1 in the SC lineage, a lag occurs. Then, mast cells and macrophages are recruited to nerve. Later, T cell/DC recruitment through CXCL10/CXCR3 drives neurofibroma initiation and sustains PNF macrophages and tumor growth. Stat3 signaling is an additional critical mediator of neurofibroma initiation, cytokine production, and PNF growth. At each stage of PNF development therapeutic benefit should be achievable through pharmacologic modulation of leukocyte recruitment and function.
Keywords: CXCR3, dendritic cells, Interferon, Neurofibromatosis type 1, STAT3, T-cells
Key Points.
1. Neurofibroma formation is initiated by loss of the NF1 gene in Schwann cells and Schwann cell precursors.
2. Macrophage and mast cell recruitment to tumors is followed by recruitment of T cells and dendritic cells, which enable tumor formation.
3. In addition to therapies that act on established tumors, therapies that block these early events might prevent tumorigenesis.
Tumors can form at sites of chronic inflammation,1,2 suggesting that inflammation may contribute to tumorigenesis.3 Accumulating evidence supporting this idea led to the inclusion of inflammation and evasion of immune system surveillance as hallmarks of cancer.4 In several systems, tumor initiation is known to trigger the production of inflammatory cytokines/chemokines, with the resulting leukocyte infiltration leading to inflammation. The consequent inflammatory environment facilitates additional genetic mutations and subsequently activates inflammatory signaling. It does so through reactive oxygen species and subsequent DNA damage, enhancing further inflammation and promoting tumor growth and progression.5 Thus, inflammation can modulate the course of each stage of tumor development, but until recently had been little-studied in nerve tumors.
Genetic “driver” mutations occur in benign (cancer precursor) lesions.6 For example, an oncogenic BRAFV600E mutation is found in ≈90% of benign melanocytic nevi and 70% of serrated polyps, precursor lesions for melanoma, and colon cancer respectively.7–9APC tumor suppressor gene mutations cause benign colorectal adenomas that are susceptible to progression to malignant colorectal carcinomas.10 In NF1, patients harbor inactivating mutations in the NF1 tumor suppressor gene and develop benign peripheral nerve lesions called neurofibromas, susceptible to progression to malignant peripheral nerve sheath tumors (MPNSTs), highly aggressive soft tissue sarcomas.11 Some genetic driver mutations induce tumorigenesis and also proinflammatory signals. In a genomic analysis of >10,000 tumors from the TCGA database, NF1 was amongst the mutated genes (including TP53, HLA-B, BRAF, PTEN, APC, and CASP8) that correlated with high levels of leukocytes across cancer types.12 In neurofibromas and MPNSTs, a remarkable 30% of cells are macrophages.13,14 This review showcases recent progress suggesting that targeting the inflammatory milieu will provide therapeutic benefit for NF1 associated neurofibroma.
NF1, the Disease
As is described elsewhere in this volume, population-based studies highlight patient predisposition to cutaneous/dermal (DNF) and plexiform neurofibromas (PNF) in Neurofibromatosis type I (NF1).15,16 Briefly, PNF associated with large nerves may be congenital, and grow most rapidly in the first decade of life.17,18 A quarter of individuals with NF1 have visible or symptomatic PNF, and whole-body MRI shows that >50% have at least one PNF. PNF growth can compress the trachea, bladder, or other vital structures, causing significant morbidity and severe pain.19–22 DNF were recently shown to originate from HOXB7 expressing SC lineage involving the Hippo pathway23 and in boundary cap cells,24 and correlate with SC hyperplasia and increased innervation of skin appendages.25 DNF are solitary lesions in normal individuals, but thousands can develop in NF1 patients, largely during puberty, and pregnancy.26–28 Unlike DNF, PNF can transform to MPNSTs.11,29–31 Although this review is focused on PNF, we recognize that inflammation is also likely to be relevant to DNF.24
Many NF1 diagnostic findings involve hyperplastic or benign neoplastic processes. Cells in these tumors show loss of the Neurofibromin 1 (NF1) tumor suppressor gene function. In DNF and PNF, only SC show bi-allelic NF1 loss of function mutations (reviewed in ref. 32) Because the protein product of the Neurofibromin 1 gene, neurofibromin, functions as an off-signal for Ras family proteins, SCs with partial (NF1+/−) or complete (NF1−/−) loss of neurofibromin function, Ras-GTP signaling is elevated after cell stimulation. Basal Ras-GTP may also be elevated.33,34 This results in activation of numerous cellular signaling pathways altering many aspects of SC function: cellular growth, proliferation, migration, differentiation, and survival.33,35 Downstream of Ras activation, the Raf/MEK/ERK mitogen-activated protein kinase (MAPK) pathway is activated,36 and is of particular importance in neurofibroma. Thus, pharmacological inhibitors of MEK signaling shrink >70% of PNF in mouse models and shows similar efficacy in NF1 patients tested in small Phase1/2 clinical trials.37–39 Other Ras effector pathways likely also contribute to altered SC function, but are less studied. Some exceptions are a role for the RRAS2/TC21-AKT-TGF-β pathway in tumor initiation and a major role for the Stat3 pathway in Nf1 SC progenitor survival, neurofibroma initiation, and tumor growth,40,41 described in more detail below.
Immune Infiltrates in Neurofibroma
Leukocytes participate in peripheral nerve repair and variety of inflammatory processes, and the relative importance of specific leukocyte populations in neurofibroma development and growth is under intense investigation. In healthy peripheral nerves SC make up about 90% of cells, and innate immune cells are scarce. Resident macrophages comprise <5% of cells, mast cells are present at <1 per HPF, and other granulocytes and lymphocytes are largely absent.42 In contrast, neurofibromas are replete with immune cells, and inflammation has long been hypothesized to contribute to neurofibroma development. The importance of inflammation to non-tumor nerve pathology is suggested by a correlation between increased mast cell abundance in mouse models of autoimmune inflammatory disease of the nerves.43 In mouse models of Charcot–Marie–Tooth disease and EAN, T cells and inflammatory macrophages promote disruption of inflamed peripheral nerves.44–46 Also, macrophages play a key role in regulating nerve repair and Schwann cell (SC) function after nerve injury.47–49
Mast Cells
The increased numbers of mast cells in neurofibromas50,51 compared with normal nerve led to testing the hypothesis that infiltrating mast cells contribute to neurofibroma growth, pruritus or neuropathic pain in NF1 patients. A 1987 study tested ketotifen, an antihistamine and “mast cell stabilizer”, in 10 patients. Neurofibroma growth was not inhibited, yet patients reported symptomatic improvement of pain and pruritus, suggesting that mast cell activation may contribute to these symptoms.52 Mast cells have also been a focus of research in mouse models of PNF.53–55Nf1-null SC secrete the potent mast cell chemoattractant SCF (Kit ligand), and Nf1 heterozygous mast cells are hyper-responsive to SCF signaling.51 W41 mice have loss of function for the SCF receptor c-kit, and in a CNPase-hEGFR mouse model W41 mice show reduced nerve pathology (mast cell recruitment, axon-glial dissociation, fibrosis).55 A recent study tested whether mast cells were necessary for PNF formation. Scf (Kit ligand) loss in neurofibroma SC prevented mast cell recruitment but not neurofibroma development in Plp-CreERT2; Nf1fl/fl; Scff/fl mice.56
Nf1 heterozygous mast cells also secrete excess TGF-β, a profibrotic growth factor that can induce c-Abl-dependent proliferation and collagen deposition in fibroblasts, providing a possible mechanism for contribution of mast cell to NF1 associated nerve pathology.57 TGFβ is profibrotic and may play additional roles in tumor stroma.58,59 This idea led to preclinical and clinical trials of Imatinib, a c-Kit, and c-Abl inhibitor. Although not specific for mast cells, this treatment reduced plexiform neurofibroma growth in Krox20-Cre;Nf1−/fl mice and a subset of human patients.54,60,61 Thus, mast cells do not appear to be necessary for tumorigenesis, but likely contribute to aspects of plexiform neurofibroma biology.
T Cells and Dendritic Cells
Recent studies demonstrate the presence of T cells and dendritic cells (DCs) in human and mouse neurofibromas.62–64 In nerves and neurofibromas from Dhh-Cre;Nf1fl/fl mice CD11c+;CD11b− DCs are present.63 CD3+ T cell populations in mouse and human neurofibromas are a mixed population of CD4+ and CD8+ T cells.64 In one study of 36 tumors, immunohistochemical analysis of HLA-A/-B/-C, B2M, and PD-L1 expression was correlated with numbers of neurofibroma and MPNST lymphocytes (CD4+ (cytotoxic T), CD8+ (cytotoxic T), FOXP3+ (suppressive T), CD45RO+ (memory T), and CD56+ (NKT). All cell types were present, but numbers showed significant heterogeneity among patient samples. Although T cells are frequently examined in the context of their antitumor functions, T cell-mediated chronic inflammation can also contribute to tumor development65; their role in PNF remains unstudied.
Macrophages
Macrophages are the dominant innate immune cell population in neurofibromas. Indeed, macrophages make up a remarkable 20–40% of mouse and human PNF cells.14 Macrophages participate in immune surveillance in normal tissues, and in this role they can help inhibit tumor formation. However, once tumors become established, local tumor-associated macrophages (TAMs) are recruited from blood monocytes and/or through proliferation of local macrophages. These TAMS can be protumorigenic, providing trophic support for tumor cells, regulating angiogenesis, invasion, and fibrosis, and suppressing antitumor immune responses.66–68 Studies in mouse neurofibroma models support the idea that macrophages initially inhibit PNF development and, later, promote growth of established PNF. Other studies demonstrate roles for T cells and/or DCs for sustaining TAMs within neurofibromas (see below).
Given that macrophages are the major immune cell population in human and mouse PNF, efforts to characterize these cells are ongoing.14,56 Markers of “M1” and “M2” macrophage polarization are useful as read-outs for these distinct macrophage functions, whereas single markers do not convey macrophage phenotypic diversity and the various mechanisms by which macrophages suppress or facilitate tumor development and growth.66–68 Expression of iNOS (an M1 marker) was detected in one PNF model.56 However, genome wide neurofibroma macrophage gene expression of sorted F4/80+;Cd11b+ macrophages did not meaningfully correlate with defined M1/M2 polarization phenotypes as described,69 but rather showed a mixed phenotype; it remains unclear whether two populations are present, or if all cells show a mixed phenotype.70 Overall, the subtypes and phenotypic identities of T cells, monocytes/macrophages, and DCs in PNF requires further analysis.
Nf1+/ − Hematopoietic Cells and Neurofibroma Formation in Mouse Models
In some mouse models of PNF an Nf1 heterozygous microenvironment is required for tumor development. For example, plexiform neurofibroma development in the Krox20-Cre model depends upon Nf1+/− bone marrow derived cells, supporting the intriguing idea that hematopoietic cells promote neurofibroma formation.54,56,71–73 However, the Nf1 heterozygous microenvironment only modestly accelerates PNF formation in other mouse models.74,75 Thus, inflammatory cells may be wild-type or NF1+/−; both can effectively contribute to neurofibroma development. This explains how PNF can form in patients who are somatic mosaic for NF1 mutation, and also, albeit rarely, in the general population.
Similarities Between Injured Nerve and Neurofibroma
After nerve cut or crush injury, and in neuritis, numbers of immune cells (mast cells, macrophages, and T cells) become elevated.43,46,48,76,77 To test if nerve injury potentiates tumorigenesis, the sciatic nerve was cut in adult Nf1 heterozygous mice. This generated pigmented melanocytes (possibly through transdifferentiation of SC) and rare neurofibromas.78 Ribeiro et al.71 demonstrated that adult P0-CreER;Nf1fl/fl mice, which do not form neurofibromas, do so after nerve crush, correlating with an influx of immune cells. Thus, injury with attendant inflammation can co-operate with Nf1 loss to drive tumor formation.
Nerve injury causes dramatic changes in SC; later the same SC re-differentiate as the injury is repaired.79 After nerve injury mature, quiescent, myelinating SCs, and Remak SCs dissociate from axons. Myelinating and nonmyelinating SCs both become “Repair” SCs, with altered gene expression, morphology, and behavior.79,80 Repair SCs have been described as trans-differentiated, as dedifferentiated, or as activated, each term reflecting the down-regulation of differentiation-associated genes and up-regulation of immature SC associated genes and of novel Repair-cell specific genes. For example, TGFβ receptor expression is up-regulated in Repair cells, and signaling through this receptor establishes the mesenchymal and invasive phenotype of the Repair SCs.80 Repair SCs also up-regulate expression and produce proinflammatory cytokines. These cytokines act on resident endoneurial macrophages, which expand up to ~10-fold in number and become activated. Repair SC proinflammatory cytokines also contribute to leukocyte recruitment from the blood, and infiltrating CCR2hi monocyte-derived macrophages significantly outnumber endoneurial macrophages; CCR2 is necessary for recruitment of these blood-derived cells.76,81
Consistent with the idea that tumors are “wounds that do not heal”, molecules that are characteristic of the injury and of the repair phase of the nerve injury response are expressed by neurofibroma SC. These include TGFβ, and cytokines and growth factors that support neuronal survival, stimulate leukocyte recruitment, and activate stromal populations.70 The persistence of inflammatory cells and cytokine expression by neurofibroma SC correlates with the increased signaling through Ras-GTP in NF1−/− SCs, due to loss of Nf1. Dysregulation of Raf/MEK/ERK signaling is also implicated in Charcot–Marie–Tooth associated peripheral nerve inflammation.82 pERK is acutely elevated after nerve injury, peaking within 24-h, and remaining activated above baseline levels for weeks.83 Importantly, transient hyperactivation of Raf1 signaling in myelinating SCs induces demyelination and inflammation,84 and increases in ERK activity driven by constitutively active MEK1DD accelerate Wallerian degeneration after nerve injury, resulting in prolonged inflammation and abnormal injury resolution.85,86 Ras-GTP leads to increased phosphorylation (activation) of activation of JNK and ERK, increasing expression of the proto-oncogenic AP-1 transcription factors (including c-Jun, FosB, and c-Fos). AP-1 transcription factors also increase after nerve injury. C-jun is indispensable for formation of Repair SC types, but is not necessary for normal SC development, SC proliferation or macrophage recruitment.80,87,88
The absence of MEK prevents developmental SC formation,89 and as noted above, blocking MEK activity shrinks 75% of neurofibromas. This suggests that activating MEK might be sufficient to drive neurofibroma formation. In the injured nerve setting, activating MEK delayed repair and functional recovery, and reduced numbers of small caliber axons per Remak bundle, a phenotype observed in neurofibromas,76 but later tumor formation was not assessed. Transgenic expression of receptor tyrosine kinase signaling by EGFR expression in SCs (in CNPase-hEGFR mice) similarly mimics early mast cell recruitment and Remak bundle disruption, but neurofibromas rarely form, and macrophages are not significantly recruited to nerve.55 Why do tumors not form? Only loss of NF1 increases signaling through all Ras proteins, and signaling pathways in addition to MEK. A possibility we favor is that level or duration of RAS/MAPK pathway activation is critical. Supporting this idea, homozygous expression of EGFR increases neurofibroma formation, in comparison to that in EGFR heterozygotes.41 Also, sustained overexpression of type III-beta3 neuregulin, a SC growth factor, is sufficient to drive both nerve pathology and neurofibroma formation in mice.90
The increase in cytokine gene expression that occurs after nerve injury correlates with immune cell recruitment. Raf/MEK/ERK activation, loss of Nf1, and elevated EGFR signaling in SCs also show elevated cytokine expression and immune cell recruitment. In each of these settings, there is increased expression of the macrophage chemoattractant Ccl2, and the mast cell chemoattractant Scf.51,54,55 In cells sorted from neurofibromas, SCs show increased expression of leukocyte chemoattractants (eg Scf, Ccl2, Ccl5), fibrosis (eg Tgfb), and angiogenesis (eg Vegf).40,56,91 Computational reconstruction of molecular networks and signaling also predicted a role for type-1 interferon (IFN), a cytokine upstream in immune response signaling in neurofibromas.70 Confirming the computational prediction, treatment of neurofibroma-bearing mice with polyethylene glycolyated (PEG)-type-1 IFN-a-2b reduced expression of many cytokines, and neurofibroma growth was slightly reduced in a Phase II trial of PEGylated IFN-alpha-2b (NCT00678951).92
Temporal Features of Neurofibroma Immune Cell Recruitment
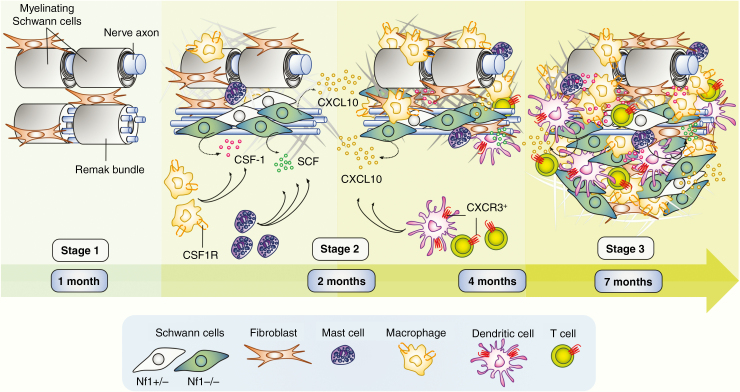
Mouse models have provided opportunities to study the timing of neurofibroma formation. After Nf1 loss in the SC lineage, the driver event in neurofibroma formation, a delay occurs. Peripheral nerves and DRG are grossly normal in 1-month old Dhh-Cre;Nf1fl/fl mice 14 and gene expression is not significantly different from controls70 (Figure 1; Stage 1). Thus, although SC lack Nf1 from mid-gestation these phenotypes, and macrophage infiltration, are absent at 1 month of age. Later, SCs and disruption of nonmyelinated axon-SC Remak bundles61,72 mast cell infiltration occurs, and fibrosis begins (Figure 1; Stage 2). By the 2 month time point, macrophages have become abundant, even though gene expression analysis in DhhCre;Nf1fl/fl70 revealed few differences from control. The macrophages present at this time point are therefore likely to be predominantly resident endoneurial macrophages.14 Also at 2 months, rare CD11c+; CD11b− DC and T cells are present in paraspinal nerve roots and ganglia, where tumors will form.14,63 Small discrete PNF are present by 4 months of age (Figure 1; Stage 3). Stage 3 tumors contain elevated numbers of DC and T cells, and show increased fibrosis and increased disruption of neuron-SC interactions.63
Figure 1.
Inflammation driven neurofibroma formation. After Nf1 loss in Schwann cells (SCs), a delay in phenotype occurs (Stage1). Subsequently, SCs show elevated growth factors/cytokine production (eg. CSF-1 and SCF), begin to show slight disruption of Remak bundles, and infiltration of mast cells. Macrophages are abundant by 2 months of age, and further cytokines are produced (eg CXCL10/IP-10), concurrent with occasional presence of CXCR3 positive dendritic cells (DCs) and T cells (Stage 2). By 4 months, small tumors form. These contain increased numbers of DCs, T cells. Macrophages remain abundant. Fibrosis is robust, and Remak bundle disruption dramatic. By 7 months, tumors enlarge; all features characterized at 4 months persist (Stage 3).
The idea that there is a window that occurs between Stage 1 and Stage 2, prior to the onset of significant inflammation, that may be useful therapeutically comes from experiments in which transient early blockade of EGFR signaling in CNPase-human EGFR mice prevented mast cell recruitment and fibrosis, Remak bundle disruption, and reduced expression of Ccl2, Scf, and Tgfb.41,93
At Stage 2 (2 months), changes in expression of only a few genes, including Cxcl10/Ip10, differentiate Dhh-Cre;Nf1fl/fl nerves from wild-type nerves, or from CNP-EGFR nerves with nerve disruption but rare neurofibroma.63Cxcl10 was the only cytokine/growth factor with detectably elevated differential expression.63 Single cell RNA sequencing localized Cxcl10 to FABP7-expressing immature and/or satellite SCs which also showed low Nf1 expression. Making it a candidate to drive neurofibroma formation, the Cxcl10 receptor, Cxcr3, was expressed only by rare CD4+ T cells, CD8+ T cells, and DCs in preneurofibromas/inflamed Dhh-Cre;Nf1fl/fl DRG. Further studies are needed to define all T cell and DC subsets, and their activation states. To test the importance of CXCL10/CXCR3 signaling in neurofibroma development, we generated Dhh-Cre;Nf1fl/fl;Cxcr3-null mice. These animals had no nerve pathology at 7 months of age and did not develop PNF. Thus, recruitment of Cxcr3 expressing T cells and DCs occurs early in disease, is a critical contributor to neurofibroma development, and the absence of Cxcr3 prevents transition to Stage 3.
An important additional finding in this study was that mast cells and macrophages are recruited to mutant nerve, even in double mutant mice lacking Cxcr3. However, in the absence of Cxcr3, macrophage recruitment was not maintained.63 This result supports the idea that after loss of Nf1 (Stage 1) and macrophage recruitment (Stage 2), macrophages are sustained in neurofibromas by T cells and/or DCs.
PNF Initiation
STAT3 signaling is dispensable for the development of normal SCs, but it is critical for the autocrine growth factor mediated growth/survival of Repair SC after nerve injury.41,94 Our recent work shows that Stat3 is important for neurofibroma initiation and neurofibroma growth.41,95 In DhhCre;Nf1fl/fl;Stat3fl/fl mice, while PNF formed, they were both significantly reduced in number and significantly smaller than PNF in DhhCre;Nf1fl/fl mice; thus Stat3 contributes to tumor initiation and tumor growth. Mechanistically, EGFR activates P-Stat3 and increases SCP/neurofibroma-initiating cell self-renewal in vitro, a surrogate for tumor initiation. Further, IL-6 reinforced Jak2/Stat3 activation in SCPs and SCs, suggesting that levels of tyrosine kinase signaling in SCPs modify neurofibroma initiation. After nerve injury repair occurs in wild-type nerves, but when SCs lack STAT3, nerves show reduced expression of c-Jun, Ngfr, ErB2/3, and other Repair associated genes.66,68 Thus, Stat3 drives nerve repair in wild-type mice,96 but elevated Stat3 in promotes neurofibroma initiation and growth. As is the case for Ras-GTP, regulated levels of Stat3 may be necessary for optimal reapir. An RRAS2/TC21-AKT-TGF-β pathway appears to play a minor role in tumor initiation, with loss of TC21 delaying neurofibroma formation by a few months.37
Stat3, CCR2, and CSF1 in PNF Macrophage Function and Neurofibroma Enlargement
Raf/MEK/ERK and STAT3 signaling, in addition to their neurofibroma SC-intrinsic functions, are likely to play important roles in shaping a protumorigenic nerve microenvironment. For example, blocking MEK signaling in neurofibroma reduced tumor cell proliferation, and also reduced numbers of blood vessels, correlating with tumor shrinkage.37 In many tumor types, STAT3-mediated signaling promotes inflammatory gene expression, causing paracrine effects on immune cells.97 Neurofibromas that formed after Stat3 deletion contained reduced numbers of Iba1+;F4/80+;CD11b+ TAMs in established tumors.96 These findings are consistent with chronic inflammation supported by macrophages promoting Stat3-mediated tumor growth (Stages 3 and 4).66–68
To test this idea, we administered FLLL32, an inhibitor of JAK2/STAT3 signaling, to mice with PNF. Pharmacological inhibition of STAT3 signaling reduced neurofibroma growth in Dhh-Cre;Nf1fl/fl mice with established disease.96 Notably, significant SC and macrophage proliferation occurs in Dhh-Cre;Nf1fl/fl neurofibromas and proliferation in both cell types was suppressed by FLLL32. Subsequent analyses showed that expression of ligands for CCR2, an important mediator of CCR2hi monocyte recruitment, are significantly reduced in animals responding to treatment.96 Sorted F4/80+;CD11b+ macrophages isolated from wild-type and Dhh-Cre;Nf1fl/fl nerves and neurofibromas express Ccr270. However, loss of CCR2 in Dhh-Cre;Nf1fl/fl;Ccr2-null mice did not prevent tumor development or reduce the number of tumor macrophages in neurofibromas,96 so that if hematopoietic macrophages are relevant in neurofibroma, they are recruited through other mechanisms. The relative contributions of resident and hematopoietic macrophages to neurofibromas, and specific macrophage functions, remain unclear.
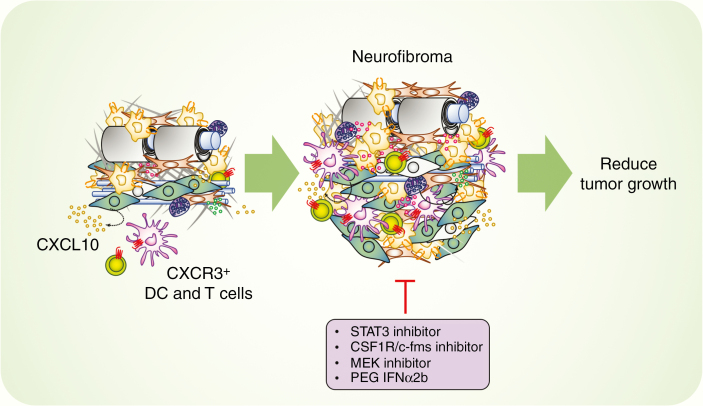
In many tumor types CSF-1/CSF1R signaling plays a central role in macrophage development, recruitment, and polarization toward a tumor-supportive phenotype.66–68 Long-term CSF1R inhibition is well-tolerated in adult mice, and CSF1R inhibitor therapy is an ongoing area of research interest.68,98 Prada et al. examined the effects of a CSF1R/c-fms inhibitor on Dhh-Cre;Nf1fl/fl mice.14 In established tumors, reduction of PNF growth correlated with macrophage depletion, but inhibition of CSF1R beginning at 1 month (prior to tumor formation), enhanced neurofibroma growth.14 This paradoxical effect of CSF1R inhibition could reflect either the inhibition of distinct macrophage populations or a global shift in macrophage function in the procession of neurofibroma development. Thus, macrophages in established neurofibromas appear to have protumor functions—consistent with other tumor macrophage populations—and the role of macrophages in neurofibroma initiation is likely to be antitumor. Overall, these data suggest that STAT3-targeted therapies—and other therapies targeting macrophages and neurofibroma growth—may be useful in PNF (Figure 2).
Figure 2.
Potential immunotherapy targets in neurofibroma. In neurofibroma mouse models, the CXCL10/CXCR3 axis involving dendritic cells and T cells is critical in the early development of neurofibroma (~2 months). Macrophages contribute to neurofibroma formation via the involvement of STAT3 and CSF-1/CSF1R signaling. Inhibitory molecules targeting STAT3, CSF-1/CSF1R, and pegylated Interferon alpha 2b in established neurofibroma modestly inhibit tumor growth. MEK inhibition significantly shrinks most neurofibromas.
Oncogenic Stress and Stages in Neurofibroma Development
What might cause the lag that occurs between loss of Nf1 in SCs and SCPs during embryogenesis and neurofibroma formation months later? High levels of cell stress, including stress driven by Ras activation, can cause cell cycle arrest, senescence or cell death, providing barriers to cancer.99 Ras activation can induce cellular senescence,100–104 and senescent cells fuel a proinflammatory and protumorigenic microenvironment by producing proteins in a so-called senescence associated secretory phenotype (SASP).105 Recent evidence shows that RAS oncogene-induced senescence drives the stimulator of interferon gene (STING) pathway, linking senescence to inflammation and cancer.106
In epithelia, even in precancerous hyperplastic lesions, replicative stress and early DNA damage are present, and correlate with cell senescence or apoptosis, delaying or preventing tumorigenesis. For example, activation of the ATM-Chk2-p53 pathway in premalignant epithelial tumors correlates with DNA damage; DNA damage activates the ATR/ATM-regulated checkpoint, reducing cell division and providing an inducible barrier against tumor progression.107 Ras-driven DNA damage in premalignant lesions may result from reduced origin licensing.108 Alterations that interfere with the DNA damage checkpoint are predicted to circumvent oncogenic stress and promote tumorigenesis. It is notable that DNA damage was a theme identified by transcriptome analysis of neurofibromas.109 In addition, downstream of oncogenic stress levels of CDKN2A increase, activating p53 to limit cell growth; in NF1 deficient mouse and human PNF CDKN2A expression is elevated.37 Also, increased Ink4a/Arf expression prevented SC proliferation and tumors in NSE-SMDF+/− mice.110
Another potential brake on neurofibroma formation downstream of Ras/MAPK signaling is suppression of the interferon response. Type 1 interferon receptor (IFNAR) deficiency allows spontaneous transformation of MEFs, and predisposes mice to DMBA/TPA-mediated papilloma formation in vivo.111 The cross talk between the Ras/MAPK and Interferon pathways is complicated. Ras/MAPK signaling stimulated by Nogo-B decreases expression of interferon (IFNRaR1)-regulated genes.112–114 IFNa-IFNaR1 signaling suppresses proliferation in cancer cells by decreasing P-ERK, independent of Ras,115 via JAK1/STAT1 signaling.116 Gene network analysis reveals genes induced by RAS/MAPK and by type I interferons; IFN-a signaling may limit transformation through co-regulated genes.117 Merging these lines of investigation, recent study suggests that Ras signaling drives DNA damage, which itself independently activates Interferon and suppresses p53, holding Ha-Ras-driven skin tumors in check.118 In this light, the reduced cytokines after treatment of neurofibroma with interferon may be of interest.92
Conclusion
Mast cell infiltration, progressive disruption of Remak bundle organization, SC hyperplasia, and collagen deposition are well-documented features of mouse and human neurofibroma. Yet, nerves showing these features do not necessarily progress to neurofibroma development, implying that additional events drive tumorigenesis. Nerve injury can elicit neurofibroma formation, and macrophages are present in large numbers in neurofibromas, supporting the idea that inflammation triggers potentiate nerve tumorigenesis driven by NF1−/− SC. Recent studies show that Stat3 signaling in SCs and tumor macrophages, and Cxcr3+ T cells and DCs recruited to neurofibromas by Cxcl10 expression in subpopulations of SCs, play critical roles neurofibroma initiation and growth, and sustain macrophage recruitment. Together these studies support a model in which NF1 mutant SC, after differentiation, are induced by local inflammation to drive tumor formation and growth.
Acknowledgements
This work was supported by the National Institutes of Heath (5 R01 NS028840-27); the Children’s Tumor Foundation; and Department of Defense—Congressionally Directed Medical Research Programs (DOD W81XWH-11-1-0057) (to N.R.), and (5 F30 NS096796-02) (to J.S.F.). Dr. Ratner receives research support from Revolution Medicine and Boehringer Ingelheim International GmbH. We thank David A. Hildeman and Jianqiang Wu (CCHMC) for critical review of the manuscript.
Conflict of interest statement
The authors have no relevant conflicts to disclose.
References
- 1. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. [DOI] [PubMed] [Google Scholar]
- 2. Virchow R. Die Krankhaften Geschwülste, Dreissig Vorlesungen Gehalten Während Des Wintersemesters 1862–1863 an Der Universität zu Berlin. Berlin: A. Hirschwald; 1863. [Google Scholar]
- 3. Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res. 2015;3(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 5. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kato S, Lippman SM, Flaherty KT, Kurzrock R. The conundrum of genetic “drivers” in benign conditions. J Natl Cancer Inst. 2016;108(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33(1):19–20. [DOI] [PubMed] [Google Scholar]
- 8. Yeh I, von Deimling A, Bastian BC. Clonal BRAF mutations in melanocytic nevi and initiating role of BRAF in melanocytic neoplasia. J Natl Cancer Inst. 2013;105(12):917–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan TL, Zhao W, Leung SY, et al. BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Res. 2003;63(16):4878–4881. [PubMed] [Google Scholar]
- 10. Esteller M, Sparks A, Toyota M, et al. Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res. 2000;60(16):4366–4371. [PubMed] [Google Scholar]
- 11. De Raedt T, Brems H, Wolkenstein P, et al. Elevated risk for MPNST in NF1 microdeletion patients. Am J Hum Genet. 2003;72(5):1288–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thorsson V, Gibbs DL, Brown SD, et al. The Immune Landscape of Cancer. Immunity. 2018;48(4):812–830.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patwardhan PP, Surriga O, Beckman MJ, et al. Sustained inhibition of receptor tyrosine kinases and macrophage depletion by PLX3397 and rapamycin as a potential new approach for the treatment of MPNSTs. Clin Cancer Res. 2014;20(12):3146–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prada CE, Jousma E, Rizvi TA, et al. Neurofibroma-associated macrophages play roles in tumor growth and response to pharmacological inhibition. Acta Neuropathol. 2013;125(1):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crowe FW. A Clinical, Pathological, and Genetic Study of Multiple Neurofibromatosis. Springfield, IL: Thomas; 1956. [Google Scholar]
- 16. Mulvihill JJ, Sorensen SA, Nielsen A. 4 Decades of neurofibromatosis (nf) (recklinghausen disease) in denmark – incidence of cancers. Am J Hum Genet. 1983;35(6):A68–A68. [Google Scholar]
- 17. Dagalakis U, Lodish M, Dombi E, et al. Puberty and plexiform neurofibroma tumor growth in patients with neurofibromatosis type I. J Pediatr. 2014;164(3):620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nguyen R, Dombi E, Widemann BC, et al. Growth dynamics of plexiform neurofibromas: a retrospective cohort study of 201 patients with neurofibromatosis 1. Orphanet J Rare Dis. 2012;7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prada CE, Rangwala FA, Martin LJ, et al. Pediatric plexiform neurofibromas: impact on morbidity and mortality in neurofibromatosis type 1. J Pediatr. 2012;160(3):461–467. [DOI] [PubMed] [Google Scholar]
- 20. Merker VL, Bredella MA, Cai W, et al. Relationship between whole-body tumor burden, clinical phenotype, and quality of life in patients with neurofibromatosis. Am J Med Genet A. 2014;164A(6):1431–1437. [DOI] [PubMed] [Google Scholar]
- 21. Plotkin SR, Davis SD, Robertson KA, et al. Sleep and pulmonary outcomes for clinical trials of airway plexiform neurofibromas in NF1. Neurology. 2016;87(7 Suppl 1):S13–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kongkriangkai AM, King C, Martin LJ, et al. Substantial pain burden in frequency, intensity, interference and chronicity among children and adults with neurofibromatosis Type 1. Am J Med Genet A. 2019;179(4):602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Z, Mo J, Brosseau JP, et al. Spatiotemporal loss of NF1 in schwann cell lineage leads to different types of cutaneous Neurofibroma susceptible to modification by the hippo pathway. Cancer Discov. 2019;9(1):114–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Radomska KJ, Coulpier F, Gresset A, et al. Cellular origin, tumor progression, and pathogenic mechanisms of cutaneous neurofibromas revealed by mice with Nf1 knockout in boundary cap cells. Cancer Discov. 2019;9(1):130–147. [DOI] [PubMed] [Google Scholar]
- 25. Rice FL, Houk G, Wymer JP, et al. The evolution and multi-molecular properties of NF1 cutaneous neurofibromas originating from C-fiber sensory endings and terminal Schwann cells at normal sites of sensory terminations in the skin. PLOS ONE. 2019;14(5):e0216527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ortonne N, Wolkenstein P, Blakeley JO, et al. Cutaneous neurofibromas: current clinical and pathologic issues. Neurology. 2018;91(2 Suppl 1):S5–S13. [DOI] [PubMed] [Google Scholar]
- 27. Duong TA, Bastuji-Garin S, Valeyrie-Allanore L, et al. Evolving pattern with age of cutaneous signs in neurofibromatosis type 1: a cross-sectional study of 728 patients. Dermatology. 2011;222(3):269–273. [DOI] [PubMed] [Google Scholar]
- 28. Dugoff L, Sujansky E. Neurofibromatosis type 1 and pregnancy. Am J Med Genet. 1996;66(1):7–10. [DOI] [PubMed] [Google Scholar]
- 29. Evans DG, O’Hara C, Wilding A, et al. Mortality in neurofibromatosis 1: in North West England: an assessment of actuarial survival in a region of the UK since 1989. Eur J Hum Genet. 2011;19(11):1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Masocco M, Kodra Y, Vichi M, et al. Mortality associated with neurofibromatosis type 1: a study based on Italian death certificates (1995–2006). Orphanet J Rare Dis. 2011;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duong TA, Sbidian E, Valeyrie-Allanore L, et al. Mortality associated with neurofibromatosis 1: a cohort study of 1895 patients in 1980-2006 in France. Orphanet J Rare Dis. 2011;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ratner N, Miller SJ. A RASopathy gene commonly mutated in cancer: the neurofibromatosis type 1 tumour suppressor. Nat Rev Cancer. 2015;15(5):290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim HA, Rosenbaum T, Marchionni MA, et al. Schwann cells from neurofibromin deficient mice exhibit activation of p21ras, inhibition of cell proliferation and morphological changes. Oncogene. 1995;11(2):325–335. [PubMed] [Google Scholar]
- 34. Sherman LS, Atit R, Rosenbaum T, et al. Single cell Ras-GTP analysis reveals altered Ras activity in a subpopulation of neurofibroma Schwann cells but not fibroblasts. J Biol Chem. 2000;275(39):30740–30745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang Y, Rangwala F, Fulkerson PC, et al. Role of TC21/R-Ras2 in enhanced migration of neurofibromin-deficient Schwann cells. Oncogene. 2004;23(2):368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simanshu DK, Nissley DV, McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170(1):17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jessen WJ, Miller SJ, Jousma E, et al. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest. 2013;123(1):340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dombi E, Baldwin A, Marcus LJ, et al. Activity of Selumetinib in Neurofibromatosis type 1-related plexiform Neurofibromas. N Engl J Med. 2016;375(26):2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jousma E, Rizvi TA, Wu J, et al. Preclinical assessments of the MEK inhibitor PD-0325901 in a mouse model of Neurofibromatosis type 1. Pediatr Blood Cancer. 2015;62(10):1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patmore DM, Welch S, Fulkerson PC, et al. In vivo regulation of TGF-β by R-Ras2 revealed through loss of the RasGAP protein NF1. Cancer Res. 2012;72(20):5317–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu J, Liu W, Williams JP, Ratner N. EGFR-Stat3 signalling in nerve glial cells modifies neurofibroma initiation. Oncogene. 2017;36(12):1669–1677 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Müller M, Leonhard C, Krauthausen M, et al. On the longevity of resident endoneurial macrophages in the peripheral nervous system: a study of physiological macrophage turnover in bone marrow chimeric mice. J Peripher Nerv Syst. 2010;15(4):357–365. [DOI] [PubMed] [Google Scholar]
- 43. Johnson D, Yasui D, Seeldrayers P. An analysis of mast cell frequency in the rodent nervous system: numbers vary between different strains and can be reconstituted in mast cell-deficient mice. J Neuropathol Exp Neurol. 1991;50(3):227–234. [DOI] [PubMed] [Google Scholar]
- 44. Kobsar I, Hasenpusch-Theil K, Wessig C, et al. Evidence for macrophage-mediated myelin disruption in an animal model for Charcot–Marie–Tooth neuropathy type 1A. J Neurosci Res. 2005;81(6):857–864. [DOI] [PubMed] [Google Scholar]
- 45. Kohl B, Fischer S, Groh J, et al. MCP-1/CCL2 modifies axon properties in a PMP22-overexpressing mouse model for Charcot–Marie–tooth 1A neuropathy. Am J Pathol. 2010;176(3):1390–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang M, Peyret C, Shi XQ, et al. Evidence from human and animal studies: pathological roles of CD8(+) T cells in autoimmune peripheral neuropathies. Front Immunol. 2015;6:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cattin AL, Burden JJ, Van Emmenis L, et al. Macrophage-induced blood vessels guide schwann cell-mediated regeneration of peripheral nerves. Cell. 2015;162(5):1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tomlinson JE, ˇygelytė E, Grenier JK, et al. Temporal changes in macrophage phenotype after peripheral nerve injury. J Neuroinflammation. 2018;15(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stratton JA, Holmes A, Rosin NL, et al. Macrophages regulate schwann cell maturation after nerve injury. Cell Rep. 2018;24(10):2561–2572.e6. [DOI] [PubMed] [Google Scholar]
- 50. Viskochil DH. It takes two to tango: mast cell and Schwann cell interactions in neurofibromas. J Clin Invest. 2003;112(12):1791–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang FC, Ingram DA, Chen S, et al. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for Nf1+/ − mast cells. J Clin Invest. 2003;112(12):1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Riccardi VM. A controlled multiphase trial of ketotifen to minimize neurofibroma-associated pain and itching. Arch Dermatol. 1993;129(5):577–581. [PubMed] [Google Scholar]
- 53. Staser K, Yang FC, Clapp DW. Mast cells and the neurofibroma microenvironment. Blood. 2010;116(2):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang FC, Ingram DA, Chen S, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/– and c-kit-dependent bone marrow. Cell. 2008;135(3):437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Monk KR, Wu J, Williams JP, et al. Mast cells can contribute to axon-glial dissociation and fibrosis in peripheral nerve. Neuron Glia Biol. 2007;3(3):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liao CP, Booker RC, Brosseau JP, et al. Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis. J Clin Invest. 2018;128(7):2848–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang FC, Chen S, Clegg T, et al. Nf1+/ − mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Hum Mol Genet. 2006;15(16):2421–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tang LY, Heller M, Meng Z, et al. Transforming growth factor-β (TGF-β) directly activates the JAK1-STAT3 axis to induce hepatic fibrosis in coordination with the SMAD pathway. J Biol Chem. 2017;292(10): 4302–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hamidi A, Song J, Thakur N, et al. TGF-beta promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85alpha. Sci Signal. 2017;10(486). doi:10.1126/scisignal.aal4186 [DOI] [PubMed] [Google Scholar]
- 60. Robertson KA, Nalepa G, Yang FC, et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial. Lancet Oncol. 2012;13(12):1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang F-C, Ingram D, Chen S, et al. Imatinib Mesylate reduces plexiform Neurofibromas by targeting the hematopoietic microenvironment. Blood. 2007;110(11):1915 LP– 1915. [Google Scholar]
- 62. Farschtschi S, Park SJ, Sawitzki B, et al. Effector T cell subclasses associate with tumor burden in neurofibromatosis type 1 patients. Cancer Immunol Immunother. 2016;65(9):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fletcher JS, Wu J, Jessen WJ, et al. Cxcr3-expressing leukocytes are necessary for neurofibroma formation in mice. JCI Insight. 2019;4(3). doi: 10.1172/jci.insight.98601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Haworth KB, Arnold MA, Pierson CR, et al. Immune profiling of NF1-associated tumors reveals histologic subtype distinctions and heterogeneity: implications for immunotherapy. Oncotarget. 2017;8(47):82037–82048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Winkler AE, Brotman JJ, Pittman ME, et al. CXCR3 enhances a T-cell-dependent epidermal proliferative response and promotes skin tumorigenesis. Cancer Res. 2011;71(17):5707–5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cortez-Retamozo V, Etzrodt M, Newton A, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci USA. 2012;109(7):2491–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li X, Yao W, Yuan Y, et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66(1):157–167. [DOI] [PubMed] [Google Scholar]
- 68. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Müller S, Kohanbash G, Liu SJ, et al. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 2017;18(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Choi K, Komurov K, Fletcher JS, et al. An inflammatory gene signature distinguishes neurofibroma Schwann cells and macrophages from cells in the normal peripheral nervous system. Sci Rep. 2017;7:43315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ribeiro S, Napoli I, White IJ, et al. Injury signals cooperate with Nf1 loss to relieve the tumor-suppressive environment of adult peripheral nerve. Cell Rep. 2013;5(1):126–136. [DOI] [PubMed] [Google Scholar]
- 72. Zheng H, Chang L, Patel N, et al. Induction of abnormal proliferation by nonmyelinating schwann cells triggers neurofibroma formation. Cancer Cell. 2008;13(2):117–128. [DOI] [PubMed] [Google Scholar]
- 73. Zhu Y, Ghosh P, Charnay P, et al. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296(5569):920–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brosseau JP, Liao CP, Wang Y, et al. NF1 heterozygosity fosters de novo tumorigenesis but impairs malignant transformation. Nat Commun. 2018;9(1):5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wu J, Williams JP, Rizvi TA, et al. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell. 2008;13(2):105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Müller M, Wacker K, Getts D, et al. Further evidence for a crucial role of resident endoneurial macrophages in peripheral nerve disorders: lessons from acrylamide-induced neuropathy. Glia. 2008;56(9):1005–1016. [DOI] [PubMed] [Google Scholar]
- 77. Moalem G, Monsonego A, Shani Y, et al. Differential T cell response in central and peripheral nerve injury: connection with immune privilege. FASEB J. 1999;13(10):1207–1217. [DOI] [PubMed] [Google Scholar]
- 78. Rizvi TA, Huang Y, Sidani A, et al. A novel cytokine pathway suppresses glial cell melanogenesis after injury to adult nerve. J Neurosci. 2002;22(22):9831–9840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594(13):3521–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Clements MP, Byrne E, Camarillo Guerrero LF, et al. The wound microenvironment reprograms schwann cells to invasive mesenchymal-like cells to drive peripheral nerve regeneration. Neuron. 2017;96(1):98–114.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mueller M, Leonhard C, Wacker K, et al. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab Invest. 2003;83(2):175–185. [DOI] [PubMed] [Google Scholar]
- 82. Fledrich R, Stassart RM, Klink A, et al. Soluble neuregulin-1 modulates disease pathogenesis in rodent models of Charcot-Marie-Tooth disease 1A. Nat Med. 2014;20(9):1055–1061. [DOI] [PubMed] [Google Scholar]
- 83. Sheu JY, Kulhanek DJ, Eckenstein FP. Differential patterns of ERK and STAT3 phosphorylation after sciatic nerve transection in the rat. Exp Neurol. 2000;166(2):392–402. [DOI] [PubMed] [Google Scholar]
- 84. Harrisingh MC, Perez-Nadales E, Parkinson DB, et al. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004;23(15):3061–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cervellini I, Galino J, Zhu N, et al. Sustained MAPK/ERK activation in adult Schwann cells impairs nerve repair. J Neurosci. 2017;38(3):679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sheean ME, McShane E, Cheret C, et al. Activation of MAPK overrides the termination of myelin growth and replaces Nrg1/ErbB3 signals during Schwann cell development and myelination. Genes Dev. 2014;28(3):290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kim S, Maynard JC, Strickland A, et al. Schwann cell O-GlcNAcylation promotes peripheral nerve remyelination via attenuation of the AP-1 transcription factor JUN. Proc Natl Acad Sci USA. 2018;115(31):8019–8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Arthur-Farraj PJ, Morgan CC, Adamowicz M, et al. Changes in the coding and non-coding transcriptome and DNA Methylome that define the schwann cell repair phenotype after nerve injury. Cell Rep. 2017;20(11):2719–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Newbern JM, Li X, Shoemaker SE, et al. Specific functions for ERK/MAPK signaling during PNS development. Neuron. 2011;69(1):91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gomez-Sanchez JA, Lopez de Armentia M, Lujan R, et al. Sustained axon-glial signaling induces Schwann cell hyperproliferation, Remak bundle myelination, and tumorigenesis. J Neurosci. 2009;29(36):11304–11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Williams JP, Wu J, Johansson G, et al. Nf1 mutation expands an EGFR-dependent peripheral nerve progenitor that confers neurofibroma tumorigenic potential. Cell Stem Cell. 2008;3(6):658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jakacki RI, Dombi E, Steinberg SM, et al. Phase II trial of pegylated interferon alfa-2b in young patients with neurofibromatosis type 1 and unresectable plexiform neurofibromas. Neuro Oncol. 2017;19(2):289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ling BC, Wu J, Miller SJ, et al. Role for the epidermal growth factor receptor in neurofibromatosis-related peripheral nerve tumorigenesis. Cancer Cell. 2005;7(1):65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bournazou E, Bromberg J. Targeting the tumor microenvironment: JAK-STAT3 signaling. JAKSTAT. 2013;2(2):e23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu J, Keng VW, Patmore DM, et al. Insertional mutagenesis identifies a STAT3/Arid1b/β-catenin pathway driving neurofibroma initiation. Cell Rep. 2016;14(8):1979–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fletcher JS, Springer MG, Choi K, et al. STAT3 inhibition reduces macrophage number and tumor growth in neurofibroma. Oncogene. 2019;38(15):2876–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sauter KA, Pridans C, Sehgal A, et al. Pleiotropic effects of extended blockade of CSF1R signaling in adult mice. J Leukoc Biol. 2014;96(2):265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136(5):823–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Michaloglou C, Vredeveld LC, Soengas MS, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436(7051):720–724. [DOI] [PubMed] [Google Scholar]
- 101. Dhomen N, Reis-Filho JS, da Rocha Dias S, et al. Oncogenic braf induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15(4):294–303. [DOI] [PubMed] [Google Scholar]
- 102. Cole AM, Ridgway RA, Derkits SE, et al. p21 loss blocks senescence following Apc loss and provokes tumourigenesis in the renal but not the intestinal epithelium. EMBO Mol Med. 2010;2(11):472–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27(20):2801–2809. [DOI] [PubMed] [Google Scholar]
- 104. Larribere L, Wu H, Novak D, et al. NF1 loss induces senescence during human melanocyte differentiation in an iPSC-based model. Pigment Cell Melanoma Res. 2015;28(4):407–416. [DOI] [PubMed] [Google Scholar]
- 105. Coppé JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLOS Biol. 2008;6(12):2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Li T, Chen ZJ. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med. 2018;215(5):1287–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bartkova J, Horejsí Z, Koed K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434(7035):864–870. [DOI] [PubMed] [Google Scholar]
- 108. Hills SA, Diffley JF. DNA replication and oncogene-induced replicative stress. Curr Biol. 2014;24(10):R435–R444. [DOI] [PubMed] [Google Scholar]
- 109. Pemov A, Hansen NF, Sindiri S, et al. Low mutation burden and frequent loss of CDKN2A/B and SMARCA2, but not PRC2, define pre-malignant neurofibromatosis type 1-associated atypical neurofibromas. Neuro Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gomez-Sanchez JA, Gomis-Coloma C, Morenilla-Palao C, et al. Epigenetic induction of the Ink4a/Arf locus prevents Schwann cell overproliferation during nerve regeneration and after tumorigenic challenge. Brain. 2013;136(Pt 7):2262–2278. [DOI] [PubMed] [Google Scholar]
- 111. Chen HM, Tanaka N, Mitani Y, et al. Critical role for constitutive type I interferon signaling in the prevention of cellular transformation. Cancer Sci. 2009;100(3):449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Battcock SM, Collier TW, Zu D, et al. Negative regulation of the alpha interferon-induced antiviral response by the Ras/Raf/MEK pathway. J Virol. 2006;80(9):4422–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Klampfer L, Huang J, Corner G, et al. Oncogenic Ki-ras inhibits the expression of interferon-responsive genes through inhibition of STAT1 and STAT2 expression. J Biol Chem. 2003;278(47): 46278–46287. [DOI] [PubMed] [Google Scholar]
- 114. Ahn DG, Sharif T, Chisholm K, et al. Ras transformation results in cleavage of reticulon protein Nogo-B that is associated with impairment of IFN response. Cell Cycle. 2015;14(14):2301–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Silvennoinen O, Schindler C, Schlessinger J, et al. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science. 1993;261(5129):1736–1739. [DOI] [PubMed] [Google Scholar]
- 116. Inamura K, Matsuzaki Y, Uematsu N, et al. Rapid inhibition of MAPK signaling and anti-proliferation effect via JAK/STAT signaling by interferon-alpha in hepatocellular carcinoma cell lines. Biochim Biophys Acta. 2005;1745(3):401–410. [DOI] [PubMed] [Google Scholar]
- 117. Komatsu Y, Hirasawa K, Christian SL. Global gene analysis identifying genes commonly regulated by the Ras/Raf/MEK and type I IFN pathways. Genom Data. 2015;4:84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Messenger ZJ, Hall JR, Jima DD, et al. C/EBPβ deletion in oncogenic Ras skin tumors is a synthetic lethal event. Cell Death Dis. 2018;9(11):1054. [DOI] [PMC free article] [PubMed] [Google Scholar]