Abstract
Background
Pediatric neurofibromatosis type 1 (NF1)–associated optic pathway gliomas (OPGs) exhibit different clinico-radiological features, treatment, and outcome compared with sporadic OPGs. While NF1-associated OPGs are caused by complete loss-of-function of the NF1 gene, other genetic alterations of the RAS-MAPK pathway are frequently described in the sporadic cases. We identified a group of patients who presented OPGs with typical radiological features of NF1-associated OPGs but without the NF1 diagnostic criteria. We aim to investigate into the possible molecular mechanisms underlying this “NF1-like” pediatric OPGs presentation.
Methods
We analyzed clinico-radiological features of 16 children with NF1-like OPGs and without NF1 diagnostic criteria. We performed targeted sequencing of the NF1 gene in constitutional samples (n = 16). The RAS-MAPK pathway major genes were sequenced in OPG tumor samples (n = 11); BRAF FISH and IHC analyses were also performed.
Results
In one patient’s blood and tumor samples, we identified a NF1 nonsense mutation (exon 50: c.7285C>T, p.Arg2429*) with ~8% and ~70% VAFs, respectively, suggesting a mosaic NF1 mutation limited to the brain (segmental NF1). This patient presented signs of neurodevelopmental disorder. We identified a somatic alteration of the RAS-MAPK pathway in eight tumors: four BRAF activating p.Val600Glu mutations, three BRAF:KIAA oncogenic fusions, and one putative gain-of-function complex KRAS indel inframe mutation.
Conclusions
NF1-like OPGs can rarely be associated with mosaic NF1 that needs specific constitutional DNA analyses for diagnosis. Further studies are warranted to explore unknown predisposition condition leading to the NF1-like OPG presentation, particularly in patients with the association of a neurodevelopmental disorder.
Keywords: BRAF fusion, mosaicism, NF1, pediatric optic pathway glioma, pilocytic astrocytoma, segmental neurofibromatosis
Key Points.
Segmental neurofibromatosis type 1 (NF1) do rarely exist in the brain of children with specific radiological presentation of their optic pathway glioma.
Ultra-deep sequencing is able to detect NF1-mosaïcism and when available NF1 mutation should be searched in the tumor as well.
Detection of NF1 in children with OPG is of paramount importance since it can modify the management of the tumor.
Importance of the Study.
Neurofibromatosis type 1 (NF1)-like radiological presentation of OPG in patients without NF1 diagnostic criteria raises the question of possible NF1 mosaicism, especially in patients with associated neurodevelopmental disabilities. The present report confirms this possibility in rare cases that does not, however, account for most of the cases studied here. Further studies are needed to better characterize these conditions. Our study also suggests that NF1 testing in the blood of OPGs patients should be performed at a higher sequencing depth than usually performed in order to evidence such conditions. Moreover, sequencing of the NF1 gene should be part of the diagnostic work-out on the tumor itself when more frequent alterations have been excluded, especially if the radiological appearance of the tumor is suggestive of a NF1-associated OPG.
Pediatric optic pathway gliomas (OPGs) account for 20% of brain tumors, usually in children under 2 years and the main challenge of treatment is the visual and developmental outcome.1,2 These low-grade tumors may develop sporadically (without a known inherited basis) or form in the context of the neurofibromatosis type 1 (NF1) tumor predisposition syndrome. Sporadic and NF1-associated OPGs have different radiological features, treatments, and outcomes,3–7 but both show activation of the MAPK (mitogen-activated protein kinase) and mTOR pathways.
In sporadic OPGs, the most common somatic genetic alteration is a genomic rearrangement resulting in the generation of a fusion transcript in which the kinase domain of the BRAF gene is fused to a gene of unknown function (KIAA1549).8–11 These fusion BRAF alterations create a BRAF molecule lacking the regulatory amino terminal domain, leading to increased BRAF activation of the downstream MEK signaling cascade. While KIAA1549 is the most commonly reported fusion partner, other genomic regions can undergo rearrangement to generate alternate fusion BRAF molecules. Further genomic studies revealed the presence of other potential driver mutations or fusions in sporadic OPGs, including the KRAS, FGFR1, PTPN11, RAF1, and NTRK2 genes.
NF1-associated OPGs harbor bi-allelic inactivation of the NF1 tumor suppressor gene (Neurofibromin 1; MIM 613113). As such, patients with NF1 are born with one mutated copy of the NF1 gene (constitutional NF1 gene mutation) and develop tumors following somatic (acquired) loss of the remaining NF1 allele in the tumor without any other recurrent oncogenic alteration.12–14 The clinical diagnosis of NF1 is based on the clinical diagnostic criteria outlined in the National Institutes of Health (NIH) consensus development conference in 1987.15 The main features of NF1 are multiple neurofibromas, café-au-lait (CAL) spots, axillary freckling, Lisch nodules, tibial pseudarthrosis, learning and attention deficits, and a predisposition to develop benign and malignant nervous system tumors.
Neuropsychological outcome of sporadic and NF1-associated pediatric OPGs is different.13,16,17 NF1-associated OPGs may even occur before other NF1 clinical features are present. In addition, the great variation in phenotypic expressivity in NF1 patients and the possible occurrence of mosaicism further complicate the diagnosis. In these cases, the molecular analysis of the NF1 gene may be helpful to recognize the condition as a form of NF1. However, the molecular diagnosis of NF1 can be challenging due to the large size of the NF1 gene, the existence of multiple pseudogenes, the lack of mutational hotspots, and the great allelic heterogeneity.14,18
In clinical practice, one can suspect an undiagnosed NF1, based only on radiological appearance of the OPG. Generally, the NF1-associated OPGs are infiltrative, involving the optic nerves, chiasm, and/or posterior optic radiations.16,19–21 Their growth involves the optic nerves in the pathways intimately. In contrast, sporadic OPGs frequently present earlier with larger tumors, are often limited to the chiasm, and have a higher risk of progression (Table 1).16,19,20
Table 1.
Comparison between sporadic and NF1-associated OPGs in children
| NF1-associated | Sporadic | |
|---|---|---|
| Age | < 6 years | < 6 years (frequently <1 year) |
| Location | Optic nerve, chiasm, and posterior optic radiations | Chiasm |
| Relation with visual pathway | Infiltration | Compression |
| Risk of progression | Around 50% | Constant |
| Spontaneous regression | Possible | No |
| Visual Acuity Impairment | More important (+++) | Less important (++) |
| Motor impairment | Less important (++) | More important (+++) |
| Histopathology | Pilocytic Astrocytoma | Pilocytic Astrocytoma, Ganglioglioma |
| Molecular alterations | NF1 loss | KIAA1549-BRAF fusion+++, Other fusions or mutations, less frequently: NTRK2, FGFR1, BRAF V600E, KRAS, RAF1, PTPN11 |
In our clinical records, we have identified a group of patients who presented OPGs with typical radiological features of NF1-associated OPGs but without the NF1 diagnostic criteria: we called this type of presentation “NF1-like” pediatric OPGs. In this study, we aimed to investigate into the possible molecular mechanisms underlying this “NF1-like” pediatric OPGs presentation affecting children who do not have the clinical criteria to formulate the diagnosis of NF1. We therefore thoroughly described clinical and radiological characteristics of these patients with NF1-like pediatric OPGs. Then, we performed next generation sequencing (NGS) analysis of the NF1 gene in blood and paired tumor DNAs. Finally, we analyzed the RAS-MAPK pathway using a large NGS panel in tumor DNAs.
Materials and Methods
Patients
We analyzed clinical and radiological data of the pediatric patients seen in consultation at Gustave Roussy (Villejuif) with diagnosis of NF1-like OPG seen followed at Gustave Roussy (Villejuif, France) between January and December 2017. All patients had an ophthalmological and dermatological screening and a genetic consultation. The written informed consents were obtained during the genetic consultation. The diagnosis of NF1-like OPGs was based on (i) histological diagnosis of low-grade glioma, (ii) the presence of radiologic features of NF1-associated OPGs (infiltrating tumors spreading thorough the optic nerves, chiasm, and/or posterior optic radiations), and (iii) the absence of NF1 diagnosis criteria (National Institutes of Health consensus development conference, 1988).15 The protocol has been approved by the IRB at Gustave Roussy and is in line with the current French legislation on genetic studies.
Clinical characteristics
We reviewed age, signs and symptoms at diagnosis, family history, treatment modalities, and evolution over time. Sign and symptoms of neuro-developmental disorder were thoroughly collected. Patients with three or more of the following symptoms were classified into the neurodevelopmental syndrome group: macrocephaly, facial dysmorphia, pigmentation abnormalities, stereotypies, epilepsy, and attention-deficit/hyperactivity disorder (ADHD).
Radiological features
Patients were classified into two groups according to the tumor characteristics on magnetic resonance imaging (MRI) scans. We considered tumor location (structures anatomically affected), the bilaterality and symmetry of the lesions, and tumor extension in the optic pathway. Tumors spreading at the entire optic pathway and in some cases with major extra-optic component were classified as radiological group 1. Tumors mainly localized at chiasm with slight bilateral changes at the optic radiation fibers were classified as radiological group 2.
Samples, Pathological Review, and DNA Extraction
All patients underwent genetic consultation and a written informed consent and blood samples were obtained. Available tumors tissue samples were centrally reviewed at Hôpital Sainte-Anne (AP-HP, France) by expert neuropathologists (A.T-.E; P.V.). Immunohistochemistry (IHC) was performed to detect BRAF p.Val600Glu mutation, as described previously.22 Fluorescence in situ hybridization (FISH) was performed to detect all BRAF fusion variants, including the FAM131B fusion, using labeled FISH probes for identification of gene split. DNA was isolated from peripheral blood mononuclear cells and tumor samples using standard proteinase K digestion followed by phenol–chloroform extraction. DNA concentrations were quantified using a Qubit 2.0 Fluorometer with the Quant-iT dsDNA HS assay kit (ThermoFisher, Saint-Aubin, France).
Next Generation Sequencing
We performed an NGS analysis of the NF1 gene in blood and tumor samples, as described previously.18 We also performed an NGS analysis of the RAS-MAPK pathway in the tumor samples.
NF1 sequencing
Amplicon sequencing libraries were prepared from 10 ng of DNA per sample according to the TruSeq Custom Amplicon Library Preparation Guide (Illumina Inc, San Diego, CA). The custom primer panel targets the entire NF1-coding exons, intron boundaries (25 bp), and the 5′ and 3′ untranslated regions (UTRs) with amplicons with an average size of 150 bp. The pooled libraries were paired-end (2 × 150) and sequenced with NextSeq 500 Mid Output Kit v2 on a NextSeq500 instrument (Illumina). After demultiplexing and generation of FASTQ files, the sequence analysis was performed according to the Genome Analysis Tool Kit (GATK) guidelines using Polyquery (Paris Descartes University, Paris, France). Assessment of variants implication was performed based on population databases (dbSNP and GnomAD), mutation databases (HGMD and COSMIC), and predictions software (Alamut and mutation taster). To confirm the results, we performed a second NGS analysis of NF1 in the entire cohort with another technique (Ion Torrent, ThermoFisher).
RAS-MAPK panel sequencing
We performed an NGS analysis of the RAS-MAPK pathway in the tumor samples. The Ion AmpliSeq custom panel was designed using the Ampliseq designer software (ThermoFisher). The panel targeted the coding sequences and IVS boundaries of the following genes encoding major RAS-MAPK pathway components and regulators: BRAF, RAF1, RRAS, HRAS, KRAS, NRAS, MAP2K1, MAP2K2, SOS1, CBL, ETV5, PTPN11, RASA1, RASA2, SPRED1, SPRED2, SPRED3, SPRY1, SPRY2, and SPRY4. NGS library preparation used the Ion AmpliSeq Library Kit 2.0 according to the manufacturer’s instructions. We used the Ion Personal Genome Machine System (ThermoFisher). Ion Reporter 5.6 (ThermoFisher) was used to perform the calling of the variants from the BAM files. Variants annotation and filtering was performed according to the Genome Analysis Tool Kit (GATK) guidelines using Polyquery (Paris Descartes University). Assessment of variants implication was performed based on population databases (dbSNP and GnomAD), mutation databases (COSMIC), and predictions software (Alamut, mutation taster, OncoKB, and Cancer Genome Interpreter).
Results
Patients Characteristics
Between January 2017 and December 2017, 16 patients met the inclusion criteria (Table 2). The median age at diagnosis was 2 years (range 0–10). Five patients (P1–P5) had a neurodevelopmental disorder as defined above, two of them (patients P3 and P4) with a very similar clinical spectrum including facial dysmorphia, macrocephaly, and stereotypies. Café-au-lait spots were found in five patients, but spots’ number and size did not meet NF1 criteria. Patient P16 presented a giant CAL lesion covering the entire right hemithorax.
Table 2.
Patients characteristics.
| Patient | Age at diagnosis (y) | Neuro-developmental abnormalities | Localization | Histopathology |
|---|---|---|---|---|
| P1 | 2 | CAL, Epilepsy, ADHD | Chiasma, Optical Nerves, Temporal Lobe, Optical Radiations | Pilocytic Astrocytoma |
| P2 | 6 | Epilepsy, ADHD, Precocious Puberty, FH | Chiasma, Temporal Lobe, Intraventricular | Ganglioglioma |
| P3 | 0 | Facial dysmorphia, Epilepsy, Macrocephaly, Stereotypies, Psychomotor Retardation, Growth Delay, FH | Chiasma, Thalamic and Peduncular lesions | Unclassifiable (PA vs GG) |
| P4 | 0 | CAL, Facial dysmorphia, Macrocephaly, Stereotypies, Leukocoria | Chiasma, Optical Nerves, Peduncular lesions, Optical Radiations | Pilocytic Astrocytoma |
| P5 | 0 | CAL, Intrauterine growth restriction, Psychomotor Retardation, Stereotypies, Growth Delay | Chiasma, Optical Nerves, Peduncular lesions, Optical Radiations | Pilocytic Astrocytoma |
| P6 | 4 | Macrocephaly, Learning difficulties | Chiasma, Optical Nerves, Peduncular lesions, Optical Radiations | Pilocytic Astrocytoma |
| P7 | 5 | Absence | Chiasma, Optical Nerves, Peduncular lesions, Optical Radiations | NSI |
| P8 | 2 | Absence | Chiasma, Optical Nerves, Peduncular lesions, Optical Radiations | NSI |
| P9 | 2 | Absence | Chiasma, Optical Nerves, Optical Tracts and Radiations | NSI |
| P10 | 10 | CAL, Precocious Puberty | Chiasma, Optical tracts | NSI |
| P11 | 3 | Absence | Chiasma, Thalamic and Peduncular lesions | Pilocytic Astrocytoma |
| P12 | 0 | Absence | Chiasma, Peduncular lesions, Temporal Lobe, Optical Radiations | NSI |
| P13 | 2 | Absence | Chiasma, Optical Nerves, Thalamic and Peduncular lesions | Pilocytic Astrocytoma |
| P14 | 2 | Absence | Chiasma, Optical Radiations | Pilocytic Astrocytoma |
| P15 | 1 | CAL, FH | Chiasma, Optical Radiations | Pilocytic Astrocytoma |
| P16 | 1 | Growth Delay | Chiasma, Optical Radiations | Pilocytic Astrocytoma |
ADHD, attention-deficit/hyperactivity disorder; CAL, café-au-lait spots; FH, family history of low-grade glioma/neurodevelopmental syndrome; GG: Ganglioglioma; NSI, no surgical intervention; PA: Pilocytic Astrocytoma.
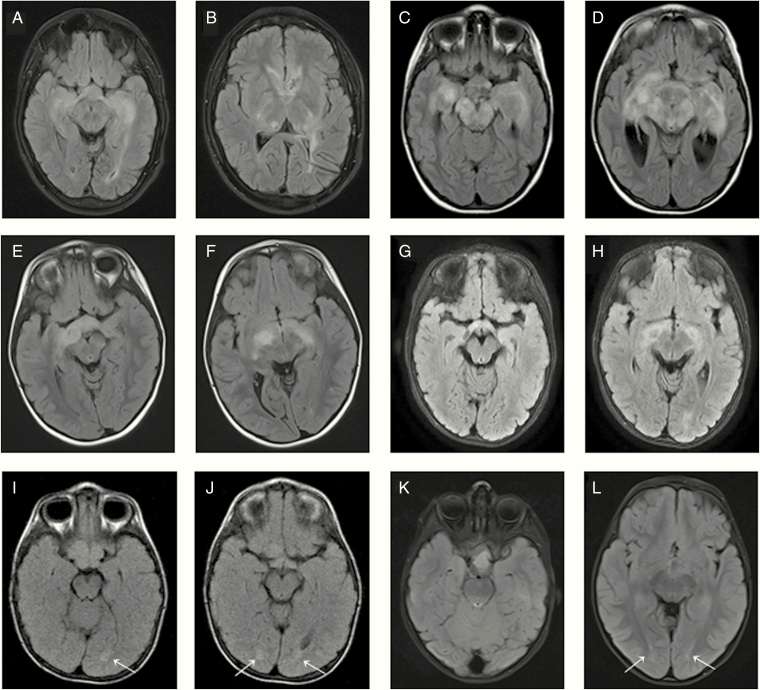
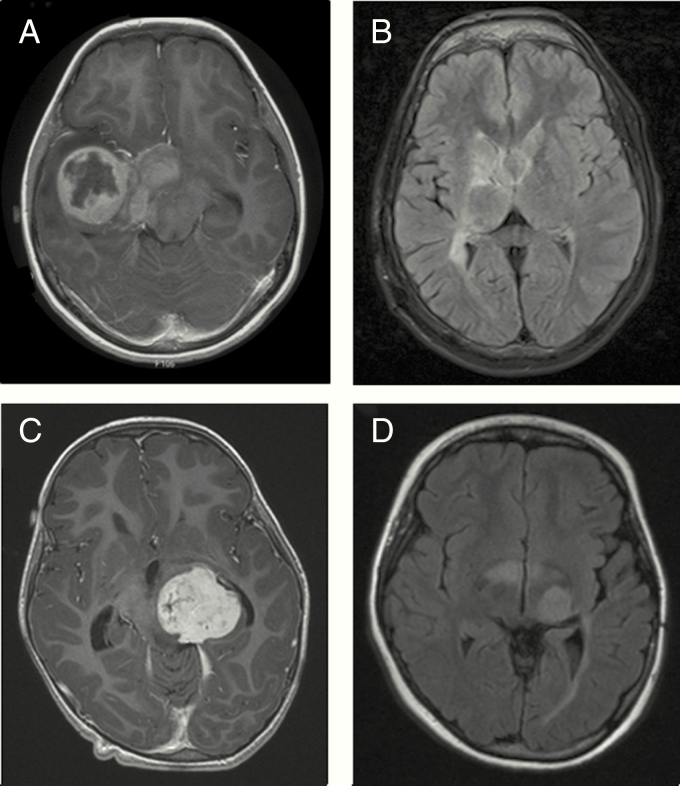
Patients P1 to P13 presented an infiltrative glioma of the entire optic pathway showing continuous bright signal on fluid attenuated inversion recovery (FLAIR) sequences (Figure 1A–1H) with a very similar spreading pattern in patients 4 to 8 (Table 2, Figure 1C–1F). In addition, patients P2, P11, P12, and P13 presented a common tendency to form extra-optical lesions (Figure 2). Patients P14, P15, and P16 presented a glioma primarily located at chiasm with very slight changes at the optic radiation fibers (Figure 1I–1L).
Figure 1.
Axial FLAIR images showing infiltrative OPG spreading at the entire optic pathway corresponding to patients P1 (A, B), P6 (C, D), P7 (E, F), and P9 (G, H). Axial FLAIR images showing OPG mainly localized at chiasm with slight bilateral changes (arrows) at the optic radiation fibers corresponding to patients P14 (I, J) and P15 (K, L).
Figure 2.
Axial FLAIR (A, B, D) and contrast-enhanced (C) images showing an OPG with major extra-optic component corresponding to patients P2 (A, B) and P11 (C, D).
Histopathological Review
Pilocytic astrocytoma was the most common histological type (Table 2). The BRAF fusion FISH analysis was performed in all but two patients’ samples (for patients P4 and P5, the samples were used up) and a BRAF fusion rearrangement was found in three patients (patients P14, P15, and P16). Moreover, the IHC analysis identified a (Figure 2) BRAF p.Val600Glu mutation in four patients (patients P2, P2, P5, and P13), which was confirmed by NGS analysis.
NF1 and RAS-MAPK NGS Analysis
Eleven patients had experienced previous surgical intervention: frozen (n = 10) and FFPE (formalin-fixed paraffin-embedded) (n = 1) samples were retrieved from tumor banks for NGS analysis. NF1 NGS analysis was performed in all DNA (blood and tumor) samples (Table 3). On average, 99% of high-quality sequencing reads (98% of bases) mapped to the reference genome for every sample. This resulted in an evenly distributed mean sequencing depth for NF1 of 656X. A good uniformity between samples and between amplicons was obtained.
Table 3.
Patients clinical and radiological group classification with NF1 and RAS-MAPK screening results
| Patient | Neurodevelopmental disorder | Radiological features | NGS analysis | |
|---|---|---|---|---|
| NF1 mutation: blood/tumor samples | Somatic mutation of the RAS-MAPK pathway | |||
| P1 | Yes | Group 1 | Yes: mosaic | NF1: c.7285C>T, p.Arg2429* |
| P2 | Yes | Group 1 | No/No | BRAF: c.1799T>A, p.Val600Glu |
| P3 | Yes | Group 1 | No/No | BRAF: c.1799T>A, p.Val600Glu |
| P4 | Yes | Group 1 | No/No | No |
| P5 | Yes | Group 1 | No/No | BRAF: c.1799T>A, p.Val600Glu |
| P6 | No | Group 1 | No/No | No |
| P7 | No | Group 1 | No/Not tested | Not tested |
| P8 | No | Group 1 | No/Not tested | Not tested |
| P9 | No | Group 1 | No/Not tested | Not tested |
| P10 | No | Group 1 | No/Not tested | Not tested |
| P11 | No | Group 1 | No/No | KRAS: c.197_203delins13, p.Ala66_Arg68delinsAspCysThrValLeu |
| P12 | No | Group 1 | No/Not tested | Not tested |
| P13 | No | Group 1 | No/No | BRAF: c.1799T>A, p.Val600Glu |
| P14 | No | Group 2 | No/No | BRAF fusion* |
| P15 | No | Group 2 | No/No | BRAF fusion* |
| P16 | No | Group 2 | No/No | BRAF fusion* |
Group 1: tumors spreading at the entire optic pathway, in some cases with major extra-optic component; Group 2: tumors mainly localized at chiasm with slight bilateral changes at the optic radiation fibers.
*Performed with Fluorescence in situ hybridization analysis.
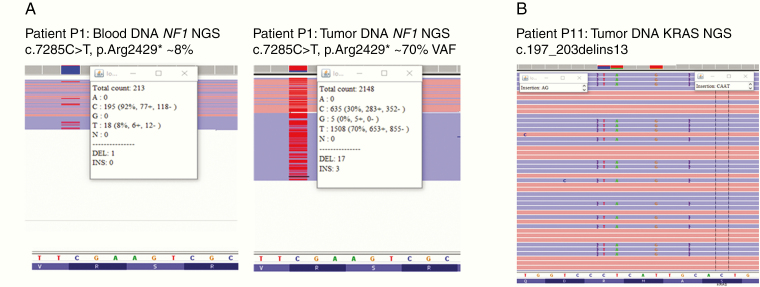
In one patient, an NF1 nonsense mutation was found in exon 50: c.7285C>T, p.Arg2429* with ~8% and ~70% VAFs (variant allele frequency) in blood and tumor samples, respectively (Patient P1, Figure 3A). No other NF1 gene alterations were identified in the rest of the samples in this cohort. Positive and negative results were confirmed with a second NF1 NGS analysis (see Materials and Methods section). The c.7285C>T, p.Arg2429* NF1 pathogenic variant was previously described in NF1 patients (rs786202457).23
Figure 3.
(A) Image extracted from Integrative Genomics Viewer showing NF1 mutation at exon 50: c.7285C>T, p.Arg2429* in blood (left) and tumor (right) samples. (B) Image extracted from IGV showing the complex KRAS exon 3 mutation: c.197_203delins13, p.Ala66_Arg68delinsAspCysThrValLeu in tumor sample of patient P11.
A large NGS panel targeting RAS-MAPK pathway components was sequenced in all tumor samples (n = 11). In four patients, the presence of the BRAF c.1799T>A, p.Val600Glu mutation was confirmed. In addition, we identified a complex KRAS exon 3 inframe indel mutation (Patient P11) which was confirmed by Sanger sequencing (Figure 3B): c.197_203delins13, p.Ala66_Arg68delinsAspCysThrValLeu.
Discussion
In the present study, we investigated into the possible molecular mechanisms underlying the presentation of NF1-like pediatric OPGs with typical radiological features of NF1-associated OPGs but without NF1 clinical diagnostic. Among the 16 patients, five showed neuro-developmental abnormalities (including macrocephaly, facial dysmorphia, pigmentation abnormalities, stereotypies, epilepsy, and attention-deficit; Table 2). We analyzed the NF1 gene in blood DNAs from a series of 16 patients with NF1-like pediatric OPGs, using a targeted NGS approach. The hypothesis that NF1-like OPGs could be caused by segmental NF1 confined to the central nervous system could be proven in only one of the patients in this cohort (patient P1; Table 3) thanks to deep sequencing of the NF1 gene, as previously described by Pasmant et al.18 This patient had a remarkable clinical presentation with sign and symptoms of neurodevelopmental disorder including café-au-lait spots, epilepsy, and attention-deficit/hyperactivity disorder (Table 2). Although most patients with NF1 harbor a constitutional mutation in NF1, some patients have been described who have so‑called segmental NF1. These patients have clinical manifestations of NF1 limited to only a single portion of their body.24–26 These postzygotic de novo mutations apparently arise in development during organogenesis, as evidenced by the topographically limited and lineage-restricted manifestations in these patients and the absence of disease in either parent. The developmental timing and cell lineage affected ultimately determine the tissue distribution of mosaicism and the patterns of disease reoccurrence within families. The mutational load and distribution of the mosaic genomic alteration can have dramatic effects on the clinical manifestation of the mutation. Interestingly, many dominantly inherited disorders, such as NF1, can have extraordinarily variable expressivity, and therefore, chance variations in expressivity can be mistaken for apparently segmental mosaicism. Segmental NF1 can, like inherited forms of the disease, be caused by a wide variety of NF1 mutations. Recently, Miklja et al. described how sequencing-based testing can meaningfully affect clinical care of children with low-grade glioma.12 Our study suggests that in NF1-like OPG, the presence of a neurodevelopmental syndrome should prompt to perform NF1 testing at a higher sequencing depth in order to evidence NF1 mosaicism. However, NF1 mosaicism was detected in only one patient of our series and we could not exclude that a NF1 cryptic variant (e.g., a deep intronic variant) was missed by our NGS approach in the negative patients.
Among the 16 patients with NF1-like pediatric OPGs, 11/16 patients had experienced surgical intervention. The NGS analysis of the major genes of the RAS-MAPK pathway and the BRAF fusion FISH detection in the tumor samples revealed genetic alterations of the RAS-MAPK pathways other than NF1 gene alterations in 8/11 patients (Table 3). In agreement with the literature,9,27 these results confirmed the great implication of the RAS-MAPK pathway in pediatric OPGs. Interestingly, the spectrum of the genetic alterations identified in these tumors is not similar to the one described in the general population of non-NF1 OPGs and other pilocytic astrocytomas3,12,28–30; there was indeed an over-representation of the BRAF pVal600Glu mutation. Of note, a BRAF fusion was identified in all patients belonging to group 2 with radiological features closer to sporadic presentation (tumors mainly localized at chiasm with slight bilateral changes at the optic radiation fibers). These two findings may suggest possible genotype–phenotype correlations in OPGs, which deserve further studies.
Since NF1-associated OPGs and sporadic OPGs have different growth and localization patterns, clinical behavior, and outcome, clinical management must take into account the molecular mechanism leading to the development of the tumor in each patient. Pediatric OPGs are chronically relapsing tumors that tend to burnout into a static state when children grow older. Consequently, the main challenge is to gain time preserving visual function by controlling tumor progression. The current treatment for pediatric OPGs may include chemotherapy, surgery, radiotherapy, or a combination of these modalities, as well as recent targeted therapy (BRAF-V600E- or MEK-inhibitors). While chemotherapy (vincristine-carboplatine based) is considered the first-choice therapy and has demonstrated to be effective in controlling progressive disease independently of NF1-status, radiotherapy is mainly indicated in older children and has been formally contraindicated in NF1 patients.31 NF1-associated OPGs have more frequently an indolent course; thus, “wait and see” can be considered as an option for nonprogressive patients more often than in sporadic cases. On the contrary, surgery may be indicated more frequently in sporadic OPGs as first option, since they are often presented as large, cystic, exophytic, or hypothalamic life-threatening tumors.
Therapy targeting the MAPK- and mTOR-pathways could be a valuable alternative to the standard treatment of pediatric OPGs. Recently, Fangusaro et al. have shown that the different genomic alterations of this pathway have implications in therapeutic responses.32 Single or combination therapy with FGFR, NTRK2, and/or MEK inhibitors represents rational treatment options.33,34 Moreover, a phase 1–2 trial assessing Selumetinib in childhood OPGs is currently opened (NCT01089101, Pediatric Brain Tumor Consortium-Memphis). Our data support that, in front of an atypical presentation of the OPG, molecular information on the tumor and constitutional NF1 status may provide crucial indications for proper management.
In addition, we identified a complex mutation of the KRAS gene in a patient presenting an OPG with greater extra-optical component and no signs and symptoms of neurodevelopment disorder (patient P11: Table 3; Figure 2 and 3). No other mutation was found in the tumor. The KRAS complex inframe indel mutation would be a gain-of-function mutation as it affects codons 66–68 (next to the KRAS hotspot codon 61) and it is predicted to be probably damaging in prediction softwares. Although other alterations of the RAS-MAPK pathway such as mutations in RAF1, KRAS, FGFR1, PTPN11, and NTRK2 have been described in OPGs, the KRAS complex mutation identified in patient P11 has been never reported.
In conclusion, we have described the clinical–radiological and molecular characteristics of NF1-like OPGs. We hypothesized that NF1-like OPGs could be caused by NF1 mosaicism and evidence for mosaicism was found in only one patient with neurodevelopmental symptoms. Additional studies are warranted to explore unknown predisposition condition leading to the OPGs with NF1-like presentation, particularly in patients with neurodevelopmental disorder. Further investigations of larger cohorts of sporadic and NF1-associated OPGs might improve our understanding of this peculiar entity and could allow exploration of sensibility to MAPK pathway inhibitors.
Acknowledgments
We thank the Genetic Laboratory at Gustave Roussy (Natacha Castor), the Necker Imagine DNA biobank (BB-033-00065), the Tumor Bank of Necker-Enfants malades Hospital (Gisèle Le Gal), the Tumor Bank of Gabriel Montpied-Clermont Ferrand Hospital (Prof. Kemeny), the Paris-Descartes Bioinformatics Platform, and the CAP NF FOUNDATION (https://www.anrfrance.fr/).
Funding
This work was funded by grant from the Fondation CAP NF awarded to MJ.L-.I and by the Fondation Gustave Roussy Pediatric Campaign to L.G.R., L.B., and J.G.
Conflict of Interest
The authors declare no conflict of interest in any matter presented in this manuscript.
Authorship statement
MJ.L-.I. collected and analyzed clinical data, performed experiments, analyzed high throughput data, wrote and prepared manuscript for publication, and submitted manuscript for publication; I.L. isolated DNA from blood and tumors and performed experiments; L.G-.R. carried out genetic consultation and analyzed clinical data; A.T-.E. performed pathology analysis of tumors; A.B-.S. analyzed high-throughput data; P.V. performed pathology analysis of tumors; D.V. contributed to experimental design and analyzed high-throughput data; M.V. contributed to experimental design and analyzed high-throughput data; L.B. carried out genetic consultation and analyzed clinical data; J.G. designed the project, collected and analyzed clinical data, and wrote part of the manuscript; E.P. contributed to experimental design, analyzed high-throughput data, supervised experiments, and wrote part of the manuscript.
References
- 1. Falzon K, Drimtzias E, Picton S, Simmons I. Visual outcomes after chemotherapy for optic pathway glioma in children with and without neurofibromatosis type 1: results of the International Society of Paediatric Oncology (SIOP) Low-Grade Glioma 2004 trial UK cohort. Br J Ophthalmol. 2018;0(1):1–5. [DOI] [PubMed] [Google Scholar]
- 2. Rakotonjanahary J, De Carli E, Delion M, et al. ; Brain Tumor Committee of SFCE Mortality in Children with Optic Pathway Glioma Treated with Up-Front BB-SFOP Chemotherapy. PLoS One. 2015;10(6):e0127676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Helfferich J, Nijmeijer R, Brouwer OF, et al. . Neurofibromatosis type 1 associated low grade gliomas: a comparison with sporadic low grade gliomas. Crit Rev Oncol Hematol. 2016;104:30–41. [DOI] [PubMed] [Google Scholar]
- 4. Walker DA, Liu J, Kieran M, et al. ; CPN Paris 2011 Conference Consensus Group A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro Oncol. 2013;15(4):462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blanchard G, Lafforgue MP, Lion-François L, et al. ; NF France network Systematic MRI in NF1 children under six years of age for the diagnosis of optic pathway gliomas. Study and outcome of a French cohort. Eur J Paediatr Neurol. 2016;20(2):275–281. [DOI] [PubMed] [Google Scholar]
- 6. Lacaze E, Kieffer V, Streri A, et al. . Neuropsychological outcome in children with optic pathway tumours when first-line treatment is chemotherapy. Br J Cancer. 2003;89(11):2038–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trevisson E, Cassina M, Opocher E, et al. . Natural history of optic pathway gliomas in a cohort of unselected patients affected by Neurofibromatosis 1. J Neurooncol. 2017;134(2):279–287. [DOI] [PubMed] [Google Scholar]
- 8. Penman CL, Faulkner C, Lowis SP, Kurian KM. Current Understanding of BRAF Alterations in Diagnosis, Prognosis, and Therapeutic Targeting in Pediatric Low-Grade Gliomas. Front Oncol. 2015;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones DT, Hutter B, Jäger N, et al. ; International Cancer Genome Consortium PedBrain Tumor Project Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45(8):927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Packer RJ, Pfister S, Bouffet E, et al. . Pediatric low-grade gliomas: implications of the biologic era. Neuro Oncol. 2017;19(6): 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma MK, Zehnbauer BA, Watson MA, Gutmann DH. RAS pathway activation and an oncogenic RAS mutation in sporadic pilocytic astrocytoma. Neurology. 2005;65:1335–1337. [DOI] [PubMed] [Google Scholar]
- 12. Miklja Z, Pasternak A, Stallard S, et al. . Molecular profiling and targeted therapy in pediatric gliomas: review and consensus recommendations. Neuro Oncol. 2019;21(8):968–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khatua S, Gutmann DH, Packer RJ. Neurofibromatosis type 1 and optic pathway glioma: molecular interplay and therapeutic insights. Pediatr Blood Cancer. 2017;65:1–7. [DOI] [PubMed] [Google Scholar]
- 14. Sabbagh A, Pasmant E, Imbard A, et al. . NF1 molecular characterization and neurofibromatosis type I genotype-phenotype correlation: the French experience. Hum Mutat. 2013;34(11):1510–1518. [DOI] [PubMed] [Google Scholar]
- 15. National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Arch Neurol Chicago. 1988;45: 575–578. [PubMed] [Google Scholar]
- 16. Robert-Boire V, Rosca L, Samson Y, Ospina LH, Perreault S. Clinical Presentation and Outcome of Patients With Optic Pathway Glioma. Pediatr Neurol. 2017;75:55–60. [DOI] [PubMed] [Google Scholar]
- 17. Sharif S, Upadhyaya M, Ferner R, et al. . A molecular analysis of individuals with neurofibromatosis type 1 (NF1) and optic pathway gliomas (OPGs), and an assessment of genotype-phenotype correlations. J Med Genet. 2011;48(4):256–260. [DOI] [PubMed] [Google Scholar]
- 18. Pasmant E, Parfait B, Luscan A, et al. . Neurofibromatosis type 1 molecular diagnosis: what can NGS do for you when you have a large gene with loss of function mutations? Eur J Hum Genet. 2015;23(5):596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shofty B, Ben-sira L, Kesler A, et al. . Isolated optic nerve gliomas: a multicenter historical cohort study. JNS Pediatr. 2017;20(6):549–555. [DOI] [PubMed] [Google Scholar]
- 20. Avery RA, Mansoor A, Idrees R, et al. . Quantitative MRI criteria for optic pathway enlargement in neurofibromatosis type 1. Neurology. 2016;86(24):2264–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prada CE, Hufnagel RB, Hummel TR, et al. . The Use of Magnetic Resonance Imaging Screening for Optic Pathway Gliomas in Children with Neurofibromatosis Type 1. J Pediatr. 2015;167(4):851–856.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Just PA, Audebourg A, Pasmant E, et al. . Immunohistochemistry versus next-generation sequencing for the routine detection of BRAF V600E mutation in melanomas. Hum Pathol. 2014;45(9):1983–1984. [DOI] [PubMed] [Google Scholar]
- 23. Fahsold R, Hoffmeyer S, Mischung C, et al. . Minor lesion mutational spectrum of the entire NF1 gene does not explain its high mutability but points to a functional domain upstream of the GAP-related domain. Am J Hum Genet. 2000;66(3):790–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maertens O, De Schepper S, Vandesompele J, et al. . Molecular dissection of isolated disease features in mosaic neurofibromatosis type 1. Am J Hum Genet. 2007;81(2):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Messiaen L, Vogt J, Bengesser K, et al. . Mosaic type-1 NF1 microdeletions as a cause of both generalized and segmental neurofibromatosis type-1 (NF1). Hum Mutat. 2011;32(2):213–219. [DOI] [PubMed] [Google Scholar]
- 26. Biesecker LG, Spinner NB. A genomic view of mosaicism and human disease. Nat Rev Genet. 2013;14(5):307–320. [DOI] [PubMed] [Google Scholar]
- 27. Chiang JC, Ellison DW. Molecular pathology of paediatric central nervous system tumours. J Pathol. 2017;241(2):159–172. [DOI] [PubMed] [Google Scholar]
- 28. Prabowo AS, Iyer AM, Veersema TJ, et al. . BRAF V600E mutation is associated with mTOR signaling activation in glioneuronal tumors. Brain Pathol. 2014;24(1):52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koelsche C, Wöhrer A, Jeibmann A, et al. . Mutant BRAF V600E protein in ganglioglioma is predominantly expressed by neuronal tumor cells. Acta Neuropathol. 2013;125(6):891–900. [DOI] [PubMed] [Google Scholar]
- 30. Schindler G, Capper D, Meyer J, et al. . Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. [DOI] [PubMed] [Google Scholar]
- 31. Grill J, Couanet D, Cappelli C, et al. . Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. 1999;45(3):393–396. [DOI] [PubMed] [Google Scholar]
- 32. Fangusaro J, Onar-thomas A, Poussaint TY, et al. . Selumetinib in paediatric patients with BRAF -aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma : a multicentre, phase 2 trial. Lancet Oncol. 2019;2045(19):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dombi E, Baldwin A, Marcus LJ, et al. . Activity of selumetinib in neurofibromatosis type 1–related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–2560. doi:10.1056/NEJMoa1605943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Drilon A, Laetsch TW, Kummar S, et al. . Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]