Dear Editor,
Hematological manifestations reported in SARS-CoV-2 infection mainly include lymphocytopenia as a poor prognosis factor [1]. Thrombocytopenia can be associated with disseminated intravascular coagulation [2] or immune process [3]. Data regarding anemia are scarce and in line with a recent report on six patients with heterogeneous hemolytic anemia [4], we herein report on two patients with SARS-CoV-2-related cold agglutinin disease (CAD).
Patient 1
A 43-year-old woman was admitted after a 10-day course of asthenia, fever, cough, diarrhea, and dyspnea. Her medical history comprised obesity and untreated multiple sclerosis. Nasopharyngeal Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) confirmed SARS-CoV-2 infection. Chest computed tomography showed a typical severe interstitial pneumonia. Hemoglobin was normal upon admission (13.1 g/dL). A treatment with oxygen support and antibiotics (ceftriaxone and azithromycin for 3 days then tazocillin for 3 days) was started, with a rapid improvement of respiratory parameters. At day 6, hemoglobin levels dropped to 6.1 g/dL, with hemolytic features (low haptoglobin, elevated bilirubin, and lactate dehydrogenase (LDH) levels). The direct antiglobulin test (4+) was positive with the presence of cold agglutinins (Fig. 1). Etiological workup for hemolysis was negative. After poor initial transfusion efficiency, hemoglobin levels and hemolytic parameters improved.
Fig. 1.
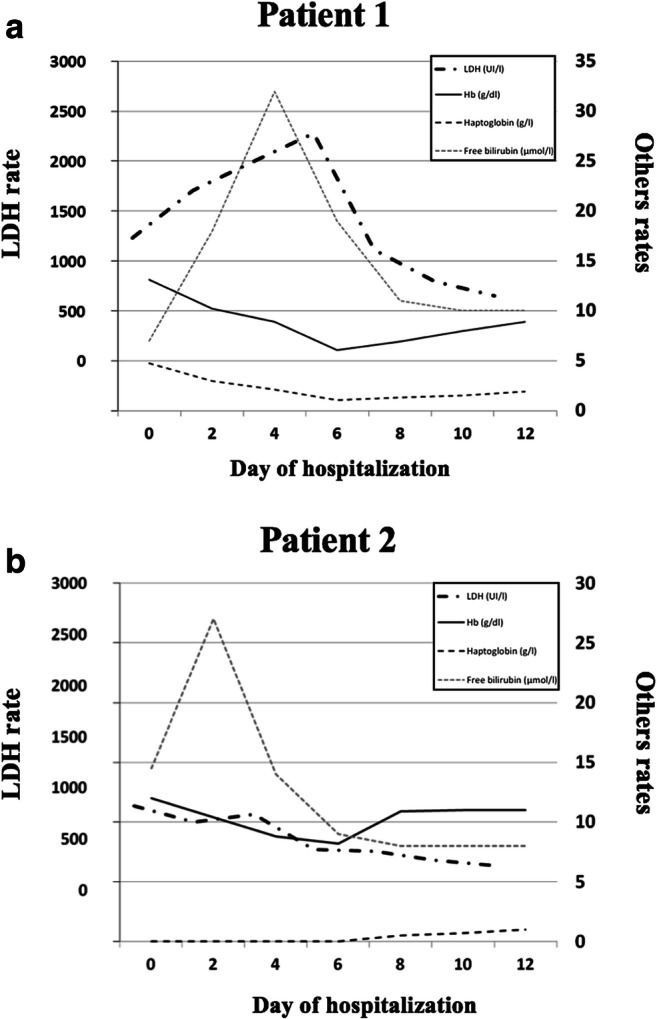
Evolution of the biological parameters of cold agglutinin-associated hemolysis. a Evolution of the biological parameters of hemolysis in patient 1; b evolution of the biological parameters of hemolysis in patient 2
Patient 2
A 63-year-old man, with a medical history of hypertension, was admitted for severe acute respiratory syndrome in intensive care unit (ICU). In the last 2 weeks, he presented with fever, cough, and progressive worsening dyspnea. The nasopharyngeal RT-PCR confirmed a SARS-CoV-2 infection. The initial blood count showed a non-regenerative normocytic anemia (10.5 g/dL). As haptoglobin levels were unmeasurable (< 0.08 g/L), contrasting with increased serum orosomucoid (2.35 g/L), a hemolytic process was suspected and the direct antiglobulin test was positive (C3 [4+] and IgG [2+]) with cold agglutinins (Fig. 1). At day 6 of hospitalization, hemoglobin level decreased to 8.2 g/dL. Etiological workup was negative. Hemoglobin improved within 9 days along with clinical improvement.
We here report two supplemental cases of SARS-CoV-2-associated autoimmune hemolytic anemia with cold agglutinins which seems to be a rare but nevertheless real complication of this condition. CAD was previously reported in association with viral infections and Mycoplasma pneumonia infection. The pathophysiological mechanism might be related to an antigen cross-reaction with red blood cell secondary to molecular mimicry, as with Influenzae disease [5]. A drug-induced CAD was considered uncommon [6].
We believe that a more extensive investigation of anemia in SARS-CoV2 patients, including the appraisal of hemolytic patterns might be useful for the detection of other cases of CAD and appropriate management of anemia during SARS-CoV2-infection.
Acknowledgments
The authors would like to acknowledge the patients and members of the APHP Lariboisière COVID Group (physicians, non-medical staff).
Author contribution
H.T., G.J., O.M., and S.D. designed the study, recruited patients, wrote, and reviewed, and R.M. and M.S. designed, reviewed, and approved the paper.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tessa Huscenot and Joris Galland contributed equally to this work.
References
- 1.Henry BM. COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8:e24. doi: 10.1016/S2213-2600(20)30119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zulfiqar AA, Lorenzo-Villalba N, Hassler P et al (2020) Immune thrombocytopenic purpura in a patient with Covid-19. N Engl J Med 382(18):e43 [DOI] [PMC free article] [PubMed]
- 4.Lazarian G, Quinquenel A, Bellal M et al (2020) Autoimmune hemolytic anemia associated with Covid-19 infection. Br J Haematol. 10.1111/bjh.16794 [DOI] [PMC free article] [PubMed]
- 5.Schoindre Y, Bollée G, Dumont M-D, Lesavre P, Servais A. Cold agglutinin syndrome associated with a 2009 influenza a H1N1 infection. Am J Med. 2011;124:e1–e2. doi: 10.1016/j.amjmed.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013;122:1114–1121. doi: 10.1182/blood-2013-02-474437. [DOI] [PubMed] [Google Scholar]


