Abstract
Background
Breast capsular contracture is a major problem following implant-based breast reconstruction, particularly in the setting of radiation therapy. Recent work has identified a fibrogenic fibroblast subpopulation characterized by CD26 surface marker expression.
Objectives
This work aimed to investigate the role of CD26-positive fibroblasts in the formation of breast implant capsules following radiation therapy.
Methods
Breast capsule specimens were obtained from irradiated and nonirradiated breasts of 10 patients following bilateral mastectomy and unilateral irradiation at the time of expander-implant exchange, under institutional review board approval. Specimens were processed for hematoxylin and eosin staining as well as for immunohistochemistry and fluorescence activated cell sorting for CD26-positive fibroblasts. Expression of fibrotic genes and production of collagen were compared between CD26-positive, CD26-negative, and unsorted fibroblasts.
Results
Capsule specimens from irradiated breast tissue were thicker and had greater CD26-postive cells on immunofluorescence imaging and on fluorescence activated cell sorting analysis than did capsule specimens from the nonirradiated breast. Compared with CD26-negative fibroblasts, CD26-positive fibroblasts produced more collagen and had increased expression of the profibrotic genes IL8, TGF-β1, COL1A1, and TIMP4.
Conclusions
CD26-positive fibroblasts were found in a significantly greater abundance in capsules of irradiated compared with nonirradiated breasts and demonstrated greater fibrotic potential. This fibrogenic fibroblast subpopulation may play an important role in the development of capsular contracture following irradiation, and its targeted depletion or moderation may represent a potential therapeutic option.
Level of Evidence: 2

Implant-based breast reconstruction is a valued treatment option for patients following mastectomy but is frequently complicated by capsular contracture. Insertion of breast implants initiates a physiological reaction that results in formation of a fibrous capsule around the foreign material. In a subset of patients, this capsule undergoes excessive fibrosis and progressively shrinks, creating a breast that is abnormally shaped, firm in texture, and often a source of significant pain. The occurrence of capsular contracture varies dramatically between patients and even between breasts in the same patient.1,2 Despite this unpredictability, capsular contracture represents a significant medical burden; symptomatic capsular contracture rates are as high as 45%,3 and more than 45,000 females are affected in the United States every year.4-7 Due to the cosmetic, physiological, and psychological disturbances, capsular contracture is one of the most common reasons for reoperation following breast reconstruction.8 Although a number of surgical techniques and nonmedical therapies have been suggested, none are truly effective, and recurrence rates range between 10% and 46%.9-11
A number of factors have been identified that increase the risk of capsular contracture, including periprosthetic infection, diabetes, postoperative hematoma, smooth vs textured implants, subglandular vs submuscular implant placement, and excessive breast motion during recovery.4,12-20 In addition, radiation therapy, a treatment frequently received by breast cancer patients, can significantly worsen capsular contracture.21,22 In women who underwent bilateral mastectomies with expander placement prior to adjuvant radiation therapy, studies have demonstrated an over 4-fold increase in capsular contracture on the irradiated side.23 The cellular and molecular events underlying the fibrotic process and how they are influenced by these contributory factors, however, remain uncertain.4 To identify potential therapeutic targets able to disrupt the fibrotic process and decrease rates of contracture, it is essential to understand its precise etiopathogenesis.
Histological studies implicate fibroblasts as one of the principle cell types responsible for depositing the connective tissue surrounding breast implants.24,25 Fibroblasts accumulate in the zone of contact between the implant and the soft tissue envelope,26-28 and fibroblast number at this capsule-implant interface is correlated with the clinical grade of capsular contracture.29 Fibroblasts deposit collagen and connective tissue, and as contracture worsens, the collagen fibers increase in thickness and become highly oriented perpendicular to the fibroblasts, suggestive of contractile fibroblast activity.30 Appropriately stimulated fibroblasts may also differentiate into myofibroblasts, which are cells capable of generating large contractile forces and depositing excessive amounts of extracellular matrix (ECM). Myofibroblasts are found in abundance in the most severely contracted capsules.31,32 The irradiated breast tissue may provide excessive stimulation for these fibrotic activities, because cytokine cascades are initiated following irradiation and culminate in elevated levels of TGF-β, a factor known to have potent fibroblastic activity.31,33
Our group has recently identified a fibrogenic fibroblast subpopulation characterized by expression of the cell surface marker CD26.34 CD26, also known as dipeptidyl peptidase-4 (DPP4), is a homodimeric type II transmembrane glycoprotein that bears resemblance to fibroblast activation protein-α. It functions as a serine exopeptidase and catalyzes a number of neuropeptides, a binding site for collagen and fibronectin, and has a key role in ECM degradation and tissue invasion.35 CD26-positive fibroblasts are responsible for the majority of scar formation in mouse skin, and targeting of these cells utilizing a direct CD26 inhibitor reduced scar formation after injury.34 We hypothesized that CD26-positive fibroblasts in breast tissue contribute to capsule formation and that the fibrogenic activity of CD26-positive fibroblasts may be increased in the irradiated, contracted breast capsules. To test this, we studied capsule tissue specimens in patients who underwent bilateral mastectomy with expander placement followed by unilateral irradiation and compared the fibroblast subpopulation activity and composition within these capsules in response to radiation therapy.
METHODS
Breast Capsule Specimens
This study was approved by Stanford University School of Medicine’s Institutional Review Board (protocol #25954). Bilateral breast implant capsule specimens were obtained from 10 adult female patients (mean age, 57.9 years; range: 43-72 years) who underwent bilateral mastectomy with expander placement followed by unilateral breast irradiation. Patients were considered for inclusion if they did not have diabetes, fibrosing conditions (eg, scleroderma), peripheral vascular disease, or autoimmune conditions and were not on chemotherapy or anti-inflammatory medications. At the time of expander-implant exchange, expander capsule tissue specimens were obtained from both the irradiated and nonirradiated sides. This study was conducted over a 15-month period from September 2017 to December 2018.
Hematoxylin and Eosin Histology
Samples of the capsules from the irradiated and nonirradiated tissue specimens were immediately fixed in 4% paraformaldehyde for 16 hours at 4°C. Samples were then washed with phosphate-buffered saline (PBS, Cat: 10010023, ThermoFisher Scientific, Waltham, MA), dehydrated in gradients of alcohols, and embedded in paraffin blocks. Blocks were sectioned into 8-μm slices and stained with hematoxylin and eosin (H&E, Cat: H-3502, Vector Laboratories, Burlingame, CA). Stained slides were imaged utilizing a Leica DM5000 B Light microscope (Leica Microsystems, Buffalo Grove, IL) and a 10× objective. Capsule thickness measurements were taken on 10 stained samples from each capsule specimen by 2 authors acting independently (M.R.B. and D.I.) employing Image J software (https://imagej.nih.gov/ij/). The capsule was defined as the fibrous layer of collagen proximal to the implant surface. Each author took 5 measurements per sample, and the mean of the total 10 measurements per sample was recorded as the capsule thickness for that sample.
Immunofluorescent Histology
Samples of each capsule specimen from the irradiated and nonirradiated tissue specimens were also immediately fixed in 4% paraformaldehyde for 16 hours at 4°C, washed in PBS, and then placed into 30% sucrose in PBS for 5 days before embedding in cryoembedding medium (OCT, Cat: 25608-930, Tissue-Tek, VWR, Radnor, PA). OCT blocks were cut into 6-μm-thick cryosections and placed onto glass slides. The slides were soaked in PBS for 5 minutes to remove OCT and allowed to air dry before hydrophobic squares were drawn around each tissue section (PAP pen, Cat: Z377821, Sigma-Aldrich, St. Louis, MO). The tissue sections were permeabilized utilizing 0.2% Triton X-100 (Cat: X100, Sigma-Aldrich) for 30 minutes, washed with 0.05% Tween-20 (Cat: 9005-64-5, Sigma-Aldrich) in PBS, and incubated with 1% blocking solution (Power Block, Cat: HK083-50K, BioGenex, Fremont, CA) for 2 hours at room temperature. Sections were then incubated with 2 primary antibodies against CD26 (Rabbit Anti-CD26 antibody, Cat: 28340, Abcam, Burlingame, CA) and vimentin (Goat Anti-Vimentin antibody, Cat: 11256, Abcam), a pan-fibroblast marker. Primary antibodies were diluted in 0.1% Power Block (1:200) for 18 hours at 4°C. The sections were washed with 0.1% Triton X-100 in PBS, and then incubated with secondary antibody (Donkey Anti-Goat IgG Alexa Fluor 647, Cat: ab150135, Abcam; Donkey Anti-Rabbit IgG Alexa Fluor 488, Cat: ab150073, Abcam) diluted in 0.1% Power Block (1:4000) for 1 hour at room temperature. Finally, the slides were washed with 1% Triton X-100 in PBS and mounted with 4′,6-diamidino-2-phenylindole (DAPI)-containing aqueous mounting medium (DAPI Fluoromount-G, Cat: 0100-20, Southern Biotech, Birmingham, AL). Immunofluorescent images were obtained utilizing a Zeiss LSM880 confocal microscope. At least 3 images per sample, 3 samples per slide, and 3 slides per patient were analyzed for co-staining of CD26 and vimentin.
Fluorescence-Activated Cell Sorting
Portions of capsule specimens were immediately processed for fluorescence-activated cell sorting (FACS). With sharp scissors, the breast capsule tissue was thoroughly minced into <1-mm fragments and enzymatically digested for 1 hour at 37°C under gentle agitation (120 rpm) utilizing collagenase (Collagenase from Clostridium histolyticum, Cat: C6685, Sigma-Aldrich) dissolved in digest buffer (0.75 mg/mL) consisting of 5% fetal bovine serum (FBS, Gibco, Cat: 10082147, ThermoFisher), 100 U/mL DNase I (Worthington, Lakewood, NJ), 0.1% Poloxamer 188 (Cat: P5556-100ML, Sigma-Aldrich), 20 mM HEPES (Cat: 15630080, Sigma-Aldrich), and 1 mM CaCl2, in Medium 199 (Cat: SH30223.02, HyClone, GE Healthcare, Chicago, IL). The digest was then quenched by adding Dulbecco’s Modified Eagle Medium-GlutMAX (Cat: 10566-016, Gibco, ThermoFisher) containing 20% FBS in a 1:1 ratio and first through a 100-μm and then a 40-μm cell strainer. The mixture was then centrifuged at 300 g for 5 minutes at 4°C. The supernatant was aspirated, and the remaining pellet was resuspended in 500 μL FACS buffer (PBS with 2% FBS, 1 mM ethylenediaminetetraacetic acid (Cat: 15575020, Invitrogen, ThermoFisher), 1% penicillin-streptomycin solution (Pen-Strep, PS, Cat: 15140122, ThermoFisher). Cells were counted utilizing a hemocytometer, and the cell suspension was brought to a concentration of 1 million cells per 100 μL volume in FACS buffer. Cells were then stained with antibodies for CD45 (Anti-human CD45-Pacific Blue, 1:100, Cat: 304029, Biolegend, San Diego, CA), CD31 (Anti-human CD31 [PECAM-1] eFluor 450, 1:100, Cat: 48-0319-42, eBioscience, San Diego, CA), CD235a (Anti-human CD235a [Glycophorin A] eFluor 450, 1:100, Cat: 48-9987-42, eBioscience), and CD26 (Anti-human CD26-Fluorescein isothiocyanate, 1:100, Cat: 302704, Biolegend). After 30 minutes incubation, the cell suspensions were diluted in FACS buffer, centrifuged (300 g, 5 minutes, 4°C), and finally resuspended in FACS buffer for FACS analysis and sorting on the BD FACS Aria II system (Becton Dickinson, East Rutherford, NJ).
Quantitative Polymerase Chain Reaction
CD26-positive, CD26-negative, and unsorted (CD45-CD235a-CD31-) fibroblasts from irradiated capsules were FACS sorted directly into TRIzol lysing solution (Cat: 15596026, ThermoFisher) and immediately frozen in dry ice and kept at −80°C until processing. Gene expression was compared with unsorted fibroblasts from irradiated capsules for transcript analysis. RNA was harvested utilizing RNeasy Mini Kit (Cat: 74104, Qiagen, Hilden, Germany). Reverse transcription was performed utilizing TaqMan Reverse Transcription Reagents (Cat: 4304134, Invitrogen, ThermoFisher), and an ABI Prism 7900HT Sequence Detection System (Cat: 4317596, ThermoFisher) was employed to perform quantitative real-time polymerase chain reaction to evaluate expression levels for genes known to be associated with enhanced fibrotic activity in fibroblasts: interleukin 8 (IL8), transforming growth factor β1 (TGF-β1), collagen, type 1, alpha 1 (COL1A1), and metalloproteinase inhibitor 4 (TIMP4). All experiments were run in triplicate and data were standardized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression for statistical analysis. Significant differences in gene expression levels between the CD26-positive, CD26-negative, and unsorted fibroblasts from irradiated breast capsule specimens were determined employing the relative threshold cycle method.36 To obtain ΔCT values, averaged CT values of the reference transcripts were subtracted from CT values of the candidate transcripts. ΔCT values of each gene in the analysis were compared to determine statistically significant differences.
Human Procollagen I Alpha 1 Production
The CD26-positive and CD26-negative fibroblasts and unsorted (CD45-CD235a-CD31-) fibroblasts were also analyzed for human procollagen I alpha 1 production by ELISA. Sorted CD26-positive and CD26-negative fibroblasts and unsorted fibroblasts were expanded in fibroblast media (10% FBS and 1% PS in Dulbecco’s Modified Eagle Medium-GlutMAX) until 70% confluency in a 100-mm plate. The growth medium was then aspirated and adherent cells were rinsed twice in PBS, solubilized on ice for 15 minutes, and then pelleted (18,000 g, 20 minutes, 4°C). Supernatants were assayed utilizing a commercially available ELISA kit (ELISA Kit, Cat: ab210966, Abcam) as per the manufacturer’s instructions.
Statistical Analysis
Continuous data were described utilizing the mean and standard deviation of the mean when parametric and with the median and the range when nonparametric. Data were reported as frequencies when categorical. A 1-way analysis of variance followed by Bonferroni multiple comparisons was used to compare means between groups. Statistical significance was defined as P < 0.05. All statistical analyses were performed utilizing Prism GraphPad 5.0 (GraphPad Software, Inc., La Jolla, CA) statistical software.
RESULTS
Hematoxylin and Eosin Histology
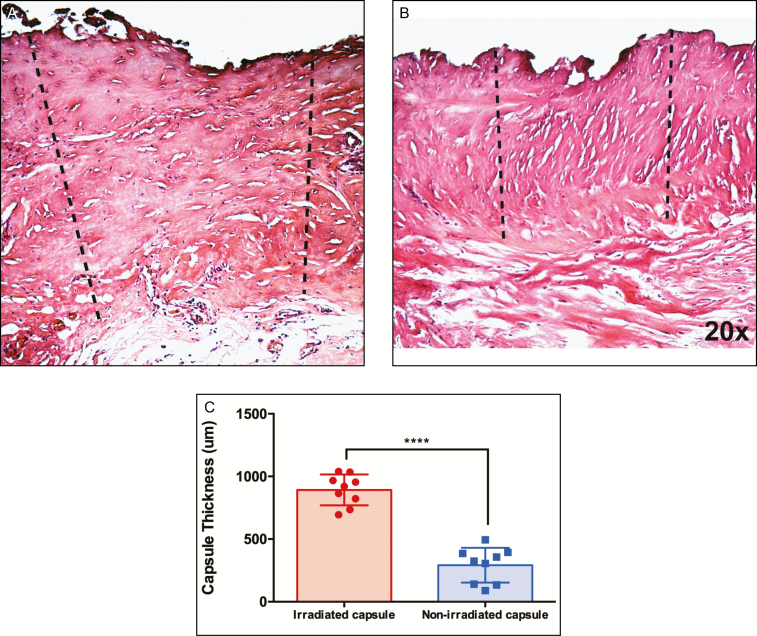
Implant capsule specimens obtained from irradiated tissue were macroscopically thicker than the nonirradiated tissue, consistent with the degree of clinical capsular contracture that was greater on the irradiated side. H&E staining of capsule specimens revealed that the capsules surrounding expanders in the nonirradiated breast appeared more uniform, with more organized collagen deposition, compared with the capsules surrounding the implants of the irradiated breasts. In addition, the capsules in the irradiated breast tissue were histologically thicker than those in the nonirradiated breast (892.2 ± 41.1 μm vs 291.7 ± 46.3 μm, P ≤ 0.0001****) (Figure 1A,B).
Figure 1.
Representative hematoxylin and eosin staining of capsule specimens from the irradiated (A) and nonirradiated (B) breast capsulotomy specimens from a single patient. Capsule thickness measurements were made by drawing a line from the capsule to the implant. The capsule was defined as the layer of collagenous tissue proximal to the implant. Measurements were made at 20× magnification. (C) The capsule of the irradiated capsule was thicker than nonirradiated capsule (P < 0.0001****).
Immunofluorescent Staining for CD26-Positive Fibroblasts
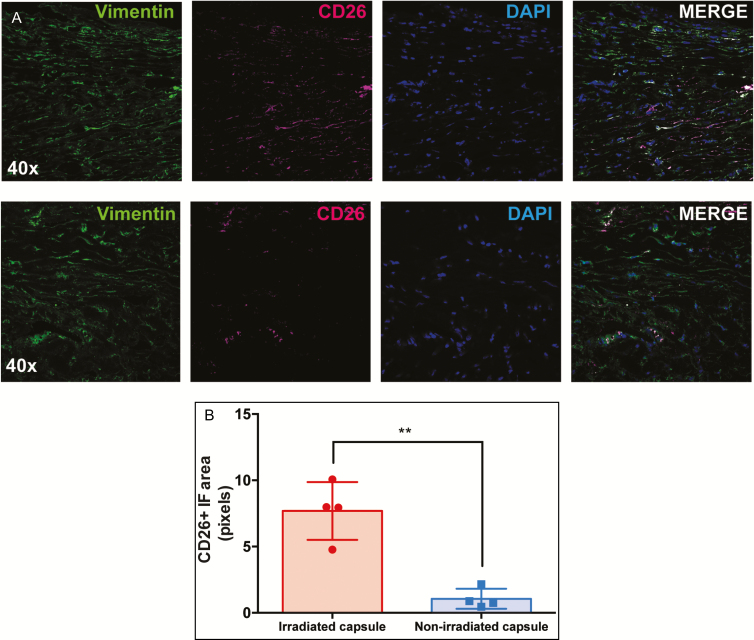
CD26 has been established in skin as a marker of fibroblasts with increased fibrotic activity.34 We therefore analyzed the abundance of CD26-positive fibroblasts in irradiated compared with nonirradiated breast capsule specimens by staining vimentin, a pan-fibroblast marker, and CD26 utilizing immunofluorescent antibodies. We found significantly more CD26-positive fibroblasts, positive for both CD26 and vimentin, in irradiated capsules vs nonirradiated breast capsules (7.69 ± 1.09 vs 1.06 ± 0.38, P = 0.0012**) (Figure 2).
Figure 2.
(A) Immunohistochemical staining of capsule specimens from irradiated (top row) and nonirradiated (bottom row) breast tissue. Cryosections were immunofluorescently stained with vimentin (green) to label fibroblasts and CD26 (magenta) to label the fibrogenic subpopulation of fibroblasts. Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). Images shown are at 40× magnification. (B) Multiple sections of each specimen were analyzed for co-staining of CD26 and vimentin, which revealed a greater number of CD26-positive fibroblasts in the irradiated capsule (P = 0.0012**).
Fluorescence-Activated Cell Sorting Analysis for CD26-Positive Fibroblasts
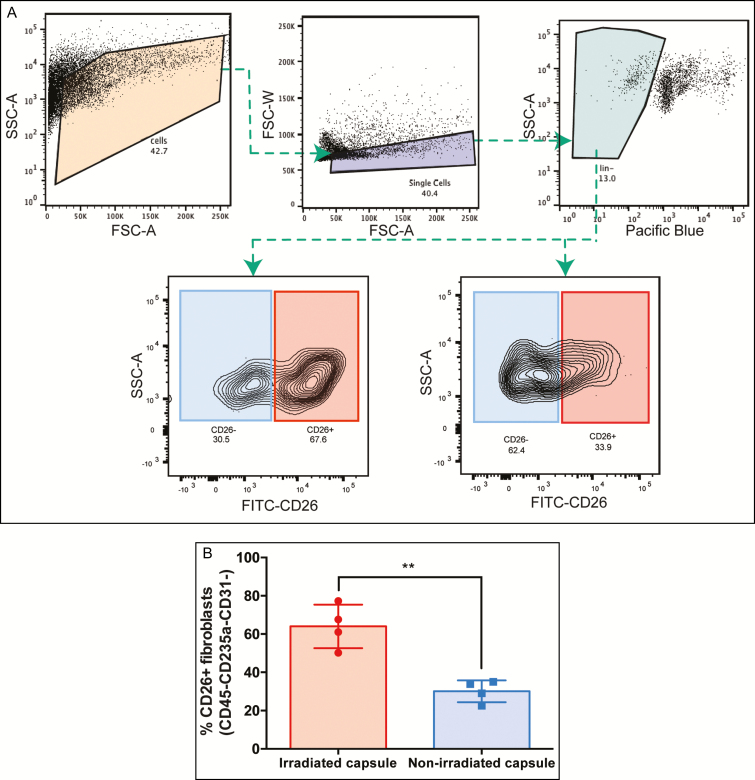
The number of CD26-positive fibroblasts in the capsule specimens was also quantified utilizing flow cytometry, and results showed that irradiated capsule specimens contained a significantly higher percentage of CD26-positive fibroblasts than nonirradiated capsule specimens (64.03 ± 5.70 vs 30.10 ± 2.85, P = 0.0018**) (Figure 3).
Figure 3.
(A) Flow cytometry plots illustrating the gating strategy used to isolate the CD26-positive and negative fibroblasts, with representative plots of the proportions of CD26-positive and CD-negative fibroblasts in irradiated (bottom left) and nonirradiated breast capsules (bottom right). (B) There was a higher percentage of CD26-positive fibroblasts in the irradiated compared with nonirradiated breast tissue (P = 0.0018**). SSC-A, side scatter area; FSC-A, forward scatter area; FSC-W, forward scatter width; FITC, Fluorescein Isothiocyanate; CD26, cluster differentiation 26.
Quantitative Polymerase Chain Reaction Indicating the CD26-Positive Breast Capsule Fibroblasts Are Pro-Fibrotic
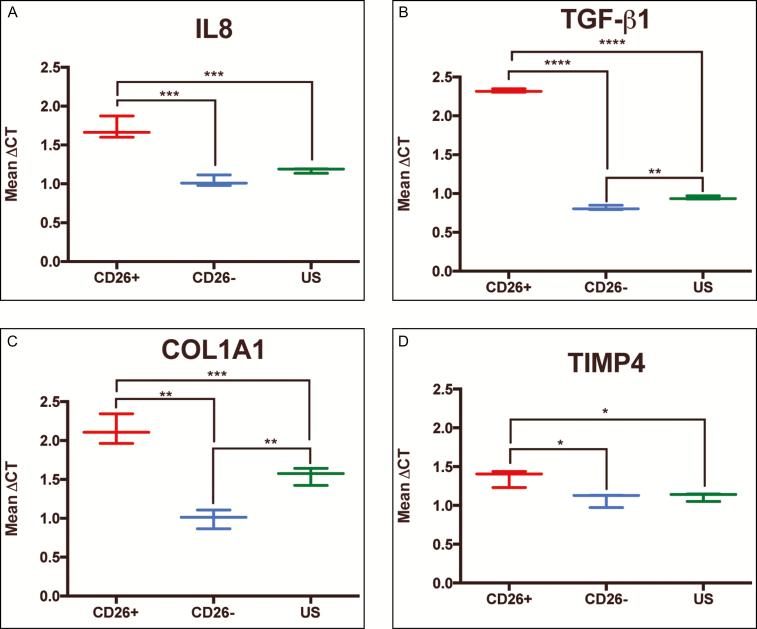
To evaluate whether CD26-expressing fibroblasts in irradiated breast implant capsules are more fibrotic than CD26-negative fibroblasts or unsorted fibroblasts, the expression levels of genes known to regulate inflammation (IL8) and ECM deposition (TGF-β1, COL1A1, and TIMP4) were compared between the fibroblast subpopulations isolated from irradiated breast capsule specimens. The analysis revealed increased expression of IL8 (mean ΔCT 1.71 vs 1.03, P ≤ 0.001***), TGF-β1 (2.32 vs 0.82, P ≤ 0.0001****), COL1A1 (2.14 vs 1.00, P ≤ 0.001***), and TIMP4 (1.36 vs 1.08, P ≤ 0.05*) in CD26-positive compared with CD26-negative fibroblasts, and increased expression of IL8 (1.71 vs 1.17, P ≤ 0.001***), TGF-β1 (2.32 vs 0.94, P ≤ 0.0001****), COL1A1 (2.14 vs 1.5, P ≤ 0.01**), and TIMP4 (1.36 vs 1.11, P ≤ 0.05*) in CD26-positive compared with unsorted fibroblasts (Figure 4).
Figure 4.
Quantitative polymerase chain reaction results comparing the relative expression of genes associated with enhanced fibrotic activity in CD26-positive, CD26-negative, and unsorted (CD45-CD235a-CD31-) fibroblasts isolated from irradiated breast tissue specimens. All experiments were run in triplicate and data were standardized to glyceraldehyde 3-phosphate dehydrogenase expression for statistical analysis. There were higher levels of expression of (A), IL8 (B), TGF-β1 (C), COL1A1, and (D) TIMP4-positive fibroblasts compared with CD26-negative, and CD26-positive fibroblasts compared with unsorted fibroblasts (P ≤ 0.05*; P ≤ 0.01**; P ≤ 0.001***; P ≤ 0.0001****).
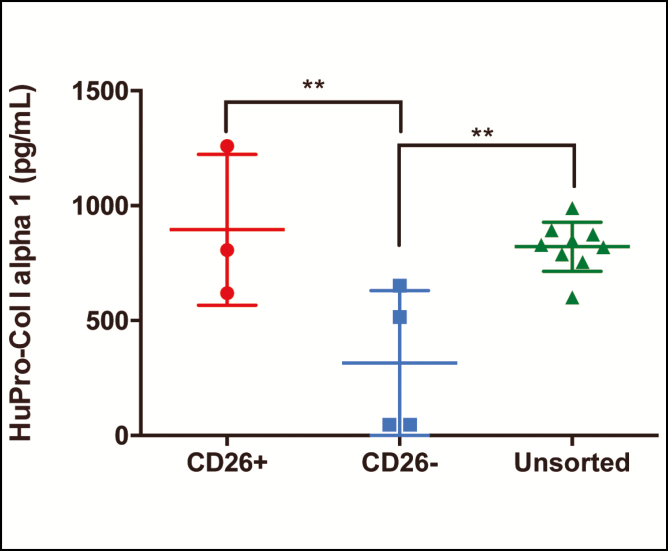
Human Procollagen I Alpha 1 Production
To compare the fibrogenic potential of CD26-positive, CD26-negative, and unsorted (CD45-CD235a-CD31-) fibroblasts, human pro-collagen I alpha 1 content was compared between these fibroblast subtypes. There was a higher percentage of human pro-collagen I alpha 1 in CD26-positive compared with CD26-negative fibroblasts (895.4 vs 315.3 ng/mL, P ≤ 0.01**) and in unsorted compared to CD26-negative fibroblasts (821.8 vs 315.3 ng/mL, P ≤ 0.01**) (Figure 5).
Figure 5.
The human pro-collagen I alpha 1 (HuProCol I alpha 1) content in human sorted and cultured CD26-positive, CD26-negative, and unsorted (CD45-CD235a-CD31-) fibroblasts. There was a higher percentage of human pro-collagen I alpha 1 in CD26-positive compared with CD26-negative fibroblasts (P < 0.01**) and in unsorted compared with CD26-negative fibroblasts (P < 0.01**).
DISCUSSION
Breast capsular contracture frequently complicates implant-based breast reconstruction and remains the most common complication of aesthetic and breast reconstructive surgery.6 Contracted breast capsules result in distortions to the breast shape and texture and can cause substantial pain and/or need for breast revision surgery. Breast irradiation is a key component of cancer therapy for many patients but significantly increases the risk of capsular contracture.21,22 Current treatment modalities for capsular contracture are limited by an incomplete understanding of the exact cellular and molecular mechanisms responsible for the excessive fibrotic reaction and how it is augmented by radiation therapy.
Fibroblasts are one of the main cell types responsible for breast capsular contracture via the deposition of ECM.29,30 Breast capsules become thicker as time following implantation increases, suggesting that fibroblasts continue to lay down collagen fibers and ECM even long after implantation surgery.32,37 This pathological phenomenon is thought to be driven by an excessive inflammatory response.38 Upon implant insertion, polymorphonuclear leukocytes migrate to the foreign material from the vasculature39 and secrete cysteinyl leukotrienes, which are potent lipid inflammatory mediators.40 The leukotrienes stimulate fibroblasts to migrate and proliferate.41,42 In turn, fibroblasts secrete TGF-β, synthesize collagen, and differentiate into myofibroblasts. These events eventually culminate in capsular contracture.43,44 Radiation therapy initiates an exaggerated fibrotic response, which may be mediated by TGF-β, a major factor/cytokine implicated in the fibrosis of irradiated tissue45; tissue levels of TGF-β rise within hours of radiation exposure and remain elevated for extensive periods of time.45-49 TGF-β may therefore strengthen the initiation, development, and persistence of fibrotic tissue in the post-irradiated breast.
In this study, we demonstrated that the irradiated breast capsule tissue was thicker and was associated with a greater degree of clinical contracture than the nonirradiated capsule, consistent with previous findings.50 A novel finding of this work was the greater abundance of CD26-positive fibroblasts in irradiated vs nonirradiated breast implant capsules. The CD26-positive fibroblasts also had an enhanced fibrogenic potential compared with the CD26-negative fibroblasts and the unsorted fibroblasts. The association between the number of CD26-positive fibroblasts and the presence of contracture suggests that CD26-positive fibroblasts may contribute to the formation of fibrotic breast capsule tissue and that this fibroblastic response is exaggerated in the post-irradiated breast. The increased production of human Pro-collagen I alpha 1 and expression of fibrogenic genes in the CD26-positive compared with CD26-negative fibroblasts supports this hypothesis. Our previous work has established the role of CD26-positive fibroblasts in cutaneous fibrobrosis. A subset of fibroblasts in the skin of mice expresses Engrailed-1 (Eng-1) during embryonic development, and in postnatal life the Eng-1 positive fibroblasts are responsible for the majority of fibrotic tissue formed after wounding and radiation. A cell surface marker screening analysis identified the surface marker CD26 to be uniquely and highly expressed on Eng-1 positive fibroblasts. Intradermal transplantation of CD26-positive fibroblasts in mice resulted in a fibrotic reaction of greater intensity with more collagen deposition than that found with transplantation of CD26-negative fibroblasts.34 The findings of this study show that CD26 marks a subset of human fibroblasts with increased fibrogenic potential and that are upregulated in the context of capsular contracture, particularly following radiation. It should be recognized, however, that capsular contracture is likely a multifactorial process, and the complexity of radiation-induced toxicity probably extends beyond the CD26-positive fibroblast. The exact role played by CD26-positive fibroblasts in the pathogenesis remains a topic of future investigation.
Breast capsule formation and contraction represent a significant challenge for the reconstructive surgeon. The gold standard treatment of established capsular contracture is total capsulectomy, which is invasive and associated with a high risk of recurrence. A number of therapeutic agents have been investigated in the treatment of contracture, including nonsteroidal anti-inflammatory drugs, chemotherapeutics, antibiotics, vitamin E, steroids, and leukotriene inhibitors, but none are able to effectively reduce the formation of breast capsules in clinical trials.6 Leukotriene receptor antagonists, including Accolate (zafirlukast) and Singulair (Montelukast), are among the most promising agents, though supportive evidence is still preliminary.51-53 Our results suggest that the CD26-positive fibroblasts may be the principle fibroblast subtype implicated in capsular contracture, and therefore their selective depletion or inhibition may be an effective strategy to minimize breast capsular contracture in irradiated breast tissue. Inhibition of CD26-positive fibroblasts has been successfully achieved utilizing a small molecule-based inhibitor of CD26/DPP4 peptidase activity (Diprotin A) in cutaneous wounds in mice, where Diprotin A-treated wounds healed with significantly reduced scars.34 A number of CD26/DPP4 inhibitors including sitagliptin (Merck) and vildagliptin (Novartis) are FDA approved and are currently being utilized for diabetes.54 Future work may consider the therapeutic benefit of these agents in breast capsular contracture. For example, breast implants could be coated with CD26/DPP4 inhibitors prior to implantation to inhibit the fibrogenic CD26-positive fibroblasts and prophylactically minimize the risk of capsular contracture. Alternatively, injections of CD26/DPP4 inhibitors could be delivered to the peri- implant tissue postoperatively. Based on the results discussed in this paper, the immediate next steps are to test the hypothesis that inhibition of CD26-positive fibroblasts reduces fibrosis surrounding implanted tissue utilizing preclinical studies. Although we have focused on the irradiated breast, future work may compare the abundance of the CD26-positive fibroblast between nonirradiated breasts with and without contracture to understand whether results can be translated to the cosmetic patient population.
CONCLUSIONS
Capsular contracture is a common complication of breast reconstructive surgery that is difficult to treat. Our results suggest that a subpopulation of fibroblasts, characterized by CD26 surface marker expression, are fibrogenic and implicated in the excessive fibrotic reaction that is associated with capsular contracture in the irradiated breast. Future work should aim to explore the therapeutic potential of selective modulation of this fibroblast subpopulation.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
This study was supported by an Aesthetic Surgery Education and Research Foundation (ASERF) grant.
REFERENCES
- 1. Rieger UM, Mesina J, Kalbermatten DF, et al. . Bacterial biofilms and capsular contracture in patients with breast implants. Br J Surg. 2013;100(6):768-774. [DOI] [PubMed] [Google Scholar]
- 2. Stevens WG, Nahabedian MY, Calobrace MB, et al. . Risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2013;132(5):1115-1123. [DOI] [PubMed] [Google Scholar]
- 3. Headon H, Kasem A, Mokbel K. P44. Capsular contracture after breast augmentation: an update for clinical practice. Eur J Surg Oncol. 2015;41(11):S280-S281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Araco A, Caruso R, Araco F, Overton J, Gravante G. Capsular contractures: a systematic review. Plast Reconstr Surg. 2009;124(6):1808-1819. [DOI] [PubMed] [Google Scholar]
- 5. Spear SL, Murphy DK, Slicton A, Walker PS; Inamed Silicone Breast Implant U.S. Study Group . Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(7 Suppl 1):8S-16S; discussion 17S. [DOI] [PubMed] [Google Scholar]
- 6. Adams WP Jr. Capsular contracture: what is it? What causes it? How can it be prevented and managed? Clin Plast Surg. 2009;36(1):119-26, vii. [DOI] [PubMed] [Google Scholar]
- 7. Momoh AO, Ahmed R, Kelley BP, et al. . A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol. 2014;21(1):118-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Handel N, Cordray T, Gutierrez J, Jensen JA. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg. 2006;117(3):757-767; discussion 768. [DOI] [PubMed] [Google Scholar]
- 9. Spear SL, Seruya M, Clemens MW, Teitelbaum S, Nahabedian MY. Acellular dermal matrix for the treatment and prevention of implant-associated breast deformities. Plast Reconstr Surg. 2011;127(3):1047-1058. [DOI] [PubMed] [Google Scholar]
- 10. Cunningham B. The mentor study on contour profile gel silicone MemoryGel breast implants. Plast Reconstr Surg. 2007;120(7 Suppl 1):33S-39S. [DOI] [PubMed] [Google Scholar]
- 11. Nava MB, Rocco N, Catanuto G, et al. . Role of Mitomycin C in preventing capsular contracture in implant-based reconstructive breast surgery: a randomized controlled trial. Plast Reconstr Surg. 2017;139(4):819-826. [DOI] [PubMed] [Google Scholar]
- 12. Chong SJ, Deva AK. Understanding the etiology and prevention of capsular contracture: translating science into practice. Clin Plast Surg. 2015;42(4):427-436. [DOI] [PubMed] [Google Scholar]
- 13. Miller KE, Hontanilla B, Cabello A, Marre D, Armendariz L, Leiva J. The effect of late infection and antibiotic treatment on capsular contracture in silicone breast implants: a rat model. J Plast Reconstr Aesthet Surg. 2016;69(1):70-76. [DOI] [PubMed] [Google Scholar]
- 14. Fischer S, Hirche C, Reichenberger MA, et al. . Silicone implants with smooth surfaces induce thinner but denser fibrotic capsules compared to those with textured surfaces in a rodent model. PLoS One. 2015;10(7):e0132131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lipa JE, Qiu W, Huang N, Alman BA, Pang CY. Pathogenesis of radiation-induced capsular contracture in tissue expander and implant breast reconstruction. Plast Reconstr Surg. 2010;125(2):437-445. [DOI] [PubMed] [Google Scholar]
- 16. Krueger EA, Wilkins EG, Strawderman M, et al. . Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49(3):713-721. [DOI] [PubMed] [Google Scholar]
- 17. Contant CM, van Geel AN, van der Holt B, Griep C, Tjong Joe Wai R, Wiggers T. Morbidity of immediate breast reconstruction (IBR) after mastectomy by a subpectorally placed silicone prosthesis: the adverse effect of radiotherapy. Eur J Surg Oncol. 2000;26(4):344-350. [DOI] [PubMed] [Google Scholar]
- 18. Tallet AV, Salem N, Moutardier V, et al. . Radiotherapy and immediate two-stage breast reconstruction with a tissue expander and implant: complications and esthetic results. Int J Radiat Oncol Biol Phys. 2003;57(1):136-142. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz MR. Discussion: risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2013;132(5):1126-1127. [DOI] [PubMed] [Google Scholar]
- 20. Stevens WG, Calobrace MB, Alizadeh K, Zeidler KR, Harrington JL, d’Incelli RC. Ten-year core study data for Sientra’s food and drug administration-approved round and shaped breast implants with cohesive silicone gel. Plast Reconstr Surg. 2018;141(4S Sientra Shaped and Round Cohesive Gel Implants):7S-19S. [DOI] [PubMed] [Google Scholar]
- 21. Rosato RM, Dowden RV. Radiation therapy as a cause of capsular contracture. Ann Plast Surg. 1994;32(4):342-345. [DOI] [PubMed] [Google Scholar]
- 22. Whitfield GA, Horan G, Irwin MS, Malata CM, Wishart GC, Wilson CB. Incidence of severe capsular contracture following implant-based immediate breast reconstruction with or without postoperative chest wall radiotherapy using 40 Gray in 15 fractions. Radiother Oncol. 2009;90(1):141-147. [DOI] [PubMed] [Google Scholar]
- 23. Chen TA, Momeni A, Lee GK. Clinical outcomes in breast cancer expander-implant reconstructive patients with radiation therapy. J Plast Reconstr Aesthet Surg. 2016;69(1):14-22. [DOI] [PubMed] [Google Scholar]
- 24. Domanskis E, Owsley JQ Jr. Histological investigation of the etiology of capsule contracture following augmentation mammaplasty. Plast Reconstr Surg. 1976;58(6):689-693. [DOI] [PubMed] [Google Scholar]
- 25. Montandon D, Gabbiani G, Ryan GB, Majno G. The contractile fibroblast. Its relevance in plastic surgery. Plast Reconstr Surg. 1973;52(3):286-290. [PubMed] [Google Scholar]
- 26. Prantl L, Angele P, Schreml S, Ulrich D, Pöppl N, Eisenmann-Klein M. Determination of serum fibrosis indexes in patients with capsular contracture after augmentation with smooth silicone gel implants. Plast Reconstr Surg. 2006;118(1):224-229. [DOI] [PubMed] [Google Scholar]
- 27. Dolores W, Christian R, Harald N, Hildegunde P, Georg W. Cellular and molecular composition of fibrous capsules formed around silicone breast implants with special focus on local immune reactions. J Autoimmun. 2004;23(1):81-91. [DOI] [PubMed] [Google Scholar]
- 28. Tan KT, Wijeratne D, Shih B, Baildam AD, Bayat A. Tumour necrosis factor-α expression is associated with increased severity of periprosthetic breast capsular contracture. Eur Surg Res. 2010;45(3-4):327-332. [DOI] [PubMed] [Google Scholar]
- 29. Brazin J, Malliaris S, Groh B, et al. . Mast cells in the periprosthetic breast capsule. Aesthetic Plast Surg. 2014;38(3):592-601. [DOI] [PubMed] [Google Scholar]
- 30. Steiert AE, Boyce M, Sorg H. Capsular contracture by silicone breast implants: possible causes, biocompatibility, and prophylactic strategies. Med Devices (Auckl). 2013;6:211-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rudolph R, Abraham J, Vecchione T, Guber S, Woodward M. Myofibroblasts and free silicon around breast implants. Plast Reconstr Surg. 1978;62(2):185-196. [DOI] [PubMed] [Google Scholar]
- 32. Bui JM, Perry T, Ren CD, Nofrey B, Teitelbaum S, Van Epps DE. Histological characterization of human breast implant capsules. Aesthetic Plast Surg. 2015;39(3):306-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gabbiani G. The myofibroblast: a key cell for wound healing and fibrocontractive diseases. Prog Clin Biol Res. 1981;54:183-194. [PubMed] [Google Scholar]
- 34. Rinkevich Y, Walmsley GG, Hu MS, et al. . Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science. 2015;348(6232):aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thielitz A, Vetter RW, Schultze B, et al. . Inhibitors of dipeptidyl peptidase IV-like activity mediate antifibrotic effects in normal and keloid-derived skin fibroblasts. J Invest Dermatol. 2008;128(4):855-866. [DOI] [PubMed] [Google Scholar]
- 36. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402-408. [DOI] [PubMed] [Google Scholar]
- 37. Siggelkow W, Faridi A, Spiritus K, Klinge U, Rath W, Klosterhalfen B. Histological analysis of silicone breast implant capsules and correlation with capsular contracture. Biomaterials. 2003;24(6):1101-1109. [DOI] [PubMed] [Google Scholar]
- 38. Jones KS. Effects of biomaterial-induced inflammation on fibrosis and rejection. Semin Immunol. 2008;20(2):130-136. [DOI] [PubMed] [Google Scholar]
- 39. Anderson JM. Mechanisms of inflammation and infection with implanted devices. Cardiovasc Pathol. 1993;2(3):33-41. [Google Scholar]
- 40. Peters-Golden M, Henderson WR Jr. Leukotrienes. N Engl J Med. 2007;357(18):1841-1854. [DOI] [PubMed] [Google Scholar]
- 41. Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143-179. [DOI] [PubMed] [Google Scholar]
- 42. Baud L, Perez J, Denis M, Ardaillou R. Modulation of fibroblast proliferation by sulfidopeptide leukotrienes: effect of indomethacin. J Immunol. 1987;138(4):1190-1195. [PubMed] [Google Scholar]
- 43. Danielpour D, Dart LL, Flanders KC, Roberts AB, Sporn MB. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989;138(1):79-86. [DOI] [PubMed] [Google Scholar]
- 44. Wahl SM. Transforming growth factor beta (TGF-beta) in inflammation: a cause and a cure. J Clin Immunol. 1992;12(2):61-74. [DOI] [PubMed] [Google Scholar]
- 45. Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47(2):277-290. [DOI] [PubMed] [Google Scholar]
- 46. Martin M, Lefaix JL, Pinton P, Crechet F, Daburon F. Temporal modulation of TGF-beta 1 and beta-actin gene expression in pig skin and muscular fibrosis after ionizing radiation. Radiat Res. 1993;134(1):63-70. [PubMed] [Google Scholar]
- 47. Randall K, Coggle JE. Expression of transforming growth factor-beta 1 in mouse skin during the acute phase of radiation damage. Int J Radiat Biol. 1995;68(3):301-309. [DOI] [PubMed] [Google Scholar]
- 48. Martin M, Vozenin MC, Gault N, Crechet F, Pfarr CM, Lefaix JL. Coactivation of AP-1 activity and TGF-beta1 gene expression in the stress response of normal skin cells to ionizing radiation. Oncogene. 1997;15(8):981-989. [DOI] [PubMed] [Google Scholar]
- 49. Delanian S, Martin M, Lefaix J. TGFb1, collagen I and III gene expression in human skin fibrosis induced by therapeutic irradiation. Br J Radiol. 1992;65:82-83. [Google Scholar]
- 50. Katzel EB, Koltz PF, Tierney R, et al. . The impact of Smad3 loss of function on TGF-β signaling and radiation-induced capsular contracture. Plast Reconstr Surg. 2011;127(6):2263-2269. [DOI] [PubMed] [Google Scholar]
- 51. Scuderi N, Mazzocchi M, Fioramonti P, Bistoni G. The effects of zafirlukast on capsular contracture: preliminary report. Aesthetic Plast Surg. 2006;30(5):513-520. [DOI] [PubMed] [Google Scholar]
- 52. Scuderi N, Mazzocchi M, Rubino C. Effects of zafirlukast on capsular contracture: controlled study measuring the mammary compliance. Int J Immunopathol Pharmacol. 2007;20(3):577-584. [DOI] [PubMed] [Google Scholar]
- 53. Lille S, Jacoby J. The potential benefit of preemptive leukotriene inhibitor treatment to breast augmentation/mastopexy surgery. Plast Reconstr Surg. 2018;142(4):610e-611e. [DOI] [PubMed] [Google Scholar]
- 54. Ahrén B. DPP-4 inhibitors. Best Pract Res Clin Endocrinol Metab. 2007;21(4):517-533. [DOI] [PubMed] [Google Scholar]