Abstract
Among 182 children with influenza infection in 2016–2017, 18% had neurologic manifestations of influenza (NMI), including seizures and encephalopathy; 85% of these children were infected with the H3N2 strain. Children with NMI had 3.5-times-higher odds of having a neurologic comorbidity than those without NMI and a 10-fold increased odds of hospitalization.
Keywords: encephalitis, encephalopathy, influenza, neurologic manifestation, pediatric, seizures
Influenza viruses cause a variety of neurologic manifestations in children, including simple and complex febrile seizures, exacerbations of underlying seizure disorders, encephalopathy, and encephalitis [1]. The type and frequency of influenza-associated neurologic diseases in children are influenced by circulating strains, predisposing host factors, and uptake and efficacy of influenza vaccinations [2–4].
In 2016–2017, an apparent increase in associated neurologic diseases during an influenza A(H3N2)-predominant season was noted at Children’s Hospital Colorado (CHCO). Our objectives for this study were to describe the spectrum of neurologic manifestations, identify predisposing host risk factors, and assess outcomes associated with circulating influenza strains during the 2016–2017 season among children who presented to a quaternary-care children’s hospital.
METHODS
We conducted a retrospective cohort study with nested matched case-control of children with neurologic manifestations of influenza (NMI) between December 27, 2016, and April 12, 2017. The study was conducted at CHCO, a large academic quaternary-care hospital with affiliated sites that serves Colorado and surrounding states. The hospital network has approximately 500 inpatient beds and has 19000 inpatient admissions and 158000 emergency department or urgent care visits per year.
The primary outcome was the presence of NMI, defined as seizure(s), altered mental status for >24 hours as the presenting complaint, or encephalopathy, encephalitis, meningitis, or seizures among the discharge diagnoses. Patients with NMI were matched 1:4 with sex- and race-matched controls using random-number assignment for children with polymerase chain reaction (PCR)-confirmed influenza infection without neurologic manifestations in the same time period. Race was categorized as white, Asian, black/African American, or other/unknown. Clinical characteristics and influenza strains between cases and controls were compared. In addition, the proportion of NMI (according to International Classification of Diseases, Ninth Revision [ICD-9] codes for seizures, encephalopathy, meningitis, and encephalitis) was calculated among PCR-confirmed influenza cases between the 2009–2010 and 2016–2017 influenza seasons to identify temporal trends. Although we did not have influenza A substrain data for these years, we reviewed the predominant circulating influenza A strain for each season.
All inpatient and outpatient children with PCR-confirmed influenza infection at CHCO during the study period were included. Children with a nurse-only visit in the emergency department or a visit unrelated to influenza infection were excluded. Influenza testing was conducted at clinician discretion according to CHCO protocols, which recommend testing for children with a medical condition that increases the risk of complications from influenza and for hospitalized patients. Respiratory specimens (nasopharyngeal swabs, nasal aspirates, and tracheal aspirates) were tested with the FilmArray (Salt Lake City, Utah) respiratory pathogen panel, which differentiates influenza subtypes H1N1, H3N2, A (not otherwise specified), and B. Influenza PCR testing of cerebrospinal fluid (CSF) was not conducted.
Children at high risk for complications from influenza were determined using Advisory Committee on Immunization Practices guidelines [5]. Vaccination status was determined from emergency department provider documentation, the admission note, and/or the Colorado Immunization Information System registry linked to the electronic health record used by approximately 75% to 85% of practices in the state. Children were considered vaccinated if they received the appropriate number of influenza vaccine doses for their age, partially vaccinated if they were aged 6 months to <9 years and received 1 vaccine (consistent with guidelines in place during the study period), or unvaccinated if they did not receive influenza vaccination for the season, and their vaccination status was considered unknown if we could not determine their true status.
Study investigators extracted data from the electronic health record via systematic retrospective chart review. Data collected included sociodemographic and clinical characteristics, vaccination status, and concurrent viral and secondary bacterial infections (pneumonia, meningitis, sinusitis, otitis media, bacteremia). All the investigators reviewed a common subset of charts to ensure standardization of processes and uniformity of data collection. A neurologist (J. M.) conducted a secondary review of all NMI cases to ensure appropriate classification. Data were collected in a standardized data-collection tool in the Research Electronic Data Capture (REDCap) system hosted at the University of Colorado [6]. Approval was obtained from the Colorado Multiple Institutional Review Board.
Descriptive statistics were used to summarize clinical, sociodemographic, and outcome variables. Conditional logistic regression was used to analyze socioeconomic and clinical characteristics associated with NMI, matching on sex and race. Multivariate logistic regression models were used to evaluate the relationship between NMI and outcomes including hospitalization, intensive care unit (ICU) admission, readmission, inpatient rehabilitation, and oseltamivir prescription controlling a priori for an underlying neurologic condition. A multivariate log gamma model was used to evaluate the relationship between NMI and length of stay, controlling for underlying neurologic condition. All statistical calculations were performed using SAS 9.4 (SAS Corp, Cary, North Carolina) using a level of significance of .05.
RESULTS
In 2016–2017, 33 (18%) of 182 influenza-positive children at CHCO who met study inclusion criteria presented with a neurologic manifestation. Of these patients with NMI, 18 had seizures only, 5 had encephalopathy only, and 10 had both seizures and encephalopathy (Table 1). Patients with NMI accounted for 43% of influenza-related hospitalizations in 2016–2017, and 54% of hospitalized patients with NMI required ICU admission. Among children with NMI, 10 (30%) of 33 had an underlying neurologic condition but did not have greater morbidity or a more severe presentation than children without an underlying neurologic condition. Abnormalities on brain magnetic resonance imaging were found in 3 (27%) of 11 children with NMI (in whom findings were consistent with posterior reversible encephalopathy syndrome, acute left middle cerebral artery territory infarct, and parameningeal enhancement in a child with meningitis). CSF pleocytosis (>10 cells/μL) was observed in 3 (23%) of 13 children.
Table 1.
Matched Analysis of Clinical and Laboratory Characteristics and Outcomes of Children Who Tested Positive for Influenza With and Those Without Neurologic Manifestations—2016–2017
| Variable | Neurologic Manifestation (n = 33)a | Unadjusted OR (95% CI) | P | Adjusted ORb/Effect Estimate (95% CI) | P | |
|---|---|---|---|---|---|---|
| Yes (n = 33) | No (n = 132) | |||||
| Clinical and laboratory characteristics | ||||||
| Age (median [IQR]) (years) | 6 (2–10) | 7 (2–13) | 0.94 (0.88–1.01) | .11 | — | — |
| Hispanic or Latino (n [%]) | 10 (30) | 48 (37) | 0.72 (0.31–1.67) | .44 | — | — |
| High-risk condition | 21 (64) | 80 (61) | 1.13 (0.52–2.48) | .75 | — | — |
| Underlying neurologic medical condition (n [%]) | 10 (30) | 13 (10) | 3.48 (1.43–8.46)c | <.01 | — | — |
| Unvaccinated against influenza vaccine (n [%]) | 19 (66) | 72 (58) | 1.49 (0.64–3.48) | .35 | — | — |
| Length of illness before presentation (n [%]) | ||||||
| 0–2 days | 23 (70) | 65 (49) | — | — | ||
| 3–5 days | 4 (12) | 43 (33) | 0.26 (0.08–0.80)c | .02 | — | — |
| >5 days | 6 (18) | 24 (18) | 0.74 (0.27–2.00) | .55 | — | — |
| Fever present | 33 (100) | 121 (92) | NA | NA | — | — |
| Upper respiratory symptoms present | 26 (79) | 125 (95) | 0.21 (0.06–0.66)c | <.01 | — | — |
| Secondary bacterial infection present (n [%]) | 10 (30) | 18 (14) | 2.81 (1.11–7.11)c | .03 | — | — |
| Influenza exacerbated underlying medical condition(s) (n [%]) | 14 (42) | 31 (23) | 2.27 (1.04–4.92)c | .04 | — | — |
| Emesis (n [%]) | 8 (24) | 34 (26) | 0.92 (0.38–2.25) | .86 | — | — |
| Influenza testing result (n [%]) | ||||||
| Type A | 29 (88) | 92 (70) | Reference | — | — | |
| Type B | 4 (12) | 40 (30) | 0.30 (0.10–0.93)c | .04 | — | — |
| Clinical outcomes | ||||||
| Hospitalized | 26 (79)c | 34 (26)c | 10.7 (4.26–26.90)c | <.01 | 10.3 (4.02–26.25)c | <.01 |
| Length of stay (median [IQR]) (days) (n = 60) | 3 (2, 5)c | 3 (2, 6)c | 1.92 (1.20–3.08)c | <.01 | 2.08 (1.29–3.34)c | <.01 |
| Admitted to ICU (n = 60) | 14 (54) | 10 (29) | 2.80 (0.96–8.14) | .06 | 2.37 (0.75–7.51) | .14 |
| Readmitted or had a repeat visit within 30 days | 1 (3) | 3 (2) | 1.34 (0.14–13.35) | .80 | 1.13 (0.10–12.42) | .92 |
| Oseltamivir prescribed | 32 (97)c | 89 (67)c | 15.5 (2.04–116.9)c | <.01 | 12.8 (1.68–98.11)c | .01 |
Abbreviations: OR, odds ratio; CI, confidence interval; ICU, intensive care unit; IQR, interquartile range; NA, not applicable.
aUnless otherwise specified.
bAdjusted for underlying high-risk medical condition.
cSignificant result.
Influenza A(H3N2) was the predominant strain associated with NMI in 2016–2017; it accounted for 29 (88%) of 33 subtyped influenza strains. The remainder of the NMI cases involved children with influenza B infection, and we found no cases of H1N1 infection in our cohort.
Among children with known vaccination status, 19 (65%) of 29 with NMI were unvaccinated or partially vaccinated against influenza for that season.
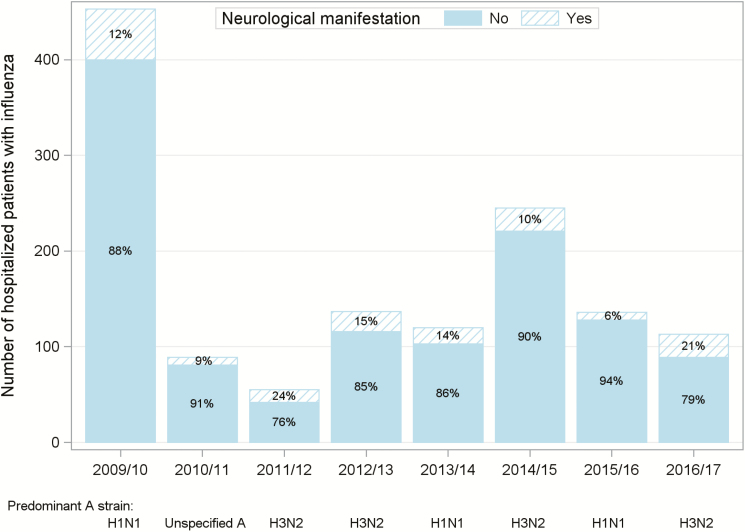
Temporal trends in influenza hospitalizations and NMI from 2009 to 2017 are shown in Figure 1. The highest proportions of children with NMI were seen in the 2016–2017 (21%) and 2011–2012 (24%) influenza seasons, when H3N2 was the predominant circulating influenza type A strain.
Figure 1.
Proportion of patients hospitalized at Children’s Hospital Colorado with influenza who presented with neurologic manifestations (altered mental status and seizures) according to season, 2009–2017.
Clinical characteristics and outcomes of influenza-infected patients with NMI versus those without NMI in the 2016–2017 influenza season are shown in Table 1. Children with NMI had higher odds of having the influenza A strain, an underlying neurologic condition, and a secondary bacterial infection than those without NMI. When we controlled for any underlying medical condition, we found that patients with NMI had a 10-times-higher risk of hospitalization than those without NMI.
DISCUSSION
During the H3N2-predominant influenza season of 2016–2017, neurologic manifestations were responsible for the second highest proportion of influenza-related hospitalizations in children over the previous 8 influenza seasons at our institution. Lengths of stay for these hospitalizations were brief, and no deaths were associated with these encounters. Children most commonly presented with febrile seizures, brief resolving encephalopathy, and increased seizure frequency among those with a known seizure disorder. The majority of children who presented with a neurologic complication were undervaccinated, which highlights an opportunity for prevention through universal influenza immunization.
This study adds to our understanding of influenza A(H3N2) infection as an important contributor to neurologic manifestations associated with influenza viruses. We found that influenza A(H3N2) infection commonly causes neurologic manifestations that lead to hospitalization even more frequently than infection with influenza A(H1N1) and influenza B but differs in manifestation type and severity. Despite their initial presentation with encephalopathy and seizures, most children in the cohort had normal neuroimaging results, CSF without pleocytosis, and normal electroencephalography results and experienced clinical resolution of their neurologic symptoms within 24 to 48 hours. More than 50% of the children required ICU admission, but most of them experienced rapid resolution of symptoms. It is not known whether this brief self-resolving encephalopathic, but not encephalitic, presentation is unique to influenza A(H3N2) infection given the lack of substrain typing in other published studies [7, 8], but it seems clinically distinct from the acute necrotizing encephalitis and severe influenza-associated encephalopathy presentations that have been described with influenza A(H1N1) and B strains and are associated with significant morbidity and death [4, 9, 10]. These data suggest that strain typing can provide important prognostic information for children with influenza infection and encephalopathy.
Several reports of NMI during the 2009 H1N1 pandemic have been published, and an apparent increased frequency of NMI cases was observed in adults and children [3, 4]. However, before the pandemic, the H3N2 strain was associated with several cases of influenza encephalopathy in Japan in the 1980s and during the 1994–1995 epidemic [11]. Although it is not known whether H3N2 is more neurotropic than other influenza strains, the presence of a variant H3N2 strain with a novel substitution at the receptor-binding site Tyr-13-Phe was found in patients with influenza A encephalopathy [12]. This finding suggests that influenza encephalopathy might be caused by a variant of influenza A(H3N2) that has neurovirulence. We were unable to evaluate this nucleotide substitution in our study.
Limitations of our study result from analyses of single-center retrospective data. Although our study included one of the largest series of patients with influenza A(H3N2) NMI, the size of our data set prevented more robust multivariate analyses. Selection bias could have resulted from our review of children who presented to a large quaternary-care pediatric center, with sicker children perhaps more likely to undergo testing for influenza, and variability in obtaining lumbar punctures, electroencephalography, and imaging resulting in limited diagnostic data available for analyses. We also do not have long-term follow-up data available for these patients to determine ongoing morbidity or long-term sequelae.
CONCLUSION
We report that neurologic manifestations were prevalent among influenza-infected children and frequently led to hospitalization during the influenza A(H3N2)-predominant 2016–2017 season. Most NMI observed were brief and reversible. Our findings suggest that influenza testing among febrile children with altered mental status/seizures should be considered and highlight the importance of influenza vaccination among children with an underlying neurologic disorder.
Notes
Financial support. This study was supported partly by National Institutes of Health (NIH)/National Center for Research Resources Colorado Clinical and Translational Science Institute grant number UL1 RR025780 and NIH grant number K23 AI128069-01.
Potential conflicts of interest. A. D. has provided consulting services to Merck, Pfizer, and Sanofi Pasteur but does not receive any research funding from these entities. S. R. receives grant support for GlaxoSmithKline. A. W. receives grant support from MedImmune. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ekstrand JJ. Neurologic complications of influenza. Semin Pediatr Neurol 2012; 19:96–100. [DOI] [PubMed] [Google Scholar]
- 2. Britton PN, Blyth CC, Macartney K, et al. ; Australian Childhood Encephalitis (ACE) Study Investigators; Influenza Complications Alert Network (FluCAN) Investigators; Paediatric Active Enhanced Disease Surveillance (PAEDS) Network The spectrum and burden of influenza-associated neurological disease in children: combined encephalitis and influenza sentinel site surveillance from Australia, 2013–2015. Clin Infect Dis 2017; 65:653–60. [DOI] [PubMed] [Google Scholar]
- 3. Wilking AN, Elliott E, Garcia MN, et al. Central nervous system manifestations in pediatric patients with influenza A H1N1 infection during the 2009 pandemic. Pediatr Neurol 2014; 51:370–6. [DOI] [PubMed] [Google Scholar]
- 4. Glaser CA, Winter K, DuBray K, et al. A population-based study of neurologic manifestations of severe influenza A(H1N1)pdm09 in California. Clin Infect Dis 2012; 55:514–20. [DOI] [PubMed] [Google Scholar]
- 5. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016; 65:1–54. [DOI] [PubMed] [Google Scholar]
- 6. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newland JG, Laurich VM, Rosenquist AW, et al. Neurologic complications in children hospitalized with influenza: characteristics, incidence, and risk factors. J Pediatr 2007; 150:306–10. [DOI] [PubMed] [Google Scholar]
- 8. Chung BH, Tsang AM, Wong VC. Neurologic complications in children hospitalized with influenza: comparison between USA and Hong Kong. J Pediatr 2007; 151:e17–8; author reply e18–9. [DOI] [PubMed] [Google Scholar]
- 9. Lin CH, Huang YC, Chiu CH, et al. Neurologic manifestations in children with influenza B virus infection. Pediatr Infect Dis J 2006; 25:1081–3. [DOI] [PubMed] [Google Scholar]
- 10. Mariotti P, Iorio R, Frisullo G, et al. Acute necrotizing encephalopathy during novel influenza A(H1N1) virus infection. Ann Neurol 2010; 68:111–4. [DOI] [PubMed] [Google Scholar]
- 11. Sugaya N. Influenza-associated encephalopathy in Japan. Semin Pediatr Infect Dis 2002; 13:79–84. [DOI] [PubMed] [Google Scholar]
- 12. Mori SI, Nagashima M, Sasaki Y, et al. A novel amino acid substitution at the receptor-binding site on the hemagglutinin of H3N2 influenza A viruses isolated from 6 cases with acute encephalopathy during the 1997–1998 season in Tokyo. Arch Virol 1999; 144:147–55. [DOI] [PubMed] [Google Scholar]