Abstract
Background
Dosing recommendations for treating childhood tuberculosis (TB) were revised by the World Health Organization, yet so far, pharmacokinetic studies that have evaluated these changes are relatively limited. We evaluated plasma drug concentrations of rifampicin (RIF), isoniazid (INH), pyrazinamide (PZA), and ethambutol (EMB) among children undergoing TB treatment in Tanzania when these dosing recommendations were being implemented.
Methods
At the end of intensive-phase TB therapy, blood was obtained 2 hours after witnessed medication administration to estimate the peak drug concentration (C2h), measured using high-performance liquid chromatography or liquid chromatography–tandem mass spectrometry methods. Differences in median drug concentrations were compared on the basis of the weight-based dosing strategy using the Mann–Whitney U test. Risk factors for low drug concentrations were analyzed using multivariate regression analysis.
Results
We enrolled 51 human immunodeficiency virus–negative children (median age, 5.3 years [range, 0.75–14 years]). The median C2hs were below the target range for each TB drug studied. Compared with children who received the “old” dosages, those who received the “revised” WHO dosages had a higher median C2h for RIF (P = .049) and PZA (P = .015) but not for INH (P = .624) or EMB (P = .143); however, these revised dosages did not result in the target range for RIF, INH, and EMB being achieved. A low starting dose was associated with a low C2h for RIF (P = .005) and PZA (P = .005). Malnutrition was associated with a low C2h for RIF (P = .001) and INH (P = .001).
Conclusions
Among this cohort of human immunodeficiency virus–negative Tanzanian children, use of the revised dosing strategy for treating childhood TB did not result in the target drug concentration for RIF, INH, or EMB being reached.
Keywords: East Africa, malnutrition, pediatric, pharmacokinetics, tuberculosis
Tuberculosis (TB) is the leading global killer, caused by the curable pathogen, Mycobacterium tuberculosis (MTB) [1]. In 2016, among the estimated 10.4 million people with TB disease, nearly 1.7 million (16%) died as a result of their illness, including an estimated 253 000 children younger than 15 years. International child health and TB stakeholders have been advocating for ways to reach “zero deaths from TB in children” [2]. An essential part of the plan for achieving this goal is the timely initiation of proper TB treatment.
The principles of treating childhood TB largely parallel those for adults; short-course treatment consists of intensive therapy for 2 months with rifampicin (RIF), isoniazid (INH), and pyrazinamide (PZA) with or without ethambutol (EMB), based on human immunodeficiency virus (HIV) status and extent of disease, followed by continuation therapy for 4 months with RIF and INH [3]. Medication dosing recommendations for children historically were based on extrapolations from pharmacokinetics (PK) data among adults [4]. Similarly, optimal drug-concentration targets for TB medications among children have been adapted from studies of their PK and pharmacodynamics (PD) in adults [5]. Because the first-line anti-TB drugs are concentration dependent in their activity, the PK parameters most predictive of efficacy are the area under the concentration curve (AUC) and the correlative peak concentration (Cmax). As a result of age-related differences in drug absorption, distribution, metabolism, and elimination, most children require different dosing strategies than adults to achieve comparable target AUC or Cmax values [6]. A systematic review of available PK/PD data from the use of first-line TB therapy in children was spearheaded by the World Health Organization (WHO) and concluded with the issuance of revised pediatric dosing guidelines that recommend increasing the doses of RIF, PZA, and EMB and doubling the dose of INH in an effort to help children reach their respective target concentrations to improve treatment outcomes [4, 7–9].
The number of PK assessments available for validating these revised doses among children is growing. Results of an early study among South African children aged ≤2 years indicated that the revised doses could result in target concentrations of RIF, INH, and PZA [10]. However, subsequent studies from South Africa and elsewhere have not found consistent results. No PK data from children in Tanzania are currently available. We conducted a longitudinal cohort study to investigate TB diagnostics among Tanzanian children at risk for TB; among the subset of children started on TB treatment, we aimed to measure the estimated peak concentrations of first-line TB drugs. Given that the revised pediatric dosing guidelines were being implemented during the recruitment period, we sought to compare the drug concentrations among children who received the “revised” doses and those who received the “old” doses.
METHODS
Setting and Population
This study was conducted at Haydom Lutheran Hospital, a rural 400-bed referral hospital in Tanzania that serves a population of 2 000 000. Children in this study represent a subset of participants enrolled in a longitudinal study investigating TB diagnostics. Participants included were aged 6 months to 15 years, diagnosed clinically or microbiologically with pulmonary and/or extrapulmonary TB, prescribed first-line TB treatment, and returned for their 2-month follow-up visit. All of the children underwent tuberculin skin test placement (5 tuberculin units of PPD-RT23 [Staten Serum Institut, Copenhagen, Denmark]); microbiologic confirmation was attempted by collecting expectorated sputum, induced sputum, aspirated gastric fluid, aspirated lymph node fluid, a biopsy specimen, or another sterile bodily fluid, as appropriate, and testing it with Ziehl–Neelsen staining, the GeneXpert MTB/RIF molecular assay (Cepheid, Sunnyvale, California), and a mycobacterial culture using the BACTEC MGIT system (Becton Dickinson, Sparks, Maryland). Per national guidelines, all children undergoing TB treatment were tested for HIV.
Treatment
Medications, daily fixed-dose combination (FDC) pills available in the public sector according to the Tanzanian National TB and Leprosy Program dosing guidelines, were prescribed by clinicians. FDC pills were available in pediatric and adult formulations, which differed on the basis of their content. Formulations available for intensive-phase treatment included film-coated pediatric FDC pills that contained 60 mg of RIF, 30 mg of INH, and 150 mg of PZA per tablet (EMB was administered separately as a 100- or 400-mg tablet, as appropriate) or film-coated adult FDC pills that contained 150 mg of RIF, 75 mg of INH, 400 mg of PZA, and 275 mg of EMB per tablet. Continuation-phase FDC pills were available in a pediatric formulation (60 mg of RIF and 30 mg of INH) and an adult formulation (150 mg of RIF and 75 mg of INH). Medications were prescribed on the basis of the child’s weight band according to the Tanzanian National TB and Leprosy Program guidelines [11]. Specific FDC formulations were dispensed on the basis of local availability, but the preference was to use the pediatric formulation in younger children to minimize the extent of pill-cutting that parents/guardians of young children or those who were unable to swallow tablets had to do; they were encouraged to crush the tablet(s) and dissolve its contents in a small amount of safe water before administering it to their child, per the national guidelines.
Specimen Collection
Two months after starting TB treatment, a timed phlebotomy was performed. Given the allowable window of time for follow-up visits, some participants had already transitioned to the continuation phase of their therapy. Depending on the child’s age and traveling distance to the study site, medications were given on an empty stomach or at least 2 hours after breakfast. TB medication administration was witnessed by research staff; 2 hours later, a single venous blood sample was collected in a cell preparation tube (CPT) (Becton Dickinson) vacutainer to determine the estimated peak drug concentration (C2h). Plasma was separated, immediately stored at −80°C for batch analysis, and later transported on dry ice to the referral laboratory.
Drug Concentration Measurements
Plasma drug concentrations were measured for all TB drugs that were administered on the day of specimen collection using validated high-performance liquid chromatography (for INH) or liquid chromatography–tandem mass spectrometry (for RIF, PZA, and EMB). The drug-specific standard curve ranges were 0.40 to 20.00 µg/mL for INH, 0.50 to 50.00 µg/mL for RIF, 2.00 to 100.00 µg/mL for PZA, and 0.20 to 10.00 µg/mL for EMB. Any result below the limit of detection (LOD) was recoded as 0. All assays met all standard quality-control criteria, including conducting procedures to ensure that CPT additives did not interfere with assay performance.
To validate drug content across each of the 4 FDC formulations, the individual tablets described earlier were crushed, weighed, and diluted in 1:1 methanol/water. The contents of each tablet were spiked into plasma samples and assayed as described earlier for RIF, INH, PZA, and EMB using the high-performance liquid chromatography or liquid chromatography–tandem mass spectrometry protocol. The target concentrations for the dilutions varied according to drug; the target concentration for RIF was 50 µg/mL, for INH, 30 µg/mL, for PZA, 100 µg/mL, and for EMB, 10 µg/mL.
Definitions
The previous recommendations for daily dosing were as follows: RIF, 10 mg/kg of body weight (range, 8–12 mg/kg); INH, 5 mg/kg (range, 4–6 mg/kg); PZA, 25 mg/kg (range, 20–30 mg/kg); and EMB, 15 mg/kg (range, 15–20 mg/kg). The revised recommendations included increasing the daily doses to 15 mg/kg (range, 10–20 mg/kg) for RIF, 10 mg/kg for INH (range, 10–15 mg/kg), 35 mg/kg (range, 30–40 mg/kg) for PZA, and 20 mg/kg (range, 15–25 mg/kg) for EMB. For purposes of this analysis, weight-based doses were defined as being consistent with the old or revised dosing recommendations if they were below or above, respectively, the following thresholds: 12.5 mg/kg for RIF, 7.5 mg/kg for INH, 30 mg/kg for PZA, and 20 mg/kg for EMB. The C2h targets accepted among adults and used to define target ranges were 8 to 24 µg/mL for RIF, 3 to 6 µg/mL for INH, 20 to 50 µg/mL for PZA, and 2 to 6 µg/mL for EMB [5]. Age, height, and weight measured on the day of timed phlebotomy were used to calculate anthropometric Z scores; height-for-age and weight-for-age Z scores were calculated among participants aged <5 years, and the body mass index-for-age Z score (BAZ) was calculated for all participants using the WHO AnthroPlus calculator. To code nutritional status, children with a Z score of less than or equal to −2 standard deviations below the mean for any indicator were defined as being malnourished.
Statistical Analyses
Demographic and clinical characteristics were evaluated using simple frequencies. Characteristics and drug concentrations were compared using the t or Mann–Whitney U test for nonparametric data. Bivariate and multivariate linear regression analyses were used to determine risk factors for low exposure to individual drugs. The model included the weight-based dose for the given drug and age as continuous variables and sex and nutritional status as dichotomous variables. All tests of significance were 2 sided; a P value of <.05 was considered significant in the final model. Data were analyzed with SPSS 24.0 (IBM, Chicago, Illinois).
Regulatory Approvals
This protocol was approved by the National Institute of Medical Research in Tanzania and the institutional review board at University of Virginia. All parents/guardians provided written permission after informed consent; children older than 7 years provided written assent.
RESULTS
Between January 2014 and January 2016, 51 children were enrolled, were started on TB therapy, and attended a 2-month follow-up visit; all of them were HIV negative. Eight additional children were enrolled and started on TB therapy, but they died before the follow-up visit and therefore are not included in this report. The median age was 5.3 years (range, 0.75–14 years); 8 (16%) were younger than 2 years. Demographic and clinical findings are listed in Table 1. Moderate/severe malnutrition was common (77%) in this population; 16 (70%) of 23 were underweight (WAZ ≤ −2), the same proportion were stunted (height-for-age Z score ≤ −2). Of the 51 children in the study, 14 (28%) had a body mass index-for-age Z score of less than or equal to −2. Twenty (39%) children had microbiologically confirmed TB, and 67% had pulmonary TB. Although persistent diarrhea was reported by 7 (14%) participants at enrollment, all of them reported resolution at the 2-month follow-up visit when their drug concentrations were measured.
Table 1.
Demographic and Clinical Features of Participants
| Feature | Value (N = 51) |
|---|---|
| Median (IQR) age (years) | 5.3 (2.3–9.6) |
| Female sex (n [%]) | 24 (47) |
| HIV positive (n [%]) | 0 (0) |
| Vaccinated against BCG (n [%]) | 45 (88) |
| Close contact with TB case reported (n [%]) | 28 (55) |
| Persistent diarrhea (n [%]) | 7 (14) |
| Any malnutrition (n [%])a | 39 (77) |
| Underweight (WAZ < −2 SDs) (n of N [%]) | 16 of 23 (70) |
| Stunted (HAZ < −2 SDs) (n of N [%]) | 16 of 23 (70) |
| BMI-for-age Z score < −2 SDs (n of N [%]) | 14 of 51 (27) |
| Underwent reactive tuberculin skin test (n [%]) | 40 (78) |
| Location of suspected TB (n [%]) | |
| Pulmonary only | 18 (35) |
| Extrapulmonary only | 17 (33) |
| Pulmonary and extrapulmonary | 16 (32) |
| Microbiologically confirmed TB (n [%]) | 20 (39) |
| Positive acid-fast bacilli smear result | 9 (18) |
| Positive GeneXpert MTB/RIF result | 14 (28) |
| Positive TB culture result | 15 (29) |
Abbreviations: BCG, bacillus Calmette–Guérin; BMI, body mass index; HAZ, height-for-age Z score; HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation; TB, tuberculosis; WAZ, weight-for-age Z score.
aThese measurements of malnutrition are independent of each other. “Any malnutrition” was defined as a WAZ, HAZ, or BMI-for-age Z score of less than −2. WAZ and HAZ were calculated only among children younger than 5 years; a BMI-for-age Z score was calculated for every participant.
Two hours after medication administration, median C2hs were below the target ranges for all the TB drugs tested. Compared with children who received the old dosages, those who received the revised WHO dosages had higher median drug C2hs, and statistically significant differences for RIF (P = .049) and PZA (P = .015) concentrations were found (Table 2). Overall, 67% (34 of 51) of the children had simultaneously low concentrations to all drugs tested; no child simultaneously met the target ranges for both RIF and INH (Table 3).
Table 2.
Observed Versus Expected Drug Concentrations According to Dosing Strategy
| Concentration Type | Concentration of Drug According to Dosing Strategy | |||||||
|---|---|---|---|---|---|---|---|---|
| Rifampicin | Isoniazid | Pyrazinamide | Ethambutol | |||||
| Old (n = 35) |
Revised (n = 16) |
Old (n = 47) |
Revised (n = 4) |
Old (n = 16) |
Revised (n = 12) | Old (n = 12) | Revised (n = 12) | |
| Median (IQR) C2h (µg/mL) | 0.68 (0.27–1.84) |
2.17 (0.59–4.61) |
1.01 (0.54–2.38) |
2.05 (0.41–4.60) |
11.78 (2.43–23.57) |
28.17 (13.33–32.52) |
0.46 (0.00–1.36) |
1.04 (0.60–1.65) |
| P | .049 | .624 | .015 | .143 | ||||
| Target value (µg/mL) | 8–24 | 3–6 | 20–50 | 2–6 | ||||
Abbreviations: C2h, peak drug concentration; IQR, interquartile range.
Table 3.
Frequency of Achieving Target Drug Concentrations
| No. of Drugs Within Target Range | RIF, INH, PZA, and EMBa Tested (n = 28) (n [%]) | RIF and INH Tested (n = 23) (n [%]) |
|---|---|---|
| 0 | 15 (54) | 19 (83) |
| 1 | 8 (28) | 4 (17) |
| 2 | 4 (14) | 0 |
| 3 | 1 (4) | NA |
| 4 | 0 | NA |
Abbreviations: EMB, ethambutol; INH, isoniazid; NA, not applicable; PZA, pyrazinamide; RIF, rifampicin.
aEMB was tested in 24 participants.
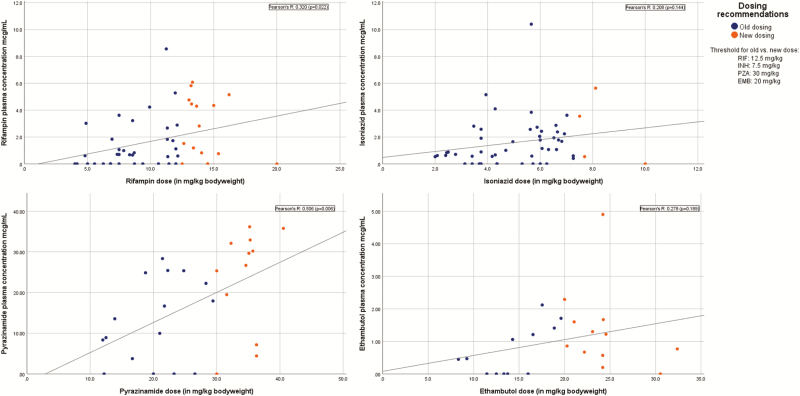
Figure 1 shows the observed concentrations according to the weight-based dose for each drug. The vast majority of children who received revised doses of RIF, INH, and EMB did not meet their target values. Target ranges were achieved more readily with revised doses of PZA, yet only 2 (7%) of 28 children who received PZA achieved a C2h of >35 µg/mL. Many children had concentrations that were below the LOD for the respective drug; RIF concentrations were below the LOD in 17 (33%) of 51 children, INH concentrations were below the LOD in 11 (22%) of 51 children, PZA concentrations were below the LOD in 5 (18%) of 28 children, and EMB concentrations were below the LOD in 6 (25%) of 24 children.
Figure 1.
Scatterplot of estimated peak drug concentrations (C2h) according to weight-based dose. Data are grouped according to dosing recommendation, and regression lines and Pearson’s correlation coefficients are shown.
The adjusted effects of weight-based dosing, age, sex, and malnutrition using multivariate linear regression analysis for each drug are listed in Table 4. This analysis identified dose (P = .005) and malnutrition (P = .001) as significant risk factors for a low RIF C2h, and we found a trend for age (P = .078). For every 1 mg/kg increase in RIF dose, the C2h increased by 0.2 µg/mL (95% confidence interval [CI], 0.1–0.4 µg/mL). Malnourished children had a 2.0 µg/mL (95% CI, 0.9–3.2 µg/mL) lower RIF C2h than nonmalnourished children. The only significant risk factor identified for a low INH C2h was malnutrition (P = .001), which was associated with a 2.1 µg/mL (95% CI, 0.9–3.2 µg/mL) lower INH C2h. Weight-based dosing (P = .005) was the sole risk factor associated with a low PZA concentration; every 1 mg/kg increase in PZA dose was associated with a 0.8 µg/mL (95% CI, 0.3–1.4 µg/mL) increase in the PZA C2h. No significant risk factors were identified for a low concentration of EMB. It should be noted that weight did not confound the effects between drug dose, malnutrition, and drug exposures for any of the drugs (data not shown).
Table 4.
Adjusted Effects of Participant Factors on Estimated C2h of First-line Tuberculosis Drugs
| Drug | Predictor | Effect on C2h | 95% CI | Significance (P) |
|---|---|---|---|---|
| Rifampicin | Dose (mg/kg of body weight) | 0.21 | 0.07 to 0.35 | .005 |
| Age (years) | 0.11 | −0.01 to 0.24 | .078 | |
| Male vs female sex | 0.54 | −0.45 to 1.53 | .275 | |
| Malnourished | −2.03 | −3.20 to −0.87 | .001 | |
| Isoniazid | Dose (in mg/kg bodyweight) | 0.22 | −0.06 to 0.49 | .123 |
| Age (years) | −0.02 | −0.14 to 0.10 | .799 | |
| Male vs female sex | −0.05 | −1.00 to 0.90 | .918 | |
| Malnourished | −2.06 | −3.19 to −0.93 | .001 | |
| Pyrazinamide | Dose (mg/kg of body weight) | 0.82 | 0.28 to 1.37 | .005 |
| Age (years) | 0.75 | −0.37 to 1.87 | .178 | |
| Male vs female sex | −0.34 | −9.63 to 8.94 | .940 | |
| Malnourished | −2.70 | −13.86 to 8.46 | .621 | |
| Ethambutol | Dose (mg/kg of body weight) | 0.04 | −0.04 to 0.14 | .291 |
| Age (years) | 0.08 | −0.04–0.21 | .186 | |
| Male vs female sex | 0.42 | −0.51 to 1.36 | .355 | |
| Malnourished | −0.80 | −1.93 to 0.34 | .159 |
Abbreviations: C2h, peak drug concentration; CI, confidence interval.
Additional quality-control studies confirmed that the CPTs imparted no effect on drug quantification, as evidenced by a <10% difference in quantification between the control and experimental samples. The drug concentrations within each tablet were found to be within the expected range of concentrations stated by the manufacturers, accounting for the expectation that the tablets do not have complete solubility within 10 mL of a 1:1 methanol/water solution (see Supplementary Table 1).
DISCUSSION
In this cohort of HIV-uninfected Tanzanian children who completed the intensive phase of TB therapy, the C2hs of first-line drugs were markedly below the lower limit of the target ranges. The concentration-dependent activity of the first-line TB drugs renders the estimated C2hs an appropriate proxy for predicting an antimycobacterial effect. Drug dose was a major risk factor for low concentrations of RIF and PZA; malnutrition was also associated with low RIF and INH concentrations. Receipt of higher doses (ie, consistent with revised treatment guidelines) corresponded to significant increases in median C2hs of RIF and PZA. However, the revised dosing strategy remained insufficient for this population to achieve the target peak drug concentrations, which indicates that additional dose increases are warranted.
The estimated peak RIF and EMB concentrations among our cohort were extremely low. When we focused on the subset of children who received revised doses, RIF concentrations rose to only 2.17 µg/mL, still far below the recommended target range of 8 to 24 µg/mL. EMB concentrations rose only enough for 15% of the participants to achieve a concentration within the target range of 2 to 5 µg/mL. Reports that describe subtarget concentrations have increased despite implementation of the revised dosing strategy. Among pediatric TB studies performed in Ghana and South Africa, peak RIF and EMB concentrations were below their target ranges in the vast majority of the children (59%–100% for RIF, 52%–94% for EMB) [12–16]. Low RIF concentrations have been associated with lower doses [12, 13, 17], as seen in our cohort. Since implementation of the revised dosing recommendations, some studies are still finding that low peak RIF or EMB concentrations are more pronounced in children coinfected with HIV [12, 13]. However, this phenomenon is not limited to HIV coinfected populations. The adjusted analysis within our HIV-negative cohort suggests that sizeable increases in weight-based dosing are needed to augment RIF concentrations. In this cohort from rural Tanzania, the impact of dose augmentation on achieving target concentrations might be impeded by the burden of malnutrition. We posit that these findings might be further generalizable to children in other locations in sub-Saharan Africa and Asia, where moderate-to-severe malnourishment might be more prevalent.
Additional drivers of low RIF concentrations include drug formulation and associated bioavailability, recent examples of which can be seen in 2 pediatric studies conducted in South Africa in which similar protocols were used [15, 16]. In addition to finding that the majority of children aged <12 years and all infants aged <12 months had a RIF concentration of <8 µg/mL, RIF concentrations were found to be 45% to 76% lower among children who received 1 formulation of RIF than among those who received another formulation. These systematic differences were detected despite confirming equivalent RIF content and stability, which indicates variable bioavailability between formulations. In our investigation, the drug content of RIF within the FDC tablets was found to be appropriate. Among the first-line TB drugs, RIF is the only one that is hydrophobic and, thus, poorly soluble in water [18]. As encouraged by the national TB guidelines, the common practice in our setting for children who were unable to swallow pills reliably was to crush the tablets and dissolve them in water before administering. However, we were unable to test the hypothesis that this process might have affected RIF bioavailability in our cohort differentially. Widespread access to dispersible tablets with proven bioavailability is a critical step toward alleviating this concern.
In our cohort, only 14% of the children who achieved a target concentration of INH, which is lower than has been reported among recent studies in which the revised dosages were used [10, 12, 14, 16, 19]. It should be noted that very few participants (8%) in our study received the revised dose for INH. The routinely available FDC tablets in Tanzania contained a 2:1 ratio of RIF/INH. Tablets that support the revised WHO dosing guidelines, at a 1.5:1 ratio of RIF/INH, were introduced in the country only recently. INH monotherapy tablets were not routinely available to supplement dosing. These circumstances resulted in many children being underdosed with INH as a means of not exceeding the weight-based dosing recommendations for RIF. We caution that such programmatic constraints might be more widespread and have demonstrated that it can have a direct deleterious effect on first-line PK.
Malnutrition was a significant risk factor for low RIF and INH concentrations among children in this study; malnourished children had an approximately 2 µg/mL lower C2h than children who were not malnourished. Although the effect size was similar for both drugs, the effect on achievement of the target concentrations is relative to the therapeutic window, which was greater for RIF (8–24 µg/mL) than for INH (3–6 µg/mL). These findings may have larger implications for the success of TB treatment in this community, given the overall burden of malnutrition [20]. TB and malnutrition have long been described as coprevalent [21, 22]. Physiologic changes that occur during malnutrition can influence drug disposition directly or indirectly [23, 24]. It has been postulated that poor absorption of RIF and INH might be related to altered intestinal permeability [25], which can be more prevalent in populations burdened by environmental enteropathy [26]. Yet, studies have had variable findings between the associations with RIF or INH drug exposure and malnutrition in children. Most studies that examined these relationships have not found malnutrition to be a significant driver of low drug exposures to RIF [10, 27, 28] or INH [10, 12, 28–30], including one that examined an intermittent dosing regimen [31]. Fewer studies have found a link between malnutrition and low peaks or exposures to RIF [12, 32] and INH [32].
PZA has been described as the one of the best absorbed TB drugs, and a clear linear correlation exists between dose and peak concentrations [5, 33]. Indeed, children in our study who received a higher dose of PZA experienced a significant increase in their C2h (P = .015). However, only 67% of them achieved a C2h greater than the proposed minimum target of 20 µg/mL, and only 7% surpassed 35 µg/mL, the threshold that has been associated with improved treatment outcomes among adults [34]. Only a few pediatric studies have assessed the effect of PK on outcomes. In 2 pediatric studies that used thrice-weekly regimens among Indian children with a high proportion of HIV co-infection, Ramachandran et al [31, 35] corroborated that the PZA threshold of 35 µg/mL significantly influences treatment outcomes. These studies also found an association between peak RIF concentration and outcome [31, 32, 35].
Our study had certain limitations. Our findings might have been affected by survival bias. Eight children who met inclusion criteria for enrollment into the study died within 2 months of their TB treatment initiation. There is concern that inadequate drug exposures could have contributed to such an unfavorable outcome so early in the treatment course for these children, in which case, the true median drug concentrations might have been even lower had they been included. Our study design included a single measurement, made 2 hours after medication administration, as a means of estimating the peak concentration. Although this is the typical time of estimated peak, some reports have indicated that INH and PZA could peak earlier, especially among children coinfected with HIV [10, 12–14, 16]. However, numerous pediatric studies found that average INH concentrations of >3 µg/mL can be achieved 2 hours after a 10 mg/kg dose [19, 32, 35–37]. This study was not designed to evaluate any effects that tablet crushing might have had on medication bioavailability. Last, although the families and children received counseling regarding fasting before TB medication administration, the timing of last food consumption was not recorded, so we cannot rule out any potential confounding effects that concomitant food administration might have had on drug absorption and subsequent drug C2h values.
In summary, despite implementation of the revised WHO dosing recommendations for childhood TB, numerous children still do not attain adequate peak concentrations. The results of our work suggest that using TB drugs with proper coformulations and proven bioavailability are important steps toward maximizing drug exposure, as is improving malnutrition in children with TB. However, as newer data emerge to indicate that higher starting doses and peak concentrations might be associated with improved microbicidal activity and patient outcomes, there is an added urgency for more complete pediatric PK/PD studies to find the optimal doses and exposure targets as they relate to treatment outcomes and the potential for shortening treatment duration [31–35, 38, 39].
Supplementary Material
Notes
Acknowledgments. This important work would not be possible without the participants and their dedicated families. We thank the administration at Haydom Lutheran Hospital and Haydom Center for Global Health Research for their support. We acknowledge the valuable assistance with pharmacokinetic testing by Carlos Alemán and statistical interpretation by Elizabeth Rogawski
Financial support. This work was funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grants K23 AI097197 to T. A. T. and U01 AI 115594 to S. K. H. and E. R. H.) and the Potts Memorial Foundation.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2017. WHO/HTM/TB/201723. Geneva, Switzerland: World Health Organization, 2017:262. [Google Scholar]
- 2. World Health Organization. Roadmap for childhood tuberculosis: towards zero deaths. Geneva, Switzerland: World Health Organization, 2013 [Google Scholar]
- 3. World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children, 2nd ed Geneva, Switzerland: World Health Organization, 2014. [PubMed] [Google Scholar]
- 4. Graham SM. Treatment of paediatric TB: revised WHO guidelines. Paediatr Respir Rev 2011; 12:22–6. [DOI] [PubMed] [Google Scholar]
- 5. Alsultan AP, Charles A. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 2014; 74:839–54. [DOI] [PubMed] [Google Scholar]
- 6. Batchelor HK, Marriott JF. Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol 2015; 79:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donald PR, Maher D, Maritz JS, Qazi S. Ethambutol dosage for the treatment of children: literature review and recommendations. Int J Tuberc Lung Dis 2006; 10:1318–30. [PubMed] [Google Scholar]
- 8. Abdel-Rahman SM, Kearns GL. Pharmacokinetic analyses of fixed-dose drug combinations for pediatric tuberculosis Available at: http://www.who.int/selection_medicines/committees/expert/17/application/TB_Children.pdf?ua=1. Accessed 23 October 2018.
- 9. World Health Organization. Rapid advice: treatment of tuberculosis in children. Geneva, Switzerland: World Health Organization, 2010:19. [PubMed] [Google Scholar]
- 10. Thee S, Seddon JA, Donald PR, et al. . Pharmacokinetics of isoniazid, rifampin, and pyrazinamide in children younger than two years of age with tuberculosis: evidence for implementation of revised World Health Organization recommendations. Antimicrob Agents Chemother 2011; 55:5560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Tuberculosis and Leprosy Programme. National Guidelines for the Management of Tuberculosis in Children, 1st ed Dar es Salaam, Tanzania: United Republic of Tanzania Ministry of Health and Social Welfare, 2012. [Google Scholar]
- 12. Kwara A, Enimil A, Gillani FS, et al. . Pharmacokinetics of first-line antituberculosis drugs using WHO revised dosage in children with tuberculosis with and without HIV coinfection. J Pediatric Infect Dis Soc 2016; 5:356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Antwi S, Yang H, Enimil A, et al. . Pharmacokinetics of the first-line antituberculosis drugs in Ghanaian children with tuberculosis with or without HIV coinfection. Antimicrob Agents Chemother 2017; 61:e01701-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hiruy H, Rogers Z, Mbowane C, et al. . Subtherapeutic concentrations of first-line anti-TB drugs in South African children treated according to current guidelines: the PHATISA study. J Antimicrob Chemother 2015; 70:1115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McIlleron H, Hundt H, Smythe W, et al. . Bioavailability of two licensed paediatric rifampicin suspensions: implications for quality control programmes. Int J Tuberc Lung Dis 2016; 20:915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bekker A, Schaaf HS, Draper HR, et al. . Pharmacokinetics of rifampin, isoniazid, pyrazinamide, and ethambutol in infants dosed according to revised WHO-recommended treatment guidelines. Antimicrob Agents Chemother 2016; 60:2171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verhagen LM, Warris A, van Soolingen D, et al. . Human immunodeficiency virus and tuberculosis coinfection in children: challenges in diagnosis and treatment. Pediatr Infect Dis J 2010; 29:e63–70. [DOI] [PubMed] [Google Scholar]
- 18. Panchagnula R, Agrawal S. Biopharmaceutic and pharmacokinetic aspects of variable bioavailability of rifampicin. Int J Pharm 2004; 271:1–4. [DOI] [PubMed] [Google Scholar]
- 19. Yang H, Enimil A, Gillani FS, et al. . Evaluation of the adequacy of the 2010 Revised World Health Organization recommended dosages of the first-line antituberculosis drugs for children: adequacy of revised dosages of TB drugs for children. Pediatr Infect Dis J 2018; 37:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mduma ER, Gratz J, Patil C, et al. . The etiology, risk factors, and interactions of enteric infections and malnutrition and the consequences for child health and development study (MAL-ED): description of the Tanzanian site. Clin Infect Dis 2014; 59:S325–30. [DOI] [PubMed] [Google Scholar]
- 21. Jaganath D, Mupere E. Childhood tuberculosis and malnutrition. J Infect Dis 2012; 206:1809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar R, Singh J, Joshi K, et al. . Co-morbidities in hospitalized children with severe acute malnutrition. Indian Pediatr 2014; 51:125–7. [DOI] [PubMed] [Google Scholar]
- 23. Oshikoya KA, Senbanjo IO. Pathophysiological changes that affect drug disposition in protein-energy malnourished children. Nutr Metab (Lond) 2009; 6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramachandran G, Kumar AK, Swaminathan S. Pharmacokinetics of anti-tuberculosis drugs in children. Indian J Pediatr 2011; 78:435–42. [DOI] [PubMed] [Google Scholar]
- 25. Pinheiro VG, Ramos LM, Monteiro HS, et al. . Intestinal permeability and malabsorption of rifampin and isoniazid in active pulmonary tuberculosis. Braz J Infect Dis 2006; 10:374–9. [DOI] [PubMed] [Google Scholar]
- 26. Owino V, Ahmed T, Freemark M, et al. . Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics 2016; 138:pii:e20160641. [DOI] [PubMed] [Google Scholar]
- 27. Schaaf HS, Willemse M, Cilliers K, et al. . Rifampin pharmacokinetics in children, with and without human immunodeficiency virus infection, hospitalized for the management of severe forms of tuberculosis. BMC Med 2009; 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mukherjee A, Velpandian T, Singla M, et al. . Pharmacokinetics of isoniazid, rifampicin, pyrazinamide and ethambutol in Indian children. BMC Infect Dis 2015; 15:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roy V, Gupta D, Gupta P, et al. . Pharmacokinetics of isoniazid in moderately malnourished children with tuberculosis. Int J Tuberc Lung Dis 2010; 14:374–6. [PubMed] [Google Scholar]
- 30. McIlleron H, Willemse M, Werely CJ, et al. . Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis 2009; 48:1547–53. [DOI] [PubMed] [Google Scholar]
- 31. Ramachandran G, Kumar AK, Bhavani PK, et al. . Pharmacokinetics of first-line antituberculosis drugs in HIV-infected children with tuberculosis treated with intermittent regimens in India. Antimicrob Agents Chemother 2015; 59:1162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramachandran G, Hemanth Kumar AK, Bhavani PK, et al. . Age, nutritional status and INH acetylator status affect pharmacokinetics of anti-tuberculosis drugs in children. Int J Tuberc Lung Dis 2013; 17:800–6. [DOI] [PubMed] [Google Scholar]
- 33. McIlleron H, Willemse M, Schaaf HS, et al. . Pyrazinamide plasma concentrations in young children with tuberculosis. Pediatr Infect Dis J 2011; 30:262–5. [DOI] [PubMed] [Google Scholar]
- 34. Chideya S, Winston CA, Peloquin CA, et al. . Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis 2009; 48:1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramachandran G, Kumar AK, Kannan T, et al. . Low serum concentrations of rifampicin and pyrazinamide associated with poor treatment outcomes in children with tuberculosis related to HIV status. Pediatr Infect Dis J 2016; 35:530–4. [DOI] [PubMed] [Google Scholar]
- 36. Schaaf HS, Parkin DP, Seifart HI, et al. . Isoniazid pharmacokinetics in children treated for respiratory tuberculosis. Arch Dis Child 2005; 90:614–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dayal R, Singh Y, Agarwal D, et al. . Pharmacokinetic study of isoniazid and pyrazinamide in children: impact of age and nutritional status. Arch Dis Child 2018. doi: 10.1136/archdischild-2017-313910 [DOI] [PubMed] [Google Scholar]
- 38. Boeree MJ, Diacon AH, Dawson R, et al. ; PanACEA Consortium A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med 2015; 191:1058–65. [DOI] [PubMed] [Google Scholar]
- 39. Boeree MJ, Heinrich N, Aarnoutse R, et al. ; PanACEA Consortium High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017; 17:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.