Abstract
In the EMPA‐REG OUTCOME trial, we explored the association between pre‐randomization uric acid level tertile (<309.30 μmol/L; 309.30 to <387.21 μmol/L; ≥387.21 μmol/L) and cardiovascular (CV) death, hospitalization for heart failure (HHF), HHF or CV death, all‐cause mortality, three‐point major adverse CV events (MACE), and incident or worsening nephropathy. Patients with type 2 diabetes and CV disease received empagliflozin or placebo. The median baseline plasma uric acid level was 344.98 μmol/L, and patients’ baseline characteristics were mainly balanced across tertiles. Baseline uric acid levels were associated with cardio‐renal outcomes: in the placebo group, for the highest versus lowest tertile, the multivariable hazard ratios for three‐point MACE, HHF or CV death, and incident or worsening nephropathy were 1.22 (95% confidence interval [CI] 0.89–1.67; P = 0.2088), 1.51 (95% CI 1.02–2.23; P = 0.0396) and 1.77 (95% CI 1.33–2.34; P < 0.0001), respectively. When tested as a continuous variable, baseline uric acid was associated with all outcomes in the placebo group. Empagliflozin improved all cardio‐renal outcomes across tertiles, with all interaction P values >0.05. Further investigation of these relationships is required.
Keywords: cardiovascular disease, clinical trial, empagliflozin, SGLT2 inhibitor, type 2 diabetes
1. INTRODUCTION
Uric acid acts as an antioxidant, particularly in the extracellular environment.1 It has been estimated that uric acid comprises approximately half of the total antioxidant capacity of biological fluids in humans. However, in cytoplasm or the acidic/hydrophobic milieu of atherosclerotic plaques, uric acid is converted to a pro‐oxidant agent that promotes oxidative stress, which may accelerate cardiovascular (CV) disease.2 Several epidemiological studies have suggested a positive relationship between elevated serum uric acid levels and risk of CV events (including coronary heart disease [CHD], stroke, and heart failure [HF]), metabolic syndrome, diabetes, and chronic kidney disease.2, 3 Yet, it remains unclear as to whether uric acid offers prognostic information in patients with established CV disease. Finally, to date, no study has evaluated whether baseline levels of uric acid modulate the efficacy of anti‐hyperglycaemic therapy. As treatment with sodium‐glucose co‐transporter‐2 inhibitors has been shown to lower uric acid levels, clinical trials evaluating this class of glucose‐lowering agents may be of particular interest to explore the interplay between serum uric acid levels and clinical outcomes.4 In an effort to address these questions, we evaluated the independent relationship between uric acid and CV outcomes in the EMPA‐REG OUTCOME trial.
2. METHODS
EMPA‐REG OUTCOME (NCT01131676, ClinicalTrials.gov) enrolled patients with type 2 diabetes, established CV disease, and an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2 at baseline. A total of 7020 patients were treated with empagliflozin 10 mg/d, empagliflozin 25 mg/d or placebo, in addition to standard of care.5 Background glucose‐lowering therapy was unchanged for the first 12 weeks and could then be adjusted to achieve glycaemic control. Throughout the study, investigators were encouraged to treat CV risk factors to achieve optimal standard of care. Post hoc, we categorized pre‐randomization baseline uric acid levels into tertiles (<309.30 μmol/L; 309.30 to <387.21 μmol/L; ≥387.21 μmol/L), and assessed differences in outcomes between tertiles. We first analysed the relationship with baseline uric acid as a continuous (vs. categorical) variable in a multivariable Cox regression model. We then explored the associations between uric acid and outcomes in the placebo and empagliflozin groups separately, using an extended multivariable Cox regression model that included the primary model adjustment of age, sex, baseline body mass index (BMI; categorical), baseline glycated haemoglobin (HbA1c) level (categorical), baseline eGFR (categorical) and region, as well as baseline use of diuretics, baseline anti‐gout medication, baseline HF, and tertile of baseline uric acid. Outcomes assessed were CV death, hospitalization for heart failure (HHF), the composite of HHF/CV death, all‐cause mortality (ACM), three‐point major adverse CV events (MACE), and incident or worsening nephropathy. We then explored the treatment effects of empagliflozin versus placebo by tertile of baseline uric acid, using Cox regression analysis for time to first event in patients treated with ≥1 dose of study drug; this model included the variables age, sex, baseline BMI (categorical), baseline HbA1c (categorical), baseline eGFR (categorical), region, treatment, tertile of baseline uric acid and interaction of treatment*tertile of baseline uric acid. The occurrence of gout (as defined by the preferred terms “gout”, “gouty arthritis”, “gouty tophus”) during treatment or within 7 days after the last dose of study drug was reported descriptively. Analyses were conducted using SAS® Version 9.4.
3. RESULTS
The median (interquartile range) baseline plasma uric acid level was 344.98 μmol/L (286.10–409.82) μmol/L (n = 7017). Empagliflozin reduced plasma uric acid levels compared to placebo during follow‐up in the overall study population, as shown previously.5 Baseline characteristics were generally balanced across tertiles, with a few exceptions: compared with the lowest tertile of uric acid, the highest tertile of uric acid contained more men (77.8% vs. 63.5%), had a lower mean eGFR (64.6 ± 19.3 vs. 83.1 ± 21.0 mL/min/1.73 m2), more prevalent history of HF (14.4% vs. 8.1%), and greater use of diuretics (58.9% vs. 30.6%) and anti‐gout medication (8.0% vs. 4.2%; Table S1). However, there was no difference in anti‐gout medication use between the empagliflozin and placebo groups at baseline. Moreover, the number of patients in the trial with gout was low, with no differences reported in the placebo versus empagliflozin treatment groups (1.5% vs. 1.1% in patients without known gout at baseline, and 12.4% vs 13.9% in those with known gout at baseline, in the placebo vs. empagliflozin groups, respectively).
Analysis of the relationship with baseline uric acid as a continuous (vs. categorical) variable in a multivariable Cox regression model confirmed that higher baseline uric acid was associated with higher risk for all outcomes in the placebo and empagliflozin groups (Table 1).
Table 1.
Multivariable Cox regression of baseline uric acid as a continuous variable to outcome in the placebo and empagliflozin groups
| Placebo | Empagliflozin | |||||
|---|---|---|---|---|---|---|
| Patients with event, n | HR (95% CI) for outcomes by increase of 1 unit in baseline uric acid (mg/dL = 59.48 μmol/L) | P | Patients with event, n | HR (95% CI) for outcomes by increase of 1 unit in baseline uric acid (mg/dL = 59.48 μmol/L) | P | |
| Three‐point MACE | 282 | 1.06 (0.98–1.15) | 0.1406 | 490 | 1.15 (1.09–1.22) | <0.0001 |
| CV death | 137 | 1.09 (0.98–1.22) | 0.1039 | 172 | 1.18 (1.08–1.30) | 0.0005 |
| HHF | 94 | 1.25 (1.10–1.41) | 0.0006 | 126 | 1.21 (1.10–1.34) | 0.0002 |
| HHF or CV death | 197 | 1.14 (1.04–1.24) | 0.0053 | 265 | 1.19 (1.11–1.28) | <0.0001 |
| ACM | 194 | 1.07 (0.98–1.17) | 0.1528 | 269 | 1.18 (1.09–1.27) | <0.0001 |
| Incident or worsening nephropathy | 387 | 1.17 (1.09–1.25) | <0.0001 | 525 | 1.14 (1.08–1.21) | <0.0001 |
Abbreviations: ACM, all‐cause mortality; BMI, body mass index; CV, cardiovascular; HF, heart failure; HHF, hospitalization for heart failure; MACE, major adverse cardiovascular events.
Cox model included age, sex, baseline BMI (categorical), baseline HbA1c (categorical), baseline eGFR (categorical), region, baseline uric acid (continuous), use of baseline diuretics, baseline anti‐gout medication and baseline HF. The composite endpoint of HHF or CV death excludes fatal stroke.
Overall, in both treatment groups, the risk of adverse outcomes was increased within the highest tertile of uric acid at baseline versus the lowest tertile, although with hazard ratios (HRs) all >1, for three‐point MACE and ACM, the relationships were less clear: in the placebo group the estimated HRs of the third versus first tertile were greater than, but close to, 1 (Figure 1).
Figure 1.
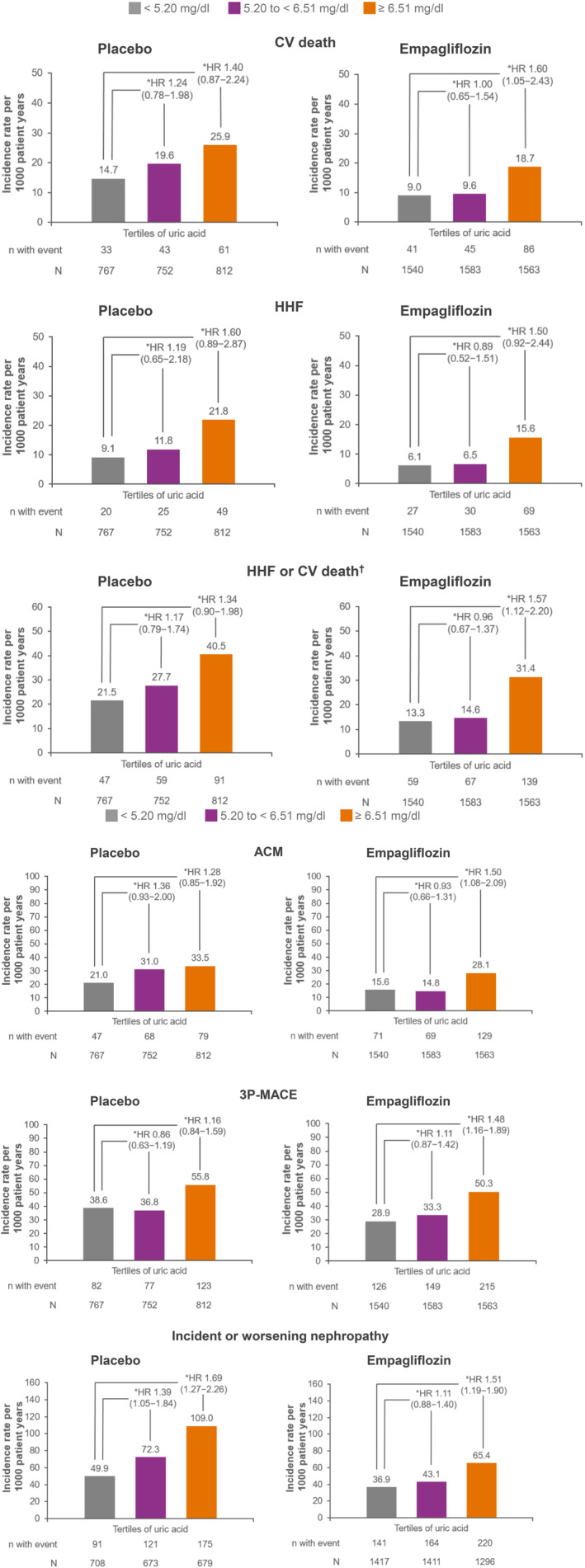
Multivariable Cox regression between tertiles of uric acid at baseline (<309.30 μmol/L, 309.30 to <387.21 μmol/L, and ≥387.21 μmol/L) for cardiovascular (CV) and kidney outcomes in the placebo and empagliflozin treatment groups. *Cox regression analysis was used to derive the hazard ratio (HR) and 95% confidence interval (CI). Cox regression model included age, sex, baseline body mass index (BMI; categorical), baseline glycated haemoglobin (HbA1c; categorical), baseline estimated glomerular filtration rate (eGFR; categorical), region, baseline use of diuretics, baseline anti‐gout medication, baseline heart failure (HF), and tertiles of baseline uric acid. †Excludes fatal stroke. 3P‐MACE, three‐point major adverse CV events; ACM, all‐cause mortality; HHF, hospitalization for heart failure
Empagliflozin improved all cardio‐renal outcomes across tertiles of baseline uric acid (Figure 2); the P values for trend for tertile of uric acid, for both placebo and empagliflozin, are shown in Table S2.
Figure 2.
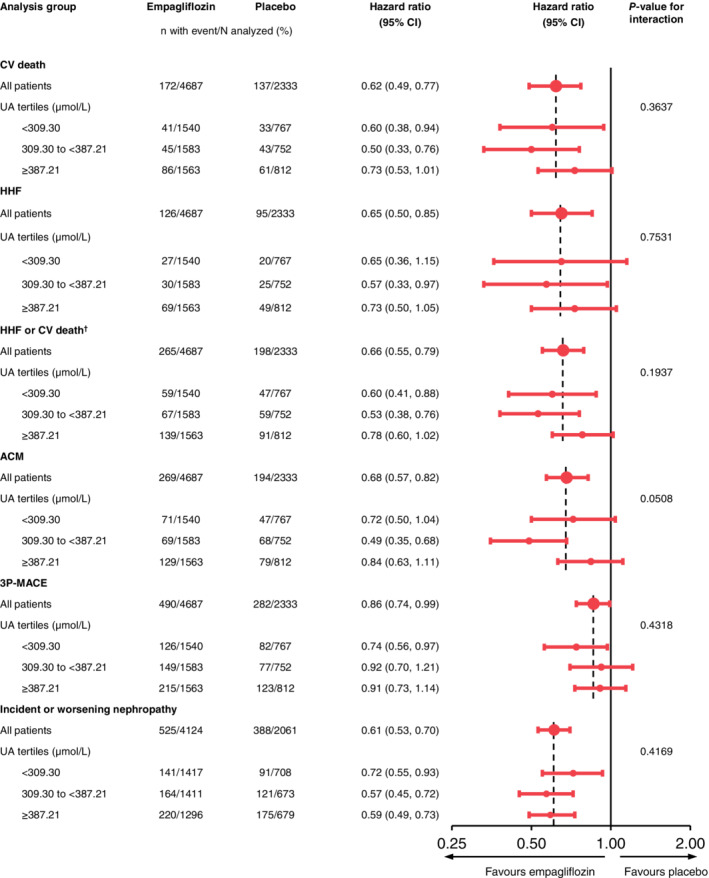
Effect of empagliflozin versus placebo on major outcomes by tertile of baseline uric acid. Cox regression analysis for time to first event in patients treated with ≥1 dose of study drug. Cox regression model included age, sex, baseline body mass index (BMI; categorical), baseline glycated haemoglobin (HbA1c; categorical), baseline estimated glomerular filtration rate (eGFR; categorical), region, treatment, tertile of baseline uric acid (UA) and interaction of treatment*tertile of baseline uric acid. P values for trend for tertiles of uric acid, for both placebo and empagliflozin, are shown in Table S2. †Excludes fatal stroke. 3P‐MACE, three‐point major adverse CV events; ACM, all‐cause mortality; CI, confidence interval; CV, cardiovascular; HHF, hospitalization for heart failure
4. DISCUSSION
This post hoc analysis of EMPA‐REG OUTCOME in patients with type 2 diabetes and established CV disease demonstrates that baseline uric acid levels are independently associated with adverse cardio‐renal outcomes. Furthermore, empagliflozin consistently reduced cardio‐renal outcomes irrespective of baseline uric acid.
Data from in vitro and animal studies indicate that elevated levels of serum uric acid are associated with an increased risk of CV and kidney disease.3 These findings are supported by evidence from a number of epidemiological studies; however, differences in the methodology, coupled with the effect of changes in kidney function on serum uric acid concentrations, make it difficult to draw firm conclusions.3 This is also the case with Mendelian randomization studies and pilot clinical trials of uric acid‐lowering therapies, where results are mixed, and interpretation is often complicated by factors such as non‐homogenous populations and study design issues.3 The conclusions of a Scientific Workshop of the National Kidney Foundation in 2016 were that the role of serum uric acid in CV and kidney disease remains to be determined and requires further investigation in large‐scale trials.3
Recent meta‐analyses have continued to explore the relationship between uric acid and risk of cardio‐renal disease. Braga et al6 reported an association between hyperuricaemia and CHD incidence (risk ratio [RR] 1.206, confidence interval [CI] 1.066–1.364; P = 0.003) and CHD mortality (RR 1.209, CI 1.003–1.457; P = 0.047), with the risk of CHD events greater in women than in men. Huang et al7 showed that elevated levels of serum uric acid independently predict the risk of ACM and the combined endpoint of readmission for, or death from, acute HF. Patients with the highest serum uric acid levels had an increased risk of ACM (RR 1.43, 95% CI 1.31–1.56) and the combined endpoint (RR 1.68, 95% CI 1.33–2.13).7
A meta‐analysis of 12 randomized controlled trials, evaluating the efficacy of uric acid‐lowering therapy on progression of chronic kidney disease, demonstrated that the risk of worsening kidney function or end‐stage renal disease or death was significantly lowered in the treatment group versus the control group (relative risk 0.39, 95% CI 0.28–0.52; P < 0.01).8
Shao et al9 conducted a meta‐analysis of 12 studies in patients with type 2 diabetes to examine the effect of serum uric acid on progression of ACM (six studies), stroke (two studies) and CHD (four studies). The risk of ACM and stroke increased with each corresponding rise in serum uric acid level of 59 μmol/L (HR 1.06, 95% CI, 1.03–1.09 and HR 1.19, 95% CI, 1.08–1.31, respectively); for CHD, there was a trend towards a higher risk of CHD with a 59‐μmol/L increase in serum uric acid, although this did not reach statistical significance.9
The results of these recent meta‐analyses lend further weight to the association between serum uric acid levels and risk of CV and kidney disease, including in patients with type 2 diabetes. Furthermore, they are supportive of the findings from the present post hoc analysis of the EMPA‐REG OUTCOME trial, which also showed that uric acid levels appear to be associated with CV and kidney outcomes in patients with type 2 diabetes and CV disease. Overall, in both treatment groups the risk of outcomes was increased within the highest versus lowest tertile of baseline uric acid, with all HRs >1, although the association was less clear for three‐point MACE and ACM in the placebo group. In these comparisons, not all 95% CIs for the HRs excluded 1, which may be attributable to the low sample size and event numbers within the subgroups and treatment arms: the study was not powered to show any associations in subgroups. For this reason, we also examined uric acid as a continuous variable and again demonstrated an association of uric acid with outcomes in both treatment groups. Empagliflozin consistently reduced these outcomes across the range of baseline uric acid levels, and this observation supports the mediation analysis undertaken in EMPA‐REG OUTCOME, demonstrating that changes in uric acid mediated ~20% to 25% of the reduction in CV death and HHF or HF death seen with empagliflozin.10, 11 Cause and effect relationships, however, will need further study.
CONFLICTS OF INTEREST
S.V. is President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not‐for‐profit physician organization, holds a Tier 1 Canada Research Chair in Cardiovascular Surgery, and has received research grants and/or speaking honoraria from Boehringer Ingelheim/Eli Lilly and Company, AstraZeneca, Janssen, Merck, Novartis, Novo Nordisk, Amgen, Sanofi, Servier, Valeant, Bayer and Pfizer. Q.J. has received travel support as an advisory board member from Merck & Co., Inc for the STRATEGY study, and has attended advisory boards and been a speaker for Eli Lilly, Novo Nordisk, Merck Sharp & Dohme China, Sanofi Aventis, Huadong Pharmaceuticals Company and Medtronic, and received research grants from Novo Nordisk, Merck Sharp & Dohme China and AstraZeneca. D.L.B. discloses the following relationships: Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS‐II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co‐leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. C.D.M. has received consulting fees from Amgen, Boehringer Ingelheim, and Octapharma. M.A. has no conflicts of interest to declare. S.E.I. has received honoraria for lectures, advisory work and/or clinical trial leadership from AstraZeneca, Boehringer Ingelheim, Eisai (TIMI), Novo Nordisk, Sanofi/Lexicon, VTV Therapeutics and Zafgen, Merck, and Abbott/Alere. C.W. reports honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, MSD and Sanofi. B.Z. has received research grants awarded to his institution from Boehringer Ingelheim, AstraZeneca and Novo Nordisk, and honoraria from Janssen, Sanofi, Eli Lilly and Company, Boehringer Ingelheim, Novo Nordisk and Merck. A.P.O., I.Z. and J.T.G are employees of Boehringer Ingelheim. D.F. has received honoraria from Sanofi, Merck & Co., Amgen, AstraZeneca, Eli Lilly and Company, and Boehringer Ingelheim, and has served on the data and safety monitoring board for Novo Nordisk.
AUTHOR CONTRIBUTIONS
S.V. and A.P.O. contributed to the interpretation of data and drafted the manuscript. D.L.B., B.Z., J.T.G., Q.J., C.D.M., M.A‐O., S.E.I., C.W. and D.F. contributed to the interpretation of data and the development of the manuscript. I.Z. contributed to the analysis and interpretation of data and the development of the manuscript. I.Z. provided statistical expertise. I.Z. and S.V. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
PRIOR PRESENTATION
Parts of this study were presented in poster form at the American College of Cardiology 68th Annual Scientific Session, New Orleans, LA, USA, March 16–18, 2019.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
The authors thank Charlie Bellinger of Elevate Scientific Solutions for medical writing assistance, limited to collation of co‐author comments, supported financially by Boehringer Ingelheim. The EMPA‐REG OUTCOME trial was funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance.
Verma S, Ji Q, Bhatt DL, et al. Association between uric acid levels and cardio‐renal outcomes and death in patients with type 2 diabetes: A subanalysis of EMPA‐REG OUTCOME. Diabetes Obes Metab. 2020;22:1207–1214. 10.1111/dom.13991
Funding information The EMPA‐REG OUTCOME trial was funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance
REFERENCES
- 1. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant‐ and radical‐caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858‐6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ndrepepa G. Uric acid and cardiovascular disease. Clin Chim Acta. 2018;484:150‐163. [DOI] [PubMed] [Google Scholar]
- 3. Johnson RJ, Bakris GL, Borghi C, et al. Hyperuricemia, Acute and Chronic Kidney Disease, Hypertension, and Cardiovascular Disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. Am J Kidney Dis. 2018;71:851‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bailey CJ. Uric acid and the cardio‐renal effects of SGLT2 inhibitors. Diabetes Obes Metab. 2019;21:1291‐1298. [DOI] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 6. Braga F, Pasqualetti S, Ferraro S, Panteghini M. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: a systematic review and meta‐analysis. Clin Chem Lab Med. 2016;54:7‐15. [DOI] [PubMed] [Google Scholar]
- 7. Huang G, Qin J, Deng X, et al. Prognostic value of serum uric acid in patients with acute heart failure: A meta‐analysis. Medicine (Baltimore). 2019;98:e14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu X, Zhai T, Ma R, Luo C, Wang H, Liu L. Effects of uric acid‐lowering therapy on the progression of chronic kidney disease: a systematic review and meta‐analysis. Ren Fail. 2018;40:289‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shao Y, Shao H, Sawhney MS, Shi L. Serum uric acid as a risk factor of all‐cause mortality and cardiovascular events among type 2 diabetes population: Meta‐analysis of correlational evidence. J Diabetes Complications. 2019;33:107409. [DOI] [PubMed] [Google Scholar]
- 10. Fitchett D, Inzucchi SE, Zinman B, et al. Mediators of the Improvement in Heart Failure Outcomes With Empagliflozin in the EMPA‐REG OUTCOME Trial. Circulation. 2017;136:A15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inzucchi SE, Zinman B, Fitchett D, et al. How Does Empagliflozin Reduce Cardiovascular Mortality? Insights From a Mediation Analysis of the EMPA‐REG OUTCOME Trial. Diabetes Care. 2018;41:356‐363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


