Abstract
We report details of the first seven equine cases of confirmed West Nile neuroinvasive disease in Austria. The cases presented during summer and autumn of 2016 (n = 2), 2017 (n = 3) and 2018 (n = 2). All horses showed gait abnormalities and 6 of 7 horses exhibited fasciculations and/or tremors, and we provide video recordings of these. Three horses also showed cranial nerve involvement. Following rapid improvement, three horses were discharged. Four horses were euthanized due to the severity of clinical signs and subjected to neuropathological examination. West Nile virus (WNV) lineage 2 nucleic acid was detected in 5 of 7 horses, and WNV‐specific neutralizing antibodies in all 7 horses. In addition, serologic evidence of WNV infection was found in two out of fourteen in‐contact horses. Horses may be considered a sentinel species for human WNV infections, integrating human and veterinary medicine and thus contributing to the one health concept.
Keywords: Austria, equid, flavivirus, horse, West Nile neuroinvasive disease, West Nile virus, zoonosis
1. INTRODUCTION
Until 2004 West Nile virus (WNV) did not play a major role in human or veterinary disease in central Europe, including Austria. A survey for WNV infections in wild birds and Austrian horses provided no evidence of WNV circulation up to 2002 (Weissenböck et al., 2003). In or slightly before 2004, a lineage 2 WNV emerged for the first time in Europe in south‐eastern Hungary (Bakonyi et al., 2006). Following a few years of adaptation, this virus strain spread to neighbouring countries (Bakonyi et al., 2013; Wodak et al., 2011) and thereafter to large parts of central, southern and eastern Europe. Besides asymptomatic infections and infections with non‐specific signs, this virus strain has also caused neurologic disease in humans (Danis et al., 2011; Papa, Danis, Tsergouli, Tsioka, & Gavana, 2011), horses and several species of birds, especially birds of prey (Bakonyi et al., 2013; Wodak et al., 2011; Ziegler et al., 2013). This contradicts the previous general opinion that lineage 2 WNVs are largely non‐pathogenic or have low pathogenicity.
In the European Union (EU), WNV infection is notifiable for humans and equids (European Commission, 2007; European Commission, 2012), specifically animal health authorities must notify cases of equine encephalomyelitis through the Animal Disease Notification System (ADNS) of the European Commission (European Commission, 2012). Recently, the European Centre for Disease Prevention and Control (ECDC) included West Nile disease (WND) outbreaks in horses in their weekly updated distribution maps of human WNV infections in the EU (https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc). So far, ten EU countries reported WND in horses: Austria, Bulgaria, France, Germany, Greece, Hungary, Italy, Portugal, Slovenia and Spain (Young, Coulombier, Domanović, Zeller, & Gossner, 2019). However, detailed descriptions of WNV lineage 2 induced neuroinvasive disease in horses which include clinical signs are scarce (Bouzalas et al., 2016; Kutasi et al., 2011).
In the eastern part of Austria, WNV lineage 2 was first diagnosed in 2008 in wild birds, mainly goshawks and other birds of prey (Bakonyi et al., 2013; Wodak et al., 2011) as well as in mosquitoes (Bakonyi et al., 2013). Three autochthonous human WNV infections were diagnosed retrospectively, two occurred in 2009 and one in 2010 (Stiasny, Aberle, & Heinzl, 2013). In 2014, the Austrian Red Cross, Blood Service for Vienna, Lower Austria and Burgenland, introduced a WNV nucleic acid screening of all blood donations, which revealed one positive donation during the first screening season (Kolodziejek et al., 2015). In follow‐up entomological investigations, WNV was identified in Culex pipiens pupae and egg rafts sampled close to the human blood donor's residence, indicating local vertical transmission of WNV in mosquitoes (Kolodziejek et al., 2015). Since 2014, WNV‐positive blood donations and human clinical cases including cases of West Nile Neuroinvasive Disease (WNND) were diagnosed in the following years in the eastern part of Austria (Kolodziejek et al., 2018) with 2018 producing the highest number of cases to date (N = 21) (Aberle et al., 2018). Infections with the closely related Usutu virus (USUV) were also found in Austrian blood donors (Aberle et al., 2018; Bakonyi et al., 2017). Interestingly, despite local WNV circulation in mosquitoes since 2008, and confirmed avian and human WND cases each year, a serosurvey for flavivirus antibodies in horses in eastern Austria in 2011 did not reveal antibodies against WNV. Instead, a high seroprevalence against tick‐borne encephalitis virus (TBEV) was detected (Rushton et al., 2013). Despite high awareness, equine clinical WNND cases were not observed in Austria up to 2016, contrary to neighbouring Hungary, where equine WNND cases have been reported since 2008 (Bakonyi et al., 2013; Kutasi et al., 2011). This is the first detailed report of WNND associated with lineage 2 WNV infections in horses in Austria.
2. MATERIALS AND METHODS
Demographic, geographic, historic, clinical, virological, serological, haematological, biochemical, and when available gross pathological and pathohistological data were collected from seven horses presented to the University Equine Hospital of the University of Veterinary Medicine Vienna and diagnosed with WNND from 2016 to 2018.
Clinical and neurological examinations were performed on all horses. EDTA, serum and heparin blood samples were collected from all cases. Paired serum samples were collected from three cases. A nasal swab was taken from one case, and post‐mortem brain tissue from all four lethal cases. Cerebrospinal fluid (CSF) was collected from the lumbosacral space in all but one case, where CSF was taken directly after euthanasia at the atlanto‐occipital space (case 2). Peripheral blood mononuclear cells (PBMCs) were obtained from heparinized blood. An automated haematology analyser (Advia 2120i) generated a complete blood count (CBC) for all cases. A standard biochemistry profile, with various enzymes, total protein, albumin and serum amyloid A was available for 6 of 7 cases (Cobas c501).
The presence of WNV nucleic acid was tested by RT‐qPCR as described previously (Kolodziejek, Marinov, Kiss, Alexe, & Nowotny, 2014). IgG and IgM antibody responses to flaviviruses in serum and CSF were tested by commercial enzyme‐linked immunosorbent assays (ELISAs) (ID Screen® Competition Multi‐species and ID Screen® West Nile IgM Capture) according to the manufacturer's protocol (ID Vet). Samples that reacted or were questionable in one or both ELISAs were subjected to neutralization microtests to determine the 80% plaque reduction neutralization titre (PRNT80), as described previously (Hubálek, Kříž, & Halouzka, 2011). Serum neutralization titres (SNT) were calculated by testing 2‐fold heat‐inactivated serum dilutions against 100 tissue culture infectious doses of virus and examining Vero cell culture for cytopathic effect after 3 days.
Fifteen days after admission of case 1, serum and EDTA blood samples were collected from 14 of 16 in‐contact horses and analysed for WNV RNA and reactive antibodies. Vaccination history was documented. Participating owners completed a questionnaire comprising horse management, disease history, residence time at the premise and mosquito management. The in‐contact horses received a short clinical and neurologic examination (P.d.H.).
An entomologist (J.V.C.) examined the stable site for sources of mosquito activity, with particular attention to identifying sources of juvenile mosquitoes, gravid mosquitoes, and resting mosquitoes. Larvae and adult mosquitoes were collected in and around the stables. Morphological and molecular identification of the mosquito species as well as the results of the molecular investigations of the mosquitoes for the presence of WNV nucleic acids were published separately (Kolodziejek et al., 2018).
3. RESULTS
Two of the seven WNND cases occurred in 2016, three in 2017 and two in 2018. Four animals were geldings and three mares with an age range of 6–22 years (Table 1). Five of the horses were born in Austria and likely never left the country, and travel information was not available for case 4 or 6. None of the horses was vaccinated against WNV. Six cases were stabled in or close to Vienna in eastern Austria and one in the federal state of Styria (Figure 1). An eighth case (third case of 2018) was retrospectively diagnosed and was not included in this study.
Table 1.
Summary of the first seven equine cases of confirmed West Nile neuroinvasive disease in Austria
| Case no. | Date of admission | Breed | Age (y) | Sex | Outcome |
|---|---|---|---|---|---|
| 1 | August 2016 | Haflinger | 6 | Ma | Survived |
| 2 | October 2016 | Haflinger | 10 | Ma | Euthanized |
| 3 | August 2017 | Haflinger | 8 | Ma | Survived |
| 4 | August 2017 | Zweibrücker | 22 | F | Euthanized |
| 5 | August 2017 | Warmblood‐mix | 18 | Ma | Survived |
| 6 | August 2018 | Dutch Warmblood | 8 | F | Euthanized |
| 7 | September 2018 | Austrian Warmblood | 11 | F | Euthanized |
Geldings.
Figure 1.
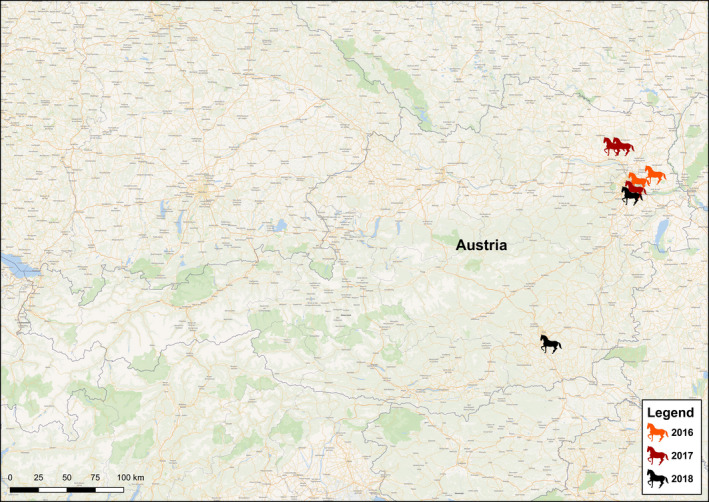
Geographic distribution of horses with West Nile neuroinvasive disease, Austria, 2016–2018
3.1. Neurologic signs associated with WNND
Gait abnormalities were the most common neurologic sign, with ataxia occurring in all cases (Table 2), four of which presented with acute ataxia. Other gait abnormalities included weakness (n = 5), sidewinding gait, bradykinesia and hind limb lameness, which was diagnosed as monoplegia (Table 2). Apart from gait abnormalities, fasciculations and tremors were present in 6 of 7 cases (Supplementary Video S1, Table 2) and manifested symmetrical and prominent in neck and triceps area, but were occasionally generalized. Increased rectal temperature was found in 4 of 7 cases. While 5 of 7 horses displayed excitation, two were lethargic during hospitalization (Table 2). Three out of seven horses showed gastrointestinal dysfunction: inappetence in cases 3, 4 and 7; colic in cases 3 and 4; and gastrointestinal impaction in cases 4 and 7. Notably, case 2 presented with persistent tachycardia and showed signs of stupor, seizure‐like activity, nystagmus and even became comatose (Table 2).
Table 2.
Summary of observed neurologic signs during the entire hospitalization period of horses diagnosed with West Nile neuroinvasive disease
| Signs | Case No. | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Ataxia | X | X | X | X | X | X | X | 7 |
| Weakness | X | X | X | X | X | 5 | ||
| Hypermetria | X | 1 | ||||||
| Bradykinesia | X | 1 | ||||||
| Femoralis paresis | X | 1 | ||||||
| Sidewinder gait | X | 1 | ||||||
| Muscle fasciculations/tremors | X | X | X | X | X | X | 6 | |
| Excitation | X | X | X | X | X | 5 | ||
| Lethargy | X | X | (X) | (X) | 4 | |||
| Hyperaesthesia | X | X | X | X | 4 | |||
| Pyrexia | X | X | X | (X) | 4 | |||
| Compulsive walking | X | X | X | 3 | ||||
| Facialis paresis | X | X | X | 3 | ||||
| Inappetence | (X) | X | (X) | 3 | ||||
| Colic | (X) | X | 2 | |||||
| Gastrointestinal impaction | X | X | 2 | |||||
| Chewing | X | X | 2 | |||||
| Nystagmus | X | X | 2 | |||||
| Recumbency | X | X | 2 | |||||
| Seizure‐like activity | X | X | 2 | |||||
| Vestibular syndrome | X | X | 2 | |||||
| Yawning | X | X | 2 | |||||
| Muscle atrophy biceps femoris | X | 1 | ||||||
Presentation of the sign for each case is indicated with an “X”, and the use of parentheses indicates the sign was anamnestic only. Signs are sorted in order of prevalence, with the first six signs (in italics) classified as gait abnormalities.
3.2. Haematological, biochemical and neuropathological examinations
Haematological and biochemical abnormalities included mild increases in creatine kinase in 5 of 6 cases, and leukocytosis or neutrophilia in three of seven cases (Tables S1–S3). Liver enzymes, glutamate dehydrogenase (GLDH) and gamma‐glutamyl transpeptidase (GGT), were above reference values in one and two out of six cases, respectively (Table S2).
Cases 2, 4, 6 and 7 were euthanized due to severe clinical signs and neuropathological examination was performed on all four horses. Pathohistology of brain tissue in all four cases showed a non‐purulent meningoencephalitis with slight to moderate lymphomonocytic perivascular cuffing and oligofocal gliosis. In three horses (cases 2, 4 and 7), the encephalitis was accentuated in the brainstem and in case 7 also in the cerebellum. However, inflammatory lesions in case 6 were mainly located in the hippocampus, but a severe unilateral haemorrhage and spongious degeneration in the spinal cord could explain the signs of severe lateralized paresis and ataxia (sidewinder). Furthermore, in case 4 moderate panmyelitis of the cervical spinal cord was detected.
West Nile virus was diagnosed by the detection of viral RNA in all but two cases (Table 3). WNV nucleic acid was detected by RT‐qPCR in the serum and PBMCs of case 1 on day one and two post‐admission as well as in brain tissues of three of the four non‐surviving horses (cases 2, 4 and 7). WNV RNA was not found in blood samples of cases 3 and 5 and in CSF of any of the seven cases (Table 3). In addition to confirming the presence of WNV nucleic acid, samples were screened against a large panel of pathogens (e.g. Equine herpes virus 1 and 4, alphaviruses, Borrelia, bacterial culture) and all gave negative results (Table S4).
Table 3.
The results of virologic and serologic testing of seven equine West Nile neuroinvasive disease cases in Austria, 2016–2018
| Case No. | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Nucleic acid test (Blood/CSF/Brain) | |||||||
| RT‐qPCR WNV | +/−/nt | −/−/+ | −/−/nt | −/−/+ | −/−/nt | nt/−/−a | nt/−/+ |
| RT‐PCR Flavivirus | −/−/nt | −/−/+ | −/−/nt | −/−/+ | −/−/nt | −/−/−a | −/−/+ |
| RT‐qPCR TBEV | −/−/nt | −/−/nt | −/−/nt | −/−/nt | −/−/nt | nt/nt/nt | nt/nt/nt |
| RT‐qPCR USUV | −/−/nt | −/−/nt | −/−/nt | −/−/nt | −/−/nt | nt/nt/nt | nt/nt/nt |
| Antibody test (Blood/CSF) | |||||||
| IgM WNVb | +/− | −/− | +/+ | +/+ | +/− | +/nt | +/nt |
| IgG WNVb | +/− | +/+ | +/+ | +/− | +/+ | +/nt | +/nt |
| PRNT80 WNVc | 1:20/nt | 1:20/− | 1:20/− | 1:20/nt | 1:40/− | nt/nt | nt/nt |
| PRNT80 TBEVc | −/nt | −/− | −/− | −/nt | −/− | nt/nt | nt/nt |
| PRNT80 USUVc | −/nt | −/− | −/− | −/nt | −/− | nt/nt | nt/nt |
| SNT WNVd | 1:20 | nt | − | 1:120 | − | 1:20 | 1:20 |
Abbreviations: +, positive; −, negative; nt, not tested; TBEV, Tick‐borne encephalitis virus; USUV, Usutu virus; WNV, West Nile virus.
Weak positive.
ID Screen® commercial ELISA kits from ID Vet.
Endpoint titres are reported for 80% plaque reduction neutralization assay (PRNT80).
Endpoint serum neutralization titres (SNT) are reported.
3.3. Virological and serological analyses
Sera from all but one of the horses (case 2) gave a positive reaction in the WNV IgM ELISA, and all seven horses were WNV IgG ELISA positive (Table 3). All horses had neutralizing antibodies against WNV, as shown by PRNT80 or SNT (Table 3). Potential cross‐reactivity caused by previous infection with TBEV or USUV (Beck et al., 2013) was excluded by the negative results from parallel testing for neutralizing antibodies against these two related flaviviruses (Table 3). A rise in neutralizing WNV antibodies was shown in all three cases for which paired serum samples were available (considering both PRNT80 and SNT, not shown). ELISA‐reactive WNV antibodies were also detected in CSF in 4 of 5 tested cases (Table 3).
Partial sequencing of the viruses derived from the equine cases 1, 2, 4, 6 and 7 reported in this paper showed lineage 2 WNV strains, closely related to each other and categorized in sublineage 2d of the Central/Southern European WNV cluster. The equine WNVs derived from cases 1 and 2 were included in a previously published (phylo)genetic analysis, in which 16 coding‐complete Austrian WNV strains from 2015 and 2016 identified in humans, horses, birds and mosquitoes were analysed and compared with published strains (Kolodziejek et al., 2018). In that study, no genetic markers were found which could be associated with disease severity. No phylogenetic analysis was performed with the 2017 and 2018 WNVs, as it was presumed that these strains were genetically similar to other European lineage 2 WNV strains (Zehender et al., 2017).
Following the definition of APHIS Veterinary Services and the World Organisation for Animal Health (OIE), all cases described in this report are confirmed equine WND cases (Table 3). All horses received anti‐inflammatory treatment with flunixin meglumine and gastric ulcer prevention with omeprazole. A complete description of treatment regimes is provided as Supplemental Material (Supplemental results). Three horses (cases 1, 3 and 5) improved and were discharged. Follow‐up telephone interviews revealed that case 1 was ridden again, but remained ataxic seven months after discharge. Cases 3 and 5 recovered fully within nine months after discharge.
3.4. Entomologic and epidemiologic investigations in the vicinity of the first equine case
Adult females of six mosquito species were trapped at the stable of the first case. A total of 116 Culex pipiens/Culex torrentium were captured, 92 of which were from a gravid trap with hay infusion (88 gravid adult females). Apart from these two ornithophilic species, adult females of the following species were collected using a BG‐Sentinel trap with CO2 or from vacuum aspiration of resting sites in stables: 87 Anopheles hyrcanus (6 from resting collections); 92 Anopheles maculipennis s.l. (89 from resting collections); and 4 Culiseta annulata (all from resting collections). Juvenile forms of these species were collected from standing water (containers and a disused 1 m2 pond) at the site, including Culex territans in addition to the aforementioned species, and all larvae identifications were confirmed by molecular barcoding, as previously described (Koloziejek et al., 2018). As the stables were 1.9 km (linear distance) from a large public park in a floodplain habitat, additional mosquito sampling was performed at the park using a CDC light trap with CO2, yielding 14 An. hyrcanus, 7 Cx. pipiens/Cx. torrentium and 4 Coquillettidia richiardii. WNV nucleic acids were detected in two pools of Cx. pipiens captured at the site, as reported previously (Kolodziejek et al., 2018).
None of the in‐contact horses of case 1 showed neurological signs apart from one with a reported sleeping disorder. Horses were residing on the premise for an average of 3.3 years (range 3 months to 5.5 years). All horses were kept outside on paddocks with shelter in small groups during summer. Ten owners used commercial insect repellents for horses, two did not use repellents and two owners did not supply information. Two owners used fly masks, but none used insect blankets on their horses. Six horses had been transported in the last 12 months, of which one horse was imported from Ireland.
Samples of serum, plasma and PBMCs of each of the 14 in‐contact horses tested negative for WNV RNA. WNV IgM ELISA was negative for all samples. Four horses tested positive in the IgG ELISA, of which two samples were confirmed positive by WNV PRNT80 (Table 4) and were also negative for neutralizing antibodies against TBEV and USUV by PRNT80. The horses with detectable WNV neutralizing antibody were housed at the premise for approximately 1.4 and 2 years, respectively. Both owners used insect repellent sprays on the horses. Both horses were diagnosed with orthopaedic problems and one had a possible neurologic problem (owner‐reported narcolepsy/sleeping disorder), as well as dermatologic and respiratory problems during the last 12 months.
Table 4.
The results of virologic and serologic testing of in‐contact horse sera housed at the same stables of the first equine WNND case in Austria in 2016
| Horse no. |
RT‐qPCR WNV |
IgMa | IgGa |
PRNT80b WNV |
PRNT80b TBEV |
PRNT80b USUV |
|---|---|---|---|---|---|---|
| 1 | − | − | − | nt | nt | nt |
| 2 | − | − | − | nt | nt | nt |
| 3 | − | − | − | nt | nt | nt |
| 4 | − | − | +/− | − | nt | nt |
| 5 | − | − | − | nt | nt | nt |
| 6 | − | − | +/− | − | nt | nt |
| 7 | − | − | +/− | − | nt | nt |
| 8 | − | − | + | − | nt | nt |
| 9 | − | − | + | 1:80 | − | − |
| 10 | − | − | − | nt | nt | nt |
| 11 | − | − | − | nt | nt | nt |
| 12 | − | − | − | nt | nt | nt |
| 13 | − | − | + | 1:160 | − | − |
| 14 | − | − | + | − | nt | nt |
+, positive; −, negative; +/−, weak positive; nt, not tested.
Commercial ELISA kits (IDVet) were used to measure the presence of IgG and IgM antibodies reactive to West Nile virus (WNV) antigen.
Endpoint titres for 80% plaque reduction neutralization assay (PRNT80) were performed for antibody‐positive samples against WNV, tick‐borne encephalitis virus (TBEV) and Usutu virus (USUV).
4. DISCUSSION
We provide a comprehensive report of the clinical presentation of equine WNND with WNV lineage 2 as the causative agent, including information on neurologic signs, blood chemistry, haematology, as well as pathological analyses when available.
Infection with WNV lineage 2 in humans leads to WNND in less than 1% of cases, compared to an 8%–10% prevalence of severe neurologic disease in horses (Bunning et al., 2002; Sejvar, 2014). Human WNND presents as a syndrome of encephalitis and meningitis, ranging in severity from behavioural changes and disorientation, to movement disorders and tremors, and may progress to coma and death in severe cases. We report similarities in clinical presentation between human and equine WNND (Table 2). Of special note were the signs of acute flaccid paralysis, muscle weakness, fever and gastrointestinal disorders (Sejvar, 2014). Of the prominent signs, gait abnormalities were the most common in our cases with ataxia in all 7 cases and muscle weakness in 5 of them. The reported neurologic abnormalities are in accordance with the reported observations of others (Kutasi et al., 2011; Ostlund et al., 2001; Porter et al., 2011). Kutasi et al. (2011) further described a noticeable high‐grade ataxia in the front limbs after an outbreak of lineage 2 WNV in Hungary. We could confirm this in 2 of our cases (1 and 4).
Although none of these signs are specific for WNND, they are likely related to the virus‐induced inflammation. Reduced gastrointestinal motility has been attributed to WNV‐induced dysfunction of the autonomic nervous system including the myenteric neurons in a hamster model (Wang, Siddharthan, Hall, & Morrey, 2011). The observed gastrointestinal signs in three of our cases and the tachycardia may thus be an inflammatory response to the presence of the virus in those tissues. This idea is supported by the finding of WNV RNA in small intestine, and inflamed heart muscle of horses with WNND by others (Bielefeldt‐Ohmann et al., 2017; Tewari, Kim, Feria, Russo, & Acland, 2004). Lesions of the autonomic nervous system may cause intestinal motility dysfunction in the horse, similar to what has been hypothesized for human cases (Bielefeldt‐Ohmann et al., 2017; Carod‐Artal, 2018; Tewari et al., 2004). As with the clinical signs, the observed haematology and biochemistry results are not specific for WNND and were used to exclude other disorders. Abnormalities such as an increased CK activity could be attributed to muscle trauma due to prolonged recumbency or falls during transport or offloading from the trailer. Increased GLDH and GGT could be the result of medication or could represent a mild WNV induced hepatitis, which has occasionally been documented in post‐mortem findings in horses and humans (Bielefeldt‐Ohmann et al., 2017; Sejvar, 2014).
Our diagnosis of WNND relied on both virological and serological evidence of recent WNV infection, as well as confirmation that WNV was present in mosquitoes within the vicinity of the index case. It is difficult to detect viraemia in naturally infected horses due to the acute, low‐level viraemia and the absence or delay of indicative clinical signs (Bielefeldt‐Ohmann et al., 2017; Bunning et al., 2002; Castillo‐Olivares et al., 2011). Nevertheless we could show WNV RNA in serum and PBMC of case 1 and in the brain tissue of 3 of 4 euthanized cases (tests were inconclusive for case 6). Sequencing revealed lineage 2 WNV in all cases where nucleic acids were detected. In addition, we could exclude other potential confounding co‐infections with flaviviruses known to be locally present (Table 3). A serum IgG response and WNV neutralizing antibodies were shown in each of the five cases in which viral nucleic acid was detected. Furthermore, specific serum neutralizing antibodies were present in all 7 cases and IgM antibodies in 6 of 7 cases. Antibodies (likely IgM) are first detected after about 1 week post‐infection (p.i.) using both PRNT and ELISA, whereas indicative clinical signs develop 3–15 days p.i. IgG antibodies in horses remain high for at least 15 months (Ostlund et al., 2001).
In the 3 cases from which no viral nucleic acid was detected, ELISA tests showed WNV antibodies in serum and CSF. Immune response was confirmed by the anamnestic reponse of neutralizing antibodies in their serum. The ELISA kits we used were not validated for antibody detection in CSF, but we could report an agreement between the presence of IgM in the blood and CSF in 3 of 5 cases. Others have reported 100% agreement between the presence of WNV‐specific IgM in serum and in CSF (Porter et al., 2004).
In addition to the clinical cases, we present an epidemiological perspective on the infection risk at the site of the index case. Previously, we demonstrated that the ornithophilic mosquito species Culex pipiens were present at the site as both juveniles and as adults, and WNV was detected in pools of these at a high rate (Aberle et al., 2018). This species is thought to be the primary vector of WNV in Europe and in North America, although the virus is often detected in other species as well (Hubálek & Halouzka, 1999). We also caught great numbers of resting mammalophilic An. maculipennis s.l., An. hyrcanus, and Cs. annulata mosquitoes. Therefore, it is likely that this site represented a focus of enzootic transmission. Two of 14 in‐contact horses had neutralizing antibodies against WNV (and not to two other endemic flaviviruses) without any previous signs of illness reported by owners. Although we evaluated owner‐reported insect management practices, the limited statistical power prevented us from drawing any inferences.
Collectivley, our efforts demonstrate that more sampling could reveal a complete picture of WNV transmission dynamics at this focus of transmission. For example, the analysis of blood samples from horse owners, riders or caretakers or other animals such as various domestic birds on the premises of the index case would have strengthened our understanding of WNV spillover. Nonetheless, our study highlights the benefit of performing follow‐up entomological and epidemiological surveys once foci of transmission are identified. Indeed, a major conclusion from our study is that horses may serve as a sufficiently sensitive indicator species (i.e. sentinel animals) for WNV activity.
5. CONCLUSION
West Nile virus is endemic in eastern Austria, and herein we show clear spillover into the equine population resulting in WNND. We describe the clinical features of WNND in horses and present various therapeutical approaches. We show evidence of seroconversion in horses without severe disease near the index case. Therefore, WNV infections in horses appear underdiagnosed, and further serological surveys are required. Because all clinical cases of equine encephalomyelitis are notifiable in Austria, WNND must be included as a differential diagnosis in equine cases of gait abnormalities, ataxia and tremors, especially in areas where WNV is endemic. Future studies may use equine cases as indicators of intense transmission activity of WNV, and employ epidemiological and entomological investigations to further understand risk factors for WNV epizootic transmission.
CONFLICT OF INTEREST
No conflicts of interest have been declared.
AUTHOR CONTRIBUTIONS
P.d.H., Z.B. and J.V.C. provided samples; N.N., R.v.d.H. and P.d.H. are responsible for the study design; P.d.H. was a treating veterinarian of the cases; R.v.d.H. and J.M.C were the supervising veterinarians of the cases; Z.B. performed histopathological analyses; J.K. and K.D. performed and interpreted the molecular and serological analyses; Z.H. carried out the PRNT; J.V.C. typed and analysed the mosquitoes; N.N., P.d.H. and J.M.C. drafted the manuscript; all authors critically revised the manuscript and approved the final version; J.V.C. improved the English.
ETHICAL APPROVAL
WNND of horses is a notifiable disease both at national (Austria) and EU level, consequently no ethical approval was required for the investigations. Regarding the entomologic and epidemiologic investigations on the home premise of the first case, the owner of the stables as well as the owners of the in‐contact horses in that stable, gave informed consent for an epidemiological investigation of the in‐contact horses as well as for an entomological investigation in and around the premises, which was approved by the Animal Ethics Committee of the University of Veterinary Medicine Vienna.
Supporting information
ACKNOWLEDGEMENTS
The authors thank the diagnostic teams of the Institute of Virology and the Institute of Pathology, both University of Veterinary Medicine Vienna. We also thank the team of the National Reference Laboratory for equine encephalomyelitis (AGES) and State Veterinarian Dr. Harald Wenzl. We thank the referring veterinarians including Mag. Isabel Hanisch, Dr. Angela Honeder and Dr. Jasmin Cermak for entrusting us their cases. We acknowledge our colleagues of the internal medicine team Sonja Berger and Hannah Junge, who were involved in treating the horses. We appreciate the help of Dominika Kapuscinska for creating the map and Dipl.‐Ing. David Meissl in compiling the clinical video. We like to express our gratitude to the stable manager of case 1 and all horse owners for their collaboration and trust.
de Heus P, Kolodziejek J, Camp JV, et al. Emergence of West Nile virus lineage 2 in Europe: Characteristics of the first seven cases of West Nile neuroinvasive disease in horses in Austria. Transbound Emerg Dis. 2020;67:1189–1197. 10.1111/tbed.13452
Advia 2120i, Siemens Healthcare GmbH, Erlangen, Germany
Cobas c501, core unit 6000, Roche Diagnostics GmbH, Vienna, Austria
REFERENCES
- Aberle, S. W. , Stiasny, K. , Aberle, J. H. , Kolodziejek, J. , Nowotny, N. , Jungbauer, C. , … Hourfar, M. K. (2018). Increase in human west nile and usutu virus infections, Austria, 2018. Eurosurveillance, 23(43), 3–8. 10.2807/1560-7917.ES.2018.23.43.1800545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakonyi, T. , Ferenczi, E. , Erdélyi, K. , Kutasi, O. , Csörgo, T. , Seidel, B. , … Nowotny, N. (2013). Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Veterinary Microbiology, 165(1–2), 61–70. 10.1016/j.vetmic.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Bakonyi, T. , Ivanics, É. , Erdélyi, K. , Ursu, K. , Ferenczi, E. , Weissenböck, H. , & Nowotny, N. (2006). Lineage 1 and 2 strains of encephalitic West Nile virus, Central Europe. Emerging Infectious Diseases, 12(4), 618–623. 10.3201/eid1204.051379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakonyi, T. , Kolodziejek, J. , Dimmel, K. , Nowotny, N. , Jungbauer, C. , Aberle, S. W. , … Allerberger, F. (2017). Usutu virus infections among blood donors, Austria, july and august 2017 – Raising awareness for diagnostic challenges. Eurosurveillance, 22(41), 1–6. 10.2807/1560-7917.ES.2017.22.41.17-00644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, C. , Jimenez‐Clavero, M. A. , Leblond, A. , Durand, B. , Nowotny, N. , Leparc‐Goffart, I. , … Lecollinet, S. (2013). Flaviviruses in Europe: Complex circulation patterns and their consequences for the diagnosis and control of west nile disease. International Journal of Environmental Research and Public Health, 10(11), 6049–6083. 10.3390/ijerph10116049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt‐Ohmann, H. , Bosco‐Lauth, A. , Hartwig, A. E. , Uddin, M. J. , Barcelon, J. , Suen, W. W. , … Bowen, R. A. (2017). Characterization of non‐lethal West Nile Virus (WNV) infection in horses: Subclinical pathology and innate immune response. Microbial Pathogenesis, 103, 71–79. 10.1016/j.micpath.2016.12.018 [DOI] [PubMed] [Google Scholar]
- Bouzalas, I. G. , Diakakis, N. , Chaintoutis, S. C. , Brellou, G. D. , Papanastassopoulou, M. , Danis, K. , Dovas, C. I. (2016). Emergence of equine West Nile virus in Central Macedonia, Greece, 2010. Transboundary and Emerging Diseases, 63(6), e219–e227. 10.1111/tbed.12334 [DOI] [PubMed] [Google Scholar]
- Bunning, M. L. , Bowen, R. A. , Bruce Cropp, C. , Sullivan, K. G. , Davis, B. S. , Komar, N. , … Mitchell, C. J. (2002). Experimental infection of horses with West Nile virus. Emerging Infectious Diseases, 8(4), 380–386. 10.3201/eid0804.010239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carod‐Artal, F. J. (2018). Infectious diseases causing autonomic dysfunction. Clinical Autonomic Research, 28(1), 67–81. 10.1007/s10286-017-0452-4 [DOI] [PubMed] [Google Scholar]
- Castillo‐Olivares, J. , Mansfield, K. L. , Phipps, L. P. , Johnson, N. , Tearle, J. , & Fooks, A. R. (2011). Antibody response in horses following experimental infection with west nile virus lineages 1 and 2. Transboundary and Emerging Diseases, 58(3), 206–212. 10.1111/j.1865-1682.2010.01197.x [DOI] [PubMed] [Google Scholar]
- Danis, K. , Papa, A. , Theocharopoulos, G. , Dougas, G. , Athanasiou, M. , Detsis, M. , … Panagiotopoulos, T. (2011). Outbreak of West Nile virus infection in Greece, 2010. Emerging Infectious Diseases, 17(10), 1868–1872. 10.3201/eid1710.110525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission (2007). 2007/782/EC: Commission Decision of 30 November 2007 approving annual and multi-annual national programmes and the financial contribution from the Community for the eradication, control and monitoring of certain animal diseases and zoonoses, presented by the Member States for 2008 and following years (notified under document number C (2007) 5776). Retrieved from: https://eur-lex.europa.eu/legal-content/EN/TXT/?qxml:id=1576843625178&uri=CELEX:32007D0782
- European Commission (2012). Commission implementing decision of 8 August 2012 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. Official Journal of the European Union. Luxembourg: Publications Office of the European Union; 27.9.2012: L 262. Retrieved from: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32012D0737 [Google Scholar]
- Hubálek, Z. , & Halouzka, J. (1999). West Nile fever–a reemerging mosquito‐borne viral disease in Europe. Emerging Infectious Diseases, 5(5), 643–650. 10.3201/eid0505.990506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubálek, Z. , Kříž, B. , & Halouzka, J. (2011). Serologic survey of humans for flavivirus West Nile in Southern Moravia (Czech Republic). Central European Journal of Public Health, 19(3), 131–133. 10.1089/vbz.2007.0283 [DOI] [PubMed] [Google Scholar]
- Kolodziejek, J. , Jungbauer, C. , Aberle, S. W. , Allerberger, F. , Bagó, Z. , Camp, J. V. , … Nowotny, N. (2018). Integrated analysis of human‐animal‐vector surveillance: West Nile virus infections in Austria, 2015–2016. Emerging Microbes and Infections, 7(1), 1–15. 10.1038/s41426-018-0021-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejek, J. , Marinov, M. , Kiss, B. J. , Alexe, V. , & Nowotny, N. (2014). The complete sequence of a West Nile virus lineage 2 Strain detected in a Hyalomma marginatum marginatum tick collected from a song Thrush (Turdus philomelos) in Eastern Romania in 2013 revealed closest genetic relationship to strain Volgograd 2007. PLoS ONE, 9(10), e109905– 10.1371/journal.pone.0109905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejek, J. , Seidel, B. , Jungbauer, C. , Dimmel, K. , Kolodziejek, M. , Rudolf, I. , … Nowotny, N. (2015). West Nile virus positive blood donation and subsequent entomological investigation, Austria, 2014. PLoS ONE, 10(5), 1–14. 10.1371/journal.pone.0126381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutasi, O. , Bakonyi, T. , Lecollinet, S. , Biksi, I. , Ferenczi, E. , Bahuon, C. , … Szenci, O. (2011). Equine encephalomyelitis outbreak caused by a genetic lineage 2 West Nile virus in Hungary. Journal of Veterinary Internal Medicine, 25(3), 586–591. 10.1111/j.1939-1676.2011.0715.x [DOI] [PubMed] [Google Scholar]
- Ostlund, E. N. , Crom, R. L. , Pedersen, D. D. , Johnson, D. J. , Williams, W. O. , & Schmitt, B. J. (2001). Equine West Nile encephalitis, United States. Emerging Infectious Diseases, 7(4), 665–669. 10.3201/eid0704.017412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa, A. , Danis, K. , Tsergouli, K. , Tsioka, K. , & Gavana, E. (2011). Development time of IgG antibodies to West Nile virus. Archives of Virology, 156(9), 1661–1663. 10.1007/s00705-011-1014-z [DOI] [PubMed] [Google Scholar]
- Porter, M. B. , Long, M. , Gosche, D. G. , Schott, H. M. , Hines, M. T. , Rossano, M. , & Sellon, D. C. (2004). Immunoglobulin M‐capture enzyme‐linked immunosorbent assay testing of cerebrospinal fluid and serum from horses exposed to West Nile virus by vaccination or natural infection. Journal of Veterinary Internal Medicine, 18(6), 866–870. [DOI] [PubMed] [Google Scholar]
- Porter, R. S. , Leblond, A. , Lecollinet, S. , Tritz, P. , Cantile, C. , Kutasi, O. , … Saegerman, C. (2011). Clinical diagnosis of west nile fever in equids by classification and regression tree (CART) analysis and comparative study of clinical appearance in three European countries. Transboundary and Emerging Diseases, 58(3), 197–205. 10.1111/j.1865-1682.2010.01196.x [DOI] [PubMed] [Google Scholar]
- Rushton, J. O. , Lecollinet, S. , Hubálek, Z. , Svobodová, P. , Lussy, H. , & Nowotny, N. (2013). Tick‐borne encephalitis virus in horses, Austria, 2011. Emerging Infectious Diseases, 19(4), 635–637. 10.3201/eid1904.121450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar, J. J. (2014). Clinical manifestations and outcomes of West Nile virus infection. Viruses, 6(2), 606–623. 10.3390/v6020606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiasny, K. , Aberle, S. W. , & Heinzl, F. X. (2013). Retrospective identification of human cases of west nile virus infection in Austria (2009 to 2010) by serological differentiation from Usutu and other flavivirus infections. Eurosurveillance, 18(43), 2061420614 10.2807/1560-7917.ES2013.18.43.20614 [DOI] [PubMed] [Google Scholar]
- Tewari, D. , Kim, H. , Feria, W. , Russo, B. , & Acland, H. (2004). Detection of West Nile virus using formalin fixed paraffin embedded tissues in crows and horses: Quantification of viral transcripts by real‐time RT‐PCR. Journal of Clinical Virology, 30(4), 320–325. 10.1016/j.jcv.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Wang, H. , Siddharthan, V. , Hall, J. O. , & Morrey, J. D. (2011). Autonomic nervous dysfunction in hamsters infected with west nile virus. PLoS ONE, 6(5), e19575 10.1371/journal.pone.0019575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenböck, H. , Hubálek, Z. , Halouzka, J. , Pichlmair, A. , Maderner, A. , Fragner, K. , … Nowotny, N. (2003). Screening for West Nile virus infections of susceptible animal species in Austria. Epidemiology and Infection, 131, 1023–1027. 10.1017/S0950268803001031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodak, E. , Richter, S. , Bagó, Z. , Revilla‐Fernández, S. , Weissenböck, H. , Nowotny, N. , & Winter, P. (2011). Detection and molecular analysis of West Nile virus infections in birds of prey in the eastern part of Austria in 2008 and 2009. Veterinary Microbiology, 149(3–4), 358–366. 10.1016/j.vetmic.2010.12.012 [DOI] [PubMed] [Google Scholar]
- Young, J. J. , Coulombier, D. , & Domanović, D. , European Union West Nile Fever Working Group , Zeller, H. , & Gossner, C. M. (2019). One Health approach for West Nile virus surveillance in the European Union: Relevance of equine data for blood safety. Eurosurveillance, 24(16). pii:1800349 10.2807/1560-7917.ES.2019.24.16.1800349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehender, G. , Veo, C. , Ebranati, E. , Carta, V. , Rovida, F. , Percivalle, E. , … Galli, M. (2017). Reconstructing the recent West Nile virus lineage 2 epidemic in Europe and Italy using discrete and continuous phylogeography. PLoS ONE, 12(7), 1–16. 10.1371/journal.pone.0179679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler, U. , Angenvoort, J. , Fischer, D. , Fast, C. , & Eiden, M. , Rodriguez, A. V. , Groschup, M. H. (2013). Pathogenesis of West Nile virus lineage 1 and 2 in experimentally infected large falcons. Veterinary Microbiology, 161(3‐4), 263–273. 10.1016/j.vetmic.2012.07.041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


