Abstract
Extracellular capsule polysaccharides increase the cellular fitness under abiotic stresses and during competition with other bacteria. They are best‐known for their role in virulence, particularly in human hosts. Specifically, capsules facilitate tissue invasion by enhancing bacterial evasion from phagocytosis and protect cells from biocidal molecules. Klebsiella pneumoniae is a worrisome nosocomial pathogen with few known virulence factors, but the most important one is its capsule. In this issue, Tan et al. assess the fitness advantage of the capsule by competing a wild‐type strain against four different mutants where capsule production is interrupted at different stages of the biosynthetic pathway. Strikingly, not all mutants provide a fitness advantage. They suggest that some mutants have secondary defects altering virulence‐associated phenotypes and blurring the role of the capsule in pathogenesis. This study indicates that the K1 capsule in K. pneumoniae is not required for gut colonization but that it is critical for bloodstream dissemination to other organs. These results contribute to clarify the contradictory literature on the role of the Klebsiella capsule during infection. Finally, the varying fitness effects of different capsule mutations observed for K. pneumoniae K1 might apply also to other capsulated diderm bacteria that are facultative or emerging pathogens.
Keywords: capsule, mutants, virulence
Diagram of the capsule biosynthesis pathway in K. pneumoniae. Capsule production is initiated by WcaJ (1) (in the K1 serotypes), or by WbaP, linking the first moiety of glucose or galactose, respectively, to an undecaprenyl phosphate (Und‐P). Other oligosaccharides are added by other glycosyltrasferases (GT) (2,3). These residues are then flipped across the inner membrane by Wzx (4) and polymerized by Wzy to other trisaccharide units (5). Capsule length is regulated by Wzc (6) and exported from the periplasm to the extracellular space via the secretin Wza (7).
1. COMMENTARY
1.1. Importance of Klebsiella pneumoniae and the role of the capsule
Klebsiella pneumoniae first described in the 1880s, when it was named the Friedlander's bacillus (Brenner, Krieg, & JT, 2005), belongs to the family Enterobacteriaceae. K. pneumoniae is ubiquitous and has been isolated from a wide range of environments including the soil, sewage and water. It is a commensal of many different hosts, from mammals to plants. In humans, it is considered an opportunistic pathogen (Struve & Krogfelt, 2004), being responsible for pneumonia, recurrent urinary infections and acute liver abscesses. In addition, K. pneumoniae is one of the most worrisome pathogens associated with high rates of acquisition of multidrug resistance determinants (MDR) (Dunn, Connor, & McNally, 2019; Wyres & Holt, 2018), compromising its treatment. The WHO defined the six most significant MDR bacteria commonly acquired in hospitals by the acronym ESKAPE, in which Klebsiella is represented by the ‘K’ (Rice, 2008). These are also recognized by the CDC as a real threat for public health (Pendleton, Gorman, & Gilmore, 2013).
Unlike other facultative pathogens, most K. pneumoniae do not have a large array of virulence factors. Its known virulence arsenal is limited to several types of fimbriae, O‐antigen, a few siderophores (aerobactin, yersiniabactin), the genotoxin colibactin and the extracellular capsule (Paczosa & Mecsas, 2016). The capsule has received a lot of attention because it increases the tolerance of bacteria to drug therapy and also masks surface antigens (Schembri, Blom, Krogfelt, & Klemm, 2005), reducing the response of the immune system to infection. At least one capsule operon was found in most – if not all – genomic sequences of K. pneumoniae available to date. Capsule production in K. pneumoniae is dependent on the Group I or Wzx/Wzy pathway (Figure 1). The production is initiated by a glycosyltransferase (GT), WcaJ or WbaP, which links the first moiety of glucose (WcaJ) or galactose (WbaP) to a lipid carrier, undecaprenyl phosphate (Und‐P). Other oligosaccharides, such as fucose and uronic acid are added at the nonreducing end of the glycosyl‐Und‐P. These lipid‐linked residues are flipped by Wzx across the inner membrane, from the cytoplasm to the periplasmic space and polymerized by virtue of the Wzy polymerase. After the length regulation of the oligosaccharidic chain by Wzc, the capsule is transferred to the outside of the cell by Wza, an outer membrane exporter. Finally, the capsule is anchored to the cell surface, most likely by Wzi (Figure 1) (Whitfield, 2006).
Figure 1.
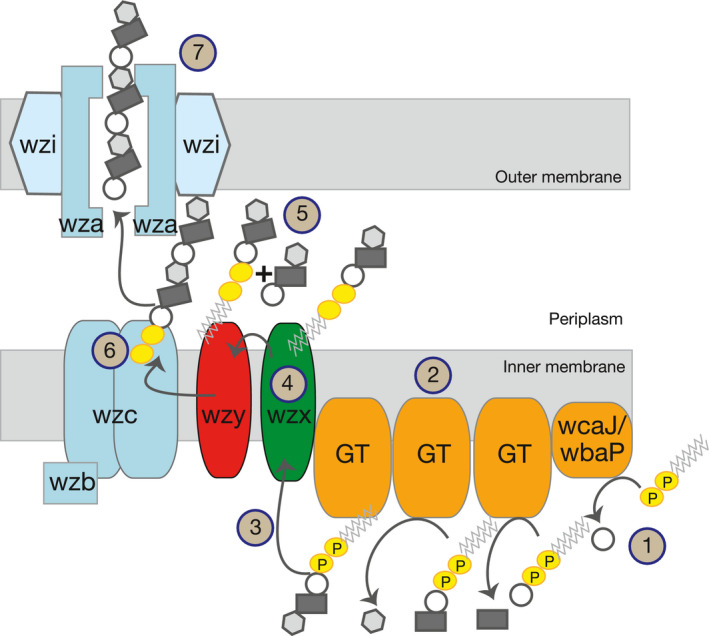
Diagram of the capsule biosynthesis pathway in K. pneumoniae. Capsule production is initiated by WcaJ (1) (in the K1 serotypes), or by WbaP, linking the first moiety of glucose or galactose, respectively, to an undecaprenyl phosphate (Und‐P). Other oligosaccharides are added by other glycosyltrasferases (GT) (2,3). These residues are then flipped across the inner membrane by Wzx (4) and polymerized by Wzy to other trisaccharide units (5). Capsule length is regulated by Wzc (6) and exported from the periplasm to the extracellular space via the secretin Wza (7)
The diversity of GT, (Figure 1), that vary between strains, results in different oligosaccharide combinations and residue modifications. These different combinations of residues result in the classification of capsules into different serotypes. At least 77 capsule serotypes are serologically defined (Mori et al., 1989; Orskov, 1955; Podschun & Ullmann, 1998), and more than 130 serotypes – including the aforementioned 77 – are identified through comparative genomics of K. pneumoniae. In this issue, Tan et al. (2020), focus on the highly prevalent K1 serotype (Figure 2). Synthesis of K1 capsule depends on WcaJ as the initial GT and the repeating unit is a trisaccharide of glucose, fucose and glucuronic acid. This serotype is associated with hypervirulence, more particularly with liver abscesses, and is prevalent in Taiwan, South Korea, Hong Kong and Southeast Asia. Strains of the K1 serotype are phylogenetically associated with the clonal‐group CG23, which is known to cause bacteremia, endophthalmitis (eye inflammation) and meningitis (Fung et al., 2002; Siu, Yeh, Lin, Fung, & Chang, 2012). A particular strain is K. pneumoniae SGH10, capsule serotype K1. It has been isolated from a patient with liver abscess induced by K. pneumoniae and possesses a conjugative element, ICEKp10 that encodes for yersiniabactin, a potent siderophore, and colibactin, a genotoxin (Lam et al., 2018). ICEKp10 is more prevalent across CG23 strains than ICEKp1, present in strain NTUH K2044, which is the one mainly used for experimental work in many laboratories. Further, NTUH K2044 does not code for a colibactin. Given its genotype and phenotype, the SGH10 strain has been proposed a novel reference for the K1 capsule serotype strains and the clonal‐group CG23 (Lam et al., 2018).
Figure 2.
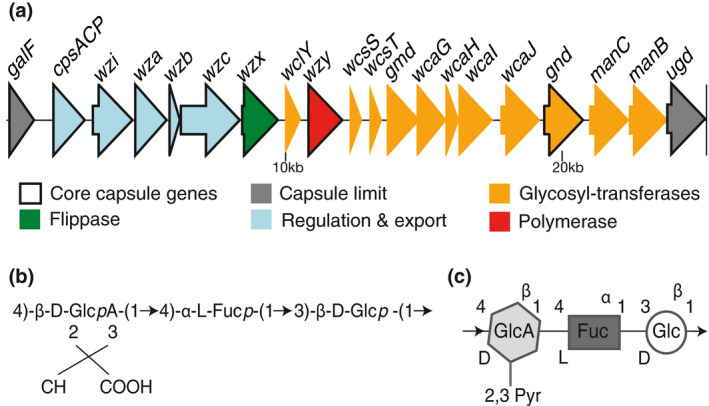
Genomic and chemical scheme of K. pneumoniae serotype K1. A. Genomic representation of the architecture of K1 serotype capsule locus. B. Schematic representation of the K1 trisaccharide repeat unit, composed of a D‐glucuronic acid, L‐fucose and a D‐glucose residue. C. Chemical representation of the trisaccharide repeat unit, adapted from iith.ac.in/K‐PAM
1.2. Not all capsule knock downs are equal
Although gut colonization of K. pneumoniae has been extensively studied, the use of different strains, capsule mutants and environments across studies has resulted in disparate conclusions regarding the role of the capsule in virulence. To clarify the fitness effect of the capsule in the gut and in bloodstream dissemination, Tan et al. used strain SGH10 and several deletion mutants that interrupt capsule production at different steps of the biosynthetic pathway (step 1, 5 and 7 in Figure 1) (Tan et al., 2020). Specifically, they constructed mutants with deletions in the wcaJ initial GT, which stops capsule production at the first possible step; the wzy gene coding for the polymerase; the secretin wza; and rmpA, a regulator that can confer hypermucoviscosity. The latter is located on the large virulence plasmid, and although its deletion does not result in capsule abolition, it significantly reduces the amount of capsule secreted.
Using the commonly studied Δwza and Δwzy capsule mutants, they show that non‐capsulated strains are less fit than the capsulated wild‐type strain in the gut. This would suggest that the capsule increases the growth rate of the strain, or most likely, it enhances cellular survival in this environment. Strikingly, the ΔwcaJ and ΔrmpA mutants show opposite phenotypes. In these mutants, the lack (or reduced amount, for ΔrmpA) of capsule is no longer detrimental, and the mutants are able to outcompete the wild type. These mutants probably have lower generation times because they are not burdened with the cost associated to the capsule production. Similarly, the authors show that Δwza and Δwzy capsule mutants, but not the ΔwcaJ mutant, have increased mortality in contact with bile salts. Such differences are not observed during exposure to human serum (Tan et al., 2020). The authors suggest that the differences observed across mutants, specifically between Δwza and Δwzy compared to ΔwcaJ, might be due to the accumulation of unpolymerized trisaccharides in the periplasmic space, in the case of Δwzy, or the polymerized capsule in the case of Δwza. This could potentially result in membrane instability affecting fitness. Scanning electron microscopy revealed that these mutants also show a different morphology: Δwza and Δwzy seem smaller and display pointed tips at the poles, whereas wild‐type cells, ΔwcaJ and ΔrmpA, appear like rods with rounded poles. This might result from membrane deformations caused by capsule accumulation, and is particularly evident in the Δwzy mutant. Overall, the authors show that the capsule is important for the dispersion of K. pneumoniae through the bloodstream to other organs, like the lung, spleen and liver. However, the use of deletion mutants with no side effects (ΔwcaJ) reveal that the capsule is not implicated in the persistence of K. pneumoniae in the gut. A similar result was reported in another study where mice were infected with spontaneous non‐capsulated mutants (Struve & Krogfelt, 2003). These results are novel and essentially different from other studies in which Δwzy mutants are used to assess the role of the capsule in pathogenesis.
Taken together, this work shows that the different non‐capsulated mutants vary in the fitness advantage they provide in one of their natural environments, the mammalian gut. These results highlight the importance of selecting with care the genes to knock out. The differential phenotypes of mutants are pernicious because they are not due to polar effects of the deletion, and are not revealed by appropriate complementation tests that restored the wild‐type phenotype. These side effects can dramatically alter some phenotypes and blur the conclusions with respect to pathogenesis. In their manuscript, Tan et al. raise awareness about the need to reevaluate and compare side‐by‐side the results reported in previous studies examining different mutants, in particular the results obtained with the traditionally used wza and wzy mutants alongside the wcaJ deletion mutants.
1.3. Capsular organization at the cellular surface
In their study, Tan and colleagues also provide novel scanning electron microscopy images of Klebsiella cells expressing the capsule (Tan et al., 2020). Capsules are thought to cover the entire surface of the cell, as typically depicted in cartoons and observed in some microscopic images (Podschun & Ullmann, 1998; Schembri et al., 2005). However, the novel images presented in the study seem to suggest that capsule production stems from discrete foci on the surface of the cell and does not diffuse around the cell surface covering it uniformly. This is further supported by the post hoc analysis of microscopic images of K. pneumoniae from previous studies (Evrard et al., 2010), and by the results from a more recent study on the production of the Escherichia coli capsule K1, which is secreted through the same biosynthesis pathway (Phanphak et al., 2019). Using super resolution microscopy and high pressuring light scattering chromatography, the latter study showed that the capsule forms rafts or discrete brushes on the surface of the cell. If confirmed, this raises a number of questions about the primary role of capsules. It is believed that the capsule protects the cell from killing by phagocytosis (Podschun, Penner, & Ullmann, 1992), prevents recognition by the human immune system by masking antigens (Schembri et al., 2005) and increases tolerance to cationic antimicrobial peptides and other bactericidal molecules (Paczosa & Mecsas, 2016). This protection could be less effective if the capsule is organized in patches or bundles on the surface instead of covering it entirely. This would support the hypothesis that capsules were not primarily selected for the fitness advantage it provides within the host but rather would be a by‐product of adaptation outside a host (Rendueles, Garcia‐Garcera, Neron, Touchon, & Rocha, 2017). Further, capsular organization in brushes would also provide an explanation to recent findings that suggested that capsules do not limit horizontal gene transfer in most bacterial species (Rendueles, Sousa, Bernheim, Touchon, & Rocha, 2018), because patches of the cell surface would still remain exposed to phages or conjugative pili.
The new finding that the capsule is distributed in discrete foci on the cell surface provides insight into one of the enigmas of capsular surface organization observed in some strains: the co‐expression of different capsules, their concurrent accommodation at the cell surface and their physical interaction in the cell envelope. Capsule multiplicity is observed in 40% of bacteria (Rendueles et al., 2017) and is very common in gut bacteria belonging to the genera Bacteroides, such as Bacteroides thetaiotamicron (Coyne & Comstock, 2008; Tzianabos et al., 1992; Xu et al., 2003). The evidence suggesting that the capsule is organized in discrete brushes leads to the hypothesis that different capsules could be secreted by different discrete foci and anchored to the surface forming alternate bundles of each capsular type thereby allowing for the coexistence of different capsular types.
Interestingly, Phanphak and colleagues also show that the capsule displays a bimodal thickness in the rafts at the cell poles, rather than the monomodal thickness that characterizes the capsule on the remaining parts of the cell envelope (Phanphak et al., 2019). This could explain the increased deformation on the poles observed in Δwza and Δwzy mutants. This deformation is also seen in Δwzx mutants (Evrard et al., 2010), and might be due to the aforementioned accumulation of capsular oligosaccharides in the periplasmic space.
1.4. Toward ‘benchmarking’ in molecular biology
In Tan et al., the authors carefully mined the literature to compile and contrast the virulence phenotypes reported according to the route of infection used and, importantly, to the capsule mutants tested (Tan et al., 2020). A major issue when studying the capsule of K. pneumoniae is the severe lack of correlation between the in vitro and in vivo results (Struve & Krogfelt, 2003). This is usually attributed to the radically different growth conditions between laboratory culture media and the in vivo environment dictacted by physiology and interactions with the host. Further, there are also striking differences in the results stemming from different studies/laboratories, which could be due to different strains used or different mutants constructed. The discovery of some of the undesirable side effects, namely the membrane instability, of the commonly used Δwza and Δwzy capsule mutants may explain some of the inconsistencies present in the literature. Indeed, one previous study agreed with the results shown by Tan et al. This study used capsule mutants that had spontaneously appeared rather than mutants engineered in the laboratory, as they have potentially no detrimental side effects due to mutant construction, to show that the capsule was not involved in gut colonization (Struve & Krogfelt, 2003). The differences across mutants might have also contributed to the difficulties in translating in vitro results to in vivo contexts.
To avoid this variability in the results across studies, application of ‘benchmarking’ techniques may be useful. Benchmarking is routinely done in business and for quality control in different fields. It has also been applied to some scientific disciplines, such as computer sciences applied to biology (Mangul et al., 2019). Yet, this is not systematic in molecular biology and gives rise to numerous problems and conflicts (Baker, 2016; Eisner, 2018; Hunter, 2017), such as the ones encountered when trying to understand the role of capsules in virulence. Using appropriate ‘benchmarking’ protocols to standardize experimental procedures would have many benefits, including the avoidance of pursuing false leads by using mutants that have not been re‐sequenced and may have off‐target mutations, or mutants that potentially have detrimental side effects dependent on the mutation that has been introduced, such as the membrane instability discussed here. Ultimately, it would contribute to the reproducibility of research and the comparability of data across laboratories.
Research in K. pneumoniae at the molecular level has been traditionally performed in only few laboratories, because K. pneumoniae was not a model organism and poses the challenges of genetically manipulating a non‐transformable bacterium (at least under laboratory conditions) with a very thick capsule. However, due to its increasing clinical importance, coupled with the development of more sophisticated methods to generate clean in‐frame deletion mutants (Dorman, Feltwell, Goulding, Parkhill, & Short, 2018; Kaniga, Delor, & Cornelis, 1991; Nyerges et al., 2016), more laboratories study K. pneumoniae leading to an exponential increase of manuscripts published about K. pneumoniae. The finding that the lack of reproducibility in Klebsiella virulence studies may be due to mutants with side effects, is thus very timely and further demands the development of ‘benchmarking’ tools to better interpret and integrate the (past and future) data and properly design future experiments.
Strong efforts are currently being deployed under the JPIAMR‐funded project (KlebNET) (https://research.pasteur.fr/fr/project/klebnet-a-one-health-network-bridging-science-and-surveillance-on-antimicrobial-resistant-klebsiella/) to bring together an international network of researchers and public health actors dedicated to reconcile knowledge and harmonize methods on K. pneumoniae genomics, antimicrobial resistance, transmission, modeling, clinical prevalence and carriage in humans, and presence in animal foods and environmental sectors. Interactions in this group presently take place most often virtually (via Google community), but will continue via the future organization of international symposia. The framework provided by such collaborative projects offers opportunities to discuss potential ‘benchmarking’ protocols for the study of complex macromolecular systems, and in this case, for the study of Klebsiella virulence and the role of its capsule.
2. CONCLUSION
The analysis of four different mutants of the capsule biosynthesis pathway has shed light on the role of the capsule in virulence of K. pneumoniae. The K1 capsule is necessary for efficient dispersal of K. pneumoniae through the bloodstream to secondary infection sites but it is not required for gut persistence (Tan et al., 2020). The side effects observed in some capsule mutants uncovered by the study of Tan et al. may not be specific to K. pneumoniae but may also be relevant for other ESKAPE microorganisms such as Acinetobacter baumannii or Enterobacter spp. These share the same biosynthetic capsule pathway, the Wzx/Wzy‐dependent pathway, but it may also hold true for pathogens that synthesize their capsule via the ABC pathway such as Neisseria meningitidis or Campylobacter jejuni. In diderms, the ABC‐dependent pathway also relies on an outer membrane exporter, commonly named KpsD that is homologous to Wza (Whitfield, 2006). Deletion of this gene may result in a similar accumulation of capsule in the periplasmic space with comparable negative effects.
Thus, the choice of the appropriate mutants to construct when studying the role of polymers, that are usually encoded in large operons, is extremely important. Ideally, the whole operon should be deleted, but this may be sometimes challenging due to its length (over 20kb for capsular operons in K. pneumoniae). Thus, the gene coding the enzyme starting the biosynthetic pathway could be the best target as it limits the risk of accumulation of secondary metabolites in the cell which may be toxic or lead to other undesired side effects. Finally, the reevaluation of results from previous studies in new genetic backgrounds, with different techniques and new mutants would greatly benefit the scientific community by expanding our understanding of the role of each gene in a synthesis pathway and, ultimately, by shedding more light into physiological diversity within a bacterial species.
ACKNOWLEDGMENTS
I thank Eduardo Rocha and Jorge Moura de Sousa for helpful comments on this MicroCommentary. This work was supported by an ANR JCJC (Agence national de recherche) grant [ANR 18 CE12 0001 01 ENCAPSULATION] and by a Laboratoire d'Excellence IBEID ‘Integrative Biology of Emerging Infectious Diseases’ grant [ANR‐10‐LABX‐62‐IBEID].
Rendueles O. Deciphering the role of the capsule of Klebsiella pneumoniae during pathogenesis: A cautionary tale. Mol Microbiol. 2020;113:883–888. 10.1111/mmi.14474
REFERENCES
- Baker, M. (2016). 1,500 scientists lift the lid on reproducibility. Nature, 533, 452–454. [DOI] [PubMed] [Google Scholar]
- Brenner, D. J. , Krieg, N. R. , & JT, S. (2005). The Proteobacteria, Bergey's manual of systematic bacteriology (Vol. XXVI, 2nd ed.). New York, NY: Springer. [Google Scholar]
- Coyne, M. J. , & Comstock, L. E. (2008). Niche‐specific features of the intestinal bacteroidales. Journal of Bacteriology, 190, 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman, M. J. , Feltwell, T. , Goulding, D. A. , Parkhill, J. , & Short, F. L. (2018). The capsule regulatory network of Klebsiella pneumoniae defined by density‐TraDISort. MBio, 9, e01863‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, S. J. , Connor, C. , & McNally, A. (2019). The evolution and transmission of multi‐drug resistant Escherichia coli and Klebsiella pneumoniae: The complexity of clones and plasmids. Current Opinion in Microbiology, 51, 51–56. [DOI] [PubMed] [Google Scholar]
- Eisner, D. A. (2018). Reproducibility of science: Fraud, impact factors and carelessness. Journal of Molecular and Cellular Cardiology, 114, 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard, B. , Balestrino, D. , Dosgilbert, A. , Bouya‐Gachancard, J. L. , Charbonnel, N. , … Tridon, A. (2010). Roles of capsule and lipopolysaccharide O antigen in interactions of human monocyte‐derived dendritic cells and Klebsiella pneumoniae . Infection and Immunity, 78, 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, C. P. , Chang, F. Y. , Lee, S. C. , Hu, B. S. , Kuo, B. I. , Liu, C. Y. , … Siu, L. K. (2002). A global emerging disease of Klebsiella pneumoniae liver abscess: Is serotype K1 an important factor for complicated endophthalmitis? Gut, 50, 420–424. 10.1136/gut.50.3.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, P. (2017). The reproducibility “crisis”: Reaction to replication crisis should not stifle innovation. EMBO Reports, 18, 1493–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniga, K. , Delor, I. , & Cornelis, G. R. (1991). A wide‐host‐range suicide vector for improving reverse genetics in gram‐negative bacteria: Inactivation of the blaA gene of Yersinia enterocolitica . Gene, 109, 137–141. [DOI] [PubMed] [Google Scholar]
- KlebNET . Retrieved from https://research.pasteur.fr/fr/project/klebnet-a-one-health-network-bridging-science-and-surveillance-on-antimicrobial-resistant-klebsiella/
- Lam, M. M. C. , Wyres, K. L. , Duchene, S. , Wick, R. R. , Judd, L. M. , Gan, Y. H. , … Koh, T. H. (2018). Population genomics of hypervirulent Klebsiella pneumoniae clonal‐group 23 reveals early emergence and rapid global dissemination. Nature Communications, 9, 2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangul, S. , Martin, L. S. , Hill, B. L. , Lam, A. K. , Distler, M. G. , Zelikovsky, A. , … Flint, J. (2019). Systematic benchmarking of omics computational tools. Nature Communications, 10, 1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, M. , Ohta, M. , Agata, N. , Kido, N. , Arakawa, Y. , Ito, H. , … Kato, N. (1989). Identification of species and capsular types of Klebsiella clinical isolates, with special reference to Klebsiella planticola . Microbiology and Immunology, 33, 887–895. [DOI] [PubMed] [Google Scholar]
- Nyerges, A. , Csorgo, B. , Nagy, I. , Balint, B. , Bihari, P. , Lazar, V. , … Pál, C. (2016). A highly precise and portable genome engineering method allows comparison of mutational effects across bacterial species. Proceedings of the National Academy of Sciences, 113, 2502–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov, I. (1955). Serological investigations in the Klebsiella group. I. New capsule types. Acta Pathologica Et Microbiologica Scandinavica, 36, 449–453. [DOI] [PubMed] [Google Scholar]
- Paczosa, M. K. , & Mecsas, J. (2016). Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiology and Molecular Biology Reviews, 80, 629–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton, J. N. , Gorman, S. P. , & Gilmore, B. F. (2013). Clinical relevance of the ESKAPE pathogens. Expert Review of Anti‐Infective Therapy, 11, 297–308. [DOI] [PubMed] [Google Scholar]
- Phanphak, S. , Georgiades, P. , Li, R. , King, J. , Roberts, I. S. , & Waigh, T. A. (2019). Super‐resolution fluorescence microscopy study of the production of K1 capsules by Escherichia coli: evidence for the differential distribution of the capsule at the poles and the equator of the cell. Langmuir, 35, 5635–5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podschun, R. , Penner, I. , & Ullmann, U. (1992). Interaction of Klebsiella capsule type 7 with human polymorphonuclear leucocytes. Microbial Pathogenesis, 13, 371–379. [DOI] [PubMed] [Google Scholar]
- Podschun, R. , & Ullmann, U. (1998). Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clinical Microbiology Reviews, 11, 589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendueles, O. , de Sousa, J. A. M. , Bernheim, A. , Touchon, M. , & Rocha, E. P. C. (2018). Genetic exchanges are more frequent in bacteria encoding capsules. PLoS Genetics, 14, e1007862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendueles, O. , Garcia‐Garcera, M. , Neron, B. , Touchon, M. , & Rocha, E. P. C. (2017). Abundance and co‐occurrence of extracellular capsules increase environmental breadth: Implications for the emergence of pathogens. PLoS Path, 13, e1006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, L. B. (2008). Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. Journal of Infectious Diseases, 197, 1079–1081. [DOI] [PubMed] [Google Scholar]
- Schembri, M. A. , Blom, J. , Krogfelt, K. A. , & Klemm, P. (2005). Capsule and fimbria interaction in Klebsiella pneumoniae . Infection and Immunity, 73, 4626–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu, L. K. , Yeh, K. M. , Lin, J. C. , Fung, C. P. , & Chang, F. Y. (2012). Klebsiella pneumoniae liver abscess: A new invasive syndrome. The Lancet Infectious Diseases, 12, 881–887. [DOI] [PubMed] [Google Scholar]
- Struve, C. , & Krogfelt, K. A. (2003). Role of capsule in Klebsiella pneumoniae virulence: Lack of correlation between in vitro and in vivo studies. FEMS Microbiology Letters, 218, 149–154. [DOI] [PubMed] [Google Scholar]
- Struve, C. , & Krogfelt, K. A. (2004). Pathogenic potential of environmental Klebsiella pneumoniae isolates. Environmental Microbiology, 6, 584–590. [DOI] [PubMed] [Google Scholar]
- Tan, Y. H. , Chen, Y. , Chu, W. , Sham, L. , & Gan, Y. H. (2020). Cell envelope defects of different capsule‐null mutants in K1 hypervirulent Klebsiella pneumoniae can affect bacterial pathogenesis. Molecular Microbiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos, A. O. , Pantosti, A. , Baumann, H. , Brisson, J. R. , Jennings, H. J. , & Kasper, D. L. (1992). The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. Journal of Biological Chemistry, 267, 18230–18235. [PubMed] [Google Scholar]
- Whitfield, C. (2006). Biosynthesis and assembly of capsular polysaccharides in Escherichia coli . Annual Review of Biochemistry, 75, 39–68. [DOI] [PubMed] [Google Scholar]
- Wyres, K. L. , & Holt, K. E. (2018). Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Current Opinion in Microbiology, 45, 131–139. [DOI] [PubMed] [Google Scholar]
- Xu, J. , Bjursell, M. K. , Himrod, J. , Deng, S. , Carmichael, L. K. , Chiang, H. C. , … Gordon, J. I. (2003). A genomic view of the human‐Bacteroides thetaiotaomicron symbiosis. Science, 299, 2074–2076. [DOI] [PubMed] [Google Scholar]


