Graphical abstract
Abbreviations: AGU, AmyloGlucosidase Unit; AHG, anhydro-L-galactose; AOAC, Association of Official Agricultural Chemists; BHU (2), Biomass Hydrolysis Unit; CBU, CelloBiase Unit; CDW, cell dry weight; dw basis, dry weight basis; FPU, Filter Paper Unit; Fr, Froude number; G. sesquipedale, Gelidium sesquipedale; H. boliviensis, Halomonas boliviensis; HMF, 5-hydroxymethyl furfural; FID, flame ionization detector; KNU, Kilo Novo alpha-amylase Unit; Mw, molecular weight; MSG, monosodium glutamate; NREL, National Renewable Energy. Laboratory; NABH, neoagarobiose hydrolase; P3HB, poly-3-hydroxybutyrate
Keywords: Gelidium sesquipedale, Halomonas boliviensis, Waste seaweed, Poly-3-hydroxybutyrate, Seaweed residues, Macroalgae residues
Highlights
-
•
Residues of red seaweed Gelidium after phycocolloid extraction still contain 44 % of upgradable carbohydrates.
-
•
Hydrolysis of Gelidium cabohydrates to monosaccharides yielded 30 % (w/w) glucose per g dry biomass.
-
•
Hydrolysates from Gelidium residues are suitable C-sources for P3HB production by Halomonas boliviensis.
-
•
Halomonas boliviensis accumulated 41 % (w/w) of P3HB based on the algal hydrolysates.
-
•
The outlined process adds value to waste seaweed and promotes circular economy.
Abstract
Agar extraction from Gelidium and Gracilaria red seaweed species produces hundred thousand ton of carbohydrate-rich residues annually. Gelidium sesquipedale waste biomass obtained after agar extraction, still contained 44.2 % w/w total carbohydrates (dry-weight basis). These residues were biologically up-graded to poly-3-hydroxybutyrate (P3HB) after saccharification of their carbohydrate fraction to simple sugars. A combined hydrolysis treatment using sulfamic acid followed by enzymatic hydrolysis with cellulases produced a glucose-rich hydrolysate with a negligible content of inhibitors. With this treatment a sugar yield of circa 30 % (g glucose/g biomass) was attained. The algal hydrolysates were assessed as carbon source for the production of P3HB by the halotolerant bacteria Halomonas boliviensis. A cell concentration of 8.3 g L−1 containing 41 % (w/w) of polymer and a yield (YP/S) of 0.16 gpolymer/gglucose were attained in shake flask assays. In this work, cellulose-rich seaweed waste was shown to be an upgradable, sustainable source of carbohydrates.
1. Introduction
Polyhydroxyalkanoates (PHAs) are a family of biodegradable polyesters with recognized advantages over synthetic plastics. These polymers are composed of hydroxyalkanoic acid monomers forming chains with variable molecular weight (50–1000 kDa). Different types of monomers and arrangements are possible conveying thus different properties and applications to PHAs. Applications can range from daily uses in packaging to niche areas in the medicine field such as the fabrication of resorbable medical devices like sutures, meshes, implants, and tissue engineering scaffolds. Drug release applications have also been reported [1]. Despite the obvious advantages, their commercialization has limited success. A high production cost caused mainly by the price of the carbon-source and a difficult downstream step encompassing either the use of high volumes of organic solvents or multiple steps when using aqueous solutions for PHA separation, remain the main weaknesses to reach a competitive price in the plastic sector [2,3]. To reduce the cost of the raw materials, several waste streams have been tested [[4], [5], [6]] including cellulose-rich agricultural residues such as sugar cane bagasse [7] and wheat straw [8]. These renewable feedstocks have been tested as carbon platforms for PHA production with promising results [9,10]. To be used as carbon-source by most microbes, cellulose and hemicellulose need to undergo hydrolysis and release simple sugars. However, due to the high lignin content of lignocellulosic biomass (approx. 15–25 % of the dry-weight) [11], the pre-treatment step aiming at delignification of the biomass (and some hemicellulose degradation), generally involves harsh conditions like high temperatures (160–240 °C) and pressures (0.7 and 4.8 MPa) (High Pressure Saturated Steam) that can be followed by an explosive decompression (Steam Explosion). Alternatively, acid (e.g. H2SO4, HCl) or alkaline (NaOH, KOH, NH4OH) pre-treatments have also been applied either to hydrolyse hemicellulose or remove the lignin, respectively [12,13]. Depending on the conditions used in the pre-treatment, toxic compounds may be released that are harmful to the microbes in the subsequent biological process. Those compounds result from the further degradation of simple monosaccharides as glucose and xylose to furan aldehydes, namely hydroxymethyl furfural (HMF) and furfural, respectively, as well as from the monomers of lignin.
Because macroalgae, also called seaweed, lack lignin and have a high carbohydrate content (50–70 % dw) they are considered potential carbon platforms for many bioprocesses [14]. Seaweeds are classified in three groups: green (Chlorophyta), brown (Phaeophyta) and red (Rhodophyta). Particularly concerning the carbohydrate fraction, seaweed includes characteristic complex polysaccharides that are typical from marine biomass: green seaweed contain ulvan, while brown seaweed present alginate and fucoidan and red species contain agar or carrageenan [15,16]. Besides these characteristic polysaccharides, seaweed have glucose polysaccharides (glucans) like cellulose, starch and laminarin. Cellulose is present in all groups while starch exists in green and red algae and laminarin is typical of brown species (Table 1). Macroalgal polysaccharides are classified according to their biological function in two groups: storage and structural polysaccharides. While starch and laminarin are examples of reserve polysaccharides of green and brown algae, respectively, structural polysaccharides like cellulose and agar (red algae) belong to the cell wall of macroalgae. Cellulose reaches approximately 15 wt % of dry biomass in red macroalgae [17].
Table 1.
Typical storage and structural polysaccharides of the three macroalgae groups and the monosugars resulting from their complete hydrolysis.
| Seaweed group |
Examples | Polysaccharides |
Major monosaccharides upon hydrolysis |
||
|---|---|---|---|---|---|
| Storage | Structural | Storage | Structural | ||
| Red |
Gelidium sesquipedale, Gracilaria sp. |
Floridean starch | Cellulose Agar |
glucose | glucose D-galactose anhydro-D-galactose (L- AHG) |
|
Chondrus crispus Gigartina papillata |
Cellulose Carrageenan |
glucose D-galactose anhydro-D-galactose (L-AHG) |
|||
| Green |
Ulva lactuca, Ulva pertusa |
Starch | Ulvan | glucose | glucose xylose L-rhamnose glucuronic acid iduronic acid |
| Cellulose | glucose | ||||
| Brown |
Laminaria hyperborea, Fucus vesiculosus, Macrocystis pyrifera |
Laminarin + mannitol |
Alginate | glucose + mannitol | mannuronic acid guluronic acid |
| Fucoidan | fucose D-xylose D-galactose D-mannose glucuronic acid |
||||
| Cellulose | glucose | ||||
Seaweed wastes from phycocolloid extraction industries are carbohydrate-rich materials which, in most cases, are underexploited. Some authors have already considered the use of waste seaweed biomass for the production of bioethanol [18,19]. These residues contain mainly cellulose and some phycocolloid leftovers that are potential sugar feedstocks for the production of value-added chemicals and materials. Their utilization using a biorefinery perspective enhances the sustainability of the seaweed industry through the complete valorisation of the carbohydrate fraction besides phycocolloid extraction.
In this work, residues of the red agarophyte Gelidium sesquipedale, also called Gelidium corneum, supplied by a local agar-extraction plant, were used. The residues still contained significant amounts of cellulose and agar which were tested as sugar platforms for the production of poly-3-hydroxybutyrate (P3HB). This red alga is abundant in the Western Europe and Morocco coasts [20] and is harvested mainly for the extraction of agar. Agar consists of two fractions: agarose and agaropectin. Agarose is the gelling component, while the agaropectin has only a low gelling ability. Industrial agar extraction is carried out in boiling water for 4 h, after which the solution is separated from the residue by filtration [20]. The extraction yield is generally not complete (60–85 %; personal communication) and thus residues of agar are present in the solid waste. To produce a sugar-rich hydrolysate, Gelidium residues must undergo hydrolysis. Unlike terrestrial biomass that contains lignin, the complete hydrolysis of the carbohydrates in seaweed residues potentially does not require a pre-treatment step, the enzymatic step alone being enough for the release of simple sugars. Yet, the presence of agar leftovers hampers the processing of high biomass loads and for that reason a dilute acid pre-treatment was carried out to break the agar to oligosaccharides. The enzymatic hydrolysis of the Gelidium solid wastes was carried out with a mixture of cellulases and β-glucosidase.
A halophilic bacterial species, able to consume the main sugars in the Gelidium hydrolysate and to accumulate high amounts of PHAs, was selected from literature. Halophiles have properties that make them desirable in industrial biotechnology processes due to their tolerance to high salt concentrations and the ability to work at high pH values. This allows to operate in non-strict sterile conditions, facilitating large-scale continuous cultivation and potentially decreases operational costs, rendering PHA production processes more competitive. Besides, the selection of a halophilic strain was a logic choice due to the high salt concentration in seaweed hydrolysates. In this work, Halomonas boliviensis [21,22] a halotolerant bacteria able to consume glucose and galactose released from Gelidium residues, was chosen.
2. Materials and methods
2.1. Raw biomass
Gelidium sesquipedale residues obtained after agar-agar extraction were a courtesy of Iberagar SA- Sociedade Luso-Espanhola de Colóides Marinhos, Portugal. Upon reception, biomass was maintained at −18 °C. When needed, it was washed several times under tap water and residues such as shell fragments and sand were removed manually. The biomass was left to dry at room temperature for 24 h and was subsequently frozen at −80 °C before lyophilisation (Christ, model Alpha 1−2 LD plus). The lyophilised biomass was subsequently blended using a coffee grinder (1 min in pulses of 10 s) to attain even pieces of biomass and was left in a desiccator till use.
2.2. Enzymes
Cellulase complex (NS 22086) and β-glucosidase (NS 22118) from Novozymes® (Bagsvaerd, Denmark), with an activity of 1000 Biomass Hydrolysis Unit (BHU (2))/g and 250 CelloBiase Unit (CBU)/g, respectively, were used. The cellulase complex activity was determined using the procedure described in the NREL/TP-510-42628 technical report [23], while the β-glucosidase activity determination followed the protocol described by [24]. NS 22086 had a filter paper activity of 111.2 Filter Paper Unit (FPU)/mL, while NS 22118 had a cellobiase activity of 20.1 CBU/mL. Filter paper Grade 1 from Whatman and cellobiose (≥99 %) from Sigma Aldrich, respectively, were used in the activity assays.
Amylolytic enzymes, namely α-amylase from Bacillus amyloliquefaciens (>250 Kilo Novo alpha-amylase Unit (KNU/g) from Sigma and amyloglucosidase (NS22035) from Novozymes (750 AmyloGlucosidase Unit (AGU /g) were used in the enzymatic assays for starch determination.
2.3. Chemicals
The chemicals used were sulphuric acid 96 % (Acros Organics), sulfamic acid (VWR Chemicals), phosphoric acid 85 % (Pancreac Quimica), di-potassium hydrogen phosphate 99 % (Pancreac Quimica), potassium di-hydrogen phosphate 98 % (Pancreac Quimica), (D)+ glucose anhydrous 99.5 % (Fischer Chemical), D(+) galactose ≥98 % (Carl Roth Chemicals), 5-hydroxymethylfurfural ≥98 % (Sigma Aldrich), hydrochloric acid min. 37 % (Riedel-de Haën) and calcium carbonate min.99 % (Merck).
2.4. Microorganism and culture media
Halomonas boliviensis DSM 15516 [22] was selected from literature for its ability to synthesize polyhydroxyalkanoates from glucose and galactose, the monosugars released upon the hydrolysis of cellulose and agar, respectively.
2.5. Strain storage
Halomonas boliviensis DSM 15516 was purchased from the DSMZ collection and activated with HM medium described in Quillaguaman, 2004 [22]. This medium had the following composition (g/L): NaCl, 45.0; MgSO4.7H2O, 0.25; CaCl2.2H2O, 0.09; KCl, 0.5; NaBr, 0.06; peptone (Difco), 5.0; yeast extract (Difco), 10.0; glucose, 1.0, and 2.0 % granulated agar (for solid medium).The pH was adjusted to 7.5 by using 1 M KOH. The solid cultures were renewed monthly to reactivate the microorganisms.
Cultures of H. boliviensis were stored at −80 °C in 2 mL micro tubes containing 300 μL of glycerol and 1500 μL of a previously grown liquid culture in the late exponential phase prepared with seed culture medium [25,26] and supplemented with 20 g/L of glucose.
2.6. Seed medium and inoculum preparation
The inoculation medium (or seed medium) for H. boliviensis contained: 45 g/L of NaCl; 25 mL/L of a 100 g/L MgSO4 x 7 H2O solution; 0.55 g/L K2HPO4; 2.3 g/L NH4Cl; 15 g/L, Tris; 3 g/L monosodium glutamate (MSG) and 0.005 g/L FeSO4 x7 H2O. Solutions of NaCl (300 g/L) and MgSO4 x7 H2O (100 g/L) were prepared and sterilized separately to avoid precipitation during the sterilization in the autoclave. After all salts were dissolved the pH was adjusted to 7.5 with 37 % (w/w) HCl.
The H. boliviensis inoculum was prepared by transferring the contents of a microtube (2 mL) to 500 mL shake flasks containing 76.5 mL of seed medium; 15 mL of a 300 g/L NaCl solution; 2.5 mL of a 100 g/L MgSO4 x 7 H2O solution and 4 mL of a 500 g/L glucose solution to attain a total volume of 100 mL. Cultivations were carried out in an orbital shaker at 30 °C and 170 rpm. After circa 24 h, when the culture reached an O.D.600nm of 5 (exponential phase), a given volume was withdrawn and used as inoculum in the production assays.
2.7. Production medium
The medium for P3HB production in shake flasks assays (medium A) contained: 45 g/L NaCl; 50 mL/L of 100 g/L MgSO4 .7 H2O; 2.2 g/L K2HPO4; 1.0 g/L NH4Cl; 0.005 g/L FeSO4 .7 H2O; 15 g/L Tris and 20 g/L of MSG. Solutions of NaCl (300 g/L) and MgSO4.7 H2O (100 g/L) were prepared and sterilized separately to avoid precipitation during the sterilization in the autoclave. In a particular study to determine the influence of the nitrogen content on P3HB production, different concentrations of NH4Cl (0 or 1 g/L) and MSG (0; 5; 10 g/L) were tested. After all salts were dissolved the pH was adjusted to 7.5 with 37 % (w/w) HCl.
For the growth on G. sesquipedale hydrolysates, a 20-fold concentrated production medium was prepared. The pH of this concentrated medium was not adjusted as previously. The concentrated NaCl and MgSO4 .7 H2O were also prepared and sterilized separately.
2.8. Algal biomass characterization
The compositional analysis of the waste seaweed biomass was determined following several established procedures. To determine ash, moisture and total solids in the algal biomass the procedure described in the Technical Report NREL/TP-5100-60956 procedure was followed [27]. These determinations were done in triplicate. A pre-conditioned crucible (575 °C in a muffle furnace overnight) was weighed and 100 mg of lyophilized Gelidium biomass added. The sample was then placed in a convection drying oven at 60 °C for at least 18 h until constant weight, thus allowing the determination of total solids and moisture.
To determine the ash content, the cooled crucible containing the dried sample was subsequently put in the muffle furnace at 575 °C using the temperature gradient according to the NREL protocol. The cooled sample was then weighed and the ash content determined.
The determination of total carbohydrates followed the procedure in Van Wychen & Laurens, 2013a [28] and was initiated by the hydrolysis of 25 mg of biomass with 250 μL of 72 % (w/w) sulphuric acid in glass tubes, at 30 °C. After an hour the hydrolysate was diluted to a final concentration of 4% (w/w) sulphuric acid with 7 mL of demineralised water. Samples were then placed in an autoclave for one hour at 121 °C. Once the samples were cooled to room temperature, an aliquot was taken for neutralization to pH 6–8 using calcium carbonate. Centrifugation was then applied (9167 g, 5 min), to separate the solids after neutralization and the supernatant collected for sugar quantification. These determinations were done in triplicate.
Total fibre, protein and lipid contents (% dry-weight) were evaluated using AOAC official methods of analysis: AOAC Method 991.43 for the determination of the total fibre, AOAC 960.52 for the determination of protein and the conventional Soxhlet extraction for lipid determination. The mineral composition was assessed by nitric-perchloric digestion and Inductively Coupled Plasma- Atomic Emission Spectrometry (ICP-AES) for P, Na, K, Ca, Fe, Cu, Zn determination, while nitric-perchloric extraction followed by ICP-AES analysis was used for Cr and Pb assessment. All these determinations were performed in duplicate.
2.9. Production of Gelidium sesquipedale hydrolysates
Mild acid hydrolysis took place at 121 °C and 1 bar. Different catalysts, catalyst concentrations, contact times and biomass concentrations were tested. Sulfuric acid, phosphoric acid and sulfamic acid were evaluated as catalysts at various concentrations namely: 0.25, 0.5 and 1.0 % (w/v) for H2SO4; 0.5, 1.0 and 1.5 % (w/v) for H3PO4 and 1.0, 1.5 and 2.0 % (w/v) in the case of sulfamic acid.
The effect of the biomass load on the hydrolysis degree was also examined. This was carried out with sulfuric acid as catalyst and a contact time of 30 min. This assay was performed with a constant working volume by changing the biomass load resulting in biomass concentrations of 2.1 %, 4.3 % and 8.6 % (w/v).
The biomass was weighed in 50 mL glass flasks and 10 mL of acid was added to each flask. These flasks were then sealed with a rubber stop and an aluminium cap and placed in the autoclave. After chemical hydrolysis, aliquots from the three samples were centrifuged (9167 g, 5 min) and supernatant samples prepared for sugar determination by HPLC.
Enzymatic hydrolysis was carried out at 50 °C and pH 4.8 for 30 h under continuous magnetic stirring (660 rpm). This was accomplished in a thermostatically controlled incubator chamber (JP Selected). The assays were performed with different biomass concentrations in 50 mL glass flasks with a total volume of 10 mL. The enzyme cocktail consisted of 10 μl cellulase /mL plus 7.5 μL β-glucosidase/mL (V enzyme solution/ V biomass suspension) using 10 x diluted enzyme solutions. This corresponded to adding 1.1 FPU/mL of cellulase complex (NS 22086) and 0.15 CBU/mL of β-glucosidase (NS 22118) to 10 mL of seaweed slurry. The best enzyme ratio (cellulase: β-glucosidase) was previously defined (results not shown). Previous assays have indicated that the enzymatic hydrolysis is complete within 30 h. Aliquots from the supernatant were taken during the hydrolysis and the sugar concentration determined by HPLC. The assays were carried out in duplicate.
Prior to use, the commercial enzyme solutions were diluted 10 times with milli Q water to ease the handling of the originally viscous solutions. The presence of glucose was detected in both commercial enzyme solutions. This reducing sugar is most probably used as preservative in the formulation. Glucose concentration was determined at the onset of each enzymatic assay and its concentration deduced from the final glucose concentration obtained after polysaccharide hydrolysis.
The combined hydrolysis of the algal biomass was performed by chemical pre-treatment followed by enzymatic hydrolysis. After pre-treatment, the pH of the algal slurry was adjusted to 4.8 with NaOH 8 M and enzyme addition took place so as to reach the concentrations given above. The enzymatic hydrolysis was carried out for 30 h at 50 °C and 660 rpm. After 30 h aliquots were taken, centrifuged at 9167 g for 5 min and prepared for sugar quantification.
2.10. Production of larger volumes of hydrolysate for growth and P3HB production assays
To produce larger volumes of Gelidium hydrolysate, a 2 L shake flask with a working volume of 700 mL and a biomass load of 8.6 % (w/v) was used. Hydrolysis, combining dilute acid pre-treatment with 0.5 % (v/v) sulfamic acid (30 min, 121 °C) followed by enzymatic hydrolysis, took place. Before enzyme addition, the biomass slurry was brought to a pH of 4.8 with NaOH 2 M. Enzyme solutions were then added to a concentration of 1.1 FPU/mL biomass suspension + 0.15 CBU/mL biomass suspension of cellulase and β-glucosidase, respectively. Enzymatic hydrolysis was conducted at 50 °C for 30 h in an orbital shaker at 200 rpm (Agitorb). The hydrolysate slurry was centrifuged to remove the solids and the supernatant autoclaved. The supernatant was then cooled overnight at 4 °C and centrifuged again, to discard formed precipitates, before use.
2.11. Shake flasks assays
The shake flask assays were carried out to follow growth and P3HB production by H. boliviensis under different conditions. These assays were performed in 500 mL shake flasks containing production medium supplemented with 20 g/L glucose or G. sesquipedale hydrolysate to a 100 mL final working volume. A 5 % (v/v) previously grown inoculum volume was added to attain an initial OD600nm of 0.5. Samples from shake flasks were periodically taken for cell growth determination by optical density and cell dry weight (CDW), sugar consumption and P3HB production. The assays were performed in duplicate and the average of the results considered.
2.12. Analytical methods
2.12.1. Biomass quantification
Cellular growth was monitored off-line by measuring the optical density (O.D.) at a wavelength of 600 nm (O.D.600nm) in a double beam spectrophotometer Hitachi U-2000.
The cell dry weight (CDW) of the microbial culture was determined by transferring 1.2 mL of the broth to a previously weighed and dried 1.5 mL microtube. These samples were centrifuged in a Sigma 1−15 P microcentrifuge (9168 x g during 4 min) and the supernatant was separated after centrifugation. The pellet was then washed by resuspension in distilled water and centrifuged for the second time. After the second centrifugation step the supernatant was discarded. The cleaned pellet was then dried in an oven (Memmert model 200) at 60 °C for at least 48 h.
2.12.2. Sugar quantification
The quantification of monosaccharides and HMF in the hydrolysates was performed by High-Performance Liquid Chromatography (HPLC) (Hitachi LaChrom Elite), using a Rezex ROA Organic acid H + 8% (30 mm x 7.8 mm) column, an autosampler (Hitachi LaChrom Elite l-2200), an HPLC pump (Hitachi LaChrom Elite L-2130) and a Hitachi L-2490 refraction index detector or a Hitachi L-2420 UV/VIS detector. The injection volume was 20 μL and elution was achieved using a 5 mM solution of H2SO4. The pump was operated at a flow rate of 0.5 mL/min. The column was kept at 65 °C in a column heater for large columns (Croco-CIL 100-040-220 P, 40cm × 8cm × 8 cm, 30–99 °C) and connected externally to the HPLC system.
Samples for sugar quantification were firstly centrifuged (1−15 P microcentrifuge, Sigma) for 5 min at 9167 g. After the first centrifugation, a sample of supernatant (200 μL) was diluted with 200 μL of 50 mM H2SO4 and the mixture vortexed and centrifuged. HPLC vials were then prepared with 100 μL of the supernatant from the second centrifugation and 900 μL of 50 mM H2SO4. Prior to the analyses, calibration curves for glucose, galactose and HMF in the adequate concentration ranges were obtained. The detection of monosaccharides and HMF was done by the RI and UV/VIS detector, respectively
Vials with the standards were prepared using the same procedure as with the samples.
2.12.3. P3HB quantification
The amount of P3HB produced was determined after centrifuging 1.2 mL of the culture broth and washing the cell pellet. To determine the concentration of P3HB produced, the polymer was converted into stable and volatile hydroxycarboxylic acid methyl esters, through acidic methanolysis and the esters were further analysed by gas chromatography (GC).
One millilitre of chloroform was added to the cell pellet, the cells were resuspended and then transferred to a glass tube. In order to initiate the acidic methanolysis, 1 mL of “solution A” containing 97 mL methanol, 3 mL of 96 % H2SO4 and 330 μL hexanoic acid, was added to each glass tube. After vortexing, the tubes were placed in an oven (Memmert model 200) at 100 °C. After 5 h the reaction was stopped by adding 1 mL of 60 g/L Na2CO3 solution to each tube. Each glass tube was vortexed and subsequently centrifuged. After centrifugation (5 min at 2 697 g in a Heraeus Labofuge 200 from Thermo Scientific), 200 μL from the bottom phase (organic phase) was transferred to a GC vial. The vials were stored at −18 °C until analysis.
The organic phase was analysed in a gas chromatograph (Hewlett Packard 5890 series II) equipped with a FID detector and a 7683B injector. The oven, injector and detector temperatures were set to 60, 120 and 150 °C, respectively. The GC column used in this study was a HP-5 from Agilent J&W Scientific, 30 m length and 0.32 mm internal diameter. The data acquisition and integration were performed by the Shimadzu CBM-102 communication Bus Module and Shimadzu GC solution software (Version 2.3).
Peak identification was achieved by using an internal standard of 3-methyl hydroxybutyrate from Sigma-Aldrich. Calibration curves were obtained using samples of P(3HB) produced previously (purity 98.2 %), which were subjected to the same methylation process as the cells.
3. Results and discussion
3.1. Biomass characterization
The chemical composition of Gelidium sesquipedale residues was determined and is shown in Table 2. As these determinations were carried out with lyophilized biomass, the moisture content was very low (6.1 %). The largest component of the dry-weight was the carbohydrate fraction (44.80 %) that is composed of cellulose, residual agar and starch.
Table 2.
Composition of Gelidium sesquipedale residues.
| Parameter | Value | Unit | Method |
|---|---|---|---|
| Dry-weight (DW) | 93.90 ± 0.83 | (%) | NREL 60956 |
| Moisture | 6.10 ± 0.83 | (%) | NREL 60956 |
| Total fiber | 76.26 | (% dry-weight) | AOAC 991.43 |
| Proteins | 0.68 | Idem | Kjeldahl method |
| Lipids | 3.36 | Idem | Soxhlet method |
| Ash | 16.39 ± 1.15 | Idem | NREL 60956 |
| Carbohydrate | 44.80 ± 1.90 | Idem | NREL 60957 |
| Agar | 7.3 | Idem | NREL60957 and Eq. (2) |
| Cellulose | 37.05 ± 0.02 | Idem | NREL60957 and Eq. (1) |
| Starch | 0.80 ± 0.33 | Idem | Megazyme total starch procedure |
| Minerals | |||
| Phosphorous | 7.16 | g/Kg dry-weight | Nitric-perchloric digestion + ICP-AES |
| Sodium | 1.68 | Idem | Idem |
| Potassium | 1.86 | Idem | Idem |
| Calcium | 31.68 | Idem | Idem |
| Iron | 1248.2 | mg/Kg dry-weight | Idem |
| Copper | 25.4 | Idem | Idem |
| Zinc | 243.3 | Idem | Idem |
| Chromium | 23.3 | Idem | Nitric-perchloric extraction + ICP-AES |
| Lead | 5.8 | Idem | Idem |
The total carbohydrate content was determined using the NREL 60957 protocol [28], considering that storage (starch) and structural (cellulose and agar) polysaccharides in the algal biomass were completely hydrolysed into monomeric subunits after a two-step hydrolysis reaction with sulfuric acid. The released monosaccharides D-glucose and D-galactose were analyzed by HPLC. The amount of starch in the residues was quantified based on the glucose released upon enzymatic hydrolysis using an amylolytic cocktail of α-amylase and amyloglucosidase and the Total Starch Megazyme (Megazyme, Bray, Ireland) procedure (including search for resistant starch). The amount of starch was calculated using Eq. (3) and it was found to be very low. The glucose released from the hydrolysis of starch was subtracted from the total amount of glucose determined using the Van Wychen and Laurens, 2013a protocol, and the value obtained used to determine the amount of cellulose (Eq. (1)). The amount of agar in the biomass was calculated from the D-galactose concentrations, using Eq. (2).
| (1) |
| (2) |
| (3) |
where: 162 is the molecular weight (Mw) of glucose and galactose monomeric units in polymeric glucan and galactan, 180 is the Mw of glucose and galactose and 1.27 is the weight ratio between L-3,6- anhydro galactose (AHG) and D-galactose in agar, i.e. 1:1.27 (0.44 % D-galactose: 0.56 % L-AHG) [29].
The results showed that G. sesquipedale residues still contain a non-negligible content of carbohydrates (44.8 % dw), the main being cellulose with circa 37. 5% of dw, followed by agar with 7.3 % dw. The later indicates that the extraction process was not complete, i.e., the waste biomass still contains a significant amount of agar. It is interesting that the cellulose content in seaweed waste is comparable to that of agricultural biomass residues of 33–47 % [30].
The protein content was determined from the total nitrogen by the Kjeldhal method multiplied by a factor of 5.0 [31]. As expected, the protein content of Gelidium residues was very low (0.68 % dry weight), because proteins are most probably removed during the agar extraction procedure. In reality, prior to agar extraction, the algae are washed with 0.5 % (w/v) sodium carbonate (90 °C, 30 min) for the removal of undesired components, namely red pigments (phycoestrine). Subsequently, the agar is extracted from the solid phase using a hot aqueous solution with pH adjusted between 4.8 and 8.0 (depending on the temperature of the extraction) [32]. Since proteins are easily extracted under alkaline conditions [33], they are being probably extracted during the hot alkaline washing step with sodium carbonate and discarded in the liquid phase.
3.2. Diluted acid pretreatment
Enzymatic saccharification aiming at the release of galactose and AHG from agar is costly because a complex cocktail of endoagarases (α -agarase and β-agarase), exoagarases (agarase II) and finally neoagarobiose hydrolase (NABH) is required. Moreover, saccharification is not efficient primarily because agarose has a low solubility in water. Yun et al., 2016 suggest the combination of enzymatic hydrolysis with acid hydrolysis for an efficient hydrolysis of agarose into monomeric sugars with high titres and yields [17].
In this work because of the low content of agar in the Gelidium residues (Table 2), enzymatic hydrolysis using solely cellulases was chosen. Nonetheless, a mild chemical pre-treatment was carried out to accomplish the hydrolysis of agar to galactose monomers.
Different acid catalysts such as sulphuric, phosphoric and sulfamic acids, catalyst concentrations and time of hydrolysis were evaluated aiming to improve agar hydrolysis while minimizing the formation of 5-hydroxymethyl furfural. HMF is formed by the dehydration of hexoses during the thermal acid treatment and it is proven to be an inhibitor of microbial cell growth [34,35]. In the particular case of agar, HMF is released from the degradation of 3,6-anhydro-L-galactose (AHG), as this monomer is acid-labile [17].
For each hydrolytic treatment the recovery yield of galactose was calculated based on the amount of agar in the biomass.
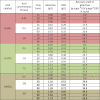
The results of the digestion of lyophilized Gelidium residues (43 g/L) by sulfuric acid are shown in Table 3. Different H2SO4 concentrations of 0.25, 0.5 and 1.0 % (w/v) and reaction times of 15, 30 and 60 min were tested. The best results were attained with 1% (w/v) sulfuric acid for 30 min, resulting in a recovery yield of galactose from agar of 54.7 %. This represents a complete hydrolysis of agar since the remaining fraction is AHG. The 5-HMF generated under these conditions reached a concentration of 0.7 g/L. A shorter treatment or a less concentrated acid treatment led to a significant decrease of the agar hydrolysis yield, with no D-galactose and negligible amounts of HMF (0.25 % w/v) being released. The hydrothermal treatment without acid addition generated neither D-galactose nor 5-HMF (results not shown). A longer treatment (60 min) with 0.5 or 1% (w/v) H2SO4 led to slightly lower sugar titres due to sugar degradation.
Table 3.
Summary of the results of the pre-hydrolysis step of Gelidium residues with different acid catalysts: biomass load 4.3 % (w/v) at 121 °C; no mixing, total working volume 10 mL.
 |
Like sulphuric acid, phosphoric acid is a strong inorganic acid that has been used as a catalyst during the hydrothermal pretreatment of lignocellulosic biomass [36]. It has the additional advantage of the formation of a phosphate salt upon neutralization of the hydrolysate, which can be used as a phosphate source for the subsequent fermentation process. Phosphoric acid was also successfully used in the saccharification of the red macroalga Gracilaria verrucosa [37] at a concentration of 1.5 % (w/v), 140 °C and 60 min reaction time.
Phosphoric acid was tested as catalyst in the hydrothermal treatment of Gelidium residues. Acid concentrations of 0.5, 1.0 and 1.5 % (w/v) were used at 121 °C, for 15, 30 and 60 min hydrolysis time and with a biomass concentration of 43 g/L. Increasing H3PO4 concentrations improved slightly the release of D-galactose. The optimal hydrolysis conditions were attained with 1.5 % (w/v) phosphoric acid and a 60 min treatment (Table 3). Under these conditions the concentrations of galactose and HMF attained were 1.3 and 0.6 g/L, respectively, resulting in a galactose recovery yield of 37.9 %. The produced HMF increased with higher acid concentrations.
Sulfamic acid (H3NSO3), also known as amidosulfonic acid, is considered a green catalyst [38]. It is a moderately strong acid (pKa = 1.01), non-corrosive and has a dual active site. It was tested as catalyst in the hydrothermal hydrolysis of the red alga Gracilaria verrucosa at a concentration of approximately 1 % (w/v) (130 °C and 90 min reaction time), resulting in a total reducing sugar yield of 39.9 % [29]. In this work sulfamic acid (H3NSO3) was also tested as catalyst in the hydrothermal hydrolysis of Gelidium residues. Acid concentrations of 0.5, 1.0 and 1.5 (w/v) were used at 121 °C, for 30, 60 and 90 min contact time (except for 0.5 % H3NSO3 where only 30 and 60 min reaction time was tested). The results on Table 3 show that the release of D-galactose improves with increasing acid concentrations and reaction time. The same happens with the HMF formed. The best results were attained with 1.5 % (w/v) H3NSO3 and a contact time of 90 min, resulting in 1.7 g/L D- galactose and 0.8 g/L HMF and a galactose recovery yield of 50 %.
During the acid-catalysed hydrothermal hydrolysis under the tested conditions only D- galactose was released from agar, no glucose being freed from the saccharification of cellulose. The generation of HMF was observed in all cases with no further inhibitory compounds being identified in the hydrolysates. Although phosphoric and sulfamic acids proved to be promising catalysts the highest yield of sugars (over 54 %) was attained with 1 % sulfuric acid after 30 min.
3.3. Sequential acid hydrolysis and enzymatic treatment
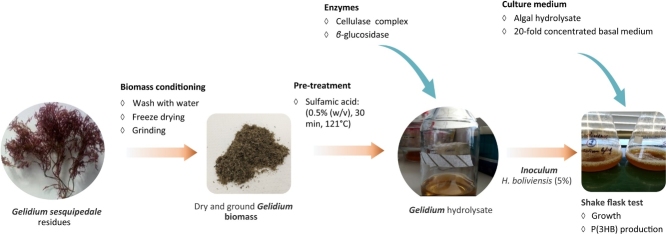
To perform the saccharification of the cellulose fraction after acid pre-treatment, hydrolysis with an enzyme cocktail of cellulases and β-glucosidase was used. Enzymatic hydrolysis was carried out after pre-treatment with sulfuric acid (1% (w/v), 30 min, 121 °C) or after treatment with sulfamic acid (0.5 % (w/v), 30 min, 121 °C). These were the chosen conditions due to their promising results concerning the yield of sugar release or the low levels of produced HMF, respectively. Different biomass concentrations, namely 21.5, 43.2 and 86.4 g/L biomass were tested aiming at increasing the sugar concentration in the hydrolysates. The enzymatic hydrolysis was carried out for 30 h using 1.1 FPU /mL biomass slurry of cellulase and 0.15 CBU/mL of β-glucosidase. Results of the combined hydrolysis assays can be seen in Fig. 1. A significant increase of the total amount of sugars released is observed with the increase of the biomass load. However, the combined treatment with sulfuric acid yielded slightly higher amounts of sugars at similar biomass concentrations as compared to that obtained with sulfamic acid. The hydrolysis yield increased in both cases when the amount of biomass doubled from 21.5 g/L to 43 g/L, but slightly decreased when the biomass concentration was again doubled in the treatment with sulfamic acid. This suggests a lower accessibility of the acid and/or the enzymes to the algae at high biomass concentrations. It might be caused by inadequate agitation conditions during the hydrolysis i.e. no agitation during the hydrothermal treatment (assays carried out in the autoclave) plus insufficient agitation during the enzymatic hydrolysis.
Fig. 1.
Effect of the biomass concentration on the amount of sugars and HMF released by the combined hydrolysis with A: sulphuric acid (1 % (w/v); 30 min) and B: sulfamic acid (0.5 % (w/v); 30 min) followed by enzymatic hydrolysis.
When sulfamic acid was used, the hydrolysis of 86.4 g/L biomass yielded a hydrolysate with 24.1 g/L total sugars and a concentration of HMF below 0.01 g/L, corresponding to 29.7 % total sugar yield (g total sugars/ g algae dry weight * 100 %). In this case glucose was predominantly released. A small concentration of 0.3 g/L of galactose was released at 21.6 g/L biomass concentration and this could be explained by the higher accessibility of the acid to the biomass, causing a partial hydrolysis of agar.
Aiming at the production of larger quantities of Gelidium hydrolysates, a scale-up of the combined hydrolysis process was conducted. The extent of hydrolysis will most probably depend on the agitation that the reaction medium experiences. To keep the degree of mixing constant in orbitally shaken bioreactors, the Froude number (Fr) should be maintained constant during scaling-up [39,40]. In an attempt to obtain comparable hydrolytic yields at increasingly higher working volumes, experimental parameters leading to a constant Fr number were thus set in the scale-up assays. For the purpose, the rotation speed N (rpm) was decreased at increasing working volumes according to Eq. (4):
| (4) |
where N is the rotational speed (rpm) and d is the characteristic length scale – flask diameter. If d increases during the scale-up, N2 should correspondingly decrease to maintain Fr constant.
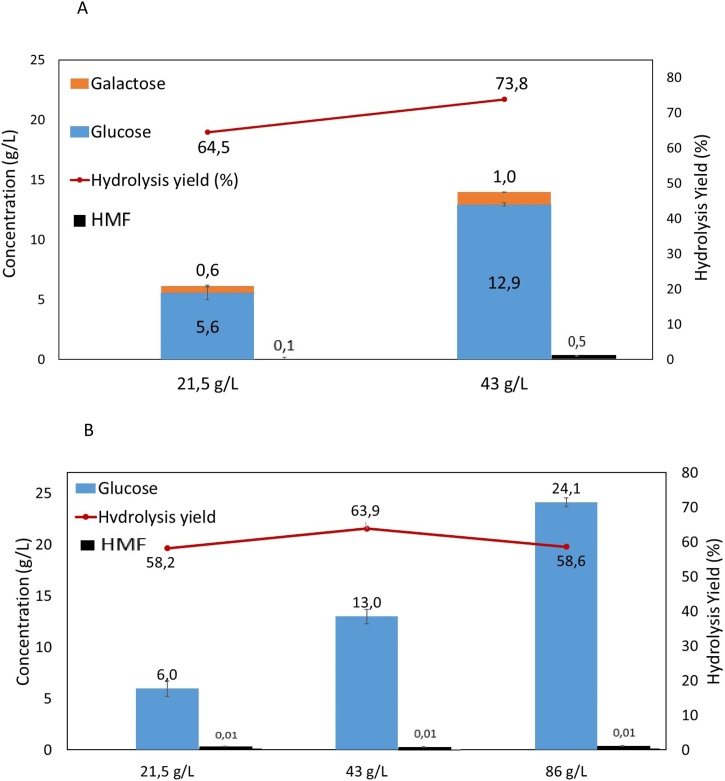
The results of the scale-up experiments are given in Fig. 2. The release of glucose obtained in the range 10 mL–700 mL was in fact approximately constant.
Fig. 2.
Scale-up of the combined hydrolysis process. Effect of the total working volume on the amount of glucose released during the combined hydrolysis with a pre-treatment with sulfamic acid (0.5 % (w/v); 30 min) followed by enzymatic hydrolysis. The biomass concentration was 86.4 g/L in all assays.
3.4. Shake flask cultivations
Halomonas boliviensis DSM15516, a halotolerant bacterium able to accumulate high P3HB amounts [21] using glucose and galactose, the monosaccharides released in the hydrolysis of Gelidium residues was selected to perform this work. The choice of a halophilic strain was based on a potentially high salt content in the algal hydrolysates caused by a high mineral content of the algal biomass itself (Table 2) and also by the salts formed upon neutralization of the biomass suspension after the acidic pre-treatment. Moreover, salt-rich media were chosen to perform the cultivations because they are less prone to contamination and thus non-strict sterile conditions can be used, leading to savings in equipment and operation costs at large scale.
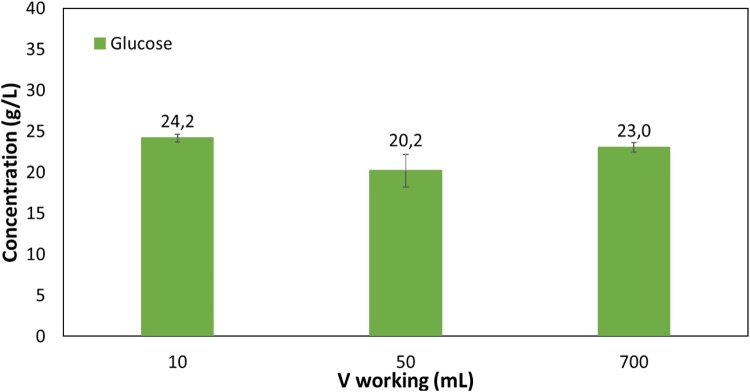
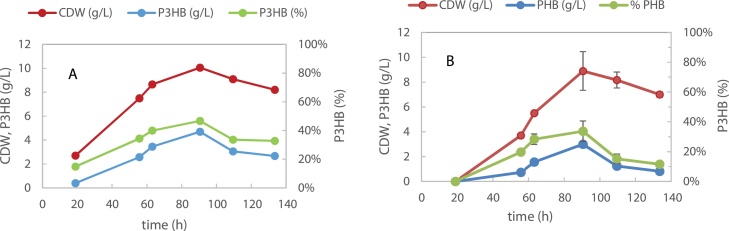
The growth and P3HB production on 20 g/L glucose or 20 g/L galactose was carried out using the production medium with 1 g/L NH4Cl and 20 g/L MSG. This medium composition was based on the one reported by Quillaguaman [25]. Maximum CDW and P3HB concentrations on glucose were attained at 90 h cultivation (Fig. 3) reaching 10.8 ± 1.5 g/L and 5.0 ± 0.2 g/L, respectively, corresponding to a 46.7 ± 6.8 % PHB cell content. These results are in accordance with previous observations in shake flasks [21] where 55 % polymer accumulation was reached. The growth and polymer production on galactose attained slightly lower results with a maximum CDW and P3HB accumulation of 8.4 ± 0.4 g/L and 35.1 ± 0.7 % P3HB, respectively, at 90 h cultivation. Both sugars were confirmed to be good substrates for P3HB production by H. boliviensis and this strain is thus a promising choice for the valorization of Gelidium hydrolysates to PHAs.
Fig. 3.
H. boliviensis growth and P3HB production on A. 20 g/L glucose; B. 20 g/L d- galactose.
3.5. Growth and production on Gelidium hydrolysates
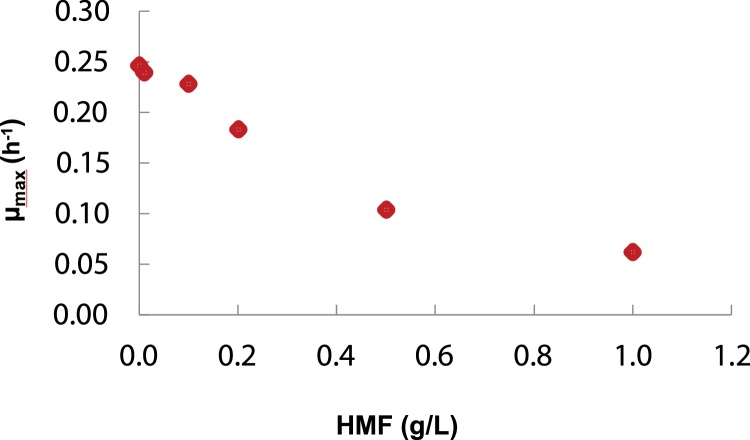
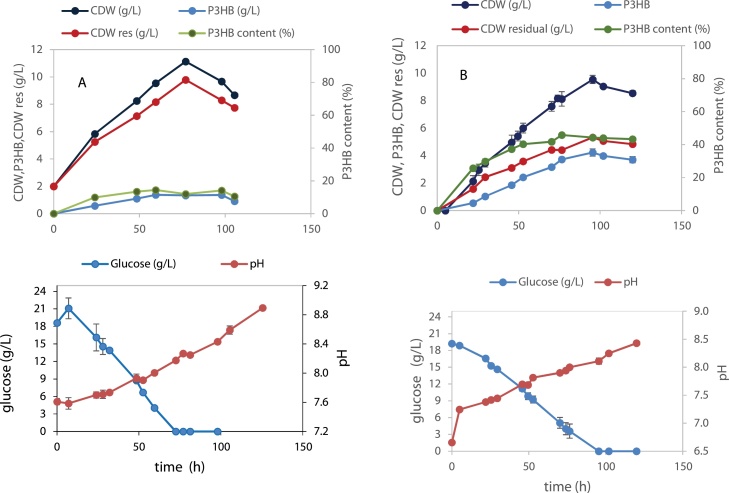
The Gelidium hydrolysates were examined as carbon source for the production of P3HB by Halomonas boliviensis in shake flask cultures. The first assays were carried out with the hydrolysate prepared with 43.2 g/L biomass using the combined treatment with sulfuric acid (1% w/v, 30 min, 121 °C) followed by enzymatic treatment. The assay was compared to a control featuring a mixture of glucose and galactose simulating the hydrolysate composition (Fig. 4). Similar biomass concentrations were attained in the control with a CDW of 10.1 g/L versus a CDW of 9.3 g/L with the hydrolysate. However the maximum concentration of polymer produced in the sugar mixture was much higher (P3HB = 4.7 g/L) compared to the hydrolysate (P3HB = 3.0 g/L), corresponding to approx. 47 % and 32 % of the P3HB cell content, respectively. Besides a lower production of polymer, a delay in cell growth and production was observed. This might be ascribed to the effect of possible inhibitors in the hydrolysate. Typical inhibitors present in seaweed hydrolysates are HMF, as well as acetic and levulinic acids [17]. The hydrolysate was tested for the presence of these inhibitors and only HMF was found. Its effect on cell growth was determined in H. boliviensis cultures containing 20 g/L initial glucose concentration and HMF in the range 0−1 g/L (Fig. 5). The results indicate that the inhibitory effect of HMF on H. boliviensis growth is significant for HMF concentrations above 0.1 g/L. At this concentration, the μmax was 92.8 % of the attained in the control with no HMF (μmax =0.25 h−1). For this reason the choice of a mild chemical pre-treatment releasing lower inhibitor titres is an asset.
Fig. 4.
H. boliviensis growth and P3HB production on A. control simulating the sugar hydrolysate composition of the hydrolysate; B. hydrolysate produced using a pre-treatment with 1% (w/v) sulfuric acid followed by enzymatic hydrolysis.
Fig. 5.
Effect of the HMF concentration on the maximum specific growth rate of H. boliviensis cultivated in production medium supplemented with 20 g/L glucose.
The hydrothermal hydrolysis with 0.5 % (w/v) sulfamic acid at 121 °C for 30 min was the chosen pre-treatment method for subsequent production of hydrolysates from the G. sesquipedale residues as, under these conditions no more than 0.01 g/L of HMF were released, even though this treatment was unable to release galactose from agar. This is acceptable for processing waste Gelidium because the amount of agar and thus of released galactose is quite low and so it might be neglected for further valorisation to P3HB. The pre-treatment step is nevertheless very important, especially at higher biomass loads (>5 % (w/v)) in order to break the gel structure of agar and ease the processing of the biomass.
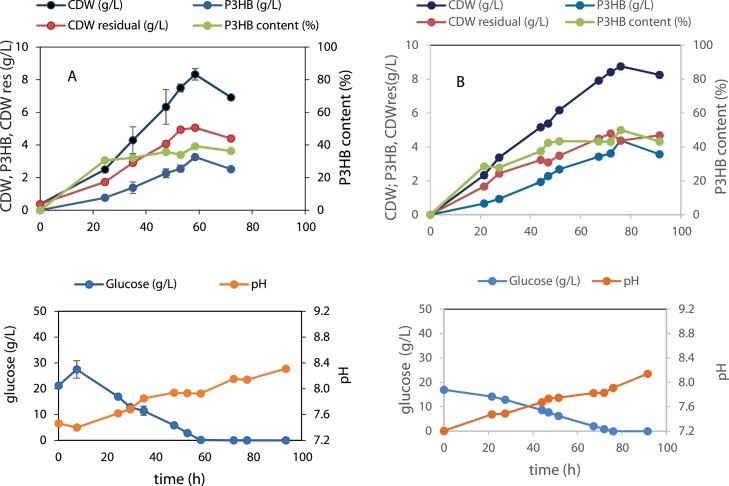
To assess the growth and P3HB production of H. boliviensis on the produced algal hydrolysate a 20-fold concentrated production medium along with magnesium and NaCl solutions (medium “A” in Table 4) was added to the algal hydrolysate to attain an initial glucose concentration of approx. 20 g/L. The results were compared to a control with the same glucose concentration. It was observed that a similar CDW of 9 g/L is attained in both conditions (Fig. 6). Concerning polymer production, the highest accumulated polymer content in H. boliviensis cells grown on algal hydrolysate was only 21 % (w/w), while this value attained 44.2 % (w/w) on the control with glucose.
Table 4.
Effect of the nitrogen content, supplemented to the cultivation media in the form of monosodium glutamate (MSG) and ammonium chloride (NH4Cl), on the biomass and polymer production. A combined hydrolysate prepared with sulfamic acid and featuring an initial glucose concentration of 20 g/L was used in all assays.
| Medium | NH4Cl (gL−1) |
MSG (gL−1) |
CDW (gL−1) |
P3HB (g L−1) |
P3HB (%) |
Y P/S (gPHB/gglu.cons) |
Prod vol max (gPHB L−1 h−1) |
|---|---|---|---|---|---|---|---|
| A | 1 | 20 | 8.6 | 1.8 | 21.3 | 0.12 | 0.028 |
| B | 1 | 0 | 4.7 | 2.7 | 56.7 | 0.11 | 0.047 |
| C | 1 | 5 | 7.1 | 3.7 | 52.2 | 0.15 | 0.057 |
| D | 1 | 10 | 8.7 | 3.5 | 40.8 | 0.16 | 0.056 |
| E | 0 | 10 | 8.3 | 3.4 | 41.1 | 0.16 | 0.056 |
Fig. 6.
Growth and P3HB production of H. boliviensis on A: Gelidium hydrolysate produced using a combined hydrolysis with 0.5 % w/v sulfamic acid for 30 min followed by enzymatic hydrolysis and B: control on glucose.
3.6. Engineering the cultivation medium based on the hydrolysate composition
The accumulation of P3HB by H. boliviensis occurs predominantly under nitrogen, phosphorous or oxygen limitation, like in many others bacterial species [41,42]. The low accumulation attained by supplementing the hydrolysate with medium “A” could be explained by an excess of nitrogen that can arise either from medium “A” or from the algal hydrolysates. To infer on the influence of the N content on the growth and P3HB cellular content, both the NH4Cl and the monosodium glutamate concentrations of the mineral medium supplemented to the hydrolysate were varied, while the other concentrations were maintained constant. The different medium compositions and the respective results attained are summarized in Table 4.
From these results it is evident the contribution of MSG to the generation of biomass in detriment of higher polymer concentrations. Medium A, the basal medium used in the shake flask assays, was clearly not the best medium to supplement the Gelidium hydrolysates when aiming at high polymer concentrations. This result might be due to the high N content of the cultivation medium resulting in a low carbon/nitrogen ratio. Some authors have described the need to have C/N ratios above 20 to obtain a significant polymer accumulation [7,43]. The composition of the hydrolysate alone was analysed (Table 5) and showed a C/N ratio of 15.1, i.e. below 20. This result indicates that the supplementation of high concentrations of an N-rich source like MSG to the cultivation medium is not beneficial to attain high polymer productions. The composition of medium C or E was considered more suitable because it generated both higher yields of product on sugar and higher polymer productivities when compared to medium A. Medium E was tested as supplement to the hydrolysate and the results compared to a control with glucose (Fig. 7). Although similar biomass concentrations were attained (approx. 8.5 g/L), higher polymer contents were observed in the control (circa 50 %) compared to the hydrolysate (circa 40 %) as already expected. Despite the lower concentration of polymer attained in the hydrolysate, the overall polymer productivities were similar (0.056 g/L.h) for both the hydrolysate and the control. The hydrolysate produced from the Gelidium residues was thus shown to be a good alternative C-source for the production of PHAs.
Table 5.
Composition of the hydrolysate prepared after a combined pre-treatment using 0.5 % (w/v) sulfamic acid at 121 °C and 30 min of 8.6 % (w/v) biomass slurry followed by an enzymatic hydrolysis with cellulolytic enzymes (50 °C; 30 h).
| Parameter | Value | Method |
|---|---|---|
| Carbon (g C /L) | 13.6 | HPLC (M&M) |
| Nitrogen Kjeldhal (g N/L) | 0.9 | M.M. 8.9 (2018-04-12) |
| Calcium (g/L) | 1.9 | ISO 11885:2007 |
| Magnesium (g/L) | 0.2 | ISO 11885:2007 |
| Phosphorous (mg P/L) | 79.0 | M.M. 4.8 (2016-05-06) |
| Sodium (mg/L) | 39.0 | ISO 11885:2007 |
Fig. 7.
H. boliviensis growth and P3HB production on Gelidium hydrolysate supplemented with medium E (A) and control on glucose (B).
4. Conclusions
The waste material resulting from the extraction of agar from Gelidium sesquipedale is an underexploited and yet valuable material still containing non-negligible amounts of carbohydrates (44.80 ± 1.90 % dw basis), of which approx. 35 % w/w is cellulose.
The present work shows that hydrolysates from Gelidium residues are suitable C-sources for P3HB production by Halomonas boliviensis. High polymer yields and productivities were attained after adjusting the composition of the cultivation media to adequate C/N ratios, pinpointing the importance of assessing the hydrolysates composition to design a suitable production medium.
The utilization of this by-product is an inspiring example of a circular economy approach with the extra-advantage of minimizing its disposal in the environment.
CRediT authorship contribution statement
S. Tůma: Visualization, Investigation. J.K. Izaguirre: Visualization, Investigation. M. Bondar: Validation. M.M. Marques: Visualization, Investigation. P. Fernandes: Supervision, Conceptualization, Writing - review & editing. M.M.R. da Fonseca: Supervision, Writing - review & editing, Project administration. M.T. Cesário: Conceptualization, Supervision, Writing - original draft, Writing - review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
This work has received funding from FCT-Fundação para a Ciência e Tecnologia under the project (PTDC/BII-BIO/29242/2017). Funding received by iBB-Institute for Bioengineering and Biosciences from FCT (grant UIDB/04565/2020) and from Programa Operacional Regional de Lisboa 2020 (Project N. 007317) is also acknowledged.
Štěpan Tůma acknowledges support from the ERASMUS Plus program (EU) and Jon Kepa Izaguirre acknowledges the Basque Government for the pre-doctoral fellowship that supported his staying at iBB-IST.
The authors would like to acknowledge IBERAGAR S.A., Portugal for kindly supplying the Gelidium residues.
References
- 1.Zinn M., Witholt B., Egli T. Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 2001;53:5–21. doi: 10.1016/S0169-409X(01)00218-6. [DOI] [PubMed] [Google Scholar]
- 2.Kunasundari B., Sudesh K. Isolation and recovery of microbial polyhydroxyalkanoates. Express Polym. Lett. 2011;5:620–634. doi: 10.3144/expresspolymlett.2011.60. [DOI] [Google Scholar]
- 3.Rosengart A., Cesário M.T., de Almeida M.C.M.D., Raposo R.S., Espert A., de Apodaca E.D., da Fonseca M.M.R. Efficient P(3HB) extraction from Burkholderia sacchari cells using non-chlorinated solvents. Biochem. Eng. J. 2015;103 doi: 10.1016/j.bej.2015.06.013. [DOI] [Google Scholar]
- 4.Koller M., Atlić A., Dias M., Reiterer A., Braunegg G. vol. 14. 2010. (Industrial Production of PHA from Waste Raw Materials). [DOI] [Google Scholar]
- 5.Lee W.S., Chua A.S.M., Yeoh H.K., Ngoh G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014;235:83–99. doi: 10.1016/j.cej.2013.09.002. [DOI] [Google Scholar]
- 6.Nikodinovic-Runic J., Guzik M., Kenny S.T., Babu R., Werker A., O’Connor K.E. 1st ed. vol. 84. Elsevier Inc.; 2013. Carbon- (Rich Wastes as Feedstocks for Biodegradable Polymer (Polyhydroxyalkanoate) Production Using Bacteria). [DOI] [PubMed] [Google Scholar]
- 7.Lopes M.S.G., Gomez J.G.C., Taciro M.K., Mendonça T.T., Silva L.F. Polyhydroxyalkanoate biosynthesis and simultaneous remotion of organic inhibitors from sugarcane bagasse hydrolysate by Burkholderia sp. J. Ind. Microbiol. Biotechnol. 2014;41:1353–1363. doi: 10.1007/s10295-014-1485-5. [DOI] [PubMed] [Google Scholar]
- 8.Cesário M.T., Raposo R.S., de Almeida M.C.M.D., van Keulen F., Ferreira B.S., da Fonseca M.M.R. Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. N. Biotechnol. 2014;31 doi: 10.1016/j.nbt.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Obruca S., Benesova P., Marsalek L., Marova I. Use of lignocellulosic materials for PHA production. Chem. Biochem. Eng. Q. 2015;29:135–144. doi: 10.15255/CABEQ.2014.2253. [DOI] [Google Scholar]
- 10.Cesário M.T.F., de Almeida M.C.M.D. Lignocellulosic hydrolysates for the production of polyhydroxyalkanoates. Microorg. Biorefineries. 2015;26:79–104. doi: 10.1007/978-3-662-45209-7. [DOI] [Google Scholar]
- 11.Tayyab M., Noman A., Islam W., Waheed S., Arafat Y., Ali F., Zaynab M., Lin S., Zhang H., Lin W. Bioethanol production from lignocellulosic biomass by environment-friendly pretreatment methods: a review. Appl. Ecol. Environ. Res. 2017 doi: 10.15666/aeer/1601. [DOI] [Google Scholar]
- 12.Mosier N., Wyman C., Dale B., Elander R., Lee Y.Y., Holtzapple M., Ladish M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005;96:673–686. doi: 10.1016/j.biortech.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Singh R., Shukla A., Tiwari S., Srivastava M. A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew. Sustain. Energy Rev. 2014;32:713–728. doi: 10.1016/j.rser.2014.01.051. [DOI] [Google Scholar]
- 14.Sadhukhan J., Gadkari S., Martinez-Hernandez E., Ng K.S., Shemfe M., Torres-Garcia E., Lynch J. Novel macroalgae (seaweed) biorefinery systems for integrated chemical, protein, salt, nutrient and mineral extractions and environmental protection by green synthesis and life cycle sustainability assessments. Green Chem. 2019;21:2635–2655. doi: 10.1039/c9gc00607a. [DOI] [Google Scholar]
- 15.Dawczynski C., Schubert R., Jahreis G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007;103:891–899. doi: 10.1016/j.foodchem.2006.09.041. [DOI] [Google Scholar]
- 16.Percival E. The polysaccharides of green, red and brown seaweeds: their basic structure, biosynthesis and function. Br. Phycol. J. 1979;14:103–117. doi: 10.1080/00071617900650121. [DOI] [Google Scholar]
- 17.Yun E.J., Kim H.T., Cho K.M., Yu S., Kim S., Choi I.G., Kim K.H. Pretreatment and saccharification of red macroalgae to produce fermentable sugars. Bioresour. Technol. 2016;199:311–318. doi: 10.1016/j.biortech.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Sudhakar M.P., Jegatheesan A., Poonam C., Perumal K., Arunkumar K. Biosaccharification and ethanol production from spent seaweed biomass using marine bacteria and yeast. Renew. Energy. 2017;105:133–139. doi: 10.1016/J.RENENE.2016.12.055. [DOI] [Google Scholar]
- 19.Tan I.S., Lee K.T. Enzymatic hydrolysis and fermentation of seaweed solid wastes for bioethanol production: an optimization study. Energy. 2014;78:53–62. doi: 10.1016/j.energy.2014.04.080. [DOI] [Google Scholar]
- 20.Armisén R., Agar Galatas F. Second Ed. 2009. Handb. Hydrocoll. pp. 82–107. [DOI] [Google Scholar]
- 21.Quillaguamán J., Delgado O., Mattiasson B., Hatti-Kaul R. Poly(β-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1. Enzyme Microb. Technol. 2006;38:148–154. doi: 10.1016/j.enzmictec.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Quillaguamán J., Hatti-Kaul R., Mattiasson B., Alvarez M.T., Delgado O. Halomonas boliviensis sp. nov., an alkalitolerant, moderate halophile isolated from soil around a Bolivian hypersaline lake. Int. J. Syst. Evol. Microbiol. 2004;54:721–725. doi: 10.1099/ijs.0.02800-0. [DOI] [PubMed] [Google Scholar]
- 23.Adney B., Baker J. 2008. Measurement of Cellulase Activities: Laboratory Analytical Procedure (LAP) Issue Date: 08/12/1996. [Google Scholar]
- 24.Ghose T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987;59:257–268. doi: 10.1351/pac198759020257. [DOI] [Google Scholar]
- 25.Quillaguamán J., Doan-Van T., Guzmán H., Guzmán D., Martín J., Everest A., Hatti-Kaul R. Poly(3-hydroxybutyrate) production by Halomonas boliviensis in fed-batch culture. Appl. Microbiol. Biotechnol. 2008;78:227–232. doi: 10.1007/s00253-007-1297-x. [DOI] [PubMed] [Google Scholar]
- 26.Guzmán D., Balderrama-Subieta A., Cardona-Ortuño C., Guevara-Martínez M., Callisaya-Quispe N., Quillaguamán J. Evolutionary patterns of carbohydrate transport and metabolism in Halomonas boliviensis as derived from its genome sequence: influences on polyester production. Aquat. Biosyst. 2012;8:9. doi: 10.1186/2046-9063-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Wychen S., Laurens L.M.L. vol. 303. 2013. (Determination of Total Solids and Ash in Algal Biomass). [Google Scholar]
- 28.Van Wychen S., Laurens L.M.L. vol. 303. 2013. (Determination of Total Carbohydrates in Algal Biomass). [Google Scholar]
- 29.Park M.R., Kim S.K., Jeong G.T. Biosugar production from Gracilaria verrucosa with sulfamic acid pretreatment and subsequent enzymatic hydrolysis. Biotechnol. Bioprocess Eng. 2018;23:302–310. doi: 10.1007/s12257-018-0090-2. [DOI] [PubMed] [Google Scholar]
- 30.Garrote G., Domínguez H., Parajó J.C. Hydrothermal processing of lignocellulosic materials. Holz Als Roh - Und Werkst. 1999;57:191–202. doi: 10.1007/s001070050039. [DOI] [Google Scholar]
- 31.Angell A.R., Mata L., de Nys R., Paul N.A. The protein content of seaweeds: a universal nitrogen-to-protein conversion factor of five. J. Appl. Phycol. 2016;28:511–524. doi: 10.1007/s10811-015-0650-1. [DOI] [Google Scholar]
- 32.McHugh D.J. 2003. Seaweeds Uses as Human Foods. https://doi.org/ISBN 92-5-104958-0. [Google Scholar]
- 33.Fleurence J., Le Coeur C., Mabeau S., Maurice M., Landrein A. Comparison of different extractive procedures for proteins from the edible seaweeds Ulva rigida and Ulva rotundata. J. Appl. Phycol. 1995;7:577–582. doi: 10.1007/BF00003945. [DOI] [Google Scholar]
- 34.Boopathy R., Bokang H., Daniels L. Biotransformation of furfural and 5-hydroxymethyl furfural by enteric bacteria. J. Ind. Microbiol. 1993;11:147–150. doi: 10.1007/BF01583715. [DOI] [Google Scholar]
- 35.Palmqvist E., Hahn-Hägerdal B. Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000 doi: 10.1016/S0960-8524(99)00161-3. [DOI] [Google Scholar]
- 36.de Vasconcelos S.M., Santos A.M.P., Rocha G.J.M., Souto-Maior A.M. Diluted phosphoric acid pretreatment for production of fermentable sugars in a sugarcane-based biorefinery. Bioresour. Technol. 2013;135:46–52. doi: 10.1016/j.biortech.2012.10.083. [DOI] [PubMed] [Google Scholar]
- 37.Kwon O.M., Kim S.K., Jeong G.T. Potential of phosphoric acid-catalyzed pretreatment and subsequent enzymatic hydrolysis for biosugar production from Gracilaria verrucosa. Bioprocess Biosyst. Eng. 2016;39:1173–1180. doi: 10.1007/s00449-016-1593-x. [DOI] [PubMed] [Google Scholar]
- 38.Montes D’Oca M.G., Soares R.M., De Moura R.R., De Freitas Granjão V. Sulfamic acid: an efficient acid catalyst for esterification of FFA. Fuel. 2012;97:884–886. doi: 10.1016/j.fuel.2012.02.038. [DOI] [Google Scholar]
- 39.Tissot S., Farhat M., Hacker D.L., Anderlei T., Kühner M., Comninellis C., Wurm F. Determination of a scale-up factor from mixing time studies in orbitally shaken bioreactors. Biochem. Eng. J. 2010;52:181–186. doi: 10.1016/j.bej.2010.08.005. [DOI] [Google Scholar]
- 40.Rodriguez G., Anderlei T., Micheletti M., Yianneskis M., Ducci A. On the measurement and scaling of mixing time in orbitally shaken bioreactors. Biochem. Eng. J. 2014;82:10–21. doi: 10.1016/j.bej.2013.10.021. [DOI] [Google Scholar]
- 41.Silva L.F., Taciro M.K., Michelin Ramos M.E., Carter J.M., Pradella J.G.C., Gomez J.G.C. Poly-3-hydroxybutyrate (P3HB) production by bacteria from xylose, glucose and sugarcane bagasse hydrolysate. J. Ind. Microbiol. Biotechnol. 2004;31:245–254. doi: 10.1007/s10295-004-0136-7. [DOI] [PubMed] [Google Scholar]
- 42.García-Torreiro M., Lu-Chau T.A., Lema J.M. Effect of nitrogen and/or oxygen concentration on poly(3-hydroxybutyrate) accumulation by Halomonas boliviensis. Bioprocess Biosyst. Eng. 2016;39:1365–1374. doi: 10.1007/s00449-016-1612-y. [DOI] [PubMed] [Google Scholar]
- 43.Singh Saharan B., Grewal A., Kumar P. Biotechnological production of polyhydroxyalkanoates: a review on trends and latest developments. Chin. J. Biol. 2014;2014:1–18. doi: 10.1155/2014/802984. [DOI] [Google Scholar]