Abstract
BACKGROUND
Despite intense research, it remains intriguing why hormonal therapies in general and progestins in particular sometimes fail in endometriosis.
OBJECTIVE AND RATIONALE
We review here the action mechanisms of progesterone receptor ligands in endometriosis, identify critical differences between the effects of progestins on normal endometrium and endometriosis and envisage pathways to escape drug resistance and improve the therapeutic response of endometriotic lesions to such treatments.
SEARCH METHODS
We performed a systematic Pubmed search covering articles published since 1958 about the use of progestins, estro-progestins and selective progesterone receptor modulators, to treat endometriosis and its related symptoms. Two reviewers screened the titles and abstracts to select articles for full-text assessment.
OUTCOMES
Progesterone receptor signalling leads to down-regulation of estrogen receptors and restrains local estradiol production through interference with aromatase and 17 beta-hydroxysteroid dehydrogenase type 1. Progestins inhibit cell proliferation, inflammation, neovascularisation and neurogenesis in endometriosis. However, progesterone receptor expression is reduced and disrupted in endometriotic lesions, with predominance of the less active isoform (PRA) over the full-length, active isoform (PRB), due to epigenetic abnormalities affecting the PGR gene transcription. Oxidative stress is another mechanism involved in progesterone resistance in endometriosis. Among the molecular targets of progesterone in the normal endometrium that resist progestin action in endometriotic cells are the nuclear transcription factor FOXO1, matrix metalloproteinases, the transmembrane gap junction protein connexin 43 and paracrine regulators of estradiol metabolism. Compared to other phenotypes, deep endometriosis appears to be more resistant to size regression upon medical treatments. Individual genetic characteristics can affect the bioavailability and pharmacodynamics of hormonal drugs used to treat endometriosis and, hence, explain part of the variability in the therapeutic response.
WIDER IMPLICATIONS
Medical treatment of endometriosis needs urgent innovation, which should start by deeper understanding of the disease core features and diverse phenotypes and idiosyncrasies, while moving from pure hormonal treatments to drug combinations or novel molecules capable of restoring the various homeostatic mechanisms disrupted by endometriotic lesions.
Keywords: progestins, endometriosis, endometrium, therapeutic failure, innovation, hormonal treatments, selective progesterone receptor modulators, new drugs
Introduction
Endometriosis-like symptoms have been alluded to in ancient medical records dating from about 4000 years ago (Nezhat et al., 2012). Chinese medicinal herbs have been prescribed to alleviate disabling pelvic pain and severe systemic symptoms related to the menstrual period since ancient times, when the pharmacological rationale for the medical treatment of dysmenorrhea was still unknown (Nezhat et al., 2012; Wieser et al., 2007). The first pathological description of endometriotic and adenomyotic lesions was made by Karl von Rokitansky in 1860 (Hudelist et al., 2009; Rokitansky, 1860), but only in the beginning of the 20th century, the morphological and clinical pictures of such diseases were better defined (Cullen, 1908; Cullen, 1920). In the twenties of the last century, John Sampson coined the name ‘endometriosis’ and proposed the ‘retrograde menstruation theory’ to explain its pathogenesis (Sampson, 1927). At that time, medical therapies for this condition were virtually non-existent and the severe pelvic symptoms were treated, even in young patients, by aggressive surgical procedures such as hysterectomy and oophorectomy (Brosens and Benagiano, 2011).
Interestingly, some of the first recommendations for endometriosis prophylaxis were early marriage and frequent childbearing (Meigs, 1953). In fact, the attempt at using hormonal therapies for symptomatic management of endometriosis, already performed occasionally, gained an impulse when the idea of inducing a state of ‘pseudo pregnancy’ came to the light (Andrews et al., 1959; Kistner, 1958). By observing the positive effect of pregnancy on endometriosis evolution, Kistner postulated that the process of decidualisation might cause necrosis and the consequent elimination of superficial ectopic implants (Kistner, 1958). Thereby, the logical reasoning for using progesterone for the clinical control of endometriosis was established.
Progestins encompass the oldest and the newest medical treatments of endometriosis. By binding to progesterone receptors (PRs), these synthetic compounds can induce anti-estrogenic, pro-apoptotic, anti-inflammatory and anti-neurogenic effects, resulting in pain relief and interruption of pathogenic mechanisms within the endometriotic lesions (Gezer and Oral, 2015). The possible routes of progestin administration include oral and vaginal preparations, intramuscular and subcutaneous injections, patches, subdermal implants and intrauterine systems. Derivates of C-21 progesterone (medroxyprogesterone acetate (MPA) and dydrogesterone) and C-19 nortestosterone (levonorgestrel, norethisterone, lynestrenol, desogestrel and dienogest) are representants of the class (Gezer and Oral, 2015). Combined estrogen–progestins were introduced as contraceptives in the 1960s. They have been widely used in patients with endometriosis since then, with good rates of patient adherence and satisfaction (Andrews, 1976; Vercellini et al., 2018b).
Preliminary studies published in 1971 indicated the use of danazol as a therapeutic possibility for endometriosis, mainly due to its alleged anti-gonadotropic properties. At that time, no direct action mechanism of danazol had been demonstrated in endometriotic implants, but some progestational-like changes induced by this substance were observed in the endometrium of estrogen-primed animals (Greenblatt et al., 1971). Later, despite being effective against endometriosis symptoms, its inconvenient androgenic side effects curbed the clinical applicability of danazol (Brosens and Benagiano, 2011). Subsequent initiatives of danazol administration by vaginal or intrauterine routes resulted in much improved tolerance while keeping the therapeutic effects (Cobellis et al., 2004; Igarashi et al., 1998; Razzi et al., 2007a), but the drug never regained its former popularity.
Progestins, either alone or conjugated with estrogens, continue to be successfully indicated to treat endometriosis (Vercellini et al., 2016b). However, some patients have only partial improvement or do not respond to this therapy at all (Barra et al., 2019). In addition, progestins and combined hormonal contraceptives do not eliminate endometriotic lesions, but induce their quiescence at best (Liang et al., 2018). Estrogen-suppressive therapies, such as gonadotropin-releasing hormone (GnRH) agonists and antagonists or aromatase inhibitors, are also far from being a panacea and are still expensive and have bothersome side effects (Brown et al., 2010). Despite intense research, it remains intriguing why hormonal therapies sometimes fail in endometriosis. Thus, the aim of this review is to evaluate the action mechanisms of PR ligands in endometriosis, to identify critical differences between the effects of progestins on normal endometrium and endometriosis and to envisage pathways to overcome the therapeutic failures.
Methods
In this narrative review, we performed a historical bibliographic search for references to the medical treatment of endometriosis and a systematic Pubmed search covering articles in any language published since 1958 with the following terms: (‘Endometriosis’[Mesh] OR ‘Endometrium’[Mesh]) AND (‘Progestins’[Mesh] OR ‘Medroxyprogesterone Acetate’[Mesh] OR ‘Norethindrone’[Mesh] OR ‘Desogestrel’[Mesh] OR ‘dienogest’ [Supplementary Concept] OR ‘Dydrogesterone’[Mesh] OR ‘Levonorgestrel’[Mesh] OR ‘Mifepristone’[Mesh] OR ‘ulipristal acetate’ [Supplementary Concept] OR vilaprisan [Supplementary Concept]). Two reviewers screened the titles and abstracts to select articles for full-text assessment. We also searched clinicaltrials.gov and EudraCT for study protocols on hormonal treatments of endometriosis with the status of ‘not yet recruiting’, ‘recruiting’, ‘active, not recruiting’ or recently (until December 2017) ‘completed’ or ‘terminated’.
Progesterone Receptors in Human Endometrium and Endometriosis
The progestational effects of natural progesterone and synthetic progestins are mediated by three categories of specific receptors: the classical nuclear PRs that include subtypes A and B (Hill et al., 2012), a mitochondrial isoform (PR-M) also derived from the same PGR gene (Price and Dai, 2015), and the cell membrane receptors that comprise progesterone receptor membrane components (PGRMC) and membrane receptors (mRPs) belonging to the progestin and adipoQ (PAQR) protein family (Gerdes et al., 1998; Kowalik et al., 2013). In addition, progesterone and progestins may have cross effects on glucocorticoid, mineralocorticoid and androgen receptors (Stanczyk et al., 2013).
Human endometrium expresses both PR isoforms during all phases of the menstrual cycle, but the total amount of receptors and the ratio between PRA and PRB change in response to variations in circulating ovarian steroids, with an overall predominance of PRA over PRB (Bedaiwy et al., 2015; Mote et al., 1999). The receptors are more abundant in the glandular epithelium than in the endometrial stroma and the maximal expression of PRA and PRB is seen at mid-cycle, followed by a gradual decrease that reaches a nadir at the late secretory phase (Fig. 1). In the secretory phase, stromal PR localises specifically in cells with morphological features of decidualisation (Mote et al., 1999), a critical mechanism of endometrial differentiation that ultimately concurs to allow embryo implantation (Gellersen and Brosens, 2014; Wu et al., 2018). Furthermore, stromal PR activation elicits paracrine mechanisms that limit epithelial proliferation (Kim et al., 2013; Wu et al., 2018). Splice variants of the PGR transcript have been found in human endometrium (Yamanaka et al., 2002) and a putative truncated isoform (PRC) was detected in term gestation decidua (Goldman and Shalev, 2007), but their functional significance remains elusive.
Figure 1.
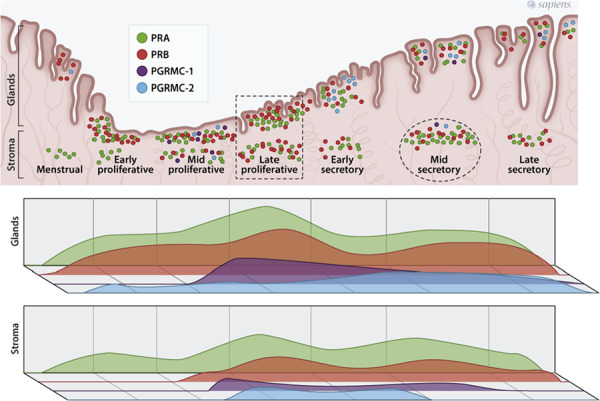
Schematic representation of nuclear progesterone receptors A (PRA) and B (PRB) and membrane receptors PGRMC-1 and PGRMC-2 during the menstrual cycle in human endometrium. The dot density indicates the abundance of the represented molecule at each cycle phase and tissue compartment (glands or stroma), according to immunolocalisation (Bedaiwy et al., 2015; Keator et al., 2012; Mote et al., 1999), western blotting (Bedaiwy et al., 2015), real-time PCR and in situ hybridisation (Keator et al., 2012). Note, the maximal expression of PRA and PRB in the late proliferative phase (dotted rectangle) and the transient increase in stromal PRA expression at mid-secretory phase (dotted circle).
PGRMC-1 transcript (Kao et al., 2002) and protein (Chen et al., 2009) are abundant in human proliferative endometrium with down-regulation in the mid-secretory phase. Conversely, a detailed study in non-human primate showed that PGRMC-2 expression increases in the mid-secretory phase, especially in the glandular epithelium of the functionalis zone (Keator et al., 2012). Once activated by the extracellular ligand, PGRMC-1 recruits the intracellular serpine mRNA-binding protein 1 and the complex ligand-receptor-binding protein activates protein kinase G to reduce Ca2+ levels in the cytosol (Kowalik et al., 2013). PGRMC-1 mediates progesterone effects on cholesterol metabolism, steroidogenesis and apoptosis in ovarian cells in vitro, and these effects have been hypothesised to contribute to progesterone actions on endometrial glands (Keator et al., 2012; Kowalik et al., 2013).
When it comes to endometriosis (Table I), studies that have evaluated superficial peritoneal lesions or ovarian endometriomas indicate that PR expression looses the menstrual cyclic pattern (Beliard et al., 2004), PRB levels are low (Bedaiwy et al., 2015; Beliard et al., 2004; Eaton et al., 2013; Hayashi et al., 2012; Wu et al., 2006), sometimes even undetectable (Attia et al., 2000), and PRA is by far the predominant PR isoform expressed within lesions (Attia et al., 2000; Bedaiwy et al., 2015; Bono et al., 2014). The PRB deficiency is much more evident in the lesions than in the eutopic endometrium of women with endometriosis (Attia et al., 2000; Bedaiwy et al., 2015; Wu et al., 2006). Deep rectal lesions express PR isoforms both at glands and stroma (Vinci et al., 2016; Zanatta et al., 2015), but, when compared to normal endometrium, PRB immunoreactivity is suppressed in DIE and even more in OMA (Liu et al., 2018) (Table I). The predominance of PRA over PRB in endometriosis is explained by the hypermethylation of PGR at the promoter region that starts the full gene transcription and generates PRB, while the downstream promoter region associated with PRA transcription remains unmethylated (Rocha-Junior et al., 2019; Wu et al., 2006). The mechanism responsible for PRB promoter methylation in endometriosis is probably the chronic inflammatory environment with increased release of proinflammatory cytokines (Wu et al., 2008). Other epigenetic mechanisms such as aberrantly expressed micro-RNAs can limit PGR transcription in endometriosis (McKinnon et al., 2018).
Table I.
Summary of studies that have evaluated progesterone receptor expression and localisation in endometriotic lesions.
| Reference | Sample | Phenotype | Findings |
|---|---|---|---|
| Bedaiwy et al., 2015 | C, E, L | SUP, OMA | PRA (Western blot): C < E > OMA > SUP PRB (Western blot): C = E > OMA > SUP |
| Beliard et al., 2004 | E, L | SUP | PRA + PRB (immunohistochemistry): C = E > L Down-regulation in the secretory phase in C and E, but not in L |
| Bono et al., 2014 | L | OMA | PRA + PRB (immunohistochemistry): detected in 10/10 samples PRB (immunohistochemistry): detected in 6/10 samples |
| Eaton et al., 2013 | C, L (stromal cells) | OMA | PRA (western blot): C > L PRB (western blot): C > L |
| Hayashi et al., 2012 | C, L | OMA | PRA (immunohistochemistry): C = L PRB (immunohistochemistry): C > L |
| Wu et al., 2006 | C, E, L | SUP, OMA | PRB mRNA (real-time PCR): C > E > L Hypermethylation or PGR at the PRB promoter region in L |
| Attia et al., 2000 | E, L | ‘extra-ovarian’ | PRA (western blot): E > L PRB (western blot): only E |
| Beranič and Rižner, 2012 | L (Z-12 cell line) | SUP | Expressed PRB mRNA (real time PCR) and protein (western blot) |
| Vinci et al., 2016 | L | DIE | PRA + PRB (immunohistochemistry): detected in 34/112 samples |
| Zanatta et al., 2015 | L | DIE | PRA + PRB (immunohistochemistry): detected in 17/18 samples PRB (immunohistochemistry): detected in 17/18 samples |
| Liu et al., 2018 | C, L | OMA, DIE | PRB (Immunohistochemistry): C>DIE>OMA |
C: control endometrium from healthy women; E: eutopic endometrium from women with endometriosis; L: endometriotic lesion; SUP: superficial peritoneal endometriosis; OMA: ovarian endometrioma; DIE: deep infiltrating endometriosis; PRA: progesterone receptor A; PRB: progesterone receptor B; PGR: progesterone receptor gene
Studies in a human myometrial cell line suggest that the balance between PRA and PRB isoforms controls the myometrial responsiveness to proinflammatory stimuli, and that an increased PRA:PRB ratio shifts the pregnant uterus from a quiescent to an active state at late gestation (Amini et al., 2016; Chen et al., 2017; Patel et al., 2018). In myometrial cells, progesterone represses interleukin (IL)-1β-induced proinflammatory genes through co-repressor molecules recruited by the PR DNA-binding domain in cooperation with the amino-terminal region that is unique to PRB (Chen et al., 2017). An increased abundance of PRA also represses PRB-mediated transcriptional activity (Amini et al., 2016; Patel et al., 2018). By analogy, one may hypothesise that the relative increase of PRA over PRB in endometriosis contributes to tissue inflammation. However, the evidence available thus far suggests that both PR isoforms mediate the anti-inflammatory actions of progesterone in the endometrium (Patel et al., 2015). More importantly, in immortalised human endometriotic epithelial cell lines expressing either PRA or PRB, dienogest inhibited the transcription of multiple pro-inflammatory genes regardless of the receptor isoform expressed by the cell (Ichioka et al., 2015). Thus, it appears that in endometriosis the PR imbalance toward PRA does not provoke the local inflammatory response.
In summary, endometriosis is characterised by down-regulation of PR in general and PRB in particular through epigenetic inhibition (promoter hypermethylation) of upstream PGR transcription by local inflammatory mediators. Endometriotic lesions also lack the menstrual cycle variation of PR distribution that characterises the normal endometrium. As we shall discuss later, PRB down-regulation is an important but not the only mechanism to explain the refractoriness of some endometriotic lesions to progestin therapy.
Therapeutic Effects of Progesterone and Progestins in Human Endometrium and Endometriosis
Progestins are synthetic compounds that mimic the effects of progesterone (Gezer and Oral, 2015). The features that all progestins share are the ability to bind PR and induce the secretory transformation of estrogen-primed uterine endometrium, the so-called progestogenic effect. Steroids with progestational activity have differences in their chemical structures that affect their profile and potency of action on hypothalamic–pituitary axis, reproductive and breast tissues and metabolic processes (Gezer and Oral, 2015). The final progestogenic activity of any substance depends also on the route, the timing and the dose administered (de Lignieres et al., 1995; Rommler et al., 1985). Progestins reduce the frequency and increase the amplitude of pulsatile GnRH release, resulting in a reduction in follicle-stimulating hormone (FSH) and luteinising hormone (LH) secretion. As a result, continuous use of progestins leads to the suppression of ovarian steroidogenesis with anovulation and low serum levels of ovarian steroids (Horne and Critchley, 2011) (Fig. 2).
Figure 2.
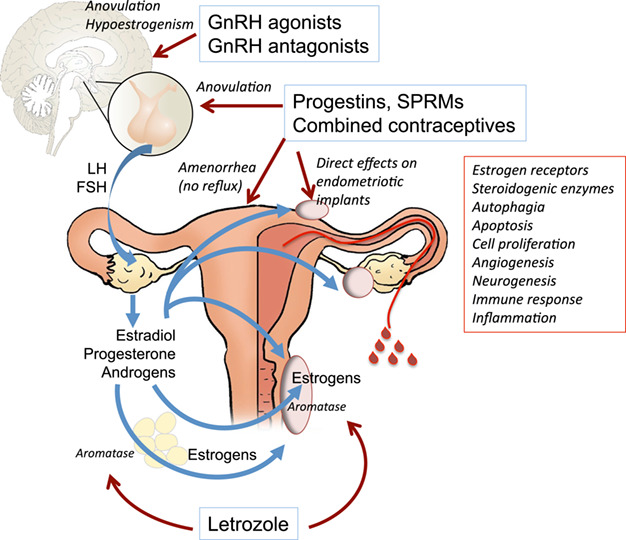
Therapeutic mechanisms of hormonal treatments for endometriosis. Progestins, selective progesterone receptor modulators (SPRMs) and combined contraceptives block ovulation through central inhibition of gonadotropin release, induce amenorrhea which prevents repeated menstrual reflux and have many direct effects on endometriotic implants. Gonadotropin-releasing hormone (GnRH) agonists and antagonists block ovulation and induce hypoestrogenism, while aromatase inhibitors such as letrozole reduce androgen aromatisation into estrogens, both in the adipose tissue and within endometriotic lesions.
The long-standing hypoestrogenic and hypergestagenic state supports the use of progestins for treating endometriosis. In fact, the condition induced by these compounds causes decidual transformation of the eutopic endometrium and the same effect is observed to some extent in ectopic lesions (Vercellini et al., 2016b). The use and effectiveness of progestins for treating endometriosis is not just related to their growth inhibiting actions, but also to their induction of anovulation, inhibition of blood vessel growth and anti-inflammatory properties, creating a steady hypoestrogenic and progestogenic milieu for disease quiescence (Vercellini et al., 2008).
Effect on hypothalamic–pituitary–ovary axis
Hormonal therapies for endometriosis inhibit gonadotropin release, reduce ovarian steroid secretion and can induce amenorrhea, thereby preventing repeated endometrial reflux (Fig. 2). Combined oral contraceptives (COCs) inhibit the pituitary production and secretion of both FSH and LH (Mishell et al., 1977; Vandenberg et al., 1974). Inhibition of pituitary gonadotropins by progestins takes place at the hypothalamus, blocking the normal production of GnRH (Kastin et al., 1972), and also at the pituitary level, as shown by lower pituitary secretion of FSH and LH in response to the administration of GnRH (Robyn et al., 1974).
It has been claimed that androgenic effects contribute to the anti-gonadotropic effects of progestins (Bullock and Bardin, 1977; Neumann, 1978). However, some 17-hydroxyprogesterone derivatives, such as MPA or cyproterone acetate, which have either synandrogenic or anti-androgenic action, also inhibit gonadotropin secretion (Bullock and Bardin, 1977; Couzinet et al., 1986). In addition, the 19-norprogesterone derivative without androgenic activity, nomegestrol acetate (NOMAC), has a similar anti-gonadotropic activity compared to the 19-nortestosterone derivative with androgenic activity, norethisterone acetate (NETA) (Couzinet et al., 1996).
Danazol has anti-gonadotropic activities, and daily oral administration of 400 mg of danazol inhibits both ovulation and LH surge (Barbieri and Ryan, 1981; Lauersen and Wilson, 1977; Wood et al., 1975). However, ovulation is not inhibited by daily vaginal administration due to the lower serum concentration of danazol reached by this route (Mizutani et al., 1995; Razzi et al., 2007a). Regarding intrauterine progestin administration, i.e., levonorgestrel (LNG)-releasing intrauterine system (LNG-IUS), plasma FSH concentrations were found below the normal upper limit of luteal phase concentrations, plasma LH secretion was normally pulsatile and preovulatory LH peaks were seen. Therefore, it seems that pituitary function remained essentially unchanged (Nilsson et al., 1980). Higher percentages of ovulatory cycles were found after 6 years of use, and no case of complete suppression of ovulation was found (Xiao et al., 1995).
Effects on estrogen receptors and estrogen synthesis
Perhaps the most remarkable therapeutic mechanisms of progestins in endometriosis derive from the fact that PR signalling leads to down-regulation of estrogen receptors (ERs). This effect has been clearly demonstrated not only in the eutopic endometrium but also in the glands and stroma of endometriotic lesions following in vivo treatment with different progestational formulations (Brichant et al., 2018; Engemise et al., 2011; Gomes et al., 2009).
Another front of anti-estrogen action of progestins is the restraint of local estradiol production through interference with certain enzymes. As shown by experimental evidence, dienogest inhibits aromataseexpression and, consequently, local estrogen production in immortalised human endometrial epithelial cells (Shimizu et al., 2011). Similarly, a decreased aromatase transcription was reported after MPA and dydrogesterone administration to nude mice implanted with human endometrial fragments (Fechner et al., 2007). Furthermore, in the Z-12 epithelial cell line of peritoneal endometriosis, different types of progestin, like MPA, dydrogesterone and dienogest, were able to inhibit the expression of 17 beta-hydroxysteroid dehydrogenase (17β-HSD) type 1, the enzyme that catalyses the reduction of estrone to estradiol (Beranič and Rižner, 2012; Mori et al., 2015). Similarly, they upregulate the expression of the oxidative 17β-HSD type 2, which inactivates estradiol (Beranič and Rižner, 2012).
However, some progestins derived from 19-nor-testosterone such as norethisterone and gestodene are metabolised into non-phenolic A-ring-reduced compounds that display estrogenic activity mediated by ERα (Larrea et al., 2001).
Effects on tissue morphology, growth, vascularisation and regeneration
Endometrial tissues undergo physiological continuous changes resulting from endogenous ovarian hormonal secretion. The use of exogenous steroids with estrogenic, androgenic and progestogenic activity induces a spectrum of histologic changes in endometrial glandular and stromal architecture, blood vessels and cytology (Dinh et al., 2015). These changes are observed not only in eutopic endometrium but also in endometriosis. The direct effects of progestins include the attenuation of the inflammatory state and the creation of a pseudo-pregnancy condition with increased apoptosis and atrophy of the endometriotic implants (Liang et al., 2018; Mabrouk et al., 2018). At the beginning of treatment, progestins induce secretory differentiation, then, following the down-regulation of the ERs after several cycles, the endometrium appears atrophic with simple tubular glands, weak secretory vacuolation and low cellular density at the stroma. There are no consistent histologic features that allow differentiation between the endometrial and vascular effects of different progestins. However, depot MPA and progestin implants early on induce decidualisation and then atrophy of both glands and stroma (Dinh et al., 2015).
During treatment with dydrogesterone in endometriosis, the ectopic tissue undergoes different changes, including decidualisation, undifferentiation or involution (Cornillie et al., 1987). Histological evaluation after a short term treatment of endometriosis with gestrinone or danazol showed a degree of cellular inactivation and degeneration of the endometriotic implants (Brosens et al., 1987). In endometriomas, dienogest induces a low frequency of proliferative cells and a high rate of apoptotic cells (Miyashita et al., 2014). A recent study evaluated the histopathological effect of short term dienogest treatment in endometriotic tissue in vivo, showing a high frequency of decidualisation and a tendency to a reduced inflammation. No differences were shown in terms of necrosis, glandular atrophy and angiogenesis between medically treated and untreated lesions (Mabrouk et al., 2018).
In vitro, dienogest induces a dose-dependent inhibition of human endometrial stromal cell (hESC) proliferation together with morphological and functional changes, including the production of prolactin, a typical marker of decidualisation (Okada et al., 2001). Furthermore, dienogest treatment of endometriotic cells suppresses protein kinase B (AKT) and extracellular signal-regulated kinase (ERK)1/2 activity, thereby inhibiting mammalian target of rapamycin (mTOR), inducing autophagy, and promoting apoptosis (Choi et al., 2015). Also, NETA showed a direct anti-proliferative effect on endometriotic stromal cells in vitro without apparent cytotoxicity, as assessed by lactate dehydrogenase release, while inducing apoptosis through increased caspase 3/7 activity (Minami et al., 2013). The role of progestins in inhibiting cell proliferation, inflammation and neovascularisation in endometriosis has been demonstrated by the lack of lesion expansion, the maintenance of ERα and PR and the attenuation in Ki67 (cell proliferation marker), CD31 (neovascularisation marker), pro-inflammatory cytokine expression and macrophage infiltration, in experimental endometriosis treated with progesterone (Li et al., 2016). Furthermore, progestins reduce nerve fibre density and nerve growth factor signalling in peritoneal endometriotic lesions (Tokushige et al., 2009) and in cultured endometriotic stromal cells (Makabe et al., 2017).
Effects on local immune response
Endometriosis is associated with changes in both cell-mediated and humoral immunity (Riccio et al., 2018). The peritoneal fluid of women with endometriosis contains a number of immune cells secreting several cytokines and growth factors, which play a role in the development and maintenance of endometriotic implants.
Progesterone, dienogest and danazol attenuate the expression of IL-8 by reducing tumour necrosis factor (TNF)-induced nuclear factor-kappa B (NF-κB) activation in endometriotic stromal cells (Horie et al., 2005; Lazzeri et al., 2010). Notably, dienogest reverses some alterations of the immune system in endometriosis, as it increases the activity of natural killer (NK) cells in the peritoneal fluid and in the spleen, decreases the number of peritoneal fluid cells, decreases IL-1β production by peritoneal macrophages (Katsuki et al., 1998) and inhibits IL-1β-induced CCL20 release by an endometriotic epithelial cell line (Mita et al., 2017). Also, danazol may interfere with the immune system involvement in endometriosis (Hill et al., 1987). The drug inhibits spontaneous peripheral blood lymphocyte-mediated cytotoxicity toward endometrial stromal cells (Vigano et al., 1994) and suppresses spontaneous and activated NK cell cytotoxicity in vitro (Vigano et al., 1992). Danazol treatment counteracts peripheral blood monocyte-mediated enhancement of endometrial cell proliferation. There are also direct effects of danazol on endometrial cells, suggesting that this drug affects both monocyte-derived growth-stimulating factors and endometrial cell response to these factors (Braun et al., 1994). Moreover, danazol suppresses the production of IL-1β by human monocytes in vitro (Mori et al., 1990) and, coherently with this mechanism, danazol administration to women with endometriosis results in a decrease in serum IL-6 and IL-1α levels (Koumantakis et al., 1994).
In short, the therapeutic effects of progestins, COCs and other hormonal drugs used in endometriosis include inhibition of estrogen synthesis and action through down-regulation of steroidogenic enzymes and ER, inhibition of endometriotic cell survival and proliferation, limitation of local angiogenesis and neurogenesis and, linking all these mechanisms, attenuation of the immune-inflammatory response (Fig. 2). Nonetheless, experimental studies comparing directly endometrial and endometriotic tissues show a net difference in their response to such drugs.
Differential effects of progesterone and progestins between normal endometrium, eutopic endometrium of females with endometriosis and endometriotic lesions
In animal models
In spite of the limitations of murine models of endometriosis to emulate the human disease, they help to understand the morphological and functional changes induced by hormonal treatments in endometriotic implants. In nude mice transplanted subcutaneously with human endometrial fragments, treatment with depot MPA induced low or atrophic surface epithelium, decidual reaction and either narrow or dilated glands. In the same mouse model, danazol treatment induced dilated glands and a medium to the high epithelium, although with variable responses within the treatment group. Interestingly, this study suggested that an intact stroma is necessary for the glandular epithelium of the endometriotic tissue to respond to hormonal treatments (Bergqvist et al., 1985). In another study with human endometrial grafting into the peritoneal cavity of nude mice, MPA administration in a dose that caused 50% reduction of uterine weight was unable to cause regression of endometriotic lesions induced with endometrial fragments of normal volunteers or women with endometriosis (Palmer et al., 2016). Using the same experimental model, the subcutaneous administration of slow-release progesterone over 2 weeks reduced the number and size of endometriotic lesions in nude mice injected with normal human endometrium, but the same treatment was ineffective when the animals had been implanted with endometrial fragments from women with endometriosis (Bruner-Tran et al., 2006).
For obvious reasons, the effects of hormonal treatments on heterotopic endometrial xenografts cannot be compared with the effects of the same treatments on the eutopic endometrium of the human donors or of the mouse host. However, in the model of autologous uterine tissue transplantation, the effects of hormonal treatments may vary considerably between the eutopic endometrium and the endometriotic lesions. For example, in rats autografted with uterine tissue in the inguinal region, treatment with MPA resulted in significant atrophy of the ectopic implants but not of the eutopic uterine horn (Pereira et al., 2015). In rats autotransplanted with uterine sections into the parietal peritoneum and mesenteric vessels, the antiprogestin onapristone induced severe atrophic changes in luminal and glandular epithelium of ectopic implants and, conversely, induced epithelial cell proliferation and hypertrophy in the eutopic endometrium (Stoeckemann et al., 1995). However, bazedoxifene administration reduced gland size and number in mouse endometrium as well as in autologous endometriotic lesions (Kulak Jr et al., 2011).
The ability of endometriosis to respond to hormonal treatments is better understood as a dynamic rather than a static phenomenon, experimental evidence suggests. The mouse model of peritoneal transplantation of uterine cell suspension from syngeneic donors revealed that progesterone treatment, if initiated 4 days before transplantation, effectively inhibits the establishment and growth of endometriotic lesions through anti-proliferative, anti-angiogenic and anti-inflammatory mechanisms. In contrast, when progesterone treatment starts later, i.e. 4 days after transplantation, the local expression of PR and its target genes is markedly reduced and the treatment effects are not seen anymore (Li et al., 2016).
In cultured human cells and tissues
Primary cultures of hESCs and epithelial cells provide an insightful model to understand the differential effects of hormonal treatments between normal endometrium, eutopic endometrium in the presence of endometriosis and endometriotic lesions. With this approach, our group found out that both stromal and epithelial cells from endometriotic lesions produce aberrantly high amounts of reactive oxygen species (ROS) such as hydrogen peroxide and, unlike normal endometrial cells, endometriotic cells fail to reduce their hydrogen peroxide production in response to danazol (Ngô et al., 2009). Another study observed that an eosinophil-attracting chemokine, CCL11, was produced by epithelial cells isolated from endometriosis when stimulated with estradiol, MPA and pro-inflammatory cytokines, but not in the absence of inflammatory stimulus, whereas normal endometrial cells did not release CCL11 in vitro regardless of hormonal and/or inflammatory stimuli (Hornung et al., 2000).
One important mechanism activated by progesterone that opposes estrogen and limits endometrial growth is the induction of phosphatase and tensin homolog deleted from chromosome 10 (PTEN), a tumour suppressor that promotes autophagy and apoptosis in normal endometrial stromal cells by inhibiting AKT and mTOR activation (Choi et al., 2017). The normal endometrium increases PTEN expression and decreases AKT phosphorylation upon progesterone exposure in vivo or in vitro. However, stromal cells derived from endometriotic cyst do not increase PTEN expression when exposed to progesterone in vitro, and eutopic endometrium from women with endometriosis lacks PTEN increase in the secretory phase of the menstrual cycle (Choi et al., 2017). Consequently, the AKT/mTOR pathway remains active during the whole cycle in the eutopic endometrium and is not inhibited by progesterone treatment in endometriotic lesions, allowing abnormal survival of endometriotic stromal cells.
As summarised in Fig. 3, a number of morphological and functional changes that follow progestin or progesterone stimulation of normal endometrial cells are not seen when the same stimulus is applied to endometriotic cells or eutopic endometrial cells from women with endometriosis (Aoyagi et al., 2017; Bruner-Tran et al., 2002; Cheng et al., 2007; Novembri et al., 2011; Sultana et al., 2017; Tsuno et al., 2009; Yu et al., 2014). Among the molecular targets in normal endometrium that resist progestin or progesterone action in endometriotic cells are the nuclear transcription factor FOXO1, whose deficiency impairs stromal cell decidualisation (Yin et al., 2012), matrix metalloproteinases (Bruner-Tran et al., 2002), the transmembrane gap junction protein connexin 43 (Yu et al., 2014) and paracrine regulators of estradiol metabolism (Cheng et al., 2007). These in vitro experiments provide useful hypotheses to explain why hormonal treatments may not affect endometriotic lesions as expected, resulting in therapeutic failure.
Figure 3.
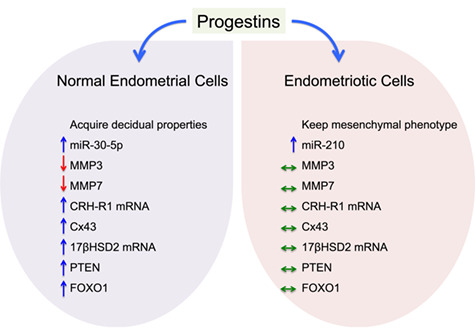
Effects of progestin treatment in vitro on normal endometrial cells compared to endometriotic cells cultured in parallel and under the same conditions. The arrows indicate that progestin treatment stimulates (↑), inhibits (↓) or has no effect (⇔) on the target gene, RNA or protein.
In human studies in vivo
Clinical studies evaluating human endometriotic lesions before and after medical treatments are rare, as current practices recommend avoiding multiple surgeries. Nevertheless, two independent clinical trials obtained samples of both eutopic endometrium and endometriotic lesions before and 6 months after the insertion of LNG-IUS in symptomatic women with endometriosis (Engemise et al., 2011; Gomes et al., 2009). Although none had a control group without endometriosis or a placebo group, these studies consistently showed that 6 months of LNG-IUS use resulted in decreased expression of estrogen and progesterone receptors, decreased cell proliferation and increased expression of an apoptosis marker in both eutopic endometrium and endometriosis. These results suggest that levonorgestrel treatment delivered into the uterus is able to induce progestin-like effects in pelvic endometriosis that are surprisingly similar to the effects induced in the eutopic endometrium that is in close contact with the drug releasing system.
Why Do Progestins Sometimes Fail in Endometriosis?
Characteristics of the type of hormone
All progestins used to treat endometriosis have been labelled for contraception and/or menopausal hormone therapy in doses and regimens sufficient to induce endometrial decidualisation and prevent endometrial hyperplasia. Direct comparisons between different types of progestins is rare in endometriosis clinical studies, with the scarce evidence suggesting that when given by the same route and the same regimen, the effectiveness may be similar. This is the case, for example, of an observational study comparing NETA versus dienogest used sequentially by the same patients, each drug for 6 months, resulting in the same rates of symptom control, health-related quality of life and overall satisfaction with treatment (Vercellini et al., 2016a). Nevertheless, it is largely unknown whether they are all equally capable of acting on endometriosis lesions to induce apoptosis or to inhibit cell proliferation, adhesion, invasiveness, angiogenesis and inflammation. In vitro studies may give some clues to this question, by comparing face-to-face the effects of different progestins on primary cell or tissue cultures derived from endometriosis.
In endometrial explants of women with endometriosis, chlormadinone acetate consistently inhibited cyclooxygenase-2 synthesis and prostaglandin F2α release, contrasting to a weaker effect of dienogest and drospirenone (Roth et al., 2019). In isolated endometrial stromal cells challenged with the proinflammatory cytokine TNF, incubation with high concentrations (10−5 M) of MPA failed to inhibit the in vitro secretion of the chemokine CCL2, while equimolar doses of dienogest or NETA were sufficient to block CCL2 release by these cells (Grandi et al., 2016). The same study, however, showed that MPA was capable of inhibiting IL-6 secretion by endometrial stromal cells despite a paradoxical increase of IL-6 mRNA transcription (Grandi et al., 2016). Still in hESCs, dienogest inhibited the in-vitro transcription of the growth factor midkine, whereas NETA and MPA did not (Nirgianakis et al., 2016).
Another reason for therapeutic failure may be the concomitant administration of an estrogen with the progestin. Face-to-face comparisons between hormonal treatments with and without estrogen have been done in randomised controlled trials (RCTs) or patient preference trials and have not shown meaningful differences between groups in terms of pain relief (Cheewadhanaraks et al., 2012; Leone Roberti Maggiore et al., 2014; Morotti et al., 2014; Razzi et al., 2007b; Vercellini et al., 2016b; Vercellini et al., 2002; Vercellini et al., 2005). However, in general, the trial participants receiving progestin treatments are more satisfied than those treated with combined hormonal therapies (Leone Roberti Maggiore et al., 2014; Morotti et al., 2014; Scala et al., 2018; Vercellini et al., 2002; Vercellini et al., 2005). In fact, for most women who do not respond to COC, changing to NETA may be a successful strategy to treat endometriosis pain and improve general well-being (Vercellini et al., 2018c).
Casper (2017) provides an empirical framework to explain why combined hormonal contraceptives may be less effective than isolated progestins to alleviate endometriosis symptoms and probably ineffective to inhibit endometriosis progression. Briefly, endometriotic lesions have endogenous estradiol production, reduced estradiol inactivation and progesterone insensitivity, while combined hormonal contraceptives have supraphysiological doses of estrogen, creating an imbalance in favour of estrogen action in the endometriotic implants (Casper, 2017). On the other hand, estrogen priming may be necessary to induce PR in endometriotic lesions (Bono et al., 2014).
It may be reasonable to avoid estrogen administration and even to inhibit estrogen synthesis to achieve maximal suppression of endometriotic implants. The later strategy has long been pursued with pituitary gonadotropin blockade by GnRH analogues (extensively reviewed by Brown et al. (2010)) and, more recently, with the use of aromatase inhibitors such as anastrozole and letrozole (Amsterdam et al., 2005; Ferrero et al., 2009). In mice, letrozole therapy is superior to MPA to induce endometriosis regression and also to inhibit stem cell migration to the endometriotic lesions (Ersoy et al., 2017). However, aromatase inhibitors promote pituitary gonadotropin release with consequent ovarian stimulation; therefore, they must be combined with a progestin or other method of gonadotropin inhibition to treat endometriosis in premenopausal women (Ailawadi et al., 2004). In two non-randomised, open-label trials, the use of letrozole (2.5 mg/day) combined with NETA (2.5 mg/day) over 6 months was more effective than NETA alone to reduce the intensity of chronic pelvic pain and deep dyspareunia associated with rectovaginal endometriosis (Ferrero et al., 2009) as well as the size of ovarian endometriomas (Ferrero et al., 2014). Nonetheless, both treatments were effective, none had lasting effects after discontinuation and the addition of letrozole to NETA did not increase patient satisfaction at the end of treatment (Ferrero et al., 2009; Ferrero et al., 2014). This evidence places letrozole as a second-line therapy for selected patients who fail to respond to progestins (Table II).
Table II.
Potential approaches for patients who have failed initial therapy for endometriosis and the currently available evidence.
| Cause of progestin failure | Potential approaches | Evidence |
|---|---|---|
| Hormone type | To change the type of progestin | Observational study: NETA and dienogest may have similar effects (Vercellini et al., 2016a). In-vitro studies: different progestins may have different mechanisms of action (Grandi et al., 2016; Nirgianakis et al., 2016; Roth et al., 2019). |
| To avoid estrogen association | RCTs did not show meaningful differences between isolated progestins and progestin-estrogen associations (Cheewadhanaraks et al., 2012; Razzi et al., 2007b; Vercellini et al., 2002; Vercellini et al., 2005). Prospective self-control study: for patients who do not respond to COC, changing to NETA may be beneficial (Vercellini et al., 2018c). |
|
| To inhibit estrogen synthesis | Non-randomised open-label trials: for patients who fail to respond to progestins, letrozole can be tried as a second line therapy (Ferrero et al., 2009; Ferrero et al., 2014). | |
| Therapeutic regimen | To change therapeutic regimen | Systematic review: continuous administration may be better than cyclic regimens (Seracchioli et al., 2009). |
| Route of administration | To change the route of administration | RCT: systemic and local progestins may be similar (Carvalho et al., 2018). |
| Progesterone resistance | To associate NSAIDs to progestin therapy | No high-quality evidence for NSAIDs effectiveness on endometriosis symptoms, but the association with hormonal treatments might diminish the inflammatory response that boosts progesterone resistance (Brown et al., 2017). |
| To associate antioxidants | Multi-centre open-label non comparative clinical trial: antioxidant preparations containing N-acetyl-cysteine may mitigate symptoms (Lete et al., 2018). |
NETA: norethisterone acetate; RCTs: randomised controlled trials; COC: combined oral contraceptive; NSAIDs: non-steroidal anti-inflammatory drugs
Characteristics of therapeutic regimen and route of administration
A retrospective cross-sectional study of deep endometriotic lesions compared women who were using different medical treatments preoperatively (COC, oral progestins or GnRH agonists) to a group without treatment, and found a large intra-lesion, intra-patient and intra-treatment variance in the expression levels of ER, which was more evident than any difference between treatments or between treated and non-treated lesions (Brichant et al., 2018). This irregular effect of systemic progestins contrasts with the consistent inhibition of ER expression in endometriotic lesions after LNG-IUS insertion (Engemise et al., 2011; Gomes et al., 2009). A more consistent drug delivery to pelvic lesions would explain why LNG-IUS was found to be more effective than oral danazol (even in standard dose of 600 mg/day) to treat endometriosis-related pain (Taneja et al., 2017). However, an open-label RCT suggested that subdermal etonogestrel implant and LNG-IUS may be equally effective to treat endometriosis-associated pelvic pain and dysmenorrhea (Carvalho et al., 2018). Independently from pain reduction, the degree of satisfaction with treatments to prevent relapse after endometriosis surgery appears to be higher among LNG-IUS users compared to women on COC (Morelli et al., 2013).
A systematic review concluded that continuous administration of COC after conservative surgery for endometriosis results in a faster reduction in frequency and severity of dysmenorrhea compared with the cyclic regimen (Seracchioli et al., 2009). A subsequent patient preference trial of a COC containing ethinyl estradiol and dienogest showed a net superiority of the continuous over the cyclic regimen to reduce pain scores at 3 months and also to decrease the prevalence of dysmenorrhea, dyspareunia, non-menstrual pelvic pain and dyschezia both at 3 months and 6 months of follow-up (Caruso et al., 2016). Not surprisingly, the continuous regimen achieved faster and better improvement in the patients’ quality of life (Caruso et al., 2016).
Characteristics of the disease phenotype
Endometriotic implants are very heterogeneous in clinical features and evolution, being currently classified into three distinct phenotypes: superficial peritoneal endometriosis, ovarian endometrioma and deep-infiltrating endometriosis (Johnson et al., 2017; Nisolle and Donnez, 1997). The overwhelming majority of clinical studies on medical therapies for endometriosis either mixes various disease phenotypes or chooses one main phenotype, making it difficult to evaluate whether a therapy is equally effective to treat superficial peritoneal disease, ovarian endometrioma or the several manifestations of deep endometriosis. Yet, knowing which hormonal treatment is more appropriate to treat each endometriosis phenotype would lead to a lesion-based therapeutic decision (Vercellini et al., 2018a) and possibly maximise patient adherence and treatment results.
Morphological endpoints
Structurally and functionally, endometriotic lesions are not all the same (Tosti et al., 2015). Nerve fibres expressing protein gene product 9.5 are much more abundant in deep-infiltrating than in superficial peritoneal endometriosis (Wang et al., 2009a) and, among the deep lesions, the intestinal forms (either at sigmoid, appendix or rectum) are more densely innervated with myelinated, unmyelinated, sensory and autonomic nerve fibres than other forms of deep endometriosis such as nodules infiltrating the uterosacral ligament (Wang et al., 2009b). Nerve fibre density in superficial peritoneal endometriosis is reduced to approximately half in women treated with progestins or combined pills compared to women not receiving hormonal treatment (Tokushige et al., 2009) but, in deep rectovaginal lesions, which are more innervated, the effect of hormone therapy on nerve fibre density seems to be even more pronounced (Tarjanne et al., 2015).
Vascularisation also differs among the various types of lesions. For instance, blood vessel density evaluated by von Willebrand factor staining is twice as higher in rectal endometriosis than in bladder or ovarian endometriotic implants (Machado et al., 2008). Progestin therapy is associated with a lower number of microvessels and a larger vascular area, suggesting inhibited angiogenesis and increased vasodilation in both superficial and deep (uterosacral, vaginal and vesical) endometriotic lesions. Again, the effect of progestins varied according to the lesion type and the reduction of blood vessels associated with the therapy was more intense in superficial than in deep endometriosis (Jondet et al., 2006). As for ovarian endometrioma, a controlled clinical trial showed that dienogest treatment for 6 months before surgery, despite inducing decidualisation, is unable to inhibit angiogenesis within the endometriotic cysts (Mabrouk et al., 2018).
Another endpoint of hormonal treatment that markedly differs according to the endometriosis phenotype is the effect on lesion size. Ovarian endometriomas tend to have a substantial reduction in volume after 6 months or more of progesting therapy, be it norethisterone (Taniguchi et al., 2017), drospirenone (Harada et al., 2017; Taniguchi et al., 2015) or dienogest (Lee et al., 2018; Park et al., 2016). Conversely, deep endometriosis appears to be more resistant to size regression upon medical treatments. A cohort study that followed 83 women with deep rectovaginal endometriosis treated over 12 months with NETA or desogestrel observed a mean reduction of 20 to 30% in the volume of the endometriotic nodules, as estimated by ultrasonography with bowel preparation (Ferrero et al., 2013). Our group noted a 50% reduction of rectovaginal plaque volume after 12 months of dienogest therapy (Razzi et al., 2007a), whereas another cohort study saw no volumetric change in deep lesions of intestine or posterior fornix with the same treatment (Leonardo-Pinto et al., 2017), although symptoms improved leading to a better quality of life. Indeed, intestinal endometriosis can be so resistant to medical therapy that in some cases it keeps growing and invading the organ wall despite continuous medication (Millochau et al., 2016).
Clinical endpoints
In a patient preference, parallel cohort trial comparing oral progestin (NETA) therapy versus second-line laparoscopic surgery for women with persistent or recurrent endometriosis after a first surgery, the outcomes varied according to the treatment chosen but also depending on the type of endometriosis. Among women with rectovaginal disease, the rate of satisfaction after 12 months was around 50% in both treatment groups, whereas among the participants with other types of endometriosis (mostly ovarian endometriomas), the satisfaction rate was much higher with progestin than with surgical treatment (Vercellini et al., 2012). The effects of 6 months progestin (NETA) therapy on pain relief were assessed in a prospective study of women with rectovaginal endometriosis (Ferrero et al., 2009) and subsequently by the same authors in another study including only women with ovarian endometrioma (Ferrero et al., 2014). When the results of the two studies are viewed together (Fig. 4), it becomes clear that patients on progestin therapy ended with the same level of pain scores in the rectovaginal endometriosis cohort as in the ovarian endometrioma cohort, but in the former the therapeutic effect was more evident because the patients had higher pain intensity at baseline.
Figure 4.
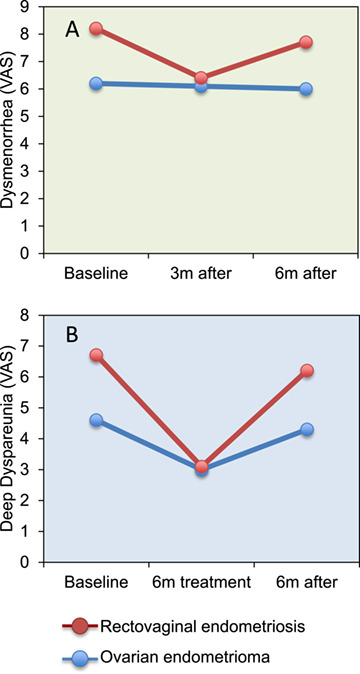
Endometriosis phenotype may affect the therapeutic response to progestins. The graphs summarise dysmenorrhea (A) and deep dyspareunia (B) scores obtained by visual analogue scale (VAS) in women with rectovaginal endometriosis (Ferrero et al., 2009) and in women with ovarian endometrioma (Ferrero et al., 2014) treated with norethisterone acetate for 6 months. Data were extracted from published tables of two separate studies and plotted together for better visualisation.
Characteristics of individuals: the pharmacogenomics
Individual genetic characteristics can affect the bioavailability of hormonal drugs used to treat endometriosis and, hence, explain part of the variability in the therapeutic response. A semiquantitative study of deep endometriosis biopsies from women undergoing hormonal treatments (progestins, COC or GnRH agonist) revealed a large heterogeneity of ERα and PR immunostaining between patients and even between endometriotic glands from the same lesion, particularly in women treated with a progestin (Brichant et al., 2018). N-Acetyltransferases (NAT) control drug kinetics by acetylating active substances and converting them into inactive metabolites. Different combinations of NAT2 alleles are associated with different acetylation activity and speed. In a sample of Russian women with adenomyosis or endometriosis, a greater rate of effective hormonal treatment was associated with genotypes that contained more than one mutant allele, which predisposes to slower inactivation of drugs (Iskhakova et al., 2006).
The cytochrome P450 3A (CYP3A) system is one of the main enzymatic pathways involved in the metabolism of progestins and anti-progestins (Jang and Benet, 1997; Moreno et al., 2012). CYP3A, CYP2C19, and other gene polymorphisms involved in hormone metabolism have been investigated in women with endometriosis (Bozdag et al., 2010; Moreno et al., 2012; Sapkota et al., 2017), but the association of such polymorphisms with the individual response to hormonal therapy remains largely unknown. In other hormone-dependent conditions, however, evidence shows that single-nucleotide polymorphisms of genes involved in steroid hormone synthesis, metabolism or transport have important influence on the therapeutic response to steroid hormones in conditions as diverse as renal transplantation (Rekers et al., 2017), nephrotic syndrome (Chiou et al., 2012), epilepsy (Grover et al., 2010), prostate cancer (Binder et al., 2016) and breast cancer (Wang et al., 2010).
The PGR gene variant PROGINS is characterised by a 306-bp insertion in intron G that renders the receptor less responsive to ligands and predisposes the carrier to progesterone insensitivity and thereby to increased risk of spontaneous abortion (Schweikert et al., 2004), endometrial cancer (Junqueira et al., 2007) and endometriosis (De Carvalho et al., 2007). An in vitro study with eutopic endometrial cells from women with endometriosis showed that cells harbouring the PROGINS variant when incubated with progesterone responded with more proliferation, more viability and less apoptosis than control cells expressing the wild-type PR (D'Amora et al., 2009).
Progesterone resistance in endometriosis: an insurmountable barrier?
Progesterone resistance is defined as subnormal cellular response to the effects of natural progesterone, but the concept can be extended to encompass a poor response to therapeutic progestins. The putative mechanisms and consequences of progesterone resistance in endometriosis have been comprehensively updated in recent reviews (McKinnon et al., 2018; Patel et al., 2017; Yilmaz and Bulun, 2019). Briefly, the central feature of the low response of endometriosis to progestins is the insufficiency of PRB transcription, translation and biological activity. This defect may be congenital, as exemplified by the PROGINS variant (D'Amora et al., 2009), or acquired, through the epigenetic influence of an inflammatory milieu (Patel et al., 2017). Hence, we may consider alternatives to revert the epigenetic mechanisms that inhibit PRB synthesis by adding an anti-inflammatory substance or an immunomodulator as adjuvant to progestin therapy.
It is surprising that the effectiveness of non-steroidal anti-inflammatory drugs to treat endometriosis symptoms remains uncertain as no high-quality evidence is available to date (Brown et al., 2017). The association of anti-inflammatories with hormonal treatments might potentially decrease the levels of proinflammatory cytokines such as TNF and IL-1β and consequently reduce PRB methylation (Fig. 5). Immunomodulators can also be useful to the same scope. For example, Ibrutinib (an FDA-approved inhibitor of the B cell receptor signalling mediator Btk) is able to skew activated B lymphocytes towards regulatory B cells and lower serum TNF levels in endometriotic mice (Riccio et al., 2019).
Figure 5.
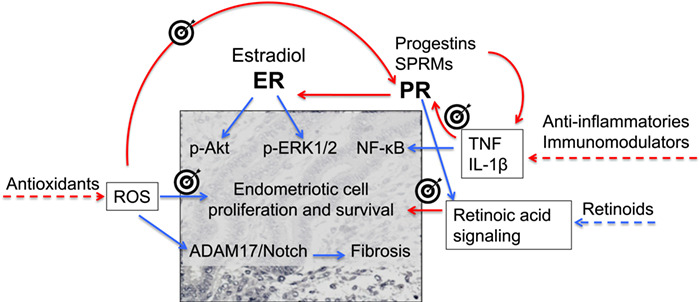
Hypothetical targets and strategies to overcome progesterone resistance in endometriosis based on currently available drugs. Progesterone receptor (PR) expression is inhibited by gene hypermethylation induced by proinflammatory cytokines and reactive oxygen species (ROS), which could be inhibited by anti-inflammatory drugs/immunomodulators and antioxidants, respectively. The same drugs and others, such as retinoids, may activate mechanisms downstream PR signalling that prevent endometriosis proliferation and fibrosis. Blue arrows: stimulation; red arrows: inhibition. ER: estrogen receptor; p-Akt: phosphorylated serine/threonine protein kinase B; p-ERK1/2: phosphorylated extracellular signal-regulated kinase 1/2; NF-κB: nuclear factor-kappa B; ADAM17: A disintegrin and metalloproteases metallopeptidase domain 17; TNF: tumour necrosis factor; IL: interleukin.
Oxidative stress is another mechanism involved in progesterone resistance in endometriosis, as endometriotic lesions and their surrounding peritoneal fluid are rich in ROS (Santulli et al., 2015), which stimulate the A disintegrin and metalloproteases metallopeptidase domain 17 (ADAM17)/Notch signalling pathway (Gonzalez-Foruria et al., 2017), and Notch signal induces PGR hypermethylation (Su et al., 2016). Antioxidants like N-acetyl-cysteine inhibit ROS production and cell proliferation in endometriotic lesions (Ngô et al., 2009) and mitigate symptoms (Giorgi et al., 2016; Lete et al., 2018), but their potential to reduce PGR methylation and overcome progesterone resistance remains to be investigated.
Moreover, strategies to bypass PR signal and directly activate its downstream targets might be a more complex, albeit rational approach (Fig. 5). These would include antioxidants because ROS are capable of promoting endometriosis growth and fibrosis by mechanisms independent of sex hormones (Gonzalez-Foruria et al., 2017). A complementary approach to antioxidants could be the use of retinoids to compensate for the deficiency of endometriotic lesions in a mechanism of apoptosis induced by PR and mediated by retinoic acid signal (Pavone et al., 2016; Pavone et al., 2010).
In summary, clinical studies support at least four explanations for a suboptimal response to progestin therapy in endometriosis (Table II): the type of progestin used may not be ideal for that patient, the presence of estrogen in the formulation may be harmful, the pill-free interval of a cyclic regimen may weaken the drug efficacy and the progesterone insensitivity of endometriotic tissues may demand the association of a non-hormonal drug to overcome this resistance.
Selective Progesterone Receptor Modulators to Treat Endometriosis: Rationale and State of the Art
Selective progesterone receptor modulators (SPRMs) comprise a relatively recent class of synthetic molecules capable of interacting with PR. The interest in these new drugs is based on their affinity to PR, especially considering their antagonist effects (Bouchard et al., 2011; Clemenza et al., 2018; Fu et al., 2017). SPRMs have a wide spectrum of action and are related to a number of therapeutic as well as adverse effects. By binding PR, SPRMs can perform an isolated agonist or antagonist function, but a mixed activity is also possible (Wagenfeld et al., 2016). For instance, onapristone acts only as an antagonist while mifepristone and asosprinil have both antagonist and agonist effects and ulipristal acetate (UPA), in its turn, has a predominant PR antagonist action in the uterus (Benagiano et al., 2014).
The effect produced by the interaction of SPRMs and PR depends on the conjunction of several factors, including the amount of PRA and PRB in each tissue and the changes in receptor conformation promoted by the different ligands. The final action also relies on a complex machinery of co-regulators and co-repressors, whose proportion will determine a greater or lesser transcriptional activity in target genes (Bouchard et al., 2011; Chabbert-Buffet et al., 2012). Apparently, the intrinsic mechanisms are distinct among the different SPRMs. In fact, a study showed that UPA and mifepristone lead to diverse gene expression patterns in endometrium (Kannan et al., 2018).
The occurrence of cross-reaction with other steroid receptors is not rare. In effect, mifepristone, the first SPRM discovered, in 1981, arose from anti-glucocorticoid studies (Chen et al., 2012). This drug is currently used in some countries for medical abortions in combination with prostaglandins such as misoprostol. Because of its several properties, other clinical applications are being studied, to cite Cushing’s disease, psychosis, contraception, Alzheimer’s disease, uterine fibroids, endometrial cancer and, lately, endometriosis (Chabbert-Buffet et al., 2012; Rozenberg et al., 2017).
The interest of developing a SPRM available for use in endometriosis lays on the selective inhibition of endometrial proliferation, without causing estrogen deprivation and its undesirable side effects. Other expected effects would be pain relief, obtained by the suppression of prostaglandin and cytokine synthesis in endometrial cells, and the action on the PRs present in the spiral arterioles, generating reversible suppression of endometrial bleeding (Merviel et al., 2013).
With this background, some basic and clinical studies have tested the putative actions of SPRMs on endometriosis. Studies with animal models demonstrated regression of endometriotic lesions with subcutaneous implants of mifepristone (Mei et al., 2010). A Cochrane systematic review including 10 RCTs with 960 women assessed the effectiveness and the safety of SPRMs for pain relief in endometriosis (Fu et al., 2017). Mifepristone was more effective than placebo against dysmenorrhea (moderate-quality evidence) and dyspareunia (low-quality evidence), at least when used at 5- or 10-mg doses. A trial excluded from the Cochrane meta-analysis suggested that post-operative treatment with mifepristone for 6 months is able to reduce endometrial thickness and alleviate pelvic pain in patients with endometriosis, being as effective as gestrinone. Spontaneous pregnancy rates after drug discontinuation were also similar between the mifepristone and the gestrinone groups (Zhang, 2016). Asoprisnil was tested in industry-sponsored phase II trials in the early 2000s and was able to induce amenorrhea, but no further drug development to treat endometriosis has been reported (Fu et al., 2017; Guo and Groothuis, 2018).
Ulipristal acetate
UPA is currently approved for emergency contraception and as a presurgical therapy for symptomatic women with uterine fibroids. For the last indication, its use for 3 months reduces uterine bleeding and fibroid size (Donnez et al., 2014). UPA inhibits human endometrial cell proliferation in vivo (Whitaker et al., 2017), and suppresses the growth and development of endometriotic implants in mice (Liang et al., 2018). In the latter study, there was a higher accumulation of antral follicles post-UPA use, probably due to a delay in follicular rupture induced by the drug. However, the regrowth of endometriosis lesions after treatment interruption was slower with UPA than with dydrogesterone or dienogest (Liang et al., 2018).
One of the consequences observed with the exposure to SPRMs is the so-called progesterone receptor modulator-associated endometrial changes (PAECs), characterised by extensive cyst formation, vascular and glandular changes. The morphologic features of PAEC may vary with different SPRMs. Exposure to UPA for a period exceeding 6 months leads to PAEC, but such alterations do not seem to contain cytological atypia and are reversible after the drug discontinuation (Whitaker et al., 2017). There is a paucity of information about the histomorphological effects of SPRMs in endometriosis (Kannan et al., 2018). A case report of a patient that underwent surgical intervention for uterine fibroids after receiving three 90-day courses of UPA showed the presence of PAEC in an endometriotic lesion incidentally found in the surgical specimen. This finding demonstrates histomorphological evidence of a pharmacological effect of UPA in endometriosis tissue (Bateman et al., 2017). Further reassurance comes from the finding that exposure to UPA for over 24 weeks did not lead to suppression of heart and neural crest derivatives expressed 2 protein (HAND2), a transcription factor whose inhibition might predispose to the development of endometrial hyperplasia and adenocarcinoma (Kannan et al., 2018). In spite of a predictable benignity of PAEC, further evaluation is needed to guarantee a safe longstanding use of UPA (De Milliano et al., 2017).
In 2018, the European Medicines Agency (EMA) provided an alert about a serious risk of liver injury caused by UPA and recommended some measures to minimise the negative outcomes. The medication is contraindicated in patients with liver disease; liver tests are required before, during and after the drug administration; repeated therapeutic courses can be offered only to women not eligible for surgery; and patients have to be widely oriented about the risks. Further studies have to be developed in order to clarify the mechanisms of UPA hepatotoxicity and to certify that such prophylactic measures are in fact effective (European Medicines Agency, 2018).
Vilaprisan
Vilaprisan is a new SPRM that differs from other drugs of the same class by a distinct metabolic clearance, with the potential benefit of not presenting liver toxicity. A 2B phase trial evaluated the safety and efficacy of the drug in patients with uterine fibroids during 12 weeks of treatment and a 24-week post-treatment follow-up. Improvement in uterine bleeding, occurrence of amenorrhea and reduction of fibroid volume were observed and a daily oral dose of 2 mg was considered safe for further investigation (Bradley et al., 2019). However, vilaprisan has an antagonistic effect on PR that is five times that of UPA (Schultze-Mosgau et al., 2018), which makes this new SPRM unlikely to become a better option to treat endometriosis.
In summary, although some studies performed with SPRMs in endometriosis showed a potential clinical use for symptom relief (Wagenfeld et al., 2016), there is no drug representative of this class currently approved for clinical use in endometriosis therapy. While mifepristone may have some benefit, mainly by inducing amenorrhea, the available evidence does not allow any conclusion about the therapeutic value and clinical safety of other SPRMs to be used in the long-term treatment of endometriosis (Fu et al., 2017).
Strategies to Overcome the Therapeutic Shortcomings: Innovative Drug Design, Better Phenotyping, Personalised Medicine
A number of PR-related treatments having endometriosis as inclusion criterion and its symptoms as primary outcomes are under investigation in registered clinical trials (Table III). It is clear that current clinical trials privilege single compounds over combined therapies, which could have synergic effects by hitting hormonal, inflammatory, oxidative stress and pro-fibrotic targets. Novel substances with the potential to gather all these mechanisms in a single product remain unavailable, but not unattainable. The recognition that endometriosis requires a multisided medical therapy contemplating its multifaceted pathophysiology may be the first step towards a real breakthrough in the field.
Table III.
New progesterone receptor modulating drugs for endometriosis treatment with study protocols registered in ClinicalTrials.gov
| Class | Substance | Route | Trial number | Completed |
|---|---|---|---|---|
| Progesterone | Progesterone | Subcutaneous | NCT02793908 | No |
| Progestin | Etonogestrel (Nexplanon) | Subcutaneous implant | NCT02669238 | No |
| SPRM? | Danazol | Vaginal ring | NCT00117481 | Yes |
| PR antagonist | PF-02413873 | Oral | NCT00800618 | Yes |
| Analgesic + progestin | Low dose naltrexone + norethindrone acetate | Oral | NCT03970330 | No |
Although the process of decidualisation involves many PR targets (Gellersen and Brosens, 2014), the transcription factor named promyelocytic leukemia zinc finger (PLZF) emerged as a critical mediator with a pivotal role in inducing the transcriptional reprogramming of endometrial stromal cells that ultimately leads to the decidual phenotype (Kommagani et al., 2016; Szwarc et al., 2018). This transcription factor exemplifies a promising, yet underused strategy to induce endometriosis regression through drugs acting beyond PR and therefore unaffected by progesterone resistance.
Future searches for new medical treatments of endometriosis should include better phenotyping of the included patients, considering the many specificities of each type of lesion and the possible variation in therapeutic results obtained with the same treatment administered to patients with different disease phenotypes (Tosti et al., 2015). Moving beyond the macroscopic disease picture, future therapeutic developments may lead us to consider molecular phenotypes to predict the effectiveness of a drug for every single patient. This personalised medicine approach makes sense in endometriosis, since endometrial stromal cells respond diversely to hormonal treatments according to their constitutive expression of the primary molecular targets of such drugs (Hou et al., 2017).
Conclusions
Progestins have been used to treat endometriosis for over 60 years with large success rates, but still some patients do not respond to this therapy as expected. Progestins depend on the expression of PR to exert their actions in the target cells, but PR expression is often blunted and disrupted in endometriotic foci, which cannot be resolved by simple dose increment or by small modifications in the molecular design of the drug. Combined hormonal contraceptives have the same limitations of isolated progestins, plus a possible counterproductive effect of the estrogenic compound.
Understanding the mechanisms of therapeutic success and failure is essential to guide clinical decisions and inform future research in this field. The development of new molecules for medical treatment of endometriosis should aim at strategies to overcome the resistance mechanisms and aim at new targets. However, current clinical trials consist of old drugs with new delivery (danazol vaginal ring), new drugs with old mechanisms (oral GnRH antagonists) or new mechanisms with old concepts (steroidogenic enzyme inhibitors) (Barra et al., 2019). Innovation is urgently needed and should start by deeper understanding of the disease core features, diverse phenotypes and idiosyncrasies, while moving from pure hormonal treatments to drug combinations or novel molecules capable of restoring the various homeostatic mechanisms disrupted by endometriotic lesions.
Authors’ roles
F.M.R., F.P. and F.B. conceived the review. F.M.R., L.M.C. and S.V. performed the bibliographic search and drafted the manuscript. F.P., F.B. and C.C. revised the manuscript for important intellectual content. All authors approved the final version.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil, # 201876/2017-5 to F.M.R.).
Conflict of interest
The authors report no conflict of interest.
References
- Ailawadi RK, Jobanputra S, Kataria M, Gurates B, Bulun SE. Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertil Steril 2004;81:290–296. [DOI] [PubMed] [Google Scholar]
- Amini P, Michniuk D, Kuo K, Yi L, Skomorovska-Prokvolit Y, Peters GA, Tan H, Wang J, Malemud CJ, Mesiano S. Human parturition involves phosphorylation of progesterone receptor-a at serine-345 in myometrial cells. Endocrinology 2016;157:4434–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam LL, Gentry W, Jobanputra S, Wolf M, Rubin SD, Bulun SE. Anastrazole and oral contraceptives: a novel treatment for endometriosis. Fertil Steril 2005;84:300–304. [DOI] [PubMed] [Google Scholar]
- Andrews MC, Andrews WC, Strauss AF. Effects of progestin-induced pseudopregnancy on endometriosis: clinical and microscopic studies. Am J Obstet Gynecol 1959;78:776–785. [DOI] [PubMed] [Google Scholar]
- Andrews WC Hormone therapy of endometriosis - estrogen, androgens, progestin estrogens. Excerpta Med 1976;368:46–59. [Google Scholar]
- Aoyagi Y, Nasu K, Kai K, Hirakawa T, Okamoto M, Kawano Y, Abe W, Tsukamoto Y, Moriyama M, Narahara H. Decidualization differentially regulates microRNA expression in eutopic and ectopic endometrial stromal cells. Reprod Sci 2017;24:445–455. [DOI] [PubMed] [Google Scholar]
- Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab 2000;85:2897–2902. [DOI] [PubMed] [Google Scholar]
- Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol 1981;141:453–463. [DOI] [PubMed] [Google Scholar]
- Barra F, Grandi G, Tantari M, Scala C, Facchinetti F, Ferrero S. A comprehensive review of hormonal and biological therapies for endometriosis: latest developments. Expert Opin Biol Ther 2019;19:343–360. [DOI] [PubMed] [Google Scholar]
- Bateman J, Bougie O, Singh S, Islam S. Histomorphological changes in endometriosis in a patient treated with ulipristal: a case report. Pathol Res Pract 2017;213:79–81. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Dahoud W, Skomorovska-Prokvolit Y, Yi L, Liu JH, Falcone T, Hurd WW, Mesiano S. Abundance and localization of progesterone receptor isoforms in endometrium in women with and without endometriosis and in peritoneal and ovarian endometriotic implants. Reprod Sci 2015;22:1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliard A, Noel A, Foidart JM. Reduction of apoptosis and proliferation in endometriosis. Fertil Steril 2004;82:80–85. [DOI] [PubMed] [Google Scholar]
- Benagiano G, Bastianelli C, Farris M, Brosens I. Selective progesterone receptor modulators: an update. Expert Opin Pharmacother 2014;15:1403–1415. [DOI] [PubMed] [Google Scholar]
- Beranič N, Rižner TL. Effects of progestins on local estradiol biosynthesis and action in the Z-12 endometriotic epithelial cell line. J Steroid Biochem Mol Biol 2012;132:303–310. [DOI] [PubMed] [Google Scholar]
- Bergqvist A, Jeppsson S, Kullander S, Ljungberg O. Human endometrium transplanted into nude mice. Histologic effects of various steroid hormones. Am J Pathol 1985;119:336–344. [PMC free article] [PubMed] [Google Scholar]
- Binder M, Zhang BY, Hillman DW, Kohli R, Kohli T, Lee A, Kohli M. Common genetic variation in CYP17A1 and response to abiraterone acetate in patients with metastatic castration-resistant prostate cancer. Int J Mol Sci 2016;17: pii: E1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono Y, Kyo S, Kiyono T, Mizumoto Y, Nakamura M, Maida Y, Takakura M, Fujiwara H. Concurrent estrogen action was essential for maximal progestin effect in oral contraceptives. Fertil Steril 2014;101:1337–1343. [DOI] [PubMed] [Google Scholar]
- Bouchard P, Chabbert-Buffet N, Fauser BC. Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fertil Steril 2011;96:1175–1189. [DOI] [PubMed] [Google Scholar]
- Bozdag G, Alp A, Saribas Z, Tuncer S, Aksu T, Gurgan T. CYP17 and CYP2C19 gene polymorphisms in patients with endometriosis. Reprod Biomed Online 2010;20:286–290. [DOI] [PubMed] [Google Scholar]
- Bradley LD, Singh SS, Simon J, Gemzell-Danielsson K, Petersdorf K, Groettrup-Wolfers E, Ren X, Zvolanek M, Seitz C. Vilaprisan in women with uterine fibroids: the randomized phase 2b ASTEROID 1 study. Fertil Steril 2019;111:240–248. [DOI] [PubMed] [Google Scholar]
- Braun DP, Gebel H, Dmowski WP. Effect of danazol in vitro and in vivo on monocyte-mediated enhancement of endometrial cell proliferation in women with endometriosis. Fertil Steril 1994;62:89–95. [PubMed] [Google Scholar]
- Brichant G, Nervo P, Albert A, Munaut C, Foidart JM, Nisolle M. Heterogeneity of estrogen receptor α and progesterone receptor distribution in lesions of deep infiltrating endometriosis of untreated women or during exposure to various hormonal treatments. Gynecol Endocrinol 2018;34:651–655. [DOI] [PubMed] [Google Scholar]
- Brosens I, Benagiano G. Endometriosis, a modern syndrome. Indian J Med Res 2011;133:581–593. [PMC free article] [PubMed] [Google Scholar]
- Brosens IA, Verleyen A, Cornillie F. The morphologic effect of short-term medical therapy of endometriosis. Am J Obstet Gynecol 1987;157:1215–1221. [DOI] [PubMed] [Google Scholar]
- Brown J, Crawford TJ, Allen C, Hopewell S, Prentice A. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev 2017;1:CD004753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Pan A, Hart RJ. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst Rev 2010;12:CD008475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab 2002;87:4782–4791. [DOI] [PubMed] [Google Scholar]
- Bruner-Tran KL, Zhang Z, Eisenberg E, Winneker RC, Osteen KG. Down-regulation of endometrial matrix metalloproteinase-3 and -7 expression in vitro and therapeutic regression of experimental endometriosis in vivo by a novel nonsteroidal progesterone receptor agonist, tanaproget. J Clin Endocrinol Metab 2006;91:1554–1560. [DOI] [PubMed] [Google Scholar]
- Bullock LP, Bardin CW. Androgenic, synandrogenic, and antiandrogenic actions of progestins. Ann N Y Acad Sci 1977;286:321–330. [DOI] [PubMed] [Google Scholar]
- Caruso S, Iraci M, Cianci S, Fava V, Casella E, Cianci A. Comparative, open-label prospective study on the quality of life and sexual function of women affected by endometriosis-associated pelvic pain on 2 mg dienogest/30 μg ethinyl estradiol continuous or 21/7 regimen oral contraceptive. J Endocrinol Invest 2016;39:923–931. [DOI] [PubMed] [Google Scholar]
- Carvalho N, Margatho D, Cursino K, Benetti-Pinto CL, Bahamondes L. Control of endometriosis-associated pain with etonogestrel-releasing contraceptive implant and 52-mg levonorgestrel-releasing intrauterine system: randomized clinical trial. Fertil Steril 2018;110:1129–1136. [DOI] [PubMed] [Google Scholar]
- Casper RF Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil Steril 2017;107:533–536. [DOI] [PubMed] [Google Scholar]
- Chabbert-Buffet N, Pintiaux A, Bouchard P. The immninent dawn of SPRMs in obstetrics and gynecology. Mol Cell Endocrinol 2012;358:232–243. [DOI] [PubMed] [Google Scholar]
- Cheewadhanaraks S, Choksuchat C, Dhanaworavibul K, Liabsuetrakul T. Postoperative depot medroxyprogesterone acetate versus continuous oral contraceptive pills in the treatment of endometriosis-associated pain: a randomized comparative trial. Gynecol Obstet Invest 2012;74:151–156. [DOI] [PubMed] [Google Scholar]
- Chen CC, Montalbano AP, Hussain I, Lee WR, Mendelson CR. The transcriptional repressor GATAD2B mediates progesterone receptor suppression of myometrial contractile gene expression. J Biol Chem 2017;292:12560–12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JI, Hannan NJ, Mak Y, Nicholls PK, Zhang J, Rainczuk A, Stanton PG, Robertson DM, Salamonsen LA, Stephens AN. Proteomic characterization of midproliferative and midsecretory human endometrium. J Proteome Res 2009;8:2032–2044. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang Y, Zhuang Y, Zhou F, Huang L. Mifepristone increases the cytotoxicity of uterine natural killer cells by acting as a glucocorticoid antagonist via ERK activation. PLoS ONE 2012;7:e36413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YH, Imir A, Fenkci V, Yilmaz MB, Bulun SE. Stromal cells of endometriosis fail to produce paracrine factors that induce epithelial 17β-hydroxysteroid dehydrogenase type 2 gene and its transcriptional regulator Sp1: a mechanism for defective estradiol metabolism. Am J Obstet Gynecol 2007;196:391 e391-391.e398. [DOI] [PubMed] [Google Scholar]
- Chiou YH, Wang LY, Wang TH, Huang SP. Genetic polymorphisms influence the steroid treatment of children with idiopathic nephrotic syndrome. Pediatr Nephrol 2012;27:1511–1517. [DOI] [PubMed] [Google Scholar]
- Choi J, Jo M, Lee E, Lee DY, Choi D. Dienogest enhances autophagy induction in endometriotic cells by impairing activation of AKT, ERK1/2, and mTOR. Fertil Steril 2015;104:655–664 e651. [DOI] [PubMed] [Google Scholar]
- Choi JY, Jo MW, Lee EY, Hwang SS, Choi DS. Aberrant PTEN expression in response to progesterone reduces endometriotic stromal cell apoptosis. Reproduction 2017;153:11–21. [DOI] [PubMed] [Google Scholar]
- Clemenza S, Sorbi F, Noci I, Capezzuoli T, Turrini I, Carriero C, Buffi N, Fambrini M, Petraglia F. From pathogenesis to clinical practice: emerging medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol 2018;51:92–101. [DOI] [PubMed] [Google Scholar]
- Cobellis L, Razzi S, Fava A, Severi FM, Igarashi M, Petraglia F. A danazol-loaded intrauterine device decreases dysmenorrhea, pelvic pain, and dyspareunia associated with endometriosis. Fertil Steril 2004;82:239–240. [DOI] [PubMed] [Google Scholar]
- Cornillie FJ, Puttemans P, Brosens IA. Histology and ultrastructure of human endometriotic tissues treated with dydrogesterone (Duphaston). Eur J Obstet Gynecol Reprod Biol 1987;26:39–55. [DOI] [PubMed] [Google Scholar]
- Couzinet B, Le Strat N, Brailly S, Schaison G. Comparative effects of cyproterone acetate or a long-acting gonadotropin-releasing hormone agonist in polycystic ovarian disease. J Clin Endocrinol Metab 1986;63:1031–1035. [DOI] [PubMed] [Google Scholar]
- Couzinet B, Young J, Brailly S, Chanson P, Thomas JL, Schaison G. The antigonadotropic activity of progestins (19-nortestosterone and 19-norprogesterone derivatives) is not mediated through the androgen receptor. J Clin Endocrinol Metab 1996;81:4218–4223. [DOI] [PubMed] [Google Scholar]
- Cullen TS Adenomyoma of the Uterus. Philadelphia/London: WB Saunders Company; 1908. [Google Scholar]
- Cullen TS The distribution of adenomyomata containing uterine mucosa. Arch Surg 1920;1:215–283. [Google Scholar]
- D'Amora P, Maciel TT, Tambellini R, Mori MA, Pesquero JB, Sato H, Girao MJ, Guerreiro da Silva ID, Schor E. Disrupted cell cycle control in cultured endometrial cells from patients with endometriosis harboring the progesterone receptor polymorphism PROGINS. Am J Pathol 2009;175:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho CV, Nogueira-De-Souza NC, Costa AM, Baracat EC, Girao MJ, D'Amora P, Schor E, da Silva ID. Genetic polymorphisms of cytochrome P450cl7alpha (CYP17) and progesterone receptor genes (PROGINS) in the assessment of endometriosis risk. Gynecol Endocrinol 2007;23:29–33. [DOI] [PubMed] [Google Scholar]
- de Lignieres B, Dennerstein L, Backstrom T. Influence of route of administration on progesterone metabolism. Maturitas 1995;21:251–257. [DOI] [PubMed] [Google Scholar]
- De Milliano I, Van Hattum D, Ket JCF, Huirne JAF, Hehenkamp WJK. Endometrial changes during ulipristal acetate use: a systematic review. Eur J Obstet Gynecol Reprod Biol 2017;214:56–64. [DOI] [PubMed] [Google Scholar]
- Dinh A, Sriprasert I, Williams AR, Archer DF. A review of the endometrial histologic effects of progestins and progesterone receptor modulators in reproductive age women. Contraception 2015;91:360–367. [DOI] [PubMed] [Google Scholar]
- Donnez J, Vazquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BC, Barlow DH, Palacios S, Donnez O, Bestel E et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril 2014;101:1565–1573 e1561-1518. [DOI] [PubMed] [Google Scholar]
- Eaton JL, Unno K, Caraveo M, Lu Z, Kim JJ. Increased AKT or MEK1/2 activity influences progesterone receptor levels and localization in endometriosis. J Clin Endocrinol Metab 2013;98:E1871–E1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engemise SL, Willets JM, Taylor AH, Emembolu JO, Konje JC. Changes in glandular and stromal estrogen and progesterone receptor isoform expression in eutopic and ectopic endometrium following treatment with the levonorgestrel-releasing intrauterine system. Eur J Obstet Gynecol Reprod Biol 2011;157:101–106. [DOI] [PubMed] [Google Scholar]
- Ersoy GS, Zolbin MM, Cosar E, Mamillapalli R, Taylor HS. Medical therapies for endometriosis differentially inhibit stem cell recruitment. Reprod Sci 2017;24:818–823. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency Esmya: new measures to minimize risk of rare but serious liver injury. 2018. https://www.ema.europa.eu/en/medicines/human/referrals/esmya
- Fechner S, Husen B, Thole H, Schmidt M, Gashaw I, Kimmig R, Winterhager E, Grümmer R. Expression and regulation of estrogen-converting enzymes in ectopic human endometrial tissue. Fertil Steril 2007;88:1029–1038. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Camerini G, Seracchioli R, Ragni N, Venturini PL, Remorgida V. Letrozole combined with norethisterone acetate compared with norethisterone acetate alone in the treatment of pain symptoms caused by endometriosis. Hum Reprod 2009;24:3033–3041. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Leone Roberti Maggiore U, Scala C, Di Luca M, Venturini PL, Remorgida V. Changes in the size of rectovaginal endometriotic nodules infiltrating the rectum during hormonal therapies. Arch Gynecol Obstet 2013;287:447–453. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Remorgida V, Venturini PL, Leone Roberti Maggiore U. Norethisterone acetate versus norethisterone acetate combined with letrozole for the treatment of ovarian endometriotic cysts: a patient preference study. Eur J Obstet Gynecol Reprod Biol 2014;174:117–122. [DOI] [PubMed] [Google Scholar]
- Fu J, Song H, Zhou M, Zhu H, Wang Y, Chen H, Huang W. Progesterone receptor modulators for endometriosis. Cochrane Database Syst Rev 2017;2017:1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev 2014;35:851–905. [DOI] [PubMed] [Google Scholar]
- Gerdes D, Wehling M, Leube B, Falkenstein E. Cloning and tissue expression of two putative steroid membrane receptors. Biol Chem 1998;379:907–911. [DOI] [PubMed] [Google Scholar]
- Gezer A, Oral E. Progestin therapy in endometriosis. Womens Health (Lond) 2015;11:643–652. [DOI] [PubMed] [Google Scholar]
- Giorgi VS, Da Broi MG, Paz CC, Ferriani RA, Navarro PA. N-Acetyl-cysteine and l-carnitine prevent meiotic oocyte damage induced by follicular fluid from infertile women with mild endometriosis. Reprod Sci 2016;23:342–351. [DOI] [PubMed] [Google Scholar]
- Goldman S, Shalev E. Progesterone receptor profile in the decidua and fetal membrane. Front Biosci 2007;12:634–648. [DOI] [PubMed] [Google Scholar]
- Gomes MKO, Rosa-E-Silva JC, Garcia SB, De Sá Rosa-E-Silva ACJ, Turatti A, Vieira CS, Ferriani RA. Effects of the levonorgestrel-releasing intrauterine system on cell proliferation, Fas expression and steroid receptors in endometriosis lesions and normal endometrium. Hum Reprod 2009;24:2736–2745. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Foruria I, Santulli P, Chouzenoux S, Carmona F, Chapron C, Batteux F. Dysregulation of the ADAM17/Notch signalling pathways in endometriosis: from oxidative stress to fibrosis. Mol Hum Reprod 2017;23:488–499. [DOI] [PubMed] [Google Scholar]
- Grandi G, Mueller M, Bersinger N, Papadia A, Nirgianakis K, Cagnacci A, McKinnon B. Progestin suppressed inflammation and cell viability of tumor necrosis factor-α-stimulated endometriotic stromal cells. Am J Reprod Immunol 2016;76:292–298. [DOI] [PubMed] [Google Scholar]
- Greenblatt RB, Dmowski WP, Mahesh VB, Scholer HF. Clinical studies with an antigonadotropin-Danazol. Fertil Steril 1971;22:102–112. [DOI] [PubMed] [Google Scholar]
- Grover S, Talwar P, Gourie-Devi M, Gupta M, Bala K, Sharma S, Baghel R, Kaur H, Sharma A, Kukreti R. Genetic polymorphisms in sex hormone metabolizing genes and drug response in women with epilepsy. Pharmacogenomics 2010;11:1525–1534. [DOI] [PubMed] [Google Scholar]
- Guo SW, Groothuis PG. Is it time for a paradigm shift in drug research and development in endometriosis/adenomyosis? Hum Reprod Update 2018;24:577–598. [DOI] [PubMed] [Google Scholar]
- Harada T, Kosaka S, Elliesen J, Yasuda M, Ito M, Momoeda M. Ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen for the management of endometriosis-associated pelvic pain: a randomized controlled trial. Fertil Steril 2017;108:798–805. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Tanabe A, Kawabe S, Hayashi M, Yuguchi H, Yamashita Y, Okuda K, Ohmichi M. Dienogest increases the progesterone receptor isoform B/A ratio in patients with ovarian endometriosis. J Ovarian Res 2012;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Barbieri RL, Anderson DJ. Immunosuppressive effects of danazol in vitro. Fertil Steril 1987;48:414–418. [PubMed] [Google Scholar]
- Hill KK, Roemer SC, Churchill ME, Edwards DP. Structural and functional analysis of domains of the progesterone receptor. Mol Cell Endocrinol 2012;348:418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie S, Harada T, Mitsunari M, Taniguchi F, Iwabe T, Terakawa N. Progesterone and progestational compounds attenuate tumor necrosis factor alpha-induced interleukin-8 production via nuclear factor kappaB inactivation in endometriotic stromal cells. Fertil Steril 2005;83:1530–1535. [DOI] [PubMed] [Google Scholar]
- Horne A, Critchley HOD. Medical therapies: progestins In: Giudice LC, Evers JLH, Healy DL (eds). Endometriosis: Science and Practice. Oxford: Blackwell Publishing, 2011,351–356 [Google Scholar]
- Hornung D, Dohrn K, Sotlar K, Greb RR, Wallwiener D, Kiesel L, Taylor RN. Localization in tissues and secretion of eotaxin by cells from normal endometrium and endometriosis. J Clin Endocrinol Metabol 2000;85:2604–2608. [DOI] [PubMed] [Google Scholar]
- Hou Z, Mamillapalli R, Taylor HS. Predictive biomarkers may allow precision therapy of endometriosis. J Endometr Pelvic Pain Disord 2017;9:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudelist G, Keckstein J, Wright JT. The migrating adenomyoma: past views on the etiology of adenomyosis and endometriosis. Fertil Steril 2009;92:1536–1543. [DOI] [PubMed] [Google Scholar]
- Ichioka M, Mita S, Shimizu Y, Imada K, Kiyono T, Bono Y, Kyo S. Dienogest, a synthetic progestin, down-regulates expression of CYP19A1 and inflammatory and neuroangiogenesis factors through progesterone receptor isoforms A and B in endometriotic cells. J Steroid Biochem Mol Biol 2015;147:103–110. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Iizuka M, Abe Y, Ibuki Y. Novel vaginal danazol ring therapy for pelvic endometriosis, in particular deeply infiltrating endometriosis. Hum Reprod 1998;13:1952–1956. [DOI] [PubMed] [Google Scholar]
- Iskhakova GM, Abazova EF, Viktorova TV, Kudakaeva LR. Polymorphism of the arylamine-n-acetyltransferase gene in endometriosis patients in the Republic of Bashkortostan. Balk J Med Genet 2006;9:55–60. [Google Scholar]
- Jang GR, Benet LZ. Antiprogestin pharmacodynamics, pharmacokinetics, and metabolism: implications for their long-term use. J Pharmacokinet Biopharm 1997;25:647–672. [DOI] [PubMed] [Google Scholar]
- Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, Bush D, Kiesel L, Tamimi R, Sharpe-Timms KL et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod 2017;32:315–324. [DOI] [PubMed] [Google Scholar]
- Jondet M, Vacher-Lavenu MC, Chapron C, Vacher-Lavenu MC, Chapron C. Image analysis measurements of the microvascularisation in endometrium, superficial and deep endometriotic tissues. Angiogenesis 2006;9:177–182. [DOI] [PubMed] [Google Scholar]
- Junqueira MG, da Silva ID, Nogueira-de-Souza NC, Carvalho CV, Leite DB, Gomes MT, Baracat EC, Lopes LA, Nicolau SM, Goncalves WJ. Progesterone receptor (PROGINS) polymorphism and the risk of endometrial cancer development. Int J Gynecol Cancer 2007;17:229–232. [DOI] [PubMed] [Google Scholar]
- Kannan A, Bhurke A, Sitruk-Ware R, Lalitkumar PG, Gemzell-Danielsson K, Williams ARW, Taylor RN, Bagchi MK, Bagchi IC. Characterization of molecular changes in endometrium associated with chronic use of progesterone receptor modulators: Ulipristal acetate versus mifepristone. Reprod Sci 2018;25:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global gene profiling in human endometrium during the window of implantation. Endocrinology 2002;143:2119–2138. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Schally AV, Gual C, Arimura A. Release of LH and FSH after administration of synthetic LH-releasing hormone. J Clin Endocrinol Metab 1972;34:753–756. [DOI] [PubMed] [Google Scholar]
- Katsuki Y, Takano Y, Futamura Y, Shibutani Y, Aoki D, Udagawa Y, Nozawa S. Effects of dienogest, a synthetic steroid, on experimental endometriosis in rats. Eur J Endocrinol 1998;138:216–226. [DOI] [PubMed] [Google Scholar]
- Keator CS, Mah K, Slayden OD. Alterations in progesterone receptor membrane component 2 (PGRMC2) in the endometrium of macaques afflicted with advanced endometriosis. Mol Hum Reprod 2012;18:308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev 2013;34:130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistner RW The use of newer progestins in the treatment of endometriosis. Am J Obstet Gynecol 1958;75:264–278. [DOI] [PubMed] [Google Scholar]
- Kommagani R, Szwarc MM, Vasquez YM, Peavey MC, Mazur EC, Gibbons WE, Lanz RB, DeMayo FJ, Lydon JP. The promyelocytic leukemia zinc finger transcription factor is critical for human endometrial stromal cell decidualization. PLoS Genet 2016;12:e1005937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumantakis E, Matalliotakis I, Neonaki M, Froudarakis G, Georgoulias V. Soluble serum interleukin-2 receptor, interleukin-6 and interleukin-1a in patients with endometriosis and in controls. Arch Gynecol Obstet 1994;255:107–112. [DOI] [PubMed] [Google Scholar]
- Kowalik MK, Rekawiecki R, Kotwica J. The putative roles of nuclear and membrane-bound progesterone receptors in the female reproductive tract. Reprod Biol 2013;13:279–289. [DOI] [PubMed] [Google Scholar]
- Kulak J Jr, Fischer C, Komm B, Taylor HS. Treatment with bazedoxifene, a selective estrogen receptor modulator, causes regression of endometriosis in a mouse model. Endocrinology 2011;152:3226–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrea F, Garcia-Becerra R, Lemus AE, Garcia GA, Perez-Palacios G, Jackson KJ, Coleman KM, Dace R, Smith CL, Cooney AJ. A-ring reduced metabolites of 19-nor synthetic progestins as subtype selective agonists for ER alpha. Endocrinology 2001;142:3791–3799. [DOI] [PubMed] [Google Scholar]
- Lauersen NH, Wilson KH. Evaluation of danazol as an oral contraceptive. Obstet Gynecol 1977;50:91–96. [PubMed] [Google Scholar]
- Lazzeri L, Luisi S, Petraglia F. Progestins for the treatment of endometriosis: an update. J Endometriosis Pelvic Pain Disord 2010;2:169–181. [Google Scholar]
- Lee SR, Yi KW, Song JY, Seo SK, Lee DY, Cho SH, Kim SH. Efficacy and safety of long-term use of dienogest in women with ovarian endometrioma. Reprod Sci 2018;25:341–346. [DOI] [PubMed] [Google Scholar]
- Leonardo-Pinto JP, Benetti-Pinto CL, Cursino K, Yela DA. Dienogest and deep infiltrating endometriosis: the remission of symptoms is not related to endometriosis nodule remission. Eur J Obstet Gynecol Reprod Biol 2017;211:108–111. [DOI] [PubMed] [Google Scholar]
- Leone Roberti Maggiore U, Remorgida V, Scala C, Tafi E, Venturini PL, Ferrero S. Desogestrel-only contraceptive pill versus sequential contraceptive vaginal ring in the treatment of rectovaginal endometriosis infiltrating the rectum: a prospective open-label comparative study. Acta Obstet Gynecol Scand 2014;93:239–247. [DOI] [PubMed] [Google Scholar]
- Lete I, Mendoza N, de la Viuda E, Carmona F. Effectiveness of an antioxidant preparation with N-acetyl cysteine, alpha lipoic acid and bromelain in the treatment of endometriosis-associated pelvic pain: LEAP study. Eur J Obstet Gynecol Reprod Biol 2018;228:221–224. [DOI] [PubMed] [Google Scholar]
- Li Y, Adur MK, Kannan A, Davila J, Zhao Y, Nowak RA, Bagchi MK, Bagchi IC, Li Q. Progesterone alleviates endometriosis via inhibition of uterine cell proliferation, inflammation and angiogenesis in an immunocompetent mouse model. PLoS ONE 2016;11:e0165347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Wu L, Xu H, Cheung CW, Fung WY, Wong SW, Wang CC. Efficacy, safety and recurrence of new progestins and selective progesterone receptor modulator for the treatment of endometriosis: a comparison study in mice. Reprod Biol Endocrinol 2018;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Q, Guo SW. Histological and Immunohistochemical Characterization of the Similarity and Difference Between Ovarian Endometriomas and Deep Infiltrating Endometriosis. Reprod Sci. 2018;25:329–40. [DOI] [PubMed] [Google Scholar]
- Mabrouk M, Paradisi R, Arena A, Del Forno S, Matteucci C, Zannoni L, Caprara G, Seracchioli R. Short-term histopathological effects of dienogest therapy on ovarian endometriomas: in vivo, nonrandomized, controlled trial. Gynecol Endocrinol 2018;34:399–403. [DOI] [PubMed] [Google Scholar]
- Machado DE, Abrao MS, Berardo PT, Takiya CM, Nasciutti LE. Vascular density and distribution of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) are significantly higher in patients with deeply infiltrating endometriosis affecting the rectum. Fertil Steril 2008;90:148–155. [DOI] [PubMed] [Google Scholar]
- Makabe T, Koga K, Miyashita M, Takeuchi A, Sue F, Taguchi A, Urata Y, Izumi G, Takamura M, Harada M et al. Drospirenone reduces inflammatory cytokines, vascular endothelial growth factor (VEGF) and nerve growth factor (NGF) expression in human endometriotic stromal cells. J Reprod Immunol 2017;119:44–48. [DOI] [PubMed] [Google Scholar]
- McKinnon B, Mueller M, Montgomery G. Progesterone resistance in endometriosis: an acquired property? Trends Endocrinol Metab 2018;29:535–548. [DOI] [PubMed] [Google Scholar]
- Mei L, Bao J, Tang L, Zhang C, Wang H, Sun L, Ma G, Huang L, Yang J, Zhang L et al. A novel mifepristone-loaded implant for long-term treatment of endometriosis: in vitro and in vivo studies. Eur J Pharm Sci 2010;39:421–427. [DOI] [PubMed] [Google Scholar]
- Meigs JV Endometriosis; etiologic role of marriage age and parity; conservative treatment. Obstet Gynecol 1953;2:46–53. [PubMed] [Google Scholar]
- Merviel P, Lourdel E, Sanguin S, Gagneur O, Cabry R, Nasreddine A. Interest of selective progesterone receptor modulators in endometriosis. Gynecologie Obstetrique et Fertilite 2013;41:524–528. [DOI] [PubMed] [Google Scholar]
- Millochau JC, Abo C, Darwish B, Huet E, Dietrich G, Roman H. Continuous amenorrhea may be insufficient to stop the progression of colorectal endometriosis. J Minim Invasive Gynecol 2016;23:839–842. [DOI] [PubMed] [Google Scholar]
- Minami T, Kosugi K, Suganuma I, Yamanaka K, Kusuki I, Oyama T, Kitawaki J. Antiproliferative and apoptotic effects of norethisterone on endometriotic stromal cells in vitro. Eur J Obstet Gynecol Reprod Biol 2013;166:76–80. [DOI] [PubMed] [Google Scholar]
- Mishell DR Jr, Kletzky OA, Brenner PF, Roy S, Nicoloff J. The effect of contraceptive steroids on hypothalamic-pituitary function. Am J Obstet Gynecol 1977;128:60–74. [DOI] [PubMed] [Google Scholar]
- Mita S, Nakakuki M, Ichioka M, Shimizu Y, Hashiba M, Miyazaki H, Kyo S. Dienogest inhibits C-C motif chemokine ligand 20 expression in human endometriotic epithelial cells. Eur J Obstet Gynecol Reprod Biol 2017;214:65–70. [DOI] [PubMed] [Google Scholar]
- Miyashita M, Koga K, Takamura M, Izumi G, Nagai M, Harada M, Hirata T, Hirota Y, Fujii T, Osuga Y. Dienogest reduces proliferation, aromatase expression and angiogenesis, and increases apoptosis in human endometriosis. Gynecol Endocrinol 2014;30:644–648. [DOI] [PubMed] [Google Scholar]
- Mizutani T, Nishiyama S, Amakawa I, Watanabe A, Nakamuro K, Terada N. Danazol concentrations in ovary, uterus, and serum and their effect on the hypothalamic-pituitary-ovarian axis during vaginal administration of a danazol suppository. Fertil Steril 1995;63:1184–1189. [DOI] [PubMed] [Google Scholar]
- Morelli M, Sacchinelli A, Venturella R, Mocciaro R, Zullo F. Postoperative administration of dienogest plus estradiol valerate versus levonorgestrel-releasing intrauterine device for prevention of pain relapse and disease recurrence in endometriosis patients. J Obstet Gynaecol Res 2013;39:985–990. [DOI] [PubMed] [Google Scholar]
- Moreno I, Quiñones L, Catalán J, Miranda C, Roco A, Sasso J, Tamayo E, Cáceres D, Tchernitchin AN, Gaete L et al. Influence of CYP3A4/5 polymorphisms in the pharmacokinetics of levonorgestrel: a pilot study. Biomedica 2012;32:570–577. [DOI] [PubMed] [Google Scholar]
- Mori H, Nakagawa M, Itoh N, Wada K, Tamaya T. Danazol suppresses the production of interleukin-1 beta and tumor necrosis factor by human monocytes. Am J Reprod Immunol 1990;24:45–50. [DOI] [PubMed] [Google Scholar]
- Mori T, Ito F, Matsushima H, Takaoka O, Koshiba A, Tanaka Y, Kusuki I, Kitawaki J. Dienogest reduces HSD17β1 expression and activity in endometriosis. J Endocrinol 2015;225:69–76. [DOI] [PubMed] [Google Scholar]
- Morotti M, Remorgida V, Venturini PL, Ferrero S. Progestogen-only contraceptive pill compared with combined oral contraceptive in the treatment of pain symptoms caused by endometriosis in patients with migraine without aura. Eur J Obstet Gynecol Reprod Biol 2014;179:63–68. [DOI] [PubMed] [Google Scholar]
- Mote PA, Balleine RL, McGowan EM, Clarke CL. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab 1999;84:2963–2971. [DOI] [PubMed] [Google Scholar]
- Neumann F The physiological action of progesterone and the pharmacological effects of progestogens--a short review. Postgrad Med J 1978;54:11–24. [PubMed] [Google Scholar]
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril 2012;98:S1–S62. [DOI] [PubMed] [Google Scholar]
- Ngô C, Chéreau C, Nicco C, Weill B, Chapron C, Batteux F. Reactive oxygen species controls endometriosis progression. Am J Pathol 2009;175:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson CG, Lahteenmaki P, Luukkainen T. Levonorgestrel plasma concentrations and hormone profiles after insertion and after one year of treatment with a levonorgestrel-IUD. Contraception 1980;21:225–233. [DOI] [PubMed] [Google Scholar]
- Nirgianakis K, Grandi G, McKinnon B, Bersinger N, Cagnacci A, Mueller M. Dienogest mediates midkine suppression in endometriosis. Hum Reprod 2016;31:1981–1986. [DOI] [PubMed] [Google Scholar]
- Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril 1997;68:585–596. [DOI] [PubMed] [Google Scholar]
- Novembri R, Borges LE, Carrarelli P, Rocha ALL, De Pascalis F, Florio P, Petraglia F. Impaired CRH and urocortin expression and function in eutopic endometrium of women with endometriosis. J Clin Endocrinol Metab 2011;96:1145–1150. [DOI] [PubMed] [Google Scholar]
- Okada H, Nakajima T, Yoshimura T, Yasuda K, Kanzaki H. The inhibitory effect of dienogest, a synthetic steroid, on the growth of human endometrial stromal cells in vitro. Mol Hum Reprod 2001;7:341–347. [DOI] [PubMed] [Google Scholar]
- Palmer SS, Altan M, Denis D, Tos EG, Gotteland JP, Osteen KG, Bruner-Tran KL, Nataraja SG. Bentamapimod (JNK inhibitor AS602801) induces regression of endometriotic lesions in animal models. Reprod Sci 2016;23:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Kim SH, Chae HD, Kim CH, Kang BM. Efficacy and safety of dienogest in patients with endometriosis: a single-center observational study over 12 months. Clin Exp Reprod Med 2016;43:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update 2015;21:155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B, Peters GA, Skomorovska-Prokvolit Y, Yi L, Tan H, Yousef A, Wang J, Mesiano S. Control of progesterone receptor-a transrepressive activity in myometrial cells: implications for the control of human parturition. Reprod Sci 2018;25:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand 2017;96:623–632. [DOI] [PubMed] [Google Scholar]
- Pavone ME, Malpani SS, Dyson M, Kim JJ, Bulun SE. Fenretinide: a potential treatment for endometriosis. Reprod Sci 2016;23:1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng YH. Altered retinoid uptake and action contributes to cell survival in endometriosis. J Clin Endocrinol Metab 2010;95:E300–E309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FE, Almeida PR, Dias BH, Vasconcelos PR, Guimaraes SB, Medeiros F. Development of a subcutaneous endometriosis rat model. Acta Cir Bras 2015;30:6–12. [DOI] [PubMed] [Google Scholar]
- Price TM, Dai Q. The role of a mitochondrial progesterone receptor (PR-M) in progesterone action. Semin Reprod Med 2015;33:185–194. [DOI] [PubMed] [Google Scholar]
- Razzi S, Luisi S, Calonaci F, Altomare A, Bocchi C, Petraglia F. Efficacy of vaginal danazol treatment in women with recurrent deeply infiltrating endometriosis. Fertil Steril 2007a;88:789–794. [DOI] [PubMed] [Google Scholar]
- Razzi S, Luisi S, Ferretti C, Calonaci F, Gabbanini M, Mazzini M, Petraglia F. Use of a progestogen only preparation containing desogestrel in the treatment of recurrent pelvic pain after conservative surgery for endometriosis. Eur J Obstet Gynecol Reprod Biol 2007b;135:188–190. [DOI] [PubMed] [Google Scholar]
- Rekers NV, Flaig TM, Mallat MJK, Spruyt-Gerritse MJ, Zandbergen M, Anholts JDH, Bajema IM, Clahsen-van Groningen MC, Yang J, de Fijter JW et al. Donor genotype and intragraft expression of CYP3A5 reflect the response to steroid treatment during acute renal allograft rejection. Transplantation 2017;101:2017–2025. [DOI] [PubMed] [Google Scholar]
- Riccio L, Santulli P, Marcellin L, Abrao MS, Batteux F, Chapron C. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol 2018;50:39–49. [DOI] [PubMed] [Google Scholar]
- Riccio LGC, Jeljeli M, Santulli P, Chouzenoux S, Doridot L, Nicco C, Reis FM, Abrao MS, Chapron C, Batteux F. Btk inhibitor Ibrutinib limits endometriosis progression in mice. Hum Reprod 2019;34:1225–1234. [DOI] [PubMed] [Google Scholar]
- Robyn C, Schondorf H, Jurgensen O, Dericks-Tan JS, Taubert HD. Oral contraception can decrease the pituitary capacity to release gonadotrophins in response to synthetic LH-releasing-hormone. Arch Gynakol 1974;216:73–80. [DOI] [PubMed] [Google Scholar]
- Rocha-Junior CV, Da Broi MG, Miranda-Furtado CL, Navarro PA, Ferriani RA, Meola J. Progesterone receptor B ( PGR-B) is partially methylated in eutopic endometrium from infertile women with endometriosis. Reprod Sci 2019;26:1568–1574. [DOI] [PubMed] [Google Scholar]
- Rokitansky C Über Uterusdrüsen-Neubildungen in Uterus- und Ovarial-Sarcomen. Zeitschrift der Kaiserlich Koenigl Gesellschaft der Aerzte zu Wien 1860;37:577–581. [Google Scholar]
- Rommler A, Baumgarten S, Moltz L, Schwartz U, Hammerstein J. Oral contraceptives and pituitary response to GnRH: comparative study of progestin-related effects. Contraception 1985;31:295–303. [DOI] [PubMed] [Google Scholar]
- Roth K, Zahradnik HP, Schäfer WR. Effects of different progestins on prostaglandin biosynthesis in human endometrial explants. Contraception 2019;99:61–66. [DOI] [PubMed] [Google Scholar]
- Rozenberg S, Praet J, Pazzaglia E, Gilles C, Manigart Y, Vandromme J. The use of selective progestin receptor modulators (SPRMs) and more specifically ulipristal acetate in the practice of gynaecology. Aust N Z J Obstet Gynaecol 2017;57:393–399. [DOI] [PubMed] [Google Scholar]
- Sampson JA Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927;14:422–469. [Google Scholar]
- Santulli P, Chouzenoux S, Fiorese M, Marcellin L, Lemarechal H, Millischer AE, Batteux F, Borderie D, Chapron C. Protein oxidative stress markers in peritoneal fluids of women with deep infiltrating endometriosis are increased. Hum Reprod 2015;30:49–60. [DOI] [PubMed] [Google Scholar]
- Sapkota Y, Steinthorsdottir V, Morris AP, Fassbender A, Rahmioglu N, De Vivo I, Buring JE, Zhang F, Edwards TL, Jones S et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun 2017;8:15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala C, Leone Roberti Maggiore U, Barra F, Venturini PL, Ferrero S. Norethindrone acetate versus extended-cycle oral contraceptive (Seasonique®) in the treatment of endometriosis symptoms: a prospective open-label comparative study. Eur J Obstet Gynecol Reprod Biol 2018;222:89–94. [DOI] [PubMed] [Google Scholar]
- Schultze-Mosgau MH, Hochel J, Prien O, Zimmermann T, Brooks A, Bush J, Rottmann A. Characterization of the pharmacokinetics of vilaprisan: bioavailability, excretion, biotransformation, and drug-drug interaction potential. Clin Pharmacokinet 2018;57:1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweikert A, Rau T, Berkholz A, Allera A, Daufeldt S, Wildt L. Association of progesterone receptor polymorphism with recurrent abortions. Eur J Obstet Gynecol Reprod Biol 2004;113:67–72. [DOI] [PubMed] [Google Scholar]
- Seracchioli R, Mabrouk M, Manuzzi L, Vicenzi C, Frasc C, Elmakky A, Venturoli S. Post-operative use of oral contraceptive pills for prevention of anatomical relapse or symptom-recurrence after conservative surgery for endometriosis. Hum Reprod 2009;24:2729–2735. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Mita S, Takeuchi T, Notsu T, Mizuguchi K, Kyo S. Dienogest, a synthetic progestin, inhibits prostaglandin E2 production and aromatase expression by human endometrial epithelial cells in a spheroid culture system. Steroids 2011;76:60–67. [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, Hapgood JP, Winer S, Mishell DR Jr. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev 2013;34:171–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckemann K, Hegele-Hartung C, Chwalisz K. Effects of the progesterone antagonists onapristone (ZK 98 299) and ZK 136 799 on surgically induced endometriosis in intact rats. Hum Reprod 1995;10:3264–3271. [DOI] [PubMed] [Google Scholar]
- Su RW, Strug MR, Jeong JW, Miele L, Fazleabas AT. Aberrant activation of canonical Notch1 signaling in the mouse uterus decreases progesterone receptor by hypermethylation and leads to infertility. Proc Natl Acad Sci U S A 2016;113:2300–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana S, Kajihara T, Mizuno Y, Sato T, Oguro T, Kimura M, Akita M, Ishihara O. Overexpression of microRNA-542-3p attenuates the differentiating capacity of endometriotic stromal cells. Reprod Med Biol 2017;16:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwarc MM, Hai L, Gibbons WE, Peavey MC, White LD, Mo Q, Lonard DM, Kommagani R, Lanz RB, DeMayo FJ et al. Human endometrial stromal cell decidualization requires transcriptional reprogramming by PLZF. Biol Reprod 2018;98:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja A, Kaur S, Soni RK, Bhanupriya KJ, Singla L. Evaluating the efficacy of levonorgestrel intrauterine system and danazol for relief of postoperative pain in endometriosis. J Clin Diagn Res 2017;11:QC10–QC12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi F, Enatsu A, Ikebuchi A, Yamane E, Moriyama M, Murakami J, Harada T, Harada T. Efficacy of norethisterone in patients with ovarian endometrioma. Yonago Acta Med 2017;60:182–185. [PMC free article] [PubMed] [Google Scholar]
- Taniguchi F, Enatsu A, Ota I, Toda T, Arata K, Harada T. Effects of low dose oral contraceptive pill containing drospirenone/ethinylestradiol in patients with endometrioma. Eur J Obstet Gynecol Reprod Biol 2015;191:116–120. [DOI] [PubMed] [Google Scholar]
- Tarjanne S, Ng CHM, Manconi F, Arola J, Mentula M, Maneck B, Fraser IS, Heikinheimo O. Use of hormonal therapy is associated with reduced nerve fiber density in deep infiltrating, rectovaginal endometriosis. Acta Obstet Gynecol Scand 2015;94:693–700. [DOI] [PubMed] [Google Scholar]
- Tokushige N, Markham R, Russell P, Fraser IS. Effect of progestogens and combined oral contraceptives on nerve fibers in peritoneal endometriosis. Fertil Steril 2009;92:1234–1239. [DOI] [PubMed] [Google Scholar]
- Tosti C, Pinzauti S, Santulli P, Chapron C, Petraglia F. Pathogenetic mechanisms of deep infiltrating endometriosis. Reprod Sci 2015;22:1053–1059. [DOI] [PubMed] [Google Scholar]
- Tsuno A, Nasu K, Yuge A, Matsumoto H, Nishida M, Narahara H. Decidualization attenuates the contractility of eutopic and ectopic endometrial stromal cells: implications for hormone therapy of endometriosis. J Clin Endocrinol Metab 2009;94:2516–2523. [DOI] [PubMed] [Google Scholar]
- Vandenberg G, DeVane G, Yen SS. Effects of exogenous estrogen and progestin on pituitary responsiveness to synthetic luteinizing hormone-releasing factor. J Clin Invest 1974;53:1750–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellini P, Bracco B, Mosconi P, Roberto A, Alberico D, Dhouha D, Somigliana E. Norethindrone acetate or dienogest for the treatment of symptomatic endometriosis: a before and after study. Fertil Steril 2016a;105:734–743 e733. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Buggio L, Berlanda N, Barbara G, Somigliana E, Bosari S. Estrogen-progestins and progestins for the management of endometriosis. Fertil Steril 2016b;106:1552–1571 e1552. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Buggio L, Frattaruolo MP, Borghi A, Dridi D, Somigliana E. Medical treatment of endometriosis-related pain. Best Pract Res Clin Obstet Gynaecol 2018a;51:68–91. [DOI] [PubMed] [Google Scholar]
- Vercellini P, De Giorgi O, Mosconi P, Stellato G, Vicentini S, Crosignani PG. Cyproterone acetate versus a continuous monophasic oral contraceptive in the treatment of recurrent pelvic pain after conservative surgery for symptomatic endometriosis. Fertil Steril 2002;77:52–61. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Donati A, Ottolini F, Frassineti A, Fiorini J, Nebuloni V, Frattaruolo MP, Roberto A, Mosconi P, Somigliana E. A stepped-care approach to symptomatic endometriosis management: a participatory research initiative. Fertil Steril 2018b;109:1086–1096. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Ottolini F, Frattaruolo MP, Buggio L, Roberto A, Somigliana E. Is shifting to a progestin worthwhile when estrogen–progestins are inefficacious for endometriosis-associated pain? Reprod Sci 2018c;25:674–682. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril 2005;84:1375–1387. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Somigliana E, Consonni D, Frattaruolo MP, De Giorgi O, Fedele L. Surgical versus medical treatment for endometriosis-associated severe deep dyspareunia: I. Effect on pain during intercourse and patient satisfaction. Hum Reprod 2012;27:3450–3459. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Somigliana E, Vigano P, Abbiati A, Daguati R, Crosignani PG. Endometriosis: current and future medical therapies. Best Pract Res Clin Obstet Gynaecol 2008;22:275–306. [DOI] [PubMed] [Google Scholar]
- Vigano P, Di Blasio AM, Busacca M, Sabbadini MG, Vignali M. Danazol suppresses both spontaneous and activated human lymphocyte-mediated cytotoxicity. Am J Reprod Immunol 1992;28:38–42. [DOI] [PubMed] [Google Scholar]
- Vigano P, Di Blasio AM, Busacca M, Vignali M. Immunosuppressive effect of danazol on lymphocyte-mediated cytotoxicity toward human endometrial stromal cells. Gynecol Endocrinol 1994;8:13–19. [DOI] [PubMed] [Google Scholar]
- Vinci G, Arkwright S, Audebourg A, Radenen B, Chapron C, Borghese B, Dousset B, Mehats C, Vaiman D, Vacher-Lavenu MC et al. Correlation between the clinical parameters and tissue phenotype in patients affected by deep-infiltrating endometriosis. Reprod Sci 2016;23:1258–1268. [DOI] [PubMed] [Google Scholar]
- Wagenfeld A, Saunders PT, Whitaker L, Critchley HO. Selective progesterone receptor modulators (SPRMs): progesterone receptor action, mode of action on the endometrium and treatment options in gynecological therapies. Expert Opin Ther Targets 2016;20:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Tokushige N, Markham R, Fraser IS. Rich innervation of deep infiltrating endometriosis. Hum Reprod 2009a;24:827–834. [DOI] [PubMed] [Google Scholar]
- Wang G, Tokushige N, Russell P, Dubinovsky S, Markham R, Fraser IS. Hyperinnervation in intestinal deep infiltrating endometriosis. J Minim Invasive Gynecol 2009b;16:713–719. [DOI] [PubMed] [Google Scholar]
- Wang L, Ellsworth KA, Moon I, Pelleymounter LL, Eckloff BW, Martin YN, Fridley BL, Jenkins GD, Batzler A, Suman VJ et al. Functional genetic polymorphisms in the aromatase gene CYP19 vary the response of breast cancer patients to neoadjuvant therapy with aromatase inhibitors. Cancer Res 2010;70:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker LH, Murray AA, Matthews R, Shaw G, Williams AR, Saunders PT, Critchley HO. Selective progesterone receptor modulator (SPRM) ulipristal acetate (UPA) and its effects on the human endometrium. Hum Reprod 2017;32:531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser F, Cohen M, Gaeddert A, Yu J, Burks-Wicks C, Berga SL, Taylor RN. Evolution of medical treatment for endometriosis: back to the roots? Hum Reprod Update 2007;13:487–499. [DOI] [PubMed] [Google Scholar]
- Wood GP, Wu CH, Flickinger GL, Mikhail G. Hormonal changes associated with danazol therapy. Obstet Gynecol 1975;45:302–304. [PubMed] [Google Scholar]
- Wu SP, Li R, DeMayo FJ. Progesterone receptor regulation of uterine adaptation for pregnancy. Trends Endocrinol Metab 2018;29:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Starzinski-Powitz A, Guo SW. Prolonged stimulation with tumor necrosis factor-alpha induced partial methylation at PR-B promoter in immortalized epithelial-like endometriotic cells. Fertil Steril 2008;90:234–237. [DOI] [PubMed] [Google Scholar]
- Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics 2006;1:106–111. [DOI] [PubMed] [Google Scholar]
- Xiao B, Zeng T, Wu S, Sun H, Xiao N. Effect of levonorgestrel-releasing intrauterine device on hormonal profile and menstrual pattern after long-term use. Contraception 1995;51:359–365. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Hirata S, Shoda T, Hoshi K. Progesterone receptor mRNA variant containing novel exon insertions between exon 4 and exon 5 in human uterine endometrium. Endocr J 2002;49:473–482. [DOI] [PubMed] [Google Scholar]
- Yilmaz BD, Bulun SE. Endometriosis and nuclear receptors. Hum Reprod Update 2019;25:473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Pavone ME, Lu Z, Wei J, Kim JJ. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J Clin Endocrinol Metab 2012;97:E35–E43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Boicea A, Barrett KL, James CO, Bagchi IC, Bagchi MK, Nezhat C, Sidell N, Taylor RN. Reduced connexin 43 in eutopic endometrium and cultured endometrial stromal cells from subjects with endometriosis. Mol Hum Reprod 2014;20:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanatta A, Pereira RM, Rocha AM, Cogliati B, Baracat EC, Taylor HS, Motta EL, Serafini PC. The relationship among HOXA10, estrogen receptor alpha, progesterone receptor, and progesterone receptor B proteins in rectosigmoid endometriosis: a tissue microarray study. Reprod Sci 2015;22:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YX Effect of mifepristone in the different treatments of endometriosis. Clin Exp Obstet Gynecol 2016;43:350–353. [PubMed] [Google Scholar]


