Abstract
BACKGROUND
Women who achieve pregnancy by ART show an increased risk of obstetric and perinatal complications compared with those with spontaneous conception (SC).
OBJECTIVE AND RATIONALE
The purpose of this systematic review and meta-analysis was to synthesize the best available evidence regarding the association between ART and gestational diabetes mellitus (GDM) in women with singleton pregnancies. The research question asked was whether the risk of GDM is higher in women achieving singleton pregnancy by ART compared with those achieving singleton pregnancy spontaneously.
SEARCH METHODS
A literature search, in MEDLINE, Scopus and Cochrane databases, covering the period 1978–2019, was performed aiming to identify studies comparing the risk of GDM in singleton pregnancies after ART versus after SC. Both matched and unmatched studies were considered eligible. Meta-analysis of weighted data was performed using the random effects model. Results were reported as risk ratio (RR) with 95% CI. Heterogeneity was quantified with the I2 index.
OUTCOMES
The study reports on 63 760 women who achieved a singleton pregnancy after ART (GDM was present in 4776) and 1 870 734 women who achieved a singleton pregnancy spontaneously (GDM in 158 526). Women with singleton pregnancy achieved by ART showed a higher risk of GDM compared with those with singleton pregnancy achieved spontaneously (RR 1.53, 95% CI 1.39–1.69; I2 78.6%, n = 37, 1 893 599 women). The direction or the magnitude of the effect observed did not change in subgroup analysis based on whether the study was matched (n = 17) or unmatched (n = 20) (matched: RR 1.42, 95% CI 1.17–1.72; I2 61.5%—unmatched: RR 1.58, 95% CI 1.40–1.78; I2 84.1%) or whether it was prospective (n = 12) or retrospective (n = 25) (prospective studies: RR 1.52, 95% CI 1.27–1.83, I2 62.2%—retrospective studies: RR 1.53, 95% CI 1.36–1.72, I2 82.5%). Regarding the method of fertilization, a higher risk of GDM after ART versus SC was observed after IVF (n = 7), but not after ICSI (n = 6), (IVF: RR 1.95, 95% CI 1.56–2.44, I2 43.1%—ICSI: RR 1.42, 95% CI 0.94–2.15, I2 73.5%). Moreover, regarding the type of embryo transfer (ET), a higher risk of GDM after ART versus SC was observed after fresh (n = 14) but not after frozen (n = 3) ET (fresh ET: RR 1.38, 95% CI 1.03–1.85, I2 75.4%—frozen ET: RR 0.46, 95% CI 0.10–2.19; I2 73.1%). A higher risk of GDM was observed after ART regardless of whether the eligible studies included patients with polycystic ovary syndrome (RR 1.49, 95% CI 1.33–1.66, I2 75.0%) or not (RR 4.12, 95% CI 2.63–6.45, I2 0%), or whether this information was unclear (RR 1.46, 95% CI 1.22–1.75, I2 77.7%).
WIDER IMPLICATIONS
The present systematic review and meta-analysis, by analysing 1 893 599 women, showed a higher risk of GDM in women achieving singleton pregnancy by ART compared with those achieving singleton pregnancy spontaneously. This finding highlights the importance of early detection of GDM in women treated by ART that could lead to timely and effective interventions, prior to ART as well as during early pregnancy.
Keywords: gestational diabetes mellitus, ART, spontaneous conception, singleton pregnancy, IVF/ICSI, embryo transfer
Introduction
The number of pregnancies resulting from ART is continuously increasing worldwide. Not unexpectedly, the interest in the potential risks to the mothers and children born after ART has also increased. Currently, a higher risk of obstetric and perinatal complications appears to be present in women achieving pregnancy after ART compared with those achieving pregnancy spontaneously (Nassar et al., 2003, Jackson et al., 2004, Pandey et al., 2012, Qin et al., 2015, Vermey et al., 2019).
One of the most common and important complications of pregnancy is gestational diabetes mellitus (GDM). GDM has been associated with a higher risk of pre-eclampsia, caesarean section in the mother as well as macrosomia, shoulder dystocia, hypoglycaemia and jaundice in the newborn (Ashrafi et al., 2014). In women undergoing ART, major risk factors for GDM, such as advanced maternal age, obesity, multiple pregnancy and polycystic ovary syndrome (PCOS) are often encountered, suggesting a potential association between GDM and ART (Szymanska et al., 2011). Support for this association was offered by a meta-analysis published in 2012 (Pandey et al., 2012), including, however, a limited number of studies (n = 7). Since the publication of that meta-analysis, several studies evaluating the association between GDM and ART have been published (Farhi et al., 2013, Stojnic et al., 2013, Ashrafi et al., 2014, Silberstein et al., 2014, Xu et al., 2014, Xu et al., 2015, Beyer and Amari, 2016, Valenzuela-Alcaraz et al., 2016, Zhu et al., 2016, Cai et al., 2017, Luke et al., 2017, Qin et al., 2017, Dayan et al., 2018, Frankenthal et al., 2018, Harlev et al., 2018, Lee et al., 2018, Nagata et al., 2019, Szymusik et al., 2019, Yang et al., 2019), with some of them including thousands of patients (Xu et al., 2014, Luke et al., 2017), allowing for more precise estimates to be obtained. Moreover, this is the first systematic review and meta-analysis evaluating the influence of various moderators, such as the method of fertilization and type of embryo transfer (ET), as well as of various confounders, such as study type, in the association between GDM and ART.
The purpose of this systematic review and meta-analysis was to synthesize the best available evidence regarding the association between ART and GDM in singleton pregnancies. The specific research question asked was whether the risk of GDM is higher in women achieving singleton pregnancy by ART compared with those achieving singleton pregnancy spontaneously. In addition, the influence of various moderators, such as the method of fertilization (IVF or ICSI) and type of embryo transfer (fresh versus frozen), as well as of various confounders, such as type of study (matched versus unmatched, prospective versus retrospective), was explored.
Methods
Identification of literature
A computerized literature search in MEDLINE, Scopus and Cochrane (CENTRAL) was performed independently by two reviewers (J.K.B and P.G.A), covering the period between 1978 and July 2019. This systematic review followed the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines (Liberati et al., 2009) (PROSPERO registration number: CRD42019124251).
Search strategy
The following PICO (Population, Intervention or exposure, Comparison, Outcome) elements were applied as inclusion criteria for this systematic review: Population: singleton pregnancies; Intervention: ART; Comparator: SC; Outcome: GDM. A search strategy with various synonyms was entered as free-text terms in the electronic databases in an attempt to maximize the sensitivity of the search strategy. The following search string was used: (microinjection[tiab] OR ‘intra-cytoplasmic sperm injection’[tiab] OR ICSI[tiab] OR ‘intracytoplasmic sperm injection’[tiab] OR IVF[tiab] OR ‘in-vitro fertilization’[tiab] OR ‘in vitro fertilization’[tiab] OR ‘in-vitro fertilization’[tiab] OR ‘in vitro fertilization’[tiab]) AND (‘Diabetes, Gestational’[MeSH] OR ‘gestational diabetes’[tiab] OR ‘pregnancy complications’[tiab] OR ‘obstetric complications’[tiab] OR (pregnancy[tiab] AND (diabet*[tiab] OR ‘hyperglycaemia’[tiab] OR ‘hyperglycemia’[tiab] OR ‘high blood glucose’[tiab] OR ‘high plasma glucose’[tiab]))) NOT (Animal[MeSH] NOT Human[MeSH]) NOT (letter[pt] OR comment[pt] OR editorial[pt] OR Review[pt] OR ‘practice guideline’[ptyp] OR ‘case reports’[ptyp]). No language limitations were applied. Institutional Board Review was not obtained as previously published data were used.
Selection of studies
Criteria for inclusion/exclusion of studies were established prior to the literature search. Studies had to fulfil the following criteria for eligibility: comparative data regarding the risk of GDM in women achieving singleton pregnancy by ART or spontaneously; ovarian stimulation, performed by gonadotropins and GnRH analogues. ART pregnancies included those achieved by IVF or ICSI, after fresh and/or frozen/thawed embryo transfer with autologous gametes. Studies were excluded if pregnancies were achieved using donor gametes, surrogacy, gamete intrafallopian transfer or zygote intrafallopian transfer. Studies performed exclusively in women with PCOS were also excluded due to the known association between PCOS and GDM (Toulis et al., 2009, Yu et al., 2016). Selection of eligible studies was performed independently by two of the reviewers (J.K.B and E.M.K). Any disagreement was resolved by discussion.
Data extraction
Data extraction was performed independently by two of the reviewers (J.K.B and E.M.K). When a study provided data separately for the method of fertilization and type of ET, the relevant datasets were used for subgroup analyses. Any disagreement between the two reviewers responsible for data extraction was resolved by discussion. In case of missing data or ambiguities in study design or trial conduction, the study authors were contacted by e-mail to request additional information.
Risk of bias and study quality assessment
The Newcastle-Ottawa Scale (NOS) was used for assessing the quality of each study. Briefly, this system evaluates studies based on three criteria: participant selection; comparability of study groups; and assessment of outcome or exposure. A study can be awarded a maximum of four stars for the selection category, a maximum of two stars for the comparability category and a maximum of three stars for the outcome/exposure category (Wells et al., 2014).
Subgroup analyses and meta-regression
The influence of various factors, such as type of study (matched versus unmatched, prospective versus retrospective) method of fertilization (IVF or ICSI), type of ET (fresh or frozen), inclusion or not of patients with PCOS and study quality (‘good quality’ versus ‘poor quality’ studies), was explored by performing pre-planned subgroup analyses and meta-regression.
Statistical analysis
The dichotomous data results for each of the eligible studies were expressed as risk ratio (RR) with 95% CI. These results were combined for meta-analysis using the random effects model (DerSimonian and Laird, 1986). Study-to-study variation was assessed by using the Chi2 statistic (the hypothesis tested was that the studies are all drawn from the same population, i.e. from a population with the same effect size). In addition, the use of the I2 statistic was employed to indicate heterogeneity between studies that could not be attributed to chance, with I2 ≥ 40% (Higgins and Green, 2011) indicating significant heterogeneity. The presence of publication bias was tested by using the Harbord–Egger’s test (Harbord et al., 2006). Statistical significance was set at a P level of 0.05. A meta-analysis of weighted average effect sizes was performed using STATA v14.0 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX, USA: StataCorp LP).
Results
Identification of literature
The initial literature search yielded 1356 studies, 73 of which were further evaluated by retrieving their full text and 34 of these were excluded (Supplementary Table SI). Eventually, 38 eligible studies were included in the systematic review, 37 of which offered extractable data for the meta-analysis. A flow diagram of this process is present in Fig. 1.
Figure 1.
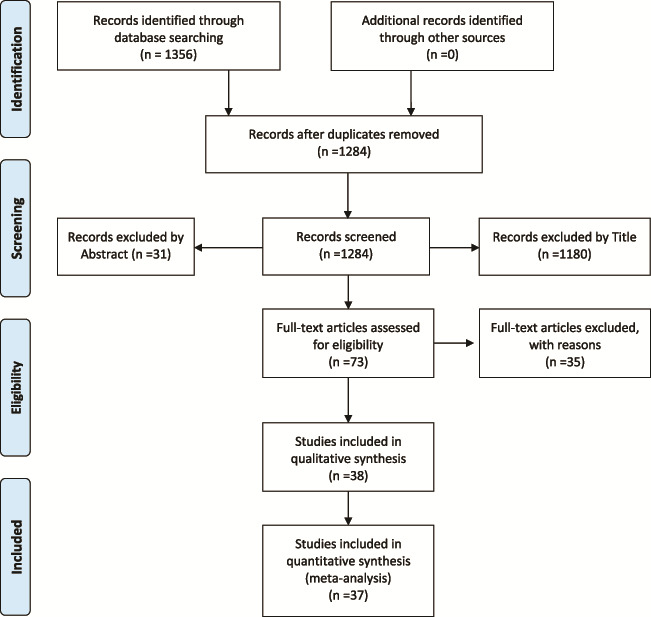
Flow diagram for selection of studies on risk of gestational diabetes mellitus after spontaneous and ART pregnancies.
Systematic review
Thirty-eight cross-sectional studies (17 matched and 21 unmatched; 13 prospective and 25 retrospective), published between 1995 and 2019, were eligible for the systematic review, including a total of 1 934 494 women. Characteristics of the studies included in the systematic review are presented in Table I. Of the 38 studies, 24 were graded as being of ‘good quality’ and 14 of ‘poor quality’, according to the NOS (Supplementary Table SII). The definition of GDM was reported in 12 out of the 38 studies. After communication with the corresponding authors, further data on the definition of GDM was obtained for 23 studies (Table I).
Table I.
Characteristics of the 38 eligible studies included in the systematic review.
| Study, country of origin, journal or meeting | Type of study/Study period |
Patients ART/SC |
Matching | Inclusion/exclusion criteria | PCOS patients included | Clear definition of GDM | Gonadotropin type (dose) in patients undergoing ART | GnRH analogue/protocol in patients undergoing ART | Criteria for hCG administration in patients undergoing ART | Fertilization method in patients undergoing ART | Fresh/Frozen ET in patients undergoing ART | Day of ET in patients undergoing ART | Luteal phase support/endometrial preparation in patients undergoing ART | Authors contacted/replied |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ashrafi et al., 2014, Iran, Eur J Obstet Gynecol Reprod Biol | Retrospective cross-sectional/September 2011–October 2012 | 95/215 | No | Women with singleton pregnancies conceived following ART or spontaneously/PCOS, age > 40 years, family history of diabetes in first-degree relatives, pre-pregnancy diabetes, glucose intolerance treated with hypoglycemic agent, history of GDM, history of stillbirth, recurrent miscarriage, history of macrosomia, parity >3, Cushing syndrome, congenital adrenal hyperplasia, hypothyroidism | No | ≥2 of the 100-g OGTT glucose levels exceeded: fasting,
>5.3 mmol/l (>95 mg/dl); 1 h, >10.0 mmol/l (>180 mg/dl);
2 h > 8.6 mmol/l (>155 mg/dl); and 3 h, >7.8 mmol/l
(>140 mg/dl) (American Diabetes Association) |
Not reported | Long agonist and antagonist protocols | Not reported | IVF/ICSI | Fresh | Not reported | Progesterone | Yes/yes |
| Barros Delgadillo et al., 2006, Mexico, Ginecol Obstet Mex | Retrospective cross-sectional/October 1999–November 2004 | 26/52 | Yes (by maternal age and the number of fetus) | Control group was selected from the institutional registry/pregnancies resolved before Week 26, diabetes mellitus, systemic chronic arterial hypertension, nephropathies, heart disease and diseases of collagen | Unclear | ≥2 altered values of the glucose tolerance curve of 180 min and by sieve of 50 g of glucose (>180 mg/dl/h). | rFSH (300–450 IU) | Leuprolide/long | ≥3 follicles ≥18 mm and E2 ≥ 500 pg/ml | IVF | Fresh | Day 3 | Progesterone vag. or I.M. gel and oral estradiol | Yes/no |
| Beyer et al, 2016, Germany, Middle East Fertility Society Journal | Retrospective cross-sectional/13-year period | 467/ 6417 |
No | ART and delivery at the university Center/Cryoconservation of 2PN oocytes resulting from IVM cycles and/or assisted hatching; delivery <24w, multiple pregnancies and incomplete data. |
Unclear | Not reported | rFSH | Cetrorelix or Decapeptyl (long) | ≥3 follicles ≥17 mm with corresponding E2 serum levels | IVF/ICSI | Fresh/frozen | Not reported | Transdermal estradiol with transvaginal progesterone | Yes/no |
| Cai et al, 2017, Singapore, Hum Reprod | Prospective cross-sectional/June 2009–September 2010 | 76/1013 | No | Aged ≥18 years at 11–14 weeks of gestation/Type 1 diabetes mellitus or were receiving chemotherapy or psychotropic drugs. | Yes | 75 g OGTT after 8–10 h of overnight fasting at 26–28 weeks’
gestation. GDM: ≥7.0 mmol/L for fasting and/or ≥7.8 mmol/L for 2-h postprandial plasma glucose levels (WHO criteria, 1999, 2013) |
Not reported | Not reported | Not reported | Undefined | Undefined | Not reported | Not reported | Yes/yes |
| Caserta et al., 2008, Italy, Acta Obstetricia et Gynecologica | Prospective cross-sectional/February 2004–October 2006 | 358/304 | Yes (parity, age, height, weight, ethnic origin, smoking and no history of infertility) | Male cause of infertility/chronic medical disorders, OHSS, female causes of infertility | Unclear | Not reported | rFSH (225 IU) | Decapeptyl (long) | ≥3 follicles reached 17 mm | ICSI | Fresh | Day 2 | Progesterone vag | Yes/no |
| Chaveeva et al, 2011, UK, Fetal Diagnosis and Therapy | Prospective cross -sectional/January 2000–December 2001 | 634/ 40 261 |
No | 11–13 + 6 weeks of gestation/pregnancies conceived by IUI, those with fetal aneuploidies or major defects | Unclear | Fasting plasma glucose level is at least 6 mmol/l or the plasma glucose level 2 h after the oral administration of 75 g glucose is ≥7.8 mmol/l | Not reported | Not reported | Not reported | IVF | Undefined | Not reported | Not reported | Yes/no |
| Dayan et al., 2018, Canada, Hum Reprod | Retrospective cross-sectional/January 2013–January 2014 | 1596/112 813 | No | A live or stillborn infant weighing ≥500 g at ≥20 weeks’ gestation/women ≤18 years or with missing maternal age, those with multiple gestations, elective terminations or ectopic or molar pregnancies, and if another form of ART was used | Yes | No: BORN birth registry (codes: D0013 & M0531) and CIHI-DAD (codes: O24.5 to O24.8) |
Not reported | Not reported | Not reported | IVF/ICSI | Fresh/frozen | Not reported | Not reported | Yes/yes |
| De Geyter et al., 2006, Switzerland, Hum Reprod | Prospective cross -sectional/August 1996–March 2004 | 261/443 | No | Pregnancies from infertile couples during the study period | Unclear | Not reported | uhMG or rFSH | Triptorelin acetate/long or Ganirelix | Not reported | IVF/ICSI | Fresh/frozen | Day 2 | Both estradiol valerate and vaginal micronized progesterone | Yes/no |
| Farhi et al., 2013, Israel, Reprod Biomed Online | Prospective cross -sectional/June 2006–December 2008 | 509/587 | No | 6–12 weeks of gestation demonstrating one gestational sac with a fetal heart pulse | Yes | No: The definition of GDM for diagnosis was based solely on patients’ report |
Not reported | Not reported | Not reported | IVF/ICSI | Fresh/frozen | Not reported | Not reported | Yes/yes |
| Frankenthal et al., 2018, Israel, Obes Res Clin Pract | Prospective cross -sectional/June 2006–December 2008 | 504/554 | No | 6–12 weeks of gestation | Yes | No: The definition of GDM for diagnosis was based solely on patients’ report |
Not reported | Not reported | Not reported | Undefined | Undefined | Not reported | Not reported | Yes/yes |
| Harlev et al., 2018, Israel, Int J Gynaecol Obstet | Retrospective cross-sectional/January 1991–December 2013 | 229/7929 | No | Women aged at least 40 years/conceived following oocyte donation, were surrogate mothers, or if they had multifetal pregnancies; aged >45 years | Yes | OGTT >200 or an OGTT of 100gr with 2 abnormal values in a non-previously diagnosed patient as diabetic |
Not reported | Not reported | Not reported | IVF/ICSI | Undefined | Not reported | Not reported | Yes/yes |
| Isaksson et al., 2002, Finland, Hum Reprod | Retrospective cross-sectional/January 1993–March 1999 | 69/345 | Yes (maternal age, parity, year of birth, mother’s residence, number of children at birth) | Pregnancies ending in birth/ Triplet pregnancies and those ending in spontaneous abortion |
Unclear | Not reported | hMG | Buserelin long | ≥3 mature follicles ≥18 mm | IVF/ICSI | Undefined | Day 2 | Progesterone vag | Yes/no |
| Katalinic et al., 2004, Germany, Fertil Steril | Prospective cross -sectional/ART: August 1998–August
2000 Control: January 1993–December 2001 |
2687/7938 | No | Pregnancies, conceived after an ICSI procedure and the transfer of fresh embryos before the 16th week of gestation. Control cohort was taken from the Congenital Malformation Monitoring-Centre Saxony-Anhalt/those who could not be contacted after inclusion, congenital malformation | Yes | No: EUROCAT (code: O24) |
Not reported | Not reported | Not reported | ICSI | Fresh | Not reported | Not reported | Yes/yes |
| Knoester et al., 2008, The Netherlands, Fertil Steril | Prospective cross -sectional/June 1996–December 1999 | 87/85 | Yes (socioeconomic status, gender and birth date) | Singletons conceived by ICSI. Regular preschools and primary
schools with zip codes that indicated social class distributions similar to the
ICSI cohort assisted in the recruitment of naturally conceived
singletons/ Oocyte or sperm donation, cryopreservation of the embryo and selective embryo reduction with medical indication |
Unclear | No: Glucose intolerance of variable degree with onset or first recognition during pregnancy |
Not reported | Not reported | Not reported | ICSI | Fresh | Not reported | Not reported | Yes/yes |
| Koivurova et al., 2002, Finland, Hum Reprod | Retrospective cross-sectional / 1990–1995 |
153/580 | Yes (sex of the child, year of birth, area, maternal age, parity, social class and fetal plurality) | All IVF live births and stillbirths after completion of week 22 of gestation or with a birth weight of ≥500 g derived from registers at the IVF outpatient clinic in the University Hospital and the Infertility Clinic of the Family Federation of Finland/not reported | Yes | Altered glucose metabolism requiring dietary or insulin treatment.
GDM was detected by a 2 h OGTT (Finnish Diabetes Association and international recommendations) |
hMG | Buserelin or Nafarelin long | Not reported | IVF/ICSI | Fresh | Day 2 | Progesterone or chorionic gonadotropin for 14 days | Yes/yes |
| Lee et al., 2018, USA, Fertil Steril | Prospective cross-sectional/not reported | 34/74 | Yes (maternal age, race, ethnicity and fetal sex) | All pregnancies at late first trimester at the time of chorionic villus sampling (CVS) and followed until delivery. | Unclear | No: Standard ACOG criteria (ICD-10-CM: O24.415) |
Not reported | Not reported | Not reported | Undefined | Fresh/frozen | Not reported | Not reported | Yes/yes |
| Luke et al., 2017, USA, AJOG | Retrospective cross-sectional/July 2004–December 2010 | 10 149/ 459 623 |
No | All live births of ≥22 weeks’ gestation and ≥350 g birth weight to Massachusetts resident women | Yes | No: ICD-9 code: 648.8 (abnormal glucose tolerance of mother, antepartum condition, or complication) |
Not reported | A range of protocols were used (aromatase inhibitors, minimal stimulation, agonist, agonist flare, antagonist) | Not reported | IVF/ICSI | Fresh/frozen | Not reported | Not reported | Yes/yes |
| Machtinger et al., 2015, USA, RBMOnline | Retrospective cross-sectional/January 2007–December 2011 | 464/1171 | No | All women with either spontaneous or IVF singleton pregnancies
followed at the outpatient clinics of the hospital during study
period/ Pregnancies from Day 5 transfers, multiple pregnancies, pregnancies with vanishing twins, cryopreserved cycles, oocyte donors and gestational carrier cycles |
Yes | No: Glucose intolerance with onset or first recognition during pregnancy |
Not reported | Not reported | Not reported | IVF/ICSI | Fresh | Day 3 | Not reported | Yes/no |
| Maman et al., 1998, Israel, Fertil Steril | Retrospective cross-sectional/1989–1994 | 169/496 | Yes (maternal age, gestational age and parity) | Pregnancies that led to a live birth (≥25 weeks’ gestation or ≥500 g birth weight) | Unclear | Abnormal fasting blood glucose level or abnormal OGTT result between 24 and 28 weeksof gestation/sequential pregnancies | Not reported | Not reported | Not reported | Undefined | Undefined | Not reported | Not reported | Yes/yes |
| Nagata et al., 2019, Japan, BMC Pregnancy and Childbirth | Prospective cross -sectional/January 2011- March | 2993/88 873 | No | All live births of ≥22 weeks’ gestation | Yes | OGTT with 75 g sugar, diagnostic criteria: blood glucose values of (i) ≥92 mg/dl in a fasted state; (ii) ≥180 mg/dl after 1 h; or (iii) ≥153 mg/dL after 2 h | Not reported | Not reported | Not reported | IVF/ICSI | Fresh/frozen | Not reported | Not reported | Yes/yes |
| Ochsenkuhnet al, 2003, Germany, Arch Gynecol Obstet | Retrospective cross-sectional/1991–1996 | 163/322 | Yes (maternal age, gestational age and parity) | Gestational age of at least 24 completed weeks and/or children with >499 g birth weight | Yes | No: Screening test with 50 g Glucose and a 100 g OGTT |
Not reported | Not reported | Not reported | Undefined | Undefined | Not reported | Not reported | Yes/yes |
| Qin et al, 2016, China, Reprod Sci. | Prospective cross -sectional/March 2013–February 2016 | 1260/2480 | No | Women who provided informed consent, belonged to singleton pregnancies, participated in the follow-up process and had a complete case report form/deliveries of women <15 years and >60 years, twin, triplet, and quadruplet) pregnancies, egg donation | Unclear | Not reported | Not reported | Not reported | Not reported | IVF/ICSI | Undefined | Not reported | Not reported | Yes/no |
| Reubinoff et al., 1997, Israel, Fertil Steril | Retrospective cross-sectional/1983–1993 | 260/260 | Yes (maternal age, parity, ethnic origin, location and date of delivery) | Pregnancies leading to live births (>25 weeks’ gestation or > 500 g birth weight) | Yes | No: Two abnormal values in OGTT |
CC + hMG or hMG alone | GnRH analogue (long luteal or follicular) | Leading follicle reached 17–20 mm and serum E2 levels >500 pg/ml | IVF | Fresh/frozen | Not reported | Progesterone I.M. | Yes/yes |
| Sazonova et al., 2011, Sweden, Hum Reprod | Retrospective cross-sectional/2002–2006 | 20 236/ 571 914 |
No | Data from 16 IVF clinics were cross-linked with the Swedish Medical Birth Registry and compared with all children born after spontaneous conception during the same time period | Yes | No: ICD-10 codes |
rFSH or hMG | Agonist or antagonist protocols | Not reported | IVF/ICSI | Fresh/frozen | Not reported | Not reported | Yes/yes |
| Schieve et al., 2007, USA, Matern Child Health J | Retrospective cross-sectional/1997–1998 | 1400/ 1400 |
Yes (birth month and year, maternal age, parity, race/ethnicity) | Restriction to singletons AND exclusion if: maternal age <20, education <high school, mother not married, public/no health insurance for prenatal care, public/no health insurance for labour and delivery; no or inadequate prenatal care or third trimester initiation of prenatal care, and data on race/ethnicity missing | Unclear | Not reported | Not reported | Not reported | Not reported | IVF/ICSI | Fresh/frozen | Not reported | Not reported | Yes/no |
| Sebastiani et al., 2009, Spain, An Pediatr (Barc). | Retrospective cross-sectional/January 1999–December 2005 | 176/185 | No | Data collected from all pregnancies that were conceived in the study period/Hereditary disease, children of alcoholic mothers, drug addicts and children of mothers who have used drugs with potential teratogenic effect during pregnancy | Unclear | Not reported | Not reported | Not reported | Not reported | IVF/ICSI | Undefined | Not reported | Not reported | Yes/no |
| Silberstein et al., 2014, Israel, J Matern Fetal Neonatal Med | Retrospective cross-sectional/1988–2006 | 1294/171 513 | No | All women who conceived and delivered singletons at the Soroka University Medical Center in the study period | Unclear | Not reported | Not reported | Not reported | Not reported | IVF | Undefined | Not reported | Not reported | Yes/no |
| Stojnic et al., 2013, Serbia, Clin Exp Obstet Gynecol | Prospective cross-sectional/January 2006–January 2010 | 634/634 | Yes (maternal age, parity, education, and BMI) | All pregnancies with duration of >26 weeks/pregnancies resulting from an oocyte donation, cryopreserved cycles or conceived as twin but continued as singleton | Yes | If at least two values of plasma glucose concentrations are ≥5.28, 10.0, 8.61 or 7.78 mmol/l for fasting, 1-, 2- and 3-h post-glucose load glucose values, after performing a 100 g OGTT (American Diabetes Association, WHO, 1999) | rFSH or hMG | GnRH agonist long | When at least of half of the dominant follicles reached 18 mm in average diameter | IVF/ICSI | Fresh | Day 2 or 3 | Micronized oral/vaginal progesterone 600 mg per day or muscular progesterone 250 mg on every second day | Yes/yes |
| Suzuki et al., 2007, Japan, Reprod Med Biol | Retrospective cross-sectional/2002–2006 | 89/849 | No | Elderly primiparous women (aged ≥35 years)/ Women who underwent GIFT, IUI and OI. |
No | A 75-g, 2-h OGTT Plasma glucose level meeting two of the following criteria: ≥100 mg/dl while fasting, ≥180 mg/dl after 1 h or ≥150 mg/dl after 2 h (Japan Society of Obstetrics & Gynecology, 1995) |
Not reported | Not reported | Not reported | IVF/ICSI | Fresh | Not reported | Not reported | Yes/yes |
| Szymusik et al., 2019, Poland, Arch Med Sci | Retrospective cross-sectional/2004–2014 | 336/308 | Yes (maternal age and parity) | Pregnancies who delivered >22 weeks of gestation/history of preterm birth, gestational hypertensive disorders or placental pathologies in the previous pregnancy, oocyte donation, frozen/thawed ET and major fetal anomalies | Unclear | OGTT of 75 g ≥92 (fasting), ≥180 (1 h) and ≥153 mg/dl (2 h) | Not reported | Not reported | Not reported | IVF/ICSI | Fresh | Not reported | Not reported | Yes/yes |
| Tomic et al., 2011, Croatia, Arch Gynecol Obstet | Prospective cross-sectional/2006–2009 | 283/283 | Yes (ethnic origin, maternal age, gravidity, smoking, BMI, weight gain in pregnancy, site and time of delivery) | Primiparous women ≥35 years of age with a birth weight at least 500 g | Unclear | Not reported | rFSH or hMG | GnRH agonist long | ≥2 follicles reached 16–17 mm in diameter | Undefined | Fresh | Day 3–5 | Progesterone vag gel or capsule | Yes/no |
| Valenzuela-Alcaraz et al., 2016, Spain, J Matern Fetal Neonatal Med | Retrospective cross-sectional/2004–2010 | 223/460 | No | Only pregnancies that were treated, followed-up and delivered at the Infertility and Assisted Reproduction Unit, Hospital Clinic | Unclear | Not reported | FSH | GnRH agonist | Not reported | IVF/ICSI | Undefined | Not reported | Not reported | Yes/no |
| Verlaenen et al., 1995, Belgium, Obstet Gynecol | Retrospective cross-sectional/January 1988–June 1994 | 140/140 | Yes (parity, maternal age, height, weight, no fertility history) | Singleton pregnancies of >20 weeks’ gestation/early pregnancy loss (<20w), embryo reduction, women referred later than 20w’ gestation due to complications | Unclear | Not reported | Not reported | Not reported | Not reported | IVF | Undefined | Not reported | Not reported | Yes/no |
| Xu et al., 2014, Australia, BMC Pregnancy and Childbirth | Retrospective cross-sectional/January 2007–December 2009 | 12 105/ 381 345 |
No | Singleton births during the study period/records that did not state ART status or gestational age | Unclear | Not reported | Not reported | Not reported | Not reported | IVF/ICSI | Undefined | Not reported | Not reported | Yes/no |
| Xu et al., 2015, China, Zhongguo Dang Dai Er Ke Za Zhi | Retrospective cross-sectional/October 2010–October 2012 | 94/164 | No | Newborns admitted to the hospital after delivery | Unclear | Not reported | Not reported | Not reported | Not reported | IVF/ICSI | Undefined | Not reported | Not reported | Yes/no |
| Yang et al., 2019, China, Gynecol Endocrinol | Retrospective cross-sectional/January 2015–January 2018 | 1663/ 3326 |
Yesv (maternal age, BMI, parity and gravidity) | Deliveries at ≥24 weeks of gestation/uterine malformation, adenomyosis, uterine myoma, submucous myoma, obesity or low weight, severe intrauterine adhesions, chronic hypertension, and diabetes | Yes | 2-h 75 g OGTT between 24 and 28 weeks of gestation, if ≥1 of the three plasma glucose concentrations equalled or exceeded the following values: fasting glucose 5.1 mmol/L, 1-h level 10.0 mmol/L and 2-h level 8.5 mmol/L | Not reported | Not reported | Not reported | IVF/ICSI | Frozen | Not reported | Not reported | Yes/no |
| Zadori et al., 2003, Hungary, J Assist Reprod Genet | Retrospective cross-sectional/January 1995–February 2002 | 185/185 | Yes (maternal age, parity, gravidity and previous obstetric outcome) | Deliveries at the Department of Obstetrics and Gynecology, University of Szeged in the study period | Unclear | Not reported | Not reported | Not reported | Not reported | Undefined | Undefined | Not reported | Not reported | Yes/no |
| Zhu et al., 2016, China, Sci Rep | Retrospective cross-sectional/2006–2014 | 1659/ 5193 |
Yes (maternal age and birth year) | Pregnancies conceived during the study period | Unclear | Not reported | Not reported | Not reported | Not reported | IVF/ICSI | Undefined | Not reported | Not reported | Yes/no |
GDM: gestational diabetes mellitus; OGTT: oral glucose tolerance test; rFSH: recombinant FSH, CC: clomiphene citrate; E2: estradiol, CC; Clomiphene citrate, ACOG: SC: spontaneous conception, ET: embryo transfer, PCOS: polycystic ovary syndrome, PN: pronuclei, WHO: World Health Organization, OHSS: ovarian hyperstimulation syndrome
In the current systematic review and meta-analysis, studies including only patients with PCOS were excluded, as per protocol. In two of the eligible studies, no patients with PCOS were included (Suzuki and Miyake, 2007, Ashrafi et al., 2014), while in 15 studies, they were included in the population analysed (Reubinoff et al., 1997, Koivurova et al., 2002, Ochsenkuhn et al., 2003, Katalinic et al., 2004, Sazonova et al., 2011, Farhi et al., 2013, Stojnic et al., 2013, Machtinger et al., 2015, Cai et al., 2017, Luke et al., 2017, Dayan et al., 2018, Frankenthal et al., 2018, Harlev et al., 2018, Nagata et al., 2019, Yang et al., 2019). In the remaining 21 eligible studies, it was unclear whether patients with PCOS were included or not (Verlaenen et al., 1995, Maman et al., 1998, Isaksson et al., 2002, Zadori et al., 2003, Barros Delgadillo et al., 2006, De Geyter et al., 2006, Schieve et al., 2007, Caserta et al., 2008, Knoester et al., 2008, Sebastiani et al., 2009, Chaveeva et al., 2011, Tomic and Tomic, 2011, Silberstein et al., 2014, Xu et al., 2014, Xu et al., 2015, Beyer and Amari, 2016, Valenzuela-Alcaraz et al., 2016, Zhu et al., 2016, Qin et al., 2017, Lee et al., 2018, Szymusik et al., 2019), although this specific information was requested from the corresponding authors (Table I). No data regarding the proportion of patients with PCOS were available in 12 out of the 15 studies that included women with PCOS, while this proportion was reported in the remaining three studies (Farhi et al., 2013: 12.5%, Machtinger et al., 2015: 2%, Frankenthal et al., 2018: 6.5%).
Diagnosis of GDM was present in 4776 out of 63 760 women who achieved singleton pregnancy after ART and in 158 526 out of 1 870 734 women who achieved singleton pregnancy spontaneously. In studies evaluating GDM after ART, IVF/ICSI was performed in 22 studies, IVF only in 5 and ICSI only in 3, whereas this information was not present in eight studies. Fresh and frozen ET were performed in 10 studies, fresh ET only in 11 and frozen ET only in 1, whereas this information was not present in 16 studies.
Maternal age (n = 16), parity (n = 11), ethnic origin (n = 7), date of delivery (n = 6) and BMI (n = 3) were the most commonly used variables for matching pregnant women after ART with their counterparts after SC. Additional matching variables included smoking (n = 3), social class (n = 3), gravidity (n = 3), fertility history (n = 3), height (n = 2), weight (n = 2), gestational age (n = 2), education (n = 1) and obstetric outcome (n = 1).
Meta-analysis
Main analysis
Thirty-seven studies (17 matched, 20 unmatched) provided data for the main comparison. Women with singleton pregnancies achieved by ART showed a higher risk of GDM compared with those women who achieved singleton pregnancy spontaneously (RR 1.53, 95% CI 1.39–1.69, I2 78.6%, 1 893 599 women) (Fig. 2). No evidence for publication bias was detected using the Harbord–Egger’s test for the primary outcome (P = 0.84).
Figure 2.
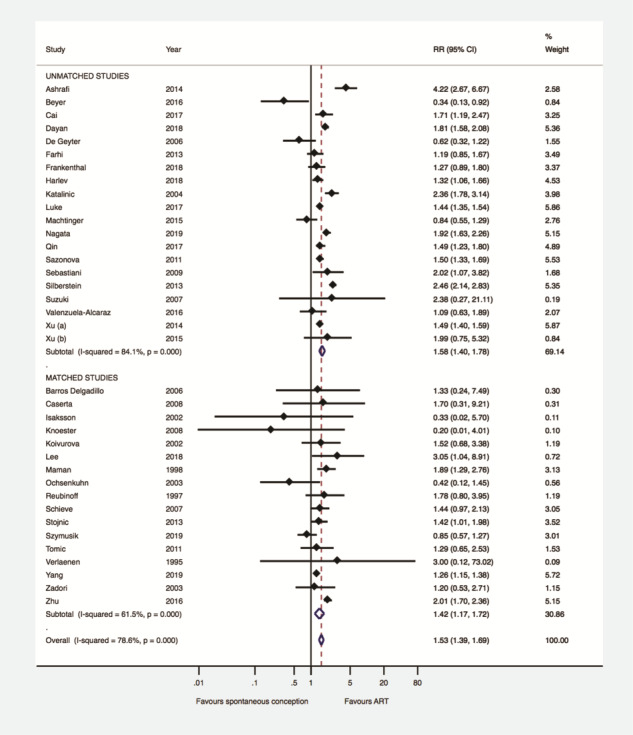
Gestational diabetes mellitus after ART versus after spontaneous conception in matched and unmatched studies. RR: risk ratio.
Subgroup analyses—meta-regression
Matched versus unmatched studies
Subgroup analysis was performed according to whether the eligible studies were matched (n = 17) or unmatched (n = 20). This, however, did not change the direction or the magnitude of the effect observed regarding the type of conception and the presence of GDM (matched studies: RR 1.42, 95% CI 1.17–1.72, I2 61.5%, 21 606 women—unmatched studies: RR 1.58, 95% CI 1.40–1.78, I2 84.1%, 1 871 993 women) (Fig. 2). Meta-regression analysis confirmed that the type of study (matched versus. unmatched) did not have a significant effect on the association between type of conception and GDM (coefficient: 0.91, 95% CI 0.67–1.22, P = 0.51).
Prospective versus retrospective cross-sectional studies
Subgroup analysis was performed according to whether eligible studies were prospective (n = 12) or retrospective (n = 25). This, however, did not change the direction or the magnitude of the effect observed regarding the type of conception and the presence of GDM (prospective studies: RR 1.52, 95% CI 1.27–1.83, I2 62.2%, 112 954 women—retrospective studies: RR 1.53, 95% CI 1.36–1.72, I2 82.5%, 1 780 645 women) (Supplementary Fig. S1). Meta-regression analysis confirmed that the type of study (prospective versus retrospective) did not have a significant effect on the association between type of conception and GDM (coefficient: 0.99, 95% CI 0.74–1.35, P = 0.99).
Type of ET
Subgroup analysis was performed according to whether pregnancies after ART were achieved exclusively either by fresh or by frozen ET (n = 17). Compared to women achieving pregnancy spontaneously, a higher risk of GDM was observed in women achieving singleton pregnancy after fresh ET (n = 14) (RR 1.38, 95% CI 1.03–1.85, I2 75.4%, 605 740 women). This association was not present when women achieving pregnancy spontaneously were compared with those achieving singleton pregnancy after frozen ET (n = 3) (RR 0.46, 95% CI 0.10–2.19; I2 73.1%, 12 186 women) (Fig. 3). Meta-regression analysis did not detect a significant effect of type of ET (fresh versus frozen) on the association between type of conception and GDM (coefficient: 0.53, 95% CI 0.19–1.44, P = 0.19).
Figure 3.
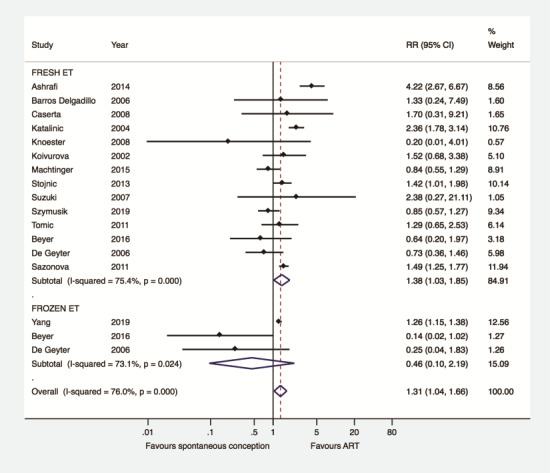
Gestational diabetes mellitus after ART versus after spontaneous conception according to type of embryo transfer. ET: embryo transfer.
Method of fertilization
Subgroup analysis was performed according to whether pregnancies were achieved exclusively after IVF or ICSI (n = 13). Compared to women achieving pregnancy spontaneously, a higher risk of GDM was observed in women achieving singleton pregnancy by IVF (n = 7) (RR 1.95, 95% CI 1.56–2.44, I2 43.1%, 265 253 women). This association was not present when women achieving singleton pregnancy spontaneously were compared with those achieving singleton pregnancy by ICSI (n = 6) (RR 1.42, 95% CI 0.94–2.15, I2 73.5%, 103 402 women) (Fig. 4). Meta-regression analysis did not detect a significant effect of method of fertilization (IVF versus ICSI) on the association between type of conception and GDM (coefficient: 0.80, 95% CI 0.45–1.41, P = 0.40).
Figure 4.
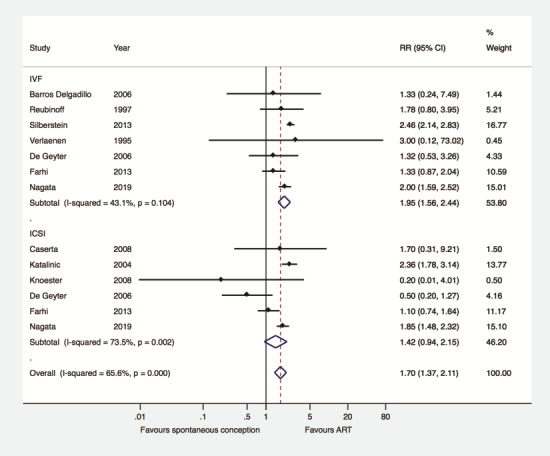
Gestational diabetes mellitus after ART versus after spontaneous conception according to method of fertilization.
Inclusion of patients with PCOS
Subgroup analysis was performed according to whether studies included patients with PCOS (n = 15), excluded specifically patients with PCOS (n = 2) or this information was unclear (n = 20). This, however, did not change the significance or the direction of the effect observed regarding the type of conception and the presence of GDM (patients with PCOS excluded: RR 4.12, 95% CI 2.63–6.45, I2 0%, − patients with PCOS included: RR 1.49, 95% CI 1.33–1.66, I2 75.0%, − unclear information: RR 1.46, 95% CI 1.22–1.75, I2 77.7%) (Fig. 5). Meta-regression analysis detected a significant effect (P < 0.03) of the population analysed on the association between type of conception and the presence of GDM. More specifically, the RR of GDM after ART compared to SC was significantly higher in studies that specifically excluded patients with PCOS compared to those which included patients with PCOS (P < 0.01) or to those in which this information was unclear (P < 0.01).
Figure 5.
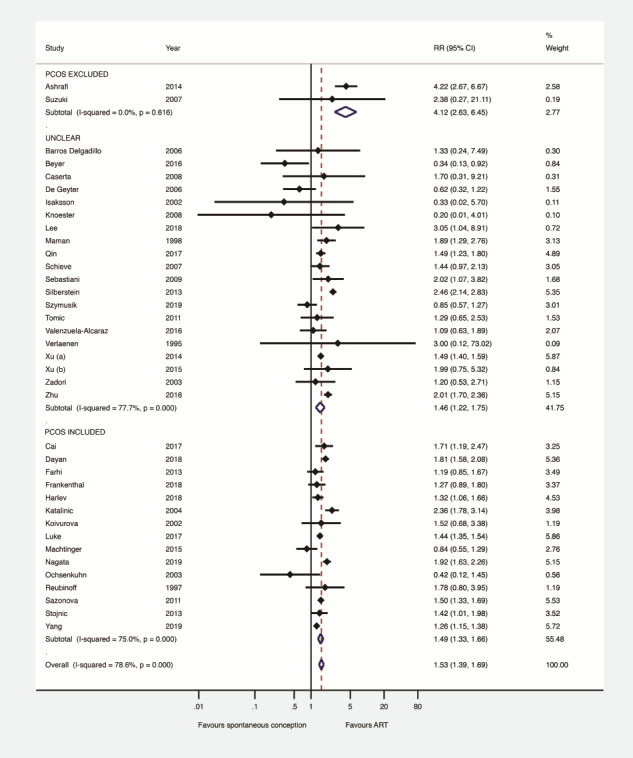
Gestational diabetes mellitus after ART versus after spontaneous conception in studies including patients with PCOS or not, or whether this information was unclear. PCOS: polycystic ovary syndrome
Quality assessment by NOS
Subgroup analysis was performed according to whether eligible studies were classified as of ‘good quality’ (n = 24) or as of ‘poor quality’ (n = 13). This, however, did not change the direction or the magnitude of the effect observed regarding the type of conception and the presence of GDM (‘good quality’ studies: RR 1.53, 95% CI 1.35–1.74, I2 74.8%, 709 503 women—‘poor quality’ studies: RR 1.50, 95% CI 1.26–1.79, I2 83.9%, 1 184 096 women) (Supplementary Fig. S2).
Discussion
Main findings
This systematic review and meta-analysis, including 1 934 494 pregnant women and 163 302 women with GDM, showed an increased risk of GDM in women achieving singleton pregnancy by ART compared with those achieving singleton pregnancy spontaneously. This higher risk was observed after IVF but not after ICSI, and after fresh but not after frozen ET. Nevertheless, meta-regression analyses did not detect any significant effect of method of fertilization or type of ET on the association between GDM and type of conception.
Strengths
To accurately evaluate the association between ART and risk of GDM, studies including exclusively women with PCOS and multiple pregnancies were excluded, since they are considered as strong risk factors for the development of GDM (Qin et al., 2015, Yu et al., 2016). To the best of our knowledge, this is the largest systematic review and meta-analysis focusing on the association between ART and risk of GDM in singleton pregnancies. The present meta-analysis is sufficiently large to provide precise risk estimates. Moreover, it allowed us to perform subgroup analyses, aiming to evaluate the impact of fertilization method and type of ET on the risk of GDM.
Limitations
The definition of GDM was not reported or was unclear in several studies, while a high degree of heterogeneity in its definition was present among those studies that offered such data. Thus, no meaningful subgroup analysis was feasible. Moreover, although the quality of most of the studies was characterised as ‘good’ by NOS, the retrospective design in the majority of the included studies, as well as the fact that most of the studies were unmatched, are potential sources of bias. Nevertheless, the higher risk of GDM in women achieving singleton pregnancy after ART as compared to those achieving pregnancy after SC did not change in subgroup analyses, evaluating whether pooled studies were prospective/retrospective or matched/unmatched.
Comparison with the literature
Two previous meta-analyses evaluated the association between ART and risk of GDM in singleton pregnancies (Jackson et al., 2004, Pandey et al., 2012). Both meta-analyses showed a higher risk for GDM, although with a limited number of studies [Jackson et al., 2004: odds ratio (OR) 2.00, 95% CI 1.36–2.99, n = 4, 2291 women; Pandey et al., 2012: RR 1.48, 95% CI 1.33–1.66, n = 6, 587 790 women]. In the present meta-analysis, the overall sample size increased from 587 790 to 1 934 494 women compared with the meta-analysis by Pandey et al. (2012).
Interpretation of the study
The underlying mechanisms regarding the increased risk of GDM in women achieving singleton pregnancy by ART compared with those achieving singleton pregnancy spontaneously remain unclear. Moreover, whether the association observed is explained by the presence of infertility per se or the ART procedure performed cannot be evaluated on the basis of the data presented (Wang et al., 2017). A potential explanation for the increased risk of GDM after ART might be the use of progesterone for luteal phase support in all ART cycles as well as during the first trimester of pregnancy (Rebarber et al., 2007, Ashrafi et al., 2014). Progesterone is known to increase insulin resistance (Branisteanu and Mathieu, 2003), which can lead to GDM.
Although a higher risk of GDM was observed after fresh but not after frozen ET, meta-regression analysis failed to detect a potential effect of the type of ET (fresh versus frozen) on the GDM risk. This might be due to the fact that the number of datasets pooled, comparing pregnancies after frozen ET versus pregnancies after SC, was limited (n = 3), in contrast to that comparing pregnancies after fresh ET versus pregnancies after SC (n = 14). Alternatively, the higher risk of GDM only after fresh ET might be due to the known adverse effects of ovarian stimulation on endometrial receptivity (Kolibianakis et al., 2002, Van Vaerenbergh et al., 2009). Endometrial quality is reported to be associated with the incidence of GDM in singleton pregnancies, since a higher probability of GDM is shown to be present after frozen ET in a hormonal replacement cycle compared with frozen ET in a natural cycle (adjusted OR 0.52, 95% CI 0.39–0.69) (Saito et al., 2019).
The higher risk of GDM, observed only after fresh ET, might be attributed to differences in the quality of placentation between fresh cycles and frozen-thawed cycles (Kansal Kalra et al., 2011), explained by differences in the hormonal peri-implantation environment in these two clinical scenarios. It has been suggested that supraphysiologic steroid hormone levels during the fresh stimulated cycles may lead to abnormal endometrial angiogenesis and abnormal placentation (Maheshwari et al., 2018). Altered placental gene regulation has been associated with GDM, probably through epigenetic mechanisms involvement (Nomura et al., 2014, Finer et al., 2015, Reichetzeder et al., 2016).
Regarding the method of fertilization, although the higher risk of GDM was statistically significant only after IVF but not after ICSI, the direction and magnitude of the effect were similar in both groups, while meta-regression analysis did not detect any significant effect of the fertilization method on the association between GDM and type of conception. Thus, it appears that the method of fertilization does not affect the association between GDM and type of conception.
The higher risk of GDM, observed only after IVF but not after ICSI, might be due to the expected higher proportion of women with female pathology associated not only with infertility, but also with GDM, such as advanced maternal age and obesity. On the contrary, in couples undergoing ICSI the expected main cause leading to infertility is male factor and the anticipated presence of the above risk factors in these couples is lower.
Due to the fact that a higher risk of GDM has been reported among women with PCOS compared to those without PCOS (Palomba et al., 2015, Azziz et al., 2016, Bahri Khomami et al., 2018), the observed association between the type of conception and GDM could be partially attributed to the inclusion of women with PCOS in many of the eligible studies. However, by performing subgroup analysis and meta-regression, the higher risk of GDM after ART compared to SC was still present in studies that specifically excluded PCOS women. In fact, the RR of GDM after ART compared to SC was significantly higher in studies that specifically excluded patients with PCOS compared to those which included them or to those in which this information was unclear. Thus, the effect of the presence of patients with PCOS in many of the eligible studies is probably negligible, which might be attributed to the relatively low proportion of women with PCOS patients in these studies.
Women achieving pregnancy after ART should be monitored for GDM, since the risk is increased compared with SC pregnancies. Early detection as well as appropriate support and care is warranted, aiming to avoid serious complications during pregnancy. Whether this risk is attributed to the underlying infertility status of the couples undergoing ART as compared with those who conceived spontaneously needs to be further elucidated.
Conclusion
In conclusion, the present systematic review and meta-analysis, by analysing 1 893 599 women, showed a higher risk of GDM in women achieving singleton pregnancy by ART compared with those achieving pregnancy spontaneously. This finding highlights the importance of early detection of GDM in women treated by ART, which could lead to timely and effective interventions, prior to ART as well as during early pregnancy.
Supplementary Material
Acknowledgements
We would like to thank the following authors for providing us with the extra information regarding their published studies: Dr Eitan Lunenfeld, Dr Judy E. Stern, Prof. Barbara Luke, Dr Sari Koivurova, Dr Mahnaz Ashrafi, Dr Chie Nagata, Prof. Alexander Katalinic, Dr Iwona Szymusik, Dr Dolly Farhi, Prof. Christina Bergh, Dr Avi Harlev, Prof. Benjamin Reubinoff, Dr Shunji Suzuki, Dr Cai Shirong, Prof. Peter Hillemanns, Prof. Margareta D. Pisarska, Dr Marjolein Knoester, Prof. Natalie Dayan and Prof. Jelena Stojnic.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Authors’ roles
J.K.B.: performed the literature search and contributed towards the data extraction, the analyses and interpretation of the data and the drafting of the manuscript. P.A.: conceived the idea for the study, reviewed the protocol, contributed towards the literature search, interpretation of the data and revised the manuscript for important intellectual content. D.G.G. and G.T.L.: reviewed the protocol and revised the manuscript for important intellectual content. B.C.T. and G.F.G.: revised the manuscript for important intellectual content. E.M.K.: constructed the protocol and contributed towards the data extraction, the analyses and interpretation of the data and the drafting of the manuscript. All authors approved the final version of the manuscript.
Funding
No financial support was received for this study.
Conflict of interest
No conflicts of interest were declared.
References
- Ashrafi M, Gosili R, Hosseini R, Arabipoor A, Ahmadi J, Chehrazi M. Risk of gestational diabetes mellitus in patients undergoing assisted reproductive techniques. Eur J Obstet Gynecol Reprod Biol 2014;176:149–152. [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- Bahri Khomami M, Boyle JA, Tay CT, Vanky E, Teede HJ, Joham AE, Moran LJ. Polycystic ovary syndrome and adverse pregnancy outcomes: current state of knowledge, challenges and potential implications for practice. Clin Endocrinol (Oxf) 2018;88:761–769. [DOI] [PubMed] [Google Scholar]
- Barros Delgadillo JC, Alvarado Mendez LM, Gorbea Chavez V, Villalobos Acosta S, Sanchez Solis V, Gavino Gavino F. [Perinatal results in pregnancies obtained with embryo transfer in vitro fertilization: a case-control study]. Ginecol Obstet Mex 2006;74:626–639. [PubMed] [Google Scholar]
- Beyer DA, Amari F. Maternal risk factors and neonatal outcomes after ART treatment – a German monocenter experience. Middle East Fertil Soc J 2016;21:155–160. [Google Scholar]
- Branisteanu DD, Mathieu C. Progesterone in gestational diabetes mellitus: guilty or not guilty? Trends Endocrinol Metab 2003;14:54–56. [DOI] [PubMed] [Google Scholar]
- Cai S, Natarajan P, Chan JKY, Wong PC, Tan KH, Godfrey KM, Gluckman PD, Shek LPC, Yap F, Kramer MS et al. Maternal hyperglycemia in singleton pregnancies conceived by IVF may be modified by first-trimester BMI. Hum Reprod 2017;32:1941–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta D, Marci R, Tatone C, Schimberni M, Vaquero E, Lazzarin N, Fazi A, Moscarini M. IVF pregnancies: neonatal outcomes after the new Italian law on assisted reproduction technology (law 40/2004). Acta Obstet Gynecol Scand 2008;87:935–939. [DOI] [PubMed] [Google Scholar]
- Chaveeva P, Carbone IF, Syngelaki A, Akolekar R, Nicolaides KH. Contribution of method of conception on pregnancy outcome after the 11-13 weeks scan. Fetal Diagn Ther 2011;30:9–22. [DOI] [PubMed] [Google Scholar]
- Dayan N, Fell DB, Guo Y, Wang H, Velez MP, Spitzer K, Laskin CA. Severe maternal morbidity in women with high BMI in IVF and unassisted singleton pregnancies. Hum Reprod 2018;33:1548–1556. [DOI] [PubMed] [Google Scholar]
- De Geyter C, De Geyter M, Steimann S, Zhang H, Holzgreve W. Comparative birth weights of singletons born after assisted reproduction and natural conception in previously infertile women. Hum Reprod 2006;21:705–712. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- Farhi A, Reichman B, Boyko V, Hourvitz A, Ron-El R, Lerner-Geva L. Maternal and neonatal health outcomes following assisted reproduction. Reprod Biomed Online 2013;26:454–461. [DOI] [PubMed] [Google Scholar]
- Finer S, Mathews C, Lowe R, Smart M, Hillman S, Foo L, Sinha A, Williams D, Rakyan VK, Hitman GA. Maternal gestational diabetes is associated with genome-wide DNA methylation variation in placenta and cord blood of exposed offspring. Hum Mol Genet 2015;24:3021–3029. [DOI] [PubMed] [Google Scholar]
- Frankenthal D, Hirsh-Yechezkel G, Boyko V, Orvieto R, Ron-El R, Lerner-Geva L, Farhi A. The effect of body mass index (BMI) and gestational weight gain on adverse obstetrical outcomes in pregnancies following assisted reproductive technology as compared to spontaneously conceived pregnancies. Obes Res Clin Pract 2018;13:150–155. [DOI] [PubMed] [Google Scholar]
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443–3457. [DOI] [PubMed] [Google Scholar]
- Harlev A, Walfisch A, Oran E, Har-Vardi I, Friger M, Lunenfeld E, Levitas E. The effect of fertility treatment on adverse perinatal outcomes in women aged at least 40 years. Int J Gynaecol Obstet 2018;140:98–104. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration 2011. [Google Scholar]
- Isaksson R, Gissler M, Tiitinen A. Obstetric outcome among women with unexplained infertility after IVF: a matched case-control study. Hum Reprod 2002;17:1755–1761. [DOI] [PubMed] [Google Scholar]
- Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol 2004;103:551–563. [DOI] [PubMed] [Google Scholar]
- Kansal Kalra S, Ratcliffe SJ, Milman L, Gracia CR, Coutifaris C, Barnhart KT. Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer. Fertil Steril 2011;95:548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katalinic A, Rösch C, Ludwig M. Pregnancy course and outcome after intracytoplasmic sperm injection: a controlled, prospective cohort study. Fertil Steril 2004;81:1604–1616. [DOI] [PubMed] [Google Scholar]
- Knoester M, Helmerhorst FM, Vandenbroucke JP, van der Westerlaken LA, Walther FJ, Veen S. Perinatal outcome, health, growth, and medical care utilization of 5- to 8-year-old intracytoplasmic sperm injection singletons. Fertil Steril 2008;89:1133–1146. [DOI] [PubMed] [Google Scholar]
- Koivurova S, Hartikainen AL, Karinen L, Gissler M, Hemminki E, Martikainen H, Tuomivaara L, Järvelin MR. The course of pregnancy and delivery and the use of maternal healthcare services after standard IVF in Northern Finland 1990-1995. Hum Reprod 2002;17:2897–2903. [DOI] [PubMed] [Google Scholar]
- Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, Devroey P. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril 2002;78:1025–1029. [DOI] [PubMed] [Google Scholar]
- Lee B, Koeppel AF, Wang ET, Gonzalez TL, Sun T, Kroener L, Lin Y, Joshi NV, Ghadiali T, Turner SD, Rich SS, Farber CR, Rotter JI, Ida Chen YD, Goodarzi MO, Guller S, Harwood B, Serna TB, van Williams J, 3rd, Pisarska MD. Differential gene expression during placentation in pregnancies conceived with different fertility treatments compared with spontaneous pregnancies. Fertil Steril 2018;111:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke B, Gopal D, Cabral H, Stern JE, Diop H. Pregnancy, birth, and infant outcomes by maternal fertility status: the Massachusetts Outcomes Study of Assisted Reproductive Technology. Am J Obstet Gynecol 2017;217: 327.e321–e327.e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger R, Zera C, Racowsky C, Missmer S, Gargiulo A, Schiff E, Wilkins-Haug L. The effect of mode of conception on obstetrical outcomes differs by body mass index. Reprod Biomed Online 2015;31:531–537. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update 2018;24:35–58. [DOI] [PubMed] [Google Scholar]
- Maman E, Lunenfeld E, Levy A, Vardi H, Potashnik G. Obstetric outcome of singleton pregnancies conceived by in vitro fertilization and ovulation induction compared with those conceived spontaneously. Fertil Steril 1998;70:240–245. [DOI] [PubMed] [Google Scholar]
- Nagata C, Yang L, Yamamoto-Hanada K, Mezawa H, Ayabe T, Ishizuka K, Konishi M, Ohya Y, Saito H, Sago H et al. Complications and adverse outcomes in pregnancy and childbirth among women who conceived by assisted reproductive technologies: a nationwide birth cohort study of Japan environment and children's study. BMC Pregnancy Childbirth 2019;19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar AH, Usta IM, Rechdan JB, Harb TS, Adra AM, Abu-Musa AA. Pregnancy outcome in spontaneous twins versus twins who were conceived through in vitro fertilization. Am J Obstet Gynecol 2003;189:513–518. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Lambertini L, Rialdi A, Lee M, Mystal EY, Grabie M, Manaster I, Huynh N, Finik J, Davey M et al. Global methylation in the placenta and umbilical cord blood from pregnancies with maternal gestational diabetes, preeclampsia, and obesity. Reprod Sci 2014;21:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenkuhn R, Strowitzki T, Gurtner M, Strauss A, Schulze A, Hepp H, Hillemanns P. Pregnancy complications, obstetric risks, and neonatal outcome in singleton and twin pregnancies after GIFT and IVF. Arch Gynecol Obstet 2003;268:256–261. [DOI] [PubMed] [Google Scholar]
- Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 2015;21:575–592. [DOI] [PubMed] [Google Scholar]
- Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update 2012;18:485–503. [DOI] [PubMed] [Google Scholar]
- Qin J, Sheng X, Wu D, Gao S, You Y, Yang T, Wang H. Adverse obstetric outcomes associated with in vitro fertilization in singleton pregnancies. Reprod Sci 2017;24:595–608. [DOI] [PubMed] [Google Scholar]
- Qin J, Wang H, Sheng X, Liang D, Tan H, Xia J. Pregnancy-related complications and adverse pregnancy outcomes in multiple pregnancies resulting from assisted reproductive technology: a meta-analysis of cohort studies. Fertil Steril 2015;103:1492–1508.e1491–1497. [DOI] [PubMed] [Google Scholar]
- Rebarber A, Istwan NB, Russo-Stieglitz K, Cleary-Goldman J, Rhea DJ, Stanziano GJ, Saltzman DH. Increased incidence of gestational diabetes in women receiving prophylactic 17alpha-hydroxyprogesterone caproate for prevention of recurrent preterm delivery. Diabetes Care 2007;30:2277–2280. [DOI] [PubMed] [Google Scholar]
- Reichetzeder C, Dwi Putra SE, Pfab T, Slowinski T, Neuber C, Kleuser B, Hocher B. Increased global placental DNA methylation levels are associated with gestational diabetes. Clin Epigenetics 2016;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubinoff BE, Samueloff A, Ben-Haim M, Friedler S, Schenker JG, Lewin A. Is the obstetric outcome of in vitro fertilized singleton gestations different from natural ones? A controlled study. Fertil Steril 1997;67:1077–1083. [DOI] [PubMed] [Google Scholar]
- Saito K, Kuwahara A, Ishikawa T, Morisaki N, Miyado M, Miyado K, Fukami M, Miyasaka N, Ishihara O, Irahara M et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum Reprod 2019. [DOI] [PubMed] [Google Scholar]
- Sazonova A, Kallen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C. Obstetric outcome after in vitro fertilization with single or double embryo transfer. Hum Reprod 2011;26:442–450. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Cohen B, Nannini A, Ferre C, Reynolds MA, Zhang Z, Jeng G, Macaluso M, Wright VC. Massachusetts Consortium for Assisted Reproductive Technology Epidemiologic R. A population-based study of maternal and perinatal outcomes associated with assisted reproductive technology in Massachusetts. Matern Child Health J 2007;11:517–525. [DOI] [PubMed] [Google Scholar]
- Sebastiani G, Pertierra Cortada A, Vidal Sorde E, Figueras Aloy J, Balasch Cortina J. [Factors associated with assisted reproduction technologies and neonatal outcomes]. An Pediatr (Barc) 2009;70: 323–332. [DOI] [PubMed] [Google Scholar]
- Silberstein T, Levy A, Harlev A, Saphier O, Sheiner E. Perinatal outcome of pregnancies following in vitro fertilization and ovulation induction. J Matern-Fetal Neo Med 2014;27:1316–1319. [DOI] [PubMed] [Google Scholar]
- Stojnic J, Radunovic N, Jeremic K, Kotlica BK, Mitrovic M, Tulic I. Perinatal outcome of singleton pregnancies following in vitro fertilization. Clin Exp Obstet Gynecol 2013;40:277–283. [PubMed] [Google Scholar]
- Suzuki S, Miyake H. Obstetric outcomes of elderly primiparous singleton pregnancies conceived by in vitro fertilization compared with those conceived spontaneously. Reprod Med Biol 2007;6:219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanska M, Horosz E, Szymusik I, Bomba-Opon D, Wielgos M. Gestational diabetes in IVF and spontaneous pregnancies. Neuro Endocrinol Lett 2011;32:885–888. [PubMed] [Google Scholar]
- Szymusik I, Kosinska-Kaczynska K, Krowicka M, Sep M, Marianowski P, Wielgos M. Perinatal outcome of in vitro fertilization singletons - 10 years' experience of one center. Arch Med Sci 2019;15: 666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic V, Tomic J. Neonatal outcome of IVF singletons versus naturally conceived in women aged 35 years and over. Arch Gynecol Obstet 2011;284:1411–1416. [DOI] [PubMed] [Google Scholar]
- Toulis KA, Goulis DG, Kolibianakis EM, Venetis CA, Tarlatzis BC, Papadimas I. Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Fertil Steril 2009;92:667–677. [DOI] [PubMed] [Google Scholar]
- Valenzuela-Alcaraz B, Crispi F, Manau D, Cruz-Lemini M, Borras A, Balasch J, Gratacos E. Differential effect of mode of conception and infertility treatment on fetal growth and prematurity. J Matern Fetal Neonatal Med 2016;29:3879–3884. [DOI] [PubMed] [Google Scholar]
- Van Vaerenbergh I, Van Lommel L, Ghislain V, In't Veld P, Schuit F, Fatemi HM, Devroey P, Bourgain C. In GnRH antagonist/rec-FSH stimulated cycles, advanced endometrial maturation on the day of oocyte retrieval correlates with altered gene expression. Hum Reprod 2009;24:1085–1091. [DOI] [PubMed] [Google Scholar]
- Verlaenen H, Cammu H, Derde MP, Amy JJ. Singleton pregnancy after in vitro fertilization: expectations and outcome. Obstet Gynecol 1995;86:906–910. [DOI] [PubMed] [Google Scholar]
- Vermey BG, Buchanan A, Chambers GM, Kolibianakis EM, Bosdou J, Chapman MG, Venetis CA. Are singleton pregnancies after assisted reproduction technology (ART) associated with a higher risk of placental anomalies compared with non-ART singleton pregnancies? A systematic review and meta-analysis. BJOG 2019;126:209–218. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang Z, Meng J, Wang X, Liu L, Chen B. History of infertility relates to increased risk of gestational diabetes mellitus: a meta-analysis. Int J Clin Exp Med 2017;10:1909–1916. [Google Scholar]
- Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://wwwohrica/programs/clinical_epidemiology/oxfordasp 2013.
- Xu XK, Wang YA, Li Z, Lui K, Sullivan EA. Risk factors associated with preterm birth among singletons following assisted reproductive technology in Australia 2007-2009-a population-based retrospective study. BMC Pregnancy Childbirth 2014;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XY, Yang JH, Ma XM, Liu AL, Liu K, He S, Mi HY, Li L. Neonatal complications and birth defects in infants conceived by in vitro fertilization. Zhongguo Dang Dai Er Ke Za Zhi 2015;17:350–355. [PubMed] [Google Scholar]
- Yang P, Kang H, Ma C, Wei Y, Tao L, Wu Z. Risk of preterm delivery in singletons conceived by in vitro fertilization. Gynecol Endocrinol 2019;35:661–664. [DOI] [PubMed] [Google Scholar]
- Yu HF, Chen HS, Rao DP, Gong J. Association between polycystic ovary syndrome and the risk of pregnancy complications: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadori J, Kozinszky Z, Orvos H, Katona M, Pal A, Kovacs L. Dilemma of increased obstetric risk in pregnancies following IVF-ET. J Assist Reprod Genet 2003;20:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Zhang Y, Liu Y, Zhang R, Wu Y, Huang Y, Liu F, Li M, Sun S, Xing L et al. Maternal and live-birth outcomes of pregnancies following assisted reproductive technology: a retrospective cohort study. Sci Rep 2016;6:35141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


