Abstract
Background
Group B Streptococcus (GBS) frequently colonizes pregnant women and can cause sepsis and meningitis in young infants. If colonization was prevented through maternal immunization, a reduction in perinatal GBS disease might be possible. A GBS type III capsular polysaccharide (CPS)-tetanus toxoid conjugate (III-TT) vaccine was evaluated for safety and efficacy in preventing acquisition of GBS colonization.
Methods
Healthy, nonpregnant women aged 18–40 years and screened to be GBS III vaginal and rectal culture negative were randomized to receive III-TT conjugate or tetanus diphtheria toxoid vaccine in a multicenter, observer-blinded trial. GBS vaginal and rectal cultures and blood were obtained bimonthly over 18 months. Serum concentrations of GBS III CPS-specific antibodies were determined using enzyme-linked immunosorbent assay.
Results
Among 1525 women screened, 650 were eligible for the intent-to-treat analysis. For time to first acquisition of vaginal GBS III, vaccine efficacy was 36% (95% confidence interval [CI], 1%–58%; P = .044), and for first rectal acquisition efficacy was 43% (95% CI, 11% to 63%; P = .014). Two months post-immunization, geometric mean concentrations of serum GBS type III CPS-specific immunoglobulin G were 12.6 µg/mL (95% CI, 9.95 to 15.81) in GBS III-TT recipients, representing a 4-fold increase from baseline in 95% of women, which persisted. Both vaccines were well tolerated.
Conclusions
GBS CPS III-TT conjugate vaccine significantly delayed acquisition of vaginal and rectal GBS III colonization. In addition to its use for maternal immunization to passively protect infants with maternally derived antibodies, a multivalent vaccine might also serve to reduce fetal and neonatal exposure to GBS.
Clinical Trials Registration
Keywords: GBS, vaccine, vaginal colonization, rectal colonization
Group B streptococcal (GBS) type III conjugate vaccine significantly decreased acquisition of vaginal and rectal colonization in women. Maternal immunization with a multivalent GBS vaccine may prevent perinatal exposure, thus augmenting prevention through placental transport of maternal GBS-specific antibodies.
Group B Streptococcus (GBS) cause substantial morbidity, mortality, and long-term neurological sequelae among infant survivors with early- (age 0–6 days) or late-onset (age 7–89 days) invasive disease, especially meningitis [1–3]. However, neonates and young infants born to mothers with sufficient concentrations of antibodies against the capsular polysaccharides (CPS) of GBS at delivery are significantly less likely to develop invasive disease [4–7]. The mechanism of protection has been attributed to placental transfer of naturally acquired maternal GBS CPS-specific immunoglobulin (Ig) G, providing passive immunity to infants.
In the absence of a vaccine, the Centers for Disease Control and Prevention adopted guidelines for prevention of early-onset GBS disease that are based on culture screening at 35 to 36 weeks’ gestation and intravenous administration of intrapartum antibiotic prophylaxis (IAP) to all GBS-colonized women, a strategy that has reduced early-onset GBS disease in the United States by more than 80% [8]. This approach has limitations including lack of impact on the incidence of late-onset disease, potential for emergence of GBS penicillin resistance [9], and disruption of the composition of the newborn gut microbiome [10]. A safe and effective vaccine for pregnant women could potentially reduce pregnancy-related morbidity such as preterm labor and stillbirth [11, 12].
A GBS conjugate vaccine could plausibly reduce genital tract colonization by increasing CPS-specific immunoglobulin G (IgG) in the cervicovaginal fluid. Some authors have posited that IgG present in the cervicovaginal secretions could represent transudate from serum IgG and IgG produced locally [13, 14]. We hypothesized that a GBS CPS conjugate vaccine would induce CPS-specific IgG concentrations sufficient to delay acquisition of GBS. The trial was designed to determine whether a monovalent GBS conjugate vaccine could decrease or delay acquisition of GBS III vaginal and rectal colonization.
METHODS
Study Design
This was a multicenter, randomized 1:1, observer-blinded study to compare GBS type III CPS (III CPS)-tetanus toxoid (TT) conjugate and tetanus-diphtheria toxoid (Td) vaccines (Salk Institute, Swiftwater, PA) for efficacy in delaying or reducing the frequency of acquisition of vaginal GBS III. After randomization, a single 0.5-mL dose of III-TT (12.55 μg of III CPS and 15.9 μg of TT [a gift from the Massachusetts Public Health Laboratory, Jamaica Plain, MA] without preservative) or Td vaccine was administered intramuscularly.
The primary outcome measure was time to first vaginal culture that yielded GBS III. The secondary outcomes included proportion of vaginal GBS III culture-positive women, proportion of women who were GBS III positive on 3 or more consecutive occasions, density of GBS III at culture-positive time points, and serum concentrations of type III CPS-specific antibodies before and at 1, 2, 4, 6, 8, 10, 12, 14, 16, and 18 months after immunization stratified by pre-vaccination antibody concentrations. An exploratory endpoint of time to first rectal GBS colonization was added since rectal and vaginal colonization are highly correlated [15].
Study Population
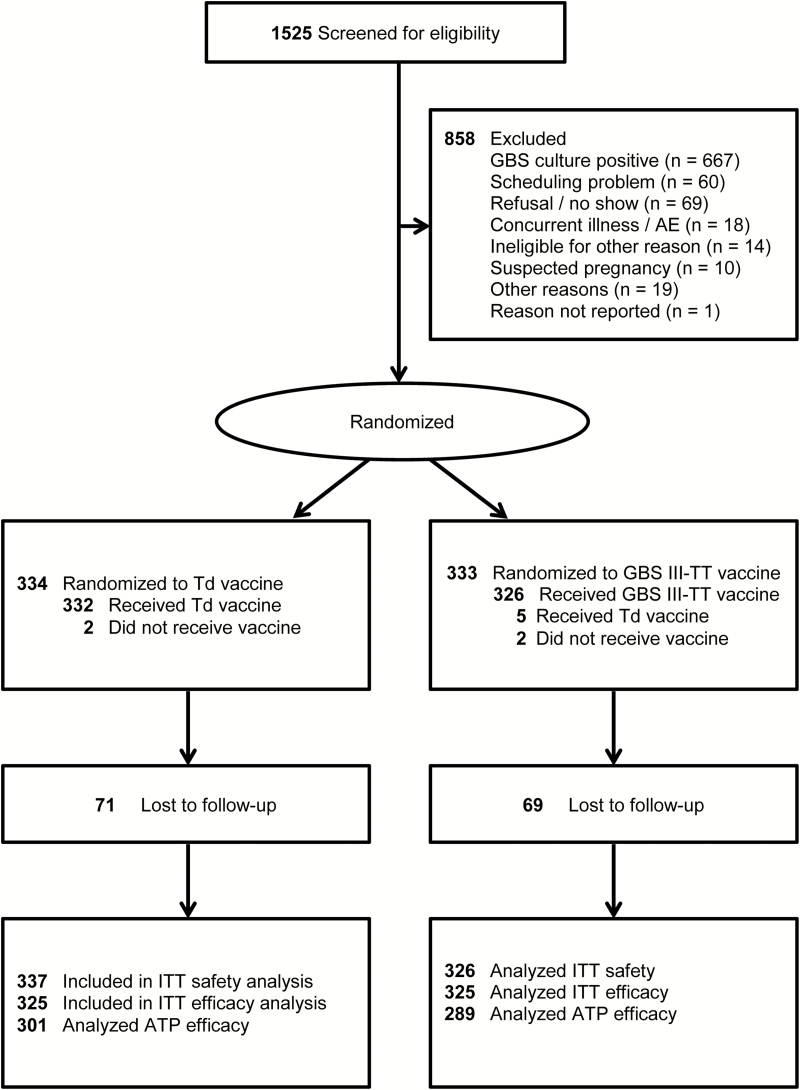
Healthy, sexually active, nonpregnant women aged 18–40 years who were using effective contraception, intending to stay in the geographical area for 18 months, and with telephone access were eligible. Written informed consent was obtained prior to screening and at randomization. There were 1525 women screened from July 2003 to August 2006 from the Magee-Womens Hospital Outpatient Clinics of the University of Pittsburgh Medical Center, University of Pittsburgh Student Health Center and Family Health Council (FHC) Clinics of Pittsburgh, Medical College of Georgia at Augusta, and Planned Parenthood of Houston and Southeast Texas, Inc. A total of 667 eligible GBS III culture-negative women were randomized, 663 were immunized and evaluable in the safety analysis, and 650 were evaluable in the intent-to-treat efficacy analysis (Figure 1).
Figure 1.
Enrollment and randomization of women to group B Streptococcus type III capsular polysaccharide-tetanus toxoid conjugate or tetanus diphtheria toxoid vaccine groups. Abbreviations: AE, adverse events; ATP, according to protocol; GBS III-TT, group B Streptococcus type III capsular polysaccharide-tetanus toxoid conjugate; ITT, intent-to-treat; Td, tetanus diphtheria toxoid.
Women were followed for up to 18 months after immunization; 2.1% in each vaccine group were lost to follow-up. Participants completed a 7-day diary card to assess occurrence of injection site and systemic symptoms. Blood samples, updated information on behavioral variables, and vaginal and rectal swab specimens for GBS culture were collected at baseline (enrollment and at immunization) and 1, 2, 4, 6, 8, 10, 12, 14, 16, and 18 months thereafter.
Randomization and Blinding
Random allocation and vaccine assignments were generated by a statistician at EMMES Corporation. All immunizations of participants from the Pittsburgh sites were administered either at Magee-Womens Hospital or FHC Clinic at Aliquippa. Stratification occurred by site (Magee vs Aliquippa) and race (white vs non-white) for a total of 4 strata, and vaccine allocations were issued in randomly varied block sizes of either 4 or 8. The Houston and Augusta sites were each issued a single randomization list without stratification by race or subsite due to logistical reasons. To minimize vaccine wastage, sites were instructed to vaccinate 12 participants each month using a fixed block of 12 with 1:1 allocation to GBS III-TT and Td vaccines.
Sample Size
From a previous evaluation of GBS serotypes from a longitudinal cohort study [15], it was estimated that the incidence of vaginal acquisition of GBS III would be 14 per 100 person-years and that immunization could reduce this rate by 80%. Through simulation studies with 500 evaluable women and 15% per year dropout rate, the primary endpoint analysis had power of 99% to detect 80% vaccine efficacy against a null hypothesis of zero efficacy, using a log-rank test with Efron’s partial likelihood for tied event times. The secondary analysis had power of >95% to detect 80% vaccine efficacy, using a Generalized Estimating Equation (GEE) model formulation and assuming the worst-case scenario in which as many as one-quarter of visits are missing.
The study enrollment plan assumed that if 1800 women were screened, 600 would consent to participate and receive immunization, which due to dropouts and exclusion criteria, would result in approximately 500 evaluable participants. However, based on blinded assessment of dropout rates, in May 2006, the Data Safety Monitoring Board recommended increasing the target for enrollment from 600 to 660 to ensure 500 evaluable participants and preserve power for the secondary efficacy analysis. At close of enrollment, 667 participants were enrolled, forming the intent-to-treat cohort.
Laboratory Detection of GBS
Vaginal and rectal swabs were transported via overnight shipping in Amies transport media (MML Diagnostics, Troutdale, OR) to a central laboratory for processing as previously described [15] using Columbia-Colistin Nalidixic Acid (CNA) agar containing colistin and nalidixic acid and selective broth medium (SBM). Three isolates from each vaginal and rectal sample were selected for serotyping. If no colonies consistent with GBS were observed on the CNA, the SBM was subcultured onto a CNA plate and 3 neomycin disks were placed on the quadrants, incubated for 24 hours, and evaluated as described above. GBS was identified using PathoDX Strep Grouping kits (Remel Microbiology Products, Lenexa, KS).
Serotyping of GBS
Three isolates per positive culture from each study participant were inoculated onto 5% sheep blood agar plates, incubated overnight, subcultured into Todd-Hewitt broth (THB) with 2% sheep blood, grown overnight at 37°C, and stored at −30°C until processing. Then, these were inoculated into THB, extracted with hot hydrochloric acid, and serotyped by Ouchterlony double immunodiffusion using monospecific hyperimmune rabbit antisera produced for types Ia, Ib, and II–IX, as described previously [16]. If the first isolate was not typeable, extracts from the second and third isolates were tested. If none were typeable, the first isolate was inoculated into broth with 1.2% glucose and 1.5% disodium phosphate (to enhance CPS production) and retested as described [16].
Serologic Methods
Serum samples obtained at baseline, enrollment, and for up to 18 months after immunization had type III CPS-specific IgG measured using a quantitative enzyme-linked immunosorbent assay (ELISA) with type III CPS covalently linked to human serum albumin (HSA) as the coating antigen, as previously described [17]. Competitive inhibition experiments indicated that the conjugation of III CPS to HSA does not alter epitope specificity. It has been shown that IgM and IgA response to GBS III-TT vaccine is below the limit of detection by this ELISA. The lower limit of quantification (LLOQ) for GBS type III CPS-specific IgG was 0.04 µg/mL. Values below the LLOQ were assigned a value of 0.02 µg/mL for statistical analyses.
Statistical Analyses
A 0.05 level of significance was used for hypothesis testing and a 95% confidence level was used for interval estimation. Vaccine efficacy was defined as 1 minus the relative risk of acquisition of vaginal GBS III. Modeling was used to calculate vaccine efficacy to adjust for missing data and incomplete follow-up [18]. Relative risk was estimated using a Cox regression model fit to the time from enrollment to first occurrence of vaginal GBS III in the primary analysis [19], and a binary generalized linear regression model with logit link fit to the proportion of participants with recurrent vaginal GBS III across repeated study visits was used in the secondary analysis [20]. The treatment effect from the model, beta, was transformed as 1-exp(beta) to obtain an estimate of vaccine efficacy. A 2-sided Fisher exact test and corresponding confidence interval (CI) for the odds ratio (OR) were used to compare dichotomous secondary efficacy endpoints [21]. Models appropriate for repeated ordinal measures were used to assess the density of GBS III cultured from vaginal swabs at culture-positive visits [22]. Serum IgG antibody concentration to GBS III CPS was assessed graphically by fitting linear mixed effects models to mean levels. Post-vaccination antibody concentrations to GBS III CPS were examined by prevaccination levels of native antibody, vaccination group, age at enrollment, and vaginal or rectal GBS III positivity by clinic visit. One-month post-vaccination serum IgG concentrations were assessed using reverse cumulative distribution curves on the log10 scale.
RESULTS
Study Population
Among 1525 women screened for GBS type III vaginal or rectal colonization, 667 (43.7%) were positive. Of the remaining 858 culture-negative women, 854 were eligible for participation. After exclusions, 667 women were enrolled and randomized; 658 received the allocated vaccine either III-TT (n = 326) or Td (n = 332). Seventy-nine percent of women enrolled (n = 527) completed the 18-month study (Figure 1).
Participant characteristics by vaccine group are summarized in Table 1. The median age was 24 years (range, 18–40 years); 61.4% of women were white, 32.3% African-American, 4.3% multiracial, 3.5% Hispanic, 0.9% Asian, and 1.1% other or unreported. No significant differences in use of birth control methods by vaccine group were found.
Table 1.
Baseline Characteristics of Study Participants by Vaccine Group and Combined
| Characteristic | Tetanus Diphtheria Toxoid Vaccine (n = 325) | Group B Streptococcus Vaccine (n = 325) | All (n = 650) |
|---|---|---|---|
| Age, median (interquartile range), years | 24.0 (21–29) | 24.0 (21–28) | 24.0 (21–29) |
| 18–30 | 261 (80.3) | 260 (80.0) | 521 (80.2) |
| 31–40 | 64 (19.7) | 65 (20.0) | 129 (19.8) |
| Ethnicity | |||
| Hispanic or Latino | 11 (3.4) | 12 (3.7) | 23 (3.5) |
| Not Hispanic or Latino | 314 (96.6) | 313 (96.3) | 627 (96.5) |
| Race | |||
| American Indian/Alaskan Native | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| Asian | 3 (0.9) | 3 (0.9) | 6 (0.9) |
| Native Hawaiian or other Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Black or African-American | 111 (34.2) | 99 (30.5) | 210 (32.3) |
| White | 195 (60.0) | 204 (62.8) | 399 (61.4) |
| Multiracial | 14 (4.3) | 14 (4.3) | 28 (4.3) |
| Race not reported | 1 (0.3) | 5 (1.5) | 6 (0.9) |
| Contraceptive methods | |||
| Depot medroxyprogesterone acetate (Depo-Provera)a | 36 (11.1) | 46 (14.2) | 82 (12.6) |
| Oral contraceptivesb | 121 (37.2) | 127 (39.1) | 248 (38.2) |
| Other hormonal | 16 (4.9) | 27 (8.3) | 43(6.6) |
| Nonhormonal | 150 (46.2) | 125 (38.5) | 275 (42.3) |
| No method indicatedc | 2 (0.6) | 0 (0.0) | 2 (0.3) |
Includes women who had at least 1 post enrollment efficacy assessment that was evaluable. Women are classified by group based on the vaccine to which they were randomly allocated (intent-to-treat analysis), not the vaccine they received. All rows are percentages unless otherwise specified.
aIncludes “pills” (for hormonal regulation).
bIncludes “morning after pill” (emergency contraception).
cParticipants answered “no” to all methods.
Immunogenicity of GBS III-TT Conjugate Vaccine
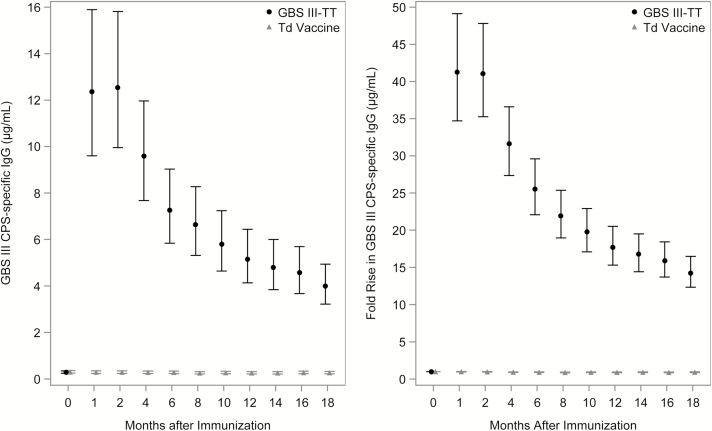
The baseline geometric mean concentrations (GMCs) of GBS type III CPS-specific IgG in sera from women in both vaccine groups were low, with 147 (22%) of 664 women having a result at or below the LLOQ. The GMC of the GBS III-TT and Td group was similar (0.29 vs 0.31). Post-immunization, women who received GBS III-TT vaccine had significantly higher serum GMCs of type III CPS-specific IgG (12–13 µg/mL) than women who received Td vaccine (P < .0001), and this difference persisted at all subsequent time intervals (Figure 2A). Recipients of GBS III-TT vaccine had a more than 40-fold rise in type III CPS-specific IgG 1-month post-vaccination compared to pre-vaccination values; no increases were observed for recipients of Td vaccine (Figure 2B).
Figure 2.
(A) Geometric mean concentrations (95% confidence intervals [CIs]) of group B Streptococcus (GBS) capsular polysaccharide (CPS) type III antibodies in sera from GBS III-TT (tetanus toxoid) and tetanus diphtheria toxoid (Td) immunized women over time. Lower limit of detection of GBS type III CPS-specific immunoglobulin (Ig) G was 0.04 µg/mL. (B) Mean fold-rise (95% CIs) in serum GBS type III CPS-specific IgG over time post immunization for GBS III-TT (black circles) and Td (gray triangles) immunized participants. The fold-rise was 1 for Td-immunized women because the concentration of GBS type III CPS-specific IgG was below the lower limit of detection. Abbreviations: CPS, capsular polysaccharide; GBS III-TT, group B Streptococcus type III capsular polysaccharide-tetanus toxoid conjugate; Ig, immunoglobulin; Td, tetanus diphtheria toxoid.
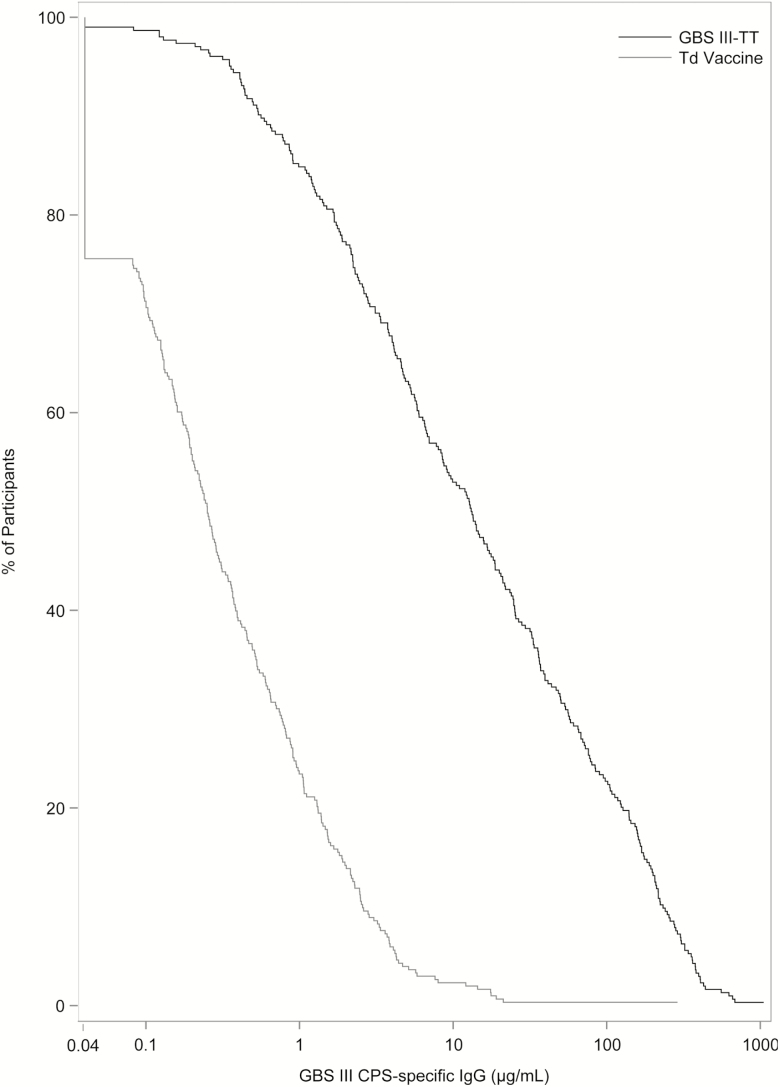
When expressed as reverse cumulative distribution plots, women immunized with GBS III-TT vaccine had a robust immune response at 1-month post-immunization when compared to those who received Td vaccine (log-rank test statistic, 414.04; P < .01; Figure 3). Non-white women and those aged 30–40 years had higher CPS III-specific serum antibody levels 1-month post-immunization compared to white women (log-rank test, 5.97; P = .01; Supplementary Figure 1) and those aged 18–21 years (log-rank test, 14.48; P < .0001; Supplementary Figure 2), respectively. A strong association between pre-vaccination serum concentrations of type III CPS-specific IgG and subsequent immune response was noted, with higher GBS type III CPS-specific IgG pre-immunization levels predictive of higher antibody responses (log-rank test, 222.8; P < .001; Supplementary Figure 3).
Figure 3.
Reverse cumulative distribution plot comparing serum group B Streptococcus (GBS) type III capsular polysaccharide (CPS)-specific immunoglobulin (Ig) G concentrations (µg/mL) from women 1 month after immunization with GBS III-TT (black line) or tetanus diphtheria toxoid (Td) vaccines (gray line). The y-axis shows the percent of women in each group with a given GBS type III CPS-specific IgG concentration or higher, demonstrating a significantly greater immune response in the GBS vaccine group. Abbreviations: CPS, capsular polysaccharide; GBS III-TT, group B Streptococcus type III capsular polysaccharide-tetanus toxoid conjugate; Ig, immunoglobulin; Td, tetanus diphtheria toxoid.
Safety of GBS III-TT Conjugate Vaccine
GBS III-TT vaccine was well tolerated, although mild to moderate tenderness (72%) or pain (54%) at the injection site were frequent. Women who received Td vaccine reported local symptoms of greater severity compared to women who received GBS III-TT vaccine (P < .0001). Solicited systemic symptoms (headache, malaise, muscle aches) reported were absent or mild (41%) in recipients of Td and GBS III-TT vaccines, respectively, and typically resolved within 2 days. Among 1868 unsolicited adverse events reported by 663 immunized women during the 18 months of follow-up, 9.8% of events were judged to be vaccine associated by the investigator. When the rates of unsolicited adverse events were compared, there was no significant difference between vaccine groups.
Vaccine Efficacy
In preventing or delaying time to first acquisition of vaginal GBS III, stratified by geographic region, GBS III-TT vaccine efficacy was 36% (95% CI, 1% to 58%; P = .044). In a secondary, unstratified analysis, efficacy was 35% (95% CI, 1% to 58%; P = .047; Table 2). While vaccination with III-TT vaccine delayed or prevented vaginal colonization with serotype III GBS, it had no effect on colonization with non-type III GBS serotypes. Supplemental Figure 4 illustrates this finding.
Table 2.
Estimate of Group B Streptococcus (GBS) Type III Capsular Polysaccharide-tetanus Toxoid Conjugate Vaccine Efficacy Against Acquisition of Vaginal and Rectal Colonization With Type III GBS
| Vaccine Efficacy | Confidence Interval | P Value | |
|---|---|---|---|
| Time to first positive swab | |||
| Vaginal | 0.35 | 0.01 to 0.58 | .047a |
| Rectal | 0.42 | 0.10 to 0.63 | .015a |
| Proportion of positive swabs | |||
| Vaginal | |||
| Not adjusted | 0.36 | −0.07 to 0.61 | .089 |
| Adjustedb | 0.36 | −0.06 to 0.62 | .085 |
| Rectal | |||
| Not adjusted | 0.41 | −0.01 to 0.66 | .055 |
| Adjusted | 0.49 | 0.13 to 0.70 | .014a |
aStatistically significant at the 0.05 level of significance.
bThe model was adjusted for a priori covariates including race, vaginal lactobacilli, sexual activity, and months since immunization.
In the secondary exploratory analysis, there was similar efficacy for time to first acquisition of rectal GBS III when stratified by geographical region (43%; 95% CI, 11% to 63%; P = .014) and without stratification (42%; 95% CI, 10% to 63%; P = .015; Table 2). A Kaplan-Meier curve comparing the 2 vaccine groups for rectal cultures yielding GBS III over time illustrates this moderate GBS III-TT efficacy (Supplemental Figure 5).
When GBS III-TT vaccine efficacy was expressed as proportion of vaginal cultures yielding GBS III, efficacy was 36% (95% CI, −6% to 62%) after adjustment for covariates including race, vaginal lactobacilli, sexual activity, and months since immunization. Vaccine efficacy for proportion of rectal swabs yielding GBS III was 49% (95% CI, 13% to 70%) after adjustment for covariates. More than 98% of vaginal and rectal isolates had serotyping results that were concordant (ie, both GBS III positive or GBS III negative).
Conditional analysis (restricted to GBS III culture-positive women) showed that those who received GBS III-TT vaccine and became colonized with GBS had similar density of organisms as did women who received Td vaccine (OR, 0.81; 95% CI, 0.39 to 1.66). However, an unconditional analysis (results from all post-immunization vaginal specimens) revealed that GBS III-TT vaccine recipients had a trend toward lower density of colonization than women in the Td vaccine group (OR, 0.62; 95% CI, 0.36 to 1.05). Other secondary endpoints did not reach statistical significance.
DISCUSSION
Preventing perinatal exposure to a pathogen would be an ideal approach to impede the transmission of GBS. Our multicenter, randomized trial is the first to demonstrate that immunization with a GBS CPS-protein conjugate vaccine has moderate efficacy in delaying or preventing vaginal and rectal acquisition of GBS, the first step in the pathogenesis of GBS sepsis and meningitis in neonates. Women who received monovalent GBS III-TT conjugate vaccine had a significant delay in time to first acquisition of vaginal type III GBS colonization, with an estimated efficacy of 36% compared to women who received Td vaccine. The vaccine also delayed time to first rectal type IIIGBS colonization, with an estimated efficacy of 43%.
The presumed mechanism for the vaccine’s effect at the vaginal site is transudation of III CPS-specific IgG from serum into vaginal fluid, although this was not evaluated in the present study. Human immunodeficiency virus (HIV)-infected women have been reported to have HIV-specific IgG in cervicovaginal and rectal lavage fluids [23]. Most women with hepatitis C infection are also reported to have hepatitis C virus–specific IgG present in the vaginal fluid; the authors concluded that this was derived from systemic rather than local production based on its monomeric structure [24]. Finally, induction of specific antibodies in cervicovaginal mucus and rectal secretions was demonstrated after administration of an HIV-1 candidate vaccine [25].
Our study provides proof-of-concept that GBS conjugate vaccine–induced antibodies against type III GBS can delay colonization at mucosal sites. These findings are consistent with and amplify those of 2 observational cohort studies in South African pregnant women that showed that women with high naturally acquired antibodies to CPS-specific IgG [26] or to surface proteins of GBS had a reduced risk of acquiring vaginal and rectal GBS colonization [27]. Decreasing GBS colonization through immunization could have a significant public health impact by reducing pregnancy loss, preterm gestation, and third trimester stillbirth, which are sequelae that cannot be prevented by IAP to prevent mother-to-infant transmission [8].
The GBS III-TT vaccine was safe and demonstrated robust immunogenicity. Injection site symptoms were common, but rates were similar in both vaccine groups. Four-fold or greater antibody increases above pre-immunization serum levels were observed in 95% of GBS vaccine recipients, and significantly increased serum levels persisted over the 18 study months. Greater immune responses occurred in women with preexisting, naturally acquired specific antibodies [28] and in those who were older [29], which is consistent with a prime-boost response.
This study has strengths including the multicenter design, large sample size, inclusion of women of diverse ethnicities, and long duration of follow-up. An additional strength was use of intensive cultivation techniques that enabled detection of multiple colony types and revealed colonization by multiple GBS serotypes. A limitation was use of a conjugate vaccine against a single GBS capsular type, leading to an unchanged overall colonization rate in the GBS vaccine group. The replacement by GBS types not targeted by the monovalent vaccine suggests that women are exposed to a range of GBS serotypes and that a successful vaccine must be multivalent to impact mucosal GBS colonization. Finally, the study was performed in the United States, limiting generalizability of the findings to other regions where GBS disease incidence is high.
In summary, we showed that a single dose of a monovalent vaccine targeting 1 serotype of GBS delayed acquisition of type III GBS vaginal and rectal colonization. In the present study, the decline in GBS III colonization at the end of the 18 months of follow-up was associated with replacement by non-type III strains, resulting in similar overall colonization rates in both vaccine groups, suggesting that a multivalent vaccine that represents the common colonizing and invasive GBS serotypes (Ia, Ib, II, III, IV, and V) would be required for optimal efficacy. Fortunately, such a vaccine has been developed and early clinical trials have been initiated.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the women who participated in this clinical trial and the study staff who contributed to its success.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIH; N01-AI-25495).
Potential conflicts of interest. M. S. E. reports receiving grant funding from Pfizer, Inc. S. L. H. reports grants from the NIH during the conduct of the study and personal fees from Merck and grants from Cepheid outside the submitted work. M. E. reports another NIH contract (HHSN272201500002C) during the conduct of the study. P. F. reports grants from the NIH during the conduct of the study. C. J. B. reports grants from the NIH during the conduct of the study. D. L. K. reports grants from the NIH during the conduct of the study. H. H. was a contract employee for the Emmes Corporation during the conduct of the study. L. C. P. reports grants from the NIH during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Phares CR, Lynfield R, Farley MM, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. Active Bacterial Core Surveillance/Emerging Infections Program Network. JAMA 2008; 299:2056–65. [DOI] [PubMed] [Google Scholar]
- 2. Libster R, Edwards KM, Levent F, et al. Long-term outcomes of group B streptococcal meningitis. Pediatrics 2012; 130:e8–15. [DOI] [PubMed] [Google Scholar]
- 3. Kohli-Lynch M, Russell NJ, Seale AC, et al. Neurodevelopmental impairment in children after group B streptococcal disease worldwide: systematic review and meta-analyses. Clin Infect Dis 2017; 65:190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baker CJ, Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med 1976; 294:753–6. [DOI] [PubMed] [Google Scholar]
- 5. Lin FY, Weisman LE, Azimi PH, et al. Level of maternal IgG anti-group B Streptococcus type III antibody correlated with protection of neonates against early-onset disease caused by this pathogen. J Infect Dis 2004; 190:928–34. [DOI] [PubMed] [Google Scholar]
- 6. Fabbrini M, Rigat F, Rinaudo CD, et al. The protective value of maternal group B Streptococcus antibodies: quantitative and functional analysis of naturally acquired responses to capsular polysaccharides and pilus proteins in European maternal sera. Clin Infect Dis 2016; 63:746–53. [DOI] [PubMed] [Google Scholar]
- 7. Baker CJ, Carey VJ, Rench MA, et al. Maternal antibody at delivery protects neonates from early onset group B streptococcal disease. J Infect Dis 2014; 209:781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease–revised guidelines from CDC, 2010. MMWR Recomm Rep 2010; 59:1–36. [PubMed] [Google Scholar]
- 9. Longtin J, Vermeiren C, Shahinas D, et al. Novel mutations in a patient isolate of Streptococcus agalactiae with reduced penicillin susceptibility emerging after long term oral suppressive therapy. Antimicrob Agents Chemother 2011; 55:2893–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aloisio I, Mazzola G, Corvaglia LT, et al. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl Microbiol Biotechnol 2014; 98:6051–60. [DOI] [PubMed] [Google Scholar]
- 11. Bianchi-Jassir F, Seale AC, Kohli-Lynch M, et al. Preterm birth associated with group B Streptococcus maternal colonization worldwide: systematic review and meta-analyses. Clin Infect Dis 2017; 65:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seale AC, Blencowe H, Bianchi-Jassir F, et al. Stillbirth with group B Streptococcus disease worldwide: systematic review and meta-analyses. Clin Infect Dis 2017; 65:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hordnes K, Tynning T, Kvam AI, Jonsson R, Haneberg B. Colonization in the rectum and uterine cervix with group B streptococci may induce specific antibody responses in cervical secretions of pregnant women. Infect Immun 1996; 64:1643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hordnes K, Digranes A, Haugen IL, et al. Systemic and mucosal antibody responses to group B Streptococci following immunization of the colonic-rectal mucosa. J Reprod Immunol 1995; 28:247–62. [DOI] [PubMed] [Google Scholar]
- 15. Meyn LA, Krohn MA, Hillier SL. Rectal colonization by group B Streptococcus as a predictor of vaginal colonization. Am J Obstet Gynecol 2009; 201:76.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diedrick MJ, Flores AE, Hillier SL, Creti R, Ferrieri P. Clonal analysis of colonizing group B Streptococcus, serotype IV, an emerging pathogen in the United States. J Clin Microbiol 2010; 48:3100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guttormsen HK, Baker CJ, Edwards MS, Paoletti LC, Kasper DL. Quantitative determination of antibodies to type III group B streptococcal polysaccharide. J Infect Dis 1996; 173:142–50. [DOI] [PubMed] [Google Scholar]
- 18. Chen HY, Little RJ. A profile conditional likelihood approach for the semiparametric transformation regression model with missing covariates. Lifetime Data Anal 2001; 7:207–24. [DOI] [PubMed] [Google Scholar]
- 19. Efron B. The efficiency of Cox’s likelihood function for censored data. J Am Statistical Assn 1977; 72:557–65. [Google Scholar]
- 20. Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988; 44:1049–60. [PubMed] [Google Scholar]
- 21. Pocock SJ. Clinical trials: a practical approach. Chichester; NY: Wiley & Sons, Inc, 1983. [Google Scholar]
- 22. Diggle PJ. An approach to the analysis of repeated measurements. Biometrics 1988; 44:959–71. [PubMed] [Google Scholar]
- 23. Mestecky J, Wei Q, Alexander R, Raska M, Novak J, Moldoveanu Z. Humoral immune responses to HIV in the mucosal secretions and sera of HIV-infected women. Am J Reprod Immunol 2014; 71:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bélec L, Legoff J, Si-Mohamed A, et al. Mucosal humoral immune response to hepatitis C virus E1/E2 surface glycoproteins and HCV shedding in saliva and cervicovaginal fluids from chronically HCV-infected patients. J Hepatol 2003; 38:833–42. [DOI] [PubMed] [Google Scholar]
- 25. Akapirat S, Karnasuta C, Vasan S, et al. ; RV305 Study Group. Characterization of HIV-1 gp120 antibody specificities induced in anogenital secretions of RV144 vaccine recipients after late boost immunizations. PLoS One 2018; 13:e0196397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kwatra G, Adrian PV, Shiri T, Buchmann EJ, Cutland CL, Madhi SA. Natural acquired humoral immunity against serotype-specific group B Streptococcus rectovaginal colonization acquisition in pregnant women. Clin Microbiol 2015; 21:568.e13–21. [DOI] [PubMed] [Google Scholar]
- 27. Dzanibe S, Kwatra G, Adrian PV, Kimaro-Mlacha SZ, Cutland CL, Madhi SA. Association between antibodies against group B Streptococcus surface proteins and recto-vaginal colonisation during pregnancy. Sci Rep 2017; 7:16454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donders GG, Halperin SA, Devlieger R, et al. Maternal immunization with an investigational trivalent group B streptococcal vaccine: a randomized controlled trial. Obstet Gynecol 2016; 127:213–21. [DOI] [PubMed] [Google Scholar]
- 29. Campbell JR, Hillier SL, Krohn MA, Ferrieri P, Zalesnik DF, Baker CJ. Group B streptococcal colonization and serotype-specific immunity in pregnant women at delivery. Obstet Gynecol 2000; 96:496–505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.