Abstract
Materials and Methods
Thirty-six male Wistar rats were divided into six groups: groups 1, 2, and 3 received vehicle, Cd (100 mg/L/day by drinking water), and A. hirtifolium extract (200 mg/kg/day; orally), respectively. Groups 4, 5, and 6 were Cd groups which were treated with A. hirtifolium extract (50, 100, and 200 mg/kg/day, respectively). After 2 weeks, liver enzymes such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) and also oxidative stress biomarkers including lipid peroxidation (LPO), total antioxidant capacity (TAC), total thiol molecule (TTM), and the histopathological changes were determined using standard procedure.
Results
The findings showed that Cd caused a remarkable rise in levels of serum hepatic enzymes such as ALT (P < 0.001), AST (P < 0.01) and ALP (P < 0.001) compared with the control group. In addition, Cd led to the decreasing of the levels of TTM (P < 0.001) and TAC (P < 0.001) and increasing of LPO (P < 0.001) in liver tissue in comparison with the control group. In this regard, remarkable vascular congestion, hepatocellular degeneration, and vacuolization were observed in hepatic tissue of Cd-treated rats. Following the administration of A. hirtifolium extract, a significant improvement was observed in the functional and oxidative stress indices of hepatic tissue alongside histopathologic changes.
Conclusion
The current study indicated that the A. hirtifolium extract might prevent hepatic oxidative injury by improving oxidant/antioxidant balance in rats exposed to Cd.
1. Introduction
Allium hirtifolium Boiss, known as Persian shallot “Mooseer,” grows wild as blackish, paper-like tunics in some areas of Iran and other Asian countries. It is traditionally used in the routine diet in countries of central Asia as a spice and flavoring agent in food, especially yoghurt. Recent research has suggested that A. hirtifolium is composed of 9-hexadecenoic acid, 11,14-eicosadienoic acid, and n-hexadecanoic acid, and its hydromethanolic extract has strong antimicrobial properties against several bacteria [1]. Allicin (diallyl thiosulfinate) is considered responsible for the antibacterial, antifungal, and antiparasite potentials of A. hirtifolium [2]. In addition to the antimicrobial property, the phenolic compound of the ethanol extract of A. hirtifolium is reported to have moderate to good antioxidant activity [3, 4], as well as immunomodulatory activity [5] and accelerates wound healing by increasing the epithelialization rate [6]. The active compound of A. hirtifolium, shallomin, is also found safe in humans; therefore, research is continued on the various effects of A. hirtifolium in different organs [7].
Special attention has been paid to the effect of A. hirtifolium on liver protection in animal studies. A. hirtifolium extract has shown protective effects against liver cell apoptosis by inhibiting the growth of human hepatoma cancer cells and BCL2 gene [8]. Also, the chloroformic extract of A. hirtifolium is found to be able to inhibit proliferation of tumor cell lines including cervical cancer, breast, adenocarcinoma, and connective cell lines [9]. Hydroalcoholic extract of A. hirtifolium can also protect rat liver cells against the effects of oxidants in alloxan-induced diabetes and reduce the serum concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) [10, 11]. The favorable result of the hydroalchoholic extract of A. hirtifolium on reducing the serum blood glucose levels, glucokinase activity, and gene expression has also been suggested by other researchers [12]. Nevertheless, in another study, it has been shown that A. hirtifolium extract could not decrease serum levels of apo-lipoproteins, AST, ALT, glucose, or insulin, while it could significantly reduce serum levels of triglyceride and cholesterol as well as atherosclerotic plaque thickness to media [13]. Accordingly, the efficacy of A. hirtifolium on liver enzymes is controversial, and further research is required in this regard.
Cadmium (Cd) is a food contaminant that increases the incidence rate of liver disease [14, 15] and induces oxidative stress in different body organs, especially liver, kidney, and blood, by generating reactive oxygen species (ROS) and impairing the antioxidant defense system [16]. Accordingly, this metal is used to induce liver failure in different animals [17]. Therefore, the aim of the study was to evaluate the therapeutic potential of A. hirtifolium in Cd-induced hepatotoxicity in rats.
2. Materials and Methods
2.1. Preparation of the Extract
The bulbs of the A. hirtifolium were collected from Hamadan in western Iran. The plant was identified by the herbarium section of School of Pharmacy, Hamadan University of Medical Sciences (HUMS), Hamadan, Iran, with code number 234. The bulbs were ground and extracted by methanol/water (50 : 50) at 25°C for 48 h in triplicate using a maceration method. Then, the extract was filtrated and evaporated to become dry in a rotary evaporator (Heidolph, Germany) under vacuum at 40°C. The resulting extract was kept in a dark place at 4°C.
2.2. Phytochemical Screening
The phytochemical analyses were performed on the A. hirtifolium extract using the standard methods to identify secondary metabolites such as alkaloids, saponins, tannins, flavonoids, steroids, terpenoids, proteins, amino acids, glycosides, and anthraquinones [18, 19].
2.3. Determination of Total Phenolic Content
The total phenolic content of the A. hirtifolium extract was determined according to the Folin–Ciocalteu procedure, 2 hours and 1, 7, and 14 days after the extraction (each experiment was performed in triplicate). The results were expressed as mg gallic acid equivalents (GAE)/g extract [20].
2.4. Determination of Total Flavonoid Content
Total flavonoid content of the A. hirtifolium extract was determined based on the method reported by Fathollahi et al. [21], 2 hours and 1, 7, and 14 days after the extraction (each experiment was performed in triplicate). The results were expressed as mg quercetin/g extract [21].
2.5. Animals and Experimental Design
Thirty-six adult male Wistar rats, weighted 210–240 g, were obtained from the animal house of Hamadan University of Medical Sciences (HUMS), kept in polypropylene cages at room temperature (25 ± 2°C), 12 h dark/12 h light cycle, and humidity of about 50%, and provided with free food and water.
After one week of acclimatization, the animals were divided randomly into six groups and treated as follows:
Group 1: the rats received normal saline (control group)
Group 2: the rats received 100 mg/L/day Cd chloride (Cd group)
Group 3: the rats received 200 mg/kg A. hirtifolium extract, orally (AhB200)
Group 4: the rats received 100 mg/L/day Cd chloride by drinking water + 50 mg/kg A. hirtifolium extract orally (AhB50 + Cd)
Group 5: the rats received 100 mg/L/day Cd chloride by drinking water + 100 mg/kg A. hirtifolium extract orally (AhB100 + Cd)
Group 6: the rats received 100 mg/L/day Cd chloride by drinking water + 200 mg/kg A. hirtifolium extract orally (AhB200 + Cd)
After 2 weeks of treating animals of the six groups as described above, the animals were anaesthetized by 50 mg/kg ketamine and 10 mg/kg xylazine. The serum samples, obtained by centrifugation of the heart's blood for 15 min at 5000 rpm, were kept at −20°C. A section of the fresh liver was separated and stored at −80°C for biochemical analysis. A part of rat's liver was excised and put in 10% formalin for histological analysis.
2.6. Liver Function Experiments
The serum samples were used for examination of ALT, AST, and ALP using colorimetric biochemical kits (Pars Azmon, Iran).
2.7. Preparation of Hepatic Tissue Homogenate
The hepatic tissue (100 mg) was homogenized with 1 mL phosphate-buffered saline (50 mM, pH 7.3) and centrifuged at 3000 g for 10 min at 4°C. The supernatant was separated for the biochemical analysis [22].
2.8. Measurement of Lipid Peroxidation
The hepatic lipid peroxidation (LPO) was evaluated by determining bioactive aldehydes using the thiobarbituric acid reactive substances (TBARS) method [23]. In brief, 100 µl of hepatic tissue homogenate was mixed with 500 µl reagent containing 2-thiobarbituric acid (TBA, 0.2%) in H2SO4 (0.05 M). The mixture was heated for 30 min at 100°C in boiling water bath. Then, the optimum absorbance was measured at 532 nm against different concentrations of malondialdehyde (MDA) as the standard, and its findings were reported as nmol/mg protein.
2.9. Measurement of Total Antioxidant Capacity
The total antioxidant capacity of tissue homogenate was measured by determining its ability to reduce Fe+3 to Fe+2 using the ferric-reducing antioxidant power (FRAP) method [24]. In brief, FRAP reagent was prepared by mixing 1 volume of 20 mM FeCl3, 10 volumes of 300 mM acetate buffer (pH 3.6), and also 1 volume of 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM HCL. The complex between Fe2+ and TPTZ, as an indicator, gives a blue color with an absorbance maximum at 593 nm. The results were presented as nmol/mg protein.
2.10. Measurement of Total Thiol Molecules
Total thiol molecules (TTM) were assayed using 5,5′dithiobis-2-nitro benzoic acid (DTNB) as the reagent, and its absorbance was read against a blank at 412 nm [25]. 200 µl of tris-EDTA buffer solution (0.25 M tris base and 20 mM EDTA, pH 8.2) was mixed with 10 µl of homogenized tissue, and its optimum absorbance was detected at 412 nm. Then, 10 µl of DTNB solution (10 mmol/l in methanol) was added to each sample and incubated at 37°C for 15 min. The absorbance of the samples (A2) and also DTNB blank (B) was read again at 412 nm. The level of thiol molecules was determined by reduced glutathione as standard and reported as µM/mg protein.
2.11. Protein Assay
At the end of each experiment, protein levels were measured in the crude homogenate of hepatic tissues by Bradford method.
2.12. Histopathologic Examination
After fixation of hepatic tissue in formalin (10%), the paraffin-embedded block was prepared and cut into 4 µm thick sections using a rotary microtome. The samples were stained with hematoxylin and eosin (H&E) dye for histopathological examination.
2.13. Statistical Analysis
All variables were quantitative. The mean values with standard error mean (SEM) were reported for each variable and compared among the six study groups using one-way ANOVA with Tukey's post hoc test. For the statistical analysis, the GraphPad Prism statistical software version 6.0 was used. P values <0.05 were considered statistically significant.
3. Results
3.1. Phytochemical Analysis
Using the phytochemical screening, we obtained that the hydroalcoholic extract of the A. hirtifolium is composed of different compounds such as phenols, flavonoids, saponins, glycosides, steroids, tannins, terpenoids, and amino acids (Table 1). The total phenolic and flavonoid contents of A. hirtifolium extract were 80.1 ± 0.9 mg GAE/g extract and 58.2 ± 0.4 mg quercetin/g extract, respectively. During the 14 days after the extraction, no significant changes were observed in the phenolic and flavonoid values, which could indicate the stability of extract during this period (data not shown).
Table 1.
Preliminary phytochemical screening of the Allium hirtifolium extract.
| Phytochemical constituents | Methanol extract |
|---|---|
| Flavonoids | +++ |
| Phenols | +++ |
| Alkaloids | − |
| Proteins | − |
| Terpenoids | + |
| Steroids | + |
| Saponins | +++ |
| Anthraquinones | − |
| Amino acids | + |
| Tannins | + |
| Glycosides | ++ |
Strongly positive (+++), moderately positive (++), slightly positive (+), and negative (−).
3.2. The Effects of A. hirtifolium Extract on Liver Function
The results of comparing liver enzymes among the six study groups are shown in Figure 1. As illustrated, ALT, AST, and ALP levels of the Cd group were significantly higher than the control group (P < 0.001, P < 0.01, and P < 0.001, respectively). The two pretreatment groups, receiving 100 and 200 mg A. hirtifolium extract, had a significantly lower ALT level compared with the Cd group (P < 0.001). The A. hirtifolium extract could significantly decrease the serum levels of AST and ALP at the dose of 200 mg/kg, compared with the Cd group (P < 0.05).
Figure 1.
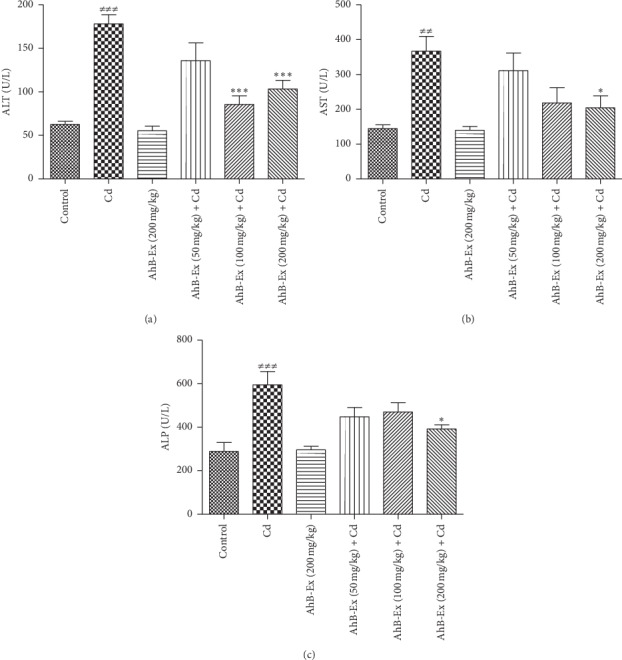
Effects of Allium hirtifolium Boiss extract on liver function biomarkers of cadmium-exposed rats. Values are expressed as mean ± SEM and compared between the groups (6 rats in each group) using one-way ANOVA with Tukey's post hoc test. ##P < 0.01 and ###P < 0.001 vs. control group; ∗P < 0.05 and ∗∗∗P < 0.01 vs. cadmium group. Cadmium was administered by drinking water (100 mg/L) for 2 weeks. Cd: cadmium, AhB-Ex: Allium hirtifolium Boiss extract, ALT: alanine aminotransferase, AST: aspartate aminotransferase, and ALP: alkaline phosphatase.
3.3. The Effects of A. hirtifolium Extract on Hepatic Oxidative Damage
The results of comparing oxidative stress biomarkers among the six study groups are shown in Figure 2. As illustrated, the Cd group had a significantly higher LPO and lower TAC and TTM than the control group (P < 0.001). The two treatment groups, receiving 100 and 200 mg A. hirtifolium extract, had a significantly lower LPO level, compared with the Cd group (P < 0.01). The A. hirtifolium extract could significantly decrease the hepatic LPO level (P < 0.01) and increase TTM levels (P < 0.05) at the doses of 100 and 200 mg/kg, compared with the Cd group (P < 0.01). In addition, a significant increase was observed in the hepatic TAC levels at the dose of 200 mg/kg (P < 0.05).
Figure 2.
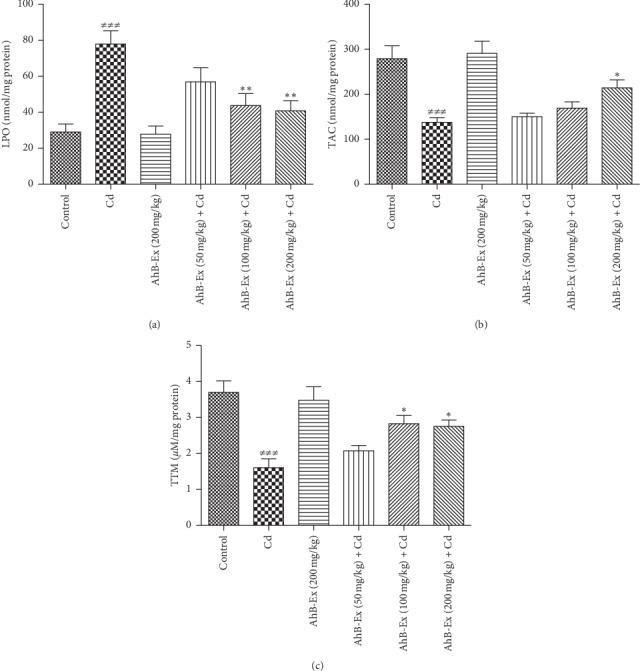
Effects of Allium hirtifolium Boiss extract on oxidative stress biomarkers in the liver of cadmium-exposed rats. Values are expressed as mean ± SEM and compared between the groups (6 rats in each group) using one-way ANOVA with Tukey's post hoc test. ###P < 0.001 vs. control group; ∗P < 0.05 and ∗∗P < 0.01 vs. cadmium group. Cadmium was administered by drinking water (100 mg/L) for 2 weeks. Cd: cadmium, AhB-Ex: Allium hirtifolium Boiss extract, LPO: lipid peroxidation, TAC: total antioxidant capacity, and TTM: total thiol molecule.
3.4. The Effects of A. hirtifolium Extract on Pathological Changes
As mentioned in Figure 3, remarkable vascular congestion, hepatocellular degeneration, and vacuolization were observed in hepatic tissue of Cd-treated rats. Following the pretreatment with different doses of A. hirtifolium extract, a remarkable improvement was found in some of the pathological alterations such as vascular congestion and hepatocellular degeneration. The optimum protective effect was observed at the dose of 200 mg/kg of the extract.
Figure 3.
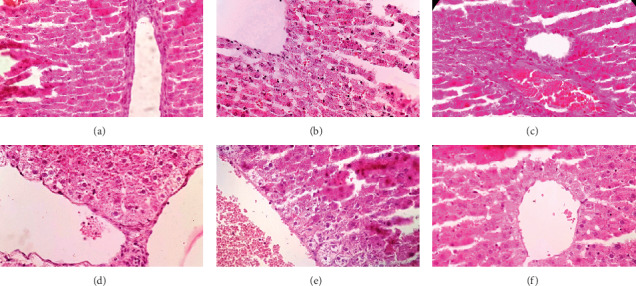
Effects of Allium hirtifolium Boiss extract on hepatic tissue of cadmium-exposed rats. The histopathological changes such as vascular congestion and accumulations of inflammatory cells were evaluated in different groups. (a) The control group showing normal appearance of hepatocytes. (b) The cadmium group showing vascular congestion in remarkable degree, hepatocellular degeneration, and vacuolization. (c) The A. hirtifolium extract (200 mg/kg) group showing normal appearance of hepatocytes. (d) The cadmium plus A. hirtifolium extract (50 mg/kg) group showing rare inflammatory cells, but vascular congestion and cellular degeneration persist. (e) The cadmium plus A. hirtifolium extract (100 mg/kg) group showing mild cellular degeneration without obvious inflammation, but congestion still persists. (f) The cadmium plus A. hirtifolium extract (200 mg/kg) group showed no pathological changes. Original magnification of all images is ×40.
4. Discussion
The results of this study emphasize the nutritional importance of A. hirtifolium Boiss in Cd-induced liver failure. Preliminary phytochemical studies showed significant amounts of phenolic and flavonoid compounds in the A. hirtifolium extract. Due to the remarkable antioxidant properties of these compounds, quantitative contents of phenols and flavonoids were determined in the extract. Based on the received dosage range of the A. hirtifolium extract for each animal (50–200 mg/kg/day), the minimum and maximum amounts received for phenolic compounds (4–16 mg/kg/day) and flavonoid compounds (2.5–10 mg/kg/day) were estimated.
In the present study, increased hepatic serum enzymes (ALT, AST, and ALP) are considered as an important biomarker in the diagnosis of liver failure [26]. Therefore, the results of the current study indicate disruption of hepatic cells' integrity caused by Cd and occurrence of hepatic failure. Several animal studies have also suggested increased levels of hepatic enzymes in the rats' serum that received Cd chloride in drinking water [27, 28], which confirm the results of the present study and emphasize on the importance of exposure to Cd. Clinical studies have also confirmed the significance of hepatotoxicity induced by Cd, which is present in soil, water, food (dietary intake), and air (smoking and air pollution), and considered an important health concern [15, 29–31]. Other studies have also shown the positive correlation of serum Cd concentrations with liver enzyme levels (ALT, AST, and ALP) in Korean adults [32] and Indonesian pregnant women [33], which is consistent with this study. According to the evidence, the body has a limited capacity to Cd exposure, and the long-term exposure to Cd accumulates Cd and causes toxicity in several organs, such as liver and kidneys and impair their function [34–36], indicating the toxic effect of Cd in different organ systems [37].
Since oxidative stress plays a fundamental role in Cd-induced hepatic failure, the oxidant/antioxidant markers were evaluated in this survey. In the current study, a significant increase in LPO and a noticeable decrease in TTM and TAC liver levels were found in the Cd group, indicating the induction of oxidative damage due to Cd toxicity. Although the accurate mechanism of the increased oxidative stress is still not well understood, review studies have indicated that Cd increases the LPO by changing the intracellular glutathione levels, induces the production of ROS and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and inhibits antioxidants [38, 39], which are consistent with the results of the present study.
Due to the significance of oxidative damage caused by Cd, researchers have studied several herbal medicines that can protect against the destructive effects of Cd in the liver with a great focus on oxidative stress [40, 41]. However, none of previous studies have studied the protective effects of A. hirtifolium extract on Cd-induced hepatotoxicity.
Another important finding of the present study, considered the main objective of our study, was the effect of A. hirtifolium on oxidative damage induced by cadmium. Our findings indicated that A. hirtifolium could improve hepatic oxidative damage by increasing the hepatic thiol group and, subsequently, decrease liver LPO. In this regard, the previous studies have suggested that administration of some Allium species such as A. tripedale and A. jesdianum can prevent the effects of hepatotoxic agents such as acetaminophen in rats [42, 43]. A. hirtifolium is reported to have the highest antioxidant property, compared with other 12 plant extracts, as well as significant radical scavenging property [44]. This plant and other garlic compounds have a high concentration of sulphur compounds (such as diallyl sulfide, S-ethylcysteine, diallyl disulfide, and N-acetylcysteine) with a strong antioxidant activity that protect against cell injury [45]. A. hirtifolium is also reported to have a high concentration of saponins, sulphur-containing compounds, and flavonoids which are responsible for its antioxidant activity [46]. Apparently, the different compositions of the plant extract, obtained from different parts of the country, can cause different degrees of antioxidative and liver-protective effects [4]. In addition, the methanolic extract of A. hirtifolium is reported to have a higher concentration of polyphenolic, flavonoid, and proanthocyanidin compounds and a stronger antioxidative property than its aqueous extract [47]. Therefore, the essential components of A. hirtifolium are important factors in determining its protective effects.
In conclusion, the present study showed that Cd induced significant liver injury and oxidative stress in rats, and oral administration of 100 and 200 mg A. hirtifolium extract could significantly reduce the destructive effect of Cd on the liver. Due to the fact that Iranians frequently use A. hirtifolium in combination with yoghurt, it would be of great importance if such a study has been performed in humans with liver dysfunction.
Acknowledgments
The authors would like to thankfully acknowledge the financial support from the Shiraz University of Medical Sciences (SUMS) and scientific support from HUMS.
Data Availability
The data supporting the findings of this study are available within the article.
Ethical Approval
The animal experiments were approved by the Ethics Committee of SUMS, Shiraz, Iran, with Grant no. 99-01-121-22558, in accordance with the guidelines of the Research Ethics Committee of the Ministry of Health and Medical Education, Iran (2019), based on the Helsinki Protocol (Helsinki, Finland, 1975).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Ismail S., Jalilian F. A., Talebpour A. H., et al. Chemical composition and antibacterial and cytotoxic activities of Allium hirtifolium Boiss. BioMed research international. 2013;2013:8. doi: 10.1155/2013/696835.696835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asgarpanah J., Ghanizadeh B. Pharmacologic and medicinal properties of Allium hirtifolium Boiss. African Journal of Pharmacy and Pharmacology. 2012;6(25):1809–1814. doi: 10.5897/ajpp12.509. [DOI] [Google Scholar]
- 3.Ghahremani-majd H., Dashti F., Dastan D., Mumivand H., Hadian J., Esna-Ashari M. Antioxidant and antimicrobial activities of Iranian mooseer (Allium hirtifolium Boiss) populations. Horticulture, Environment, and Biotechnology. 2012;53(2):116–122. doi: 10.1007/s13580-012-0131-2. [DOI] [Google Scholar]
- 4.Pirbalouti A. G., Ahmadzadeh Y., Malekpoor F. Variation in antioxidant, and antibacterial activities and total phenolic content of the bulbs of mooseer (Allium hirtifolium Boiss) Acta Agriculturae Slovenica. 2015;105(1):15–22. [Google Scholar]
- 5.Jafarian A., Ghannadi A., Elyasi A. The effects of Allium hirtifolium Boiss on cell-mediated immune response in mice. Iranian Journal of Pharmaceutical Research. 2010;2(1):51–55. [Google Scholar]
- 6.Ghodrati Azadi H., Fathi B., Kazemi Mehrjerdi H., Maleki M., Shaterzadeh H., Abyazi M. Macroscopic evaluation of wound healing activity of the Persian shallot, Allium hirtifolium in rat. Iranian Journal of Veterinary Science and Technology. 2012;3(1):31–38. [Google Scholar]
- 7.Amin M., Pipelzadeh M. H., Mehdinejad M., Rashidi I. An in vivo toxicological study upon shallomin, the active antimicrobial constitute of Persian shallot (Allium hirtifolium Boiss) extract. Jundishapur Journal of Natural Pharmaceutical Products. 2012;7(1):17–21. doi: 10.17795/jjnpp-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosseini F. S., Falahati-Pour S. K., Hajizadeh M. R., et al. Persian shallot, Allium hirtifolium Boiss, induced apoptosis in human hepatocellular carcinoma cells. Cytotechnology. 2017;69(4):551–563. doi: 10.1007/s10616-017-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azadi H. G., Ghaffari S. M., Riazi G. H., Ahmadian S., Vahedi F. Antiproliferative activity of chloroformic extract of Persian shallot, Allium hirtifolium, on tumor cell lines. Cytotechnology. 2008;56(3):179–185. doi: 10.1007/s10616-008-9145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Javad H., Seyed-Mostafa H.-Z., Farhad O., et al. Hepatoprotective effects of hydroalcoholic extract of Allium Hirtifolium (Persian shallot) in diabetic rats. Journal of Basic and Clinical Physiology and Pharmacology. 2012;2(1) doi: 10.1515/jbcpp-2012-0017. [DOI] [PubMed] [Google Scholar]
- 11.Kazemi S., Asgary S., Moshtaghian J., Rafieian M., Adelnia A., Shamsi F. Liver-protective effects of hydroalcoholic extract of Allium hirtifolium boiss in rats with alloxan-induced diabetes mellitus. Arya Atherosclerosis. 2010;6(1):11–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmoodi M., Zarei S., Rezaeian M., et al. Persian shallot (<>Allium hirtifolium<> boiss) extract elevates glucokinase (GCK) activity and gene expression in diabetic rats. American Journal of Plant Sciences. 2013;4(7):1393–1399. doi: 10.4236/ajps.2013.47170. [DOI] [Google Scholar]
- 13.Rafieian-Kopaei M., Keshvari M., Asgary S., Salimi M., Heidarian E. Potential role of a nutraceutical spice (Allium hirtifolium) in reduction of atherosclerotic plaques. Journal of HerbMed Pharmacology. 2013;2 [Google Scholar]
- 14.Heshmati A., Karami-Momtaz J., Nili-Ahmadabadi A., Ghadimi S. Dietary exposure to toxic and essential trace elements by consumption of wild and farmed carp (Cyprinus carpio) and Caspian kutum (Rutilus frisii kutum) in Iran. Chemosphere. 2017;173:207–215. doi: 10.1016/j.chemosphere.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Hyder O., Chung M., Cosgrove D., et al. Cadmium exposure and liver disease among US adults. Journal of Gastrointestinal Surgery. 2013;17(7):1265–1273. doi: 10.1007/s11605-013-2210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matović V., Buha A., Ðukić-Ćosić D., Bulat Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association. 2015;78:130–140. doi: 10.1016/j.fct.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Renugadevi J., Prabu S. M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Experimental and Toxicologic Pathology. 2010;62(2):171–181. doi: 10.1016/j.etp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 18.kumar Bargah R. Preliminary test of phytochemical screening of crude ethanolic and aqueous extract of Moringa pterygosperma Gaertn. Journal of Pharmacognosy and Phytochemistry. 2015;4(1) [Google Scholar]
- 19.Ugochukwu S. C., Uche A., Ifeanyi O. Preliminary phytochemical screening of different solvent extracts of stem bark and roots of Dennetia tripetala G. Baker. Asian Journal of Plant Science and Research. 2013;3(3):10–13. [Google Scholar]
- 20.Dastan D., Salehi P., Maroofi H. Chemical composition, antioxidant, and antimicrobial activities onLaserpitium carduchorumHedge&LamondEssential oil and extracts during various growing stages. Chemistry & Biodiversity. 2016;13(10):1397–1403. doi: 10.1002/cbdv.201600087. [DOI] [PubMed] [Google Scholar]
- 21.Fathollahi R., Dastan D., Lari J., Masoudi S. Chemical composition, antimicrobial and antioxidant activities of Crupina crupinastrum as a medicinal plant growing wild in west of Iran. Journal of Reports in Pharmaceutical Sciences. 2018;7(2):174–182. [Google Scholar]
- 22.Zeinvand-Lorestani H., Nili-Ahmadabadi A., Balak F., Hasanzadeh G., Sabzevari O. Protective role of thymoquinone against paraquat-induced hepatotoxicity in mice. Pesticide Biochemistry and Physiology. 2018;148:16–21. doi: 10.1016/j.pestbp.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Benzie I. F. F., Strain J. J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 25.Hu M.-L. Measurement of protein thiol groups and glutathione in plasma. Methods in Enzymology. 1994;233:380–385. doi: 10.1016/s0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- 26.Nili-Ahmadabadi A., Alibolandi P., Ranjbar A., et al. Thymoquinone attenuates hepatotoxicity and oxidative damage caused by diazinon: an in vivo study. Research in Pharmaceutical Sciences. 2018;13(6):p. 500. doi: 10.4103/1735-5362.245962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alhazzi I. Cadmium induced hepatotoxicity and oxidative stress in rats: protection by selenium. Research Journal of Environmental Sciences. 2008;2(4):305–309. doi: 10.3923/rjes.2008.305.309. [DOI] [Google Scholar]
- 28.Asagba S., Eriyamremu G. Oral cadmium exposure alters haematological and liver function parameters of rats fed a Nigerian‐like diet. Journal of Nutritional & Environmental Medicine. 2007;16(3-4):267–274. doi: 10.1080/13590840701775403. [DOI] [Google Scholar]
- 29.Anetor J. I., Osibanjo O., Benedo Osadolor H., A Idomeh F., Osazee Igiewe W., Uzoma Kalikwu O. Liver damage risk assessment study in workers occupationally exposed to E-waste in Benin city, south-south Nigeria. Journal of Chemical Health Risks. 2015;5(3) [Google Scholar]
- 30.Sand S., Becker W. Assessment of dietary cadmium exposure in Sweden and population health concern including scenario analysis. Food and Chemical Toxicology. 2012;50(3-4):536–544. doi: 10.1016/j.fct.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 31.Satarug S., Vesey D. A., Gobe G. C. Health risk assessment of dietary cadmium intake: do current guidelines indicate how much is safe? Environmental Health Perspectives. 2017;125(3):284–288. doi: 10.1289/ehp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang M.-Y., Cho S.-H., Lim Y.-H., Seo J.-C., Hong Y.-C. Effects of environmental cadmium exposure on liver function in adults. Occupational and Environmental Medicine. 2013;70(4):268–273. doi: 10.1136/oemed-2012-101063. [DOI] [PubMed] [Google Scholar]
- 33.Wibowo A., Rahaju F. A., Iskandar E., Suhartono E. The role of urinary cadmium on liver function and erythrocytes cell count in pregnancy. International Journal of Bioscience, Biochemistry and Bioinformatics. 2014;4(4):224–228. doi: 10.7763/ijbbb.2014.v4.344. [DOI] [Google Scholar]
- 34.Dastan D., Karimi S., Larki-Harchegani A., Nili-Ahmadabadi A. Protective effects of Allium hirtifolium Boiss extract on cadmium-induced renal failure in rats. Environmental Science and Pollution Research. 2019;23(18):18886–18892. doi: 10.1007/s11356-019-04656-7. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar A., Ravindran G., Krishnamurthy V. A brief review on the effect of cadmium toxicity: from cellular to organ level. International Journal of Biotechnology Research. 2013;3(1):17–36. [Google Scholar]
- 36.Wallia A., Allen N. B., Badon S., El Muayed M. Association between urinary cadmium levels and prediabetes in the NHANES 2005-2010 population. International Journal of Hygiene and Environmental Health. 2014;217(8):854–860. doi: 10.1016/j.ijheh.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arroyo V., Flores K., Ortiz L., Gómez-Quiroz L., Gutiérrez-Ruiz M. Liver and cadmium toxicity. Journal of Drug Metabolism and Toxicology. 2012;5(1) [Google Scholar]
- 38.Cuypers A., Plusquin M., Remans T., et al. Cadmium stress: an oxidative challenge. Biometals. 2010;23(5):927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- 39.Patra R., Rautray A. K., Swarup D. Oxidative stress in lead and cadmium toxicity and its amelioration. Veterinary Medicine International. 2011;2011:9. doi: 10.4061/2011/457327.457327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andleeb S., Shaukat S., Ara C. Protection against cadmium-induced abnormalities and hepatotoxicity in ovo by Allium sativum. Punjab University Journal of Zoology. 2018;33(1):34–41. doi: 10.17582/pujz/2018.33.1.34.41. [DOI] [Google Scholar]
- 41.Obioha U. E., Suru S. M., Ola-Mudathir K. F., Faremi T. Y. Hepatoprotective potentials of onion and garlic extracts on cadmium-induced oxidative damage in rats. Biological Trace Element Research. 2009;129(1-3):p. 143. doi: 10.1007/s12011-008-8276-7. [DOI] [PubMed] [Google Scholar]
- 42.Ghobadi S., Dastan D., Soleimani M., Nili-Ahmadabadi A. Hepatoprotective potential and antioxidant activity of Allium tripedale in acetaminophen-induced oxidative damage. Research in Pharmaceutical Sciences. 2019;14(6):p. 488. doi: 10.4103/1735-5362.272535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sohrabinezhad Z., Dastan D., Asl S. S., Nili-Ahmadabadi A. Allium jesdianum extract improve acetaminophen-induced hepatic failure through inhibition of oxidative/nitrosative stress. Journal of Pharmacopuncture. 2019;22(4):p. 239. doi: 10.3831/KPI.2019.22.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souri E., AMIN G. R., Jalalizadeh H., Barezi S. Screening of Thirteen Medicinal Plant Extracts for Antioxidant Activity. Life Sciences. 2008;73(2):167–179. doi: 10.1016/s0024-3205(03)00259-5. [DOI] [PubMed] [Google Scholar]
- 45.Omar S. H., Al-Wabel N. A. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharmaceutical Journal. 2010;18(1):51–58. doi: 10.1016/j.jsps.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hajian N., Rezayatmand Z., Shahanipur K. Preventive effects of Allium hirtifolium Boiss methanolic and aqueous extracts on renal injury induced by lead in rats. Journal of HerbMed Pharmacology. 2018;7(3) doi: 10.15171/jhp.2018.26. [DOI] [Google Scholar]
- 47.Siahpoosh A., Souhangir S. Antioxidative and free radical scavenging activities of aqueous and methanolic bulbs extracts of Allium hirtifolium. International Journal of Biosciences. 2014;5(9):379–392. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available within the article.


