Summary
Objectives
To investigate the importance of time in pregnancy and neonatal sex on the association between maternal metabolic parameters and neonatal sum of skinfolds.
Methods
This was a longitudinal, secondary analysis of the vitamin D and lifestyle intervention for gestational diabetes mellitus study, conducted in nine European countries during 2012 to 2015. Pregnant women with a pre‐pregnancy body mass index (BMI) of ≥29 kg/m2 were invited to participate. We measured 14 maternal metabolic parameters at three times during pregnancy: <20 weeks, 24 to 28 weeks, and 35 to 37 weeks of gestation. The sum of four skinfolds assessed within 2 days after birth was the measure of neonatal adiposity.
Results
In total, 458 mother‐infant pairs (50.2% female infants) were included. Insulin resistance (fasting insulin and HOMA‐index of insulin resistance) in early pregnancy was an important predictor for boys' sum of skinfolds, in addition to fasting glucose and maternal adiposity (leptin, BMI and neck circumference) throughout pregnancy. In girls, maternal lipids (triglycerides and fatty acids) in the first half of pregnancy were important predictors of sum of skinfolds, as well as fasting glucose in the second half of pregnancy.
Conclusions
Associations between maternal metabolic parameters and neonatal adiposity vary between different periods during pregnancy. This time‐dependency is different between sexes, suggesting different growth strategies.
Keywords: foetal growth, foetal programming, maternal health, metabolic syndrome, neonatal body composition, pregnancy
Abbreviations
- BMI
body mass index
- DALI
vitamin D and lifestyle intervention for gestational diabetes mellitus prevention: an European multicentre randomized trial
- GDM
gestational diabetes
- HAPO
hyperglycemia and adverse pregnancy outcome
- HOMA‐IR
HOMA‐index of insulin resistance
1. INTRODUCTION
Globally, in 2017 an estimated 38 million children under 5 years of age presented either overweight or obesity. This is a major public health concern.1 Hence, strategies have to be developed to reduce this burden and prevent childhood obesity. This requires a thorough identification and understanding of the underlying determinants.
Maternal obesity in pregnancy is linked with the development of neonatal and childhood adiposity.2, 3, 4, 5 Although there is much interest in the relationship between maternal obesity and childhood adiposity, maternal metabolic factors driving the increase in offspring adiposity have not been fully understood.
The Hyperglycemia and Adverse Pregnancy Outcome study showed a continuous positive relationship of maternal glucose in the second trimester with measures of neonatal body fat.6 Findings from the Healthy Start Study support this and, in addition, showed temporal changes in the association of glucose and neonatal fat, with stronger associations in the second half of pregnancy compared to early pregnancy.7, 8 However, other studies clearly indicate that the influence of maternal phenotype on foetal growth can already be demonstrated in the early pregnancy period: foetal abdominal circumference, as proxy for foetal overgrowth, is increased in women with obesity and/or gestational diabetes (GDM) already at 20 weeks of gestation or even before9, 10; maternal insulin resistance in the first half of pregnancy is related to neonatal fat percentage,7 and fasting glucose in the first trimester with the risk of a large‐for‐gestational age baby.11 Although these studies suggest an influence of the early pregnancy period, surprisingly little is known about temporal relations between maternal metabolic measures and neonatal fat accrual.12
Female neonates are known to be more insulin resistant,13, 14 which might explain why males are more affected by in utero exposure to gestational diabetes.9 Therefore, it is mandatory to assess temporal relations of maternal metabolism with neonatal adiposity in a sex‐dependent manner.
In this study, we investigated the association between maternal health measures at different times during pregnancy and neonatal sum of skinfolds in a sex‐specific manner. We hypothesized that: (a) associations between maternal metabolic parameters and neonatal adiposity differ at different time points, and (b) these temporal differences are sex‐specific. We tested these hypotheses in 458 mother‐infant pairs in the vitamin D and lifestyle intervention for gestational diabetes mellitus (DALI) study, a pan‐European study originally designed for the prevention of gestational diabetes.15
2. METHODS
2.1. Design and participants
This is a longitudinal, secondary analysis of the DALI study, which was a multicentre parallel randomized trial conducted in nine European countries (Austria, Belgium, Denmark, Ireland, Italy, Netherlands, Poland, Spain and United Kingdom) during 2012 to 2015. The study was prospectively registered as a randomized clinical trial (RCT) with the primary aim to prevent gestational diabetes mellitus on November 21, 2011 (ISRCTN70595832). Local ethics committee approval and written informed consent of all women was obtained. Pregnant women with a pre‐pregnancy body mass index (BMI) of ≥29 kg/m2, <20 weeks of gestation, a singleton pregnancy and aged ≥18 years were invited to participate.16 Exclusion criteria included diagnosis with early gestational diabetes mellitus,17, 18 pre‐existing diabetes, and chronic medical conditions.
2.2. Design and procedures
The study was originally designed as an RCT with the following groups, pre‐stratified for site: (a) healthy eating; (b) physical activity; (c) healthy eating + physical activity; (d) healthy eating + physical activity + vitamin D; (e) healthy eating + physical activity + placebo; (f) vitamin D; (g) placebo, (h) control. Staff involved with measurements, but not participants, were blinded to the lifestyle intervention. Both staff and participants were blinded to vitamin D intervention. For the purpose of this analysis, the data were analyzed as a longitudinal cohort. Maternal measurements took place at baseline (<20 weeks), at 24 to 28 and at 35 to 37 weeks of gestation. Since methodology has been extensively described elsewhere,16 only variables of interest will be detailed in this manuscript.
2.3. Measurements
2.3.1. Neonatal outcome
Triceps, subscapular, supra‐iliac and quadriceps skinfolds were measured within 48 hours of birth with a Harpenden skinfold calliper (Baty, UK), and the values summed to obtain the primary neonatal outcome measure, the sum of skinfolds. Each skinfold measurement was measured twice and if a difference of more than 0.2 mm was registered, a third measurement was performed and the average of the three was taken. The neonatal age at the measurement was the time between birth and measurements, which was registered in hours.
2.3.2. Maternal metabolic and adiposity parameters
Maternal height was determined at baseline with a stadiometer (SECA 206; SECA, UK). Women were weighed on calibrated electronic scales (SECA 888 and 877, SECA, UK) at baseline (<20 weeks), 24 to 28 weeks, and at 35 to 37 weeks of gestation. BMI was calculated as weight in kilogram divided by the square of height in metres. Gestational weight gain was defined as the change in objectively measured weight from pre‐pregnancy to <20, <20 to 24‐28 weeks and from 24‐28 to 35‐37 weeks. Neck circumference was obtained in a standing relaxed upright position between mid‐cervical spine and mid‐anterior neck, to within 1 mm.19
After fasting for 10 hours, blood was collected and mothers consumed a 250 mL 75 g glucose drink (within a period of 5 minutes). Further blood collections took place after 60 and 120 minutes. All the samples were centrifuged and separated aliquots of plasma (1000 μL or 250 μL) placed in microrack tubes and stored at −20°C or −80°C in the central trial laboratory, prior to analysis, in Graz, Austria. The maternal concentrations of plasma fasting glucose, fasting insulin, triglycerides, free fatty acids and leptin were quantified. For insulin and leptin commercially available Enzyme‐Linked Immuno Sorbent Assays were used. In the samples taken 60 and 120 minutes after glucose load, only glucose (1‐hour glucose and 2‐hour glucose) and insulin (1‐hour insulin and 2‐hour insulin) were assessed. Fasting insulin resistance was derived from homeostasis model assessment (HOMA‐index of insulin resistance – HOMA‐IR).20 Insulin secretion after glucose load were calculated with the Stumvoll first phase and Stumvoll second phase validated equations.21
2.3.3. Covariates
Information on possible covariates was collected in the baseline questionnaire or from medical files: national site(s) of recruitment, maternal age, gestational age during pregnancy (<20, 24 to 28 and 35 to 37 weeks), maternal ethnicity (European or non‐European descent), maternal education (low, medium and high), smoking status at 35 to 37 weeks of gestation (yes/no), pre‐pregnancy BMI and gestational age at birth.
2.4. Statistical analyses
All analyses were performed in STATA version 13 for windows (StataCorp LP, College Station, Texas) and a 5% type I error rate was used for the analyses. We have 80% statistical power to detect associations with small effect sizes (f 2 = 0.04) considering the number of participants evaluated (n = 458) and the number of exposures and confounding variables in each of our models. Skewed variables were log‐transformed before analyses (fasting insulin, 1‐hour insulin, 2‐hour insulin, HOMA‐IR, Stumvoll first phase, Stumvoll second phase, triglycerides, leptin and BMI). Independent t tests were performed to evaluate descriptive differences in the mother's metabolic and adiposity parameters for each pregnancy period in relation to their child's sex (results shown in Table 1). Independent t tests also evaluated sex‐differences in neonatal skinfolds.
Table 1.
Mean and SD of the mothers' variables included in the main analyses for different gestational time and stratified by sex
| Maternal parameters | <20 weeks | 24‐28 weeks | 35‐37 weeks | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | P | Boys | Girls | P | Boys | Girls | P | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Fasting glucose (mmol/L) | 4.58 | 0.38 | 4.61 | 0.36 | .39 | 4.54 | 0.40 | 4.61 | 0.43 | .07 | 4.47 | 0.45 | 4.56 | 0.43 | .05 |
| 1‐h glucose (mmol/L) | 6.60 | 1.38 | 6.80 | 1.47 | .15 | 7.56 | 1.70 | 7.77 | 1.60 | .19 | 7.93 | 1.55 | 8.12 | 1.45 | .25 |
| 2‐h glucose (mmol/L) | 5.72 | 1.11 | 5.81 | 1.18 | .45 | 6.17 | 1.25 | 6.15 | 1.27 | .87 | 6.44 | 1.25 | 6.49 | 1.12 | .70 |
| Fasting insulin (μU/mL) | 12.69 | 1.03 | 13.12 | 1.03 | .46 | 13.98 | 1.03 | 15.46 | 1.03 | .02 | 17.05 | 1.04 | 17.97 | 1.04 | .37 |
| 1‐h insulin (μU/mL) | 85.48 | 1.05 | 84.98 | 1.05 | .93 | 112.62 | 1.04 | 124.58 | 1.04 | .09 | 157.47 | 1.05 | 166.11 | 1.05 | .43 |
| 2‐h insulin (μU/mL) | 60.87 | 1.05 | 53.62 | 1.06 | .43 | 72.44 | 1.05 | 76.22 | 1.05 | .47 | 107.99 | 1.06 | 110.62 | 1.06 | .75 |
| HOMA‐IR ([μU/mL]*[mmol/L]) | 2.57 | 1.03 | 2.68 | 1.03 | .40 | 2.84 | 1.03 | 3.15 | 1.03 | .03 | 3.31 | 1.04 | 3.62 | 1.05 | .16 |
| Stumvoll first phase (pmol/L) | 1639.26 | 1.02 | 1616.57 | 1.02 | .68 | 1740.14 | 1.02 | 1872.26 | 1.03 | .04 | 2556.15 | 1.03 | 2676.00 | 1.03 | .31 |
| Stumvoll second phase (pmol/L) | 421.81 | 1.02 | 416.57 | 1.02 | .70 | 449.52 | 1.02 | 482.72 | 1.02 | .03 | 650.25 | 1.03 | 680.23 | 1.03 | .30 |
| Triglycerides (mmol/L) | 1.23 | 1.02 | 1.26 | 1.02 | .48 | 1.76 | 1.02 | 1.70 | 1.02 | .25 | 2.21 | 1.03 | 2.13 | 1.03 | .33 |
| Fatty acids (mmol/L) | 0.63 | 0.23 | 0.64 | 0.21 | .48 | 0.59 | 0.23 | 0.58 | 0.19 | .37 | 0.59 | 0.23 | 0.61 | 0.21 | .40 |
| Leptin (pg/mL) | 33.63 | 1.03 | 34.16 | 1.03 | .74 | 32.59 | 1.03 | 34.29 | 1.04 | .30 | 32.07 | 1.04 | 33.02 | 1.04 | .59 |
| Neck circumference (cm) | 36.18 | 2.06 | 36.21 | 2.17 | .89 | 36.29 | 2.12 | 36.28 | 2.23 | .98 | 36.53 | 2.17 | 36.60 | 2.22 | .74 |
| BMI (kg/m2) | 34.13 | 1.01 | 34.17 | 1.01 | .91 | 35.63 | 1.01 | 35.64 | 1.01 | .97 | 36.88 | 1.01 | 36.96 | 1.01 | .84 |
| Gestational weight gain (kg) | 2.02 | 3.92 | 1.84 | 4.48 | .64 | 4.06 | 3.28 | 3.92 | 2.74 | .63 | 3.82 | 2.78 | 3.76 | 2.82 | .82 |
Note: P value from independent t test; Fasting insulin, 1‐hour insulin, 2‐hour insulin, HOMA‐IR, Stumvoll first phase, Stumvoll second phase, triglycerides, leptin and BMI were log‐transformed for the analyses, but the values in the table were exponentiated for clarity.
Abbreviation: BMI, body mass index.
We conducted principal component factor analyses for each measurement time during pregnancy to group the exposures with shared variances in the same factor. All components from the mother's health profile were included in the factor analyses: fasting glucose, 1‐hour glucose, 2‐hour glucose, fasting insulin, 1‐hour insulin, 2‐hour insulin, HOMA‐IR, Stumvoll first phase, Stumvoll second phase, triglycerides, free fatty acids, leptin, neck circumference, BMI and gestational weight gain. Independent of the pregnancy period, the scree plot determined that four factors were relevant to be estimated. Thus, four factors were estimated for each time during pregnancy by the following criteria:
Independent of the time during pregnancy, each factor should be composed of the same variables;
Each factor was composed of variables with high loading factors (≥0.4) in at least two measuring points after varimax rotation of the correlation matrix;
Components with high loading factors (≥0.4) in more than one factor were only included in the factor with the highest loading factor;
Factors were estimated using the linear regression prediction score.
At all three time points during pregnancy, factor 1 was composed of fasting insulin and HOMA‐IR (representing insulin resistance); factor 2 was composed of 1‐hour insulin, 2‐hour insulin, Stumvoll first phase and Stumvoll second phase (representing insulin response); factor 3 was constituted of 1‐hour glucose and 2‐hour glucose (representing glucose tolerance), and, factor 4 was composed of leptin, BMI and neck circumference (representing maternal adiposity). Table S1 presents the cumulative variance of each factor in the three periods of gestation. Fasting glucose, triglycerides, free fatty acids and gestational weight gain did not have a high loading factor for any of the factors, nor did they built a separate factor, and were not included in any of the factors. Thus, they were analyzed as single exposures in relation to neonatal sum of skinfolds.
The association of each resulting factor from the mother's health profile with neonatal sum of skinfolds was assessed using linear multilevel analyses, adjusted for intervention group, site of recruitment, gestational age during pregnancy, gestational age at birth, neonatal age at measurement, maternal ethnicity, maternal education, BMI (except in factor 4 since BMI was part of this factor), maternal age, smoking status and the cluster structure. Sex‐effects were estimated by inclusion of an interaction term between the factor and sex. We used robust correction for estimating the standard errors and estimated P‐values using the Bonferroni adjustment for multiple testing. The association between the mother's health profile not included in any of the factors (fasting glucose, triglycerides, free fatty acids and gestational weight gain) and neonatal sum of skinfolds was evaluated following the same procedure abovementioned. Note that we tested the same analysis replacing BMI for gestational weight gain as a confounder. The conclusions were similar, so we only present the results with BMI adjustment.
2.5. Supplementary analysis
Multiple linear multilevel analyses tested the association between each of the maternal measurements during pregnancy and neonatal sum of skinfolds (see Table S2). Sex‐effects were estimated by inclusion of an interaction term between the exposures and sex. All analyses were adjusted for intervention group, site of recruitment, maternal ethnicity, education, BMI (except when BMI was the exposure), age, smoking status, gestational age during pregnancy (<20, 24 to 28 and 35 to 37 weeks), gestational age at birth (weeks) and neonatal age at measurement (hours post birth). The cluster structure of data was also taken into account with individuals nested into the site of recruitment. We used robust correction for estimating the standard errors and used the Bonferroni adjustment for multiple testing. Analyses with triglycerides and fatty acids as exposures were further adjusted for HOMA‐IR.
3. RESULTS
In total, 458 mother‐infant pairs (50.2% female infants) were included in the study. On average, the mothers were 32.1 (±5.3) years of age and their infants were measured 19.9 (±30.5) hours after birth. Mothers gave birth after 39.7 (±1.4) weeks of gestation, and most mothers perceived themselves as of European descent (84.6%) and did not smoke during pregnancy (85.6%). More than half of the mothers (57.6%) reported high educational level, whereas 32.0% reported medium educational level. Neonatal boys exhibited lower sum of skinfolds compared to girls (Mean sum of skinfoldsboys = 20.3 mm [±5.2]; Mean sum of skinfoldsgirls = 21.5 mm [±5.4]; P = .02).
3.1. Maternal metabolic parameters at three time points
Table 1 describes the unadjusted sex‐differences in the mothers' metabolic profile for each gestational time. In the first measurement prior to 20 weeks of gestation, no differences were observed in maternal metabolic measurements between mothers of boys and girls. At 24 to 28 weeks of gestation, mothers of girls had higher fasting insulin, HOMA‐IR and Stumvoll first and second phases compared to mothers of boys. At 35 to 37 weeks of gestation, mothers of girls had higher fasting glucose compared to those of boys (Table 1).
3.2. Associations of maternal metabolic parameters with neonatal adiposity
Associations of the four factors, and fasting glucose, triglycerides, fatty acids and gestational weight gain at the three time points with neonatal adiposity are described in Table 2. Factor 1 (fasting insulin and HOMA‐IR) at <20 weeks of gestation was associated with boys' sum of skinfolds, and with girls' sum of skinfolds at 35 to 37 weeks of gestation. Factor 2 (1‐hour insulin, 2‐hour insulin, Stumvoll first phase, Stumvoll second phase) was associated with boys' sum of skinfolds at 35 to 37 weeks of gestation, and no association was found of this factor with girls' sum of skinfolds. Factor 3 (1‐hour glucose and 2‐hour glucose) at 35 to 37 weeks of gestation was associated with boys' sum of skinfolds and at 24 to 28 and 35 to 37 weeks of gestation with girls' sum of skinfolds. Finally, factor 4 (leptin, BMI and neck circumference) was associated with boys' sum of skinfolds in all pregnancy periods, but not with girls' sum of skinfolds (Figure 1).
Table 2.
Multilevel regression coefficients of the association between maternal health profile components and child's sum of skinfolds (mm) by sex in three periods of gestation
| Maternal parameters | Boys | Girls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <20 weeks | 24‐28 weeks | 35‐37 weeks | <20 weeks | 24‐28 weeks | 35‐37 weeks | |||||||
| β | P | β | P | β | P | β | P | β | P | β | P | |
|
Factor 1 Fasting insulin and HOMA‐IR |
1.091 | .006 | .130 | .713 | .445 | .103 | .536 | .094 | .339 | .372 | .347 | .044 |
|
Factor 2 1‐h and 2‐h insulin, and Stumvoll first and second phases |
.458 | .163 | .305 | .256 | .673 | .009 | .389 | .228 | .449 | .215 | .208 | .521 |
|
Factor 3 1‐h and 2‐h glucose |
.384 | .362 | −.241 | .617 | 1.083 | .022 | .529 | .280 | 1.387 | .012 | 1.520 | .020 |
|
Factor 4 Leptin, BMI and neck circumference |
1.136 | .004 | 1.114 | .008 | 1.342 | .008 | .349 | .196 | .505 | .100 | .596 | .152 |
| Fasting glucose (mmol/L) | 1.792 | <.001 | 1.406 | .011 | 2.085 | .019 | −.045 | .968 | 2.475 | .008 | 1.361 | .035 |
| Triglycerides (log) | 1.428 | .423 | .235 | .854 | 2.740 | .005 | 2.005 | .031 | 1.907 | .119 | 1.395 | .235 |
| Fatty acids (mmol/L) | 1.555 | .152 | .462 | .787 | 1.179 | .544 | 3.886 | <.001 | 4.942 | .011 | −1.678 | .203 |
| Gestational weight gain (kg) | .197 | .033 | −.003 | .975 | .059 | .645 | .021 | .646 | .125 | .345 | .010 | .526 |
Note: Adjusted for intervention group, site of recruitment, maternal ethnicity, education, BMI (except when BMI was the exposure), age and smoking status, gestational age during pregnancy (weeks), gestational age at birth (weeks) and neonatal age at measurement (hours), and cluster structure (individuals nested in site of measurement). Besides the aforementioned adjustments, HOMA‐index was also an adjustment when triglycerides and fatty acids were the exposures. P values are Bonferroni adjusted.
Abbreviation: BMI, body mass index.
Figure 1.
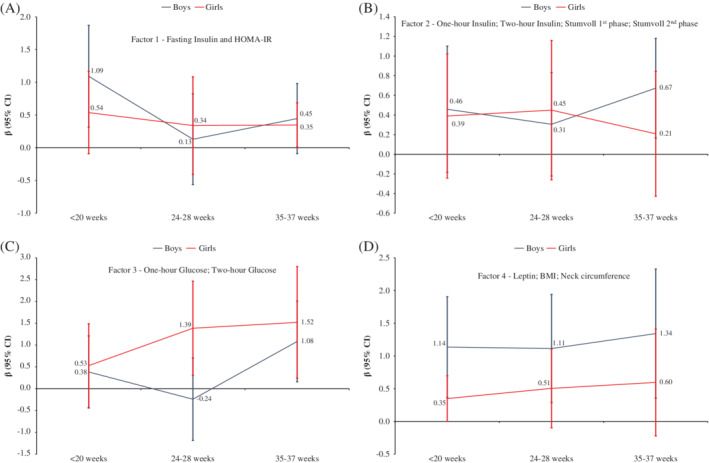
Multilevel regression coefficients of the association between (A) factor 1 (fasting insulin and HOMA‐index); (B) factor 2 (1‐hour insulin, 2‐hour insulin, Stumvoll phase 1 and Stumvoll phase 2); (C) factor 3 (1‐hour glucose and 2‐hour glucose) and (D) factor 4 (leptin, BMI and neck circumference) and neonatal sum of skinfolds (mm) by sex in three periods of gestation. Adjusted intervention group, site of recruitment, gestational age during pregnancy, gestational age at birth, neonatal age at measurement, maternal ethnicity, maternal education, body mass index (BMI), except in the factor 4 since BMI was part of this factor, maternal age, smoking status and cluster structure (individuals nested in site of measurement)
Fasting glucose at <20, 24 to 28 and 35 to 37 weeks of gestation was associated with boys' sum of skinfolds, and at 24 to 28 and 35 to 37 weeks of gestation with girls' sum of skinfolds. Triglycerides at <20 weeks were positively associated with the sum of skinfolds in girls, and 35 to 37 weeks with sum of skinfolds of boys. Fatty acids were associated with sum of skinfolds in girls at <20 and 24 to 28 weeks. Gestational weight gain at <20 weeks was positively associated with boys' sum of skinfolds (Table 2). Table S2 describes the associations of maternal parameters that were included in the factors with neonatal adiposity.
4. DISCUSSION
The main aim of the present study was to identify the effect of some maternal metabolism on neonatal skinfolds at different times in pregnancy in a sex‐dependent manner. Results confirmed our hypotheses that associations of metabolic parameters with neonatal adiposity change between different time‐periods and differ between sexes. We demonstrate a complex pattern of metabolic metabolism and its association with neonatal fat measured as sum of skinfolds, as illustrated in Figure 2. In summary, insulin resistance in early pregnancy is an important predictor for boys' sum of skinfolds, in addition to fasting glucose throughout pregnancy. In girls, maternal lipids in the first half of pregnancy period play a role for sum of skinfolds, as well as fasting glucose in the second half of pregnancy.
Figure 2.
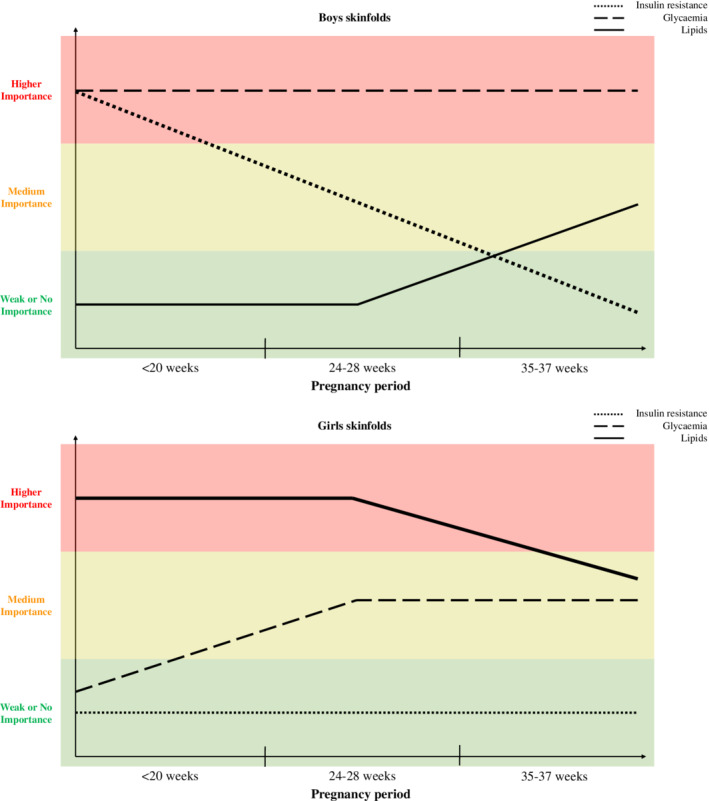
Summarized visualization of the degree of importance of some maternal parameters in relation to neonatal sum of skinfolds by sex
In both sexes, the early pregnancy period is relevant for neonatal adiposity, although for different metabolic parameters (ie, insulin resistance in boys and lipid levels in girls). Although a later intervention was effective in reducing neonatal adiposity,22 our data suggest that ideally maternal metabolism shall be normalized already early in pregnancy to reduce the risk of neonatal adiposity. Hence, interventions beginning prior to or in very early pregnancy might be even more effective than those initiated later, and achieved a 9% reduction in neonatal fat.22 Our results also indicate that both types of nutrients, glucose and lipids, are related to neonatal adiposity, although associations are time‐ and sex‐dependent. This argues for future time‐ and sex‐specific studies to delineate metabolic pathways between maternal adiposity, insulin resistance, the different nutrients and neonatal adiposity.
The physiological pathways influencing neonatal adiposity originate from maternal insulin resistance, which is higher with increasing maternal BMI. Indeed, in the Healthy Start Study7 maternal insulin resistance in the first half of pregnancy was an independent predictor of neonatal adiposity. This is in principle in line with our findings of insulin resistance (represented by factor 1) in early pregnancy playing a role, but in our study, this was limited to boys only. Later in pregnancy, the positive association of insulin resistance with neonatal adiposity was found in both boys and girls. The strong determining role of early insulin resistance for neonatal adiposity is not without precedent and extends to the pre‐pregnancy period, in which insulin resistance had the strongest association with neonatal fat mass, more so than insulin resistance in late pregnancy.23 However, neither of those previous studies tested for sex differences in this association or, specifically, in pregnant women with obesity. Two studies that analyzed boys and girls separately24, 25 found associations of insulin resistance late in pregnancy with measures of adiposity in girls, but not in boys, which is not fully in line with our findings. The different finding might be explained by differences in maternal BMI or the participants' level of glucose tolerance, since those other studies were not limited to women with obesity without GDM at baseline, as in our study sample.24, 25
The pathway from insulin resistance to neonatal adiposity might be through one of the nutrients glucose or lipids.8 Although found in various populations,7, 24, 25, 26 the role of fasting glucose for neonatal adiposity in women with obesity is a novel finding to the best of our knowledge. Maternal fasting glucose was a driver of fat accretion throughout pregnancy in boys and mainly in later pregnancy in girls. The sex difference in early pregnancy might explain why the Healthy Start Study7 only found an association of fasting glucose after 20 weeks of gestation, but not before, with neonatal adiposity. The reasons for the sex‐difference in the timing of the association of fasting glucose with neonatal fat remain speculative. The glucose steal phenomenon posits foetal hyperinsulinemia, the main driver of fat accretion, as the result of maternal hyperglycemia from early on.27 A sex‐difference in the timing of the glucose steal may be one explanation for our findings, but this remains to be studied.
Interestingly, independent of maternal insulin sensitivity, lipids in the first half of the pregnancy period were associated with sum of skinfolds in girls. In boys, only triglycerides at 35 to 37 weeks were related with neonatal skinfolds. This sex interaction in the association of lipids with neonatal skinfolds might explain the lack of association in previous studies not distinguishing between sexes.7, 19, 20 To complicate matters further, the association of maternal lipids with neonatal adiposity might also depend on maternal metabolic status and/or weight. Crume et al7 observed that maternal triglycerides, in middle to late pregnancy, were associated with neonatal fat mass only in women with obesity before pregnancy. In a study including women with a normal glucose metabolism and those with gestational diabetes, maternal lipids, triglycerides and free fatty acids in late pregnancy were positively associated with neonatal fat mass only in women with gestational diabetes, but not in women with normal glycaemia.28 This complex interaction between maternal phenotype, neonatal sex and maternal lipid profile could be a reason why in a Mendelian randomization approach no causal effect of maternal triglyceride concentrations on offspring birth weight was found.29 Mendelian randomization is a powerful method for establishing causal exposure‐outcome relations. The present results call for using this method in a sex‐specific manner, and at the same time distinguishing between women with different weight status. Moreover, neonatal fat percentage is the relevant offspring outcome more sensitive to in utero influences, and hence, more variable than birth weight.
While analysis of the insulin secretory response (factor 2) did not result in a clear picture, glucose tolerance (factor 3) at the end of pregnancy was related to neonatal adiposity for boys and girls. Conflicting results have been found on the association of glucose tolerance with neonatal adiposity. Most,7, 25, 30 but not all,23 studies reported positive associations. None of those assessed sex differences, and importantly, only one study evaluated exclusively pregnant women with obesity. Thus, more information is needed to draw any conclusions on the role of insulin secretory response or glucose tolerance for neonatal adiposity.
Maternal adiposity, mostly assessed by the pre‐pregnancy BMI, has been related with neonatal adiposity in numerous studies,4, 5, 25, 31, 32, 33 although one reported no association.34 We found maternal adiposity to be associated with neonatal adiposity in boys throughout pregnancy, but no association was found in girls. Although, when analyzed individually, maternal BMI in the second half of pregnancy period was associated with the girls' adiposity. Different from many of the previously mentioned studies, we only had women with obesity in our sample, which might have precluded finding strong associations with maternal adiposity. Reports on sex differences in the association of maternal adiposity and neonatal adiposity are conflicting: Some find associations only in boys,35 but other studies only in girls.36, 37 Prior studies in pregnant women with diabetes have reported an interaction of foetal sex with a number of maternal variables in the prediction of large or small‐for‐gestational age new‐borns but results are not comparable since the outcome variable was not new‐born adiposity and maternal variables did not include lipids or insulin sensitivity.38
4.1. Sex and time differences
Conceptually, male and female foetuses follow different growth strategies in utero.39, 40 Mechanisms are not well understood, but male embryos have more rapid cell divisions compared to females,41 which could lead to a more rapid growth.39, 40 Their higher growth rate might explain why male foetuses are also more responsive to changes in nutrient supply.39, 40 In addition, the peak growth velocity of males seems to be later in pregnancy compared to female foetuses.39 These different growth strategies might result in differences in nutritional needs, at different times in pregnancy. The results of our study provide strong evidence to support this concept. However, more systematic assessments of time‐ and sex‐dependent associations of maternal metabolism with neonatal adiposity are needed in future studies.
Although foetal fat is mostly accumulated late in pregnancy,42, 43 our data highlight that also the early pregnancy period is important for neonatal adiposity. This finding is in line with other studies, showing that maternal metabolism that led to gestational diabetes diagnosis later in pregnancy, had an influence on foetal abdominal circumference already at 17 weeks of pregnancy, with a more pronounced effect in males.9 Also maternal obesity was related to accelerated growth at 20 weeks of gestation,10 without sex differences studied.
4.2. Strengths and limitations
The European representativeness of our study sample with trials sites spread over Europe is a strength, but the restriction to women with obesity can be seen as a limitation of our study. However, since the prevalence of pregnant women with obesity is alarming,44 studying the consequences of maternal obesity is of public health relevance. A strength of our study is the measurements of maternal metabolic parameters at three time points in pregnancy. Without this elaborate approach, identification of time‐dependent effects had not been possible.
DALI study was designed to evaluate the effects of different interventions on preventing gestational diabetes in pregnant women with obesity. Although we adjusted the analyses for the various interventions, we cannot fully exclude a minor influence on the results. While more direct measures of neonatal adiposity by for example, air displacement plethysmography, would have been preferred, we recently reported an association of sum of skinfolds with cord blood leptin in this cohort,22 supporting the validity of the skinfold measures as proxy for neonatal adiposity.
5. CONCLUSION
Our results show time‐dependent associations of maternal metabolic parameters with neonatal adiposity. Importantly, and a novel key result, we demonstrate that this time‐dependence varies between sexes. Thus, the study highlights the urgent need for inclusion of neonatal sex in all analyses, not as confounder, but as major determinant and modifying factor. This has been recommended previously.45, 46 The present study can inform future studies to include larger sample sizes, because of the sex differences, but also measurements of maternal metabolism at more than one time point during pregnancy.
CONFLICT OF INTEREST
No conflict of interest was declared.
AUTHOR CONTRIBUTIONS
R.L. designed this study, conducted the statistical analysis, contributed to the interpretation of the results and drafted the manuscript. G.D. and M.v.P. designed this study, contributed to the interpretation of the results and drafted the manuscript. All authors, except R.L., made substantial contribution to the conception of the DALI study and acquisition of data. All authors revised the manuscript and contributed to the content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Supporting information
Table S1 Cumulative explained variance of each factor in three periods of gestation.
Table S2 Multilevel regression coefficients of the association between maternal health profile components and child's sum of skinfolds (mm) by sex in three periods of gestation.
Legend: Adjusted for intervention group, site of recruitment, maternal ethnicity, education, BMI (except when BMI was the exposure), age and smoking status, gestational age during pregnancy (weeks), gestational age at birth (weeks) and neonatal age at measurement (hours), and cluster structure (individuals nested in site of measurement). Besides the aforementioned adjustments, HOMA‐index was also an adjustment when triglycerides and fatty acids were the exposures. p values are Bonferroni adjusted
ACKNOWLEDGEMENTS
The authors would like to thank all participants for their time on participating in this study. The project described received funding from the European Union seventh framework (FP7/2007‐2013) under Grant Agreement no. 242187. In the Netherlands, additional funding was provided by the Netherlands Organisation for Health Research and Development (ZonMw; grant 200310013). In Poland, additional funding was obtained from Polish Ministry of Science (grants 2203/7, PR/2011/2). In Denmark, additional funding was provided by Odense University Free Research Fund. In the United Kingdom, the DALI team acknowledges the support received from the NIHR Clinical Research Network: Eastern, especially the local diabetes clinical and research teams based in Cambridge. In Spain, additional funding was provided by CAIBER 1527‐B‐226. The funders had no role in any aspect of the study beyond funding.
Lima RA, Desoye G, Simmons D, et al. Temporal relationships between maternal metabolic parameters with neonatal adiposity in women with obesity differ by neonatal sex: Secondary analysis of the DALI study. Pediatric Obesity. 2020;15:e12628 10.1111/ijpo.12628
Funding information CAIBER, Grant/Award Number: 1527‐B‐226; Odense University Free Research Fund; Polish Ministry of Science, Grant/Award Numbers: 2203/7, PR/2011/2; Seventh Framework Programme, Grant/Award Number: 242187; ZonMw, Grant/Award Number: 200310013
REFERENCES
- 1. UNICEF, WHO, World Bank . Joint Child Malnutrition Estimates ‐ Levels and Trends. New York: UNICEF; 2018. [Google Scholar]
- 2. Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes. JAMA. 2017;317(21):2207 10.1001/jama.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gillman MW, Ludwig DS. How early should obesity prevention start? N Engl J Med. 2013;369(23):2173‐2175. 10.1056/NEJMp1310577. [DOI] [PubMed] [Google Scholar]
- 4. Sewell MF, Huston‐Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195(4):1100‐1103. 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 5. Starling AP, Brinton JT, Glueck DH, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the healthy start study. Am J Clin Nutr. 2015;101(2):302‐309. 10.3945/ajcn.114.094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcome (HAPO) study: associations with neonatal anthropometrics. Diabetes. 2009;58(2):453‐459. 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crume TL, Shapiro AL, Brinton JT, et al. Maternal fuels and metabolic measures during pregnancy and neonatal body composition: the healthy start study. J Clin Endocrinol Metab. 2015;100(4):1672‐1680. 10.1210/jc.2014-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shapiro ALB, Schmiege SJ, Brinton JT, et al. Testing the fuel‐mediated hypothesis: maternal insulin resistance and glucose mediate the association between maternal and neonatal adiposity, the healthy start study. Diabetologia. 2015;58(5):937‐941. 10.1007/s00125-015-3505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Macaulay S, Munthali RJ, Dunger DB, Norris SA. The effects of gestational diabetes mellitus on fetal growth and neonatal birth measures in an African cohort. Diabet Med. 2018;35(10):1425‐1433. 10.1111/dme.13668. [DOI] [PubMed] [Google Scholar]
- 10. Sovio U, Murphy HR, Smith GCS. Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women. Diabetes Care. 2016;39(6):982‐987. 10.2337/dc16-0160. [DOI] [PubMed] [Google Scholar]
- 11. Riskin‐Mashiah S, Younes G, Damti A, Auslender R. First‐trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care. 2009;32(9):1639‐1643. 10.2337/dc09-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liang N, Zhu H, Cai X, et al. The high maternal TG level at early trimester was associated with the increased risk of LGA newborn in non‐obesity pregnant women. Lipids Health Dis. 2018;17(1):294 10.1186/s12944-018-0936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilkin TJ, Murphy MJ. The gender insulin hypothesis: why girls are born lighter than boys and the implications for insulin resistance. Int J Obes (Lond). 2006;30(7):1056‐1061. 10.1038/sj.ijo.0803317. [DOI] [PubMed] [Google Scholar]
- 14. Walsh JM, Segurado R, Mahony RM, Foley ME, McAuliffe FM. The effects of fetal gender on maternal and fetal insulin resistance. PLoS One. 2015;10(9):e0137215 10.1371/journal.pone.0137215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simmons D, Jelsma JGM, Galjaard S, et al. Results from a European multicenter randomized trial of physical activity and/or healthy eating to reduce the risk of gestational diabetes mellitus: the DALI lifestyle pilot. Diabetes Care. 2015;38(9):1650‐1656. 10.2337/dc15-0360. [DOI] [PubMed] [Google Scholar]
- 16. Jelsma JGM, van Poppel MNM, Galjaard S, et al. DALI: vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention: an European multicentre, randomised trial—study protocol. BMC Pregnancy Childbirth. 2013;13(1):1 10.1186/1471-2393-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization . Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Geneva: WHO; 2013. [PubMed] [Google Scholar]
- 18. Egan AM, Vellinga A, Harreiter J, et al. Epidemiology of gestational diabetes mellitus according to IADPSG/WHO 2013 criteria among obese pregnant women in Europe. Diabetologia. 2017;60(10):1913‐1921. 10.1007/s00125-017-4353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ben‐Noun LL, Sohar E, Laor A. Neck circumference as a simple screening measure for identifying overweight and obese patients. Obes Res. 2001;9(8):470‐477. 10.1038/oby.2001.61. [DOI] [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412‐419. [DOI] [PubMed] [Google Scholar]
- 21. Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23(3):295‐301. [DOI] [PubMed] [Google Scholar]
- 22. van Poppel MNM, Simmons D, Devlieger R, et al. A reduction in sedentary behaviour in obese women during pregnancy reduces neonatal adiposity: the DALI randomised controlled trial. Diabetologia. 2019;62:915‐925. 10.1007/s00125-019-4842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Catalano PM, Drago NM, Amini SB. Maternal carbohydrate metabolism and its relationship to fetal growth and body composition. Am J Obstet Gynecol. 1995;172(5):1464‐1470. [DOI] [PubMed] [Google Scholar]
- 24. Sommer C, Sletner L, Mørkrid K, Jenum AK, Birkeland KI. Effects of early pregnancy BMI, mid‐gestational weight gain, glucose and lipid levels in pregnancy on offspring's birth weight and subcutaneous fat: a population‐based cohort study. BMC Pregnancy Childbirth. 2015;15(1):84 10.1186/s12884-015-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lowe LP, Metzger BE, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcome (HAPO) study: associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care. 2012;35(3):574‐580. 10.2337/dc11-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uvena‐Celebrezze J, Fung C, Thomas AJ, et al. Relationship of neonatal body composition to maternal glucose control in women with gestational diabetes mellitus. J Matern Neonatal Med. 2002;12(6):396‐401. 10.1080/jmf.12.6.396.401. [DOI] [PubMed] [Google Scholar]
- 27. Desoye G, Nolan CJ. The fetal glucose steal: an underappreciated phenomenon in diabetic pregnancy. Diabetologia. 2016;59(6):1089‐1094. 10.1007/s00125-016-3931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ortega‐Senovilla H, Schaefer‐Graf U, Meitzner K, Abou‐Dakn M, Herrera E. Decreased concentrations of the lipoprotein lipase inhibitor angiopoietin‐like protein 4 and increased serum triacylglycerol are associated with increased neonatal fat mass in pregnant women with gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98(8):3430‐3437. 10.1210/jc.2013-1614. [DOI] [PubMed] [Google Scholar]
- 29. Hwang L‐D, Lawlor DA, Freathy RM, Evans DM, Warrington NM. Using a two‐sample Mendelian randomization design to investigate a possible causal effect of maternal lipid concentrations on offspring birth weight. Int J Epidemiol. 2019;48:1457‐1467. 10.1093/ije/dyz160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carlsen EM, Renault KM, Nørgaard K, et al. Glucose tolerance in obese pregnant women determines newborn fat mass. Acta Obstet Gynecol Scand. 2016;95(4):429‐435. 10.1111/aogs.12839. [DOI] [PubMed] [Google Scholar]
- 31. Breij LM, Steegers‐Theunissen RPM, Briceno D, Hokken‐Koelega ACS. Maternal and fetal determinants of neonatal body composition. Horm Res Paediatr. 2015;84(6):388‐395. 10.1159/000441298. [DOI] [PubMed] [Google Scholar]
- 32. Pacce S, Saure C, Mazza CS, et al. Impact of maternal nutritional status before and during pregnancy on neonatal body composition: a cross‐sectional study. Diabetes Metab Syndr Clin Res Rev. 2016;10(1):S7‐S12. 10.1016/j.dsx.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 33. Carlsen E, Renault K, Nørgaard K, et al. Newborn regional body composition is influenced by maternal obesity, gestational weight gain and the birthweight standard score. Acta Paediatr. 2014;103(9):939‐945. 10.1111/apa.12713. [DOI] [PubMed] [Google Scholar]
- 34. Eriksson B, Löf M, Forsum E. Body composition in full‐term healthy infants measured with air displacement plethysmography at 1 and 12 weeks of age. Acta Paediatr. 2010;99(4):563‐568. 10.1111/j.1651-2227.2009.01665.x. [DOI] [PubMed] [Google Scholar]
- 35. Pereira‐da‐Silva L, Cabo C, Moreira A, et al. The adjusted effect of maternal body mass index, energy and macronutrient intakes during pregnancy, and gestational weight gain on body composition of full‐term neonates. Am J Perinatol. 2013;31(10):875‐882. 10.1055/s-0033-1363502. [DOI] [PubMed] [Google Scholar]
- 36. Henriksson P, Löf M, Forsum E. Parental fat‐free mass is related to the fat‐free mass of infants and maternal fat mass is related to the fat mass of infant girls. Acta Paediatr. 2015;104(5):491‐497. 10.1111/apa.12939. [DOI] [PubMed] [Google Scholar]
- 37. Lingwood BE, Henry AM, d'Emden MC, et al. Determinants of body fat in infants of women with gestational diabetes mellitus differ with fetal sex. Diabetes Care. 2011;34(12):2581‐2585. 10.2337/dc11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sojo L, Garcia‐Patterson A, María M‐A, et al. Are birth weight predictors in diabetic pregnancy the same in boys and girls? Eur J Obstet Gynecol Reprod Biol. 2010;153(1):32‐37. 10.1016/j.ejogrb.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 39. Lampl M, Jeanty P. Timing is everything: a reconsideration of fetal growth velocity patterns identifies the importance of individual and sex differences. Am J Hum Biol. 2003;15(5):667‐680. 10.1002/ajhb.10204. [DOI] [PubMed] [Google Scholar]
- 40. Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJP. Boys live dangerously in the womb. Am J Hum Biol. 2010;22(3):330‐335. 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mittwoch U. Blastocysts prepare for the race to be male. Hum Reprod. 1993;8:1550‐1555. 10.1093/oxfordjournals.humrep.a137889. [DOI] [PubMed] [Google Scholar]
- 42. Anblagan D, Deshpande R, Jones NW, et al. Measurement of fetal fat in utero in normal and diabetic pregnancies using magnetic resonance imaging. Ultrasound Obstet Gynecol. 2013;42(3):335‐340. 10.1002/uog.12382. [DOI] [PubMed] [Google Scholar]
- 43. Wells JCK, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66(3):423‐434. 10.1017/S0029665107005691. [DOI] [PubMed] [Google Scholar]
- 44. Chen C, Xu X, Yan Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS One. 2018;13(8):e0202183 10.1371/journal.pone.0202183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505(7485):612‐613. 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schaefer‐Graf U, Napoli A, Nolan CJ. Group the DPS. Diabetes in pregnancy: a new decade of challenges ahead. Diabetologia. 2018;61(5):1012‐1021. 10.1007/s00125-018-4545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Cumulative explained variance of each factor in three periods of gestation.
Table S2 Multilevel regression coefficients of the association between maternal health profile components and child's sum of skinfolds (mm) by sex in three periods of gestation.
Legend: Adjusted for intervention group, site of recruitment, maternal ethnicity, education, BMI (except when BMI was the exposure), age and smoking status, gestational age during pregnancy (weeks), gestational age at birth (weeks) and neonatal age at measurement (hours), and cluster structure (individuals nested in site of measurement). Besides the aforementioned adjustments, HOMA‐index was also an adjustment when triglycerides and fatty acids were the exposures. p values are Bonferroni adjusted


