Abstract
Litomosoides sigmodontis is the only filarial nematode where the full life cycle, from larval delivery to the skin through to circulating microfilaria, can be completed in immunocompetent laboratory mice. It is thus an invaluable tool for the study of filariasis. It has been used for the study of novel anti‐helminthic therapeutics, the development of vaccines against filariasis, the development of immunomodulatory drugs for the treatment of inflammatory disease and the study of basic immune responses to filarial nematodes. This review will focus on the latter and aims to summarize how the L sigmodontis model has advanced our basic understanding of immune responses to helminths, led to major discoveries in macrophage biology and provided new insights into the immunological functions of the pleural cavity. Finally, and most importantly L sigmodontis represents a suitable platform to study how host genotype affects immune responses, with the potential for further discovery in myeloid cell biology and beyond.
Keywords: helminths, Litomosoides sigmodontis, macrophages, Th2 cells
1. INTRODUCTION
Filarial nematodes are thread‐like tissue‐dwelling roundworms that are transmitted by arthropod vectors and infect over 120 million people worldwide. 1 , 2 , 3 The most well‐known of human filarial nematodes, Wuchereria bancrofti and Brugia malayi, reside in the lymphatics and are thus referred to as lymphatic filaria. The other major filarial nematode of human consequence, Onchocerca volvulus, forms nodules in subcutaneous tissue. However, all filaria including the focus of this review, Litomosoides sigmodontis, migrate through the lymphatics as part of their life cycle. 4 A clinically important feature of filarial life cycles is that sexually mature female worms produce microfilaria (mF) a larval form that are acquired by the arthropod vector via the blood or skin. 5 Infections which lead to detectable circulating microfilaraemia in the mammalian host are said to be patent.
Filarial nematodes are best known as the causative agents of the disfiguring and disabling lymphatic filariasis (lymphoedema, hydrocele and elephantiasis) and onchocerciasis (river blindness/sowda), as well as the less severe loasis. 1 , 6 , 7 Clinical symptoms are generally a consequence of damage to lymphatic vessels or chronic immunopathology in infected tissue. 6 Infected individuals also suffer from periodic attacks of fever. 6 However, the simplistic causal relationship between filarial nematodes and immunopathology fails to capture the reality of infection on a population level. 8 In areas endemic for filarial diseases, there are a variety of outcomes to infection. Some ‘resistant’ individuals clear worms prior to the onset of patency, while others develop long‐lasting infections with or without microfilaraemia. Asymptomatic (or subclinical) infection is typically associated with high levels of circulating microfilariae. Progression to the characteristic clinical pathologies is also highly variable. In lymphatic filariasis, pathology can occur in the presence or absence of detectable parasitaemia. In onchocerciasis, pathology is more common in infected individuals which do not have mF. 1 , 6 , 8 , 9 , 10 These divergent clinical outcomes highlight the importance of understanding how host genotype impacts upon the immune response to filarial nematodes.
While L sigmodontis infection of mice does not result in the characteristic pathology seen in humans, it does model some of the diversity of immune responses. Different inbred strains of mice exhibit distinct susceptibility/resistance to infection, particularly in relation to mF, along with remarkably different immune responses to the parasite. Here, we wish to provide a cell‐by‐cell resource that summarizes what we know about the immune response to L sigmodontis infection with respect to the divergent nature of immune responses in susceptible and resistant hosts. We also highlight the power of this strain comparison for new discovery in filariasis, type 2 immune responses and myeloid biology.
2. L sigmodontis
L sigmodontis is a filarial nematode of the Onchocercidae family first isolated from the cotton rat Sigmodon hispidus. 11 The site of infection is the pleural cavity which resembles the relatively rare human diseases caused by Mansonella ozzardi and M perstans. 3 L sigmodontis is transmitted to the primary host via an arthropod vector, the tropical mite Ornithonyssus bacoti, which acts as the intermediate host and a reservoir for infective L sigmodontis L3 larvae. The life cycle and maintenance of L sigmodontis in a laboratory setting have been described in detail elsewhere. 12 For experiments with laboratory mice, infection can be achieved by allowing infected mites to feed naturally, with L3 larvae entering the skin when the mite performs a blood meal (natural infection). Alternatively, a known number of L3 larvae can be isolated from mites and injected directly into mice (subcutaneous infection). 13 Although natural infection includes the activation of innate immune responses in the skin that follow mite feeding, subcutaneous infection allows the infective dose to be known, and the immunological findings from laboratories that use the different routes have not fundamentally differed.
Many L3 larvae are destroyed in the skin. 14 , 15 Surviving larvae forcibly enter lymphatic vessels 15 , 16 and migrate to the pleural cavity by about day 4 post‐infection (p.i.). The exact route the worms take on their way to the pleural cavity is not well understood but may involve translocation through the lung. 17 At around day 8, the worm undergoes a moult to become an L4 larva and another final moult at circa day 28‐30 to become an adult. However, it takes another 30 days for the worms to become sexually mature and produce mF. It remains the only filarial nematode in which infection with larvae leads to circulating mF in the immunocompetent murine host.
3. THE SITE OF INFECTION: THE PLEURAL CAVITY
A major advantage of the L sigmodontis model is that the site of infection is simple in terms of tissue architecture. Isolation of worms and immune cells is performed by lavage of the pleural cavity, without the need for destructive tissue homogenization or digestion.
The pleura is a serous membrane with a two‐layer membrane structure folded back on itself made up of a layer of mesothelial cells (the mesothelium). 18 Lining the lung is the visceral membrane, and lining the chest wall and diaphragm is the parietal membrane. 19 The space between the two membranes is only in potential a cavity. In reality, it is a thin layer of fluid kept at negative pressure which allows the lungs to remain ‘attached’ to the parietal membrane when the chest expands, thus catering for lung inflation. 19 , 20 The pleural fluid contains lysozyme, antibody, complement and proteins such as surfactants, that reduce the friction of breathing. 19 , 20 Many of these factors, including clotting factors and complement, are locally produced rather than entering the cavity via the serum. Pleural fluid enters via capillaries lining the parietal membrane and is drained via lymphatics within the cavity (Figure 1). 20 The pleural fluid is highly cellular and due to breathing, is in constant motion. The cells of the pleural fluid are almost exclusively CD45+ immune cells. In naïve mice, these are comprised of, in order of abundance, B cells (B1 then B2 cells), macrophages (F4/80high resident macrophages then F4/80low monocyte‐derived macrophages), T cells, and small numbers of dendritic cells, NK cells and mast cells (Figure 1) (CM Finlay, personal observations).
FIGURE 1.
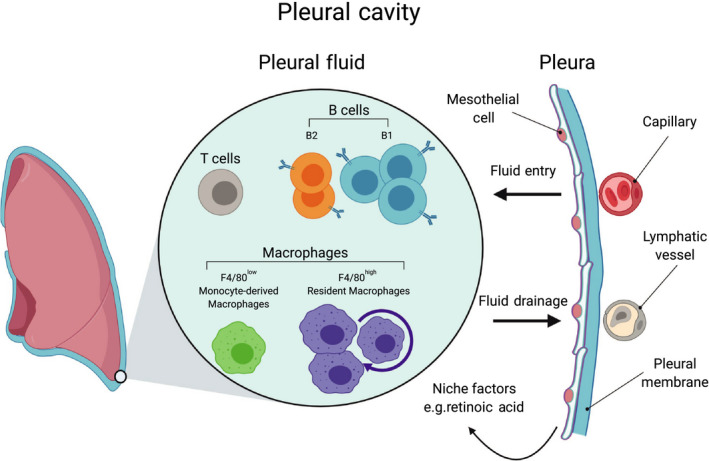
The Pleural Cavity. The pleural cavity is a fluid‐filled space created by a two‐membrane (pleura) structure that lines the lung and chest wall. The pleural fluid supports breathing by providing lubrication and by allowing close apposition of the inner pleura, covering the lung, with the outer pleura, covering the chest wall. Pleural fluid enters the cavity via the intercostal arteries and is drained by the lymphatics. The pleural fluid is cell dense with CD45+ immune cells. In mice, these include populations of B cells (B1 and B2 cells), macrophages (F480high and F4/80low) and T cells with smaller numbers of mast cells and dendritic cells
Infection with L sigmodontis results in oedema of the pleural cavity (pleural effusion) which is accompanied by the expansion of immune cells in the fluid through proliferation or recruitment. Eosinophils and neutrophils are absent from the pleural fluid of naïve mice but are recruited during infection. Hence, for the duration of infection, worms remain in immediate proximity to the immune cells of the pleural fluid. L sigmodontis, like other filaria, perform continuous whip like movements known as the ‘filarial dance’ that may act as a method to prevent the attachment of such immune cells. 21
4. STRAIN DEPENDENCY
L sigmodontis was first used to infect ‘albino’ mice in 1946, but it was not widely used as a murine model until the early 1990s. 22 The establishment of L sigmodontis as a mouse model to study filarial nematode infection and strain differences owes much to the pioneering work of Odile Bain's laboratory at Muséum National d'Histoire Naturelle, Paris. Most notably, their paper from 1992 which investigated the susceptibility of various strains of mice to L sigmodontis using a number of quantitative readouts of worm health and sexual maturity at a later stage of infection. 23 We have compiled and reanalysed the parasitology data from that study to provide an overview of relative susceptibility across host genotype (Figure 2. the method of analysis is described in the figure legend). Strains of mice can be subdivided into mice which are susceptible and microfilaraemic (BALB sublines), semi‐resistant (C3H, CBA and DBA), or resistant (C57BL/10 sublines) (Figure 2).
FIGURE 2.
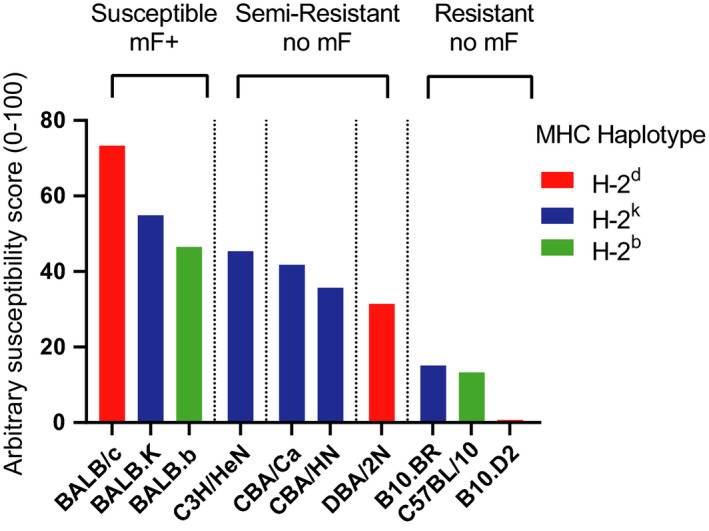
Resistance to L sigmodontis infection varies across host genotype. Data from Tables 2 (readouts of L sigmodontis infection in ten strains of mice) and III (readouts of morphology of L sigmodontis worms taken from ten strains of mice) from Petit et al 23 were reanalysed to create a relative susceptibility score. Only data for male mice were included. The following readouts were collated and weighted as follows: mF/10 μL of blood was scaled 0‐100 with 100 being given to the strain with the highest mF numbers (weighted 2), percentage mF+ (weighted 2), percentage worm+ (weighted 1), percentage localization of worms in the pleural cavity (weighted 0.5), number of worms recovered as a percentage of injected L3 larvae (weighted 1.5), length worms scaled to longest worm recovered from any strain (weighted 0.25 each for male and female worms), width of worms scaled to widest worm recovered (weighted 0.25 each for male and female worms), uterine egg score for recovered worms (weighted 0.25), divided egg score for recovered worms (weighted 0.25), percentage of worms with internal mF (weighted 1) and mF score for recovered worms (weighted 0.5). Weighted scores were added together to give a total possible score of 1000, and this number was divided by 100 to give scores shown in the graph above. Bar colour represents MHC haplotype for each strain
Notably, resistance does not segregate with MHC haplotype as all BALB mice despite MHC subtype (H‐2c, H‐2k or H‐2d) are poor controllers of infection while C57BL/10 (H‐2b) or B10.D2 (H‐2d) are highly resistant. 23 BALB/c v B10.D2 are the most divergent in terms of susceptibility/resistance yet share the same MHC haplotype (H‐2d) (Figure 2). 5 , 23 The more widely available C57BL/6 strain shares the resistance phenotype seen in C57BL/10 sublines. 24 , 25 , 26 The C57BL/6‐resistant vs BALB/c‐susceptible comparison is now the established model for strain comparison in L sigmodontis.
Only about one third or less L3 survive the 4 day journey from the skin to the pleural cavity. 24 , 26 Prior to day 20 p.i., L4 larvae are significantly larger in susceptible BALB/c mice than C57BL/6 mice 26 with a small difference in worm number (Figure 3). 24 Despite these differences, there is little observable worm killing in the pleural cavity in either strain before the adult moult around day 28‐30 p.i. (Figure 3). 24 , 26 Later in infection, obvious differences emerge. C57BL/6 mice begin to kill worms around day 35 p.i., and from day 40 p.i. onwards, dead and granulomatous worms are readily detectable in the pleural lavage. In contrast, worms in BALB/c mice continue to grow, undergo embryogenesis and by day 56 p.i. start to produce mF which are detectable in the bloodstream (Figure 3). 24 , 26
FIGURE 3.
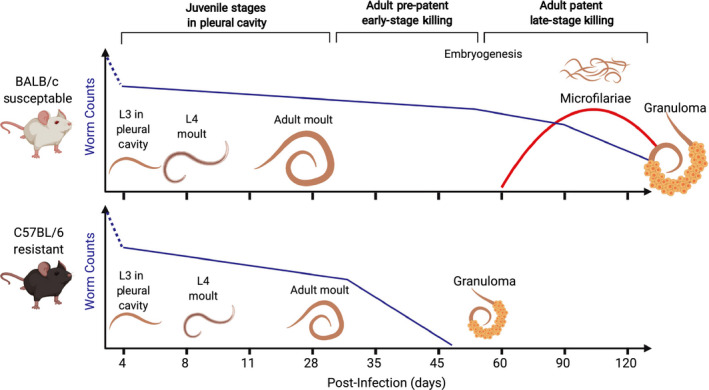
Timeline of experimental L sigmodontis infection. Mice are infected by allowing L sigmodontis‐infected O bacoti mites to blood feed on mice (natural infection) or by direct injection of a known number of L sigmodontis L3 larvae subcutaneously. Most L3 larvae are killed in the skin but those that do survive enter the lymphatics and typically appear in the pleural cavity by day 4 p.i. In the pleural cavity, L sigmodontis undergo a moult to an L4 larva around day 8 p.i. and another moult to adulthood around day 28‐30 p.i. Few worms are killed between arrival and adult moult in either strain. At day 35 p.i., worms are significantly larger in BALB/c‐susceptible mice than in resistant C57BL/6 mice. At this time point, worms begin to die in C57BL/6 mice whereas they continue to survive in BALB/c mice. Between day 35 and day 60 p.i. in BALB/c mice, worms become sexually mature, mate and undergo embryogenesis. Beginning around day 55 p.i., female worms in BALB/c mice produce mF which are detectable in the blood stream. Worms are gradually killed from day 80 onwards, and blood microfilaraemia is correspondingly reduced
Patency has a strong sexual dimorphism. 23 , 24 Depending on host sex, approximately 20% (male) to 70% (female) of BALB/c mice will develop patent infection. The presence of mF‐ individuals and the wide range in mF numbers is reflective of human filarial infections. Female mice harbour more adult worms than males and are over four times more prone to be mF+ than males, independent of the number of adult worms borne. 24 Nonetheless, in all BALB/c mice, worms are eventually killed between days 90 and 120 p.i. Thus, while they are represented as ‘susceptible’, BALB/c mice are more accurately semi‐susceptible in comparison with the cotton rat or the Mongolian jird (Meriones unguiculatus) which can harbour patent infections for years. 27 C57BL/6 mice are rightly considered resistant because they effectively kill worms prior to the emergence of patent infection. The same strain dependence for primary infection largely extends to survival of the mF in the bloodstream, demonstrated by injection of naïve mice with mF taken from patent hosts. C57BL/6 mice eliminate mF rapidly from the bloodstream whereas BALB/c take months to fully clear transplanted mF. 25 , 28 This indicates that C57BL/6 mice are more resistant to a number of life cycle stages of L sigmodontis, from L4 to adult worms to mF.
5. ROLE OF LYMPHOCYTES
It is clear that mice require an adaptive immune system to control L sigmodontis infection and mF. 29 Infected RAG2−/−IL2Rγ−/− mice which lack T, B and NK cells, harbour more worms than wild‐type (WT) C57BL/6 controls and have microfilaraemia in the blood at day 72 p.i. which is never seen in WT C57BL/6 mice. 29 T cells are required for worm killing in both resistant and susceptible mice. Depletion of CD4+ T cells from infected BALB/c mice increases worm burden and blood mF. 30 We have completed similar experiments in C57BL/6 mice and found that depletion of CD4+ T cells resulted in a significant delay in worm killing (unpublished observation) (Tables 1 and 2).
TABLE 1.
Outcome of reductionist studies in L sigmodontis infection using susceptible BALB/c mice and semi‐resistant strains (129/S and C3H)
| Deficiency | Outcome | References |
|---|---|---|
| Lymphocytes (RAG2−/−IL4−/−) | ↓ Worm killing, ↑ mF ↓ PC cells | [46] |
| B1 cells (‘Xid’ mice) | ↓ Worm killing, ↑ mF, ↓ Th2 cytokines ↓ IgM ↓ IgG | [40] |
| B cells (‘B‐less’ mice) | = Worm killing, ↑ mF | [36] |
| B cells (‘μMT’ mice) | = Worm killing, ↓ mF, ↓ Worm development, ↓ Th1, ↓ and Th2 | [31] |
| CD4+ T cells | ↓ Worm killing, ↑ mF, ↑ IFN‐γ, ↓ Th2 cytokines ↓ IgE, ↓ EΦ | [30] |
| IFN‐γ | ↓ Worm killing, ↓ Granulomas, ↓ NΦ, ↑ MΦ | [80] |
| IL4 or L‐4Rα | = Worm killing, ↑ mF, ↑ mF incidence, ↓ EΦ, ↑ NΦ, ↓ B‐cell proliferation, ↓ IgM, ↓ Lung inflammation | [36, 41, 44, 115] |
| IL‐5 | ↓ Worm killing, ↑ mF, ↓ EΦ, ↓ NΦ | [41, 44, 82] |
| IL‐4R and IL‐5 | ↓ Worm killing, ↑ mF, ↑ mF incidence ↑ Fibrosis, ↑ Th1 skewed | [43, 44] |
| IL‐5 + IFN‐γ | ↓ Worm killing, ↓ NΦ, ↓ EΦ, ↓ Granulomas | [50] |
| IL‐12 | = Worm killing | [80] |
| IL‐21 | ↓ mF, ↑ Th2 responses, ↓ Th1, ↑ IgM, ↑ IgG1. | [62] |
| IL‐10/IL‐10R | = Worm killing, ↑ BΦ IL‐4 production, ↑ IFN‐γ | [60, 75] |
| CD25/GITR/CTLA4 | ↑ Worm killing, ↓ Worm fitness | [60, 65] |
| PD1/PD‐L2 | = worm killing, ↓ mF, ↑ Th2 | [61] |
| NK cells | ↓ Worm killing, ↓ mF killing/clearance | [73] |
| NKT cells | = Worm killing | [73] |
| Eosinophils | ↓ Worm killing, ↑ mF, ↓ Pleural fibrosis, ↑ Worm development | [44, 46, 115] |
| EPO and MBP | ↓ Worm killing (129/SvJ mice) | [83] |
| ST2 | = Worm killing, ↑ mF (due to reduced splenic clearance), ↓ PC cells | [78] |
| IL‐33 | = MΦ proliferation, ↓ M(IL‐4), worm readouts unknown | [120] |
| Basophils | = Worm killing | [74, 76] |
| CXCL12 | = Worm killing | [117] |
| CCL17 | ↓ Worm killing | [79] |
| TLR4 | ↑ mF | [122] |
| TLR4 | = mF | [123] |
| IL‐6 | (Skin Phase) ↑ Early worm burden, ↑ EΦ, ↑ IgE, ↓ CCL17, ↓ NΦ, ↓ Mast cells | [128] |
| Eotaxin‐1 | (Skin phase) ↓ Worm killing (in PC in late stage), = mF, ↓ EΦ IL‐6 production | [84] |
| Histamine‐R‐1 |
(Skin phase) ↓ larval establishment ↑ Worm killing (EΦ‐dependent), ↑ EΦ, ↓ IgE, ↓ IL‐5, ↓ IFN‐γ |
[79, 85] |
| Mast cells | (Skin phase) ↑ larval establishment | [79] |
Abbreviations: =, no change; ↑, increased; ↓, decreased; BΦ, basophil; EΦ, eosinophil; M(IL‐4), alternatively activated macrophages; MΦ, macrophage; NΦ, neutrophil; PC, .
TABLE 2.
Outcome of reductionist studies in L sigmodontis infection using resistant C57BL/6 mice
| Deficiency | Outcome | References |
|---|---|---|
| Lymphocytes (RAG2−/− mice) | ↓ MΦ proliferation, ↓ M(IL4) activation, worm readouts unknown | [98] |
| Lymphocytes (RAG2−/−IL2rg−/−mice) | ↓ Worm killing, mice become mF+ | [29] |
| B cells (Total, μMT mice) | = Worm Killing | [31, 34] |
| CD4+ T cells | ↓ Worm killing ↓ MΦ proliferation ↓ M(IL‐4), ↓ EΦ | (UO) |
| IL‐4 | ↓ Worm killing, mice become mF+, ↑Th1 | [34] |
| IL‐5 | = Worm killing | [45, 46] |
| IL‐10/IL‐10R | = worm killing, ↑ T cell cytokines, ↑ Worm killing in IL‐4‐/‐ mice, ↑ clearance of mF challenge | [28, 67, 68, 69] |
| TGF‐β | ↑ antigen‐specific T cell responses (via IL‐10), worm readouts unknown | [69] |
| IL‐17A | ↓ Worms, ↑ Worm fitness, ↑ Th1‐skewed | [154] |
| TRIF | ↓ Worm killing | [124] |
| NOD2 | ↓ Worm killing | [125] |
| CXCL12 | ↓ Worm Killing, ↓ PC cells | [117] |
| Granzyme A/B | ↑ Worm killing, ↓ lymphocyte cell death, ↑ Th2 polarization, ↑ IgM | [71] |
| Neutrophils | = Worm killing, ↑ Worm killing in skin of mice with high basal skin NΦ (CXCR4+/ 13) | [129] |
Abbreviations: =, no change; ↑, increased; ↓, decreased; M(IL‐4), alternatively activated macrophages; MΦ, macrophage; PC, peritoneal cavity; UO, unpublished observations.
Although B cells have a clear protective role in mediating vaccine‐induced immunity, 31 the contribution of B cells to primary infection is less clear and may differ by strain. B cells are the most numerous cells of the pleural fluid and they expand greatly during L sigmodontis infection. 32 This includes antibody‐producing B2 cells and innate‐like B1 cells. 33 Parasite‐specific IgE and IgG1 are produced by both resistant and susceptible L sigmodontis‐infected mice. 34 Although differences in B‐cell numbers between strains are not apparent in the pleural lavage, C57BL/6 mice exhibit sustained increases in all B‐cell subsets within the fat‐associated lymphoid clusters (FALCs) of the cavity. 33 In contrast, BALB/c mice exhibit only a transient increase and fail to maintain B1b and B2 cells, which produce the majority of antigen‐specific IgM in the pleural cavity. The result is that BALB/c mice have far less L sigmodontis‐specific IgM than C57BL/6 mice by day 18 p.i. 33 Release of IgM in the serous cavity by FALC B cells may represent a crucial source of protective antibodies, as serum IgM cannot diffuse into the body cavities. The potential importance of IgM for elimination of filarial larvae has been demonstrated in a closely related model of filarial infection, using sIgM−/− mice. 35
In an apparent contradiction, C57BL/6 ‘μMT’ mice which lack B cells are still able to kill worms and control mF. 34 The same mutation backcrossed onto a BALB/c background showed no defect in late‐stage worm killing, but there were reduced mF counts and worm maturation, suggesting that B cells are somewhat protective. However, infected BALB/c μMT also exhibit reduced Th1 and Th2 response and macrophage accumulation. 31 In contrast, ‘B‐less’, another B cell–deficient mouse on the BALB/c background, actually has increased mF (but no change in worm numbers) suggesting B cells play a role in control of patent infections. 36 Total B‐cell deficiencies may paradoxically obscure the role of antibodies. Inconsistent results between different genetically altered B cell–deficient mouse strains maybe explained by the differential role of distinct B‐cell subsets. B cells, particularly B1 cells, have innate immune functions 37 which include immune regulation. 38 Thus, removing B cells from the C57BL/6 strain may alter worm killing even in the absence of host‐protective antibody. Supporting this hypothesis, BALB.Xid mice which have reduced B1 cells, particularly CD5+ B1a which are responsible for the production of antibodies to T‐independent antigens, harbour significantly more worms and higher blood mF than WT controls 39 , 40 (Tables 1 and 2).
5.1. T cells: key role for IL‐4
Immune responses to L sigmodontis are highly Th2 polarized in both resistant and susceptible strains but infected C57BL/6 mice exhibit dramatically higher local cytokine responses in the pleural cavity than BALB/c mice. 26 IL‐4 is critical for resistance to L sigmodontis as IL‐4–deficient C57BL/6 mice resemble susceptible BALB/c mice, with equivalent adult parasite survival and circulating mF 34 (Table 2).
The role of IL‐4 in susceptible BALB/c mice is not as straightforward. IL‐4–deficient or IL‐4Rα–deficient BALB/c mice, harbour similar numbers of worms as WT BALB/c mice late in infection indicating that IL‐4 is not responsible for killing of adult worms in this strain. 36 , 41 , 42 However, IL‐4 greatly limits the mF counts in BALB/c mice. 36 , 41 , 42 Infected IL‐4–deficient BALB/c mice also have reduced eosinophils but increased neutrophils. 36 In contrast to IL‐4 deficiency, IL‐5 deficiency on the BALB/c background does increase adult survival, 41 and a dramatic effect is seen in IL‐4Rα−/−/IL‐5 mice which harbour more worms and at least a hundred‐fold increase in blood mF. 43 , 44 In contrast, C57BL/6 IL‐5–deficient mice are equally resistant to WT controls, 45 , 46 highlighting another significant strain difference, as discussed below with regard to eosinophils. It is important to note that IL‐13 has not been specifically investigated in L sigmodontis infection and that IL‐4–deficient mice on the C57BL/6 background have a profound IL‐13 defect while IL‐4Rα–deficient mice do not distinguish the contribution of IL‐4 and IL‐13 (Table 1). Given that GWAS studies of onchocerciasis show association of IL‐13 polymorphisms with the development of pathology, 47 , 48 an investigation of the role of IL‐13 is warranted.
Patients with onchocerciasis and lymphatic filariasis typically present with a Th2‐polorized phenotype, but many patients have a more mixed Th1/Th2 T‐cell phenotype, especially those with pathology. 9 , 10 , 49 IFN‐γ and Th1 cells increase along with Th2 cytokines in both resistant and susceptible strains of mice infected with L sigmodontis. 26 , 50 Interestingly, transcripts for IFN‐γ peak when mF are first produced in BALB/c mice. 51 IFN‐γ and IL‐5 synergize to enhance worm killing in late‐stage infection of BALB/c mice, indicating a cooperation of Th1 and Th2 cells leading to worm killing. 50
5.2. T‐cell regulation and Hyporesponsiveness
Immune regulation and immune hyporesponsiveness are features of chronic helminth infection including filariasis. 52 , 53 , 54 , 55 The most common outcome in human filariasis is mild/asymptomatic disease despite the presence of adult worms and mF, reflecting a remarkable degree of tolerance by the host. 1 , 6 , 8 , 9 This can be explained by active immunoregulation on part of the parasite but also host regulatory networks, which act in concert to prevent immunopathology. 56 , 57 , 58 Pathological symptoms in onchocerciasis and lymphatic filariasis are often associated with immune responses to dead/dying worms which remain in the tissue, indicating that tolerance to established parasites can be more beneficial to the host than ‘protective’ worm killing. 1 , 6 , 8 , 9 , 10
Filarial nematode infection of humans is associated with regulatory and hyporesponsive Th1 and Th2 responses, especially in heavily infected individuals without pathology. 49 , 54 , 58 , 59 GWAS studies have linked polymorphisms in CTLA4 and TGFB1, genes linked with T‐cell regulation, to susceptibility to filariasis. 48 L sigmodontis has proved a powerful model system to study regulation of Th2 responses. Th2 cells in BALB/c mice infected with L sigmodontis acquire features of hyporesponsiveness that are consistent with human studies. 51 , 60 , 61 , 62 In BALB/c mice, Th2 cytokine production is relatively high in early‐stage infection (up to day 40). However, by the mid‐stage infection (days 40‐60), prior to the appearance of mF, Th2 cytokine production is notably reduced. 51 , 60 , 61 , 62 Following the production of mF however, there is a recovery in IFN‐γ and Th2 cytokines which coincide with worm killing in BALB/c mice (Figure 4). 51
FIGURE 4.
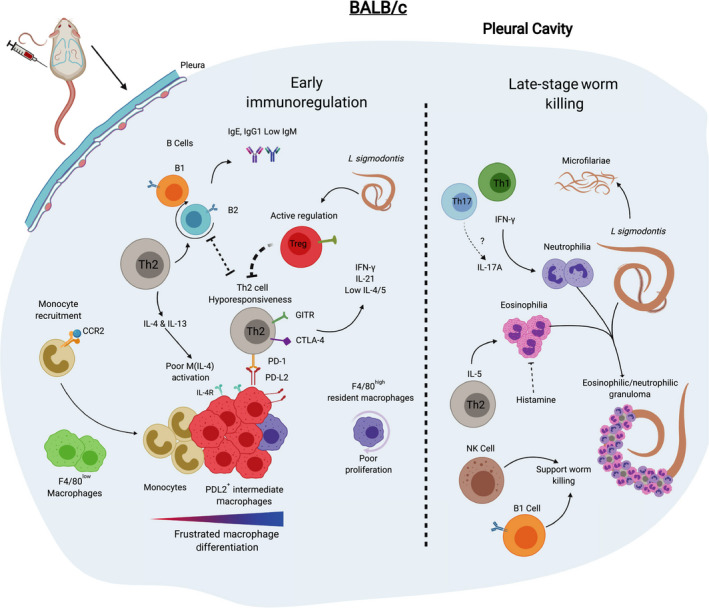
Immune response to L sigmodontis infection in BALB/c is characterized by immunoregulation and delayed worm killing. In infected BALB/c mice, there is a relatively weaker accumulation of immune cells in the pleural cavity in comparison with C57BL/6 mice. The immune response is initially Th2‐biased with associated eosinophilia and B‐cell production of IgG1 and IgE. The T‐cell response shifts towards a hyporesponsive state by day 40 p.i. Th2 cells express GITR, CTLA‐4 and PD‐1 which facilitate worm survival. Regulatory T cells also limit T‐cell responses and worm killing. In BALB/c mice, there is comparatively less F4/80high macrophage proliferation than in C57BL/6 mice and these are instead outnumbered by incoming CCR2‐dependent monocytes which develop into PD‐L2+ macrophages with a phenotype that is intermediate between F4/80low and F4/80high macrophages. Sexually mature L sigmodontis female worms produce mF beginning around day 55 p.i., and late‐stage (day 80 p.i. onwards) worm killing occurs via the gradual encasement of worms in granulomas. B1 and NK cells play a supporting role in worm killing. IFN‐γ supports neutrophilia and granuloma formation. IL‐5 and eosinophils are required for worm killing in BALB/c mice
Mechanistically, T‐cell hyporesponsiveness can be viewed as being cell‐intrinsic (hyporesponsiveness of Th2 cells themselves) or cell‐extrinsic (active regulation by other factors). A recent study has provided evidence that T‐cell hyporesponsiveness is intrinsic to the Th2 cell. 62 By day 60 p.i., Th2 cells in infected BALB/c mice cells acquire a dysfunctional phenotype that is IL‐21+Egr2+c‐Maf+Blimp‐1+IL‐4loIL‐5loT‐bet+IFN‐γ+. Critically, this phenotype is maintained upon transfer of these cells to naïve mice. 62 An intriguing feature of these cells is the production of IL‐21, which appears to contribute to mF survival while limiting germinal centre B cells and parasite‐specific IgM and IgG1. 62
There is also evidence of extrinsic regulation of T cells in L sigmodontis‐infected BALB/c mice. Th2 cells acquire PD1 expression, and signalling from PD‐L2 reduces Th2 cytokine production, and increases blood mF and worm fecundity. 61 Monocyte‐derived pleural cavity macrophages are the likely source of PD‐L2. 32 In addition, pleural cavity F4/80+ macrophages (but not DC or B cells) suppress T cells, at least partially, via TGF‐β. 63 Likewise, Treg cells play a key role in susceptibility of BALB/c mice to infection with L sigmodontis. CD25+ Treg cells expand in infected BALB/c mice. 60 , 64 , 65 Moreover, Foxp3+ CD4+ T cells enhance their expression of CD25, Foxp3, GITR, ICOS and CTLA‐4. 64 , 65 Anti‐CD25 and anti‐GITR treatment leads to an increase in Th2 cytokines and a significant increase in worm killing. 60 In vivo neutralization of CTLA‐4 and depletion of CD25+ cells also enhances worm killing. 64 Furthermore, a single depletion of CD25+ cells prior to infection increases worm killing, decreases worm fecundity and reduces blood mF in late‐stage infection (Figure 4, Table 1). 65 Collectively, these results reveal that T‐cell hyporesponsiveness and regulation plays a critical role in susceptibility to L sigmodontis infection in BALB/c mice (Figure 4).
C57BL/6 mice clear the infection before the onset of the hyporesponsive phenotype seen in BALB/c mice. Th2 cytokine concentrations are higher in the infected tissue of C57BL/6 than BALB/c mice indicating a more vigorous effector response in those animals. 26 Although less well studied than BALB/c mice in this context, C57BL/6 mice do acquire some immunoregulatory features. Treg cells expand in infected C57BL/6 mice, 66 although they proliferate less than Treg cells in BALB/c mice. 65 IL‐10–deficent C57BL/6 mice display exaggerated worm specific Th1 and Th2 responses. 67 While IL‐10–deficient animals do not harbour more worms, 67 the failure to kill worms in IL‐4–deficient C57BL/6 mice is reversed if those mice also lack IL‐10. 68 Moreover, TGF‐β–induced IL‐10 production by T cells in L sigmodontis‐infected C57BL/6 mice mediates bystander suppression of vaccine‐induced immune responses. 69 Granzyme A/B expression is a feature of activation‐induced autologous T cells associated with immunosuppressive responses to dying worms in onchocerciasis. 70 In C57BL/6 mice, granzymes A and B may promote early worm survival, suppressing type 2 immune responses and limiting parasite‐specific IgM. 71
Together, these data provide evidence that the susceptibility phenotype in BALB/c mice relies on a robust T regulatory and hyporesponsive Th2 response that is less evident in C57BL/6 mice. This divergence in effector Th2 vs regulated Th2 responses positions L sigmodontis as a model system to study the genetic basis of the control of Th2 cell fate.
5.3. ILC, NK cells, basophils and mast cells
The numbers of NK cells, basophils and type 2 innate lymphoid cells (ILC2s) increase over the course of infection in BALB/c mice, 72 , 73 , 74 , 75 , 76 but little is known about their function. These cells are present when CD4+ T cells are depleted, which enhances worm survival, and thus they are not sufficient to kill the parasite. However, they might play a supporting role in the establishment or maintenance of protective Th2 responses. Depletion of NK cells delays late‐stage worm killing and enhances mF survival, 73 but depletion of basophils has no effect on worm killing or mF counts. 74 , 76 Basophil depletion does, however, reduce parasite‐specific IgE, circulating eosinophils and IL‐4 production by T cells. 74 ILC2s and basophils are targets of IL‐33. 77 Loss of the IL‐33 receptor (ST2) in BALB/c mice does not affect worm killing although these mice do harbour more mF due to a defect in splenic clearance of blood mF. 78 L sigmodontis‐infected ST2‐deficient mice have normal Th2 responses but reduced cell recruitment to the site of infection. 78 Another target of IL‐33, mast cells, are increased following infection of semi‐susceptible C3H/HeN Mice. Moreover, mice deficient in CCL17, a chemotactic factor for mast cells had a fourfold increase in parasite burden. 79 That study suggests that mast cell degranulation, which enhances vascular permeability, may facilitate larval migration. 79 Mast cells may thus play a supporting role in establishment of early L sigmodontis infection, but we do not know whether they affect the outcome of infection in the pleural cavity (Table 1). The absence of studies for these innate cells, or the IL‐33‐ST2 axis, in C57BL/6 mice means we are currently ignorant of their contribution to innate resistance.
6. WORM KILLING, GRANULOMAS, EOSINOPHILS AND NEUTROPHILS
We do not yet have a detailed understanding of the mechanisms by which L sigmodontis parasites are killed but it does involve the gradual encasement of the worm within a granuloma. Cuticles that are shed during moulting are rapidly encased in granulomas formed of eosinophils. 27 However, the living worm represents a more difficult target due to its high motility and selective pressure to avoid exposing residues that enable immune cell attachment. 27
In the fully permissive Mongolian jird, granulomas contain neutrophils as well as eosinophils. 27 In BALB/c mice granuloma formation around young adult worms is rare but develops later in infection around larger adult worms and contains neutrophils which form the immediate layer of the granuloma exposed to the worm. 27 In BALB/c mice neutrophils are recruited relatively late in infection prior to the onset of worm killing. 50 , 80 Late‐stage granuloma formation and worm killing is attenuated in IFN‐γ–deficient animals which have a deficiency in neutrophil recruitment, but exhibit no difference in mF counts. 80 Neutrophils from infected IFN‐γ–deficient mice have reduced chemotactic and phagocytotic ability ex vivo and pleural cavity cells produce less TNF‐α and NO in response to M1 stimuli. 80 These data are strongly suggestive of a role for neutrophils in the eventual killing of the adult parasites in BALB/c mice. Notably, neutrophils are not a significant feature of L sigmodontis infection in resistant C57BL/6 mice (Table 1).
In both C57BL/6 and BALB/c mice, eosinophils increase in the pleural cavity as early as 11 days p.i., 32 and recruitment is much reduced in mice lacking CD4+ T cells. 29 , 30 Hyper‐eosinophilic IL‐5‐transgenic mice on the semi‐susceptible CBA background are more resistant to infection 81 supporting a role for eosinophils in filarial killing. Further evidence comes from primary infection of IL‐5–deficent BALB/c mice, which have much greater late‐stage worm numbers and higher blood mF. 41 In fact IL‐5 has a greater effect on worm/mF numbers than the absence of IL‐4. 41 Infected BALB/c mice also harbour more late‐stage worms and mF following administration of an anti‐IL‐5 antibody. 82 In addition to low eosinophil numbers, IL‐5–deficient mice have significantly less B‐cell accumulation and IgM production at the site of infection, 41 which might further support a role for IgM as an anti‐worm effector. A direct role for eosinophils is supported by infection of semi‐resistant 129/SvJ mice that lack key eosinophil granule proteins major basic protein (MBP) or eosinophil peroxidase (EPO). These mice harbour more worms than even susceptible BALB/c mice at day 28 p.i. implicating eosinophil degranulation as an important factor in worm killing in BALB/c mice. 83 Notably, BALB/c mice deficient in Eotaxin‐1 have increased late‐stage worm counts without any change of mF counts. 84 Further evidence comes from the finding that IL‐4Rα−/−, IL‐5−/−, dblGATA (eosinophil deficient) and IL‐4R−/−/IL‐5−/− mice on BALB/c background all display increased worm burdens and mF in the blood in late‐stage L sigmodontis infection. 44 Of the four strains assayed, IL‐4R−/−/IL‐5−/− display the greatest increase in susceptibility (Tables 1 and 2). 44 Finally, administration of anti‐histamine to infected BALB/c enhances clearance of adult worms in an eosinophil‐dependent manner, indicating that parasite‐induced histamine impairs anti‐parasite immune responses by limiting eosinophil activation. 85
Granuloma formation is less apparent in BALB/c mice lacking both IFN‐γ and IL‐5 50 and neutrophil recruitment and activity is markedly decreased in these mice, with greater worm burdens than mice deficient in either cytokine alone. 50 GM‐CSF a key survival and proliferative factor for both eosinophils and neutrophils has a greater inhibitory effect on worm killing than IL‐5. 82 Interestingly, neutralization of IL‐5 in infected BALB/c mice, which reduces worm killing, is associated with reduced neutrophil accumulation, 82 indicating an unexpected cooperation between neutrophils and eosinophils in late‐stage worm killing in BALB/c mice (Figure 4, Table 1).
Evidence for eosinophils mediating L sigmodontis worm killing in resistant C57BL/6 is less clear. In C57BL/6 mice, granulomas form along the lateral lines of small newly moulted adult worms. 27 Granulomas in these mice are predominantly eosinophilic (unpublished observation). However, there is no deficit in worm killing following primary infection of IL‐5–deficient C57BL/6 mice (Figure 5). 45 , 46 This finding, that infected IL‐5–deficent C57BL/6 mice, which cannot attract eosinophils have no defect in worm killing is hard to square with the idea that granulomas are required to kill worms (Table 2). 45 This raises the possibility that granulomas in C57BL/6 may form around already dead worms, like they do around shed cuticles rather than being a key component in worm killing itself.
FIGURE 5.
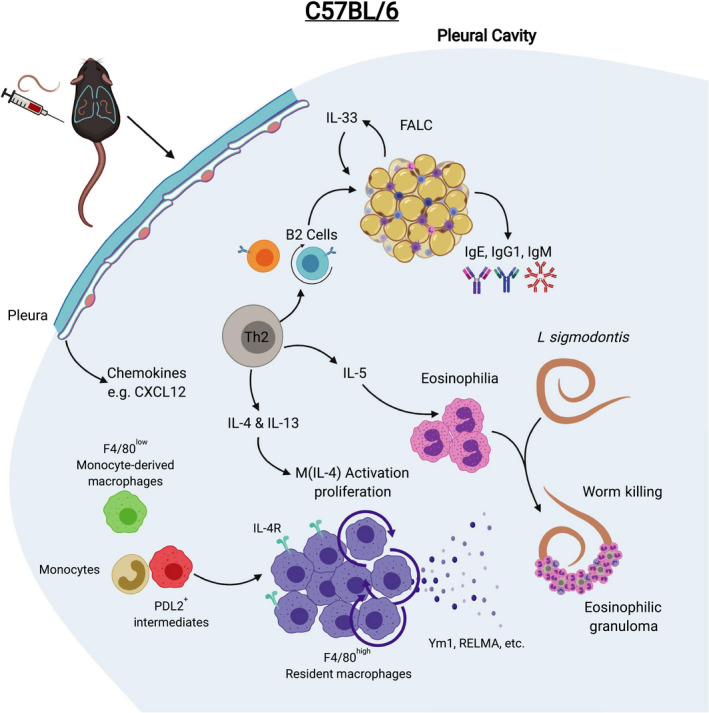
Immune response to L sigmodontis infection in C57BL/6 mice leads early to worm killing in the pleural cavity. C57BL/6 mice mount an early and sustained Th2‐biased immune response to L sigmodontis characterized by greater cell accumulation in the pleural cavity than in BALB/c mice. This is associated with eosinophilia, proliferation of B2 cells and the development of FALCs within the pleural and pericardial cavities and the production of parasite‐specific IgE, IgG1 and IgM. The pleura supports resistance by the production of chemokines including CXCL12. F4/80high macrophages proliferate and greatly expand in number and become M(IL‐4) activated producing Ym1 and RELM‐α. Monocytes, F480low macrophages and PD‐L2+ intermediate macrophages are present but outnumbered by F4/80high macrophages. Dead worms encased in eosinophilic granulomas are detectable by day 35 p.i., and infection is rapidly cleared thereafter
A far more straightforward role for eosinophils exists in killing of larval parasites during secondary infection. In both C57BL/6 and BALB/c mice, eosinophils are rapidly recruited to the skin and degranulate where they mediate larval killing; IL‐5–deficent mice of both strains lose protection following vaccination. 45 , 86 This is consistent with a greater role for eosinophils in larval killing over adult worm killing seen for other helminths. 87 The evolutionary importance of eosinophils in parasite control is perhaps best illustrated by the finding that, in both BALB/c and C57BL/6 mice, L sigmodontis L3 larvae respond to the presence of eosinophils by accelerating their development. 46 Thus, the parasite appears able to detect cues that a host is mounting an effective immune response.
Taken together, these results show that killing of adult L sigmodontis is highly strain dependent. Both eosinophils and neutrophils contribute to worm killing and mF control in late‐stage infection in BALB/c mice but killing of immature worms in resistant C57BL/6 is still poorly defined.
7. MACROPHAGES OF THE PLEURAL CAVITY
There are two distinct macrophage populations in the pleural cavity, F4/80high and F4/80low macrophages which are similar to macrophages of the other serous cavities, the pericardial and peritoneal cavities (Figure 1). The F4/80high and F4/80low macrophages correspond to the large peritoneal/pleural macrophages (LPM) and small peritoneal/pleural macrophages (SPM) respectively, well described for the peritoneal cavity 88 but more recently described in the pleural cavity. 32 , 89 , 90 , 91 However, peritoneal macrophages remain better studied and much of the basic biology of the pleural cavity cells must be extrapolated from peritoneal studies.
Serous cavity F4/80low macrophages are monocyte‐derived and develop only after birth. There is some heterogeneity within the population which might represent stages of development into a terminally differentiated CD11c+ CD226+ cell. 91 F4/80low macrophages have a half‐life of about 2 weeks in vivo being replaced by CCR2+ monocytes but there is a subset that are CD115−CD11c+ and not replaced via CCR2‐mediated recruitment, with overlapping features of DC. 92 The major phenotypic feature which discriminates F4/80low from F4/80high macrophages is their high expression of MHC‐II. F4/80low macrophages also express more genes involved in antigen presentation and are capable APCs, a feature lacking in F4/80high macrophages. 93 , 94 F4/80high resident macrophages are seeded to the serous cavities before birth from foetal hematopoietic stem cells. 95 , 96 , 97 F4/80high macrophages have the capacity to self‐renew by proliferation in the serous cavities, which relies on CSF1. 92 , 98 CSF1R–deficient mice have reduced basal macrophage proliferation and lower F4/80high macrophage numbers. 98 However, it is now clear that F4/80high macrophages are eventually replaced by cells of bone‐marrow origin. 92 , 96 , 99 Replacement is gradual and requires incoming monocytes. 100 In CCR2−/− mice, which lack monocyte‐derived cells, there is a loss in the number of F4/80high macrophages suggesting that in naïve mice basal proliferation of embryonically derived F4/80high macrophages is insufficient to maintain the population. 92 Monocytes that develop into F4/80high macrophages do so via a cell which resembles an F4/80low macrophage but with intermediate phenotypic features of an F4/80high macrophage. 92 , 101 Newly differentiated F4/80high macrophages largely phenocopy embryonically derived F4/80high macrophages save for a failure in some cells to express Tim4, a marker of residency in other tissues. 92 , 102
F4/80high macrophages survive in the tissue for much longer than F4/80low macrophages with a half‐life of 12 weeks for peritoneal macrophages. 89 , 92 GATA6+ is the key transcription factor for control of the serous cavity ‘residency’ transcriptional programme. 103 , 104 , 105 Loss of GATA6 from myeloid cells impairs the development and proliferative capacity of F4/80high macrophages. The macrophages that do develop in GATA6−/− mice have a partially differentiated phenotype, resembling macrophages that are differentiating into F4/80high macrophages from monocytes in naïve mice with a failure to express ‘residency’ genes. 103 , 104 F4/80high macrophage development also requires C/EBP‐β and a similar partially differentiated phenotype is observed in C/EBPβ‐deficient animals. 16
7.1. M(IL‐4) activation and proliferation in L sigmodontis infection
Leaving aside lymphocytes, the most abundant cells in the pleural cavity of infected C57BL/6 mice are macrophages and eosinophils. The fact that IL‐5 was found to be dispensable for worm killing in C57BL/6 mice while IL‐4 is required highlights the potential importance of macrophages in L sigmodontis infection due the capacity of IL‐4Rα signalling to potently activate macrophages to become M(IL‐4) cells. 17
L sigmodontis infection induces M(IL‐4) cells in both C57BL/6 and BALB/c mice. 29 , 98 , 108 , 109 In late‐stage BALB/c infection macrophages have a marked M(IL‐4) phenotype with high expression of arginase‐1, Ym1 and RELM‐α. 19 However, our unpublished data have revealed that F4/80high macrophages from BALB/c mice, on a cell‐to‐cell basis, are much less M(IL‐4)‐polarized than in C57BL/6 mice. They have similar expression of RELM‐α and arginase‐1 but lack many of the genes that are characteristic of M(IL‐4) activation in F4/80high macrophages. 110 The relative lack of M(IL‐4) polarization may be a factor in the susceptibility of BALB/c mice to L sigmodontis infection.
In the pleural cavity of L sigmodontis‐infected C57BL/6 mice, F4/80high macrophages greatly expand in number, beginning early in infection and continuing throughout infection, eventually becoming the most abundant cell type. 32 , 111 This is accompanied by only limited recruitment of monocytes and relative loss in the proportion of F4/80low macrophages. 111 The pleural cavity F4/80high macrophages undergo a burst of proliferation peaking at 10 days p.i. which is dependent on IL‐4 and occurs without the input of blood monocytes (Figure 5). 111 This finding was the first demonstration that macrophages could expand dramatically in number through local proliferation rather than blood cell recruitment during an inflammatory response. The paradigm shift in macrophage biology that resulted was a direct consequence of the L sigmodontis model. Macrophages that can respond to IL‐4 outcompete, through proliferation, macrophages which lack IL‐4Rα 98 and proliferation is independent of the CSF1R highlighting the direct role of IL‐4 in macrophage proliferation. 98 The cellular source of IL‐4 is not known but given that macrophages fail to proliferate in L sigmodontis‐infected RAG‐deficient mice, an adaptive immune cell is involved. 98 Our unpublished data indicate that the proliferation and expansion of F4/80high macrophages is dependent on the recruitment and activation of CD4+ T cells. This suggests that innate sources of IL‐4, such as basophils, eosinophils and ILC2 are unlikely to be capable of inducing the proliferation of macrophages during infection.
The situation in BALB/c mice is markedly different. F4/80high macrophage proliferation is relatively weaker in L sigmodontis‐infected BALB/c mice. There is an accumulation of monocytes and monocyte‐derived F4/80low macrophages These macrophages are highly heterogenous and appear to contain partially differentiated macrophages. This results in a much smaller accumulation of F4/80high macrophages at the site of infection in BALB/c mice when compared to C57BL/6 mice (Figure 4). 32
F4/80high and F4/80low serous cavity macrophages respond differently to IL‐4Rα signalling. 112 For example, F4/80high macrophages uniquely upregulate Ucp‐1 and cell cycle genes while F4/80low macrophages have greater PD‐L2 expression, retinoic acid production and the unique ability to induce the differentiation of Foxp3+ Treg cells. 112 These unique responses to IL‐4 suggest that F4/80high and F4/80low macrophages will play different roles in L sigmodontis infection. Indeed, macrophages from BALB/c mice are immunoregulatory and promote worm survival. Infection of BALB/c mice leads to the recruitment of monocyte‐derived macrophages which express PD‐L2 (Figure 4). 32 Blockade of monocyte recruitment in infected BALB/c mice increases T‐cell IL‐4 production with enhanced worm killing. 32 This is highly consistent with previous data showing that antagonism of the PD‐L2/PD1 pathway reverses Th2 cell exhaustion and decreases worm fitness and mF counts in late‐stage L sigmodontis infection of BALB/c mice. 61 Thus, it appears that monocyte‐derived macrophages in BALB/c mice promote worm survival by limiting Th2 cell activity. F4/80high resident macrophages from BALB/c mice, although far fewer in number, may also be immunoregulatory as arginase‐1+F4/80high resident macrophages taken from infected BALB/c mice potently inhibit the proliferation of T cells in vitro, partially via TGF‐β. 63
Efforts to address the contribution of F4/80high resident M(IL‐4) macrophages to worm killing have been hampered by difficulties in effectively deleting IL‐4Rα due to lack of penetrance using Cre recombinase technology. In the strong IL‐4 environment of L sigmodontis infection, the few cells that still express the receptor outcompete the IL‐4Rα–deficient cells by local proliferation. 113 However, in a study of BALB/c mice using LysMCre+ X IL‐4Rαfl/fl, mF numbers were significantly higher than controls, despite the fact that the macrophages had reverted to a WT phenotype by the time the infection became patent. 113 This suggests that early in infection M(IL‐4) macrophages contribute to a pathway leading to containment of mF later in infection.
8. THE PLEURAL NICHE
Given the presence of inflammatory infiltrate, oedema and motile worms, it is not surprising that the stroma of the tissue niche undergoes transformations during L sigmodontis infection. 17 , 43 , 114 , 115 Progressive fibrotic pathologies develop in the pleura of BALB/c mice infected with L sigmodontis 43 , 115 along with inflammatory foci in the lungs. 17 , 43 , 114
The effect of the pleura itself upon pleural immune cells and the outcome of infection is largely unknown. We have previously shown that tissue‐specific niche‐derived factors support IL‐4Rα–dependent proliferation of tissue‐resident macrophages. 116 C1q, a complement component, acts to enhance F4/80high peritoneal macrophage proliferation and activation in the peritoneal cavity in response to IL‐4, while surfactant protein A performs the same function in the lung. Both factors act through the atypical myosin, Myo18A However, pleural cavity F4/80high macrophages did not express Myo18A. 116 Nevertheless, the concept that the niche can provide factors which support macrophage proliferation and M(IL‐4) activation is relevant to L sigmodontis infection.
The pleura and in particular the mesothelium play an active role in the maintenance of macrophages in the serous cavities. For example, retinoic acid (RA) produced by WT1+ mesothelial cells maintains GATA6 expression in F4/80high macrophages, which is required for its ‘residency’ programme. 89 RA and IL‐4 mediate the conversion of thioglycolate‐elicited F4/80low macrophages into F4/80high macrophages. 11 In the absence of RA, macrophages do not fully convert into F4/80high macrophages remaining in an intermediate PD‐L2+ phenotype. 11 This phenotype resembles the phenotype of PD‐L2+ F4/80low macrophages that we find in BALB/c mice infected with L sigmodontis. 32 This suggests that RA signalling deficiencies may contribute to the accumulation of this immunoregulatory cell in infected BALB/c mice.
In evidence for the role of the tissue niche in L sigmodontis infection, mesothelium was shown to be critical for the recruitment of cells to the pleural cavity. CXCL12 is produced by the mesothelium in infected‐C57BL/6 but not BALB/c mice. 117 Chemical blockade of the CXCL12‐CXCR4 pathway significantly reduced immune cell recruitment and reduced worm killing in C57BL/6 mice but had no effect in BALB/c mice. 117 Another evident difference between strains is the expansion of the FALCs, which is significantly greater in infected C57BL/6 mice in comparison to BALB/c mice. 33 IL‐33 is produced by FALC stroma, 33 and thus, IL‐33 may be more abundant in C57BL/6 mice during L sigmodontis infection. IL‐33, which is also expressed in the lining of the lung and by mesothelial cells in other organs, 77 , 118 is a diagnostic marker of pleural effusion. 119 Our unpublished data indicate that macrophages from C57BL/6 but not BALB/c mice upregulate ST2 during infection. Consistent with this finding and the potentially lower availability of IL‐33 in BALB/c mice, 33 ST2 or IL‐33‐deficency in BALB/c mice does not affect worm killing in BALB/c mice, although ST2‐deficient mice have less recruitment of eosinophils, CD4+ T cells and macrophages to the site of infection. 78 , 120 In BALB/c mice IL‐33 is not required for macrophage proliferation with L sigmodontis infection. 120 However, BALB/c mice may not have been a relevant strain to investigate macrophage proliferation as they display reduced macrophage proliferation in comparison to C57BL/6. 32 Given its potential to produce cytokines, chemokines or other factors such as RA which support F4/80high macrophages, the pleura certainly has the potential to impact upon the outcome of infection. Fundamentally, we do yet not know whether resistance to infection seen in different strains of mice is mediated by immune cells or if the tissue niche dictates the outcome.
8.1. Beyond the pleural cavity
This review has focused on the local immune responses to L sigmodontis in the pleural cavity. Thus, we have neglected to mention responses which occur early in infection in the skin and evidence for L sigmodontis having effects on immune responses in distal sites.
Most L sigmodontis L3 larvae do not survive their journey from the skin and relatively little is known about the responses in the skin apart from the aforementioned contribution of mast cells in primary infection 79 and eosinophils in secondary infection. 9 , 45 , 86 L sigmodontis contain Wolbachia, bacterial endosymbionts which contain ligands that may activate innate sensing pathways in the skin, 29 and there is evidence that TLR2, 121 TLR4, 122 , 123 TRIF 124 and NOD2 125 all contribute to the immune response to L sigmodontis infection. Innate lymphoid cells and γδ T cells are major players in skin barrier function during type 2 immunity, 126 , 127 but have not been investigated in the context of infection by filarial larvae (Tables 1 and 2). Combined data from two papers suggest that mast cells via IL‐6 activation and CCL17 recruitment may contribute to killing of L sigmodontis larvae in the skin following primary infection. 79 , 128 In addition, neutrophils have the capacity to kill L3 larvae in the skin and are recruited to the skin following infection. 125 , 129 However, neutrophil depletion in WT C57BL/6 mice had no effect on adult worm numbers, 129 leaving open the contribution of neutrophils to early resistance. The contribution of the skin to innate resistance, and perhaps more importantly, the impact the immune response to the migrating larvae in the skin/lymphatics has upon the later protective immune responses in the different strains remains an unresolved question.
In keeping with the extensively studied immune regulatory/suppressive effects of helminth infection, 53 L sigmodontis infection or products protects mice against E‐coli–induced sepsis, 121 diet‐induced glucose intolerance, 130 type‐1 diabetes, 131 , 132 atherosclerosis 18 and allergic responses. 133 , 134 These studies provide insights into new helminth‐mediated regulatory pathways such as the suppression T‐cell response by interference with the development of cytotoxic CD8+ T cells, 135 follicular helper T cells, 136 or by TGF‐β. 69 , 132 These results highlight the capacity of L sigmodontis to induce global immune alterations in the host best exemplified by the parasites ability to interfere with vaccine‐induced protective immunity against, Plasmodium, 135 Friend virus 137 and influenza. 66 Fitting these findings, and perhaps partly explaining them, is the remarkable finding that peritoneal implantation of a single adult female (but not male) delays the elimination of exogenously delivered blood mF, suggesting that the parasite acts systemically to protect her offspring. 28
9. FUTURE PERSPECTIVES
We believe that the unique features of the L sigmodontis model will reveal further insights into myeloid cell function, as well cellular communication across tissues and between immune and stromal cells. Results from our laboratory strongly suggest a key role for pleural cavity macrophages in L sigmodontis infection but the heterogeneity of these cells has yet to be described accurately. In both resistant and susceptible mice, we observe cells which fall under the banner of M(IL‐4) cells but in fact these are an amalgam of cells of diverse origins and activation states. New technologies such single‐cell sequencing, mass spectrometry and high‐parameter cytometry will allow us to better describe complex myeloid populations without the bias of ‘gates’. The pleural cavity is a relatively simple organ system which also represents a model to study niche‐immune cell interactions such as ligand‐receptor analysis that will provide a more holistic understanding of immune cell function and interactions. More importantly, we have yet to convincingly link these macrophages to worm killing. The use of novel genetically modified mice may help to answer these questions. In particular, Ms4a3‐reporter mice will allow for fate mapping of monocyte‐derived cells, 10 macrophage‐specific GATA6‐deficent mice will allow us to ascertain the role of the residency programme in F4/80high cells in resistance, 15 and mice with a cell‐specific deletion in Bhlhe40 can be used to ascertain the importance of F4/80high macrophage proliferation 138 to infection outcome.
The variation in susceptibility across many mouse strains (Figure 2) paints L sigmodontis infection as a suitable candidate for mapping susceptibility to genetic loci using haplotype lineage analysis or quantitative trait locus. The fact that only mice of the BALB lineage develop mF after infection limits susceptibility loci to that mouse. Many common mouse strains are historically derived from 19th‐century mouse fanciers who bred mice for behavioural and aesthetic traits. Thus, rather than being truly separate, inbred mouse lines share much common ancestry and are predominantly derived from the Mus musculus domesticus subspecies. 139 , 140 , 141 The most common strains of mice only represent a small portion of the diversity in alleles amongst wild mice. 142 The resistance of relatively new wild‐derived strains to L sigmodontis is of considerable interest, as this may provide more accurate determination of genetic loci, without the need for genotyping. 139 , 143 This would be achieved by the use of recombinantly inbred mouse lines which make use of wild‐derived mice, such as the Collaborative Cross, that have been created to minimize the number of strains required to capture maximal genetic diversity. 142
There are now thought to be in the region of 450 mouse strains. 144 However, if anything the number of strains in common use has been in steep decline for decades. One reason is the wide availability of genetically altered mice only on certain backgrounds and the fact that the C57BL/6J mouse is the reference genome. 145 This gradual acceptance of the dominant position of C57BL/6 mice raises the issue that many basic discoveries in immunology may be mouse strain‐specific. Indeed, it was the L sigmodontis model in C57BL/6 mice that allowed the paradigm‐shifting discovery that macrophages could increase dramatically in number through local proliferation, without requirement for blood‐derived cells during an inflammatory response. 111 Similar experiments in BALB/c mice would not have revealed the same myeloid cell dynamics. Nonetheless, the basic biology holds true in other strains but to widely varying extent 32 and the comparison itself is now proving powerful. It is thus imperative to be aware that recent breakthroughs in myeloid biology have largely been achieved using only C57BL/6 mice. 95 , 96 , 97 , 138 Since the first extension of the Th1‐Th2 dichotomy to macrophages, 146 the genetic underpinnings of these phenotypes have remained largely elusive. Despite their hybridized ancestry, inbred strains of mice harbour an astonishing amount of loss of function mutations. 147 , 148 , 149 Ultimately, cell identity and function in a type 2 immune environment will depend less on simple allelic differences and more upon transcription factor interactions. 150 , 151 The biological consequences provided by differences in cis‐regulatory elements in different strains of mice have already been shown to have profound effects on macrophage transcription factor interactions and cell function. 152 , 153 We are unlikely to be able to model the functional consequences of such genomic differences any time soon. Until such time, we will need to pay attention to the repertoire of genetic diversity provided by extant inbred mouse strains.
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
We thank Brian Chan, Alistair Chenery and Pedro Papotto for their critical review of the manuscript. We thank the editor of this issue, Dominik Rückerl, and the anonymous referees, for very comprehensive and helpful review of the manuscript. Figures made with the aid of biorender.com.
Finlay CM, Allen JE. The immune response of inbred laboratory mice to Litomosoides sigmodontis: A route to discovery in myeloid cell biology. Parasite Immunol. 2020;42:e12708 10.1111/pim.12708
The peer review history for this article is available at https://publons.com/publon/10.1111/pim.12708
REFERENCES
- 1. Chandy A, Thakur AS, Singh MP, Manigauha A. A review of neglected tropical diseases: Filariasis. Asian Pac J Trop Med. 2011;4:581‐586. [DOI] [PubMed] [Google Scholar]
- 2. John H. Cross. Fialarial Nematodes In: Baron S. (ed). Medical Microbiology. 4th edn. Galveston (TX): University of Texas Medical Branch at Galveston, 1996. https://www.ncbi.nlm.nih.gov/books/NBK7627/ [PubMed] [Google Scholar]
- 3. Paily KP, Hoti SL, Das PK. A review of the complexity of biology of lymphatic filarial parasites. J Parasit Dis. 2009;33:3‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bain O, Babayan S. Behaviour of filariae: Morphological and anatomical signatures of their life style within the arthropod and vertebrate hosts. Filaria J. 2003;2:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maréchal P, Le Goff L, Petit G, Diagne M, Taylor DW, Bain O. The fate of the filaria Litomosoides sigmodontis in susceptible and naturally resistant mice. Parasite. 1996;3:25‐31. [DOI] [PubMed] [Google Scholar]
- 6. Nutman TB. Insights into the pathogenesis of disease in human lymphatic filariasis. Lymphat Res Biol. 2013;11:144‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allen JE, Adjei O, Bain O, et al. Of mice, cattle, and humans: The immunology and treatment of river blindness. PLoS Negl Trop Dis. 2008;2:e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ravindran B. Aping Jane Goodall: insights into human lymphatic filariasis. Trends Parasitol. 2003;19:105‐109. [DOI] [PubMed] [Google Scholar]
- 9. Hoerauf A, Brattig N. Resistance and susceptibility in human onchocerciasis ‐ Beyond Th1 vs Th2. Trends Parasitol. 2002;18:25‐31. [DOI] [PubMed] [Google Scholar]
- 10. Babu S, Nutman TB. Immunology of lymphatic filariasis. Parasite Immunol. 2014;36:338‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scott JA. Observations on the rate of growth and maturity of Litomosoides carinii, a filarial worm of the cotton rat. J Parasitol. 1946;32:570‐573. [PubMed] [Google Scholar]
- 12. Fulton A, Babayan SA, Taylor MD. Use of the Litomosoides sigmodontis Infection Model of Filariasis to Study Type 2 Immunity BT ‐ Type 2 Immunity: Methods and Protocols In: Reinhardt RL, ed. Springer. New York: New York, NY; 2018:11‐26. [DOI] [PubMed] [Google Scholar]
- 13. Babayan S, Attout T, Specht S, et al. Increased early local immune responses and altered worm development in high‐dose infections of mice susceptible to the filaria Litomosoides sigmodontis. Med Microbiol Immunol. 2005;194:151‐162. [DOI] [PubMed] [Google Scholar]
- 14. Le Goff L, Martin C, Oswald IP, et al. Parasitology and immunology of mice vaccinated with irradiated Litomosoides sigmodontis larvae. Parasitology. 2000;120:271‐280. [DOI] [PubMed] [Google Scholar]
- 15. Bain O, Wanji S, Vuong PN, et al. Larval biology of six filariae of the sub‐family Onchocercinae in a vertebrate host. Parasite. 1994;1:241‐254. [DOI] [PubMed] [Google Scholar]
- 16. Kilarski WW, Martin C, Pisano M, Bain O, Babayan SA, Swartz MA. Inherent biomechanical traits enable infective filariae to disseminate through collecting lymphatic vessels. Nat Commun. 2019;10:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karadjian G, Fercoq F, Pionnier N, et al. Migratory phase of Litomosoides sigmodontis filarial infective larvae is associated with pathology and transient increase of S100A9 expressing neutrophils in the lung. PLoS Negl Trop Dis. 2017;11:1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hiriart E, Deepe R, Wessels A. Mesothelium and malignant mesothelioma. J Dev Biol. 2019;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Charalampidis C, Youroukou A, Lazaridis G, et al. Pleura space anatomy. J Thorac Dis. 2015;7:S27‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219‐225. [DOI] [PubMed] [Google Scholar]
- 21. Dietrich CF, Chaubal N, Hoerauf A, et al. Review of dancing parasites in lymphatic filariasis. Ultrasound Int Open. 2019;05:E65‐E74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hawking F, Burroughs ANNM. Transmission of Litomosoides carinii to Mice and Hamsters. Nature. 1946;158:98.20993338 [Google Scholar]
- 23. Petit G, Diagne M, Maréchal P, et al. Maturation of the filaria Litomosoides sigmodontis in BALB/c mice; comparative susceptibility of nine other inbred strains. Ann Parasitol Hum comparée. 1992;67:144‐150. [DOI] [PubMed] [Google Scholar]
- 24. Graham AL, Taylor MD, Le Goff L, Lamb TJ, Magennis M, Allen JE. Quantitative appraisal of murine filariasis confirms host strain differences but reveals that BALB/c females are more susceptible than males to Litomosoides sigmodontis. Microbes Infect. 2005;7:612‐618. [DOI] [PubMed] [Google Scholar]
- 25. Pfaff AW, Schulz‐Key H, Soboslay PT, Geiger SM, Hoffmann WH Litomosoides sigmodontis: dynamics of the survival of microfilariae in resistant and susceptible strains of mice. Exp Parasitol. 2000;94:67‐74. [DOI] [PubMed] [Google Scholar]
- 26. Babayan S, Ungeheuer MN, Martin C, et al. Resistance and susceptibility to filarial infection with Litomosoides sigmodontis are associated with early differences in parasite development and in localized immune reactions. Infect Immun. 2003;71:6820‐6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Attout T, Martin C, Babayan SA, et al. Pleural cellular reaction to the filarial infection Litomosoides sigmodontis is determined by the moulting process, the worm alteration, and the host strain. Parasitol Int. 2008;57:201‐211. [DOI] [PubMed] [Google Scholar]
- 28. Hoffmann WH, Pfaff AW, Schulz‐Key H, Soboslav PT. Determinants for resistance and susceptibility to microfilaraemia in Litomosoides sigmodontis filariasis. Parasitology. 2001;122:641‐649. [DOI] [PubMed] [Google Scholar]
- 29. Layland LE, Ajendra J, Ritter M, Wiszniewsky A, Hoerauf A, Hübner MP. Development of patent Litomosoides sigmodontis infections in semi‐susceptible C57BL/6 mice in the absence of adaptive immune responses. Parasit Vectors. 2015;8:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al‐Qaoud KM, Taubert A, Zahner H, Fleischer B, Hoerauf A. Infection of BALB/c mice with the filarial nematode Litomosoides sigmodontis: Role of CD4+T cells in controlling larval development. Infect Immun. 1997;65:2457‐2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martin C, Saeftel M, Vuong PN, et al. B‐cell deficiency suppresses vaccine‐induced protection against murine filariasis but does not increase the recovery rate for primary infection. Infect Immun. 2001;69:7067‐7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Campbell SM, Knipper JA, Ruckerl D, et al. Myeloid cell recruitment versus local proliferation differentiates susceptibility from resistance to filarial infection. Elife. 2018;7:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jackson‐Jones LH, Duncan SM, Magalhaes MS, et al. Fat‐associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat Commun. 2016;7:12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le Goff L, Lamb TJ, Graham AL, Harcus Y, Allen JE. IL‐4 is required to prevent filarial nematode development in resistant but not susceptible strains of mice. Int J Parasitol. 2002;32:1277‐1284. [DOI] [PubMed] [Google Scholar]
- 35. Rajan B, Ramalingam T, Rajan TV. Critical role for IgM in host protection in experimental filarial infection. J Immunol. 2005;175:1827‐1833. [DOI] [PubMed] [Google Scholar]
- 36. Volkmann L, Saeftel M, Bain O, Fischer K, Fleischer B, Hoerauf A. Interleukin‐4 is essential for the control of microfilariae in murine infection with the filaria litomosoides sigmodontis. Infect Immun. 2001;69:2950‐2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baumgarth N. A hard(y) look at B‐1 cell development and function. J Immunol. 2017;199:3387‐3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cerqueira C, Manfroi B, Fillatreau S. IL‐10‐producing regulatory B cells and plasmocytes: Molecular mechanisms and disease relevance. Semin. Immunol. 2019;101323. [DOI] [PubMed] [Google Scholar]
- 39. Mohanty MC, Ravindran B. Deficiency of antibody responses to T‐independent antigens in gerbils ‐ Meriones unguiculatus. Dev Comp Immunol. 2002;26:385‐391. [DOI] [PubMed] [Google Scholar]
- 40. Ai‐Qaoud KM, Fleischer B, Hoerauf A. The Xid defect imparts susceptibility to experimental murine filariosis ‐ Association with a lack of antibody and IL‐10 production by B cells in response to phosphorylcholine. Int Immunol. 1998;10:17‐25. [DOI] [PubMed] [Google Scholar]
- 41. Volkmann L, Bain O, Saeftel M, et al. Murine filariasis: Interleukin 4 and interleukin 5 lead to containment of different worm developmental stages. Med Microbiol Immunol. 2003;192:23‐31. [DOI] [PubMed] [Google Scholar]
- 42. Rückerl D, Allen JE. Macrophage proliferation, provenance, and plasticity in macroparasite infection. Immunol Rev. 2014;262:113‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ritter M, Tamadaho RS, Feid J, et al. IL‐4/5 signalling plays an important role during Litomosoides sigmodontis infection, influencing both immune system regulation and tissue pathology in the thoracic cavity. Int J Parasitol. 2017;47:951‐960. [DOI] [PubMed] [Google Scholar]
- 44. Frohberger SJ, Ajendra J, Surendar J, et al. Susceptibility to L sigmodontis infection is highest in animals lacking IL‐4R/IL‐5 compared to single knockouts of IL‐4R, IL‐5 or eosinophils. Parasit Vectors. 2019;12:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Le Goff L, Loke P, Ali HF, Taylor DW, Allen JE. Interleukin‐5 is essential for vaccine‐mediated immunity but not innate resistance to a filarial parasite. Infect Immun. 2000;68:2513‐2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Babayan SA, Read AF, Lawrence RA, Bain O, Allen JE. Filarial parasites develop faster and reproduce earlier in response to host immune effectors that determine filarial life expectancy. PLoS Biol. 2010;8:2‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mangano VD, Modiano D. Host genetics and parasitic infections. Clin Microbiol Infect. 2014;20:1265‐1275. [DOI] [PubMed] [Google Scholar]
- 48. Hoerauf A, Kruse S, Brattig NW, Heinzmann A, Mueller‐Myhsok B, Deichmann KA. The variant Arg110Gln of human IL‐13 is associated with an immunologically hyper‐reactive form of onchocerciasis (sowda). Microbes Infect. 2002;4:37‐42. [DOI] [PubMed] [Google Scholar]
- 49. Gentil K, Hoerauf A, Pearlman E. Differential induction of Th2‐ And Th1‐associated responses by filarial antigens and endosymbiotic Wolbachia in a murine model of river blindness. Eur J Microbiol Immunol. 2012;2:134‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saeftel M, Arndt M, Specht S, Volkmann L, Hoerauf A. Synergism of Gamma Interferon and Interleukin‐5 in the Control of Murine Filariasis. Infect Immun. 2003;71:6978‐6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Taubert A, Zahner H. Cellular immune responses of filaria (Litomosoides sigmodontis) infected BALB/c mice detected on the level of cytokine transcription. Parasite Immunol. 2001;23:453‐462. [DOI] [PubMed] [Google Scholar]
- 52. Taylor MD, van der Werf N, Maizels RM. T cells in helminth infection: The regulators and the regulated. Trends Immunol. 2012;33:181‐189. [DOI] [PubMed] [Google Scholar]
- 53. Finlay CM, Walsh KP, Mills KHG. Induction of regulatory cells by helminth parasites: Exploitation for the treatment of inflammatory diseases. Immunol Rev. 2014;259:206‐230. [DOI] [PubMed] [Google Scholar]
- 54. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176:3248‐3256. [DOI] [PubMed] [Google Scholar]
- 55. Maizels RM, Smits HH, McSorley HJ. Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules. Immunity. 2018;49:801‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maizels RM, Balic A, Gomez‐Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites ‐ Masters of regulation. Immunol Rev. 2004;201(1):89‐116. [DOI] [PubMed] [Google Scholar]
- 57. Maizels RM, Yazdanbakhsh M. Immune Regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733. [DOI] [PubMed] [Google Scholar]
- 58. Hoerauf A, Satoguina J, Saeftel M, Specht S. Immunomodulation by filarial nematodes. Parasit Immunol. 2005;27:417‐429. [DOI] [PubMed] [Google Scholar]
- 59. Sartono E, Kruize YC, Kurniawan A, Maizels RM, Yazdanbakhsh M. Depression of antigen‐specific interleukin‐5 and interferon‐gamma responses in human lymphatic filariasis as a function of clinical status and age. J Infect Dis. 1997;175:1276‐1280. [DOI] [PubMed] [Google Scholar]
- 60. Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005;174:4924‐4933. [DOI] [PubMed] [Google Scholar]
- 61. van der Werf N, Redpath SA, Azuma M, Yagita H, Taylor MD. Th2 cell‐intrinsic hypo‐responsiveness determines susceptibility to helminth infection. PLoS Pathog. 2013;9:e1003215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Knipper JA, Ivens A, Taylor MD. Helminth‐induced Th2 cell dysfunction is distinct from exhaustion and is maintained in the absence of antigen. PLoS Negl Trop Dis. 2019;13:e0007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Taylor MD, Harris A, Nair MG, Maizels RM, Allen JE. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J Immunol. 2006;176:6918‐6927. [DOI] [PubMed] [Google Scholar]
- 64. Taylor MD, Harris A, Babayan SA, et al. CTLA‐4 and CD4 + CD25 + regulatory T cells inhibit protective immunity to filarial parasites in vivo. J Immunol. 2007;179:4626‐4634. [DOI] [PubMed] [Google Scholar]
- 65. Taylor MD, van der Werf N, Harris A, et al. Early recruitment of natural CD4+Foxp3+ Treg cells by infective larvae determines the outcome of filarial infection. Eur J Immunol. 2009;39:192‐206. [DOI] [PubMed] [Google Scholar]
- 66. Hartmann W, Brunn ML, Stetter N, et al. Helminth infections suppress the efficacy of vaccination against seasonal influenza. Cell Rep. 2019;29(2243–2256):e4. [DOI] [PubMed] [Google Scholar]
- 67. Haben I, Hartmann W, Specht S, et al. T‐cell‐derived, but not B‐cell‐derived, IL‐10 suppresses antigen‐specific T‐cell responses in Litomosoides sigmodontis‐infected mice. Eur J Immunol. 2013;43:1799‐1805. [DOI] [PubMed] [Google Scholar]
- 68. Specht S, Volkmann L, Wynn T, Hoerauf A. Interleukin‐10 (IL‐10) counterregulates IL‐4‐dependent effector mechanisms in murine filariasis. Infect Immun. 2004;72:6287‐6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hartmann W, Schramm C, Breloer M. Litomosoides sigmodontis induces TGF‐β receptor responsive, IL‐10‐producing T cells that suppress bystander T‐cell proliferation in mice. Eur J Immunol. 2015;45:2568‐2581. [DOI] [PubMed] [Google Scholar]
- 70. Korten S, Badusche M, Büttner DW, Hoerauf A, Brattig N, Fleischer B. Natural death of adult Onchocerca volvulus and filaricidal effects of doxycycline induce local FOXP3+/CD4+ regulatory T cells and granzyme expression. Microbes Infect. 2008;10:313‐324. [DOI] [PubMed] [Google Scholar]
- 71. Hartmann W, Marsland BJ, Otto B, Urny J, Fleischer B, Korten S. A Novel and divergent role of granzyme A and B in resistance to helminth infection. J Immunol. 2011;186:2472‐2481. [DOI] [PubMed] [Google Scholar]
- 72. Boyd A, Killoran K, Mitre E, Nutman TB. Pleural cavity type 2 innate lymphoid cells precede Th2 expansion in murine Litomosoides sigmodontis infection. Exp Parasitol. 2015;159:118‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Korten S, Volkmann L, Saeftel M, et al. Expansion of NK cells with reduction of their inhibitory Ly‐49A, Ly‐49C, and Ly‐49G2 receptor‐expressing subsets in a murine helminth infection: contribution to parasite control. J Immunol. 2002;168:5199‐5206. [DOI] [PubMed] [Google Scholar]
- 74. Torrero MN, Hübner MP, Larson D, Karasuyama H, Mitre E. Basophils amplify type 2 immune responses, but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodontis . J Immunol. 2010;185:7426 LP‐7434. [DOI] [PubMed] [Google Scholar]
- 75. Larson D, Hübner MP, Torrero MN, et al. Chronic helminth infection reduces basophil responsiveness in an IL‐10–dependent manner. J Immunol. 2012;188:4188‐4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hartmann W, Linnemann LC, Reitz M, Specht S, Voehringer D, Breloer M. Basophils are dispensable for the control of a filarial infection. ImmunoHorizons. 2018;2:296‐304. [DOI] [PubMed] [Google Scholar]
- 77. Cayrol C, Girard JP. Interleukin‐33 (IL‐33): A nuclear cytokine from the IL‐1 family. Immunol Rev. 2018;281:154‐168. [DOI] [PubMed] [Google Scholar]
- 78. Ajendra J, Specht S, Neumann AL, et al. ST2 deficiency does not impair type 2 immune responses during chronic filarial infection but leads to an increased microfilaremia due to an impaired splenic microfilarial clearance. PLoS One. 2014;9:e93072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Specht S, Frank JK, Alferink J, et al. CCL17 controls mast cells for the defense against filarial larval entry. J Immunol. 2011;186:4845‐4852. [DOI] [PubMed] [Google Scholar]
- 80. Saeftel M, Volkmann L, Korten S, et al. Lack of interferon‐γ confers impaired neutrophil granulocyte function and imparts prolonged survival of adult filarial worms in murine filariasis. Microbes Infect. 2001;3:203‐213. [DOI] [PubMed] [Google Scholar]
- 81. Martin C, Le Goff L, Ungeheuer MN, Vuong PN, Bain O. Drastic reduction of a filarial infection in eosinophilic interleukin‐5 transgenic mice. Infect Immun. 2000;68:3651‐3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Al‐Qaoud KM, Pearlman E, Hartung T, Klukowski J, Fleischer B, Hoerauf A. A new mechanism for IL‐5‐dependent helminth control: Neutrophil accumulation and neutrophil‐mediated worm encapsulation in murine filariasis are abolished in the absence of IL‐5. Int Immunol. 2000;12:899‐908. [DOI] [PubMed] [Google Scholar]
- 83. Specht S, Saeftel M, Arndt M, et al. Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect Immun. 2006;74:5236‐5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Turner JD, Pionnier N, Furlong‐Silva J, et al. Interleukin‐4 activated macrophages mediate immunity to filarial helminth infection by sustaining CCR3‐dependent eosinophilia. PLoS Pathog. 2018;14:1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fox EM, Morris CP, Hübner MP, Mitre E. Histamine 1 receptor blockade enhances eosinophil‐mediated clearance of adult filarial worms. PLoS Negl Trop Dis. 2015;9:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Martin C, Al‐Qaoud KM, Ungeheuer MN, et al. IL‐5 is essential for vaccine‐induced protection and for resolution of primary infection in murine filariasis. Med Microbiol Immunol. 2000;189:67‐74. [DOI] [PubMed] [Google Scholar]
- 87. Huang L, Appleton JA. Eosinophils in Helminth Infection: Defenders and Dupes. Trends Parasitol. 2016;32:798‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bou Ghosn EE, Cassado AA, Govoni GR, et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci USA. 2010;107:2568‐2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Buechler MB, Kim KW, Onufer EJ, et al. A stromal niche defined by expression of the transcription factor WT1 mediates programming and homeostasis of cavity‐resident macrophages. Immunity. 2019;51(119–130):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bain CC, Gibson DA, Steers N, Boufea K, Louwe PA. Origin and microenvironment contribute to the sexually dimorphic phenotype and function of peritoneal macrophages. bioRxiv.2019;1‐40. [DOI] [PMC free article] [PubMed]
- 91. Kim KW, Williams JW, Wang YT, et al. MHC II + resident peritoneal and pleural macrophages rely on IRF4 for development from circulating monocytes. J Exp Med. 2016;213:1951‐1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bain CC, Hawley CA, Garner H, et al. Long‐lived self‐renewing bone marrow‐derived macrophages displace embryo‐derived cells to inhabit adult serous cavities. Nat Commun. 2016;7:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Takenaka E, Van Vo A, Yamashita‐Kanemaru Y, Shibuya A, Shibuya K. Selective DNAM‐1 expression on small peritoneal macrophages contributes to CD4+ T cell costimulation. Sci Rep. 2018;8:15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Te LC, Rosas M, Davies LC, et al. IL‐10 differentially controls the infiltration of inflammatory macrophages and antigen‐presenting cells during inflammation. Eur J Immunol. 2016;46:2222‐2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sheng J, Ruedl C, Karjalainen K. Most tissue‐resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity. 2015;43:382‐393. [DOI] [PubMed] [Google Scholar]
- 97. Hoeffel G, Chen J, Lavin Y, et al. C‐Myb+ erythro‐myeloid progenitor‐derived fetal monocytes give rise to adult tissue‐resident macrophages. Immunity. 2015;42:665‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jenkins SJ, Ruckerl D, Thomas GD, et al. IL‐4 directly signals tissue‐resident macrophages to proliferate beyond homeostatic levels controlled by CSF‐1. J Exp Med. 2013;210:2477‐2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu Z, Gu Y, Chakarov S, Bleriot C, Chen X, Shin A, et al. Fate mapping via Ms4a3 expression history traces monocyte‐derived cells. bioRxiv.2019;652032. [DOI] [PubMed]
- 100. Liu Z, Gu Y, Chakarov S, et al. Fate mapping via Ms4a3‐expression history traces monocyte‐derived cells. Cell. 2019;178(1509–1525):e19. [DOI] [PubMed] [Google Scholar]
- 101. Gundra UM, Girgis NM, Gonzalez MA, et al. Vitamin A mediates conversion of monocyte‐derived macrophages into tissue‐resident macrophages during alternative activation. Nat Immunol. 2017;18:642‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shaw TN, Houston SA, Wemyss K, et al. Tissue‐resident macrophages in the intestine are long lived and defined by Tim‐4 and CD4 expression. J Exp Med. 2018;215:1507‐1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rosas M, Davies LC, Giles PJ, et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science (80‐ ). 2014;344:645‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Okabe Y, Medzhitov R. Tissue‐specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gautier EL, Ivanov S, Williams JW, et al. Gata6 regulates aspartoacylase expression in resident peritoneal macrophages and controls their survival. J Exp Med. 2014;211:1525‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cain DW, O’Koren EG, Kan MJ, et al. Identification of a tissue‐specific, C/EBPβ‐dependent pathway of differentiation for murine peritoneal macrophages. J Immunol. 2013;191:4665‐4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kuehn C, Tauchi M, Furtmair R, et al. Filarial extract of Litomosoides sigmodontis induces a type 2 immune response and attenuates plaque development in hyperlipidemic ApoE‐knockout mice. FASEB J. 2019;33:6497‐6513. [DOI] [PubMed] [Google Scholar]
- 109. Nair MG, Gallagher IJ, Taylor MD, et al. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen‐presenting cells. Infect Immun. 2005;73:385‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL‐4 dependent alternatively‐activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jenkins SJ, Ruckerl D, Cook PC, et al. Local Macrophage Proliferation, Rather than Recruitment from the Blood, Is a Signature of TH2 Inflammation. Science (80‐). 2011;332:1284‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gundra UM, Girgis NM, Ruckerl D, et al. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood. 2014;123:e110‐e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rückerl D, Campbell SM, Duncan S, et al. Macrophage origin limits functional plasticity in helminth‐bacterial co‐infection. PLoS Pathog. 2017;13:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Karadjian G, Berrebi D, Dogna N, et al. Co‐infection restrains Litomosoides sigmodontis filarial load and plasmodial P yoelii but not P chabaudi parasitaemia in mice. Parasite. 2014;21:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fercoq F, Remion E, Frohberger SJ, et al. IL‐4 receptor dependent expansion of lung CD169+ macrophages in microfilaria‐driven inflammation. PLoS Negl Trop Dis. 2019;13:e0007691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Minutti CM, Jackson‐Jones LH, García‐Fojeda B, et al. Local amplifiers of IL‐4Rα‐mediated macrophage activation promote repair in lung and liver. Science. 2017;356:1076‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bouchery T, Dénécé G, Attout T, et al. The chemokine CXCL12 is essential for the clearance of the filaria Litomosoides sigmodontis in resistant mice. PLoS One. 2012;7:e34971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mahlakõiv T, Flamar AL, Johnston LK, et al. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin‐33. Sci Immunol. 2019;4:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Al‐Aarag ASH, Kamel MH, Abdelgawad ER, et al. Diagnostic role of interleukin ‐33 in the differentiation of pleural effusions especially tuberculous and malignant effusions. BMC Pulm Med. 2019;19:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Jackson‐Jones LH, Ruckerl D, Svedberg F, et al. IL‐33 delivery induces serous cavity macrophage proliferation independent of interleukin‐4 receptor alpha. Eur J Immunol. 2016;46:2311‐2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gondorf F, Berbudi A, Buerfent BC, et al. Chronic filarial infection provides protection against bacterial sepsis by functionally reprogramming macrophages. PLoS Pathog. 2015;11:1‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pfarr KM, Fischer K, Hoerauf A. Involvement of Toll‐like receptor 4 in the embryogenesis of the rodent filaria Litomosoides sigmodontis. Med Microbiol Immunol. 2003;192:53‐56. [DOI] [PubMed] [Google Scholar]
- 123. Rodrigo MB, Schulz S, Krupp V, et al. Patency of Litomosoides sigmodontis infection depends on Toll‐like receptor 4 whereas Toll‐like receptor 2 signalling influences filarial‐specific CD4+ T‐cell responses. Immunology. 2016;147:429‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wiszniewsky A, Ritter M, Krupp V, et al. The central adaptor molecule TRIF influences L sigmodontis worm development. Parasitol Res. 2019;118:539‐549. [DOI] [PubMed] [Google Scholar]
- 125. Ajendra J, Specht S, Ziewer S, et al. NOD2 dependent neutrophil recruitment is required for early protective immune responses against infectious Litomosoides sigmodontis L3 larvae. Sci Rep. 2016;6:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kim BS. Innate lymphoid cells in the skin. J Invest Dermatol. 2015;135:673‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cruz MS, Diamond A, Russell A, Jameson JM. Human αβ and γδ T cells in skin immunity and disease. Front Immunol. 2018;9:1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Muhsin M, Ajendra J, Gentil K, et al. IL‐6 is required for protective immune responses against early filarial infection. Int J Parasitol. 2018;48:925‐935. [DOI] [PubMed] [Google Scholar]
- 129. Pionnier N, Brotin E, Karadjian G, et al. Neutropenic mice provide insight into the role of skin‐infiltrating neutrophils in the host protective immunity against filarial infective larvae. PLoS Negl Trop Dis. 2016;10:1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Berbudi A, Surendar J, Ajendra J, et al. Filarial infection or antigen administration improves glucose tolerance in diet‐induced obese mice. J Innate Immun. 2016;8:601‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hübner MP, Thomas Stocker J, Mitre E. Inhibition of type 1 diabetes in filaria‐infected non‐obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3+ regulatory T cells. Immunology. 2009;127:512‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hübner MP, Shi Y, Torrero MN, et al. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF‐β. J Immunol. 2012;188:559‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Dittrich AM, Erbacher A, Specht S, et al. Helminth infection with Litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and hyperreactivity in a murine asthma model. J Immunol. 2008;180:1792‐1799. [DOI] [PubMed] [Google Scholar]
- 134. Evans H, Killoran KE, Mitre BK, Morris CP, Kim S‐Y, Mitre E. Ten weeks of infection with a tissue‐invasive helminth protects against local immune complex‐mediated inflammation, but not cutaneous type I hypersensitivity, in previously sensitized mice. J Immunol. 2015;195:2973‐2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kolbaum J, Tartz S, Hartmann W, et al. Nematode‐induced interference with the anti‐Plasmodium CD8 + T‐cell response can be overcome by optimizing antigen administration. Eur J Immunol. 2012;42:890‐900. [DOI] [PubMed] [Google Scholar]
- 136. Haben I, Hartmann W, Breloer M. Nematode‐induced interference with vaccination efficacy targets follicular T helper cell induction and is preserved after termination of infection. PLoS Negl Trop Dis. 2014;8:e3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Dietze KK, Dittmer U, Koudaimi DK, et al. Filariae‐retrovirus co‐infection in mice is associated with suppressed virus‐specific IgG immune response and higher viral loads. PLoS Negl Trop Dis. 2016;10:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Jarjour NN, Schwarzkopf EA, Bradstreet TR, et al. Bhlhe40 mediates tissue‐specific control of macrophage proliferation in homeostasis and type 2 immunity. Nat Immunol. 2019;20:687‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Frazer KA, Eskin E, Kang HM, et al. A sequence‐based variation map of 8.27 million SNPs in inbred mouse strains. Nature. 2007;448:1050‐1053. [DOI] [PubMed] [Google Scholar]
- 140. Yang H, Wang JR, Didion JP, et al. Subspecific origin and haplotype diversity in the laboratory mouse. Nat Genet. 2011;43:648‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Atchley WR, Fitch WM. Gene trees and the origins of inbred strains of mice. Science (80‐). 1991;254(5031):554‐558. [DOI] [PubMed] [Google Scholar]
- 142. Roberts A, de Villena F, Wang W, McMillan L, Threadgill DW. The polymorphism architecture of mouse genetic resources elucidated using genome‐wide resequencing data: implications for QTL discovery and systems genetics. Mamm Genome. 2007;18:473‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yang H, Bell TA, Churchill GA, Pardo‐Manuel de Villena F. On the subspecific origin of the laboratory mouse. Nat Genet. 2007;39:1100‐1107. [DOI] [PubMed] [Google Scholar]
- 144. Beck JA, Lloyd S, Hafezparast M, et al. Genealogies of mouse inbred strains. Nat Genet. 2000;24:23‐25. [DOI] [PubMed] [Google Scholar]
- 145. Chinwalla AT, Cook LL, Delehaunty KD, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520‐562. [DOI] [PubMed] [Google Scholar]
- 146. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M‐1/M‐2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166‐6173. [DOI] [PubMed] [Google Scholar]
- 147. Timmermans S, Van Montagu M, Libert C. Complete overview of protein‐inactivating sequence variations in 36 sequenced mouse inbred strains. Proc Natl Acad Sci. 2017;114(34):9158‐9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet Pathol. 2011;49:32‐43. [DOI] [PubMed] [Google Scholar]
- 149. Tsukuba T, Yanagawa M, Okamoto K, et al. Impaired chemotaxis and cell adhesion due to decrease in several cell‐surface receptors in cathepsin E‐deficient macrophages. J Biochem. 2009;145:565‐573. [DOI] [PubMed] [Google Scholar]
- 150. Czimmerer Z, Daniel B, Horvath A, et al. The transcription factor STAT6 mediates direct repression of inflammatory enhancers and limits activation of alternatively polarized macrophages. Immunity. 2018;48(75–90):e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Czimmerer Z, Varga T, Kiss M, et al. The IL‐4/STAT6 signaling axis establishes a conserved microRNA signature in human and mouse macrophages regulating cell survival via miR‐342‐3p. Genome Med. 2016;8:1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Link VM, Duttke SH, Chun HB, et al. Analysis of genetically diverse macrophages reveals local and domain‐wide mechanisms that control transcription factor binding and function. Cell. 2018;173(1796–1809):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Heinz S, Romanoski CE, Benner C, et al. Effect of natural genetic variation on enhancer selection and function. Nature. 2013;503:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ritter M, Krupp V, Wiszniewsky K, et al. Absence of IL‐17A in Litomosoides sigmodontis‐infected mice influences worm development and drives elevated filarial‐specific IFN‐γ. Parasitol Res. 2018;117:2665‐2675. [DOI] [PMC free article] [PubMed] [Google Scholar]


