Abstract
Hypervirulent Klebsiella pneumoniae (hvKP) causes Klebsiella‐induced liver abscess. Capsule is important for the pathogenesis of Klebsiella in systemic infection, but its role in gut colonisation is not well understood. By generating ΔwcaJ, Δwza and Δwzy capsule‐null mutants in a prototypical K1 hypervirulent isolate, we show that inactivation of wza (capsule exportase) and wzy (capsule polymerase) confer cell envelope defects in addition to capsule loss, making them susceptible to bile salts and detergent stress. Bile salt resistance is restored when the initial glycosyltransferase wcaJ was inactivated together with wzy, indicating that build‐up of capsule intermediates contribute to cell envelope defects. Mouse gut colonisation competition assays show that the capsule and its regulator RmpA were not required for hvKP to persist in the gut, although initial colonisation was decreased in the mutants. Both ΔrmpA and ΔwcaJ mutants gradually outcompeted the wild type in the gut, whereas Δwza and Δwzy mutants were less fit than wild type. Together, our results advise caution in using the right capsule‐null mutant for determination of capsule's role in bacterial pathogenesis. With the use of ΔwcaJ mutant, we found that although the capsule is important for bacterial survival outside the gut environment, it imposes a fitness cost in the gut.
Keywords: bile salt resistance, capsule, cell envelope defects, hypervirulent, intestinal colonisation, Klebsiella pneumoniae
The capsule of hypervirulent Klebsiella pneumoniae is important for causing severe disease. Hypervirulent K. pneumoniae colonises the gut and is thought to circulate in the population by the faecal–oral route. However, the use of different mutants defective in capsule production cause confusion as we found that some mutants have additional cell envelope stability issues besides not having a capsule that make interpretations on their contribution to disease difficult. We show that using the appropriate capsule null mutant, capsule is important for causing systemic disease but not intestinal colonisation.
1. INTRODUCTION
Community‐acquired, hypervirulent Klebsiella pneumoniae (hvKP) has become the leading cause of microbial‐associated pyogenic liver abscess (PLA) in Singapore, Taiwan and other parts of Asia (Alsaif, Sudhakar, Chan, & Archuleta, 2011; Bilal, Volz, Schneider, Fideler, & Podschun, 2014; Lo et al., 2015; Siu, Yeh, Lin, Fung, & Chang, 2012; Wang et al., 1998). HvKP typically possesses a thick, hypermucoid K1 or K2 capsule and colonies on agar give a positive string test (Li, Zhao, Liu, Chen, & Zhou, 2014; Shon, Bajwa, & Russo, 2013). HvKP likely circulates in the population via the faecal–oral route (Chung et al., 2012; Siu et al., 2011) and colonises the gut of both healthy and susceptible individuals. In more susceptible individuals, such as diabetic patients, hvKP disseminates from the gut to the bloodstream and results in liver abscess and bacteremia (Fung et al., 2012; Kontopoulou et al., 2019). A recent analysis has shown that over 80% of liver abscess isolates belong to a CG23 group 1 sublineage that has disseminated globally in the human population (Lam et al., 2018). This sublineage is distinguished by the presence of a genomic island encoding colibactin (ICEKp10) and the loss of function mutations in the fimbrial protein KpcC and ethanolamine Eat population (Lam et al., 2018). All CG23 group 1 isolates also possess the K1 capsule.
The capsule is a key virulence factor of hvKP and is important for resistance to complement (Alvarez, Merino, Tomas, Benedi, & Alberti, 2000; Cortés et al., 2002; Tan, Gamage, & Gan, 2017), dissemination to the liver (Fang, Chuang, Shun, Chang, & Wang, 2004) and during systemic infection (Cheng et al. 2010; Ho et al. 2011; Palacios et al. 2018; Yeh et al. 2007, 2016). The bacterial capsule could potentially protect hvKP from the assault of the antimicrobial peptides such as cathelicidins, defensins and bile salts present in colonic mucus (Antoni et al., 2013; Campos et al., 2004). The capsule may also aid in the formation of K. pneumoniae biofilms, which are more resistant to factors such as to antibiotics and starvation (Wu et al., 2011; Zheng et al., 2018).
The production of the K1 capsule in hvKP is mediated by the Wzx/Wzy‐dependent pathway (Ho et al., 2011; Pan et al., 2015). Briefly, the initial glycosyltransferase WcaJ links the first glucose moiety to the universal lipid carrier undecaprenyl phosphate (Und‐P). Fucose and uronic acid are subsequently added to the nonreducing end of the glucosyl‐P‐Und to synthesise the trisaccharide repeating unit (Ho et al., 2011; Pan et al., 2015). The lipid‐linked intermediate is then flipped across the cytoplasmic membrane, presumably mediated by the transporter Wzx, and then polymerised by Wzy (Whitfield, 2010). Finally, the completed polymer is exported through the pore formed by the secretin Wza and displayed on the surface of the hvKP outer membrane (Li et al., 2014; Whitfield, 2006).
Blocking late steps of the Wzy‐dependent pathway is often lethal to the cell. If the cells survive, they are usually associated with cell shape defects. This phenomenon is likely due to the sequestration of Und‐P (Jorgenson, Kannan, Laubacher, & Young, 2017), which eventually blocks the synthesis of cell wall peptidoglycan. Simultaneously inactivating the initial glycosyltransferase alleviates the cell shape and envelope defects, possibly by restoring the Und‐P lipid carrier pool. To investigate the role of capsule in virulence, one should therefore inactivate the initial glycosyltransferase in the pathway in order to avoid any unwanted deleterious effects on the biogenesis of the cell envelope layer (Manat et al., 2014).
By contrast, studies demonstrating the importance of the K1 capsule in pathogenesis of hvKP were mostly done in a ∆wzy deletion genetic background (formerly known as the ∆magA mutant, which stands for the mucoviscosity associated gene A) (Fang et al., 2004; Fang, Lai, Yi, Hsueh, & Liu, 2010; Wu et al., 2008). It is unclear if the ∆wzy deletion in hvKP causes secondary cell shape defects, which were observed in many other bacterial species (D'Elia et al., 2006; Jorgenson et al., 2017; Jorgenson & Young, 2016; Sham, Zheng, Yakhnina, Kruse, & Bernhardt, 2018; Xayarath & Yother, 2007). If this is the case, the phenotypes exhibited by the ∆wzy mutant must be interpreted with caution.
In this work, we use a K1 clinical isolate SGH10 that has been designated as the reference strain for the CG23 group 1 lineage for our study (Lam et al., 2018). We generated mutants that block different steps in capsule synthesis, regulation and export such as wcaJ, wzy, wza, as well as regulator of mucoidy phenotype A (rmpA). In addition to the lack of capsule production, wzy and wza mutants show severe defects in cell envelope stability, which complicates the interpretations of their phenotypes in any infection models. This phenotype can be rescued by inactivation of wcaJ, showing that the defects are likely due to the sequestration of Und‐P. We reasoned that results generated from the wcaJ mutant are more representative of the effect of losing the capsule in hvKP as this mutant does not demonstrate cell envelope stability issues. Using this mutant in an in vivo competition assay, we demonstrated that the K1 capsule has no detectable role in colonisation persistence in the gastrointestinal tract but is necessary for bacterial pathogenesis during systemic infection.
2. RESULTS
2.1. Deletions of wcaJ, wza and wzy abolish capsule production
SGH10 belongs to serotype K1 and produces a thick capsule (Lam et al., 2018; Lee et al., 2016). The organisation of the capsule locus in SGH10 is illustrated in Figure 1a. SGH10 carries two copies of rmpA on the large virulent plasmid, rmpA and rmpA2. Plasmid rmpA2 is truncated and therefore unlikely to be a functional regulator of capsule synthesis, whereas rmpA is likely to be the active copy. We examined whether the amount of capsule produced was altered using the Percoll density gradient centrifugation assay. Production of the K1 capsule increases the buoyancy of the cells in Percoll and therefore encapsulated bacteria are retained at the top of the centrifuge tube (Figure 1b, SGH10 lane). In contrast, deletion mutants of wcaJ, wza and wzy exhibited a significantly impaired capsule synthesis. Ectopic expression of wcaJ, wza and wzy in the shuttle vector pUCP28T rescued the capsule‐null phenotype in the corresponding mutant, indicating the deletions had no polar effects.
Figure 1.
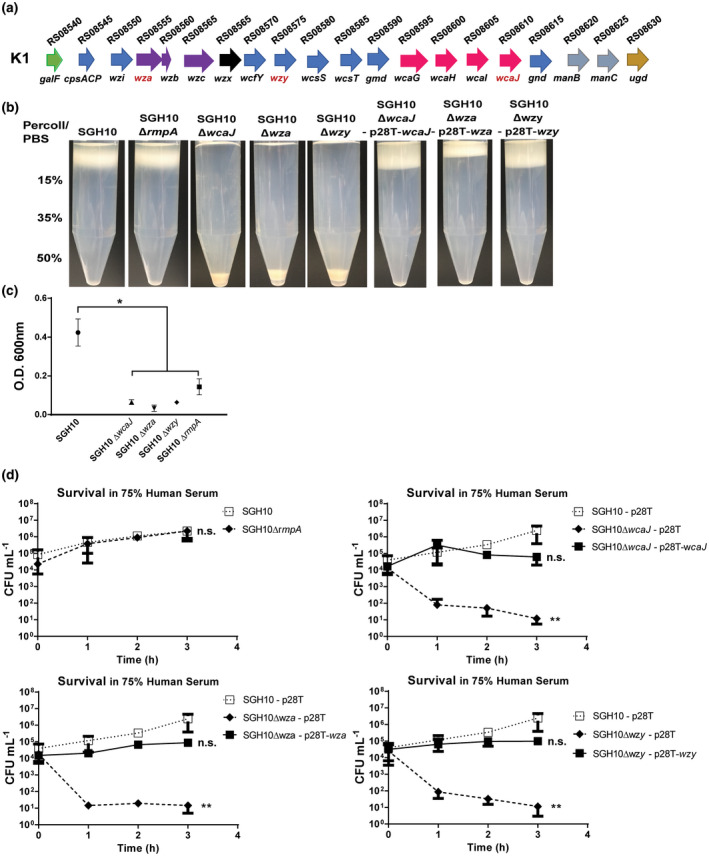
Capsule production of hvKP SGH10. The capsule regulator mutant ∆rmpA, and the capsule‐null mutants ∆wza, ∆wzy and ∆wcaJ were created and the mutants were complemented by the expression of the corresponding gene in a pUCP28T(p28T) backbone. (a) The capsule gene cluster of SGH10 (b) Capsule production in stationary phase bacteria SGH10 and the above mutants was measured semi‐quantitatively by density‐dependent separation on a Percoll gradient. (c) Strain mucoidy was measured by low‐speed centrifugation of bacteria in LB. The optical density at 600 nm (O.D. 600) of the supernatant above pellet was measured, and mean ± SD are plotted (n = 3) and * denotes p < .05, while n.s. denotes not significant. (d) Capsule mutants and their complemented counterparts were grown in 75% human serum to determine if they were resistant to complement mediated killing. Mean ± SD are plotted (n = 3) and Dunnett's multiple comparison test was conducted for CFU values relative to SGH10 at 3 hr. * denotes p < .05, ** denotes p < .001, while n.s. denotes not significant [Colour figure can be viewed at wileyonlinelibrary.com]
In line with the previous report (Hsu et al., 2011), deletion of rmpA did not alter the amount of capsule significantly, as there was no detectable effect on the cell buoyancy in Percoll (Figure 1b, SGH10∆rmpA). However, we noticed that strain SGH10∆rmpA did not produce a positive string test. When centrifuged, cells of ∆rmpA produced a clearer supernatant compared to wild‐type bacteria (Figure 1c).
Next, we examined whether the deletions of rmpA, wcaJ, wza and wzy alter the sensitivity to human serum. As expected, ∆rmpA did not show any change in serum sensitivity (Figure 1d). Unencapsulated mutants (∆wcaJ, ∆wza and ∆wzy) were readily killed by serum, but grew in heat‐treated serum (Figure S1). Serum resistance in the capsule‐null strains was restored by complementation with the plasmids harbouring the corresponding capsule genes. We conclude that the ∆rmpA deletion has no effect on capsule production while inactivation of wcaJ, wza and wzy abolished capsule synthesis. However, rmpA deletion affects the mucoviscosity of hvKP capsule.
2.2. hvKP capsule impedes phagocytosis and adhesion
Previous studies have demonstrated that K. pneumoniae capsule is essential for resistance to phagocytosis (Fang et al., 2004; Yeh et al., 2010). In concordance, ∆wcaJ and ∆wzy were susceptible to uptake by RAW 264.7 macrophages and their complemented counterparts regained resistance (Figure 2a). However, ∆rmpA was phagocytosed at a similar rate as wild type. Intriguingly, the ∆wza mutant was more resistant to phagocytosis compared to other capsule‐null mutants, suggesting that the capsule production, but not its export, is required for resistance of phagocytosis.
Figure 2.
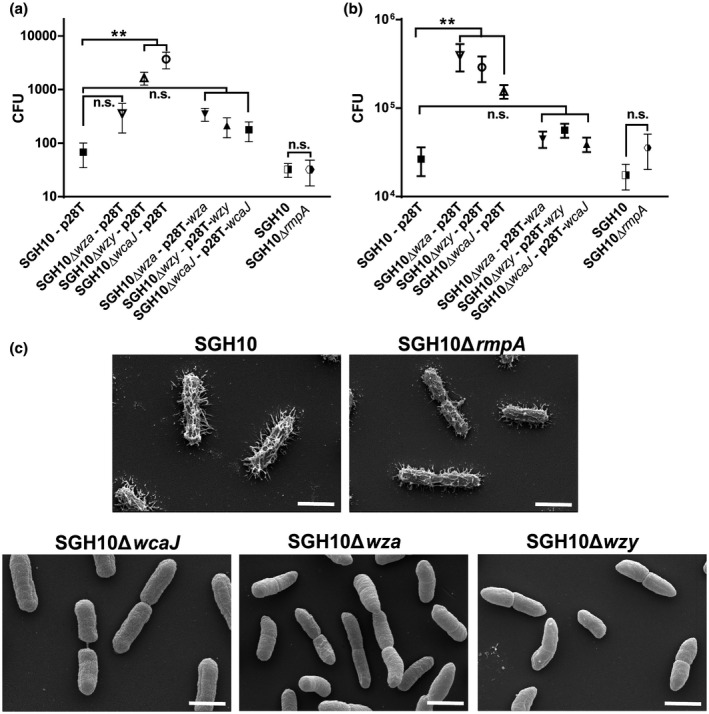
Effect of capsule on association with phagocytic and nonphagocytic cells. (a) Uptake of SGH10, SGH10∆rmpA, SGH10∆wza, SGH10∆wzy, SGH10∆wcaJ and the complemented mutants by RAW264.7 macrophages at 2 hr post infection. (b) Adhesion of SGH10 and capsule mutants, as well as the complemented capsule mutants to Caco‐2 cells at 30 min post infection. Mean ± SD are plotted (n = 3). * denotes p < .05 and ** denotes p < .001, while n.s. denotes not significant. (c) Scanning electron microscopy images of fixed, log‐phase cultures of SGH10, SGH10∆rmpA, SGH10∆wcaJ, SGH10∆wza and SGH10∆wzy cells. The scale bar is 1 μm
The ability of bacteria to adhere to the intestines contributes to persistence in the gut. Although one study suggests that the K. pneumoniae capsule could decrease adhesion to ileocecal and bladder epithelial cells (Sahly et al., 2000), other studies have shown that K. pneumoniae capsule could enhance association to lung epithelial cells and intestinal epithelial cell lines (Clements et al. 2008; Favre‐Bonte, Joly, & Forestier, 1999). The capsule‐null mutants SGH10∆wcaJ, SGH10∆wza and SGH10∆wzy demonstrated significantly increased adhesion to Caco‐2 cells, while SGH10∆rmpA did not (Figure 2b). Complementation of the capsule‐null mutants decreased adhesion to that of wild‐type bacteria.
2.3. ∆wza and ∆wzy mutants exhibit cell envelope defects
Scanning electron microscopy (SEM) reveals that the capsule of SGH10 spreads out from the surface in multiple directions. Likewise, the SGH10∆rmpA mutant is coated with capsule (Figure 2c). SGH10∆wcaJ, SGH10∆wza and SGH10∆wzy cells do not possess the layer of capsular polysaccharide evident on the surface of wild‐type bacteria. Strikingly, while the wild‐type and SGH10∆wcaJ cells are rod shaped, SGH10∆wza and SGH10∆wzy cells exhibit morphological defects. This morphology suggests that their cell envelopes are compromised. The ∆wza and ∆wzy cells are curved, tapered and thinner at the ends and do not seem to be evenly rod shaped (Figure 2c). When visualised using the FITC dextran exclusion assay, the wild‐type and ∆rmpA bacteria were surrounded by a grey halo which indicates the presence of capsule, whereas the capsule‐null mutants did not, further supporting the loss of capsule (Figure S2).
As bacteria progress down the GI tract, they encounter two main stressors, pH and bile stress. Successful pathogenesis requires hvKP to survive under the low‐pH environment of the stomach before traversing the intestines, where the pH is relatively higher. The capsule mutants did not exhibit differences in survival relative to the wild type at pH 4, which is the pH of the mouse stomach (Figure S3), indicating that capsule or the cell shape defects do not affect survival in pH stresses.
Bile salts are known to modulate the gut microbiome and have a bactericidal effect by disrupting bacterial membrane even below their critical micelle concentrations (Merritt & Donaldson, 2009; Urdaneta & Casadesús, 2017). Moreover, hvKP causes Klebsiella‐associated liver abscess and would hence have to be resistant to bile salts in the liver. When SGH10∆wza and SGH10∆wzy were plated on LB agar supplemented with the secondary bile salt sodium deoxycholate (DOC) at concentrations approximating the physiological concentration of 0.1% in the gut, SGH10∆wza and SGH10∆wzy exhibited a 100‐fold difference in survival compared to the wild‐type SGH10 (Figure 3a), which suggests that these strains possess an impaired membrane integrity phenotype. SGH10∆wcaJ and SGH10∆rmpA did not exhibit a difference in survival on DOC relative to wild type (Figure 3a), indicating that the capsule does not affect DOC resistance. The results are similar when the experiment was repeated with the primary bile salt sodium cholate at a concentration of 1% (Figure 3b). Bile salt resistance can be restored with complementation in the ∆wza and ∆wzy mutants (Figure 3d,e).
Figure 3.
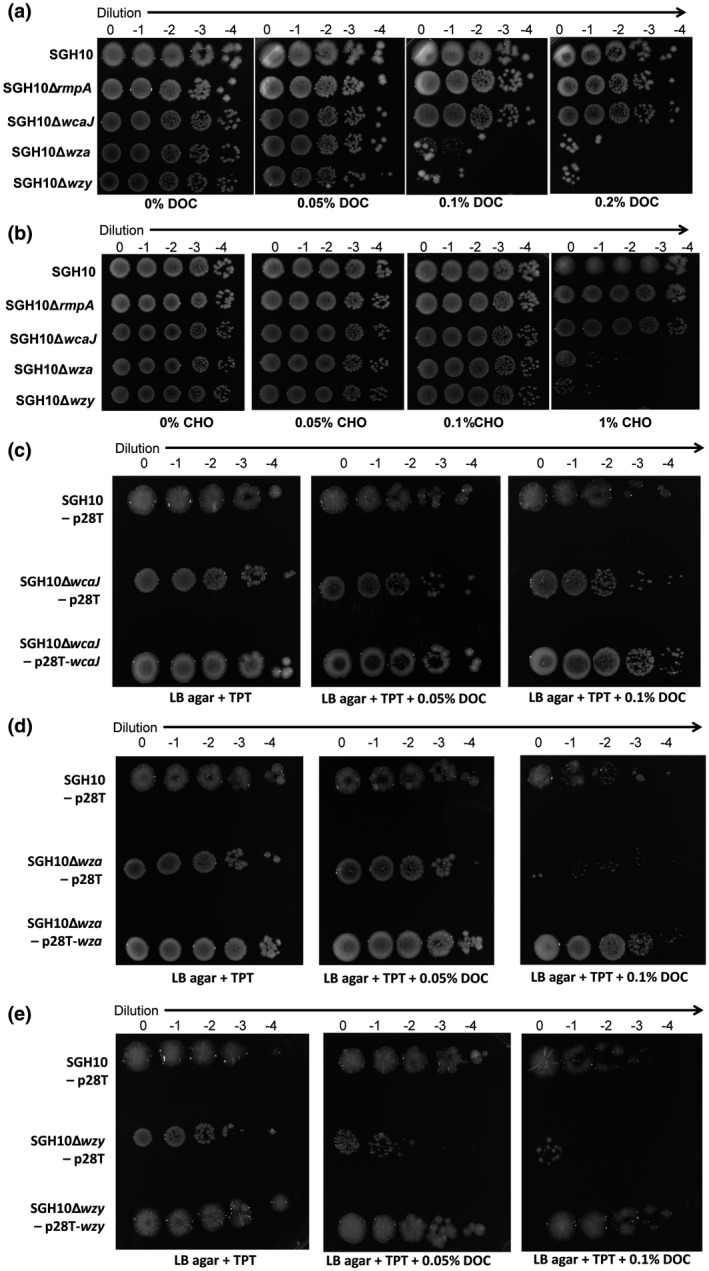
Role of capsule in bile salt resistance. SGH10 and capsule mutants were plated on LB agar supplemented with increasing concentrations of (a) secondary bile salt, sodium deoxycholate (DOC) and (b) primary bile salt, sodium cholate (CHO). Experiments were repeated (n = 3), with similar results. (c) Resistance to bile salt is not affected by complementation in ∆wcaJ mutants but was restored by complementation in (d) ∆wza and (e) ∆wzy mutants. SGH10 and complemented capsule mutants were plated on LB agar supplemented with trimethoprim (TPT) and increasing concentrations of DOC
We reasoned that if the cell envelope defects of the ∆wza and ∆wzy mutants are due to the sequestration of Und‐P or accumulation of toxic capsule intermediates, inactivating wcaJ, the first step in capsule synthesis in these mutants would relieve and rescue the defect. Indeed, the ∆wcaJ and ∆wzy double mutant is as resistant to bile salt as the ∆wcaJ mutant, which is similar to the wild‐type bacteria (Figure 4a). Furthermore, when we complemented wcaJ into the double mutant, the strain showed increased sensitivity to bile salt (Figure 4b). Conversely, complementing wzy into the double mutant did not change the bile salt sensitivity of the strain compared to that of the double mutant. We also show that the ∆wza and ∆wzy mutants were more sensitive to perturbation with the detergent sodium dodecyl sulfate (SDS) compared to ∆wcaJ, ∆wcaJ ∆wzy mutants and the wild‐type bacteria (Figure 4c). This supports our hypothesis that cell envelope defects are likely a result of sequestration of Und‐P and its unavailability for other processes which support membrane integrity, or due to toxic build‐up of capsule intermediates downstream of wcaJ.
Figure 4.
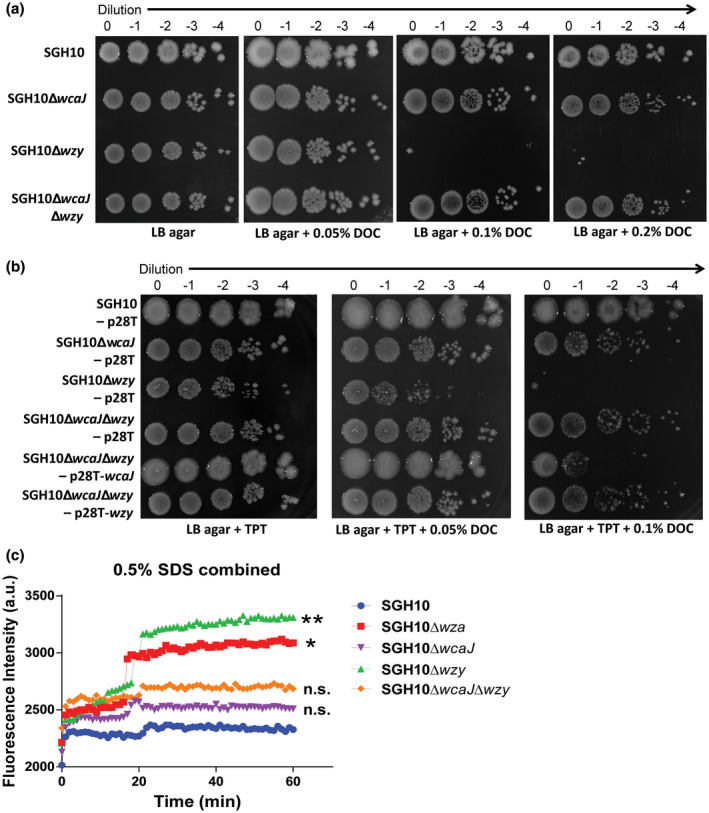
Resistance to bile salt can be rescued in ∆wzy mutant through inactivation of wcaJ. SGH10 and complemented capsule mutants were plated on LB agar supplemented with trimethoprim (TPT) and increasing concentrations of DOC. (a) Bile salt resistance of the ∆wcaJ/wzy mutant compared to the single mutant (b) the ∆wcaJ/wzy mutant complemented with wcaJ or wzy. Experiments were repeated (n = 3), with similar results. (c) Propidium iodide accumulation of SGH10 and capsule mutants during sodium dodecyl sulfate (SDS) stress. Mean ± SD are plotted (n = 3) and Dunnett's multiple comparison test was conducted for a.u. values of the capsule mutants relative to SGH10 wild type at 60 min. * denotes p < .05, ** denotes p < .001, while n.s. denotes not significant [Colour figure can be viewed at wileyonlinelibrary.com]
2.4. Capsule is not required for persistence in the gut
While capsule production may be beneficial for hvKP to survive in the host, its synthesis may pose a significant metabolic burden (Lin et al., 2013). To investigate the role of capsule in hvKP virulence, we compared the fitness of each mutant relative to SGH10∆lacZ in the nutrient‐rich LB media or in PBS supplemented with 10 g/L pig mucin that more closely mimics the condition in the mammalian gut. SGH10∆lacZ was used in place of SGH10 during competitive assays to facilitate blue‐white colony selection. SGH10∆lacZ was first shown to be a viable surrogate for wild type in this in vitro competition assay, as the competitive index of wild type to SGH10∆lacZ was close to 1 throughout the assay (Figure 5a). Using the same in vitro assay, we show that all capsule mutants were as fit as SGH10∆lacZ with the competitive index close to 1 and grew at a similar rate as the SGH10∆lacZ control (Figure 5b–e).
Figure 5.
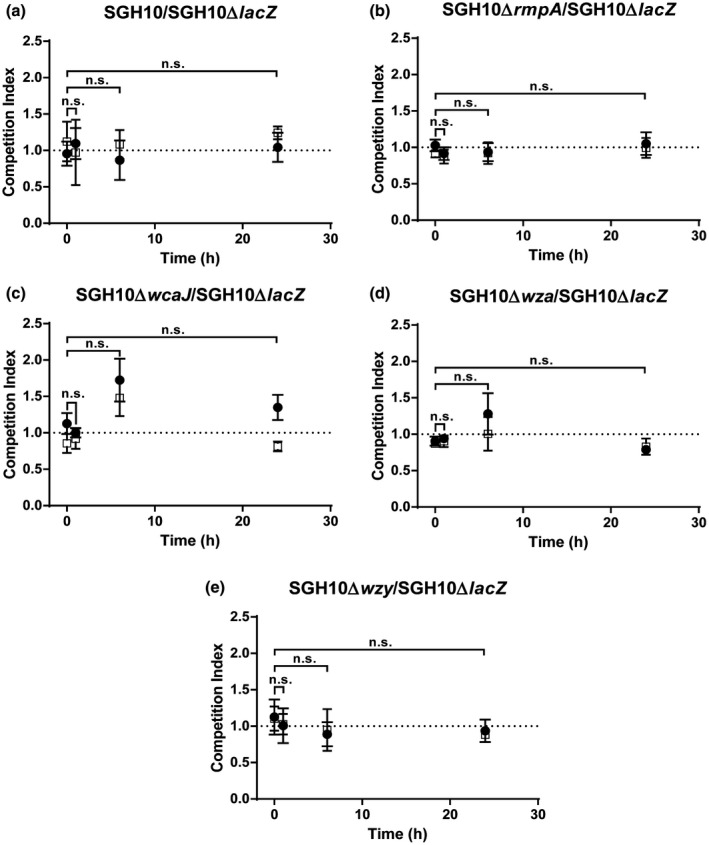
In vitro competition assays to determine relative fitness of mutants was performed between (a) SGH10 and SGH10ΔlacZ, (b) SGH10ΔrmpA and SGH10ΔlacZ, (c) SGH10ΔwcaJ and SGH10ΔlacZ, (d) SGH10Δwza and SGH10ΔlacZ and (e) SGH10Δwzy and SGH10ΔlacZ. Bacteria were inoculated into LB (circles) and 10 g/L pig mucin in PBS (open squares) at a starting ratio of 1:1, and mutant of interest and SGH10ΔlacZ were quantified at appropriate intervals to calculate competitive index (CI). Mean ± SD are plotted (n = 3) and * denotes p < .05, while n.s. denotes not significant. A CI value of 1 is taken to denote that the strains are equally fit. A CI value of <1 indicates that the mutant of interest is less fit than SGH10ΔlacZ, while a CI value of >1 indicates that the mutant is more fit than SGH10ΔlacZ. There is no statistically significant differences in all comparisons
Next, we compared the ability of the capsule mutants and SGH10∆lacZ to persist in the murine gut by conducting an in vivo competition assay. In this competition assay, ampicillin pretreated mice were gavaged with the mutant of interest and SGH10∆lacZ in a ratio of 1:1, and bacteria were plated from stools over the course of the assay. SGH10 was as fit as SGH10∆lacZ in the mouse gut as the competitive index was about 1, which indicates that SGH10∆lacZ is a valid surrogate control for this assay (Figure 6a). SGH10∆rmpA outcompeted SGH10∆lacZ in the mouse gut; the initial competitive index of SGH10∆rmpA: SGH10∆lacZ was 1, but progressively increased to ≈4 over 6 days (Figure 6b).
Figure 6.
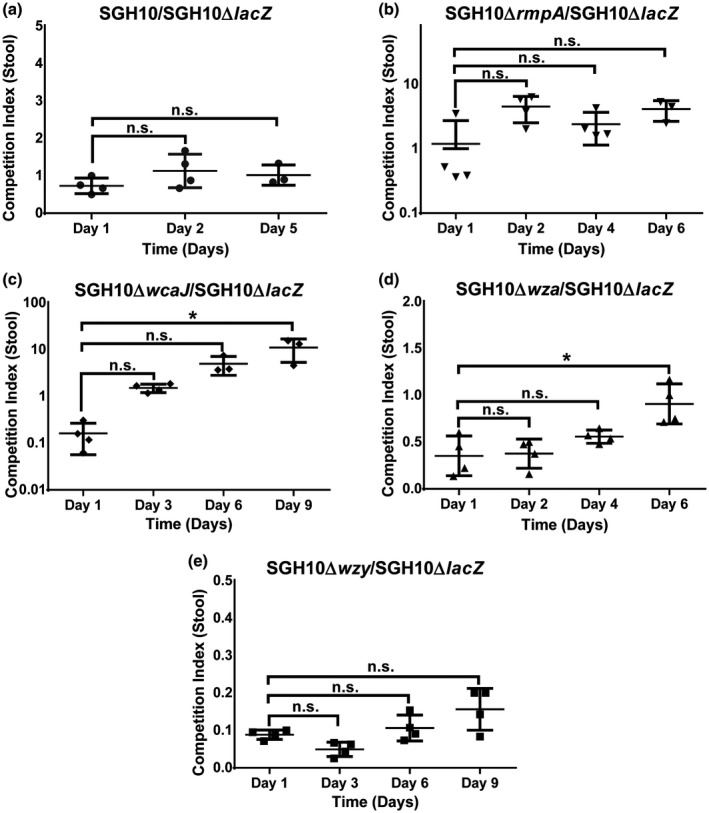
An in vivo competition assay to determine relative fitness of mutants in the mouse gut. (a) SGH10 and SGH10ΔlacZ, (b) SGH10ΔrmpA and SGH10ΔlacZ, (c) SGH10ΔwcaJ and SGH10ΔlacZ, (d) SGH10Δwza and SGH10ΔlacZ and (e) SGH10Δwzy and SGH10ΔlacZ. Bacteria were inoculated into wild‐type C57/BL6J mice via oral gavage at a starting ratio of 1:1 in order to determine if the mutant of interest is less fit than, or outcompetes SGH10ΔlacZ. At appropriate intervals, the mutant of interest and SGH10ΔlacZ were quantified in mouse stool to determine the CI of these strains in the gut. Mean ± Standard Error of the Mean (SEM) were plotted and * denotes p < .05, while n.s. denotes not significant. A CI value of 1 is taken to denote that the strains are equally fit. A CI value of <1 indicates that the mutant of interest is less fit than SGH10ΔlacZ, while a CI value of >1 indicates that the mutant is more fit than SGH10ΔlacZ
SGH10∆wcaJ was initially less fit than wild type as the competitive index of SGH10∆wcaJ: SGH10∆lacZ was 0.1 on day 1. SGH10∆wcaJ eventually outcompeted SGH10∆lacZ; the competitive index was 10 after 9 days, which indicates that capsule is not required to maintain colonisation in the gut, although it aids in initial establishment of colonisation (Figure 6c). SGH10∆wza and SGH10∆wzy were less fit than SGH10∆lacZ. Throughout the in vivo competition assay, the competitive indices of SGH10∆wza and SGH10∆wzy to SGH10∆lacZ were less than 1 (Figure 6d,e). This effect is likely due to SGH10∆wza and SGH10∆wzy harbouring cell envelope defects that result in bile sensitivity, rather than the loss of capsule production or export.
2.5. Role of capsule and rmpA in systemic infection
In many pathogenic bacteria, capsular polysaccharide is important for survival during systemic infection (Geno et al., 2015; Lawlor, Hsu, Rick, & Miller, 2005; Lee et al., 1987; Sahin et al., 2017). In hvKP, this has been shown using ∆wzy mutants (Fang et al., 2004, 2010; Wu et al., 2008). Since this mutant confers additional cell envelope defects, we therefore revisited the effect of capsular polysaccharide on systemic dissemination and survival in the murine model with the ∆wcaJ mutant. C57/BL6J mice were infected with wild‐type, SGH10∆rmpA and SGH10∆wcaJ bacteria via intraperitoneal injection. After 30 hr, the mice were sacrificed and the lungs, liver and spleen were harvested, homogenised and plated to enumerate the number of hvKP in each organ. Our results show that SGH10∆rmpA could disseminate in the bloodstream and was detected at comparable organ loads with SGH10 WT except in the liver (Figure 7a–c). SGH10∆wcaJ organ loads were significantly less than wild type, indicating that this strain is more susceptible to clearance by the immune system during systemic infection (Figure 7a–c). All wild type‐infected mice were hunched and exhibited limited mobility and had to be euthanised according to our disease scoring system, whereas the capsule‐null mutant‐infected mice were healthy at the end of the experiment. The ∆rmpA mutant‐infected mice had an intermediate phenotype, with only two out of six mice appearing to be hunched and with limited mobility. Together, the results confirm the critical role of the K1 capsule in hvKP pathogenesis.
Figure 7.
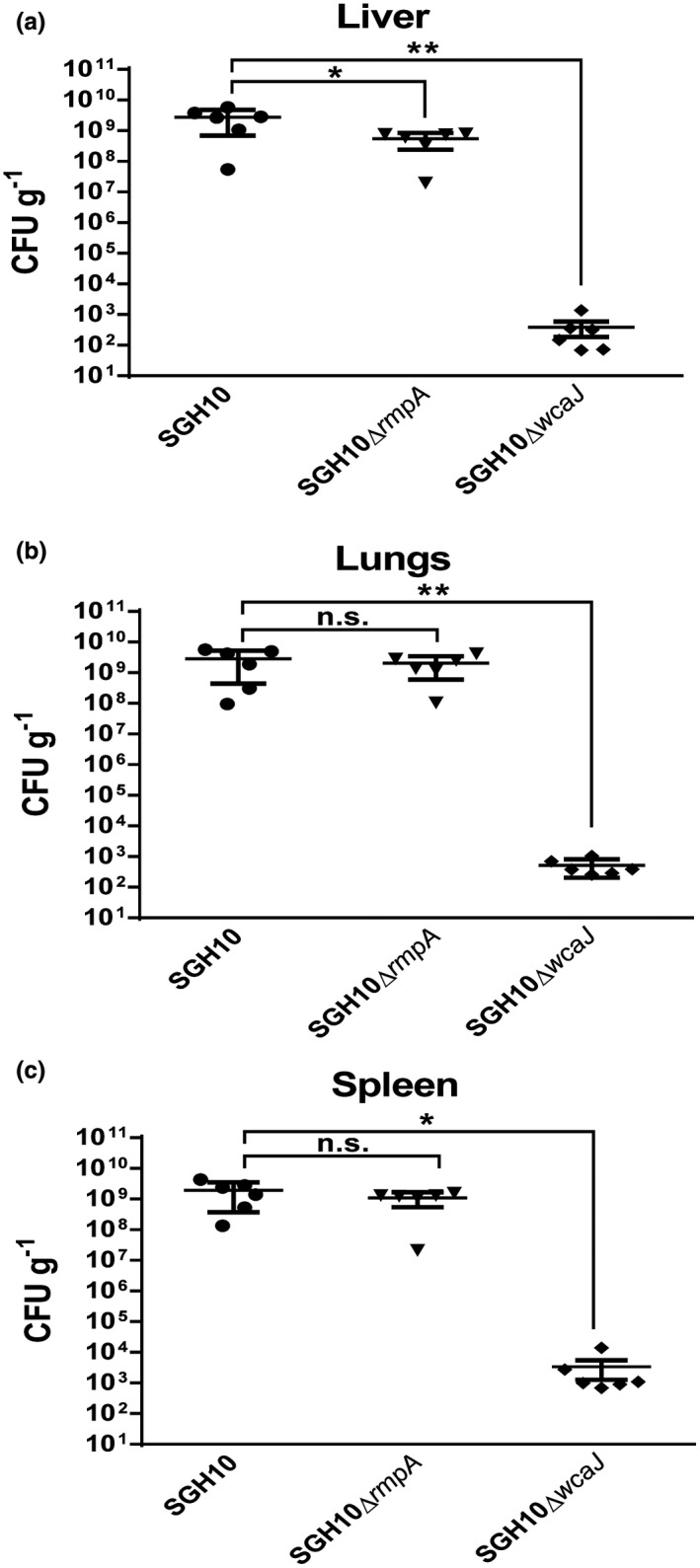
Intraperitoneal infection of C57BL/6J mice with SGH10, SGH10ΔrmpA and SGH10ΔwcaJ. (a) Bacterial loads in the liver, (b) lungs and (c) spleen were measured by plating at 30 hr post infection. Mean ± SEM are plotted and * denotes p < .05, while ** denotes p < .001
3. DISCUSSION
K. pneumoniae is proposed to be of “critical priority” for research and development of new antibiotics on the list of the World Health Organization priority pathogens (Tacconelli et al., 2018). Among various strains and isolates of K. pneumoniae, hvKP is particularly dangerous because it has the potential to acquire multi‐drug resistance and is capable of causing serious diseases like liver abscess, pneumonia and sepsis in relatively healthy individuals (Araújo et al., 2018; Chew, Lin, Teo, & Holt, 2017; Lee, Lee, Park, & Jeon, 2017; Roulston et al., 2018; Shon et al., 2013; Yao et al., 2015). The capsule of hvKP is a key virulence factor that aids dissemination to the host bloodstream and systemic infection (Alvarez et al., 2000; Cortés et al., 2002; Fang et al., 2004). Both spontaneous and specific capsule mutants for various capsule serotypes and mouse infection models have been used to study the role of K. pneumoniae capsule (Supporting Information Table S1). Different capsule mutants could give rise to divergent phenotypes due to different infection models as well as the properties of the mutants, given that capsule synthesis and export is a multi‐gene and complicated assembly line. We decided to examine the effect of using different isogenic capsule‐null mutants, particularly the commonly employed wzy and wza deletion mutants, in comparison with the wcaJ mutant, on bacterial pathogenesis, particularly in gut colonisation, as this has not been well established.
The thick capsule of hvKP could potentially prevent hvKP from adhering to the gut mucosa, causing it to be easily dislodged by peristalsis. While one study suggested that K. pneumoniae capsule reduced adhesion to ileocecal and bladder cells (Sahly et al., 2000), others have shown that K. pneumoniae capsule could enhance association to lung epithelial cells and intestinal epithelial cell lines (Clements et al. 2008; Favre‐Bonte et al., 1999). We show that the thick capsule of hvKP impedes its adhesion to colonic cell line Caco‐2 (Figure 2b). However, as hvKP grows at a relatively fast rate, clearance of weakly adhering bacteria through peristalsis may not significantly reduce colonisation of the gut. Struve and Krogfelt previously used noncapsulated variants of K. pneumoniae to show that capsule is nonessential for gastrointestinal colonisation of streptomycin‐treated mice (Struve & Krogfelt, 2003).
To definitively answer the question of whether the capsule is essential for gut colonisation, we constructed three isogenic capsule‐null mutants SGH10∆wcaJ, SGH10∆wza and SGH10∆wzy, as well as the capsule regulator mutant SGH10∆rmpA. By comparing these mutants and their phenotypes in different infection models, we sought to identify the changes that are solely attributed to the loss of capsule. Deletion of wza will lead to the inability to export capsular polysaccharide (Wei, Yuminaga, Shi, & Jian, 2018), while ∆wzy deletion will stop polymerisation of the capsule precursor (Ho et al., 2011). Both the ∆wza and ∆wzy mutants will accumulate capsule intermediates. However, in SGH10∆wcaJ, the capsule intermediates are not synthesised, and hence cells are less likely to exhibit membrane stress. Indeed, SEM showed that ∆wza and ∆wzy mutants exhibited aberrant cell shapes. These alterations are absent in the wild‐type as well as the ∆wcaJ mutant, suggesting inhibition of intermediate steps in capsule synthesis will result in cell envelope defects. Consistent with this observation, ∆wza and ∆wzy mutants are sensitive to bile salts, whereas the ∆wcaJ mutant is not.
We hypothesise that either the accumulation of dead‐end capsular polysaccharide precursors or sequestration of Und‐P caused the envelope defect in hvKP. In E. coli, the enterobacterial common antigen (ECA), O‐antigen and peptidoglycan synthesis pathways all compete for a single pool of Und‐P (Hartley & Imperiali, 2012; Hug & Feldman, 2011; Valvano, 2008). Genetic inactivation of factors required for intermediate steps of the ECA pathway induces morphological defects and swelling. This is due to the build‐up of ECA lipid II that sequesters the pool of Und‐P for peptidoglycan synthesis (Jorgenson & Young, 2016) because overexpression of Und‐P synthase UppS largely rescued the phenotype. Similarly, E. coli spheroplasts can resynthesise an intact cell wall unless colanic acid biosynthesis is interrupted by mutations downstream of the initial glycosyltransferase wcaJ, leading to spheroplast lysis (Ranjit & Young, 2016). In this case, it is the accumulation of colanic acid intermediates that inhibits spheroplast recovery because an increased level of UppS did not prevent lysis but deletion of wcaJ did. In K. pneumoniae, we found that the aberrant cell envelope in wzy and wza mutants did not change the growth rates, which indicates hvKP somehow can tolerate a lower level of Und‐P or there is an unidentified feedback loop that stops capsule production if the level of Und‐P becomes dangerously low. Our data with the double mutant of wzy and wcaJ, as well as complementation of the double mutant with either wzy or wcaJ show that the cell envelope defects are due to the accumulation of capsule intermediates. Whether the defects are due to toxic capsule intermediates, or to the sequestration and therefore depletion of Und‐P for peptidoglycan synthesis is unclear. Interestingly, the ∆wza mutant showed more resistance to bile salt and greater fitness in the gut compared to the ∆wzy mutant. If sequestration of Und‐P is the sole reason for cell envelope defects, these two mutants should have the same phenotype. Therefore, it is possible that the capsule intermediates in the ∆wzy mutant are contributing to cell wall defects. Together, our results show that ∆wza and ∆wzy deletion mutants should not be used to investigate the role of capsule in virulence due to secondary effects on cell envelope stability.
In this study, we clearly observed differences between the ∆wcaJ and the ∆wza or ∆wzy mutants in the gut. Strains SGH10∆wza and SGH10∆wzy have a competitive index <1 throughout the in vivo competition assay, demonstrating their poor ability to colonise and persist in the gut. On the other hand, SGH10∆wcaJ showed an initial disadvantage compared to the wild‐type hvKP, but this handicap was quickly rectified and eventually the unencapsulated mutant outcompeted the wild‐type bacteria in the gut. This outgrowth of the unencapsulated mutant at later points could be due to the fitness cost associated with the synthesis of the capsule. In contrast, the intraperitoneal infection model showed that the ∆wcaJ mutant had a drastic reduction in virulence and was readily cleared by the host immune system. The data agree with the ∆wcaJ mutant in a K. pneumoniae K7 strain, where it showed a decrease in virulence in the murine model of intranasal infection (Liu, Han, & Gu, 2019).
The hypermucoid nature of hvKP is contributed by rmpA which is typically encoded on the large virulence plasmid in hvKP, although it can also be found on the chromosome (Cheng et al., 2010; Hsu et al., 2011; Struve et al., 2015). RmpA regulates the expression of capsule genes by interacting with the phosphorelay response regulator RcsB (Hsu et al., 2011; Nassif, Fournier, Arondel, & Sansonetti, 1989). Plasmid‐encoded rmpA has also been shown to increase capsule mucoviscosity by increasing the expression of colanic acid in Escherichia coli (Nassif, Honore, Vasselon, Cole, & Sansonetti, 1989). As such, it has been postulated that this gene increases the expression of group I capsule in K. pneumoniae since the expression of colanic acid and group I capsule are mutually exclusive and the same initial rcsB binding site is present (Cheng et al., 2010; Hsu et al., 2011; Lai, Peng, & Chang, 2003). Previous work with the serotype K2 strain CG43 and the K1 strain NTUH‐K2044 concluded that RmpA is important for systemic infection in the former but not the latter (Cheng et al., 2010; Hsu et al., 2011; Nassif, Fournier, et al., 1989). The intraperitoneal infection by NTUH‐K2044 was performed in a competitive assay with wild‐type bacteria, showing that a competitive index of >5 when bacteria were plated from the liver at 24 hr post infection. This competitive advantage of the ∆rmpA mutant is similar to our observations with the SGH10∆rmpA mutant in competition with wild‐type bacteria during gut colonisation. This indicates that the ∆rmpA mutant could have a growth advantage not only in the gut but also in the liver. However, in our intraperitoneal infection of 30 hr, we found that the bacterial loads in the liver were lower in the ∆rmpA mutant compared to the wild type‐infected mice, although the bacterial loads were similar in other organs. This discrepancy could be due to strain differences because NTUH2044 has a functional chromosomal copy of ∆rmpA as well as the plasmid copy. The competitive advantage of ∆rmpA in the liver could be due to initial bystander protection mediated by the wild type. Our intraperitoneal infection data on the ∆rmpA mutant is in line with a previous study using intraperitoneal infection (Nassif, Fournier, et al., 1989). Walker et al. (2019) showed that the ∆rmpA mutant was less virulent than wild type during intranasal infection and bacterial loads in the lungs were significantly reduced. In the intraperitoneal route of infection, we did not see a decrease in lung bacterial loads with the ∆rmpA mutant, but overall the disease was less severe in these mice compared with those infected wild‐type SGH10. This suggests that rmpA plays a smaller role in the pathogenesis of SGH10.
Bile salts are produced in the liver and subsequently transported to the intestinal tract (Pellicoro & Faber, 2007; Russell, 2009). Given hvKP’s propensity to colonise the gut and cause liver disease, the ability to resist bile stress is likely crucial. For most Enterobacteriaceae including Klebsiella, resistance to bile stress requires an intact outer membrane (Merritt & Donaldson, 2009; Urdaneta & Casadesús, 2017; Vianney, Lazzaroni, & Germon, 1999), as no bile salt hydrolases have been reported thus far in Klebsiella. SGH10∆wcaJ is resistant to bile stress, which indicates that the capsule is not required for resistance to bile stress. Both ∆rmpA and ∆wcaJ mutants exhibited a competitive index of <1 in less than one day post inoculation. This suggests that the lower ability to initiate colonisation without a capsule or with a nonhypermucoid capsule is not related to bile salt stress but could be due to other physical or chemical factors initially encountered in the gut environment. However, the mutants were able to gain the upper hand quickly and outcompeted the wild‐type bacteria, indicating that capsule is not required for colonisation persistence, and in fact might represent a metabolic burden in the gut environment. Given that RmpA is able to regulate many other genes besides capsule, these other genes may additionally impede colonisation persistence (Hsu et al., 2011; Walker et al., 2019).
In conclusion, we advise caution in the interpretations of phenotypic results of K. pneumoniae ∆wza and ∆wzy mutants that are attributable to loss of capsule as these mutants have additional cell envelope defects due to accumulation of capsule intermediates that was not previously appreciated. Our work establishes that capsule is not essential for the persistence of K1 hvKP in the gut although it is important for systemic infection.
4. EXPERIMENTAL PROCEDURES
4.1. Maintenance of bacterial cultures
SGH10 was originally isolated from a liver abscess patient and is a reference strain for K. pneumoniae CG23 sublineage 1 deposited at the National Collection of Type Cultures at the United Kingdom (NCTC 14052) (Lam et al., 2018; Lee et al., 2016).
SGH10ΔrmpA, SGH10Δwza, SGH10Δwzy, SGH10ΔwcaJ and SGH10ΔlacZ are clean deletion mutants generated in this study. All bacterial strains were cultured in Lysogeny broth (LB) and on LB agar plates and 30 μg/ml trimethoprim (Sigma‐Aldrich) was used to maintain the pUCP28T plasmid (p28T) (West et al., 1994).
4.2. Density‐based separation of bacteria
Two millilitres of 50%, 35% and 15% Percoll in 1× PBS (Sigma‐Aldrich) were aliquoted into a 15 ml falcon tube from highest to lowest density. The strain of interest measuring 1.8 × 1011 CFU in 600 μl of 1× PBS was added dropwise to the tube. The tubes were then centrifuged at 3,000 × g for 30 min and imaged against a black background.
4.3. Low‐speed centrifugation of bacteria
Six millilitres of 109 CFU/ml of the strain of interest in LB were prepared in 15 ml falcon tubes. The tubes were then centrifuged at 2,000 × g for 10 min and imaged against a black background. Optical density 600 nm (O.D. 600 nm) readings of the supernatant were then taken using a BioMateTM spectrophotometer (Thermo Scientific).
4.4. FITC–dextran exclusion assays
Overnight cultures of the strain of interest were grown in LB and resuspended in 1× PBS at 1010 CFU/ml. One microlitre of bacterial culture and 1 μl of 10 mg/ml Fluorescein isothiocyanate (FITC)–dextran in 1× PBS (Sigma Aldrich) were mixed and spotted onto a glass slide and covered with a glass coverslip. Bacteria were imaged for differential contrast (Brightfield) and fluorescence using an Olympus IX81 inverted microscope equipped with an Olympus DP26 camera for image acquisition with a 100X oil immersion objective lens. Images were formatted and analysed using ImageJ. Bacterial capsule appears as a grey shadow against the green background of FITC–dextran in the merged image.
4.5. Mammalian cell culture
Mammalian cells were maintained in a CO2 incubator (Themo Scientific) at 37°C with 5% CO2. RAW 264.7 cells (ATCC) were grown in Dulbecco's modified Eagle's medium (DMEM) (Life Technologies) supplemented with 10% fetal bovine serum (FBS) (Singlab) and 1X penicillin–streptomycin (Singlab). Caco‐2 cells (ATCC) were grown in DMEM supplemented with 20% FBS, 1X penicillin–streptomycin and 1X nonessential amino acids (Sigma‐Aldrich).
4.6. Serum resistance assay
Twenty‐five microlitres of 2.5 × 105 CFU of K. pneumoniae in 1× PBS (Thermo Scientific) were inoculated into wells of a 96‐well plate containing 75 μl of 100% pooled normal human serum (Sigma‐Aldrich). Control serum was inactivated by heating at 56°C for 30 min prior to incubation of bacteria. The plate was then incubated at 37°C. At 0, 1, 2 and 3 hr post inoculation, appropriate dilutions were plated on LB agar to enumerate surviving bacterial colony‐forming units (CFU).
4.7. Bacterial phagocytosis and adhesion assays
For phagocytosis assays, RAW 264.7 cells were seeded at 0.125 million cells in a 24‐well plate and allowed to attach overnight. Cells were then washed with 1× PBS and 0.5 ml fresh DMEM + 10% FBS was added. K. pneumoniae was added to achieve a Multiplicity of Infection (MOI) of 1:1 and the plate was centrifuged at 250 × g for 5 min. After 1 hr, kanamycin was added to give a final concentration of 500 μg/ml kanamycin. At 2 hr post infection, cells were lysed with 0.2% Triton‐X100 in 1× PBS (Sigma‐Aldrich) and appropriate dilutions were plated on LB agar to enumerate bacterial CFU.
For cell adhesion assays, 0.25 million Caco‐2 cells/well were seeded in a 24‐well plate and allowed to attach overnight. Cells were then washed with 1× PBS and fresh DMEM + 10% FBS was added. K. pneumoniae was added to the well to give an MOI of 10:1 and the plate was centrifuged at 250 × g for 5 min. After a 30 min incubation, cells were washed twice with 1× PBS and lysed with 0.2% Triton‐X100 in 1× PBS (Sigma‐Aldrich). Appropriate dilutions were plated on LB agar to enumerate bacterial CFU.
4.8. In vitro bacterial competition assays
SGH10ΔlacZ, 103 CFU, and 103 CFU of the mutant of interest (initial ratio of 1:1) were inoculated in 5 ml of 10 g/L mucin in 1× PBS or LB in a 50 ml falcon tube and incubated at 37°C with shaking. At 0, 1, 6 and 24 hr, appropriate dilutions of the bacterial culture were plated on LB agar spread with 40 μl of 1 mM Isopropyl β‐D‐1‐thiogalactopyranoside (IPTG) and 40 μl of 20 mg/ml 5‐bromo‐4‐chloro‐3‐indolyl‐D‐galactopyranoside (X‐gal). SGH10ΔlacZ is unable to degrade X‐gal and hence produces white colonies, while the competing strain appears as blue colonies. The ratio of competing strain: SGH10ΔlacZ was then calculated to give the Competitive Index (CI) value. A CI value of <1 indicates that the mutant of interest is less fit than wild type, while a CI value of >1 indicates that the mutant of interest outcompetes the wild type.
4.9. Bacterial colony‐forming unit assay
LB agar plates supplemented with appropriate concentrations of sodium deoxycholate (DOC), sodium cholate (CHO) and LB agar adjusted to pH4 with hydrochloric acid were prepared by boiling agar with the appropriate detergent in a water bath. Overnight cultures of bacteria were diluted in 1× PBS and 5 μl of bacterial suspension were spotted on each plate. Tenfold dilutions were plated in each drop starting with 105 CFU–10 CFU, for a minimum of eight plates per condition. The plates were allowed to dry before overnight incubation in a 37°C incubator and imaged using a BioRad ChemiDocTM MP Imaging System.
4.10. Propidium iodide accumulation assays
Bacteria were grown on LB agar plates to mid‐log phase (4–5 hr) and washed off with 1× PBS. Bacterial concentration was adjusted to O.D. 600 nm = 0.5. RNase A (final concentration of 4 μg/ml) and propidium iodide (final concentration of 2 μg/ml) were added to bacterial suspensions. The suspensions were aliquoted in Corning Clear Bottom Black Side plate at 90 μl per well in triplicates. Sodium dodecyl sulfate (SDS) was added to each well to achieve a final concentration of 0.5%. The plate was read and recorded every minute for 1 hr (excitation 305 nm, emission 617 nm) and fluorescence intensity was captured in arbitrary units (a.u.).
4.11. Construction of gene deletion mutants in K. pneumoniae
Clean deletion mutants were generated in rmpA (SGH10_RS27810), wza (SGH10_RS08555), wzy (SGH10_RS08575), wcaJ (SGH10_RS08610) and lacZ (SGH10_RS14285). For generation of gene deletion mutants in K. pneumoniae, a conditional suicide vector was constructed by replacing the pMB1 replication origin in pK18mobsacB (Kvitko & Collmer, 2011) with the R6K replication origin from EZ‐Tn5™ <R6Kγ ori/KAN‐2 > Transposon (Epicentre) to generate pR6KmobsacB. To delete a gene in K. pneumoniae, ∼1000‐bp fragments upstream and downstream from the gene of interest were PCR amplified from genomic DNA templates with Q5 High‐Fidelity DNA Polymerase (New England Biolabs) and then assembled in the pR6KmobsacB vector with NEBuilder® HiFi DNA Assembly Master Mix (New England Biolabs). A list of all primers used and their sequences can be found in Table 1.
Table 1.
List of the primers used for cloning and mutant generation
| Primer name | Sequence |
|---|---|
| wcaJ Up‐for | ACATGATTACGAATTCTGCCTCTGGTAAAGGAGTGC |
| wcaJ Up‐rev | GCATCTTAGAAGATTTCCTTAAAATATTGAACCTT |
| wcaJ Dn‐for | AAATCTTCTAAGATGCTGCTTAAGATAAGCATTGT |
| wcaJ Dn‐rev | TTGCATGCCTGCAGAGCTCTGGCTGGTCCACTTA |
| wza Up‐for | ACATGATTACGAATTCTCTCCGCCAGCCAAATGAAT |
| wza Up‐rev | ATTTAAACATAATGTCACATCATTAGTAAACCAAGATTGC |
| wza Dn‐for | TGTGACATTATGTTTAAATCAGTTTTAGTTGTTTGTATTGG |
| wza Dn‐rev | CTTGCATGCCTGCAGTCGCCGGCATCATCAAGATT |
| wzy Up‐for | ACATGATTACGAATTCTGCCCTGATGCCTTTAGTTT |
| wzy Up‐rev | CTTTTTGCATCAAATAAAATCACACATTGCATCATTACC |
| wzy Dn‐for | TTTATTTGATGCAAAAAGATTTAAAATAATAGAGGAAGAACAAT |
| wzy Dn‐rev | CTTGCATGCCTGCAGCAACCCGGTCTGTAATGCCT |
| lacZ Up‐for | TATGACATGATTACGAATTCGCCATCTGATCGTTTGCCAC |
| lacZ Up‐rev | CCACGGATTACATATTAAACCCCGGTAAGT |
| lacZ Dn‐for | GTTTAATATGTAATCCGTGGGGGCGACAGC |
| lacZ Dn‐rev | CCAAGCTTGCATGCCTGCAGCCGAGAATACGCACCGACAT |
| rmpA Up‐for | CTTGCATGCCTGCAGCTTTAGTTAAGGCGGCCTTCG |
| rmpA Up‐rev | CTAGAGGATCCCCGGGTACCCATAGAAACAGTAACTTTGATCCATCAATATTCATCC |
| rmpA Dn‐for | CCCGGGGATCCTCTAGAGTCTAGGTAAAAAAGGGGAGGGGATG |
| rmpA Dn‐rev | ACATGATTACGAATTCTGAGCCAAATGTATGCCAAGG |
| wcaJ‐for | ACATGATTACGAATTCCGCATCACTTGATATGAATAATGTACT |
| wcaJ_rev | TTGCATGCCTGCAGGGTTGAAAACGGAGACGGTA |
| wza‐for | ACATGATTACGAATTCCCAGCGCAGGGATAGAAATA |
| wza‐rev | TTGCATGCCTGCAGCCGATCTTGAAATCTTTCAGTCTT |
| wzy‐for | ACATGATTACGAATTCAACTTGAACGAGCAATTCAATC |
| wzy‐rev | TTGCATGCCTGCAGATGGCCATTTGCGTTAGTACA |
Plasmids were introduced into K. pneumoniae via conjugation from E. coli donor strain S17‐1λpir. Single crossover transconjugants were selected on medium containing kanamycin (50 μg/ml) and donor E. coli was removed by carbenicillin (100 μg/ml) in medium. Kanamycin‐resistant, single crossovers were passaged in LB medium lacking sodium chloride plus 20% sucrose to counter select the sacB gene on the pR6KmobsacB backbone. Kanamycin‐sensitive, double crossovers were screened for successful deletion of the gene of interest by polymerase chain reaction. To create ΔwcaJ Δwzy double mutant, the wzy deletion construction in pR6K plasmid was introduced into ΔwcaJ single mutant. Sucrose‐counterselection and double‐crossover screening were carried out as above.
4.12. Scanning electron microscopy
Overnight cultures of bacteria were pelleted at 2,000 × g for 7 min and washed with 1× PBS (pH 7.3). The bacteria were then fixed in 2.5% glutaraldehyde (Sigma‐Aldrich) in 1× PBS (pH 7.3) for 2 hr at 4°C. Subsequently, bacteria were washed with 1× PBS (pH 7.3) twice with same centrifugal conditions at 2,000 × g, 7 min. Samples were stored at 4°C. The bacteria samples were prepared by spinning at 2,000 × g for 7 min, fixed onto sample stub for gold sputtering and imaged using scanning electron microscopy. Microscopy imaging was done with the assistance of the Electron Microscopy Unit of Yong Loo Lin School of Medicine, National University of Singapore.
4.13. Animal work
For all experiments, female C57 BL/6 JAX® mice aged 7–8 weeks were purchased from InVivos. For the in vivo bacterial competition assays, mice were orally gavaged with 2.5 mg of ampicillin sodium salt (Sigma‐Aldrich) in 100 μl 1× PBS daily for 5 days. On the subsequent day, 2.5 × 106 CFU of SGH10ΔlacZ and 2.5 × 106 CFU of the mutant of interest (a ratio of 1:1) was prepared in 100 μl 1× PBS and inoculated into mice via oral gavage. At suitable time points, stools were collected from each mouse. Serial dilutions of homogenised stool in 1× PBS were plated on Klebsiella‐selective agar (Sigma‐Aldrich) spread with 40 μl of 1 mM IPTG and 40 μl of 20 mg/ml X‐gal. SGH10ΔlacZ is unable to degrade X‐gal and hence produces white colonies, while the competing strain appears as blue colonies. The ratio of competing strain: SGH10ΔlacZ was then calculated to give the CI value.
For intraperitoneal infection of mice, 105 CFU of each strain was prepared in 100 μl 1× PBS and inoculated into mice via intraperitoneal injection. At 30 hr post infection, mice were sacrificed and serial dilutions of homogenised liver, lungs and spleen in 1× PBS were plated on Klebsiella‐selective agar.
4.14. Ethics statement
The protocol and procedures employed for animal work in this study were ethically reviewed and approved by the NUS Institutional Animal Care and Use Committee (IACUC) in the animal facility at Comparative Medicine, National University of Singapore (NUS). The care and use of animals for research and teaching in NUS is bound by the Singapore Animals and Birds Act, Animals and Birds (Care and Use of Animals for Scientific Purposes) Rules 2004 and is carried out in accordance with the National Advisory Committee for Laboratory Animal Research (NACLAR) Guidelines. For this study, animals were used under Protocols R15‐135 and R18‐0252 as approved by the NUS IACUC.
4.15. Statistical methods
All graphical and numerical data were plotted using Graphpad Prism 6.0 (GraphPad Software, La Jolla California USA, www.graphpad.com). Student's t‐test was used to compare means and standard deviations (SD) and Dunnett's multiple comparison's test was used to compare means relative to wild‐type bacteria. A p value of < .05 is considered statistically significant and denoted as * and a p value < .001 is denoted as **.
AUTHOR CONTRIBUTIONS
YHT and YHG conceptualised and designed the study. YHT performed all the experiments with the aid of YC for the generation of bacterial mutants and WHWC for animal work and SEM. YHT, LTS and YHG analysed data. YHT and YHG wrote the manuscript with contributions from the rest. All authors approved and vetted this manuscript.
Supporting information
ACKNOWLEDGMENTS
The work is supported by the National University of Singapore, Yong Loo Lin School of Medicine Aspiration Fund (NUHSRO/2014/068/AF New Idea/03) and Ministry of Education (MOE2013‐T3‐1‐002) to Y‐H G. L‐T S. is supported by the start‐up grant from the National University of Singapore and the National Research Foundation Singapore (NRF‐NRFF‐2019‐0005). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors have declared that no competing interests exist. We would like to thank the Electron Microscopy Unit of Yong Loo Lin School of Medicine, National University of Singapore for assistance with Scanning Electron Microscopy.
Tan YH, Chen Y, Chu WHW, Sham L‐T, Gan Y‐H. Cell envelope defects of different capsule‐null mutants in K1 hypervirulent Klebsiella pneumoniae can affect bacterial pathogenesis. Mol Microbiol. 2020;113:889–905. 10.1111/mmi.14447
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alsaif, H. S. , Sudhakar, K. S. K. , Chan, D. S. G. , & Archuleta, S. (2011). CT appearance of pyogenic liver abscesses caused by Klebsiella pneumoniae . Radiology, 260(1), 129–138. [DOI] [PubMed] [Google Scholar]
- Alvarez, D. , Merino, S. , Tomas, J. M. , Benedi, V. J. , & Alberti, S. (2000). Capsular polysaccharide is a major complement resistance factor in lipopolysaccharide O side chain‐deficient Klebsiella pneumoniae clinical isolates. Infection and Immunity, 68(2), 953–955. 10.1128/IAI.68.2.953-955.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni, L. , Nuding, S. , Weller, D. , Gersemann, M. , Ott, G. , Wehkamp, J. , & Stange, E. F. (2013). Human colonic mucus is a reservoir for antimicrobial peptides. Journal of Crohn’s and Colitis, 7(12), e652–e664. 10.1016/j.crohns.2013.05.006 [DOI] [PubMed] [Google Scholar]
- Araújo, B. F. , Ferreira, M. L. , Campos, P. A. D. , Royer, S. , Gonçalves, I. R. , da Fonseca Batistão, D. W. , … Ribas, R. M. (2018). Hypervirulence and biofilm production in KPC‐2‐producing Klebsiella pneumoniae CG258 isolated in Brazil. Journal of Medical Microbiology, 67, 523–528. 10.1099/jmm.0.000711 [DOI] [PubMed] [Google Scholar]
- Bilal, S. , Volz, M. S. , Schneider, T. , Fideler, T. , & Podschun, R. (2014). Klebsiella pneumoniae—Induced liver abscesses, and implications for blood supply safety, Australia. Emerging Infectious Diseases, 20(11), 1939–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos, M. A. , Vargas, M. A. , Regueiro, V. , Llompart, C. M. , Alberti, S. , & Bengoechea, J. A. (2004). Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infection and Immunity, 72(12), 7107–7114. 10.1128/IAI.72.12.7107-7114.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H. Y. , Chen, Y. S. , Wu, C. Y. , Chang, H. Y. , Lai, Y. C. , & Peng, H. L. (2010). RmpA regulation of capsular polysaccharide biosynthesis in Klebsiella pneumoniae CG43. Journal of Bacteriology, 192(12), 3144–3158. 10.1128/JB.00031-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew, K. L. , Lin, R. , Teo, J. W. P. , & Holt, K. E. (2017). Klebsiella Pneumoniae in Singapore: Hypervirulent infections and the carbapenemase threat. Frontiers in Cellular and Infection Microbiology, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, D. R. , Lee, H. , Park, M. H. , Jung, S.‐I. , Chang, H.‐H. , Kim, Y.‐S. , … Song, J.‐H. (2012). Fecal carriage of serotype K1 Klebsiella pneumoniae ST23 strains closely related to liver abscess isolates in Koreans living in Korea. Europe Journal of Clinical Microbiology and Infectious Disease, 31, 481–486. 10.1007/s10096-011-1334-7 [DOI] [PubMed] [Google Scholar]
- Clements, A. , Gaboriaud, F. , Duval, J. F. L. , Farn, J. L. , Jenney, A. W. , Lithgow, T. , … Strugnell, R. A. (2008). The major surface‐associated saccharides of Klebsiella pneumoniae contribute to host cell association. PLoS ONE, 3(11), 1–10. 10.1371/journal.pone.0003817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés, G. , Borrell, N. , de Astorza, B. , Gómez, C. , Sauleda, J. , & Albertí, S. (2002). Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infection and Immunity, 70(5), 2583–2590. 10.1128/IAI.70.5.2583-2590.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elia, M. A. , Pereira, M. P. , Chung, Y. S. , Zhao, W. , Chau, A. , Kenney, T. J. , … Brown, E. D. (2006). Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. Journal of Bacteriology, 188(12), 4183–4189. 10.1128/JB.00197-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, C.‐T. , Chuang, Y.‐P. , Shun, C.‐T. , Chang, S.‐C. , & Wang, J.‐T. (2004). A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. The Journal of Experimental Medicine, 199(5), 697–705. 10.1084/jem.20030857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, C.‐T. , Lai, S.‐Y. , Yi, W.‐C. , Hsueh, P.‐R. , & Liu, K.‐L. (2010). The function of Wzy_K1 (MagA), the serotype K1 polymerase gene in Klebsiella pneumoniae Cps gene cluster. The Journal of Infectious Diseases, 201(8), 1268–1269. [DOI] [PubMed] [Google Scholar]
- Favre‐Bonte, S. , Joly, B. , & Forestier, C. (1999). Consequences of reduction of Klebsiella pneumoniae capsule expression on interactions of this bacterium with epithelial cells. Infection and Immunity, 67(2), 554–561. 10.1128/IAI.67.2.554-561.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, C.‐P. , Lin, Y. T. , Lin, J. C. , Chen, T. L. , Yeh, K. M. , Chang, F. Y. , … Siu, L. K. (2012). Klebsiella pneumoniae in gastrointestinal tract and pyogenic liver abscess. Emerging Infectious Diseases, 18(8), 1322–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geno, K. A. , Gilbert, G. L. , Song, J. Y. , Skovsted, I. C. , Klugman, K. P. , Jones, C. , … Nahm, M. H. (2015). Pneumococcal capsules and their types: Past, present, and future. Clinical Microbiology Reviews, 28(3), 871–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley, M. D. , & Imperiali, B. (2012). At the membrane frontier: A prospectus on the remarkable evolutionary conservation of polyprenols and polyprenyl‐phosphates. Archives of Biochemistry and Biophysics, 517(2), 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, J.‐Y. , Lin, T.‐L. , Li, C.‐Y. , Lee, A. , Cheng, A.‐N. , Chen, M.‐C. , … Tsai, M.‐D. (2011). Functions of some capsular polysaccharide biosynthetic genes in Klebsiella pneumoniae NTUH K‐2044. PLoS ONE, 6(7), 1–9. 10.1371/journal.pone.0021664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, C.‐R. , Lin, T.‐L. , Chen, Y.‐C. , Chou, H.‐C. , & Wang, J.‐T. (2011). The role of Klebsiella pneumoniae RmpA in capsular polysaccharide synthesis and virulence revisited. Microbiology, 157(12), 3446–3457. 10.1099/mic.0.050336-0 [DOI] [PubMed] [Google Scholar]
- Hug, I. , & Feldman, M. F. (2011). Analogies and homologies in lipopolysaccharide and glycoprotein biosynthesis in bacteria. Glycobiology, 21(2), 138–151. 10.1093/glycob/cwq148 [DOI] [PubMed] [Google Scholar]
- Jorgenson, M. A. , Kannan, S. , Laubacher, M. E. , & Young, K. D. (2017). Dead‐end intermediates in the enterobacterial common antigen pathway induce morphological defects in Escherichia coli by competing for undecaprenyl phosphate. Molecular Microbiology, 100(1), 501–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson, M. A. , & Young, K. D. (2016). Interrupting biosynthesis of O antigen or the lipopolysaccharide core produces morphological defects in Escherichia coli by sequestering undecaprenyl phosphate. Journal of Bacteriology, 198(22), 3070–3079. 10.1128/JB.00550-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontopoulou, K. , Iosifidis, E. , Antoniadou, E. , Tasioudis, P. , Petinaki, E. , Malli, E. , … Malisiovas, N. (2019). The clinical significance of carbapenem‐resistant Klebsiella pneumoniae rectal colonization in critically ill patients: From colonization to bloodstream infection. Journal of Medical Microbiology, 1–10. 10.1099/jmm.0.000921 [DOI] [PubMed] [Google Scholar]
- Kvitko, B. H. , & Collmer, A. (2011). Construction of Pseudomonas syringae pv. tomato DC3000 mutant and polymutant strains, plant immunity. Springer Protocols, 712, 109–128. [DOI] [PubMed] [Google Scholar]
- Lai, Y.‐C. , Peng, H.‐L. , & Chang, H. Y. (2003). RmpA2, an activator of capsule biosynthesis in Klebsiella pneumoniae CG43, regulates K2 Cps gene expression at the transcriptional level. Journal of Bacteriology, 185(3), 788–800. 10.1128/JB.185.3.788-800.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, M. M. C. , Wyres, K. L. , Duchêne, S. , Wick, R. R. , Judd, L. M. , Gan, Y.‐H. , … Holt, K. E. (2018). Population genomics of hypervirulent Klebsiella pneumoniae clonal‐group 23 reveals early emergence and rapid global dissemination. Nature Communications, 9(1). 10.1038/s41467-018-05114-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor, M. S. , Hsu, J. , Rick, P. D. , & Miller, V. L. (2005). Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Molecular Microbiology, 58(4), 1054–1073. [DOI] [PubMed] [Google Scholar]
- Lee, C.‐R. , Lee, J. H. , Park, K. S. , & Jeon, J. H. (2017). Antimicrobial resistance of hypervirulent Klebsiella pneumoniae: Epidemiology, determinants, and resistance mechanisms. Frontiers in Cellular and Infection Microbiology, 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, I. R. , Molton, J. S. , Wyres, K. L. , Gorrie, C. , Wong, J. , Hoh, C. H. , … Gan, Y.‐H. (2016). Differential host susceptibility and bacterial virulence factors driving Klebsiella liver abscess in an ethnically diverse population. Scientific Reports, 6, 1–12. 10.1038/srep29316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. C. , Betley, M. J. , Hopkins, C. A. , Perez, N. E. , & Pier, G. B. (1987). Virulence studies, in mice, of transposon‐induced mutants of Staphylococcus aureus differing in capsule size. The Journal of Infectious Diseases, 156(5), 741–750. 10.1093/infdis/156.5.741 [DOI] [PubMed] [Google Scholar]
- Li, B. , Zhao, Y. , Liu, C. , Chen, Z. , & Zhou, D. (2014). Molecular pathogenesis of Klebsiella pneumoniae . Future Microbiology, 9(9), 1071–1081. [DOI] [PubMed] [Google Scholar]
- Lin, C.‐T. , Chen, Y.‐C. , Jinn, T.‐R. , Wu, C.‐C. , Hong, Y.‐M. , & Wu, W.‐H. (2013). Role of the cAMP‐dependent carbon catabolite repression in capsular polysaccharide biosynthesis in Klebsiella pneumoniae . PLoS ONE, 8(2), 1–14. 10.1371/journal.pone.0054430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Han, W. , & Gu, J. (2019). Three capsular polysaccharide synthesis related glucosyltransferases, GT‐1, GT‐2 and WcaJ, are associated with virulence and phage sensitivity of Klebsiella pneumoniae . Frontiers in Microbiology, 10(May), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, J. Z. W. , Leow, J. J. J. , Ng, P. L. F. , Lee, H. Q. , Mohd Noor, N. A. , Low, J. K. , … Woon, W. W. L. (2015). Predictors of therapy failure in a series of 741 adult pyogenic liver abscesses. Journal of Hepato‐Biliary‐Pancreatic Sciences, 22(2), 156–165. 10.1002/jhbp.174 [DOI] [PubMed] [Google Scholar]
- Manat, G. , Roure, S. , Auger, R. , Bouhss, A. , Barreteau, H. , Mengin‐Lecreulx, D. , & Touzé, T. (2014). Deciphering the metabolism of undecaprenyl‐phosphate: The bacterial cell‐wall unit carrier at the membrane frontier. Microbial Drug Resistance, 20(3), 199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt, M. E. , & Donaldson, J. R. (2009). Effect of bile salts on the DNA and membrane integrity of enteric bacteria. Journal of Medical Microbiology, 58(2009), 1533–1541. 10.1099/jmm.0.014092-0 [DOI] [PubMed] [Google Scholar]
- Nassif, X. , Fournier, J.‐M. , Arondel, J. , & Sansonetti, P. J. (1989). Mucoid phenotype of Klebsiella pneumoniae is virulence factor plasmid‐encoded. Infection and Immunity, 12(2), 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif, X. , Honore, N. , Vasselon, T. , Cole, S. T. , & Sansonetti, P. J. (1989). Positive control of colanic acid synthesis in Escherichia coli by RmpA and RmpB, two virulence‐plasmid genes of Klebsiella pneumoniae . Molecular Microbiology, 3(10), 1349–1359. [DOI] [PubMed] [Google Scholar]
- Palacios, M. , Miner, T. A. , Frederick, D. R. , Sepulveda, V. E. , Quinn, J. D. , Walker, K. A. , & Miller, V. L. (2018). Identification of two regulators of virulence that are conserved in Klebsiella pneumoniae classical and hypervirulent strains. mBio, 9(4), e01443‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Y.‐J. , Lin, T.‐L. , Chen, C.‐T. , Chen, Y.‐Y. , Hsieh, P.‐F. , Hsu, C.‐R. , … Wang, J.‐T. (2015). Genetic analysis of capsular polysaccharide synthesis gene clusters in 79 capsular types of Klebsiella spp. Scientific Reports, 9, 1–10. 10.1038/srep15573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicoro, A. , & Faber, K. N. (2007). Review article: The function and regulation of proteins involved in bile salt biosynthesis and transport. Alimentary Pharmacology and Therapeutics, 26(9), 149–160. [DOI] [PubMed] [Google Scholar]
- Ranjit, D. K. , & Young, K. D. (2016). Colanic acid intermediates prevent de novo shape recovery of Escherichia coli spheroplasts, calling into question biological roles previously attributed to colanic acid. Journal of Bacteriology, 198(8), 1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulston, K. J. , Bharucha, T. , Turton, J. F. , Hopkins, K. L. , & Mack, D. J. F. (2018). A case of NDM‐carbapenemase‐producing hypervirulent Klebsiella pneumoniae sequence type 23 from the UK. JMM Case Reports, 1–3. 10.1099/jmmcr.0.005130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, D. W. (2009). Fifty years of advances in bile acid synthesis and metabolism. Journal of Lipid Research, 50(Supplement), S120–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin, O. , Terhorst, S. A. , Burrough, E. R. , Shen, Z. , Wu, Z. , Dai, L. , … Zhang, Q. (2017). Key role of capsular polysaccharide in the induction of systemic infection and abortion by hypervirulent Campylobacter jejuni . Infection and Immunity, 85(6), 1–15. 10.1128/IAI.00001-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahly, H. , Podschun, R. , Oelschlaeger, T. A. , Greiwe, M. , Parolis, H. , Hasty, D. , … Sela, S. (2000). Capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae . Infection and Immunity, 68(12), 6744–6749. 10.1128/IAI.68.12.6744-6749.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham, L.‐T. , Zheng, S. , Yakhnina, A. A. , Kruse, A. C. , & Bernhardt, T. G. (2018). Loss of specificity variants of WzxC suggest that substrate recognition is coupled with transporter opening in MOP‐family flippases. Molecular Microbiology, 109(September), 633–641. 10.1111/mmi.14002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shon, A. S. , Bajwa, R. P. S. , & Russo, T. A. (2013). Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence, 4(2), 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu, L. K. , Fung, C.‐P. , Chang, F.‐Y. , Lee, N. , Yeh, K.‐M. , Koh, T. H. , & Ip, M. (2011). Molecular typing and virulence analysis of serotype K1 Klebsiella pneumoniae strains isolated from liver abscess patients and stool samples from noninfectious subjects in Hong Kong, Singapore, and Taiwan. Journal of Clinical Microbiology, 49(11), 3761–3765. 10.1128/JCM.00977-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu, L. K. , Yeh, K.‐M. , Lin, J.‐C. , Fung, C.‐P. , & Chang, F.‐Y. (2012). Klebsiella pneumoniae liver abscess: A new invasive syndrome. The Lancet Infectious Diseases, 12(11), 881–885. 10.1016/S1473-3099(12)70205-0 [DOI] [PubMed] [Google Scholar]
- Struve, C. , & Krogfelt, K. A. (2003). Role of capsule in Klebsiella pneumoniae virulence: Lack of correlation between in vitro and in vivo studies. FEMS Microbiology Letters, 218(1), 149–154. [DOI] [PubMed] [Google Scholar]
- Struve, C. , Roe, C. C. , Stegger, M. , Stahlhut, S. G. , Hansen, D. S. , Engelthaler, D. M. , … Krogfelt, K. A. (2015). Mapping the evolution of hypervirulent Klebsiella pneumoniae . MBio, 6(4), 1–12. 10.1128/mBio.00630-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconelli, E. , Carrara, E. , Savoldi, A. , Harbarth, S. , Mendelson, M. , Monnet, D. L. , … Ouellette, M. (2018). Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. The Lancet Infectious Diseases, 18(3), 318–327. [DOI] [PubMed] [Google Scholar]
- Tan, Y. H. , Gamage, A. , & Gan, Y.‐H. (2017). Complement—Activated vitronectin enhances the invasion of nonphagocytic cells by bacterial pathogens Burkholderia and Klebsiella . Cellular Microbiology, 4, 1–13. [DOI] [PubMed] [Google Scholar]
- Urdaneta, V. , & Casadesús, J. (2017). Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Frontiers in Medicine, 4(10), 1–13. 10.3389/fmed.2017.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano, M. A. (2008). Undecaprenyl phosphate recycling comes out of age. Molecular Microbiology, 67(12), 232–235. 10.1111/j.1365-2958.2007.06052.x [DOI] [PubMed] [Google Scholar]
- Vianney, A. , Lazzaroni, J. C. , & Germon, P. (1999). The tol proteins of Escherichia coli and their involvement in the uptake of biomolecules and outer membrane stability. FEMS Microbiology Letters, 177, 191–197. [DOI] [PubMed] [Google Scholar]
- Walker, K. A. , Miner, T. A. , Palacios, M. , Trzilova, D. , Frederick, D. R. , Broberg, C. A. , … Miller, V. L. (2019). A Klebsiella pneumoniae regulatory mutant has reduced capsule expression but retains hypermucoviscosity. MBio, 10(2), e00089‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. H. , Liu, Y.‐C. , Lee, S.‐ S.‐J. , Yen, M.‐Y. , Chen, Y.‐S. , Wang, J.‐H. , & Lin, H.‐H. (1998). Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clinical Infectious Diseases, 26(6), 1434–1438. [DOI] [PubMed] [Google Scholar]
- Wei, D. , Yuminaga, Y. , Shi, J. , & Jian, H. (2018). Non‐capsulated mutants of a chemical‐producing Klebsiella pneumoniae strain. Biotechnology Letters, 40(4), 679–687. 10.1007/s10529-018-2524-5 [DOI] [PubMed] [Google Scholar]
- West, S. E. H. , Schweizer, H. P. , Dall, C. , Sample, A. K. , & Runyen‐Janecky, L. J. (1994). Construction of improved Escherichia‐Pseudomonas shuttle vectors derived from PUC 18/l9 and sequence of the region required for their replication in Pseudomonas aeruginosa . Gene, 128, 81–86. [DOI] [PubMed] [Google Scholar]
- Whitfield, C. (2006). Biosynthesis and assembly of capsular polysaccharides in Escherichia coli . Annual Reviews of Biochemistry, 75, 39–68. [DOI] [PubMed] [Google Scholar]
- Whitfield, C. (2010). Glycan chain‐length control. Nature Chemical Biology, 6(6), 403–404. 10.1038/nchembio.376 [DOI] [PubMed] [Google Scholar]
- Wu, J. H. , Wu, A. M. , Tsai, C. G. , Chang, X. Y. , Tsai, S. F. , & Wu, T. S. (2008). Contribution of fucose‐containing capsules in Klebsiella pneumoniae to bacterial virulence in mice. Experimental Biology and Medicine, 233(1), 64–70. [DOI] [PubMed] [Google Scholar]
- Wu, M.‐C. , Lin, T.‐L. , Hsieh, P.‐F. , Yang, H.‐C. , & Wang, J.‐T. (2011). Isolation of genes involved in biofilm formation of a Klebsiella pneumoniae strain causing pyogenic liver abscess. PLoS ONE, 6(8). 10.1371/journal.pone.0023500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xayarath, B. , & Yother, J. (2007). Mutations blocking side chain assembly, polymerization, or transport of a Wzy‐dependent Streptococcus pneumoniae capsule are lethal in the absence of suppressor mutations and can affect polymer transfer to the cell wall. Journal of Bacteriology, 189(9), 3369–3381. 10.1128/JB.01938-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, B. , Xiao, X. , Wang, F. , Zhou, L. , Zhang, X. , & Zhang, J. (2015). Clinical and molecular characteristics of multi‐clone carbapenem‐resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in a Tertiary Hospital in Beijing, China. International Journal of Infectious Diseases, 37, 107–112. 10.1016/j.ijid.2015.06.023 [DOI] [PubMed] [Google Scholar]
- Yeh, K. M. (2010). Revisiting the importance of virulence determinant MagA and its surrounding genes in Klebsiella pneumoniae causing pyogenic liver abscesses: Exact role in serotype K1 capsule formation. Journal of Infectious Diseases, 201, 1259–1267. [DOI] [PubMed] [Google Scholar]
- Yeh, K.‐M. , Chiu, S.‐K. , Lin, C.‐L. , Huang, L.‐Y. , Tsai, Y.‐K. , Chang, J.‐C. , … Siu, L.‐K. (2016). Surface antigens contribute differently to the pathophysiological features in serotype K1 and K2 Klebsiella pneumoniae strains isolated from liver abscesses. Gut Pathogens, 8(4), 1–9. 10.1186/s13099-016-0085-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, K.‐M. , Kurup, A. , Siu, L. K. , Koh, Y. L. , Fung, C.‐P. , Lin, J.‐C. , … Koh, T.‐H. (2007). Capsular serotype K1 or K2, rather than MagA and RmpA, is a major virulence determinant for Klebsiella pneumoniae liver abscess in Singapore and Taiwan. Journal of Clinical Microbiology, 45(2), 466–471. 10.1128/JCM.01150-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J.‐X. , Lin, Z.‐W. , Chen, C. , Chen, Z. , Lin, F.‐J. , Wu, Y. , … Deng, Q.‐W. (2018). Biofilm formation in Klebsiella pneumoniae bacteremia strains was found to be associated with CC23 and the presence of WcaG. Frontiers in Cellular and Infection Microbiology, 8, 1–9. 10.3389/fcimb.2018.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


