Summary
Background
Patients with psoriasis value rapid and complete skin clearance. No head‐to‐head studies have focused on early responses to interleukin (IL)‐17 vs. IL‐23 inhibitors.
Objectives
To compare early and complete skin clearance by the IL‐17A inhibitor ixekizumab vs. the IL‐23p19 inhibitor guselkumab.
Methods
IXORA‐R, a 24‐week, randomized, double‐blinded study, enrolled adults with moderate‐to‐severe plaque psoriasis [static Physician's Global Assessment of Disease (sPGA) score of ≥ 3, Psoriasis Area and Severity Index (PASI) ≥ 12, and ≥ 10% body surface area]. Patients were randomized (1 : 1) to receive the approved dose of subcutaneous ixekizumab or guselkumab. Primary end point was 100% improvement in PASI (PASI 100) at week 12. Major secondary end points included other levels of improved PASI and sPGA at different time points. Comparisons were made using the Cochran–Mantel–Haenszel test with a multiple testing strategy. Nonresponder imputation was used for missing data. After the completion of the study, the final secondary end point (PASI 100 at 24 weeks) and safety data through week 24 will be reported.
Results
In total, 1027 patients were randomized. The primary end point PASI 100 at week 12 was met [215/520 ixekizumab (41%); 126/507 guselkumab (25%); P < 0·001]. All major secondary end points measured up to week 12 were met, including PASI 50 at week 1 and PASI 75 at week 2. Serious adverse event frequency was 3% for each group; no new safety signals were identified.
Conclusions
Ixekizumab was superior to guselkumab for rapidly improving signs and symptoms in patients with moderate‐to‐severe plaque psoriasis by week 12. Adverse events were similar to previous ixekizumab and guselkumab studies. Compared with the IL‐23 inhibitor guselkumab, ixekizumab can offer complete skin clearance more rapidly to patients with moderate‐to‐severe plaque psoriasis.
What's already known about this topic?
Patients with plaque psoriasis desire both high levels of clearance and rapid onset of treatment effects.
Ixekizumab, a high‐affinity monoclonal antibody that selectively targets interleukin (IL)‐17A, has demonstrated greater and faster skin clearance than etanercept and ustekinumab, with consistent long‐term efficacy, safety and durability of response.
Clinical trial data and systematic reviews have suggested that IL‐17 inhibitors can improve a patient's psoriasis more rapidly than IL‐23 inhibitors.
What does this study add?
The head‐to‐head study design directly compares the efficacy and speed of response of ixekizumab and the IL‐23 inhibitor guselkumab in moderate‐to‐severe plaque psoriasis.
The primary end point was met, showing superiority of ixekizumab over guselkumab for achieving complete skin clearance at week 12.
The safety profile of ixekizumab was consistent with previous studies.
Ixekizumab can deliver patients complete skin clearance and improved quality of life more rapidly than guselkumab.
Short abstract
Linked Comment: Veysey. Br J Dermatol 2020; 182:1321–1322.
Plain language summary available online
Plaque psoriasis is a chronic, immune‐mediated, inflammatory condition that causes uncomfortable and disfiguring changes in the skin.1 Symptoms of psoriasis often affect patients both physically and psychologically, leading to a reduced quality of life.2
Several lines of evidence indicate that achieving completely clear skin rapidly is an important goal in psoriasis treatment. First and foremost, patients desire both high levels of clearance and rapid onset of treatment effects.3, 4, 5, 6 Patients with psoriasis may also experience symptoms that disrupt their everyday lives. In particular, itch (pruritus) affects up to 80% of patients, who describe it as a severe and bothersome psoriasis symptom, with a negative impact on mood, concentration, sleep and overall quality of life.7, 8, 9 Thus, rapid resolution of itch and other psoriasis symptoms would lead to a quick improvement in quality of life. Also, quicker efficacy could lead to increased patient compliance. In another recent study, the lack of efficacy was the most common reason why patients with psoriasis discontinued a biologic treatment.10
New biologic treatments that specifically target molecules involved in the pathogenesis of psoriasis such as interleukin (IL)‐17 and IL‐23 are associated with high levels of skin improvement.11 Within 1 year of treatment, up to 80% of patients treated with IL‐17 or IL‐23 inhibitors can expect almost clear skin, as indicated by a 90% improvement in Psoriasis Area and Severity Index (PASI 90), and 50–60% of patients can expect completely clear skin (PASI 100).12, 13 Although clinical trial data and systemic reviews have suggested that IL‐17 inhibitors can improve a patient's psoriasis more rapidly than IL‐23 inhibitors,14, 15, 16, 17, 18 no trials have directly tested the speed of efficacy of IL‐17 vs. IL‐23 inhibitors in inducing complete plaque psoriasis clearance.
Ixekizumab, a high‐affinity monoclonal antibody that selectively targets IL‐17A, has demonstrated greater and faster skin clearance than etanercept17 and ustekinumab,19 with consistent long‐term efficacy, safety and durability of response for up to 5 years of continuous treatment.20, 21, 22 Here, we report the primary 12‐week results of IXORA‐R, which compared the efficacy, safety and speed of response of ixekizumab vs. guselkumab, an IL‐23p19 inhibitor, in patients with moderate‐to‐severe plaque psoriasis.
Patients and methods
Study design
IXORA‐R was a multicentre, randomized, double‐blinded, parallel‐group, phase IV study with the primary end point at 12 weeks and the blinded study continuing to 24 weeks. The study design is shown in Figure S1 (see Supporting Information). The results reported here were obtained between 9 November 2018 and 15 July 2019 by 124 investigators at 124 sites in the U.S.A. and Canada (for a list of investigators see Appendix 2, for a list of investigators by study site see File S1 in the Supporting Information).
All patients were required to give informed consent for participation in the study. The IXORA‐R protocol was approved by local ethical review boards and was conducted according to the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. Two amendments were made to the protocol and are described in File S2 (see Supporting Information).
Randomization and masking
Patients were allocated to treatment by a computer‐generated random sequence. Patients were randomly assigned (1 : 1) to receive subcutaneous injections of ixekizumab or guselkumab at the approved dosing. For ixekizumab, patients received a 160‐mg starting dose at week 0 (two 80‐mg injections), followed by 80 mg every 2 weeks from weeks 2 to 12. For guselkumab, patients received 100 mg injections at weeks 0, 4 and 12. To maintain blinding, patients on guselkumab received one placebo injection at weeks 0, 2, 6, 8 and 10.
Patients, investigators and all other personnel involved in the conduct of this ongoing study are to remain blinded to individual treatment assignments until all patients have completed the study. Additional details on randomization and masking are provided in File S2 (see Supporting Information).
Participants
Complete inclusion and exclusion criteria are provided in File S2 (see Supporting Information). Eligible patients were ≥ 18 years of age with chronic plaque psoriasis based on a diagnosis for at least 6 months before baseline, as determined by the investigator, were a candidate for phototherapy and/or systemic therapy, and had a static Physician's Global Assessment of Disease (sPGA) score of ≥ 3, a PASI ≥ 12 and ≥ 10% body surface area involvement at screening and baseline. Patients were excluded if they had a predominant pattern of pustular, erythrodermic and/or guttate forms of psoriasis, a history of drug‐induced psoriasis or a clinically significant flare of psoriasis during the 12 weeks before baseline. In addition, the study excluded patients who had used tanning booths 4 weeks before baseline, any biological agent within specified periods prior to baseline, any use of IL‐23p19 antagonists, or had any condition or contraindication as addressed in the local labelling for guselkumab. Prior use of an IL‐17 antagonist other than ixekizumab was allowed if the patient had not failed to respond to the therapy.
Procedures
Treatments were administered subcutaneously with prefilled syringes. Study visits occurred during screening and at week 0 (baseline), 1, 2, 4, 6, 8, 10 and 12. The primary end point for this study was assessed at 12 weeks. Assessments of study outcomes were completed at screening and during each study visit with the exception of Dermatology Life Quality Index (DLQI), which was done at weeks 0, 2, 4, 6, 8 and 12. Full descriptions of the assessments are provided in File S2 (see Supporting Information).
Outcomes
The primary efficacy end point of this trial was the percentage of patients reaching 100% improvement from baseline (complete clearance) in PASI, as demonstrated by PASI 100 at week 12. Major secondary end points included the proportion of patients who achieved PASI 50 at week 1, PASI 75 at week 2, PASI 90 at weeks 4 and 8, PASI 100 at weeks 4, 8 and 24, and a sPGA score of 0 at week 12 (PASI 100 at week 24 will be provided in a future publication after the final database lock).
Additionally, patient‐reported outcomes were assessed using Patient's Global Assessment of Disease Severity (PatGA), DLQI, skin pain visual analogue scale (VAS), and the itch numeric rating scale (NRS). Exploratory outcomes not included in the multiple testing procedure are listed in Table S1 (see Supporting Information). Outcomes listed in File S2 that were not reported here will be disclosed in future publications. Safety outcomes were assessed at every visit. See File S2 for detailed descriptions of outcome measures.
Statistical analyses
The sample size was estimated to have 98% power for testing the superiority of ixekizumab to guselkumab for the PASI 100 outcome at week 12 at a two‐sided 5% type I error rate (for details see File S2; see Supporting Information).
Efficacy analyses for the blinded treatment dosing period included all randomized patients according to the treatment to which they were assigned (intent‐to‐treat population). Safety data up to the week 12 database lock were summarized using the safety population (all randomized patients who received one or more dose of a trial drug) per the assigned treatments.
For the primary and major secondary end points, odds ratios and P‐values were obtained using the Cochran–Mantel–Haenszel test stratified by pooled site. Missing data were imputed using a nonresponder imputation method. A multiple testing strategy was implemented for primary and major secondary end points to control the overall familywise type I error rate at a two‐sided alpha level of 0·05. Exploratory analyses were not adjusted for multiple comparisons. One interim analysis was planned and executed when all patients completed their week 12 visit or early termination visit. This paper presents the results of this interim analysis and is considered the primary report of the trial. Additional details regarding statistical analyses are in File S2 (see Supporting Information).
Safety data on terms related to cerebrocardiovascular events and suspected inflammatory bowel disease (IBD) were adjudicated by external clinical event committees (for details see File S2 in the Supporting Information). Because this study is ongoing, some efficacy and safety data are not described in this article to maintain study blinding. These details will be included in future publications. The trial was registered with ClinicalTrials.gov (NCT03573323).
Results
Of the 1393 patients screened for the study, 1027 patients were randomized to receive treatment: 507 to guselkumab and 520 to ixekizumab, including 209 (20%) patients from Canada and 818 (80%) from the U.S.A. The completion rate was 94% (970/1027) for the first 12 weeks. More details are provided in Figure 1.
Figure 1.
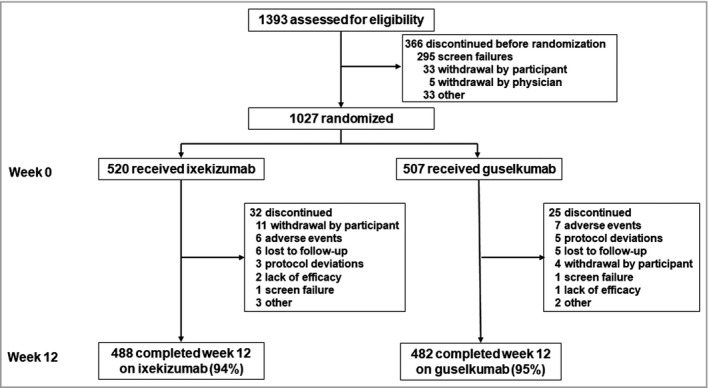
Disposition of the patients. Details are given according to the CONSORT statement for reporting randomized controlled trials.
Baseline characteristics were well‐balanced between the randomized treatment groups (Table 1). The patients had a mean (± SD) age of 49·0 ± 14·4 years and 37% (375/1027) were women. The primary outcome was complete skin clearance, as measured by PASI 100 response at week 12. PASI 100 was achieved in 41% (215/520) of patients in the ixekizumab group vs. 25% (126/507) of patients in the guselkumab group with an odds ratio (OR) of 2·14 [95% confidence interval (CI) 1·63–2·81, P < 0·001; Fig. 2a, Table 2].
Table 1.
Demographics and baseline disease characteristicsa
| Ixekizumab (n = 520) | Guselkumab (n = 507) | |
|---|---|---|
| Age, years | 49·0 ± 13·9 | 49·0 ± 14·9 |
| Women, n (%) | 182 (35) | 193 (38) |
| White ethnicity, n (%) | 439 (85) | 431 (85) |
| Weight (kg) | 96·6 ± 24·9 | 94·6 ± 24·9 |
| ≥ 100 kg, n (%) | 197 (38) | 171 (34) |
| Body mass index (kg/m2) | 32·9 ± 7·9 | 32·8 ± 7·9 |
| Country, n (%) | ||
| Canada | 103 (20) | 106 (21) |
| U.S.A. | 417 (80) | 401 (79) |
| Years since diagnosis | 17·5 ± 13·8 | 16·3 ± 13·8 |
| PASI (range 0–72) | 19·5 ± 7·9 | 19·3 ± 7·1 |
| PASI (range 0–72), median (IQR) | 17·0 (7·7) | 17·4 (7·5) |
| sPGA score, n (%) | ||
| 3 | 266 (51) | 252 (50) |
| 4 | 224 (43) | 232 (46) |
| 5 | 29 (6) | 23 (5) |
| % Body surface area | 24·1 ± 16·1 | 23·8 ± 15·4 |
| DLQI | 12·8 ± 6·9 | 13·2 ± 7·4 |
| Skin pain VAS | 47·0 ± 29·9 | 47·2 ± 30·5 |
| Itch NRS | 6·9 ± 2·4 | 7·1 ± 2·5 |
| Previous therapy, n (%) | ||
| Nonbiologic systemic | 170 (33) | 140 (28) |
| Topical therapy | 373 (72) | 352 (69) |
| Phototherapy | 77 (15) | 63 (12) |
| Biologic | 137 (26) | 133 (26) |
| Number of prior biologics, n (%) | ||
| 1 | 95 (18) | 96 (19) |
| 2 | 28 (5) | 27 (5) |
| ≥ 3 | 14 (3) | 10 (2) |
| Prior biologic class, n (%) | ||
| Anti‐IL‐17 | 25 (5) | 29 (6) |
| Anti‐IL‐17 only | 11 (2) | 16 (3) |
| Anti‐IL‐12/IL‐23 only | 11 (2) | 14 (3) |
| Anti‐TNF only | 84 (16) | 67 (13) |
| Other | 2 (0·4) | 10 (2) |
| Multiple | 29 (6) | 26 (5) |
| Prior biologic failures, n (%) | 41 (8) | 36 (7) |
Data are mean ± SD, unless otherwise indicated. PASI, Psoriasis Area and Severity Index; sPGA, static Physician's Global Assessment; DLQI, Dermatology Life Quality Index; VAS, visual analogue scale; NRS, numeric rating scale; IL, interleukin; TNF, tumour necrosis factor‐alpha; aPercentages were calculated based on the number of patients with nonmissing values.
Figure 2.

Primary and major secondary end points through week 12 in the ixekizumab (IXE, N = 520) and guselkumab (GUS, N = 507) groups. Data are percentages with 95% confidence interval. (a) Proportion of patients achieving 100% improvement in Psoriasis Area and Severity Index (PASI 100) and (b) PASI 50/75/90 and static Physician's Global Assessment (sPGA) (0). These end points were tested after adjusting for multiplicity. The prespecified testing order is given in File S2 (see Supporting Information). There is one remaining major secondary outcome (PASI 100 at week 24) to be tested when the final database lock occurs, which will not have an impact on the results shown. Nonresponder imputation was used for missing data. The 95% confidence intervals were constructed using the asymptotic method, without continuity correction (i.e. normal approximation to the binomial distribution). Listed below the x‐axes are the numbers of patients with nonmissing data for each outcome and time point. * P < 0·001 vs. guselkumab. Wk, week.
Table 2.
Primary and major secondary end points through week 12a
| Ixekizumab, n (%) (n = 520) | Guselkumab, n (%) (n = 507) | P | Guselkumab vs. ixekizumab, difference (95% CI) | Guselkumab vs. ixekizumab, odds ratio (95% CI) | |
|---|---|---|---|---|---|
| Primary outcome | |||||
| PASI 100, week 12 | 215 (41) | 126 (25) | < 0·001 | 16·5 (10·8–22·2) | 2·14 (1·63–2·81) |
| Major secondary outcomes | |||||
| PASI 50, week 1 | 143 (28) | 47 (9) | < 0·001 | 18·2 (13·6–22·8) | 4·73 (3·13–7·13) |
| PASI 75, week 2 | 119 (23) | 26 (5) | < 0·001 | 17·8 (13·7–21·8) | 6·26 (3·89–10·08) |
| PASI 90, week 4 | 109 (21) | 40 (8) | < 0·001 | 13·1 (8·9–17·3) | 3·21 (2·15–4·78) |
| PASI 100, week 4 | 35 (7) | 7 (1) | < 0·001 | 5·4 (3·0–7·7) | 5·35 (2·33–12·28) |
| PASI 90, week 8 | 304 (58) | 182 (36) | < 0·001 | 22·6 (16·6–28·5) | 2·51 (1·94–3·25) |
| PASI 100, week 8 | 154 (30) | 69 (14) | < 0·001 | 16·0 (11·1–20·9) | 2·69 (1·95–3·72) |
| sPGA 0, week 12 | 218 (42) | 128 (25) | < 0·001 | 16·7 (11·0–22·4) | 2·15 (1·64–2·82) |
CI, confidence interval; PASI, Psoriasis Area and Severity Index; sPGA, static Physician's Global Assessment. aThese end points were tested after adjusting for multiplicity. The prespecified testing order is given in File S2 (see Supporting Information). There is one remaining major secondary outcome (PASI 100 at week 24) to be tested when the final database lock occurs, which will not have an impact on the results shown.
To examine the early responses to treatment, the major secondary end points included PASI responses at early time points. Significantly more patients in the ixekizumab group than the guselkumab group achieved a PASI 50 response at week 1 [28% (143/520) for ixekizumab vs. 9% (47/507) for guselkumab; OR 4·73, 95% CI 3·13–7·13; P < 0·001] and a PASI 75 response at week 2 [23% (119/520) for ixekizumab vs. 5% (26/507) for guselkumab; OR 6·26, 95% CI 3·89–10·08; P < 0·001, Fig. 2b, Table 2]. At week 4, more patients in the ixekizumab group than the guselkumab group achieved a PASI 90 response [21% (109/520) for ixekizumab vs. 8% (40/507) for guselkumab, OR 3·21, 95% CI 2·15–4·78, P < 0·001, Fig. 2b; Table 2].
Additionally, at week 4, more patients in the ixekizumab group than the guselkumab group achieved a PASI 100 response [7% (35/520) for ixekizumab vs. 1% (7/507) for guselkumab; OR 5·35, 95% CI 2·33–12·28; P < 0·001, Fig. 2a, Table 2]. The results for complete clearance measured by sPGA were very similar to those for PASI 100. At week 12, an sPGA score of 0 was achieved by 42% (218/520) of patients in the ixekizumab group vs. 25% (128/507) of patients in the guselkumab group (OR 2·15, 95% CI 1·64–2·82; P < 0·001) (Fig. 2b, Table 2).
Another major secondary end point included in the multiple testing scheme, PASI 100 at week 24, will be reported in a future publication once the data are available. Nonetheless, for the first 12 weeks of the study, all the prespecified primary and major secondary end points included in the multiple testing scheme showed statistically significantly greater improvements in the ixekizumab group vs. the guselkumab group (Fig. 2, Table 2).
To further determine the speed at which PASI responses were attained, the median percentage improvement in PASI by time and patient group was assessed (Fig. 3). At week 1, patients on ixekizumab had twice the relative PASI improvement vs. guselkumab [median PASI improvement was 34% (interquartile range, IQR 39) for ixekizumab vs. 17% (IQR 31) for guselkumab]. At week 2, patients on ixekizumab had 1·6‐times the relative PASI improvement vs. guselkumab [median PASI improvement was 55% (IQR 37) for ixekizumab vs. 35% (IQR 39) for guselkumab].
Figure 3.
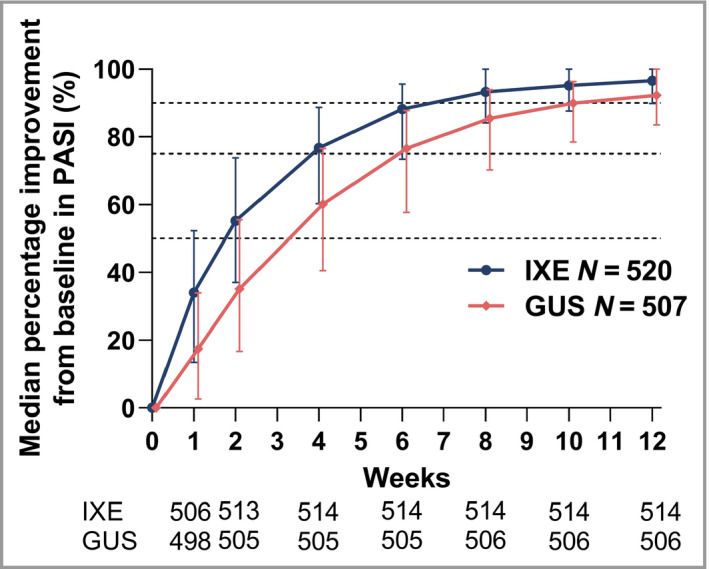
Median percentage improvement from baseline in Psoriasis Area and Severity Index (PASI). Data are shown as median percentage (with interquartile range). Listed below the x‐axis are the numbers of patients with nonmissing data for each time point. Modified baseline observation carried forward was used for missing data. Dashed lines mark 50%, 75% and 90% thresholds for improvement in PASI. GUS, guselkumab; IXE, ixekizumab.
The early responses to ixekizumab and guselkumab were also compared for patient‐reported global assessment of disease severity, quality of life, skin pain and itch, which were exploratory end points in this study. More patients on ixekizumab reported PatGA scores of 0 or 1 [PatGA (0, 1)], compared with guselkumab at week 1 [ixekizumab 7% (36/520) vs. guselkumab 2% (10/507), P < 0·001; Fig. 4a]. The proportions had the greatest differences between ixekizumab and guselkumab at weeks 4 and 6 (P < 0·001) and remained significant through week 12 (P = 0·011). Response rates for PatGA (0, 1) are in agreement with the proportions of patients with sPGA scores of 0 or 1, suggesting that patients and investigators agreed on the rates of clear or almost clear skin observed during the study (Fig. 4a).
Figure 4.
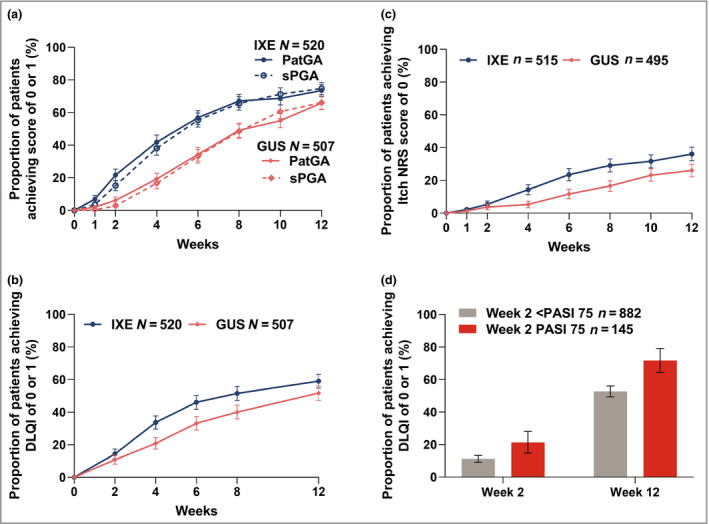
Proportion of patients achieving resolution of patient‐reported outcomes for the ixekizumab (IXE, N = 520) and guselkumab (GUS, N = 507) groups. Data are percentage (with 95% confidence interval). Proportion of patients achieving a score of (a) 0 or 1 for the Patient's Global Assessment (PatGA) and static Physician's Global Assessment (sPGA); (b) 0 or 1 for the Dermatology Life Quality Index (DLQI); and (c) 0 for the itch numeric rating scale (NRS). In (c), only patients with baseline Itch NRS score > 0 were included (IXE n = 515 and GUS n = 495). (d) The proportion of patients achieving DLQI of 0 or 1 based on week 2 75% improvement in Psoriasis Area and Severity Index (PASI 75) is shown after treatment groups were pooled. Nonresponder imputation was used for missing data. The 95% confidence intervals were constructed using the asymptotic method, without continuity correction (i.e. normal approximation to the binomial distribution).
More ixekizumab‐treated patients vs. guselkumab‐treated patients reported DLQI of 0 or 1 [DLQI (0, 1); i.e. no impact of disease on quality of life] as early as week 4 [ixekizumab 34% (175/520) vs. guselkumab 21% (106/507), P < 0·001, Fig. 4b]. Of patients with baseline skin pain VAS scores > 0, more ixekizumab‐treated patients vs. guselkumab‐treated patients reported skin pain VAS score of 0 (no skin pain) at week 8 [ixekizumab 23% (118/505) vs. guselkumab 15% (70/479), P < 0·001, Fig. S2; see Supporting Information].
More patients on ixekizumab than guselkumab reported an itch NRS score of 0, indicating complete resolution of itch symptoms as early as week 4 [ixekizumab 14% (74/515) vs. guselkumab 5% (26/495), P < 0·001, Fig. 4c]. Although there were not significant differences at week 12 for skin pain VAS (0), significant differences were reported through week 12 for PatGA (0, 1) (week 12, P < 0·001), DLQI (0, 1) (week 12, P = 0·029) and for itch NRS score of 0 (week 12, P < 0·001).
To assess the value of having early improvements in PASI, we performed a post hoc analysis of the association of early achievement of PASI 75 with low DLQI. Overall, 145 patients (119 on ixekizumab and 26 on guselkumab) achieved PASI 75 at week 2. Compared with those who did not achieve PASI 75 at week 2, more patients who had achieved PASI 75 also achieved DLQI of 0 or 1 at both week 2 and week 12 (Fig. 4d).
Similar frequencies of treatment‐emergent adverse events (TEAE), serious adverse events and adverse events leading to discontinuation were reported for guselkumab‐ and ixekizumab‐treated patients from the start of the study to the database lock (Table 3). Study drug exposure for over 80% of patients exceeded 12 weeks (mean exposure 18·6 ± 5·2 weeks). Overall, the most frequent TEAE was upper respiratory tract infection [7% (36/519) of ixekizumab‐treated patients and 7% (36/506) of guselkumab‐treated patients]. The frequency of injection‐site reactions was greater in ixekizumab‐treated patients, reported by 13% (67/519) of ixekizumab‐treated patients and 3% (17/506) of guselkumab‐treated patients. All injection‐site reactions were mild to moderate in severity.
Table 3.
Safety outcomes
| Ixekizumab (n = 519) | Guselkumab (n = 506) | |
|---|---|---|
| Treatment‐emergent adverse events | 293 (56) | 277 (55) |
| Severea | 17 (3) | 18 (4) |
| Discontinuation because of adverse events | 12 (2) | 8 (2) |
| Serious adverse events | 16 (3) | 13 (3) |
| Death | 0 | 0 |
| Common treatment‐emergent adverse eventsb | ||
| Upper respiratory tract infection | 36 (7) | 36 (7) |
| Nasopharyngitis | 31 (6) | 25 (5) |
| Injection‐site reactionc | 49 (9) | 5 (1) |
| Headache | 21 (4) | 13 (3) |
| Diarrhoea | 15 (3) | 16 (3) |
| Treatment‐emergent adverse events of special interestd | ||
| Neutropenias | 1 (0·2) | 1 (0·2) |
| Infections | 137 (26) | 130 (26) |
| Opportunistic infectionse | 2 (0·4) | 1 (0·2) |
| Reactivated tuberculosis | 0 | 0 |
| Depression | 3 (1) | 4 (1) |
| Malignancies | 2 (0·4) | 2 (0·4) |
| Allergic reactions | 15 (3) | 11 (2) |
| Injection‐site reactionsf | 67 (13) | 17 (3) |
| Severe | 0 | 0 |
| Major adverse cardiac eventg | 4 (0·8) | 1 (0·2) |
| Cerebrocardiovascular eventsg | 5 (1) | 2 (0·4) |
| Hepatic eventsh | 1 (0·2) | 8 (2) |
Data are n (%) of patients in the safety population. aPatients with multiple occurrences of the same event are counted under the highest severity. bCommon treatment‐emergent adverse events (TEAEs) are defined as those that occurred at a frequency of ≥ 2% overall. cNumbers reported here only include TEAEs with the Medical Dictionary for Regulatory Activities (MedDRA) low‐level term ‘injection‐site reaction’. dFor TEAEs of special interest, serious infections, potential anaphylaxis and inflammatory bowel disease (IBD) are not listed because there was only one report each of serious infection and anaphylaxis related to use of amoxicillin, and IBD case adjudication was not complete as of the database lock. eThe three opportunistic infections identified as such by investigators were not systemic infections (two cases of mucocutaneous candidiasis and one case of herpes zoster). fNumbers reported here are for the high‐level MedDRA term ‘injection‐site reactions’ that includes multiple lower‐level MedDRA terms, including but not limited to, injection‐site reaction, injection‐site pain, injection‐site erythema, injection‐site swelling, injection‐site pruritus, injection‐site discomfort, injection‐site oedema and injection‐site warmth. gAdjudicated by external committee. Numbers reflect patients for which adjudication was complete at the time of the database lock. hPatients with at least one hepatic‐related TEAE.
To protect the blinding in this ongoing study, we are unable to identify the treatment groups for TEAEs that only occurred in one group. We can note that there was one case of suspected IBD, which had not been adjudicated as of the database lock, and one case of anaphylaxis reported that was related to use of amoxicillin. No deaths were reported.
Discussion
IXORA‐R is a head‐to‐head trial of ixekizumab, an IL‐17 inhibitor, vs. guselkumab, an IL‐23 inhibitor, examining responses as early as week 1 in patients with moderate‐to‐severe plaque psoriasis. More ixekizumab‐treated patients than guselkumab‐treated patients achieved all primary and major secondary measures up to week 12, and the differences were statistically significant. In addition to showing more rapid achievement of clinical measures of efficacy, ixekizumab also demonstrated that it can offer faster resolution of itching and faster improvement of patients’ quality of life.
A strength of this study is that this was a head‐to‐head comparison of ixekizumab and guselkumab, an IL‐17 inhibitor vs. an IL‐23 inhibitor. To our knowledge, this is only the second head‐to‐head trial testing IL‐17 vs. IL‐23 classes of drug, the first being the ECLIPSE trial.23 However, the ECLIPSE trial, which compared the efficacy of guselkumab with the IL‐17 inhibitor secukinumab, focused on later time points, with week 12 as the earliest time point included in the multiple testing scheme. By contrast, IXORA‐R was designed to assess eight of the nine primary and major secondary time points in the first 12 weeks of the study, with one major secondary time point (PASI 100 at week 24) remaining to be disclosed after the trial finishes. IXORA‐R did not measure long‐term efficacy and safety because previous trials have demonstrated efficacy and safety of ixekizumab with up to 4 and 5 years of continuous treatment, respectively.20, 21, 22 The focus on early responses allowed direct comparison of the speed at which improvements in psoriasis occur for patients treated with ixekizumab vs. guselkumab.
Patient surveys have determined that patients with psoriasis value speed of improvement. In a survey of patients with psoriasis in Germany, 95% of patients surveyed listed ‘to get better skin quickly’ as an important treatment goal.3 Other studies have confirmed the desire of patients to have rapid improvements in skin.4, 5, 6 In a stated preference experiment in England, patients preferred treatment that had a ‘shorter time to achieve a moderate improvement’ over ‘a longer time to relapse’.6 Of the six attributes tested in that study [time to moderate (50%) improvement, relapse and risks of experiencing skin irritation, high blood pressure, liver damage and skin cancer], more patients ranked ‘time to moderate improvement’ as the most important attribute.6 In a survey of patients with psoriasis treated in an outpatient setting, a majority highly valued the ‘rapid improvement of psoriasis’.4 In a more recent survey, 90% of patients with moderate‐to‐severe psoriasis reported that they assigned high importance for rapid response.5 The patients in this survey expected 50% clear skin in about 2 weeks and completely clear skin in about 4 weeks.5
Because the added value of a rapid response may not be as obvious to the clinicians, we performed an analysis to determine the relationship between early improvement and patient quality of life. Patients who achieved PASI 75 at week 2 were more likely to rate that psoriasis had no impact on their quality of life (Fig. 4d). Furthermore, early improvement was not just associated with quality of life at week 2, but also at week 12 (Fig. 4d).
Similarly, measurement of patient‐reported outcomes of global disease severity, itch and quality of life showed patient‐reported evidence of rapid improvement. In a large, multinational, population‐based survey, patients with psoriasis identified itch as their most bothersome symptom.8 More patients receiving ixekizumab vs. guselkumab achieved resolution of itch starting at week 4 (Fig. 4c). A similar pattern of early improvement was seen in health‐related life‐quality measures. Of note, more patients receiving ixekizumab achieved DLQI of 0 or 1 (a DLQI response indicating that psoriasis was having no impact on a patient's quality of life)24 at week 4.
The primary end point, PASI 100 at week 12, was selected specifically to investigate the speed at which ixekizumab not only improves psoriasis symptoms, but also offers complete skin clearance, when compared with guselkumab. With the development of biologic treatments that offered higher levels of clearance, a shift in treatment goals for clinical trials from PASI 75 to PASI 90 was recently proposed.25 Because IL‐17 and IL‐23 inhibitors have further elevated treatment responses, offering patients the opportunity to achieve completely clear skin, the value of complete clearance to patients has recently been investigated.26, 27, 28 Patients who achieve almost clear skin (PASI 90 or sPGA 1) continue to deal with substantial residual disease, including symptoms such as itching, redness, scaling and flaking.26, 27, 28 These residual symptoms have an impact on patients’ quality of life. In one post hoc study, an incremental improvement in DLQI 0 or 1 of 18% was measured between patients achieving completely clear skin (PASI 100) vs. almost clear skin (PASI 90 to < 100).27 Furthermore, patients who had achieved complete skin clearance experienced more 100% symptom‐free days than those who had achieved PASI 75 to < 100 (42·3% vs. 10·5%).27 Thus, completely clear skin is valuable to patients, especially if it can be achieved rapidly.
The power of the study was 98% for detection of a difference of 12% between ixekizumab and guselkumab for the proportion of patients achieving PASI 100 at week 12. The advantage of a highly powered study is the ability to detect multiple end points with statistical significance.
The safety data presented here are consistent with previously published studies of ixekizumab and guselkumab in psoriasis.12, 20 The frequencies for injection‐site reactions were similar to previous clinical trials, and none of the reactions were severe. Although a total of three opportunistic infections were reported by study investigators, each was either a case of mucocutaneous candidiasis or herpes zoster, with no systemic opportunistic infections reported.
A limitation of this study is not all efficacy and safety data can be disclosed until it is complete. Disclosure of all efficacy and safety data will occur after the final database lock, including the final secondary end point (PASI 100 at 24 weeks) and safety data through week 24. Other limitations include not being powered to allow a comparison of differences in the frequency of safety events, and that patients were only from U.S.A. and Canada.
In conclusion, ixekizumab was superior to guselkumab in the proportion of patients achieving complete skin clearance by week 12. All the primary and major secondary end points as of week 12 were met. Safety results were consistent with previous studies. The results suggest ixekizumab can deliver patients more rapid complete skin clearance and improved quality of life compared with guselkumab.
Supporting information
File S1 List of IXORA‐R study investigators by study site.
File S2 Supplementary methods.
Table S1 Primary, major secondary and exploratory end points.
Fig S1. Study design.
Fig S2. Proportion of patients achieving resolution of skin pain for the ixekizumab (N = 520) and guselkumab (N = 507) groups.
Video S1 Author video.
Acknowledgments
Eli Lilly and Company (Indianapolis, IN, U.S.A.) supported this study. Requests for access to study data and protocol can be submitted at www.vivli.org, per the sharing policy of Eli Lilly and Company. We thank Emily K. Blue, an employee of Eli Lilly and Company, for her writing support. We also thank the contributions of the investigators of IXORA‐R, who are listed in Appendix 2, and the patients who participated in IXORA‐R.
Appendix 1.
Conflicts of interest: A.B. has served as a scientific adviser and/or clinical study investigator for AbbVie, Aclaris, Almirall, Arena, Athenex, Boehringer Ingelheim, Bristol‐Myers Squibb, Dermavant, Dermira, Eli Lilly and Company, FLX Bio, Forte, Galderma, Janssen, LEO, Novartis, Ortho, Pfizer, Regeneron, Sandoz, Sanofi Genzyme, Sun Pharma and UCB Pharma, and as a paid speaker for AbbVie. K.P. has served as a scientific adviser and/or clinical study investigator for AbbVie, Akros, Allergan, Almirall, Amgen, Arcutis, Avillion, Bausch Health, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly and Company, Galderma, Genentech/Roche, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO, Meiji, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, Takeda, UCB and Valeant; and as a paid speaker for AbbVie, Akros, Allergan, Almirall, Amgen, Bausch Health, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly and Company, Galderma, Genentech/Roche, Janssen, Kyowa Kirin, LEO, Meiji, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, Takeda, UCB and Valeant. A.G. has served as a consultant or speaker for Janssen, Celgene, Beiersdorf, Bristol‐Myers Squibb, AbbVie, UCB, Novartis, Incyte, Eli Lilly and Company, Allergan, Sun Pharmaceutical Industries, Xbiotech, LEO, Avotres Therapeutics and Boehringer Ingelheim; and received research/educational grants from Janssen, Incyte, Novartis, Xbiotech, UCB and Boehringer Ingelheim. A.J. has served as scientific advisor or clinical study investigator for AbbVie, Asana Biosciences, Castle Biosciences, Inc., Bristol‐Myers Squibb, Celgene, Dermira, Eli Lilly and Company, Galderma, Genentech/Roche, GlaxoSmithKline, LEO Pharma, Novartis, Pfizer, Purdue Pharma, Regeneron, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma and UCB Pharma, and as a paid speaker for Castle Biosciences, Inc., Eli Lilly and Company, Novartis, Regeneron and Sanofi Genzyme. K.R. has served as an advisor and paid speaker and has participated in clinical trials for AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Celgene, Covagen, Forward Pharma, Fresenius Medical Care, GlaxoSmithKline, Janssen, Janssen‐Cilag, Kyowa Kirin, LEO Pharma, Eli Lilly and Company, Medac, Merck Sharp & Dohme, Novartis, Miltenyi Biotech, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant, XBiotech and Xenoport. C.M. has served as principal investigator, as a speaker or on a scientific advisory board for and received compensation in the form of honoraria from AbbVie, Amgen, Celgene, Janssen, LEO Pharma, GlaxoSmithKline, Bausch Health, Eli Lilly and Company, Novartis, Pfizer and UCB Pharma. K.B.G. has consulting relationships with AbbVie, Amgen, Celgene, Eli Lilly and Company, Janssen, Novartis, Pfizer, Dermira and Boehringer Ingelheim and has received grants from AbbVie, Amgen, Celgene and Janssen. L.K.F. has been an investigator and consultant for Eli Lilly and Company, Janssen and Pfizer; a consultant for UCB; and an investigator for AbbVie, Amgen, Galderma, LEO Pharma and Regeneron. R.G. Langley has served as principal investigator, as a speaker and on the scientific advisory board for and received compensation in the form of honoraria from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Eli Lilly and Company, Merck, Novartis, Pfizer, Sun and UCB Pharma. Y.T. received grants for research from Maruho, LEO Pharma, Eisai, AbbVie, Kyowa Hakko Kirin, Taiho Pharmaceutical, Celgene, and Eli Lilly and Company, and honoraria for lectures from Torii Pharmaceutical, Maruho, LEO Pharma, Eisai, AbbVie, Kyowa Hakko Kirin, Eli Lilly and Company, Taiho Pharmaceutical, Mitsubishi Tanabe Pharma and Janssen. R.G. Lima, H.E., G.G., L.R., S.Y.P. and R.B. are employees and stockholders of Eli Lilly and Company. J.B. is a speaker and investigator for AbbVie, Celgene, Eli Lilly and Company, Janssen, Novartis and Ortho Dermatologics. He is an investigator for Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb and LEO Pharma.
Appendix 2.
List of IXORA‐R study investigators (see File S1 in the Supporting Information for a list of IXORA‐R study investigators by study site): Alim Devani; Ronald Vender; Mark A. Lomaga; Isabelle Delorme; Chih‐Ho Hong; Richard L. Langley; Lorne Albrecht; Lyn Guenther; Catherine Maari; Kim Papp; Kamal K. Singh Ohson; Kirk Barber; Charles Lynde; Aditya Gupta; Leslie Rosoph; Jean‐Sébastien Gauthier; Melinda Gooderham; Norman Wasel; Mani Raman; Marni Wiseman; David Greenstein; Abel Jarell; Charles Moon; Lani Clark; Sadra Sasha Jazayeri; Michael Bukhalo; Angela Moore; Tiffani K. Hamilton; Aron Gewirtzman; Lydie Hazan; Jeffrey Crowley; Craig Teller; Matthew Zirwas; Stacy R. Smith; M. Christine Lee; Stephen Tyring; Patricia Lee; Sunil Dhawan; Craig Leonardi; Amarilis Perez‐De Jesus; Wendy McFalda; Ellen Frankel; Paul Yamauchi; Scott Fretzin; Rocco Serrao; Todd Schlesinger; Scott Gottlieb; Peter Jenkin; Rola Gharib; Steven Davis; Navid Nami; Zoe Diana Draelos; Lloyd Godwin; Cindy Owen; Megan Landis; William Abramovits; Samuel Sanchez‐Rivera; Abby Van Voorhees; David Fivenson; Francisco Kerdel; Seth B. Forman; Jeffrey Weinberg; Jose Gonzalez‐Chavez; Brent Boyce; Linda Stein‐Gold; Charles Hudson; Constance Brown; James Coggi; Christina Feser; Rion Forconi; Sandra Johnson; Mark McCune; Lawrence Green; Vandana Madkan; Dana Maxwell Shipp; Kenneth Gordon; Jill Waibel; Oscar Soto‐Raices; Jennifer Cather; Scott Miller; John Scott; Douglas Young; Jessica Kaffenberger; Kelley Yokum; Matthew Zook; Andrew Blauvelt; Artis Truett; George Schmieder; Gary McCracken; Patrick McElgunn; James Herrmann; Jeffery M. Suchniak; James Appel; Elizabeth Barranco; Mark Lee; Jerry Bagel; Lawrence Osman; Ashley Cauthen; Neil Sadick; Eneida De La Torre; Kelly Taylor; David Cohen; Holly Hake Harris; Jennifer Soung; Vassilios Dimitropoulos; Stephen Miller; Cathy Barnes; Rawan Jumean‐Haddad; Suzanne Bruce; Lawrence Cheung; Scott Guenthner; Anthony Gaspari; Vivian Laquer; James M. Krell; Shahram Jacobs; Walter Nahm; Neil Korman; Boni Elewski; Laura Ferris; Kristina Callis Duffin; David Pariser; Brian Johnson; Paul Wallace; Jeffrey Travers; Richard Fried
Funding sources Funding for this study was provided by Eli Lilly and Company, Indianapolis, IN, U.S.A. Eli Lilly and Company contributed to study design, data collection, data analysis, data interpretation, manuscript preparation and the decision to submit the paper for publication. An advisory committee was involved in the study design and data interpretation, together with authors from Eli Lilly and Company. Authors had full access to all group‐level data in the study, but not individual‐level data that would risk unblinding those authors who were also study investigators. Authors had final responsibility for the decision to submit for publication.
Conflicts of interest Conflicts of interest statements are listed in Appendix 1.
The members of the IXORA‐R Study Group are listed in Appendix 2.
Plain language summary available online
Contributor Information
A. Blauvelt, Email: ablauvelt@oregonmedicalresearch.com.
the IXORA‐R Study Group:
Ronald Vender, Mark A. Lomaga, Isabelle Delorme, Chih‐Ho Hong, Richard L. Langley, Lorne Albrecht, Lyn Guenther, Catherine Maari, Kim Papp, Kamal K. Singh Ohson, Kirk Barber, Charles Lynde, Aditya Gupta, Leslie Rosoph, Jean‐Sébastien Gauthier, Melinda Gooderham, Norman Wasel, Mani Raman, Marni Wiseman, David Greenstein, Abel Jarell, Charles Moon, Lani Clark, Sadra Sasha Jazayeri, Michael Bukhalo, Angela Moore, Tiffani K. Hamilton, Aron Gewirtzman, Lydie Hazan, Jeffrey Crowley, Craig Teller, Matthew Zirwas, Stacy R. Smith, M. Christine Lee, Stephen Tyring, Patricia Lee, Sunil Dhawan, Craig Leonardi, Amarilis Perez‐De Jesus, Wendy McFalda, Ellen Frankel, Paul Yamauchi, Scott Fretzin, Rocco Serrao, Todd Schlesinger, Scott Gottlieb, Peter Jenkin, Rola Gharib, Steven Davis, Navid Nami, Zoe Diana Draelos, Lloyd Godwin, Cindy Owen, Megan Landis, William Abramovits, Samuel Sanchez‐Rivera, Abby Van Voorhees, David Fivenson, Francisco Kerdel, Seth B. Forman, Jeffrey Weinberg, Jose Gonzalez‐Chavez, Brent Boyce, Linda Stein‐Gold, Charles Hudson, Constance Brown, James Coggi, Christina Feser, Rion Forconi, Sandra Johnson, Mark McCune, Lawrence Green, Vandana Madkan, Dana Maxwell Shipp, Kenneth Gordon, Jill Waibel, Oscar Soto‐Raices, Jennifer Cather, Scott Miller, John Scott, Douglas Young, Jessica Kaffenberger, Kelley Yokum, Matthew Zook, Andrew Blauvelt, Artis Truett, George Schmieder, Gary McCracken, Patrick McElgunn, James Herrmann, Jeffery M. Suchniak, James Appel, Elizabeth Barranco, Mark Lee, Jerry Bagel, Lawrence Osman, Ashley Cauthen, Neil Sadick, Eneida De La Torre, Kelly Taylor, David Cohen, Holly Hake Harris, Jennifer Soung, Vassilios Dimitropoulos, Stephen Miller, Cathy Barnes, Rawan Jumean‐Haddad, Suzanne Bruce, Lawrence Cheung, Scott Guenthner, Anthony Gaspari, Vivian Laquer, James M. Krell, Shahram Jacobs, Walter Nahm, Neil Korman, Boni Elewski, Laura Ferris, Kristina Callis Duffin, David Pariser, Brian Johnson, Paul Wallace, Jeffrey Travers, and Richard Fried
References
- 1. Boehncke WH, Schon MP. Psoriasis. Lancet 2015; 386:983–94. [DOI] [PubMed] [Google Scholar]
- 2. Warren RB, Kleyn CE, Gulliver WP. Cumulative life course impairment in psoriasis: patient perception of disease‐related impairment throughout the life course. Br J Dermatol 2011; 164 (Suppl 1):1–14. [DOI] [PubMed] [Google Scholar]
- 3. Blome C, Gosau R, Radtke MA et al Patient‐relevant treatment goals in psoriasis. Arch Dermatolog Res 2016; 308:69–78. [DOI] [PubMed] [Google Scholar]
- 4. Carrascosa JM, de la Cueva P, Herranz P et al Perception of psoriasis treatment in the outpatient setting: survey of patients and their prescribing physicians. J Dermatol Treat 2017; 28:188–99. [DOI] [PubMed] [Google Scholar]
- 5. Gorelick J, Shrom D, Sikand K et al Understanding treatment preferences in patients with moderate to severe plaque psoriasis in the USA: results from a cross‐sectional patient survey. Dermatol Ther (Heidelb) 2019; 9:785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seston EM, Ashcroft DM, Griffiths CE. Balancing the benefits and risks of drug treatment: a stated‐preference, discrete choice experiment with patients with psoriasis. Arch Dermatol 2007; 143:1175–9. [DOI] [PubMed] [Google Scholar]
- 7. Globe D, Bayliss MS, Harrison DJ. The impact of itch symptoms in psoriasis: results from physician interviews and patient focus groups. Health Qual Life Out 2009; 7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lebwohl MG, Bachelez H, Barker J et al Patient perspectives in the management of psoriasis: results from the population‐based multinational assessment of psoriasis and psoriatic arthritis survey. J Amer Acad Dermatol 2014; 70:871–81.e1‐30. [DOI] [PubMed] [Google Scholar]
- 9. Amatya B, Wennersten G, Nordlind K. Patients’ perspective of pruritus in chronic plaque psoriasis: a questionnaire‐based study. J Eur Acad Dermatol Venereol 2008; 22:822–6. [DOI] [PubMed] [Google Scholar]
- 10. Murage MJ, Tongbram V, Feldman SR et al Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence 2018; 12:1483–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim HJ, Lebwohl MG. Biologics and psoriasis: the beat goes on. Dermatol Clin 2019; 37:29–36. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura M, Lee K, Jeon C et al Guselkumab for the treatment of psoriasis: a review of phase iii trials. Dermatol Ther (Heidelb) 2017; 7:281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gordon KB, Blauvelt A, Papp KA et al Phase 3 trials of ixekizumab in moderate‐to‐severe plaque psoriasis. N Engl J Med 2016; 375:345–56. [DOI] [PubMed] [Google Scholar]
- 14. Egeberg A, Andersen YMF, Halling‐Overgaard AS et al Systematic review on rapidity of onset of action for interleukin‐17 and interleukin‐23 inhibitors for psoriasis. J Eur Acad Dermatol Venereol 2019; DOI: 10.1111/jdv.15920. [DOI] [PubMed] [Google Scholar]
- 15. Leonardi CL, Blauvelt A, Sofen HL et al Rapid improvements in health‐related quality of life and itch with ixekizumab treatment in randomized phase 3 trials: results from UNCOVER‐2 and UNCOVER‐3. J Eur Acad Dermatol Venereol 2017; 31:1483–90. [DOI] [PubMed] [Google Scholar]
- 16. Blauvelt A, Papp KA, Griffiths CE et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. J Amer Acad Dermatol 2017; 76:405–17. [DOI] [PubMed] [Google Scholar]
- 17. Griffiths CE, Reich K, Lebwohl M et al Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet 2015; 386:541–51. [DOI] [PubMed] [Google Scholar]
- 18. Warren RB, See K, Burge R et al Rapid response of biologic treatments of moderate‐to‐severe plaque psoriasis: a comprehensive investigation using Bayesian and frequentist network meta‐analyses. Dermatol Ther (Heidelb) 2019; DOI: 10.1007/s13555-019-00337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reich K, Pinter A, Lacour JP et al Comparison of ixekizumab with ustekinumab in moderate‐to‐severe psoriasis: 24‐week results from IXORA‐S, a phase III study. Br J Dermatol 2017; 177:1014–23. [DOI] [PubMed] [Google Scholar]
- 20. Langley RG, Kimball AB, Nak H et al Long‐term safety profile of ixekizumab in patients with moderate‐to‐severe plaque psoriasis: an integrated analysis from 11 clinical trials. J Eur Acad Dermatol Venereol 2019; 33:333–9. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong A, Paul C, Puig L et al Safety of ixekizumab treatment for up to 5 years in adult patients with moderate‐to‐severe psoriasis: results from greater than 17,000 patient‐years of exposure. Dermatol Ther (Heidelb) 2019; DOI: 10.1007/s13555-019-00340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lebwohl MG, Gordon KB, Gallo G et al Ixekizumab sustains high level of efficacy and favourable safety profile over 4 years in patients with moderate psoriasis: results from UNCOVER‐3 study. J Eur Acad Dermatol Venereol 2019; DOI: 10.1111/jdv.15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reich K, Armstrong AW, Langley RG et al Guselkumab versus secukinumab for the treatment of moderate‐to‐severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet 2019; 394:831–9. [DOI] [PubMed] [Google Scholar]
- 24. Hongbo Y, Thomas CL, Harrison MA et al Translating the science of quality of life into practice: what do Dermatology Life Quality Index scores mean? J Invest Dermatol 2005; 125:659–64. [DOI] [PubMed] [Google Scholar]
- 25. Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol 2015; 29:645–8. [DOI] [PubMed] [Google Scholar]
- 26. Feldman SR, Bushnell DM, Klekotka PA et al Differences in psoriasis signs and symptom severity between patients with clear and almost clear skin in clinical practice. J Dermatolog Treat 2016; 27:224–7. [DOI] [PubMed] [Google Scholar]
- 27. Strober B, Papp KA, Lebwohl M et al Clinical meaningfulness of complete skin clearance in psoriasis. J Am Acad Dermatol 2016; 75:77–82.e7. [DOI] [PubMed] [Google Scholar]
- 28. Viswanathan HN, Chau D, Milmont CE et al Total skin clearance results in improvements in health‐related quality of life and reduced symptom severity among patients with moderate to severe psoriasis. J Dermatolog Treat 2015; 26:235–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1 List of IXORA‐R study investigators by study site.
File S2 Supplementary methods.
Table S1 Primary, major secondary and exploratory end points.
Fig S1. Study design.
Fig S2. Proportion of patients achieving resolution of skin pain for the ixekizumab (N = 520) and guselkumab (N = 507) groups.
Video S1 Author video.


