Abstract
Within the current paradigm of the myonuclear domain theory, it is postulated that a linear relationship exists between muscle fibre size and myonuclear content. The myonuclear domain is kept (relatively) constant by adding additional nuclei (supplied by muscle satellite cells) during muscle fibre hypertrophy and nuclear loss (by apoptosis) during muscle fibre atrophy. However, data from recent animal studies suggest that myonuclei that are added to support muscle fibre hypertrophy are not lost within various muscle atrophy models. Such myonuclear permanence has been suggested to constitute a mechanism allowing the muscle fibre to (re)grow more efficiently during retraining, a phenomenon referred to as “muscle memory.” The concept of “muscle memory by myonuclear permanence” has mainly been based on data attained from rodent experimental models. Whether the postulated mechanism also holds true in humans remains largely ambiguous. Nevertheless, there are several studies in humans that provide evidence to potentially support or contradict (parts of) the muscle memory hypothesis. The goal of the present review was to discuss the evidence for the existence of “muscle memory” in both animal and human models of muscle fibre hypertrophy as well as atrophy. Furthermore, to provide additional insight in the potential presence of muscle memory by myonuclear permanence in humans, we present new data on previously performed exercise training studies. Finally, suggestions for future research are provided to establish whether muscle memory really exists in humans.
Keywords: muscle adaptation, muscle memory, myonuclear domain size, myonuclei, satellite cell
1. INTRODUCTION
Skeletal muscle fibres are large multinucleated cells, with every muscle fibre containing hundreds to thousands of nuclei. For example, a single human biceps muscle fibre of 10 cm in length contains about 3000 nuclei. 1 Whereas in most cells the nucleus occupies the centre of the cell body, within muscle fibres the nuclei are positioned peripherally adjacent to the plasma membrane. Only under certain conditions like development, repair/regeneration, or specific pathologies, nuclei can be found migrating to the centre of a muscle fibre. Myonuclei are post‐mitotic, and have a flattened and elongated shape with the long‐axis typically running parallel to the longitudinal axis of the muscle fibre. Nuclei are not randomly distributed within the muscle fibre. In fact, nuclei appear to repel each other during positioning resulting in an evenly distributed configuration within the muscle fibre. 2 As early as the 19th century, 3 it was hypothesized that every nucleus holds jurisdiction over a certain volume of the muscle fibre cytoplasm, initially referred to as the “karyoplasmatic” ratio. More recently, the concept of a muscle fibre being divided into evenly distributed compartments, each under the control of a single myonucleus, has been referred to as the “DNA unit” 4 or “myonuclear domain”. 5 According to the myonuclear domain theory, every nucleus has a limited transcriptional capacity, only synthesizing proteins for use in the immediate vicinity surrounding the nucleus. 5 , 6
Based on the concept of the myonuclear domain theory, an approximate linear relationship must exist between total myonuclear number and muscle fibre size and/or volume. Although the myonuclear domain theory was quickly adopted by many investigators, it continues to be intensely debated within the field. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 For instance, based on the paradigm, the myonuclear domain should be kept (relatively) constant by adding additional nuclei (supplied by muscle satellite cells) during muscle fibre hypertrophy and by nuclear loss (through apoptosis) during muscle fibre atrophy. 14 However, more recent animal studies demonstrated that myonuclei do not appear to be lost with various muscle atrophy models. 7 Furthermore, it has been hypothesized that myonuclei that are added to support muscle fibre hypertrophy are not lost during detraining. 15 , 16 Such a “myonuclear permanence” could constitute a mechanism allowing the muscle fibre to grow more efficiently during retraining, as the myonuclear number would remain in an elevated or “trained” state. This potential phenomenon is referred to as “muscle memory”. 8 The proposed existence of a “muscle memory” to hypertrophic stimuli may hold a number of consequences across athletic and clinical settings. For instance, anabolic steroids are used across a variety of elite sporting competitions to induce muscle growth and enhance recovery. In this setting, the ability to permanently add nuclei to muscle fibres may create an unfair competitive advantage to previously caught and suspended doping offenders when returning back to competition. However, muscle memory may also promote quicker muscle (re)growth in older adults who participated in resistance‐type exercise training earlier in life, potentially providing a clinical benefit when aiming to combat age‐related muscle loss. Importantly though, the “muscle memory” hypothesis has mainly been based on data attained from experimental rodent models. As clear differences in muscle architecture and metabolism exist between species, 17 , 18 translating these results to humans is challenging. Thus far, any evidence from human studies to either support or refute the muscle memory hypothesis has largely been ignored. The goal of the present review was to showcase any evidence for the presence or absence of the proposed “muscle memory” in both animal and human models of muscle fibre atrophy and hypertrophy. To provide additional insight on the potential presence of muscle memory by myonuclear permanence in vivo in humans, we have re‐analysed some of our previously performed exercise training studies. Finally, we provide suggestions for future research to establish whether muscle memory exists in humans.
2. CURRENT EVIDENCE FROM ANIMAL STUDIES
According to the original hypothesis of a constant myonuclear domain, myonuclei are added during muscle fibre growth and lost during muscle atrophy. 14 In contrast, the muscle memory theory postulates that myonuclei are never lost from the skeletal muscle fibre, resulting in a reduction in myonuclear domain size during muscle fibre atrophy. A large number of animal studies, however, have reported a reduction in myonuclear number in atrophy‐inducing models such as denervation, 19 , 20 , 21 , 22 spinal cord transection, 23 , 24 , 25 mechanical unloading 26 , 27 , 28 , 29 , 30 and spaceflight 31 , 32 , 33 (see Table 1). Importantly though, many of these studies have used muscle cross‐sections to count myonuclei, and the determination of myonuclear content and apoptosis within muscle cryosections is not without uncertainty. 7 , 15 , 34 Using conventional histology, in which nuclei are labelled (eg DAPI or Hoechst dye) for light or fluorescent microscopy on muscle cross sections, there may be difficulties to distinguish true myonuclei from other nuclei, in particular muscle satellite cells. Until recently, 35 no antibody was available that specifically stains for myonuclei. Therefore, a staining approach in which a nuclear stain is combined with specific antibodies that delineates the muscle cell border would be an absolute requirement to allow clear distinction of nuclei located in or outside the muscle fibre. Staining against laminin or dystrophin protein is currently most frequently used to visualize muscle fibre cell borders. Some have suggested that staining of dystrophin may be more accurate in the assessment of myonuclear content than laminin, 36 , 37 however, this remains to be more firmly established in both rodent as well as human skeletal muscle cross‐sections. Besides delineation of the muscle fibre border, it is critical to exclude muscle satellite cells from the myonuclear count as these cells are also located inside (between sarcolemma and basal lamina) the muscle fibre. Although muscle satellite cells account for only 2%‐4% of total myonuclei within muscle fibres, their response can be dramatically different in various experimental conditions. Distinction of muscle satellite cell from myonuclei based on anatomical reference is possible using electron microscopy, 38 but remains very challenging with light or fluorescent microscopy. Hence, best practice would be to use a specific antibody that stains myonuclei only, and promising results have been shown using PCM1 labelling. 35 However, when nuclear labelling by for example DAPI or Hoechst is used, co‐staining should be performed to delineate the cell border (eg laminin, dystrophin) and satellite cells (eg NCAM or Pax7) to make a reliable and valid estimation of myonuclear number. In the past, some 30 , 39 , 40 , 41 but certainly not all, 15 , 16 , 21 , 23 , 24 , 26 , 28 , 29 , 32 , 33 , 34 , 36 , 42 , 43 , 44 studies have used this approach to evaluate myonuclear content in response to different animal muscle atrophy models. Caution should also be taken when myonuclear number per fibre length is inferred from muscle cross‐sectional data, as myonuclear shape and size have been observed to change in various experimental models. 22 Alternatively, myonuclear number can also be determined in mechanically isolated muscle fibres, which has the advantage that non‐muscle nuclei are removed 15 , 19 , 20 , 25 , 26 , 27 , 28 , 31 , 34 , 36 , 40 , 41 , 44 , 45 , 46 , 47 , 48 (Table 1). Although fibre isolation allows inclusion of a relatively high number of myonuclei per fibre, the total number of evaluated fibres is often low, which may limit accurate representation of muscle tissue.
TABLE 1.
Changes in myonuclear content and apoptosis in response to various muscle atrophy models in rodents
| Study | Specie | Muscle | Atrophy model | Atrophy duration | Muscle cross‐section | Single muscle fibre | Muscle homogenate | Myonuclear content | Myonuclear apoptosis |
|---|---|---|---|---|---|---|---|---|---|
| Nuclear (ie Haematoxylin, TUNEL, Caspase, Bcl, Propidium idodide, Hoechst, DAPI, or Arcidine) staining only | |||||||||
| Tews et al 57 | Rat | Levator labii | Denervation | 2, 6, 7, 10, 20 wk | x | Yes | |||
| Viguie et al 20 | Rat | EDL | Denervation | 2, 4, 7, 12, 18 mon | x | Decline | |||
| Yoshimura et al 43 , * | Rat | Grac | Denervation | 7, 14, 21, 28 d | x | Yes | |||
| Schmalbruch et al 22 , * | Rat | EDL, Sol | Denervation | 1, 2, 8, 22 wk | x | Decline | |||
| Borisov et al 21 | Rat | TA, EDL, Sol | Denervation | 2, 4, 7 mon | x | Yes | |||
| Jin et al 42 , * | Rat | Brach. triceps | Denervation | 16 wk | x | Yes | |||
| Wada et al 119 | Mouse | Plan | Denervation | 4 mon | x | No change | |||
| Aravamudan et al 19 | Rat | Dia | Denervation | 2 wk | x | Decline | |||
| Bruusgaard et al 34 | Mouse | EDL, Sol |
Denervation/ mechanical unloading/ nerve impulse block |
7, 14, 21 d | x | No change | |||
| Lim et al 56 | Rat | Gas | Denervation | 8 wk | x | Yes | |||
| Bruusgaard et al 15 , * | Mouse | EDL | Denervation | 14 d | x | No change | |||
| Lee et al 54 , * | Rat | Gas, Sol | Denervation | 4 wk | x | Yes | |||
| Allen et al 25 | Cat | Sol | Spinal cord transection | 6 mon | x | Decline | |||
| Dupont‐Versteegden et al 23 , * | Rat | Sol | Spinal cord transection | 10 d | x | Yes | |||
| Zhong et al 48 | Rat | Sol | Spinal cord transection | 4, 60 d | x | No change | |||
| Darr et al 26 | Rat | EDL, Sol | Mechanical unloading | 3, 10 d | x | x | Decline | Yes | |
| Kasper et al 45 | Rat | Gas, TA | Mechanical unloading | 28 d | x | No change | |||
| Allen et al 27 | Rat | Sol | Mechanical unloading | 14 d | x | Decline | Yes | ||
| Allen et al 28 , * | Rat | Sol | Mechanical unloading | 14 d | x | Decline | Yes | ||
| Mozdziak et al 47 | Rat | Sol | Mechanical unloading | 28 d | x | Decline | |||
| Leeuwenburgh et al 29 , * | Rat | Sol | Mechanical unloading | 14 d | x | Yes | |||
| Dupont‐Versteegden et al 51 , * | Rat | Sol | Mechanical unloading | 7 d | x | Yes | |||
| Jackson et al 44 , * | Mouse | Sol, Gas | Mechanical unloading | 14 d | x | No change | |||
| Allen et al 31 | Rat | Sol | Space flight | 14 d | x | Decline | |||
| Kasper et al 46 | Rat | Gas, TA | Space flight | 5.4 d | x | Increase | |||
| Lee et al 41 , * | Rat | FHL | Detraining | 20 wk | x | No change | |||
| Dungan et al 40 , * | Mouse | Plan | Detraining | 12 wk | x | Decline | |||
| Winje et al 36 , * | Mouse | EDL, Sol, TA | Prostate cancer xenograft | 6 wk | x | No change | NO | ||
| Nuclear (ie Dapi, Hoechst dye, TUNEL, EndoG) staining with cell border (ie Laminin or dystrophin) identification | |||||||||
| Bruusgaard et al 15 , * | Mouse | EDL | Denervation | 14 d | x | No change | No | ||
| Bruusgaard et al 34 , * | Mouse | EDL, Sol |
Denervation/ mechanical unloading/ nerve impulse block |
7, 14, 21 d | x | No change | No | ||
| Dupont‐Versteegden et al 23 , * | Rat | Sol | Spinal cord transection | 10 d | x | Decline | |||
| Dupont‐Versteegden et al 24 | Rat | Sol, Plan | Spinal cord transection | 8 wk | x | Decline | |||
| Allen et al 28 , * | Rat | Sol | Mechanical unloading | 14 d | x | Decline | Yes | ||
| Leeuwenburgh et al 29 , * | Rat | Sol | Mechanical unloading | 14 d | x | Decline | Yes | ||
| Hao et al 55 | Rat | Sol, Plan | Mechanical unloading | 14 d | x | Yes | |||
| Bruusgaard et al 15 , * | Rat | EDL, Sol | Mechanical unloading | 14 d | x | No change | No | ||
| Jackson et al 44 , * | Mouse | Sol, Gas | Mechanical unloading | 14 d | x | No change | |||
| Hikida et al 32 | Rat | Sol | Space flight | 10 d | x | Decline | |||
| Sandona et al 33 | Mouse | EDL, Sol | Space flight | 20 d | x | Decline | |||
| Egner et al 16 , * | Mouse | EDL, Sol | Detraining | 14 d, 3 mon | x | No change | No | ||
| Winje et al 36 , * | Mouse | EDL, Sol, TA | Prostate cancer xenograft | 6 wk | x | No change | No | ||
| Nuclear staining with cell border and satellite cell (ie Pax7 or NCAM) identification | |||||||||
| Adhihetty et al 39 , * | Rat | TA, EDL | Denervation | 5, 7, 14, 21, 42 d | x | Yes | |||
| Matsuba et al 30 | Mouse | Sol | Mechanical unloading | 14 d | x | No change | |||
| Lee et al 41 , * | Rat | FHL | Detraining | 20 wk | x | No change | |||
| Dungan et al 40 , * | Mouse | Plan | Detraining | 12 wk | x | Decline | |||
| Biochemical assay of muscle homogenate (ie RT‐Pcr, Western blotting, Elisa) | |||||||||
| Yoshimura et al 43 , * | Rat | Grac | Denervation | 7, 14, 21, 28 d | x | Yes | |||
| Tang et al 53 | Rat | Gas | Denervation | 30 d | x | Yes | |||
| Jin et al 42 , * | Rat | Brach. tri | Denervation | 16 wk | x | Yes | |||
| Alway et al 49 | Rat | Sol, Gas | Denervation | 14 d | x | Yes | |||
| Adhihetty et al 39 , * | Rat | TA, EDL | Denervation | 5, 7, 14, 21, 42 d | x | Yes | |||
| Lim et al 56 , * | Rat | Gas | Denervation | 8 wk | x | Yes | |||
| Lee et al 54 , * | Rat | Gas, Sol | Denervation | 4 wk | x | Yes | |||
| Alway et al 50 | Quail | Patagialis | Mechanical unloading | 14 d | x | Yes | |||
| Siu et al 120 | RAT | Gas | Mechanical unloading | 14 d | x | Yes | |||
| Dupont‐Versteegden et al 51 , * | Rat | Sol | Mechanical unloading | 7 d | x | Yes | |||
| Hao et al 55 | Rat | Sol, Plan | Mechanical unloading | 14 d | x | Yes | |||
| In vivo imaging | |||||||||
| Bruusgaard et al 34 , * | Mouse | EDL, Sol | Denervation/Mechanical unloading/ nerve impulse block | 7, 14, 21 d | x | No change | No | ||
| Bruusgaard et al 15 , * | Mouse | EDL | Denervation | 14 d | x | No change | no | ||
| Egner et al 16 , * | Mouse | EDL, Sol | Detraining | 14 d, 3 mon | x | No change | No | ||
| Winje et al 36 , * | Mouse | EDL, Sol, TA | Prostate cancer xenograft | 6 wk | x | No change | No | ||
This table provides an overview of studies that have assessed the change in myonuclear content and presences of (myo)nuclear apoptosis in response to various muscle atrophy models in animals. Studies are organized by analytical technique to assess (myo)nuclear content, apoptosis or both. EDL, extensor digitorum longus muscle; FHL, flexor hallucis longis; Sol, soleus muscle; Plan, plantaris muscle; TA, tibialis anterior muscle; Gas, gastrocnemius muscle; Grac, gracillis muscle.
Note that these studies appear multiple times in the table, as multiple analyses were performed within the same study.
The loss of myonuclear content during atrophy appears to be in line with the frequently reported increase in the number of apoptotic (myo)nuclei in several animal studies 39 , 42 , 43 , 49 , 50 , 51 , 52 , 53 (Table 1). Apoptosis, or programmed cell death, is currently the (only) suggested mechanism through which myonuclei are removed from skeletal muscle tissue. Yet, evidence for myonuclear apoptosis during muscle atrophy remains rather ambiguous. For example, the increase in apoptotic markers (eg TUNEL, EndoG, Bcl, Caspase) observed following experimental animal atrophy models using biochemical assays (eg western blot, Elisa, RT‐Pcr) is evaluated in muscle homogenates from which up to 50% of the nuclei are not myonuclei. 39 , 42 , 43 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 Most studies that reported an increase in the number of apoptotic nuclei on muscle cross‐sections following atrophy were not able to accurately assess whether the nuclei were located in or outside the muscle fibre, due to the lack of a cell border markers. 21 , 23 , 26 , 27 , 28 , 42 , 43 , 54 , 56 , 57 When proper staining for the cell border is performed, only very low numbers of apoptotic (0.015%) myonuclei has been observed. 15 , 34 , 58 Nuclear destruction during apoptosis is, however, very rapid and comprises only 2‐3 hours, 59 as such, some apoptotic activity in intact muscle fibres could not be entirely be ruled out. When apoptotic myonuclei are detected though, a small number of apoptotic myonuclei may likely reflect significant removal of myonuclei from skeletal muscle tissue. When nuclei are properly identified as myonuclei in muscle cross‐sections (ie, nuclei located inside the muscle fibre), some do, 28 , 29 , 39 , 55 whereas others do not 15 , 16 , 34 , 36 , 58 report an increase in apoptotic myonuclei following muscle fibre atrophy in rodent muscle (Table 1). Yet, whether this discrepancy can be explained by the different markers used to identify apoptosis, the in/exclusion of satellite cells, and/or the model or duration of the induced atrophy remains to be further established.
2.1. A potential paradigm shift
One of the first studies to challenge the supposedly established concept that myonuclei are lost during atrophy in animal skeletal muscle was performed by Bruusgaard et al 34 using direct in vivo time‐lapse imaging of single muscle fibres. In this study, myonuclei were labelled with green‐fluorescent protein (GFP) by somatic gene transfer using electroporation or intracellular injection. As the GFP label is based on water soluble oligonucleotides, and no gap junctions are present between satellite cells and the muscle fibre, muscle satellite cells are not labelled and, as such, automatically excluded from the analyses. 60 Using repeated exposure and imaging of the same fibre segments, changes in myonuclear content were assessed, in vivo, up to 4 weeks after initiating muscle atrophy. Although denervation, nerve blockage, or hindlimb suspension caused as much as 50% muscle fibre atrophy in adult mice, no changes in myonuclear content were observed. 34 Furthermore, the authors argued that nuclear apoptosis observed during atrophy was confined to muscle satellite and stromal cells, instead of myonuclei. 34 The ability to re‐assess the same muscle fibre segments in vivo by time‐lapse imaging may be the most accurate measurement to date to assess changes in myonuclear content during muscle fibre atrophy. However, it is important to note that the in vivo time‐lapse imaging findings used in this study were in line with the change in myonuclear content determined by conventional histology (staining for dystrophin and Hoechst) on muscle cross‐sections, the latter of which clearly contradicts earlier studies showing a loss in myonuclear content during atrophy using such a histological staining approach. 23 , 24 , 27 , 30 , 32 , 33 , 39 , 61 In line with these experimental muscle atrophy models, Schwartz et al 62 showed that myonuclear content also remains unchanged during naturally occurring hormonally triggered muscle atrophy (49% decrease in fibre CSA within 3 days) in the intersegmental muscle from the tobacco hawkmoth. The strength of this model is that the intersegmental muscle does not contain capillaries, satellite cells, endothelial cells or pericytes, meaning that all nuclei may be considered myonuclei. 63 However, the suggestion that the loss of 49% muscle mass within 3 days represents a model for the muscle mass loss during human aging over a period of 30 years would be too simplistic. 12 An alternative model of muscle fibre atrophy over a more prolonged period of time is cancer cachexia. Prostate cancer xenografting in mice has been reported to induce substantial muscle fibre atrophy (21%) over a 6‐week period. 36 In line with the above findings, this study reported no changes in myonuclear content assessed by in vivo imaging in single muscle fibres, as well as histological analyses of muscle cross sections. 36
2.2. Muscle memory by myonuclear permanence
The potential lack of myonuclear loss (by apoptosis) during extreme muscle atrophy models does not yet constitute convincing evidence for myonuclear permanence and the existence of muscle memory. The first experimental evidence for myonuclear retention following muscle hypertrophy was published by the Gundersen research group. 58 In this study, the extensor digitorum longus (EDL) muscle of mice was overloaded by synergistic ablation resulting in a significant increase in muscle fibre size and myonuclear content. More importantly, the elevated myonuclear number persisted during 3 months of subsequent denervation which was accompanied by severe muscle fibre atrophy. 58 Though a high degree of apoptosis was observed following 3 months of denervation, both long‐standing as well as newly acquired myonuclei were excluded from this process. 58 Additional studies in mice demonstrated no changes in myonuclear content during 2 weeks of hindlimb suspension. 15 , 44 During 2 weeks of subsequent reloading, muscle fibre size was recovered back to baseline without any change in myonuclear content, suggesting that myonuclear addition may only occur in muscle fibres that undergo growth beyond their “baseline” size. 15 , 44 Further evidence supporting the muscle memory hypothesis was provided when the same group investigated the impact of anabolic steroid‐induced muscle fibre growth in female mice. 16 Here, Egner et al 16 implanted pellets releasing testosterone propionate (or sham) subcutaneously for 14 days, with or without overload of the Soleus (Sol) and EDL muscle. A robust increase in muscle fibre size and myonuclear content were observed in response to the steroid treatment in both overloaded and non‐overloaded muscles. One week after the steroid treatment was withdrawn, blood testosterone concentrations were back to baseline (undetectable levels). When the muscle was assessed three weeks later, myonuclear content remained 42% higher compared with the sham treatment group, whereas muscle fibre size had returned back to baseline levels. 16 When overload was subsequently introduced for a 14‐day period, the group who had undergone previous steroid treatment showed a muscle fibre hypertrophy response which was more than double compared with sham‐treated mice. Similar results were also observed when a 6‐day overload period was introduced 3 months (constituting about ~12% of a mouse life span) after the testosterone propionate‐releasing implant was removed in these mice. 16 Although muscle fibre hypertrophy was reversible in this model, a previous hypertrophic condition appeared to convey a lasting imprint on muscle fibres in the form of an elevated number of myonuclei, which contributed to the ability to regain muscle mass more quickly during a subsequent overload stimulus. In other words, a single episode of anabolic steroid use may have a long lasting, if not permanent, effect on the ability for muscle to (re)grow during training. This may pose a performance advantage for suspended doping offenders upon a return to elite competition and, as such, these findings led to several discussions related to the negative aspects of unfairness in sports. On a more positive note though, myonuclear permanence from prior training may, in theory, enhance treatment strategies to combat the loss of muscle mass later in life, whether it is associated with aging per se (i.e,. sarcopenia), or as a consequence of various clinical conditions. Importantly though, it still remains to be established whether age‐related muscle fibre atrophy (obviously representing a process that takes place over a prolonged period of time) is accompanied by the concomitant loss of myonuclear content as this has been reported by some, 2 , 64 , 65 but not all 66 , 67 rodent studies. Overall, although more research is warranted to explain the apparent discrepancies within the literature, the existing evidence from animal studies definitely gives room for the possibility that myonuclei gained during muscle hypertrophic episodes earlier in life may at least partly be preserved to promote muscle tissue (re)growth later in life.
2.3. Limitation of animal models
While the animal studies described above provide valuable biological insight, translating results to in vivo human settings remains a challenge. After all, as elegantly described by Demetrius, “mice are not just small humans”. 18 For example, the time‐frame in which the different animal models induce severe muscle atrophy (40%‐50% loss in muscle tissue mass within 2‐3 weeks) and/or hypertrophy (40%‐50% gain in muscle tissue mass within 2‐3 weeks) is something that does not occur in humans. As a reference, our research group has consistently demonstrated that quadriceps muscle cross‐sectional area increases by merely 6%‐10% following 12 weeks of progressive resistance exercise training. 10 , 68 , 69 , 70 , 71 , 72 Furthermore, the surgical procedure required to induce the atrophy/hypertrophy stimulus in rodents is stressful and can induce a robust immune response or cause degeneration/regeneration of muscle fibres. Finally, whereas overload induced by synergistic ablation in animals provides a persistent stimulus, exercise training in humans is characterized by relatively short episodes of anabolic stimuli interchanged with subsequent recovery periods. In an attempt to address some of these issues, a number of studies have been performed in animals that aimed to resemble a more physiological situation of exercise training as what would occur in the human situation. A study by Lee et al 41 used a rodent version of weighted “Jacobs ladder climbing” as an exercise modality to assess the change in muscle mass/fibre size and myonuclear content (single fibre and muscle cross‐sections) following 8 weeks of so‐called “pre‐training,” followed by 20 weeks of detraining and 8 weeks of subsequent retraining. Pre‐training resulted in a significant increase in Flexor Hallucis Longis (FHL) muscle mass/fibre size and myonuclear content. 41 Whereas muscle mass/fibre size was lost, myonuclear content remained unchanged during the subsequent detraining period. 41 During retraining, the increase in muscle mass/fibre size (+14.8%) was significantly greater compared with the pre‐training period (+8.9%), with no further increase in myonuclear content, 41 much in line with myonuclear permanence paradigm. Contrasting results are, however, presented by Dungan et al 40 who exposed mice to 8 weeks of progressive weighted wheel running to induce muscle fibre hypertrophy, followed by 12 weeks of subsequent detraining. Here, the authors showed substantial (17%) muscle fibre hypertrophy and myonuclear accretion (~30%) in the plantaris muscle following the initial 8 weeks of exercise training. During the subsequent 12 weeks of detraining, both muscle fibre size and myonuclear content returned back to baseline levels. 40 Myonuclear content was assessed on both single muscle fibres as well as muscle cross‐sections, in which muscle satellite cells were excluded from the myonuclear counts. Unfortunately, no final “retraining,” nor any measurements of apoptosis were included in this study. 40 Whether these contradicting findings on the loss of myonuclear content during detraining can be explained by the model used to induce muscle fibre hypertrophy and myonuclear accretion, or any other study‐related differences, remains to be further established.
Despite these discrepancies with regards to the muscle memory paradigm in a more physiological situation of muscle fibre hypertrophy and atrophy, the ultimate goal was to translate the observations on muscle memory to the in vivo human situation. As such, it is critical that these and other results are discussed in the light of observations made in experimental trials performed in humans.
3. EVIDENCE FROM HUMAN STUDIES
The phenomenon that “skeletal muscle may hold some kind of memory” originates from observations in humans showing that previously trained individuals acquire muscle mass and strength more quickly upon retraining. 73 , 74 , 75 Staron et al 74 was the first to show that women regained their muscle strength and fibre size during 6 weeks of retraining as quickly as compared with the initial 20 weeks of strength training. Together with subsequent observations, 73 , 74 , 75 this has led to the suggestion that there may be some sort of local muscle memory responsible for such rapid muscle re‐gain. However, it was much later before the first evidence from animal studies showing that myonuclei are not necessarily lost during atrophic conditions, 34 lead the authors to speculate on the “muscle memory by myonuclear permanence” hypothesis. 58 This hypothesis has since then been investigated and discussed almost exclusively based on studies performed in animals. Whether the postulated mechanisms also hold true in humans remains largely ambiguous. Nevertheless, there are several studies in humans that provide evidence to potentially support or contradict (parts of) the muscle memory hypothesis. For example, short‐term (local) physical inactivity models in humans provide insight into whether myonuclei are lost during (acute) muscle fibre atrophy. Whereas some have reported no changes in myonuclear content when atrophy was induced by short‐term single leg knee immobilization, 76 , 77 , 78 bed‐rest, 79 or step reduction 80 protocols, others have shown that muscle fibre atrophy was accompanied by a small (5%‐10%), yet significant, decline in myonuclear content following 14 days of bed rest 81 or micro‐gravity exposure. 82 Previously, we have failed to detect any myonuclear content loss in critically ill patients despite severe muscle fibre atrophy (~20%) within as little as 7 days of being fully‐sedated. 83 The general lack of consensus between studies may be explained in part by differences in the severity of the physical inactivity model applied (eg absoluteness, duration) resulting in differences in the observed muscle atrophy. Furthermore, myonuclear content in human studies is almost exclusively evaluated using muscle cross sections which may have, as discussed earlier, a limited ability to accurately detect small changes in myonuclear content over time. However, the studies performed in our laboratory 76 , 77 , 78 , 83 as well as others 80 , 81 , 82 appear to suggest that myonuclei are not lost in large amounts following muscle fibre atrophy induced by short‐term physical inactivity.
3.1. Muscle fibre size to myonuclear number ratio in humans
The myonuclear domain theory postulates that a linear relationship exists between muscle fibre size and myonuclear content, which has consistently been reported within human skeletal muscle tissue. 84 , 85 , 86 , 87 , 88 , 89 These findings are in contrast to animal data, which suggest that the relationship between muscle fibre size and myonuclear content is not consistent throughout the life span. 2 Over the past decade, we have evaluated type I and type II muscle fibre size and myonuclear content (excluding muscle satellite cells by Pax7 or NCAM co‐staining) in over 400 untrained human volunteers with a wide age range from 18 to 89 years (Figure 1). A positive linear relationship exists between muscle fibre size and myonuclear content (Figure 1A and B). Interestingly, a similar positive association is also present between muscle fibre size and myonuclear domain size, is this case being expressed as a logarithmic correlation (Figure 1C and D). Post‐hoc analysis shows that the linear relationships between type I and type II muscle fibre size and myonuclear content are largely preserved with increasing age (see Figure S1A‐D). As ageing is accompanied by a decline in muscle fibre size, which occurs predominately in type II muscle fibres, these data suggest that myonuclear content is flexible and unlikely to be retained indefinitely throughout the human lifespan. Most studies investigating muscle fibre characteristics are based on muscle biopsy samples obtained from male adults. Kramer et al 89 is one of the few studies performed in women, showing a positive association between muscle fibre size and myonuclear content in both healthy young (23 year) and older (82 year) women. Interestingly, the positive association was not observed in age‐matched older (83 year) women suffering from a hip‐fracture due to a low‐energy fall (ie defined as falling from a standing height or less). Type II muscle fibre cross‐sectional area was significantly smaller in the hip fracture patients as compared to age‐matched healthy controls, but no differences were observed in myonuclear content. As a result, no relationship between myonuclear content and muscle fibre size was observed in the frail older women, who suffered a hip fracture. 89 Though these data are cross‐sectional in nature, they may suggest that myonuclear content is retained to some degree when extensive muscle atrophy occurs in, in this case, frail older adults.
FIGURE 1.
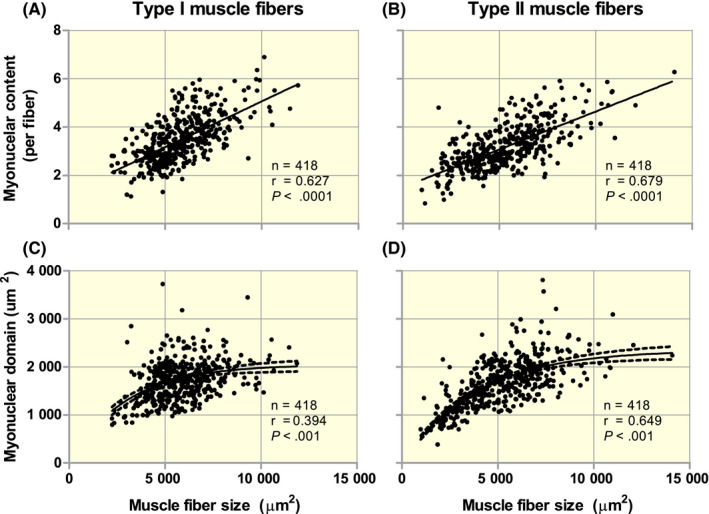
Correlation analysis between type I and type II muscle fibre size and number of myonuclei per fibre (A and B; linear relation) and myonuclear domain size (C and D; logarithmic relation) in percutaneous biopsy samples taken from the vastus lateralis of both healthy adult men (n = 330) and women (n = 88). All samples were collected with subjects at rest following an overnight fast. Muscle fibre size and myonuclear content were determined by immunofluorescent microscopy of muscle cross‐sections. Staining included antibodies for laminin (cell border), MHCI (type I muscle fibres), Dapi (nuclei), Pax7 or NCAM (satellite cells). A Dapi + cell was considered to be a myonucleus when at least 50% of the staining was present within the muscle fibre identified by laminin staining. Muscle satellite cells were identified by Pax7 or NCAM staining and excluded from the myonuclear counts. At least 100 type I and 100 type II muscle fibres per subject were included to make a reliable estimation of myonuclear content
3.2. Long‐term myonuclear permanence in humans
Within the theoretical framework of muscle memory, it is postulated that myonuclei are maintained for an extensive period of time or maybe even indefinitely. This may subsequently represent a biological advantage when baseline muscle fibre size needs to be re‐established after a period of disuse‐induced muscle mass loss. Cross‐sectional studies in humans provide additional evidence that potentially negates the proposition of indefinite myonuclear retention throughout life. For example, we have shown that type I and type II muscle fibre size as well as myonuclear content is substantially lower in severely atrophied muscle of spinal cord injured patients (~9 year after injury) compared with healthy age‐matched controls. 90 Likewise, type II muscle fibre size and myonuclear content tends to be lower in patients suffering from multiple sclerosis compared with age‐matched controls. 91 By far most studies in humans have focused on the comparison of relatively healthy young and older individuals. Whereas some studies showed a lower myonuclear content in old (>60 year) compared with young (18‐29 year) participants, 89 , 90 , 92 , 93 , 94 others did not detect any differences, 9 , 87 , 95 , 96 , 97 (see Table 2). This discrepancy may be explained in part by the different age categories and/or the relatively low number of subjects included within some of the studies. Figure 2 shows the evaluation of type I and type II muscle fibre size, myonuclear content and myonuclear domain size in a large group (n = 302) of healthy men in different age categories. Here we show that type II muscle fibres are significantly smaller in older adults, which is accompanied by a lower myonuclear content as well as smaller myonuclear domain size (Figure 2). Again, although these data are cross‐sectional, they do suggest that myonuclear content is not maintained indefinitely throughout the human lifespan. We have recently provided further evidence for this within a longitudinal exercise training and detraining study. 98 In this study, healthy older adults (n = 35) were subjected to 6 months of supervised resistance exercise training, resulting in a significant increase in muscle fibre size and myonuclear content. Following 1 year of detraining, muscle fibre size as well as myonuclear content returned back to baseline levels. 98 These results appear to be in line with the earlier discussed study performed in animals, demonstrating a decline in myonuclear content during detraining. 40
TABLE 2.
The effects of age on muscle fibre characteristics in humans
| Study | Sex | Age, yrs (n) | Muscle fibre size | Myonuclear content | Myonuclear domain size |
|---|---|---|---|---|---|
| Cristea et al 121 | M1 | 21‐32 (6) vs 72‐96 (9) |
I: ↑ (23%) IIa: ↓ (31%) |
I: ↑ IIa: ↔ |
I: ↔ IIa: ↓ (33%) |
| W1 | 24‐32 (6) vs 65‐96 (9) |
I: ↑ (38%) IIa: ↓ (15%) |
I: ↑ IIa: ↑ |
I: ↔ IIa: ↓ (41%) |
|
| Dreyer et al 95 | M1 | 21‐35 (10) vs ≥ 60 (9) |
I: ↔ II: ↓ (25%) |
Mixed: ↔ | |
| Hikida et al 122 | M1 | 23 ± 6 (7) vs 65 ± 6 (8) | Mixed: ↓ (31%) | Mixed: ↔ | |
| Kadi et al 123 | M2 | 26 ± 3 (15) vs 74 ± 4 (13) | Mixed: ↑ (23%) | ||
| W1 | 23 ± 3 (16) vs 76 ± 3 (14) | Mixed: ↑ (23%) | |||
| Kelly et al 124 | M/W1 | 26 ± 4 (27) vs 66 ± 4 (91) |
I: ↔ II: ↓ |
I: ↔ II: ↔ |
I: ↔ II: ↓ |
| Kramer et al 89 | W1 | 18‐25 (15) vs ≥ 65 (15) |
I: ↔ II: ↓ (30%) |
I: ↔ II: ↓ (23%) |
I: ↑ (31%) II: ↔ |
| Mackey et al 113 | M1 | 24 ± 3 (12) vs 66 ± 4 (12) |
I: ↔ II: ↔ |
I: ↔ II: ↔ |
I: ↔ II: ↔ |
| Manta et al 125 | M/W1 | 17‐30 (4) vs 31‐60 (4) | Mixed: ↓ | Mixed: ↓ | Mixed: ↔ |
| 17‐30 (4) vs > 60 (7) | Mixed: ↓ | Mixed: ↓ | Mixed: ↑ (19%) | ||
| 31‐60 (4) vs > 60 (7) | Mixed: ↔ | Mixed: ↔ | Mixed: ↑ (27%) | ||
| McKay et al 126 | M1 | 21 ± 3 (9) vs 70 ± 4 (9) |
I: ↔ II: ↓ (21%) |
I: ↔ II: ↓ (19%) |
|
| Petrella et al 9 | M1 | 20‐35 (15) vs 60‐75 (13) | Mixed: ↔ | Mixed: ↔ | Mixed: ↔ |
| W1 | 20‐35 (16) vs 60‐75 (14) | Mixed: ↔ | Mixed: ↔ | Mixed: ↔ | |
| Renault et al 127 | M/W3 | 23 ± 1 (6) vs 74 ± 4 (6) | Mixed: ↔ | ||
| Verdijk et al 96 | M1 | 20 ± 1 (8) vs 76 ± 1 (8) |
I: ↔ II: ↓ (27%) |
I: ↑ (17%) II: ↑ (13%) |
I: ↓(16%) II: ↓ (31%) |
| Verdijk et al 90 | M1 | 31 ± 3 (8) vs 75 ± 2 (8) |
I: ↔ II: ↓ |
I: ↔ II: ↓ (40%) |
I: ↔ II: ↔ |
| Verdijk et al 92 | M1 | 18‐49 (50) vs 50‐69 (53) |
I: ↔ II: ↓ (18%) |
I: ↔ II: ↔ |
I: ↔ II: ↔ |
| 18‐49 (50) vs ≥ 70 (49) |
I: ↔ II: ↓ (29%) |
I: ↔ II: ↓ (24%) |
I: ↔ II: ↔ |
||
| 50‐69 (53) vs ≥ 70 (49) |
I: ↔ II: ↔ |
I: ↓ (15%) II: ↓ (20%) |
I: ↔ II: ↔ |
||
| Verdijk et al 94 | M1 | 26 ± 2 (14) vs 72 ± 1 (16) |
I: ↔ II: ↓ (26%) |
I: ↓ (21%) II: ↓ (30%) |
|
| Walker et al 128 | M1 | 27 ± 2 (5) vs 70 ± 2 (6) |
I: ↔ II: ↓ (28%) |
Mixed: ↔ |
Mixed: ↓ |
| W1 | 27 ± 2 (5) vs 70 ± 2 (5) |
I: ↔ II: ↔ |
Mixed: ↔ |
Abbreviations: ↑, significantly higher compared with younger age category; ↓, significantly lower compared with younger age category; I, type I muscle fibres; II(a), type II(a) muscle fibres; M, men; M/W, men and women combined, ↔, no difference between age category; Mixed, mixed muscle fibre type; n, number of subjects included. Analyses were performed in the 1vastus lateralis, 2tibialis anterior or 3biceps brachii/masseter muscle; W, women.
FIGURE 2.
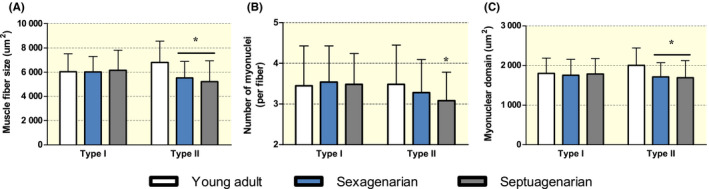
Type I and type II muscle fibre size (A), myonuclear content (B) and myonuclear domain size (C) in healthy young adults (18‐29 y; n = 119), sexuagenarian (60‐69 y; n = 91) and septuagenarian (70‐79 y; n = 93). Data were analysed by means of a one‐way ANOVA and are expressed as means ± SD. * Significantly different compared with young, P < .01. Horizontal line indicates that the differences are present in both groups
Overall, most studies in humans do not appear to support the idea that myonuclei are retained indefinitely throughout the lifespan. Yet, this does not necessarily negate the possibility that myonuclear domain size is flexible and/or that a relatively small myonuclear domain size (ie each myonuclei controls a smaller muscle fibre volume) may augment the muscle fibre (re)growth capacity. Figure 1 shows a large range in myonuclear domain sizes (from ~350 to ~3800 µm2) measured in different individuals. Part of the muscle biopsy samples depicted in this figure actually constitute baseline samples of both young and older adults prior to performing a prolonged exercise training. This provides an opportunity to investigate whether myonuclear domain size at baseline may be a determining factor in the muscle fibre growth response induced by prolonged resistance exercise training. In a previous study, we showed a significant increase in muscle fibre size and myonuclear content in young men, which was not accompanied by changes in myonuclear domain size at any time point (2‐4‐8‐12 weaks) during the 12‐week resistance training program. 10 We concluded that large changes in myonuclear domain size are not apparent in a physiological situation when hypertrophy is induced by prolonged resistance exercise training. 10 We now re‐analysed these data 10 and compared the hypertrophy response between individuals with a relatively small (<1700 µm2, Small group (n = 9); mean ± SD myonuclear domain size of 1627 ± 91 µm2) and large (>2000 µm2, Large group (n = 13); mean ± SD myonuclear domain size of 2173 ± 223 µm2) myonuclear domain size at baseline. Figure 3 shows muscle fibre hypertrophy following 2, 4, 8, and 12 weeks of resistance exercise training, with no differences between the Small and Large baseline myonuclear domain size groups. Interestingly though, myonuclear content increased earlier and to a greater extent in the group with large myonuclear domain size at baseline compared with the group with smaller myonuclear domain size at baseline. This is in line with the concept that (in humans) the myonuclear domain size may need to expand beyond a certain threshold before additional myonuclei are committed to the fibre. 99 Although myonuclear domain size was significantly different at baseline between the two groups, it is interesting to observe that following 12 weeks of exercise training, both groups end up with a myonuclear domain size around 1900 µm2. These data are consistent with other studies performed in humans showing no relationship between baseline myonuclear domain size and muscle fibre hypertrophy response during exercise training. 99 , 100 Contrasting results are, however, observed when the same analytical approach is taken in one of our previous training studies in healthy older men. 70 In this study, muscle biopsies were only taken at baseline and following 12‐weeks of progressive resistance exercise training. Interestingly, whereas a significant increase in muscle fibre size is observed in older men with a relatively small myonuclear domain size at baseline (<1600 µm2 (n = 15); mean ± SD myonuclear domain size of 1431 ± 196 µm2), no changes were observed in the Large group (>2000 µm2 (n = 12) mean ± SD myonuclear domain size of 2046 ± 304 µm2) following exercise training (Figure 4). Though we cannot determine the reason(s) why certain older adults have a smaller myonuclear domain size than others, these results may suggest that fibres with a small myonuclear domain size at baseline may undergo muscle fibre hypertrophy at a faster rate or greater extent during exercise training. Whether this effect is specific to older populations, who generally suffer from age‐related type II muscle fibre atrophy, remains to be further established.
FIGURE 3.
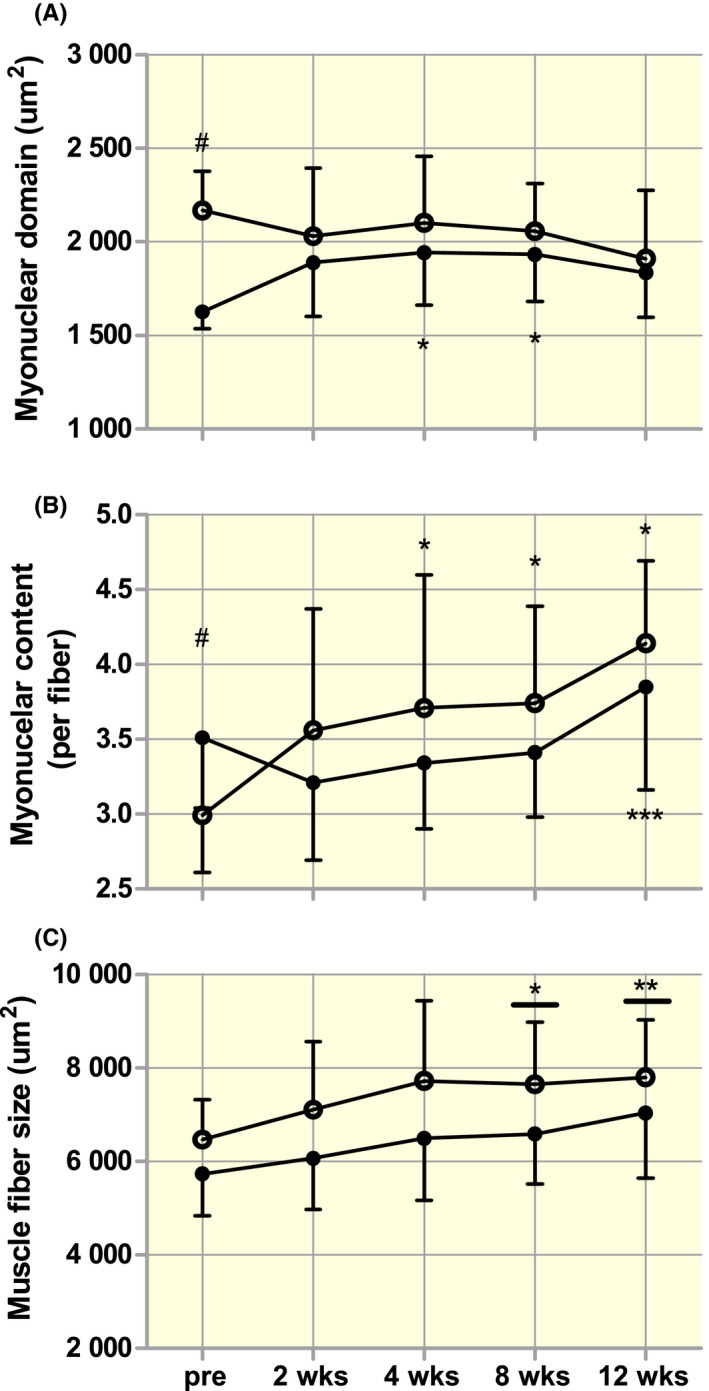
Changes in myonuclear domain size (A), myonuclear content (B) and muscle fibre size (C) in response to 2, 4, 8 and 12 weeks of progressive resistance exercise training in healthy young men with a relatively small (<1700 µm2; Small group; n = 13) or large (>2000 µm2; Large group Large group; n = 10) myonuclear domain size at baseline. Myonuclear content and fibre size were determined by immunofluorescent microscopy as described previously. 10 Muscle satellite cells were identified by NCAM staining and excluded from the myonuclear counts. Data were analysed with a two‐way repeated measures ANOVA with time (pre, 2, 4, 8 and 12 weeks) as within subject factor and group (Small vs Large) as between subject factor. A significant group × time interaction was observed for myonuclear content (P < .05) as well as myonuclear domain (P < .05), resulting in separate one‐way repeated measures ANOVAs and pairwise comparisons being performed to identify within‐group effects. Data are expressed as means ± SD. *Significantly different compared with pre, P < .05. **Significantly different compared with pre and 2 weeks, P < .05. *** Significantly different compared with 2 and 4 weeks, P < .05. # Significantly different between groups pre, P < .05. Horizontal line indicates that the effect is present for both groups
FIGURE 4.
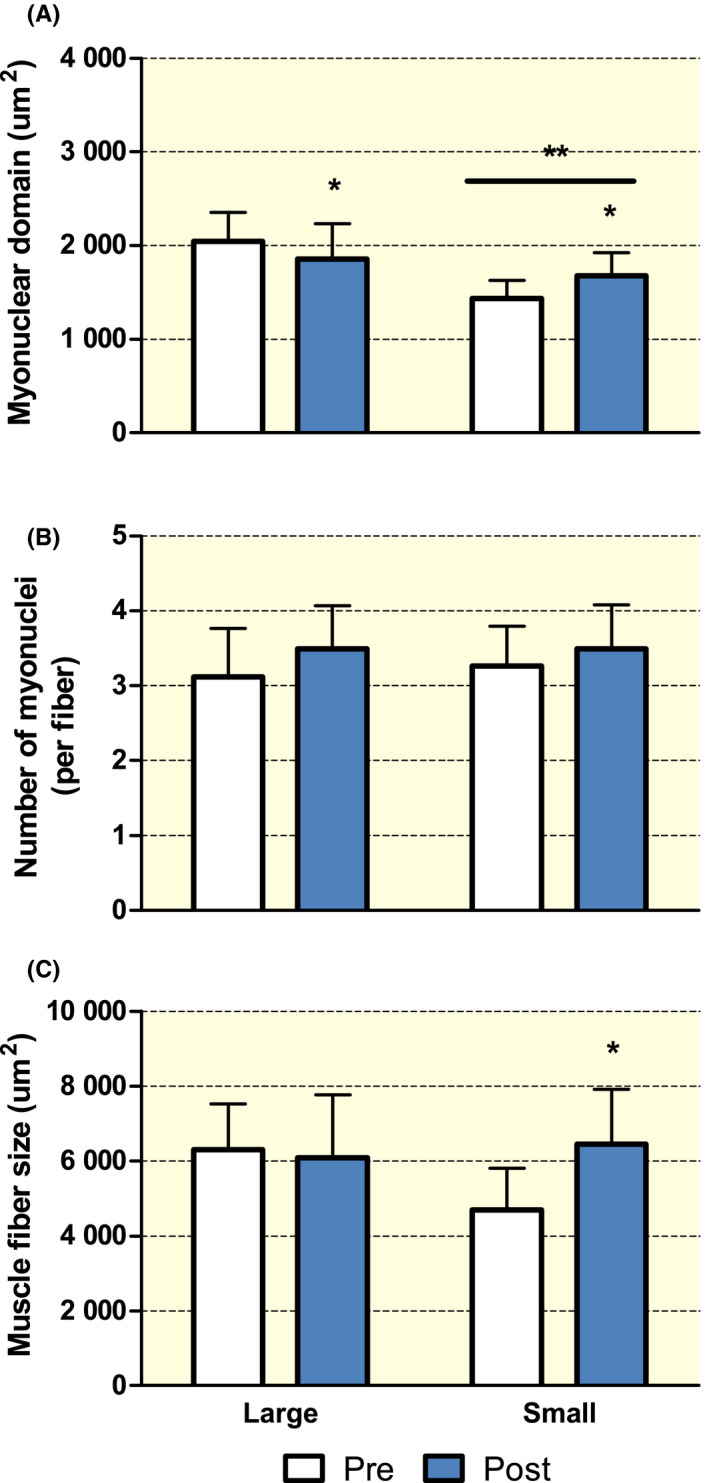
Changes in myonuclear domain size (A), myonuclear content (B) and muscle fibre size (C) in healthy older adults with a relatively small (<1600 µm2; Small group; n = 15) or large (>1800 µm2; Large group; n = 12) myonuclear domain size at baseline (pre) and after (post) 12 weeks of progressive resistance exercise training. Myonuclear content and fibre size were determined by immunofluorescent microscopy as described previously10. Muscle satellite cells were identified by NCAM staining and excluded from the myonuclear counts. Data were analysed with a two‐way repeated measures ANOVA with time (Pre vs Post) as within subject factor and group (Small vs Large) as between subject factor. A significant group × time interaction was observed for muscle fibre size (P < .05) as well as myonuclear domain size (P < .05), resulting in separate one‐way repeated measures ANOVAs and pairwise comparisons being performed to identify within‐group effects. Data are expressed as means ± SD. *Significantly different compared with Pre, P < .05. **Significantly different compared with Large group, P < .05. Horizontal line indicates that the effect is present for both time points
Of note, comparing the physiological response of exercise training based on “extreme” baseline characteristics can provide insight into potential mechanisms, but care should be taken when extrapolating these kind of observations. As discussed earlier, determining myonuclear content (and as such myonuclear domain size) in human muscle cross‐section is not without uncertainty. Furthermore, comparing individuals based on “small” vs “large” values at baseline opens up the possibility of statistical regression towards the mean, which is a concern that should be taken into consideration when interpreting the observations made. Nonetheless, the human data currently available appear to indicate that although some myonuclei may be lost throughout life, a low myonuclear domain size (whether or not the result of myonuclear permanence) may beneficially affect the muscle fibre (re)growth capacity.
3.3. Testing muscle memory by myonuclear permanence in humans
Currently there is only one study that attempted to directly test the hypothesis of muscle memory by myonuclear permanence in humans. In the study by Psilander et al, 11 healthy young men performed 10 weeks of unilateral resistance exercise training, followed by 20 weeks of detraining and a subsequent 5 weeks of bilateral retraining. Skeletal muscle thickness and strength increased significantly in response to the initial exercise training period, which was mostly lost during the successive 20 weeks of detraining. During retraining, however, no differences were observed in muscle thickness or strength gains between the previously trained leg and the untrained leg. Muscle biopsy samples were collected before and after the initial 10‐week training period and from both legs prior to and following 5 weeks of retraining. In line with the muscle thickness and strength data, both type I and type II muscle fibre size increased significantly in response to the initial 10 weeks of exercise training (+13 and + 17% respectively). However, muscle fibre size remained unchanged during detraining as well as 5 weeks of bilateral retraining. Furthermore, no changes in myonuclear content could be detected in response to the initial training, detraining, or retraining period. 11 The degree of muscle fibre growth observed during the initial 10 weeks of exercise training may have been too small to elicit a group mean increase in myonuclear content which is required to firmly establish whether muscle memory by myonuclear retention exists in humans. As Psilander et al 11 published their raw study data, a secondary analysis on these data was recently performed by Murach and colleagues. Here, Murach et al 12 retrospectively divided the participants in two groups, based on the immunohistochemical analyses of both muscle cross sections and single muscle fibres: one group in which a significant increase in myonuclear content was observed (n = 11) following the initial training program, and one group in which no change was observed (n = 8). Since nine out of the eleven subjects who demonstrated an exercise induced increase in myonuclear content, subsequently showed a decline during detraining, the authors suggested that the “newly acquired myonuclei” during exercise training are not being retained during detraining. 12 However, the findings might easily also be explained by a regression towards the mean, without any underlying loss of myonuclei as was also rightfully addressed by the authors of the original paper in their recent letter‐to‐the‐editor 13 as a response to this “secondary analysis.” Based on this, as well as the other discussed studies, it is safe to say that there is currently no consensus within the scientific community on the existence of muscle memory by myonuclear permanence in human skeletal muscle and more research is warranted using properly designed intervention studies.
3.4. Muscle fibre size cluster approach
Data presented here, as well as in most other papers, report the mean in muscle fibre size, myonuclear content and domain size of a muscle biopsy sample. However, it has previously been shown that grouping muscle fibres of similar sizes into clusters may provide a more detailed insight in the relationship between muscle fibre size and myonuclear content during muscle fibre growth. 64 , 87 , 93 The application of this approach has already shown that the smallest muscle fibres (<3000 µm2) have a disproportionally small myonuclear domain size compared with larger (3000‐7000 µm2) muscle fibres in humans. 87 , 93 Hence, it has been speculated that smaller muscle fibres with a (disproportionally) small myonuclear domain may have a greater muscle fibre hypertrophy potential in response to resistance exercise training when compared to larger muscle fibres. This may be of particular relevance in older adults who have a relative high number of smaller muscle fibres due to age‐related muscle fibre atrophy. However, thus far only one study has reported on the myocellular changes in different muscle fibre size clusters in response to exercise training. Karlsen et al 93 evaluated muscle tissue samples taken from very old older adults (83‐94 year) who performed 12 weeks of resistance exercise training. Although the exercise training program resulted in a significant increase in muscle strength, no changes in muscle fibre size, myonuclear content and/or domain size were observed in the muscle biopsy samples. 93 The lack of muscle fibre hypertrophy limited the authors’ ability to draw any firm conclusions on whether the smaller muscle fibres (with a disproportionately small myonuclear domain size) may have a larger muscle fibre growth potential in response to exercise training. Most human studies, including the discussed studies of Karlsen et al, 87 , 93 evaluate myonuclear content using muscle cross‐sections which is, as discussed earlier in this review, not without uncertainty. However, analysing myonuclear content in a large number of cross‐sectional muscle fibres does allow to account for the apparent heterogeneity in myonuclear to muscle fibre ratio observed in different muscle fibre size clusters. In contrast, though in vivo imaging techniques or single fibre isolations may provide a more accurate assessment of myonuclear content per se, these techniques are limited by the inclusion of only “a few surface fibres” or a relatively low number of fibres, respectively, which hampers the ability to account for fibre heterogeneity. 34 Obviously, such pro's and con's should be taken into consideration when evaluating data using these different techniques. Clearly more research is warranted, but the muscle fibre cluster approach may be a valuable tool to provide more insight in the role of myonuclei during muscle reconditioning, and as such, in the potential existence of muscle memory by myonuclear permanence.
4. FUTURE RESEARCH DIRECTIONS
Further experimentation is clearly warranted to more firmly establish whether muscle memory by myonuclear retention exists and, more importantly, whether it plays a relevant role in muscle reconditioning in humans. The most effective study designs should be comparable to that of the recently published study by Psilander and colleagues, 11 who applied a unilateral approach. Moreover it is critical that exercise training intensity, volume and/or duration progresses enough to induce considerable (and measurable) muscle fibre hypertrophy as well as myonuclear accretion. For example, 12 weeks of lower‐body training, three times weekly with weight progression re‐assessed weekly has consistently been shown to induce an ~25% increase in muscle fibre CSA in younger and older participants. 9 , 10 , 70 , 104 , 105 , 106 Such an exercise training period should then be followed by a detraining period of at least equivalent duration, allowing muscle fibre size to return almost entirely to baseline values (with or without a decline in myonuclear content). Subsequently, volunteers should then undergo retraining in both the prior‐trained and untrained leg separately to assess whether the increase in muscle fibre size (as well as muscle mass and strength) is more pronounced in the prior‐trained leg when compared with the initial training programme response, as well as when compared with the increase observed in the previously untrained leg.
4.1. Methodological considerations
To further advance this field of research, the accurate assessment of myonuclear content in muscle tissue is also of critical importance. Since in vivo time lapse imaging of myonuclear content is limited to animal models, determining myonuclear content in humans will most likely rely on histological analyses of muscle cross sections and/or single muscle fibres. It is essential to differentiate between nuclei that are located in‐ and outside the muscle fibres, and it is best practice to exclude muscle satellite cells from the myonuclear counts. As such, a staining approach should be used that combines a nuclear stain (eg DAPI or Hoechst dye) with specific antibodies that delineate the muscle cell border (eg laminin or dystrophin antibody) and identify muscle satellite cells (eg NCAM or Pax7 antibody). The validation of myonuclear specific antibodies, like PCM1, 35 will most likely also provide a substantial improvement in the reliability and sensitivity of myonuclear content measurement required to accurately assess changes in response to muscle fibre hypertrophy and atrophy models. As myonuclear content has been shown to differ between smaller and larger muscle fibres within single muscle cross‐sections, detailed muscle fibre size cluster analyses on myonuclear content and domain size may also provide further insight in the regulation of myonuclear content during muscle fibre reconditioning. Finally, more research is required (especially in humans) that can establish through which mechanism(s) myonuclei are potentially lost (if at all) under conditions of muscle fibre atrophy. Although myonuclear apoptosis has been suggested for many years as the mechanisms for the removal of myonuclei from intact muscle fibres, the evidence for this remains rather circumstantial. It will remain critical to determine whether the nuclear apoptosis typically observed during muscle atrophy represents true myonuclei or other cell types like stromal and satellite cells.
4.2. Measuring DNA synthesis rates
While histological imaging is applied to assess static changes in myonuclear content between time points, alternative methods have been applied recently to provide dynamic insight in myonuclear regulation. The process of myonuclear addition to a muscle fibre requires satellite cell activation, replication and differentiation, during which DNA synthesis occurs. 11 Stable isotope tracer approaches have been successfully applied to label nucleotide base pairs (ie, pyridine deoxyribonucleotides) and measure DNA synthesis rates in the blood and muscle tissue of rodents and humans. 107 , 108 , 109 , 110 , 111 Robinson et al 18 were the first to orally administer deuterated water (2H2O) and assess muscle DNA synthesis rates over several weeks in humans. The investigators reported that DNA synthesis rates were greater in a group of older males during 6 weeks of high‐intensity endurance training (0.15%/d) when compared with a group of young males who remained at rest. The elevated DNA synthesis rates suggest that new myonuclei are synthesized in muscle during exercise training, which aligns with several studies demonstrating an increase in myonuclear content over prolonged exercise training. 9 , 10 , 98 , 99 , 104 , 112 , 113 , 114 , 115 In the young, rested males, DNA synthesis rates were not statistically greater than zero, meaning that DNA synthesis was undetectable. 18 This finding is perhaps relevant to the myonuclear permanence hypothesis as it seems to provide some indication that myonuclear synthesis is either dormant or too low to detect in (untrained) humans under resting conditions. These findings may explain why myonuclear content remains relatively stable until older age and senescence. 92 Whether or not the training‐induced elevation in DNA synthesis rate returns to “dormancy” during a period of detraining and then remains dormant during re‐training (which would be supportive of the muscle memory paradigm) remains to be established. Such data, coupled with histological myonuclear content and cellular apoptosis measurements, would provide better insight into the underlying regulation of myonuclear content and add to our understanding of myonuclear permanency and the muscle memory hypothesis. It is important to note that DNA isolation from muscle currently represents a mixed DNA pool, derived not only from satellite cells, but also from innate immune cell infiltration and fibroblast activity among other muscle‐borne cell types. While Robinson et al. 18 argue that the DNA synthesis measurement primarily represents DNA synthesis derived from the satellite cell pool, future work should also aim to further refine the DNA extraction protocol in muscle tissue to specifically isolate the satellite cell and/or myonuclear pools (and note that fractional synthetic rate is only part of the balance between synthesis and repair, and never predicts absolute hypertrophy or in this case net myonuclear accretion).
4.3. Epigenetic muscle memory
Other than the existence of muscle memory by myonuclear retention, it has been hypothesized that muscle memory may also find its origin at the epigenetic level. Seaborne et al 75 demonstrated that 7 weeks of resistance exercise training‐induced muscle fibre hypertrophy was accompanied by DNA hypomethylation. Whereas muscle mass returned back to baseline during subsequent detraining, DNA hypomethylation was maintained. Moreover, this hypomethylation was also maintained during a succeeding 7‐week period of resistance exercise re‐training, during which muscle mass and strength gains were significantly greater as compared with the initial 7‐week period of exercise training. 75 Hypomethylation generally promotes enhanced gene expression due to the removal of methyl groups from the DNA, allowing improved access of the transcriptional machinery and RNA polymerases that enable transcription. Together this would suggest that exercise training leads to an enhanced hypomethylated state of genes, which is then maintained during detraining, enabling greater transcription of these genes during retraining and, as such, allowing an enhanced muscle fibre growth response. It remains to be determined whether or not training‐induced DNA hypomethylation persists over a more prolonged period, independent of changes in myonuclear content, and promotes greater muscle fibre hypertrophy during retraining after a longer period of detraining (even up to decades).
5. EVOLUTION VS TELEOLOGY
It has been speculated that muscle memory by myonuclear permanence may have an evolutionary origin. 37 Permanent retention of myonuclei might represent an adaptive mechanism to individuals who had the need for increased muscle performance (ie strength) in the past to regain muscle mass faster in the future, as a more long‐term advantage or adaptation. Hence, during less labour intensive time periods, maintenance of a large amount of muscle mass can be avoided, but the capacity to regain that muscle mass when necessary is maintained. In other words, the experience from the past becomes useful when the same task arises again, similar as observed with memory of the brain. However, since there are quite a number of studies that currently appear to contradict the myonuclear permanence hypothesis (as discussed within the review) and it also poses several challenges to conventional scientific thought of the “use it or lose it” principle, as such, it is also worth considering a more teleological perspective. As a rule, efficiency is an underpinning of basic biology. Cells/tissues/organs have an ability to adapt to stress by adding organelles or even anatomical structures to support demand. Classic examples include the addition of mitochondria and capillaries following aerobic training. 116 , 117 These are essential adaptations designed to promote greater oxygen delivery and optimize substrate use during exercise. Importantly, however, mitochondria and capillaries are rapidly lost when exercise training is not continued. 118 These are two classic examples of biological efficiency in the context of exercise science. Therefore, it is important to ask the question: does it make sense that a muscle cell would keep myonuclei when they are not needed? This is a particularly relevant question when one considers that muscle cells have a readily available source of nuclei (by the means of satellite cells) that can be rapidly mobilized following a stressful event like the early stages of an exercise training program. Although the teleological perspective may be equally thought‐provoking, it also largely remains speculation. Particularly, the costs of keeping a high number of myonuclei in a small muscle fibre has never been quantified, nor compared with the cost of eliminating and recreating myonuclei during muscle adaptation. Hence, despite the fact that new myonuclei can be generated, it may be equally “efficient” to simply maintain previously generated myonuclei, which would then be readily available for future (re)use.
The epigenetic hypothesis of muscle memory may, however, be an alternative plausible mechanism to consider. Indeed, epigenetics, the modification of genes to enhance or repress gene expression, may proof to be a credible mechanism to explain the concept of muscle memory. Furthermore, muscle memory by myonuclear permanence and epigenetics are also not mutually exclusive. Ultimately, it will be the collection of definitive data using appropriate models that will shed light on this issue. At present, though it has potential as a theory, muscle memory by myonuclear permanence fails to pass (at least in humans) the litmus test at this stage.
6. CONCLUSION
The current scientific evidence provides no consensus on the existence of muscle memory by myonuclear permanence in both animal or human skeletal muscle tissue. Clearly, more research is warranted, particularly in humans, to experimentally test the muscle memory hypothesis. Developing more sensitive methods to evaluate changes in myonuclear content and function in muscle biopsy samples may be required to fully establish the intricate role that myonuclei play in muscle reconditioning.
CONFLICT OF INTEREST
None of the manuscript authors have any conflicts of interest to declare.
Supporting information
Fig S1
Snijders T, Aussieker T, Holwerda A, Parise G, van Loon LJC, Verdijk LB. The concept of skeletal muscle memory: Evidence from animal and human studies. Acta Physiol. 2020;229:e13465 10.1111/apha.13465
REFERENCES
- 1. Schmalbruch H. Skeletal Muscle. Berlin: Springer‐Verlag; 1985. [Google Scholar]
- 2. Bruusgaard JC, Liestol K, Gundersen K. Distribution of myonuclei and microtubules in live muscle fibers of young, middle‐aged, and old mice. J Appl Physiol. (1985). 2006;100(6):2024‐2030. [DOI] [PubMed] [Google Scholar]
- 3. Strassburger E. Über die wirkungssphäre der kerne und die zelgrösse. Histol Beitr. 1893;5:91‐124. [Google Scholar]
- 4. Cheek DB. The control of cell mass and replication. The DNA unit–a personal 20‐year study. Early Hum Dev. 1985;12(3):211‐239. [DOI] [PubMed] [Google Scholar]
- 5. Hall ZW, Ralston E. Nuclear domains in muscle cells. Cell. 1989;59(5):771‐772. [DOI] [PubMed] [Google Scholar]
- 6. Pavlath GK, Rich K, Webster SG, Blau HM. Localization of muscle gene products in nuclear domains. Nature. 1989;337(6207):570‐573. [DOI] [PubMed] [Google Scholar]
- 7. Gundersen K, Bruusgaard JC. Nuclear domains during muscle atrophy: nuclei lost or paradigm lost? J Physiol. 2008;586(11):2675‐2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gundersen K, Bruusgaard JC, Egner IM, Eftestol E, Bengtsen M. Muscle memory: virtues of your youth? J Physiol. 2018;596(18):4289‐4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance‐trained young and older men and women. Am J Physiol Endocrinol Metab. 2006;291(5):E937‐946. [DOI] [PubMed] [Google Scholar]
- 10. Snijders T, Smeets JS, van Kranenburg J, Kies AK, van Loon LJ, Verdijk LB. Changes in myonuclear domain size do not precede muscle hypertrophy during prolonged resistance‐type exercise training. Acta Physiol (Oxf). 2016;216(2):231‐239. [DOI] [PubMed] [Google Scholar]
- 11. Snijders T, Nederveen JP, McKay BR, et al. Satellite cells in human skeletal muscle plasticity. Front Physiol. 2015;6:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwartz LM. Skeletal muscles do not undergo apoptosis during either atrophy or programmed cell death‐revisiting the myonuclear domain hypothesis. Front Physiol. 2018;9:1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dirks AJ, Leeuwenburgh C. The role of apoptosis in age‐related skeletal muscle atrophy. Sports Med. 2005;35(6):473‐483. [DOI] [PubMed] [Google Scholar]
- 14. Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 1999;22(10):1350‐1360. [DOI] [PubMed] [Google Scholar]
- 15. Bruusgaard JC, Egner IM, Larsen TK, Dupre‐Aucouturier S, Desplanches D, Gundersen K. No change in myonuclear number during muscle unloading and reloading. J Appl Physiol. (1985). 2012;113(2):290‐296. [DOI] [PubMed] [Google Scholar]
- 16. Egner IM, Bruusgaard JC, Eftestol E, Gundersen K. A cellular memory mechanism aids overload hypertrophy in muscle long after an episodic exposure to anabolic steroids. J Physiol. 2013;591(24):6221‐6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev. 1991;71(2):541‐585. [DOI] [PubMed] [Google Scholar]
- 18. Demetrius L. Of mice and men. When it comes to studying ageing and the means to slow it down, mice are not just small humans. EMBO Rep. 2005;6 Spec No: S39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aravamudan B, Mantilla CB, Zhan WZ, Sieck GC. Denervation effects on myonuclear domain size of rat diaphragm fibers. J Appl Physiol. (1985). 2006;100(5):1617‐1622. [DOI] [PubMed] [Google Scholar]
- 20. Viguie CA, Lu DX, Huang SK, Rengen H, Carlson BM. Quantitative study of the effects of long‐term denervation on the extensor digitorum longus muscle of the rat. Anat Rec. 1997;248(3):346‐354. [DOI] [PubMed] [Google Scholar]
- 21. Borisov AB, Carlson BM. Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec. 2000;258(3):305‐318. [DOI] [PubMed] [Google Scholar]
- 22. Schmalbruch H, Lewis DM. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve. 2000;23(4):617‐626. [DOI] [PubMed] [Google Scholar]
- 23. Dupont‐Versteegden EE, Murphy RJ, Houle JD, Gurley CM, Peterson CA. Activated satellite cells fail to restore myonuclear number in spinal cord transected and exercised rats. Am J Physiol. 1999;277(3):C589‐597. [DOI] [PubMed] [Google Scholar]
- 24. Dupont‐Versteegden EE, Murphy RJ, Houle JD, Gurley CM, Peterson CA. Mechanisms leading to restoration of muscle size with exercise and transplantation after spinal cord injury. Am J Physiol Cell Physiol. 2000;279(6):C1677‐1684. [DOI] [PubMed] [Google Scholar]
- 25. Allen DL, Monke SR, Talmadge RJ, Roy RR, Edgerton VR. Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J Appl Physiol. (1985). 1995;78(5):1969‐1976. [DOI] [PubMed] [Google Scholar]
- 26. Darr KC, Schultz E. Hindlimb suspension suppresses muscle growth and satellite cell proliferation. J Appl Physiol. (1985). 1989;67(5):1827‐1834. [DOI] [PubMed] [Google Scholar]
- 27. Allen DL, Linderman JK, Roy RR, et al. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol. 1997;273(2 Pt 1):C579‐587. [DOI] [PubMed] [Google Scholar]
- 28. Allen DL, Linderman JK, Roy RR, Grindeland RE, Mukku V, Edgerton VR. Growth hormone/IGF‐I and/or resistive exercise maintains myonuclear number in hindlimb unweighted muscles. J Appl Physiol. (1985). 1997;83(6):1857‐1861. [DOI] [PubMed] [Google Scholar]
- 29. Leeuwenburgh C, Gurley CM, Strotman BA, Dupont‐Versteegden EE. Age‐related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1288‐1296. [DOI] [PubMed] [Google Scholar]
- 30. Matsuba Y, Goto K, Morioka S, et al. Gravitational unloading inhibits the regenerative potential of atrophied soleus muscle in mice. Acta Physiol (Oxf). 2009;196(3):329‐339. [DOI] [PubMed] [Google Scholar]
- 31. Allen DL, Yasui W, Tanaka T, et al. Myonuclear number and myosin heavy chain expression in rat soleus single muscle fibers after spaceflight. J Appl Physiol. (1985). 1996;81(1):145‐151. [DOI] [PubMed] [Google Scholar]
- 32. Hikida RS, Van Nostran S, Murray JD, Staron RS, Gordon SE, Kraemer WJ. Myonuclear loss in atrophied soleus muscle fibers. Anat Rec. 1997;247(3):350‐354. [DOI] [PubMed] [Google Scholar]
- 33. Sandonà D, Desaphy J‐F, Camerino GM, et al. Adaptation of mouse skeletal muscle to long‐term microgravity in the MDS mission. PLoS ONE. 2012;7(3):e33232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruusgaard JC, Gundersen K. In vivo time‐lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. J Clin Invest. 2008;118(4):1450‐1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Winje IM, Bengtsen M, Eftestol E, Juvkam I, Bruusgaard JC, Gundersen K. Specific labelling of myonuclei by an antibody against pericentriolar material 1 on skeletal muscle tissue sections. Acta Physiol (Oxf). 2018;223(4):e13034. [DOI] [PubMed] [Google Scholar]
- 36. Winje IM, Sheng X, Hansson K‐A, et al. Cachexia does not induce loss of myonuclei or muscle fibres during xenografted prostate cancer in mice. Acta Physiol (Oxf). 2019;225(3):e13204. [DOI] [PubMed] [Google Scholar]
- 37. Gundersen K. Muscle memory and a new cellular model for muscle atrophy and hypertrophy. J Exp Biol. 2016;219(Pt 2):235‐242. [DOI] [PubMed] [Google Scholar]
- 38. Moss FP, Leblond CP. Nature of dividing nuclei in skeletal muscle of growing rats. J Cell Biol. 1970;44(2):459‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adhihetty PJ, O'Leary MF, Chabi B, Wicks KL, Hood DA. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol. (1985). 2007;102(3):1143‐1151. [DOI] [PubMed] [Google Scholar]
- 40. Dungan CM, Murach KA, Frick KK, et al. Elevated myonuclear density During skeletal muscle hypertrophy In response to training is reversed during detraining. Am J Physiol Cell Physiol. 2019;316(5):C649‐C654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee H, Kim K, Kim B, et al. A cellular mechanism of muscle memory facilitates mitochondrial remodelling following resistance training. J Physiol. 2018;596(18):4413‐4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jin H, Wu Z, Tian T, Gu Y. Apoptosis in atrophic skeletal muscle induced by brachial plexus injury in rats. J Trauma. 2001;50(1):31‐35. [DOI] [PubMed] [Google Scholar]
- 43. Yoshimura K, Harii K. A regenerative change during muscle adaptation to denervation in rats. J Surg Res. 1999;81(2):139‐146. [DOI] [PubMed] [Google Scholar]
- 44. Jackson JR, Mula J, Kirby TJ, et al. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading‐induced atrophy. Am J Physiol Cell Physiol. 2012;303(8):C854‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kasper CE, Xun L. Cytoplasm‐to‐myonucleus ratios in plantaris and soleus muscle fibres following hindlimb suspension. J Muscle Res Cell Motil. 1996;17(5):603‐610. [DOI] [PubMed] [Google Scholar]
- 46. Kasper CE, Xun L. Cytoplasm‐to‐myonucleus ratios following microgravity. J Muscle Res Cell Motil. 1996;17(5):595‐602. [DOI] [PubMed] [Google Scholar]
- 47. Mozdziak PE, Pulvermacher PM, Schultz E. Unloading of juvenile muscle results in a reduced muscle size 9 wk after reloading. J Appl Physiol. (1985). 2000;88(1):158‐164. [DOI] [PubMed] [Google Scholar]
- 48. Zhong H, Roy RR, Siengthai B, Edgerton VR. Effects of inactivity on fiber size and myonuclear number in rat soleus muscle. J Appl Physiol. (1985). 2005;99(4):1494‐1499. [DOI] [PubMed] [Google Scholar]
- 49. Alway SE, Degens H, Krishnamurthy G, Chaudhrai A. Denervation stimulates apoptosis but not Id2 expression in hindlimb muscles of aged rats. J Gerontol A Biol Sci Med Sci. 2003;58(8):687‐697. [DOI] [PubMed] [Google Scholar]
- 50. Alway SE, Martyn JK, Ouyang J, Chaudhrai A, Murlasits ZS. Id2 expression during apoptosis and satellite cell activation in unloaded and loaded quail skeletal muscles. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R540‐549. [DOI] [PubMed] [Google Scholar]
- 51. Dupont‐Versteegden EE, Fluckey JD, Knox M, Gaddy D, Peterson CA. Effect of flywheel‐based resistance exercise on processes contributing to muscle atrophy during unloading in adult rats. J Appl Physiol. (1985). 2006;101(1):202‐212. [DOI] [PubMed] [Google Scholar]
- 52. Siu PM, Pistilli EE, Alway SE. Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young adult and aged rats. Am J Physiol‐Reg I. 2005;289(4):R1015‐R1026. [DOI] [PubMed] [Google Scholar]
- 53. Tang H, Cheung WM, Ip FC, Ip NY. Identification and characterization of differentially expressed genes in denervated muscle. Mol Cell Neurosci. 2000;16(2):127‐140. [DOI] [PubMed] [Google Scholar]
- 54. Lee G, Lim JY, Frontera WR. Apoptosis in young and old denervated rat skeletal muscle. Muscle Nerve. 2017;55(2):262‐269. [DOI] [PubMed] [Google Scholar]
- 55. Hao Y, Jackson JR, Wang Y, Edens N, Pereira SL, Alway SE. beta‐Hydroxy‐beta‐methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension‐induced muscle fiber atrophy in aged rats. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R701‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lim JY, Han TR. Effect of electromyostimulation on apoptosis‐related factors in denervation and reinnervation of rat skeletal muscles. Muscle Nerve. 2010;42(3):422‐430. [DOI] [PubMed] [Google Scholar]
- 57. Tews DS, Goebel HH, Meinck HM. DNA‐fragmentation and apoptosis‐related proteins of muscle cells in motor neuron disorders. Acta Neurol Scand. 1997;96(6):380‐386. [DOI] [PubMed] [Google Scholar]
- 58. Bruusgaard JC, Johansen IB, Egner IM, Rana ZA, Gundersen K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci USA. 2010;107(34):15111‐15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45(3):528‐537. [DOI] [PubMed] [Google Scholar]
- 60. Bruusgaard JC, Liestol K, Ekmark M, Kollstad K, Gundersen K. Number and spatial distribution of nuclei in the muscle fibres of normal mice studied in vivo. J Physiol. 2003;551(Pt 2):467‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dupont‐Versteegden EE, Strotman BA, Gurley CM, et al. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1730‐1740. [DOI] [PubMed] [Google Scholar]
- 62. Schwartz LM, Brown C, McLaughlin K, Smith W, Bigelow C. The myonuclear domain is not maintained in skeletal muscle during either atrophy or programmed cell death. Am J Physiol Cell Physiol. 2016;311(4):C607‐C615. [DOI] [PubMed] [Google Scholar]
- 63. Beaulaton J, Lockshin RA. Ultrastructural study of the normal degeneration of the intersegmental muscles of Anthereae polyphemus and Manduca sexta (Insecta, Lepidoptera) with particular reference of cellular autophagy. J Morphol. 1977;154(1):39‐57. [DOI] [PubMed] [Google Scholar]
- 64. Brack AS, Bildsoe H, Hughes SM. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age‐related muscle atrophy. J Cell Sci. 2005;118(Pt 20):4813‐4821. [DOI] [PubMed] [Google Scholar]
- 65. Wada KI, Katsuta S, Soya H. Natural occurrence of myofiber cytoplasmic enlargement accompanied by decrease in myonuclear number. Jpn J Physiol. 2003;53(2):145‐150. [DOI] [PubMed] [Google Scholar]
- 66. Brooks NE, Schuenke MD, Hikida RS. Ageing influences myonuclear domain size differently in fast and slow skeletal muscle of rats. Acta Physiol (Oxf). 2009;197(1):55‐63. [DOI] [PubMed] [Google Scholar]
- 67. Fry CS, Noehren B, Mula J, et al. Fibre type‐specific satellite cell response to aerobic training in sedentary adults. J Physiol. 2014;592(12):2625‐2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Snijders T, Res PT, Smeets JSJ, et al. Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance‐type exercise training in healthy young men. J Nutr. 2015;145(6):1178‐1184. [DOI] [PubMed] [Google Scholar]
- 69. Verdijk LB, Jonkers RAM, Gleeson BG, et al. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009;89(2):608‐616. [DOI] [PubMed] [Google Scholar]
- 70. Holwerda AM, Overkamp M, Paulussen KJM, et al. Protein supplementation after exercise and before sleep does not further augment muscle mass and strength gains during resistance exercise training in active older men. J Nutr. 2018;148(11):1723‐1732. [DOI] [PubMed] [Google Scholar]
- 71. Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon LJ. Elderly men and women benefit equally from prolonged resistance‐type exercise training. J Gerontol A Biol Sci Med Sci. 2013;68(7):769‐779. [DOI] [PubMed] [Google Scholar]
- 72. Leenders M, Verdijk LB, Van der hoeven L, et al. Protein supplementation during resistance‐type exercise training in the elderly. Med Sci Sports Exerc. 2013;45(3):542‐552. [DOI] [PubMed] [Google Scholar]
- 73. Taaffe DR, Marcus R. Dynamic muscle strength alterations to detraining and retraining in elderly men. Clin Physiol. 1997;17(3):311‐324. [DOI] [PubMed] [Google Scholar]
- 74. Staron RS, Leonardi MJ, Karapondo DL, et al. Strength and skeletal muscle adaptations in heavy‐resistance‐trained women after detraining and retraining. J Appl Physiol. (1985). 1991;70(2):631‐640. [DOI] [PubMed] [Google Scholar]
- 75. Seaborne RA, Strauss J, Cocks M, et al. Human skeletal muscle possesses an epigenetic memory of hypertrophy. Sci Rep. 2018;8(1):1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Snijders T, Wall B, Dirks M, et al. Muscle disuse atrophy is not accompanied by changes in skeletal muscle satellite cell content. Clin Sci (Lond). 2014;126(8):557‐566. [DOI] [PubMed] [Google Scholar]
- 77. Dirks ML, Wall BT, Snijders T, Ottenbros CL, Verdijk LB, van Loon LJ. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol (Oxf). 2014;210(3):628‐641. [DOI] [PubMed] [Google Scholar]
- 78. Dirks ML, Wall BT, Nilwik R, Weerts DH, Verdijk LB, van Loon LJ. Skeletal muscle disuse atrophy is not attenuated by dietary protein supplementation in healthy older men. J Nutr. 2014;144(8):1196‐1203. [DOI] [PubMed] [Google Scholar]
- 79. Dirks ML, Wall BT, van de Valk B, et al. One week of bed rest leads to substantial muscle atrophy and induces whole‐body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016;65(10):2862‐2875. [DOI] [PubMed] [Google Scholar]
- 80. Moore DR, Kelly RP, Devries MC, et al. Low‐load resistance exercise during inactivity is associated with greater fibre area and satellite cell expression in older skeletal muscle. J Cachexia Sarcopenia Muscle. 2018;9(4):747‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Arentson‐Lantz EJ, English KL, Paddon‐Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle‐aged adults. J Appl Physiol. (1985). 2016;120(8):965‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Day MK, Allen DL, Mohajerani L, Greenisen MC, Roy RR, Edgerton VR. Adaptations of human skeletal muscle fibers to spaceflight. J Gravit Physiol. 1995;2(1):P47‐50. [PubMed] [Google Scholar]
- 83. Dirks ML, Hansen D, Van Assche A, Dendale P, Van Loon LJ. Neuromuscular electrical stimulation prevents muscle wasting in critically ill comatose patients. Clin Sci (Lond). 2015;128(6):357‐365. [DOI] [PubMed] [Google Scholar]
- 84. Eriksson A, Kadi F, Malm C, Thornell LE. Skeletal muscle morphology in power‐lifters with and without anabolic steroids. Histochem Cell Biol. 2005;124(2):167‐175. [DOI] [PubMed] [Google Scholar]
- 85. Kadi F, Charifi N, Henriksson J. The number of satellite cells in slow and fast fibres from human vastus lateralis muscle. Histochem Cell Biol. 2006;126(1):83‐87. [DOI] [PubMed] [Google Scholar]
- 86. Kadi F, Eriksson A, Holmner S, Thornell LE. Effects of anabolic steroids on the muscle cells of strength‐trained athletes. Med Sci Sports Exerc. 1999;31(11):1528‐1534. [DOI] [PubMed] [Google Scholar]
- 87. Karlsen A, Couppé C, Andersen JL, et al. Matters of fiber size and myonuclear domain: Does size matter more than age? Muscle Nerve. 2015;52(6):1040‐1046. [DOI] [PubMed] [Google Scholar]
- 88. Verdijk LB, Snijders T, Beelen M, et al. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc. 2010;58(11):2069‐2075. [DOI] [PubMed] [Google Scholar]
- 89. Kramer IF, Snijders T, Smeets JSJ, et al. Extensive type ii muscle fiber atrophy in elderly female hip fracture patients. J Gerontol A Biol Sci Med Sci. 2017;72(10):1369‐1375. [DOI] [PubMed] [Google Scholar]
- 90. Verdijk LB, Dirks ML, Snijders T, et al. Reduced satellite cell numbers with spinal cord injury and aging in humans. Med Sci Sports Exerc. 2012;44(12):2322‐2330. [DOI] [PubMed] [Google Scholar]
- 91. Farup J, Dalgas U, Keytsman C, Eijnde BO, Wens I. High intensity training may reverse the fiber type specific decline in myogenic stem cells in multiple sclerosis patients. Front Physiol. 2016;7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr). 2014;36(2):545‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Karlsen A, Bechshøft RL, Malmgaard‐Clausen NM, et al. Lack of muscle fibre hypertrophy, myonuclear addition, and satellite cell pool expansion with resistance training in 83–94‐year‐old men and women. Acta Physiol (Oxf). 2019;e13271. [DOI] [PubMed] [Google Scholar]
- 94. Verdijk LB, Snijders T, Holloway TM, van Kranenburg J, van Loon LJ. Resistance training increases skeletal muscle capillarization in healthy older men. Med Sci Sports Exerc. 2016;48(11):2157‐2164. [DOI] [PubMed] [Google Scholar]
- 95. Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve. 2006;33(2):242‐253. [DOI] [PubMed] [Google Scholar]
- 96. Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007;292(1):E151‐157. [DOI] [PubMed] [Google Scholar]
- 97. Mackey AL, Karlsen A, Couppé C, et al. Differential satellite cell density of type I and II fibres with lifelong endurance running in old men. Acta Physiol (Oxf). 2014;210(3):612‐627. [DOI] [PubMed] [Google Scholar]
- 98. Snijders T, Leenders M, de Groot L, van Loon LJC, Verdijk LB. Muscle mass and strength gains following 6months of resistance type exercise training are only partly preserved within one year with autonomous exercise continuation in older adults. Exp Gerontol. 2019;121:71‐78. [DOI] [PubMed] [Google Scholar]
- 99. Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell‐mediated myonuclear addition: a cluster analysis. J Appl Physiol. (1985). 2008;104(6):1736‐1742. [DOI] [PubMed] [Google Scholar]
- 100. Haun CT, Vann CG, Mobley CB, et al. Pre‐training skeletal muscle fiber size and predominant fiber type best predict hypertrophic responses to 6 weeks of resistance training in previously trained young men. Front Physiol. 2019;10:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Psilander N, Eftestøl E, Cumming KT, et al. Effects of training, detraining, and retraining on strength, hypertrophy, and myonuclear number in human skeletal muscle. J Appl Physiol. 1985;2019. [DOI] [PubMed] [Google Scholar]
- 102. Murach KA, Dungan CM, Dupont‐Versteegden EE, McCarthy JJ, Peterson CA. "Muscle memory" not mediated by myonuclear number?: secondary analysis of human detraining data. J Appl Physiol. 1985;2019;127(6):1814‐1816. [DOI] [PubMed] [Google Scholar]
- 103. Eftestol E, Psilander N, Cumming KT, et al. Muscle memory: Are myonuclei ever lost? J Appl Physiol. 1985;2019. [DOI] [PubMed] [Google Scholar]
- 104. Verdijk LB, Gleeson BG, Jonkers RAM, et al. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type‐specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64(3):332‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nederveen JP, Snijders T, Joanisse S, et al. Altered muscle satellite cell activation following 16 wk of resistance training in young men. Am J Physiol Regul Integr Comp Physiol. 2017;312(1):R85‐R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Trappe TA, Ratchford SM, Brower BE, et al. COX Inhibitor Influence on skeletal muscle fiber size and metabolic adaptations to resistance exercise in older adults. J Gerontol A Biol Sci Med Sci. 2016;71(10):1289‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Neese RA, Misell LM, Turner S, et al. Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc Natl Acad Sci USA. 2002;99(24):15345‐15350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Robinson MM, Turner SM, Hellerstein MK, Hamilton KL, Miller BF. Long‐term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J. 2011;25(9):3240‐3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Miller BF, Robinson MM, Bruss MD, Hellerstein M, Hamilton KL. A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell. 2012;11(1):150‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Drake JC, Bruns DR, Peelor FF, et al. Long‐lived crowded‐litter mice have an age‐dependent increase in protein synthesis to DNA synthesis ratio and mTORC1 substrate phosphorylation. Am J Physiol Endocrinol Metab. 2014;307(9):E813‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Konopka AR, Laurin JL, Musci RV, et al. Influence of Nrf2 activators on subcellular skeletal muscle protein and DNA synthesis rates after 6 weeks of milk protein feeding in older adults. Geroscience. 2017;39(2):175‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol. 2000;113(2):99‐103. [DOI] [PubMed] [Google Scholar]
- 113. Mackey AL, Esmarck B, Kadi F, et al. Enhanced satellite cell proliferation with resistance training in elderly men and women. Scand J Med Sci Sports. 2007;17(1):34‐42. [DOI] [PubMed] [Google Scholar]
- 114. Olsen S, Aagaard P, Kadi F, et al. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol. 2006;573(Pt 2):525‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Verney J, Kadi F, Charifi N, et al. Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle Nerve. 2008;38(3):1147‐1154. [DOI] [PubMed] [Google Scholar]
- 116. Gollnick PD, Armstrong RB, Saltin B, Saubert CW, Sembrowich WL, Shepherd RE. Saubert CWt, Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973;34(1):107‐111. [DOI] [PubMed] [Google Scholar]
- 117. Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977;270(3):677‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mujika I, Padilla S. Muscular characteristics of detraining in humans. Med Sci Sports Exerc. 2001;33(8):1297‐1303. [DOI] [PubMed] [Google Scholar]
- 119. Wada KI, Takahashi H, Katsuta S, Soya H. No decrease in myonuclear number after long‐term denervation in mature mice. Am J Physiol Cell Physiol. 2002;283(2):C484‐488. [DOI] [PubMed] [Google Scholar]
- 120. Siu P, Alway SE. Differential responses of apoptotic factors to unloading‐induced atrophy following muscle hypertrophy in adult and aged quail muscles. Med Sci Sport Exer. 2004;36(5):S146‐S146. [Google Scholar]
- 121. Cristea A, Qaisar R, Edlund PK, Lindblad J, Bengtsson E, Larsson L. Effects of aging and gender on the spatial organization of nuclei in single human skeletal muscle cells. Aging Cell. 2010;9(5):685‐697. [DOI] [PubMed] [Google Scholar]
- 122. Hikida RS, Walsh W, Barylski N, Campos G, Hagerman FC, Staron RS. Is hypertrophy limited in elderly muscle fibers? A comparison of elderly and young strength‐trained men. Basic Appl Myol. 1998;8(6):419‐427. [Google Scholar]
- 123. Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve. 2004;29(1):120‐127. [DOI] [PubMed] [Google Scholar]
- 124. Kelly NA, Hammond KG, Stec MJ, et al. Quantification and characterization of grouped type I myofibers in human aging. Muscle Nerve. 2018;57(1):E52‐E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Manta P, Vassilopoulos D, Spengos M. Nucleo‐cytoplasmic ratio in ageing skeletal muscle. Eur Arch Psychiatry Neurol Sci. 1987;236(4):235‐236. [DOI] [PubMed] [Google Scholar]
- 126. McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age‐related human muscle stem cell dysfunction. FASEB J. 2012;26(6):2509‐2521. [DOI] [PubMed] [Google Scholar]
- 127. Renault V, Thornell LE, Eriksson PO, Butler‐Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1(2):132‐139. [DOI] [PubMed] [Google Scholar]
- 128. Walker DK, Fry CS, Drummond MJ, et al. PAX7+ satellite cells in young and older adults following resistance exercise. Muscle Nerve. 2012;46(1):51‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


