Summary
Sleep disturbances and anxiety disorders exhibit high comorbidity levels, but it remains unclear whether sleep problems are causes or consequences of increased anxiety. To experimentally probe the aetiological role of sleep disturbances in anxiety, we investigated in healthy participants how total sleep deprivation influences fear expression in a conditioning paradigm. In a fear conditioning procedure, one face stimulus (conditioned stimulus [CS+]) was paired with electric shock, whereas another face stimulus was not (unpaired stimulus [CS−]). Fear expression was tested the next morning using the two face stimuli from the training phase and a generalization stimulus (i.e. a morph between the CS+ and CS− stimuli). Between fear conditioning and test, participants were either kept awake in the laboratory for 12 hr (n = 20) or had a night of sleep at home (n = 20). Irrespective of stimulus type, subjective threat expectancies, but not skin conductance responses, were enhanced after sleep deprivation, relative to regular sleep. These results suggest that sleep disturbances may play a role in anxiety disorders by increasing perceived threat.
Keywords: associative learning, generalization, safety learning, sleep quality
1. INTRODUCTION
Anxiety disorders are among the most common psychiatric disorders with a lifetime prevalence of up to 29% (Kessler et al., 2005). These disorders often severely impair a person's functioning and quality of life, with a high economic burden due to the resulting use of healthcare systems and loss of productivity at work (Greenberg et al., 1999). This makes anxiety disorders, and in particular the factors associated with their development, a crucial target for research.
Previous studies have revealed that patients with an anxiety disorder, relative to healthy controls, exhibit stronger fear expression as indexed by enhanced generalization of fear (Lissek et al., 2011, 2014). Generalization refers to the observation that fear does not stay confined to threatening stimuli that were involved in an aversive learning incident, but spreads to other stimuli (Boddez, Bennett, van Esch, & Beckers, 2017). For instance, a survivor of a car accident may not only respond fearfully to the T‐junction where the accident happened, but also to T‐junctions at new, unfamiliar places. Moreover, and relatedly, patients with an anxiety disorder, relative to healthy controls, express stronger fear in situations previously experienced as safe (Lissek et al., 2005, 2009), such as when the car accident survivor would respond fearfully to familiar T‐junctions where he or she never experienced any trouble before.
In addition to increased fear expression, patients with anxiety disorders often display sleep disturbances (Marcks, Weisberg, Edelen, & Keller, 2010). These sleep disturbances may not merely be a symptom but also an aetiological factor in the development of clinical anxiety. Prospective studies indeed show that sleep disturbances at baseline increase the likelihood of developing anxiety symptoms at a later time point (Breslau, Roth, Rosenthal, & Andreski, 1996; Jansson‐Fröjmark & Lindblom, 2008). However, prospective studies do not allow strong conclusions concerning causality. In the present study, we therefore used an experimental design to assess the effect of sleep deprivation on fear expression in healthy participants.
While the interplay between sleep and fear has been well investigated by means of experimental procedures in recent years (Davidson, Carlsson, Jönsson, & Johansson, 2016, 2018; Feng, Becker, Feng, & Zheng, 2018; Menz, Rihm, & Büchel, 2016; Menz et al., 2013; Peters et al., 2014; Straus, Acheson, Risbrough, & Drummond, 2017; see Discussion for a summary of these studies), a study that specifically focuses on the effect of sleep deprivation on fear after it has been acquired has, to our knowledge, not been carried out yet. This is not only surprising in view of the promising prospective studies, but also in view of a variety of theoretical considerations, which suggest that there might be an aetiological pathway from sleep disturbances to increased fear expression. More precisely, in case of poor or no sleep, memory may not get consolidated as well as during a good night of sleep, leading to difficulties in distinguishing between threatening and non‐threatening stimuli because of forgetting of specific stimulus attributes (Lenaert et al., 2012; Riccio, Ackil, & Burch‐Vernon, 1992). In addition, a sleep‐deprived state could reduce inhibitory regulation (Drummond, Paulus, & Tapert, 2006), which, in turn, has been associated with enhanced fear expression (Lissek et al., 2005). It has been found that sleep deprivation amplifies preemptive amygdala responding (Goldstein et al., 2013), which could also increase fear expression.
An influential model to study anxiety in the laboratory is the human fear conditioning procedure (Beckers, Krypotos, Boddez, Effting, & Kindt, 2013). In differential fear conditioning, a neutral stimulus (e.g. a picture of a face; conditioned stimulus [CS+]) is paired with an aversive stimulus (e.g. an electric shock; unconditioned stimulus [US]), while another image (e.g. of another face; unpaired stimulus [CS−]) is never paired with the aversive stimulus. To test for fear generalization, fear responses (e.g. US expectancy ratings or skin conductance responses [SCRs]) to one or more images related to the CS+ (e.g. morphed faces; generalization stimuli [GSs]) can be measured. Fear expression in situations previously experienced as safe can be evaluated by measuring fear responses to the CS−.
In the present study, participants were either subjected to a night of sleep deprivation or allowed to sleep normally between differential fear conditioning (in the evening) and test (the next morning). We hypothesized that, relative to normal sleep, sleep deprivation would yield enhanced fear expression to the GS and CS− stimuli as indicated by threat expectancy ratings and SCRs.
2. MATERIALS AND METHODS
2.1. Participants
Forty participants (M = 21.75, SD = 3.39, age range: 17–33 years) gave written informed consent, in accordance with the Declaration of Helsinki, to participate in this study, which was approved by the Social and Societal Ethics Committee of KU Leuven (see Table 1 for participants’ characteristics). Half of them were assigned to the sleep deprivation condition. For practical reasons, we decided prior to advertising the study which nights would be sleep deprivation or regular sleep nights. However, participants did not know their assigned condition beforehand and we had no influence on who would sign up for which condition either. Participants received either partial course credit or 40 euros for participation. They were screened for the following exclusion criteria based on self‐report: pregnancy, current or past history of severe medical conditions or psychiatric disorders (including sleep disorders), medical advice to avoid stressful situations, pain or disorders related to the hand or wrist, presence of an electronic implant, and age < 17 years at the time of the study. They were also asked to sleep at least 7 hr per night, get up before 10:00 hours and avoid daytime napping starting 2 days prior to the experiment, and to refrain from consumption of caffeine, tobacco and alcohol starting 6 hr before the training phase in the evening until the end of the study.
Table 1.
Sex, age, self‐reported usual daytime sleepiness (ESS), sleep habits (PSQI), circadian preference (MEQ) and trait anxiety (STAI‐T) for both conditions
| Characteristic | Regular sleep (n = 20, 16 females) | Sleep deprivation (n = 20, 16 females) | t 38 | p |
|---|---|---|---|---|
| M (SD) | M (SD) | |||
| Age | 22.10 (3.35) | 22.4 (3.47) | 0.65 | 0.52 |
| ESS | 7.90 (3.82) | 9.05 (4.10) | −0.92 | 0.37 |
| PSQI | 4.35 (2.30) | 5.65 (2.37) | −1.76 | 0.09 |
| MEQ | 48.15 (8.23) | 48.65 (8.36) | −0.19 | 0.85 |
| STAI‐T | 34.70 (6.84) | 37.10 (8.73) | −0.97 | 0.34 |
2.2. Apparatus and stimuli
The fear conditioning task was presented with Affect 4.0 software (Spruyt, Clarysse, Vansteenwegen, Baeyens, & Hermans, 2009) in a separate cubicle, which served as the experimental room. Two pictures, each of a different neutral human male face (from the Radboud Faces Database; Langner et al., 2010), were used as the CSs. The allocation of the pictures to the role of CS+ and CS− was counterbalanced within sleep conditions. A 50% morph between the CS+ and CS− served as the GS and was created with specialized software (Lenaert et al., 2012; Figure 1). A 2‐ms electrocutaneous stimulus was used as the US. It was generated by a Digitimer Constant Current Stimulator (model DS7A; Hertfordshire, UK) and administered to the wrist of the dominant hand by two 8‐mm Ag/AgCl electrodes that were filled with K‐Y lubricating jelly.
Figure 1.
Images used as CS+, GS and CS−. The CS+ and CS− images were counterbalanced within sleep conditions. CS, conditioned stimulus; GS, generalization stimulus
2.3. Measures
2.3.1. Threat expectancy ratings
During each stimulus presentation (i.e. CS+, CS−, GS or blank screen), participants′ expectancies that an electric shock would follow in the subsequent seconds were assessed on an 11‐point scale (ranging from 0 = certainly no electric shock to 10 = certainly an electric shock). Participants indicated their ratings with a single mouse click, operated by their dominant hand, and were instructed to respond as quickly as possible.
2.3.2. Skin conductance responses
To measure electrodermal activity, a Coulbourn Isolated Skin Conductance Coupler (model V71‐23: Coulbourn Instruments, Allentown, PA, USA) was used. A constant voltage of 0.5 V was transmitted through two 8‐mm Ag/AgCl electrodes, which were filled with K‐Y lubricating jelly and attached to the hypothenar surface of the palm of the non‐dominant hand. The analogue signal was digitized at 10 Hz.
2.3.3. Fear potentiated startle responses
Startle‐blink electromyography (EMG) was measured using three 4‐mm Ag/AgCl electrodes filled with electrolyte gel. Two electrodes were attached to the lower orbital portion of the left orbicularis oculi muscle, and a third electrode was attached to the participants′ forehead. The raw EMG signal was amplified with a Coulbourn Isolated Bioamplifier with bandpass filter (model V75‐04; Coulbourn Instruments, Allentown, PA, USA) set at 13–500 Hz. With a time constant of 20 ms, a Coulbourn 4‐Channel Integrator (model V76‐24; Coulbourn Instruments, Allentown, PA, USA) rectified and smoothed the amplified and filtered EMG signal. The sample rate was set to 1,000 Hz. As a result of technical problems, EMG could not be reliably recorded and the fear potentiated startle response results will not be reported.
2.4. Procedure
The protocol consisted of a fear conditioning training (i.e. evening of day 1) and a test (i.e. morning of day 2) session, which were 12 hr apart for each participant (Table 2). Participants arrived in the lab between 19:00 hours and 22:00 hours on day 1. After checking the exclusion criteria, participants gave informed consent and were then informed about their assignment to either the sleep or sleep deprivation condition. They completed questionnaires regarding their sleep habits (Pittsburgh Sleep Quality Index [PSQI]; Buysse, Reynolds, & Monk, 1989), usual daytime sleepiness (Epworth Sleepiness Scale [ESS]; Johns, 1991), circadian preference (Morningness–Eveningness Questionnaire [MEQ]; Horne & Ostberg, 1976), trait anxiety (State‐Trait Anxiety Inventory‐Trait [STAI‐T]; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) and current sleepiness level (Stanford Sleepiness Scale [SSS]; Hoddes, Zarcone, Smythe, Phillips, & Dement, 1973) to assure that there were no significant baseline differences between conditions on these measures (see Table 1 and Section 3.1). Electrodes were then attached, and the electric shock intensity was individually determined by gradually increasing the intensity until the participant indicated that the shock was “uncomfortable, but not painful” (see Supporting Information for the objective and subjective shock intensity of both days). Subsequently, the training phase of the fear conditioning task commenced.
Table 2.
Overview of the experimental design
| Day 1: Evening | Night | Day 2: Morning | |
|---|---|---|---|
| Pre‐training phase | Training phase | Sleep manipulation | Test phase |
| CS+ (3) | CS+ (8) | Regular sleep versus Sleep deprivation | CS+ (1) |
| CS− (3) | CS− (8) | CS− (1) | |
| Blank screen (3) | Blank screen (8) | Blank screen (1) | |
| – | – | GS (1) | |
Startle probe habituation phases are not displayed. The number of trials is indicated in parentheses.
CS, conditioned stimulus; GS, generalization stimulus.
During each trial of the conditioning task, participants were presented with a fixation cross on the screen for 2 s. The fixation cross was followed by the presentation of a CS/GS for 8 s. On startle probe trials, the probe occurred 7 s after CS/GS onset. On trials with an electric shock, the shock was delivered 7.5 s after CS+ onset. A fixation cross was presented during the inter‐trial interval (ITI), which ranged between 9 and 13 s (average ITI: 11 s). In addition to the CS/GS trials, there were also blank screen trials. A blank screen trial started with a fixation cross, but did not include a subsequent stimulus presentation as in the CS/GS trials (i.e. the screen remained blank apart from the fixation cross and was impossible to discriminate from the ITI). However, a startle probe could appear at the same time as it would in a CS/GS trial. In CS/GS and blank screen trials, the rating scale appeared after 2 s and disappeared after 8 s (concurring with CS/GS onset and offset, respectively, for CS/GS trials).
On day 1, the conditioning task included a startle probe habituation, pre‐training and training phase. During the habituation phase, participants were presented with nine startle probes with an ITI ranging between 18 and 25 s (average ITI: 21.5 s). The pre‐training phase comprised three CS+, CS− and blank screen trials, which were all presented without shock. The training phase consisted of two blocks. Each block comprised four CS+, CS− and blank screen trials. All CS+ presentations were paired with shock, while all CS− and blank screen presentations were without shock. The trial order was pseudo‐random with at most two consecutive trials of the same stimulus type. The startle probes were administered in two out of three presentations of the same stimulus type.
After the training phase, all electrodes were detached. Depending on the experimental condition, participants either spent the following night sleeping at home (sleep condition) or were kept awake for 12 hr in the psychology library (sleep deprivation condition). Participants in the sleep deprivation condition were kept awake in groups of five–six participants, and were monitored by an experimenter until the beginning of the experimental session on day 2. During the night of sleep deprivation, participants were allowed to engage in activities, such as watching movies, talking to other participants and the experimenter, or reading. Participants in either condition were asked to not talk about the experiment during the course of the study.
On the morning of day 2, all participants returned to the laboratory between 07:00 hours and 10:00 hours, and again completed the SSS (Hoddes et al., 1973) as a manipulation check. Afterwards, electrodes were attached and the electric shock intensity was again individually determined. Participants then continued with a startle probe habituation phase (identical to day 1) and the test phase of the conditioning task, which included one CS+, CS−, blank screen and GS trial, all without shock. The trial order was random and startle probes were administered on all trials. At the end of the test phase, electrodes were removed and participants were debriefed.
2.5. Data reduction and analysis
Skin conductance data were pre‐processed with the Psychophysiological Analysis (PSPHA) software package (De Clercq, Verschuere, de Vlieger, & Crombez, 2006). To compute the SCRs for each trial, the mean baseline value (i.e. 2‐s interval prior to CS/GS onset) was subtracted from the maximum value during the 7‐s CS/GS presentation. Although the CS/GS presentation lasted 8 s, we used the 7‐s interval to not include the possible startle probe and shock onset. Negative SCR values were recoded to zero before all SCR values were range‐corrected within participants (i.e. participants’ SCRs for days 1 and 2 were divided by the highest SCR value during day 1 for each participant) and then log10(SCR + 1)‐transformed.
We conducted a mixed‐design analysis of variance (ANOVA) with sleep condition (sleep condition, sleep deprivation condition) as between‐subjects factor and day (day 1, day 2) as within‐subjects factor on the SSS as a manipulation check. Threat expectancy ratings and SCRs were also analysed using mixed‐design ANOVAs with sleep condition as between‐subjects factor, and stimulus (CS−, GS, CS+; the blank screen trials were not included) and trial as within‐subjects factors. Follow‐up analyses were conducted using paired and independent t‐tests. Occasional failure to provide a threat expectancy rating within the provided response window resulted in missing values in the analyses, which are reflected in the degree of freedoms reported. Greenhouse–Geisser corrections were applied if the sphericity assumption was violated. The level of significance was fixed at α = 0.05. As the effect size, partial eta squared is reported.
One participant in the sleep deprivation condition failed to complete the SSS after the experimental sleep manipulation, but his or her data were included in all other analyses. Moreover, two participants in the sleep deprivation condition did not comply with the instructions to refrain from the consumption of caffeine less than 6 hr before the beginning of the study (n = 1) or to get up before 10:00 hours on the day of the study (n = 1). In the present study, the analyses on the complete sample (N = 40) will be reported. However, the conclusions remained the same when we reran the main analyses excluding those two participants (N = 38; see Supporting Information for details on these analyses and for further analyses including the PSQI scores on the complete sample).
3. RESULTS
3.1. Manipulation check
As expected, the Sleep condition × Day interaction was significant (F 1,37 = 17.32, p < 0.001, ). Follow‐up analyses revealed that there was no significant difference in sleepiness prior to the experimental manipulation between the sleep condition (M = 2.75, SD = 1.02) and the sleep deprivation condition (M = 3.05, SD = 1.54) as indicated by the SSS (t 38 = −0.73, p = 0.47). As expected, participants in the sleep deprivation condition (M = 5.00, SD = 1.20) were significantly sleepier than participants in the sleep condition (M = 3.00, SD = 1.03) following the night of sleep deprivation (t 37 = −5.60, p < 0.001).
3.2. Fear conditioning task
3.2.1. Threat expectancies
Pre‐training phase (day 1)
There was a significant main effect of trial (F 1.42,42.54 = 25.03, p < 0.001, ), indicating a reduction over trials (trial 1: M = 4.63, SD = 1.43; trial 3: M = 1.98, SD = 2.11). The Sleep condition × CS type × Trial interaction was non‐significant (F 1.57,47.17 = 0.79, p = 0.43, ), suggesting that sleep conditions did not significantly differ in their reduction over trials.
Training phase (day 1)
The main effect of CS type (F 1,35 = 132.90, p < 0.001, ) and the CS type × Trial interaction (F 3.66,128.08 = 73.73, p < 0.001, ) were significant, indicating successful fear acquisition (Figure 2a). The non‐significant main effect of sleep condition (F 1,35 = 0.47, p = 0.50, ) and non‐significant CS type × Trial × Sleep condition interaction (F 3.66,128.08 = 0.19, p = 0.93, ) suggest that conditions did not significantly differ in fear learning.
Figure 2.
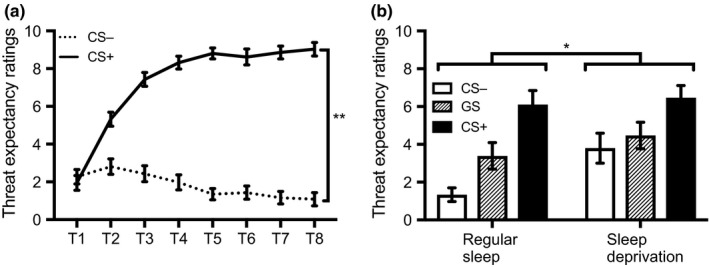
(a) Threat expectancy ratings (mean ± SEM) across all participants during the training phase. **p < 0.001, T, trial number. (b) Threat expectancy ratings (mean ± SEM) for each condition during the test phase. *p < 0.05, CS, conditioned stimulus; GS, generalization stimulus
Test phase (day 2)
The main effect of sleep condition was significant (F 1,35 = 6.06, p = 0.02, ), with the sleep deprivation group reporting higher threat expectancy ratings than the sleep group (Figure 2b). A significant main effect of stimulus type (F 2,70 = 15.23, p < 0.001, ) was also observed. Follow‐up analyses revealed that the difference between the CS− and GS (t 37 = −2.35, p = 0.02), between the CS− and CS+ (t 37 = −4.57, p < 0.001), and between the GS and CS+ (t 37 = −4.14, p < 0.001) were all significant. The Sleep condition × Stimulus type interaction was not significant (F 2,70 = 1.21, p = 0.31, ). For explorative reasons, we nonetheless tested the difference between conditions for each stimulus type separately. The sleep deprivation group (M = 3.85, SD = 3.38) provided higher threat expectancy ratings for the CS− than the sleep group (M = 1.37, SD = 1.50; t 26.5 = −2.99, p = 0.01). This indicates that sleep‐deprived participants have a higher sense of threat when confronted with a CS− that was previously experienced as safe. Differences between conditions were non‐significant for the CS+ (t 37 = −0.26, p = 0.80) and the GS (t 37 = −1.02, p = 0.32). Importantly, the difference between conditions for the CS− remained significant once we controlled for the family‐wise error rate with a Bonferroni‐alpha of 0.05 ÷ 3 = 0.02 (as three contrasts were tested).
As suggested by an anonymous reviewer, we ran additional correlational analyses between the SSS score of day 2 and the stimuli at test. We found a significant positive correlation between the SSS and the CS−, suggesting that sleepiness was associated with heightened responding to the CS− in particular (r = 0.44, p = 0.01), but not with the CS+ (r = −0.10, p = 0.56) or the GS (r = 0.28, p = 0.09).
3.2.2. Skin conductance responses
Pre‐training phase (day 1)
The main effect of trial was significant (F 1.60,60.76 = 34.76, p < 0.001, ). There was no significant Sleep condition × Trial interaction (F 1.60,60.76 = 1.52, p = 0.23, ). Accordingly, habituation occurred (trial 1: M = 0.14, SD = 0.07; trial 3: M = 0.05, SD = 0.05) and sleep conditions did not significantly differ in habituation.
Training phase (day 1)
The CS type main effect (F 1,38 = 12.54, p < 0.001, ) and the CS type × Trial interaction, (F 7,266 = 3.50, p < 0.001, ) were significant, suggesting that differential fear acquisition was successful (Figure 3a). A non‐significant main effect of sleep condition (F 1,38 = 0.02, p = 0.89, ) and a non‐significant CS type × Trial × Sleep condition interaction (F 7,266 = 0.49, p = 0.85, ) indicate that the groups did not significantly differ in acquisition.
Figure 3.
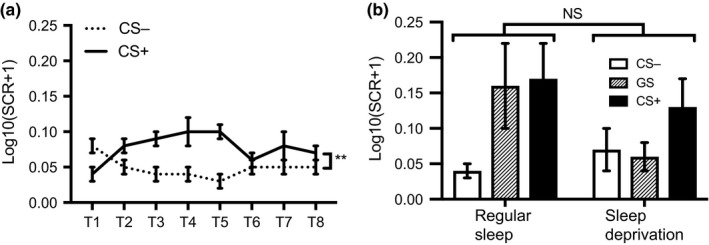
(a) Log10(SCR + 1) (mean ± SEM), where SCR values are obtained in microSiemens, across all participants during the training phase. **p < 0.001, T, trial number. (b) Log10(SCR + 1) (mean ± SEM), where SCR values are obtained in microSiemens, for each condition during the test phase. NS, not significant, CS, conditioned stimulus; GS, generalization stimulus; SCR, skin conductance response
Test phase (day 2)
The main effect of sleep condition was non‐significant (F 1,38 = 0.71, p = 0.40, ). There was a main effect of stimulus type (F 2,76 = 4.89, p = 0.01, ). Follow‐up analyses revealed that the difference between the CS− and CS+ (t 39 = −3.16, p < 0.001) was significant, while the difference between the CS− and GS (t 39 = −1.53, p = 0.13), and between the GS and CS+ (t 39 = −1.49, p = 0.14) were non‐significant. The Sleep condition × Stimulus type interaction was not significant (F 2,76 = 2.52, p = 0.09, ; Figure 3b). Analysis of the differences between conditions for each stimulus type separately failed to reveal significant differences between conditions for the CS+ (t 38 = 0.67, p = 0.51), the GS (t 23.7 = 1.49, p = 0.15) and the CS− (t 38 = −0.96, p = 0.34).
4. DISCUSSION
The present study investigated the effect of a full night of sleep deprivation on the expression of fear, using a fear conditioning procedure. Results showed that sleep deprivation, relative to sleep, increases overall subjective threat expectancies. Post hoc analyses suggest that this effect may be driven by increased threat expectancy ratings for the CS− after sleep deprivation. That is, participants in the sleep deprivation group seem to display increased fear expression when confronted with stimuli previously experienced as safe. However, because these analyses were conducted in the absence of a significant stimulus by group interaction, they should be interpreted with caution.
Adding to previous prospective research (Breslau et al., 1996; Jansson‐Fröjmark & Lindblom, 2008), our results provide evidence that sleep disturbances may play a causal role in the development of anxiety disorders by increasing the extent to which a person expects danger. Moreover, as illustrated below, they also add to the growing body of experimental research on the interplay between sleep and fear.
For example, in two studies, Davidson et al. (2016, 2018) invited participants to either take a nap or stay awake between fear learning and a test of fear expression (including a generalization test), with the aim to assess whether sleep enhances the memory consolidation of fear learning. While the first study found no differences between a nap and a wake group, the second study found larger responses to the CS+ than the CS− after a short period of wakefulness, but not after a nap. Although these studies focused on fear expression, the nap paradigm does not allow any conclusions concerning the effects of sleep deprivation. Studies by Peters et al. (2014) and Feng et al. (2018) did induce partial and total sleep deprivation, respectively; however, they focused on the effects of sleep deprivation on initial fear learning and not on fear expression following fear learning as was done in the current study. Feng et al. found increased responding to CS+ presentations in the sleep deprivation group, while Peters et al. only found a non‐specific failure to habituate in the sleep deprivation group.
Research that did focus on fear expression and how it is influenced by sleep and sleep deprivation — and therefore comes closer to the current study — was conducted by Menz et al. (2013, 2016). They found that sleep, and particularly rapid eye movement sleep, improved discrimination between threatening and non‐threatening stimuli (for related correlational evidence, see Marshall, Acheson, Risbrough, Straus, & Drummond, 2014). However, their study design included an immediate extinction phase (i.e. the CS+ was presented without the US), which occurred after fear learning and before the sleep manipulation. Although they used two CS+, of which one was extinguished and the other was left unextinguished, the extinction of the former may have still affected the latter (Liljeholm & Balleine, 2009). In another extinction study, Straus et al. (2017) found that sleep deprivation immediately before extinction impaired extinction recall on the subsequent day. In contrast to these studies, the present study provides an assessment of the effect of sleep deprivation on fear expression without any possible influence of extinction learning.
We only found effects in threat expectancy ratings. While threat expectancies have been shown to be externally valid with respect to anxiety disorders (Boddez et al., 2013), they do not capture all aspects of pathological anxiety. For example, physiological arousal is not captured by this measure, and is typically quantified by SCRs. In the present study, we did not find an effect of sleep deprivation in SCRs. One may thus speculate that sleep disturbances predominantly affect perceived threat while leaving physiological arousal unaffected (but see Feng et al., 2018; Peters et al., 2014). Alternatively, the SCR measure in the present study may not have been sensitive enough to detect an effect of the manipulation.
We found no evidence for a difference in fear generalization in either threat expectancies or SCRs. That is, our analyses did not yield evidence for significant differences in GS responding between conditions. Generalization may nonetheless serve to explain the higher threat expectancy ratings to the CS− in the sleep deprivation group (Haddad, Pritchett, Lissek, & Lau, 2012). That is, increased fear responses to the CS− in this group could be due to the aversive learning experiences with the CS+ (Boddez et al., 2017). For instance, generalization between the CS+ and the CS− could be driven by their perceptual similarity (McLaren & Mackintosh, 2000; Pearce, 1987; Rescorla & Furrow, 1977) or by their sharing of the same context during the training phase (cf. intersection of regularities; Hughes, De Houwer, & Perugini, 2016).
The former hypothesis could be investigated, for example, by using a fear conditioning procedure that makes use of one CS+ (e.g. an image of a male face) and two different CS−, one perceptually similar to the CS+ (e.g. an image of another male face) and the other perceptually dissimilar (e.g. an image of an oval; Haddad et al., 2012). In this setting, fear generalization to “safe” stimuli would be evidenced if participants in the sleep deprivation condition score higher on the perceptually similar but not on the perceptually dissimilar CS−, showing that perceptual similarity to the CS+ matters. Still, this leaves open the question as to why higher responding was observed for the CS− but not for the GS itself in our study.
According to the functional‐cognitive framework (De Houwer, 2011), one needs to differentiate between the effect of a procedure (e.g. sleep deprivation) on observable responses (e.g. SCRs, threat expectancy ratings) and mental processes that might mediate this effect. The design of the present study, however, does not allow disentangling whether the observed effects are due to the impact of sleep deprivation on processes occurring: (a) immediately after fear learning (e.g. lacking memory consolidation of specific stimulus attributes; Lenaert et al., 2012; Riccio et al., 1992); (b) during the sleep‐deprived state in which participants were tested on day 2 (e.g. impaired inhibitory regulation; Drummond et al., 2006); or (c) both. Participants in the sleep deprivation condition were indeed both sleep deprived after learning and in a sleep‐deprived state at test. Our finding that sleepiness on day 2 was associated with increased CS− responding indicates that the latter may have caused our effect.
However, future research needs to systematically disentangle these potential candidate processes. For example, introducing an additional night of (recovery) sleep before the test phase (Menz et al., 2013) would allow isolating the effect of sleep deprivation after learning because all participants would be well‐rested during test. Conversely, allowing all participants to sleep during the night following the training phase and manipulating sleep during the night before the test phase would allow isolating the effect of the sleep‐deprived state during test because memory consolidation after fear learning should have occurred to the same extent for all participants.
A potential limitation of our study is the absence of a sleep diary and of actigraphy measures to assess the regularity of the sleep–wake cycle prior to the experiment and during the sleep manipulation night. Still, all participants in the sleep condition reported to have slept at least 7 hr, while it was ensured that none of the participants in the sleep deprivation group could sleep. Accordingly, sleepiness was higher after sleep deprivation than normal sleep, suggesting that the sleep manipulation was successful (Hoddes et al., 1973).
In summary, our results demonstrate that sleep deprivation results in an increase in subjective threat anticipation, as shown by threat expectancies. Although future research is needed to determine whether these effects are due to sleep deprivation after fear learning or a sleep‐deprived state at the time of testing, our results suggest that sleep disturbances may play a role in the development of clinical anxiety by increasing perceived threats.
CONFLICT OF INTEREST
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
Y. B. designed the experiment with critical feedback by B. L., P. P. and T. B. B. L. and Y. B. programmed the experiment. A.‐K. Z. and Y. B. acquired the data. A.‐K. Z. and Y. B. analysed the data. A.‐K. Z. and Y. B. drafted the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Supporting information
ACKNOWLEGEMENT
This study was supported by grant G076015N of the Research Foundation – Flanders (FWO).
Zenses A‐K, Lenaert B, Peigneux P, Beckers T, Boddez Y. Sleep deprivation increases threat beliefs in human fear conditioning. J Sleep Res. 2020;29:e12873 10.1111/jsr.12873
REFERENCES
- Beckers, T. , Krypotos, A.‐M. , Boddez, Y. , Effting, M. , & Kindt, M. (2013). What's wrong with fear conditioning? Biological Psychology, 92, 90–96. [DOI] [PubMed] [Google Scholar]
- Boddez, Y. , Baeyens, F. , Luyten, L. , Vansteenwegen, D. , Hermans, D. , & Beckers, T. (2013). Rating data are underrated: Validity of US expectancy in human fear conditioning. Journal of Behavior Therapy and Experimental Psychiatry, 44, 201–206. [DOI] [PubMed] [Google Scholar]
- Boddez, Y. , Bennett, M. P. , van Esch, S. , & Beckers, T. (2017). Bending rules: The shape of the perceptual generalisation gradient is sensitive to inference rules. Cognition and Emotion, 31, 1444–1452. [DOI] [PubMed] [Google Scholar]
- Breslau, N. , Roth, T. , Rosenthal, L. , & Andreski, P. (1996). Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biological Psychiatry, 39, 411–418. [DOI] [PubMed] [Google Scholar]
- Buysse, D. , Reynolds, C. , & Monk, T. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Davidson, P. , Carlsson, I. , Jönsson, P. , & Johansson, M. (2016). Sleep and the generalization of fear learning. Journal of Sleep Research, 25, 88–95. [DOI] [PubMed] [Google Scholar]
- Davidson, P. , Carlsson, I. , Jönsson, P. , & Johansson, M. (2018). A more generalized fear response after a daytime nap. Neurobiology of Learning and Memory, 151, 18–27. [DOI] [PubMed] [Google Scholar]
- De Clercq, A. , Verschuere, B. , de Vlieger, P. , & Crombez, G. (2006). Psychophysiological analysis (PSPHA): A modular script‐based program for analyzing psychophysiological data. Behavior Research Methods, 38, 504–510. [DOI] [PubMed] [Google Scholar]
- De Houwer, J. (2011). Why the cognitive approach in psychology would profit from a functional approach and vice versa. Perspectives on Psychological Science, 6, 202–209. [DOI] [PubMed] [Google Scholar]
- Drummond, S. P. A. , Paulus, M. P. , & Tapert, S. F. (2006). Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. Journal of Sleep Research, 15, 261–265. [DOI] [PubMed] [Google Scholar]
- Feng, P. , Becker, B. , Feng, T. , & Zheng, Y. (2018). Alter spontaneous activity in amygdala and vmPFC during fear consolidation following 24 h sleep deprivation. NeuroImage, 172, 461–469. [DOI] [PubMed] [Google Scholar]
- Goldstein, A. N. , Greer, S. M. , Saletin, J. M. , Harvey, A. G. , Nitschke, J. B. , & Walker, M. P. (2013). Tired and apprehensive: Anxiety amplifies the impact of sleep loss on aversive brain anticipation. Journal of Neuroscience, 33, 10,607–10,615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, P. E. , Sisitsky, T. , Kessler, R. C. , Finkelstein, S. N. , Berndt, E. R. , Davidson, J. R. , … Fyer, A. J. (1999). The economic burden of anxiety disorders in the 1990s. Journal of Clinical Psychiatry, 60, 427–435. [DOI] [PubMed] [Google Scholar]
- Haddad, A. D. M. , Pritchett, D. , Lissek, S. , & Lau, J. Y. F. (2012). Trait anxiety and fear responses to safety cues: Stimulus generalization or sensitization? Journal of Psychopathological and Behavioral Assessment, 34, 323–331. [Google Scholar]
- Hoddes, E. , Zarcone, V. , Smythe, H. , Phillips, R. , & Dement, W. C. (1973). Quantification of sleepiness: A new approach. Psychophysiology, 10, 431–436. [DOI] [PubMed] [Google Scholar]
- Horne, J. A. , & Ostberg, O. (1976). A self‐assessment questionnaire to determine morningness‐eveningness in human circadian rhythms. International Journal of Chronobiology, 4, 97–110. [PubMed] [Google Scholar]
- Hughes, S. , De Houwer, J. , & Perugini, M. (2016). Expanding the boundaries of evaluative learning research: How intersecting regularities shape our likes and dislikes. Journal of Experimental Psychology: General, 145, 731–754. [DOI] [PubMed] [Google Scholar]
- Jansson‐Fröjmark, M. , & Lindblom, K. (2008). A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. Journal of Psychosomatic Research, 64, 443–449. [DOI] [PubMed] [Google Scholar]
- Johns, M. W. (1991). A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep, 14, 540–545. [DOI] [PubMed] [Google Scholar]
- Kessler, R. C. , Berglund, P. , Demler, O. , Jin, R. , Merikangas, K. R. , & Walters, E. E. (2005). Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the national comorbidity survey replication. Archives of General Psychiatry, 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Langner, O. , Dotsch, R. , Bijlstra, G. , Wigboldus, D. H. J. , Hawk, S. T. , & van Knippenberg, A. (2010). Presentation and validation of the Radboud Faces Database. Cognition and Emotion, 24, 1377–1388. [Google Scholar]
- Lenaert, B. , Claes, S. , Raes, F. , Boddez, Y. , Joos, E. , Vervliet, B. , & Hermans, D. (2012). Generalization of conditioned responding: Effects of autobiographical memory specificity. Journal of Behavior Therapy and Experimental Psychiatry, 43, S60–S66. [DOI] [PubMed] [Google Scholar]
- Liljeholm, M. , & Balleine, B. W. (2009). Mediated conditioning versus retrospective revaluation in humans : The influence of physical and functional similarity of cues. The Quarterly Journal of Experimental Psychology, 62, 470–482. [DOI] [PubMed] [Google Scholar]
- Lissek, S. , Kaczkurkin, A. N. , Rabin, S. , Geraci, M. , Pine, D. S. , & Grillon, C. (2014). Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry, 75, 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek, S. , Powers, A. S. , McClure, E. B. , Phelps, E. A. , Woldehawariat, G. , Grillon, C. , & Pine, D. S. (2005). Classical fear conditioning in the anxiety disorders: A meta‐analysis. Behavior Research and Therapy, 43, 1391–1424. [DOI] [PubMed] [Google Scholar]
- Lissek, S. , Rabin, S. , Heller, R. E. , Lukenbaugh, D. , Geraci, M. , Pine, D. S. , & Grillon, C. (2011). Overgeneralization of conditioned fear as a pathogenetic marker of panic disorder. American Journal of Psychiatry, 167, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek, S. , Rabin, S. J. , McDowell, D. J. , Dvir, S. , Bradford, D. E. , Geraci, M. , … Grillon, C. (2009). Impaired discriminative fear‐conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behavior Research and Therapy, 47, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcks, B. A. , Weisberg, R. B. , Edelen, M. O. , & Keller, M. B. (2010). The relationship between sleep disturbance and the course of anxiety disorders in primary care patients. Psychiatry Research, 178, 487–492. [DOI] [PubMed] [Google Scholar]
- Marshall, A. J. , Acheson, D. T. , Risbrough, V. B. , Straus, L. D. , & Drummond, S. P. (2014). Fear conditioning, safety learning, and sleep in humans. Journal of Neuroscience, 34, 11,754–11,760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren, I. P. L. , & Mackintosh, N. J. (2000). An elemental model of associative learning: I. Latent inhibition and perceptual learning. Animal Behavior, 28, 211–246. [Google Scholar]
- Menz, M. M. , Rihm, J. S. , & Büchel, C. (2016). REM sleep is causal to successful consolidation of dangerous and safety stimuli and reduces return of fear after extinction. Journal of Neuroscience, 36, 2148–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menz, M. M. , Rihm, J. S. , Salari, N. , Born, J. , Kalisch, R. , Pape, H. C. , … Büchel, C. (2013). The role of sleep and sleep deprivation in consolidating fear memories. NeuroImage, 75, 87–96. [DOI] [PubMed] [Google Scholar]
- Pearce, J. M. (1987). A model for stimulus generalization in Pavlovian conditioning. Psychological Review, 94, 61–73. [PubMed] [Google Scholar]
- Peters, A. C. , Blechert, J. , Sämann, P. G. , Eidner, I. , Czisch, M. , & Spoormaker, V. I. (2014). One night of partial sleep deprivation affects habituation of hypothalamus and skin conductance responses. Journal of Neurophysiology, 28, 1267–1276. [DOI] [PubMed] [Google Scholar]
- Rescorla, R. A. , & Furrow, D. R. (1977). Stimulus similarity as a determinant of Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavior Processes, 3, 203–215. [DOI] [PubMed] [Google Scholar]
- Riccio, D. C. , Ackil, J. , & Burch‐Vernon, A. (1992). Forgetting of stimulus attributes: Methodological implications for assessing associative phenomena. Psychological Bulletin, 112, 433–445. [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , Lushene, P. R. , Vagg, P. R. , & Jacobs, A. G. (1983). Manual for the State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Spruyt, A. , Clarysse, J. , Vansteenwegen, D. , Baeyens, F. , & Hermans, D. (2009). Affect 4.0: A free software package for implementing psychological and psychophysiological experiments. Experimental Psychology, 57, 36–45. [DOI] [PubMed] [Google Scholar]
- Straus, L. D. , Acheson, D. T. , Risbrough, V. B. , & Drummond, S. P. A. (2017). Sleep deprivation disrupts recall of conditioned fear extinction. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2, 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


