Abstract
The co‐evolving tumour cells and the systemic immune environment are mutually dysregulated. Tumours affect the immune response in a complex manner. For example, although lymphocytes are mobilized in response to tumours, their function is impaired by tumour progression. This study aimed to explore how the baseline and dynamic renal cell carcinoma (RCC) tumour burdens affect the T‐cell repertoire, and whether the baseline T‐cell receptor β‐chain (TCRB) diversity predicts prognosis. To characterise the TCRB repertoire, the baseline and follow‐up peripheral TCRB repertoires of 45 patients with RCC and 2 patients with benign renal disease patients were examined using high‐throughput TCRB sequencing. To explain the significance of TCRB diversity, 56 peripheral leukocyte samples from 28 patients before and after surgery were subjected to transcriptome sequencing. To validate the results, an advanced RCC patient's sample was subjected to single‐cell RNA sequencing (scRNA, 10x Genomics). Higher TCRB diversity was found to be correlated with a higher lymphocyte‐to‐neutrophil ratio, especially indicating more naïve T cells. High‐baseline TCRB diversity predicted a better prognosis for stage IV patients, and different tumour burdens exerted distinct effects on the immune status. The pre‐operative TCRB diversity was significantly higher in benign and stage I (low tumour burden) RCC patients than in stage IV (high tumour burden) patients. After the tumour burden of advanced patients was mostly relieved, we observed that the TCRB diversity was restored, T‐cell exhaustion was reduced, and naïve T‐cells were mobilized. It was demonstrated that the circulating TCRB repertoire could reflect the immune status and predict prognosis, and to some extent that cytoreductive nephrectomy (CN) reduces the burden of the immune system in advanced patients, which might provide a good opportunity for immunotherapy. © 2020 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: T‐cell receptor repertoire, renal cell carcinoma, tumour burden, nephrectomy, prognosis
Introduction
Accumulating evidence suggests that cancer is not only a genetic disorder but also a manifestation of immune dysfunction 1. Although renal cell carcinoma (RCC) is considered a strongly immunogenic cancer 2, immunosuppression frequently occurs when immune cells are exhausted and inhibited by uncontrolled tumour progression. The dysfunction of T cells, which play an important role in adaptive immunity, is involved in carcinogenesis and tumour progression. A unique T‐cell receptor (TCR) composed of heterodimers (α:β or γ:δ) is expressed on the surface of each T cell 3. Along with the composition of T cell subsets, the diversity of the TCR repertoire reflects the adaptive immune status. The TCR repertoire is composed of more than 90% TCRαβ, which recognizes self‐antigen peptides and mutated or foreign antigen peptides 4, such as neoantigens on cancer cells. The most abundant TCR β chain (TCRB) comprises variable (V), diversity (D), joining (J), and constant (C) regions. The amount of TCRB diversity arises from the recombination of VDJ regions and additional nucleotide deletions and/or insertions, specifically in the complementarity‐determining regions (CDRs). CDR3 is the primary CDR responsible for recognizing antigen peptides; it straddles the V(D)J junction, composed of the 3′‐end of the V region, the whole D region, and a partial J region. Profiling of the TCR repertoire is crucial for understanding the processes of carcinogenesis and cancer progression, especially in tumours with unknown antigenic triggers 5. Several studies have suggested that TCR diversity might be a good biomarker for therapeutic monitoring and prognostic assessment 6, 7, 8.
However, the relationship between TCR diversity and prognosis is unclear. Several studies have shown that high infiltrating or circulating TCRB diversity predicts better prognosis in cervical and lung cancer 6, 8, whereas low infiltrating TCRB diversity predicts better prognosis in bladder cancer 9. These contradictory results highlight the need for further studies to confirm if the connection between TCR diversity and prognosis is cancer specific. Therefore, a precise method for TCR repertoire construction and sequencing is required. Currently, the construction of a TCR library for sequencing utilizes multiplex polymerase chain reaction (PCR) with multiple V and J gene primers based on genomic DNA 10 or complementary DNA (cDNA) 11. Although these systems are considered bias‐controlled during the matching of multiple primers for V and J genes, mismatch induced by polymerase chain reaction (PCR) amplification is inevitable. In 2014, Chudakov et al 12, 13 reported a Switch Mechanism At the 5′‐end of RNA Templates (SMART)–based error‐free immune repertoires detection method, which was adopted by many commercial VDJ kits (e.g., SMARTer Human scTCR a/b Profiling Kit of TaKaRa) afterwards. This method uses the unique molecular index (UMI) –inserted template‐switching oligo (TSO) to correct the amplification‐induced mismatch and quantitative bias. We modified this method by introducing two universal TCR C gene primers to guarantee the specificity during the reverse transcription and the PCR amplification, and by simplifying the sequencing library construction steps.
Tumours exert pressure on the immune response, mobilizing lymphocytes while impairing their function. However, little is known about alterations in the repertoire of responding T cells and at what threshold the tumour load begins to impact the TCR repertoire. Furthermore, to explore the effects of tumours on TCRB diversity, we assessed the tumour burden by the tumor‐node‐metastasis (TNM) stage according to the American Joint Committee on Cancer staging system and tumour burden changes following nephron‐sparing surgery, radical nephrectomy, or cytoreductive nephrectomy (CN). In the era of targeted therapy, the treatment of synchronous metastatic RCC patients with CN is controversial. We first evaluated the influence of CN by examining alterations in TCR diversity and immune cell subtype activity. Compared with tumour‐infiltrating lymphocytes, peripheral T cells are not only easily obtained but also fundamental to sustaining adult human naïve T cells 14. Although the intratumour heterogeneity of the infiltrating TCRB repertoire in RCC has been reported 10, the characteristics of the circulating TCRB repertoire, especially the dynamic changes that occur after surgery, are unclear.
This study is the first to profile the baseline and dynamic characteristics of the peripheral TCRB repertoire in patients with RCC treated with surgery or targeted therapy and to estimate the prognostic value using a SMART‐based UMI‐corrected TCR library.
Materials and methods
Details of the methods used are provided in supplementary material, Supplementary materials and methods.
Eighty‐seven samples of peripheral blood leukocytes were obtained from 45 patients with RCC and 2 patients with benign kidney disease, after which the RNA was extracted, and the SMART‐based UMI‐corrected TCRB libraries for sequencing were constructed. Among these patients, 40 of the 42 treatment‐naïve patients with different tumour burdens had paired pre‐ and post‐operative (seventh day after surgery) peripheral TCRB sequencing data and were grouped to analyse the effect of surgery (alteration of the tumour burden) on the immune status. This group is hereafter referred to as the surgery‐associated cohort. Baseline TCRB sequencing data were obtained from 18 advanced RCC patients enrolled in this study and were used to predict prognosis. The detailed demographic information of the enrolled patients is shown in supplementary material, Table S1. The use of human samples and the experiments in the study were approved by the ethics committee of the Cancer Hospital of the Chinese Academy of Medical Sciences (CAMS, approval number NCC2016XQ‐22). In addition, the patients were informed of the study and thoroughly understood the research.
The proportion of immune cells was calculated by deconvoluting the bulk RNA‐seq data and validated by flow cytometry and single‐cell RNA sequencing (scRNA) data. Data were analysed by the MIGEC, MiXCR, and CellRanger software programmes and the R packages ‘tcR’, ‘GSVA’, ‘CIBERSORT’, and ‘Seurat’.
The raw TCRB repertoire and transcriptome sequencing data generated in this study are deposited in the Genome Sequence Archive 15 at the BIG Data Center 16, Beijing Institute of Genomics (BIG), Chinese Academy of Sciences, under the accession number HRA000049 [http://bigd.big.ac.cn/gsa-human]. The bulk data and the raw scRNA sequencing data (not uploaded) are also available from the corresponding authors upon reasonable request.
Results
TCRB repertoire information of enrolled patients
To minimize PCR amplification–induced bias and error, universal primers containing UMIs were used rather than multiplexed primers. All reads are grouped by their UMI, after which read groups with at least four to six reads (depends on the sequencing depth of each sample) are assembled. For the 80 samples in the surgery‐associated cohort, a total of 10 072 661 TCRB clonotypes were detected, which included 115 428 ± 62 953 (mean ± standard deviation, SD) TCRB clonotypes per sample in the pre‐operative TCRB repertoire and 136 388 ± 48 815 (mean ± SD) TCRB clonotypes in the post‐operative TCRB repertoire. All 64 V genes and 13 J genes were detected. The detailed quality control result of the SMART‐based UMI‐corrected TCRB sequencing platform developed in house is shown in supplementary material, Figure S1. It is feasible to quantify the TCRB clonotype abundance by this method.
The immune status is reflected by the baseline TCRB diversity
It is unclear whether high or low TCR diversity corresponds to a better immune status. We herein defined the immune status as the physiological homeostasis maintained by the immune system. To investigate how the TCRB diversity reflects the immune status in the baseline samples, we evaluated the relationships among the Malta's stemness index 17, immunophenotypic scores obtained from bulk RNA‐seq data analysis, and the TCRB diversity index (represented by the Efron–Thisted estimator) of 28 pre‐operative treatment‐naïve patients.
We observed that a higher peripheral blood stemness index was correlated with higher TCRB diversity (Pearson correlation analysis, R = 0.54, P = 0.0027, Figure 1A), which indicates that a high TCRB diversity might be related to the diverse naïve T cells, which are characterised with multipotent differentiation potential and proliferative capacity. The gene set enrichment analysis (GSEA) scores of the c7 gene sets from Molecular Signatures Database also revealed that wealthy TCRB diversity is associated with high numbers of T cells, especially CD4‐ and CD8‐positive naïve T cells, whereas more neutrophils or pro‐tumour Treg cells appeared when TCRB decreased in the peripheral blood (Figure 1B). Consistently, genes that were positively correlated with TCRB diversity were clustered into 162 gene ontology (GO) terms, and the top 20 significant terms were found to be related to biosynthesis and metabolic process. Especially, the genes in the top five terms are mostly ribosomal genes, which were reported to be highly expressed in naïve T cells 18 (Figure 1C). Furthermore, some terms associated with T‐cell selection and development were also included in the enriched GO terms (supplementary material, Table S2). In addition, the scRNA sequencing data suggested that TCR clonotype of naïve T cells is unique, but TCR clonotype of effector T cells was expanded (detail results are shown below). These results suggest that a higher TCRB diversity may accord with a relatively more powerful anti‐tumour potential given that diverse naïve T cells are the reserve force for identifying neoantigens on tumour cells.
Figure 1.
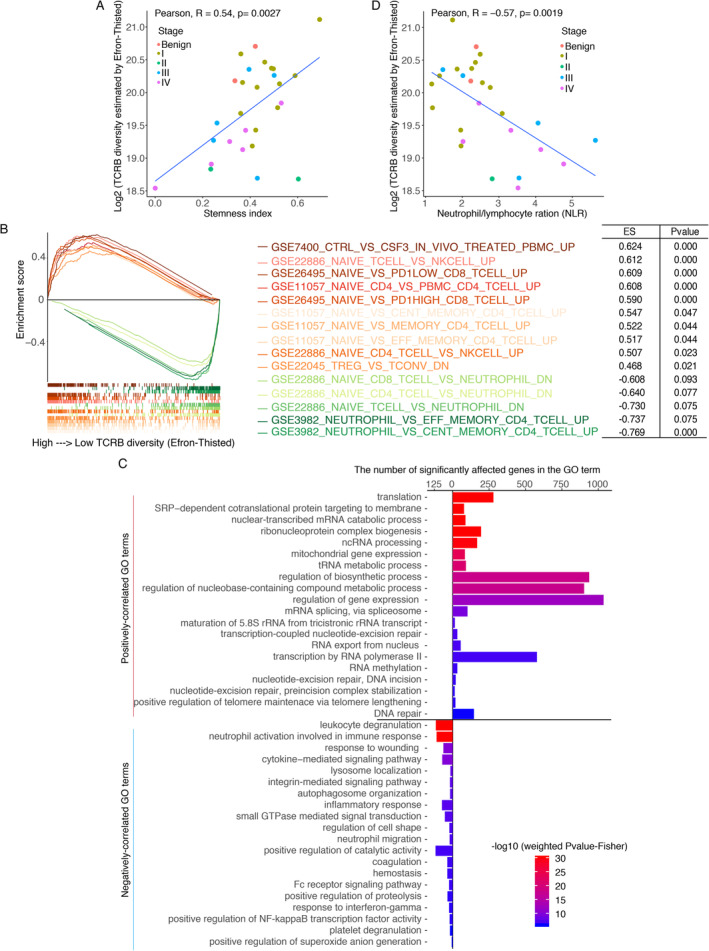
Correlation between basic TCRB diversity and other indexes. (A) The relationship between TCRB diversity and stemness indexes. (B) Correlation between the GSVA scores of c7 immune terms and TCRB diversity. Multiple significant GSEA mountain plots were merged into a single plot, with the orange lines indicating positively correlated terms and the green lines representing negatively correlated terms. Each term was attached to an enrichment score (ES) and a P value. (C) Enriched GO terms for genes correlated with TCRB diversity. The plot includes the top 20 positive and top 20 negative GO terms according to the weighted Fisher's P value. (D) Relationship between TCRB diversity and the NLR.
Conversely, genes that were negatively correlated with TCRB diversity were enriched in the inflammatory response and coagulation process, especially in the activation of neutrophil and platelet (Figure 1C), which have been reported as risk factors for worse prognosis by the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) 19 and supporters serve to circulating tumour cells survive 20. In addition, the TCRB diversity and neutrophil/lymphocyte ratio (NLR) were negatively correlated (Pearson's correlation analysis, R = −0.57, P = 0.0019, Figure 1D); the NLR predicts a poor prognosis 21.
In summary, patients with low TCRB diversity may have severe inflammatory response and relatively hypercoagulable state; conversely, high TCRB diversity indicates a homeostatically balanced immune system armed with abundant naïve T cells. Accordingly, it is possible that the difference in TCR diversity is correlated with their tumour burden.
Differences in the baseline immune statuses of patients with different tumour burdens
Although tumours with antigenic characteristics could recruit T cells, the accumulated T cells have defective killing functions, termed tumour‐induced T‐cell dysfunction 22. The tumour burden at different clinical stages may have a distinct immunogenic effect on the host. The circulating T‐cell repertoire is a fundamental source for recruitment 23. Thus to explore the pressures exerted by different tumour burdens on the immune system, we profiled and compared the baseline circulating TCRB diversities and the functional statuses of various immune cell subtypes in treatment‐naïve RCC patients with different tumour burdens in the surgery‐associated cohort.
Four types of indicators for T‐cell diversity unanimously represented pre‐operative TCRB diversity in benign and stage I RCC patients, which was significantly more abundant than that in stage IV patients. For instance, the counts of all observed clonotypes in stage I patients were higher than those in stage IV patients (Wilcoxon test, P = 0.0057, Figure 2A,B). Furthermore, the total proportion of the top 25 000 clonotypes in stage I patients was lower than that in stage IV patients (Wilcoxon test, P = 0.025, Figure 2C,D), and the TCRB diversity estimated by Efron–Thisted and Chao1 in stage I patients was consistently higher than that in stage IV patients (Wilcoxon test, P = 0.0011 and P = 0.0014, respectively, Figure 2E and supplementary material, Figure S2A). In addition, the pre‐operative TCRB diversity estimated by the Shannon–Wiener index showed a gradually decreasing trend from stages I to IV, but the differences were not significant (supplementary material, Figure S2A). Overall, the pre‐operative TCRB diversity of stage I RCC patients was significantly higher than that of stage IV patients. However, for the post‐operative TCRB diversity, there were no statistically significant differences in any of the five indicators (Figure 2A–E and supplementary material, Figure S2A).
Figure 2.
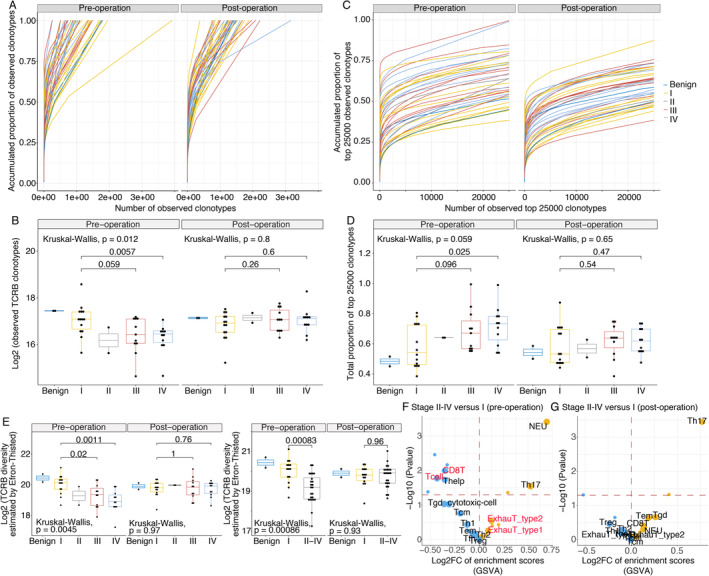
The peripheral immune cells in treatment‐naïve patients with stage II–IV disease are less diversified than those in patients with stage I disease before surgery. The total observed TCRB clonotype count (A and B), accumulated proportion of the top 25 000 clonotypes (C and D) and the TCRB diversity index (Efron–Thisted) (E) were compared among treatment‐naïve patients with different disease stages before and after surgery. Multiple group statistical analysis was performed using the Kruskal–Wallis test, and pairwise comparison was performed using a Wilcoxon test. These results suggested that the TCRB diversity in stage I patients was significantly more abundant than that in stage IV patients. Volcano plot comparing the GSVA‐based immunophenotypic scores in disease stages II–IV with that in stage I of pre‐operation (F) and post‐operation (G). In the volcano plot, the dots that represent T‐cell subtypes, neutrophils, and monocytes, are enlarged and labelled with the corresponding text. The activated and depressed immune cell subsets are shown in yellow and blue, respectively. Statistical P values less than 0.05 are indicated above the horizontal red dashed line.
Consistently, the immunophenotypic scores of the immune cell subsets demonstrated the same result: The activities of T cells and CD8‐positive T cells were lower in patients with stage II–IV tumours than in patients with stage I tumours before surgery and were not different 7 days after surgery. In addition, RNA‐seq analysis revealed an increased proportion of exhausted T cells in stage II–IV patients than in stage I patients before surgery (Figure 2F). The same differentiated immune status was found when comparing stage IV and I patients (supplementary material, Figure S2B).
In short, the immunogenic burden produced by the tumour in stage IV patients disrupts the function of the immune system.
The baseline TCRB diversity is associated with prognosis
Similar to many other cancers, patients with RCC at the same clinical stage may have different prognoses. The baseline TCRB diversity, indicating the reserved power of tumour immune surveillance, might be a promising index for predicting their prognoses. In our study, high baseline TCRB diversity in stage IV patients (due to the limited follow‐up period, the final outcomes only happened in stage IV patients) predicted high overall survival (log‐rank test, P = 0.0214; Cox proportional hazards, hazard ratio (HR) = 0.195, P = 0.037; Efron–Thisted index, Figure 3A,D). Similar results were obtained using the Chao1 index (Figure 3B) and the Shannon–Wiener index (Figure 3C). A longer follow‐up period is needed to confirm whether TCRB diversity could predict the prognosis of local RCC (stage I–III). Furthermore, to confirm this speculation, implementation of a large‐scale, rigorous trial for peripheral TCRB repertoire sequencing in RCC patients is necessary.
Figure 3.
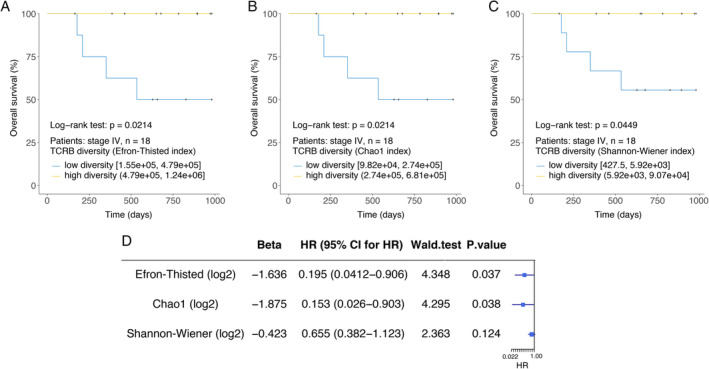
Higher baseline TCRB diversity is associated with better prognosis of in stage IV patients. Kaplan–Meier plots of the overall survival of patients with stage IV RCC (A–C, n = 18) stratified by the baseline TCRB diversity index. Cox regression analysis for overall survival of patients with stage IV RCC (D).
The reduced tumour burden restores the immune statuses of patients with advanced RCC
Next, to investigate the impact of tumour burden alterations on the TCR repertoire and global immune status, we compared alterations in paired circulating TCRB diversity levels to the activities of immune cell subsets before and after surgery. Different diversity indexes consistently indicated that the TCRB diversity in stage IV patients significantly increased after most of the tumour burden (immunogenicity) was relieved by surgery. For example, the number of observed TCRB clonotypes increased after surgery (paired Wilcoxon test, P = 0.0039, Figure 4A), and the TCRB diversity indexes (Efron–Thisted, Chao1, and Shannon–Wiener) also consistently increased after surgery (paired Wilcoxon test, P = 0.0039, P = 0.0039, and P = 0.0067, Figure 4B and supplementary material, Figure S3) in stage IV patients. Similarly, the stemness indexes indicated more stem naïve T cells after surgery in stage IV patients (Figure 4C). The immunophenotypic scores of immune cell subsets estimated by bulk RNA‐seq showed similar results, as exhausted T‐cell numbers were reduced (Figure 4E). These findings indicated that the TCR repertoire was restored when the overwhelming number of immunogens was removed by CN in stage IV patients.
Figure 4.
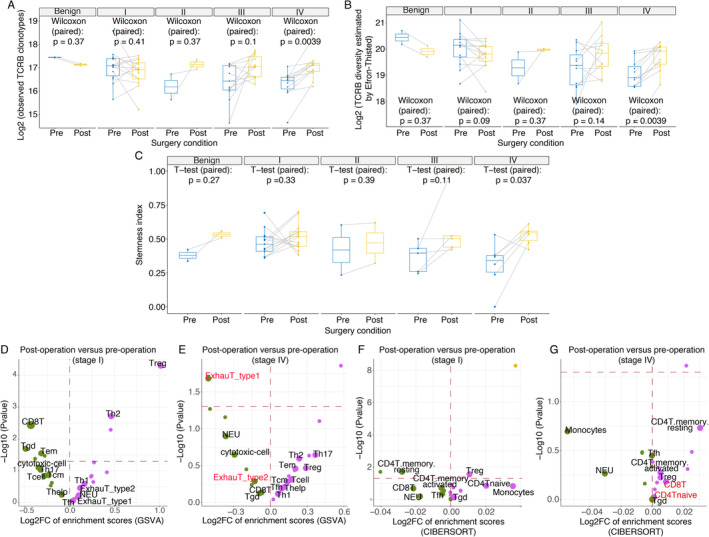
Changes in the TCRB diversity, stemness indexes, and bulk RNA‐seq–based immunophenotype before and after surgery. (A) Changes in paired observed TCRB clonotypes, (B) the TCRB diversity Efron–Thisted index, and (C) stemness indexes in pre‐ and post‐operative blood from patients with different clinical stages. Volcano plot of the GSVA‐based immunophenotypic scores before after surgery for (D) stage I and (E) stage IV patients. Volcano plot of the CIBERSORT‐based immunophenotypic scores before and after surgery for (F) stage I and (G) stage IV patients. In the volcano plot, the dots that represent T‐cell subtypes, neutrophils, and monocytes, are enlarged and labelled with the corresponding text. The magenta and green dots represent activated and depressed immune cell subtypes after surgery, respectively. In addition, the horizontal red dashes mark the boundary for a P value equal to 0.05.
To validate these results, another stage III RCC patient with inferior vena cava tumour thrombosis was enrolled to analyse the changes in exhausted T cells before and after surgery. Our results suggested that the proportions of PD‐1‐ and TIM3‐positive cells among CD8‐positive T cells were decreased after surgery (supplementary material, Figure S4E, F). In addition, the immune repertoire and gene‐expression profiling detected by scRNA‐seq (n = 8998, details available in the supplementary materials and methods) of a treatment‐naïve stage IV patient indicated that the proportions of CD4‐ and CD8‐positive naïve T cells were increased after CN (Figure 5A, B). The key feature markers used to confirm the identified clusters are shown in supplementary material (Figure S5). Integrated with the corresponding single‐cell immune repertoire data, we confirmed the high proportion of clonal expansion (defined as more than one T cells shared an identical α‐β TCR pair) in the helper T cells and effector T cells and the high clonal diversity of naïve T cells (Figure 5C).
Figure 5.
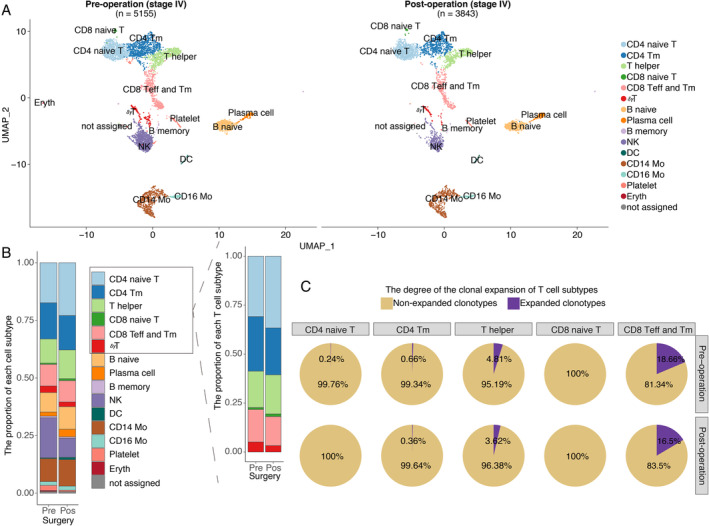
The proportion of naïve T cells increased after CN as determined by scRNA. scRNA data from a validated stage IV treatment‐naïve patient are shown. (A) Pre‐operative peripheral blood mononuclear cell (PBMC, n = 5155 cells) and post‐operative PBMC (n = 3843 cells) samples from a validated stage IV treatment‐naïve RCC patient are shown. Each dot represents a cell. Clusters were identified by principal component analysis and visualized with uniform manifold approximation and projection (UMAP). CD4 naive T: CD4 naïve T cells; CD4 Tm: CD4‐positive memory T cells; T helper: T helper cells; CD8 naive T: CD8 naïve T cells; CD8 Teff and Tm: a cluster including CD8‐positive effector T cells and memory T cells; γδT: T cells with TCRγ and δ chains; B naive: naïve B cells; Plasma cell: plasma cells; B memory: memory B cells; NK: nature killer cells; DC: dendritic cell; CD14 Mo: classic monocyte; CD16 Mo: monocyte expressing CD16; Platelet: platelet; Eryth: erythrocyte; not assigned: cells were not assigned. (B) The stack bar plot shows the proportions of all subtypes in all peripheral blood and T cell populations. (C) Pre‐operative (n = 2592) and post‐operative (n = 2208) αβT cells are shown. The pie chart represents the distribution of TCRB clonotypes with different abundances in different T‐cell subtypes. A clonotype that is detected more than once is defined as a clonotype that has undergone clonal expansion. Clonal expansion occurs in the memory and effector T cells and the naïve T cells exhibited high clonal diversity.
TCRB diversity was, in addition, positively correlated with CD4 naïve T cells (estimated by deconvolution of the RNA‐seq data, the model for estimating CD8 naïve T cells was not provided) (supplementary material, Figure S6), which indicated that higher TCRB diversity was associated with more naïve T cells. The increased TCRB diversity in stage IV patients after removal of the primary tumour with CN was potentially attributed to tumour‐associated antigens being released into the blood upon the destruction of tumour cells during surgery, and to the fact that more naïve T cells were mobilized to enhance the probability of recognizing the newly released antigens, which further initiate an immune response.
Alterations in persistent and emerging clonotypes before and after surgery with different tumour burdens and surgical strategies
In addition to the macroscopic information provided by TCRB diversity, microscopic changes in each TCRB clonotype are also important. Therefore, we defined clonotypes with the same CDR3 amino acid (CDR3aa) sequence plus the Vβ and Jβ genes in pre‐operative and post‐operative samples from the same patient as persistent rather than emerging. Subsequently, we profiled the differences in the alterations in persistent and emerging clonotypes before and after surgery with different tumour burdens and surgical strategies.
Each clonotype was ordered from high to low abundance, and the ordered clonotypes were segmented into five parts according to their cumulative frequency. All patients in the surgery‐associated cohort exhibited the same phenomenon that most high‐abundance clonotypes (in the top 60%) were persistent, which obeys the random sampling law (Figure 6A). To graphically illustrate this phenomenon, four samples at different clinical stages were selected as representative patients (Figure 6B). Statistically, the mean concentration of persistent clonotypes in each patient was significantly higher than that of emerging clonotypes (Wilcoxon test, P < 2.2 × 10–16, Figure 6C). In addition, the proportion of emerging clonotypes in stage IV patients significantly increased after removal of the primary tumours (Wilcoxon test, P = 0.0051, Figure 6D). Combined with the scRNA results, these findings imply that low‐abundance clonotypes, especially the changes of proportion of naïve T cells, are critical for expanding TCRB diversity.
Figure 6.
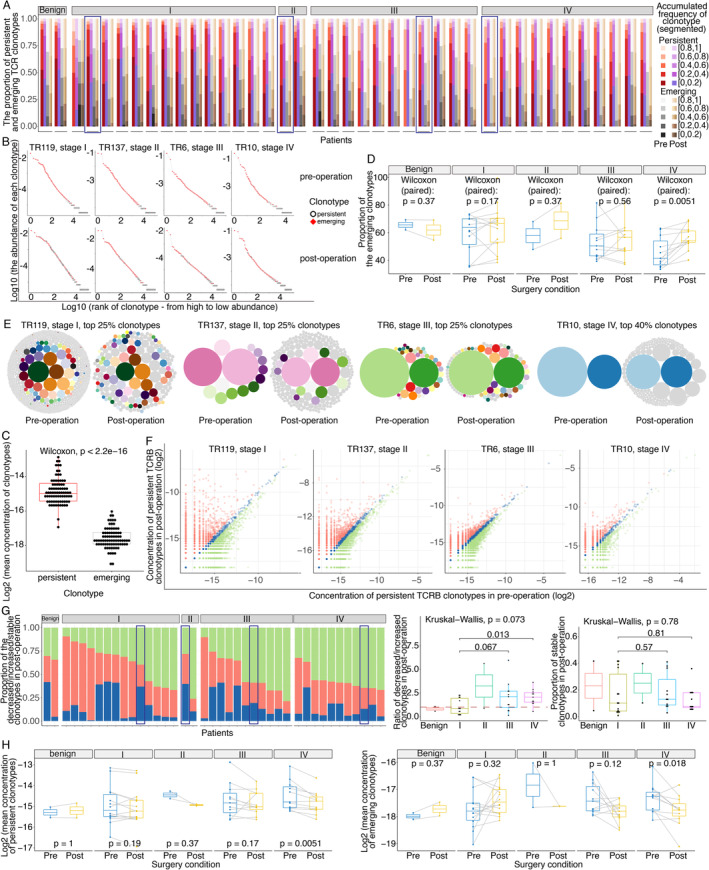
Characteristics of persistent and emerging clonotypes before and after surgery. (A) The composition ratios of the proportions of persistent and emerging clonotypes in segments with different cumulative frequencies. The red, purple, grey, and brown gradient‐filled bar represents the segmented cumulative frequencies of clonotypes ranked from high to low abundance as follows: persistent clonotypes pre‐operation, persistent clonotypes post‐operation, emerging clonotypes pre‐operation, and emerging clonotypes post‐operation. Each dash represents a patient, and the detailed order of the patients is TR183, TR95, TR116, TR119, TR186, TR45, TR55, TR56, TR58, TR59, TR60, TR65, TR66, TR79, TR86, TR88, TR137, TR169, TR128, TR16, TR17, TR182, TR184, TR22, TR23, TR6, TR70, TRPT180, TRT141, TR10, TR102, TR123, TR15, TR156, TR179, TR24, TR27, TR63, TR83, and TRML21. The four representative patients are marked with rectangular frame. (B) Abundances of persistent clonotypes in four representative patients. The plot in the first row shows pre‐operative TCRB clonotypes, and the plot in the second row shows post‐operative clonotypes. The abscissa represents each individual clonotype, ranked from high abundance to low abundance, and the ordinate depicts the frequency (log10‐transformed) of each clonotype. Red squares indicate persistent clonotypes, and white hollow circles indicate emerging clonotypes. The marked persistent clonotypes were concentrated among high‐frequency clonotypes in both pre‐ and post‐operative samples. (C) Difference in the mean concentrations of persistent and emerging clonotypes. (D) Paired changed proportions of emerging clonotypes before and after surgery in patients with different stages. (E) Changes in high‐abundance clonotypes in the four representative patients before and after surgery. Each circle represents a clonotype. The diameter of the circle indicates the concentration of the clonotype. Persistent clonotypes are marked as colourful and bright circles, and a single colour represents one clonotype. Emerging clonotypes are uniformly marked as grey circles. For the representative patients in stages II–IV, the predominant high‐abundance clonotypes that existed before surgery remained predominant high‐abundance clonotypes after surgery, but their concentrations were reduced after surgery, especially in stage IV patients. (F) Concentration changes in persistent clonotypes in four representative patients before and after surgery. Each dot represents a persistent clonotype that is present in both pre‐ and post‐operative samples. Post‐ versus pre‐operative samples showing persistent clonotypes with decreased or increased abundance are marked as green and red dots, respectively, and stable persistent clonotypes are marked as blue dots. (G) The proportions of changes in persistent clonotypes post‐ versus pre‐operation. The proportions of decreased, increased, and stable clonotypes are marked with green, red, and blue bars, respectively. The order of patients is TR183, TR95, TR66, TR65, TR58, TR116, TR86, TR56, TR79, TR60, TR55, TR119, TR88, TR186, TR59, TR45, TR137, TR169, TR128, TR182, TR17, TRT141, TR16, TRPT180, TR6, TR23, TR184, TR22, TR70, TR102, TRML21, TR156, TR27, TR83, TR15, TR123, TR179, TR10, TR24, and TR63. The middle boxplot shows the ratio of the proportions of decreased and increased clonotypes in post‐operative samples in patients with different stages. The right boxplot shows the proportions of stable clonotypes in patients at different stages. The four representative patients are marked with rectangular frame. (H) Changes in the mean concentrations of persistent (left plot) and emerging (right plot) clonotypes before and after surgery in patients with different stages as determined by the paired Wilcoxon test.
To explore whether the abundance of persistent clonotypes was significantly different before and after removal of the tumour, the dynamic changes in each clonotype in the top 25 or 40% (for the patient in stage IV) in the four representative patients are graphically represented (Figure 6E), which suggested that the high‐abundance overwhelming clonotypes decreased in stage IV patients after surgery. To further confirm whether this pattern existed universally in all persistent clonotypes, we assessed alterations in all persistent clonotypes among the 40 patients. We defined persistent clonotypes with a fold change of no more than 1.2 (post‐ to pre‐operative concentrations) as stable clonotypes; conversely, clonotypes with a fold increase of more than 1.2 were defined as increased, and the rest clonotypes were defined as decreased. Examples are shown in Figure 6F. We found that the proportion of decreased clonotypes was greater than that of increased clonotypes in stage IV patients, which was distinct from the observed trend in stage I patients (Wilcoxon test, P = 0.013, Figure 6G). However, no differences in the proportions of stable clonotypes were observed among the different stages (Figure 6G). Moreover, the mean concentrations of both the persistent and emerging clonotypes were significantly decreased in stage IV patients after surgery (paired Wilcoxon test, P = 0.0051 and P = 0.018, respectively, Figure 6H).
Overall, after the immunogenic burden was reduced by surgery, the concentration of persistent clonotypes (high abundance) in stage IV patients decreased, and the proportion and number of emerging clonotypes (moderate and low abundance) in stage IV patients increased, which was accompanied by decreases in the concentration of each clonotype.
Discussion
The treatment of synchronous metastatic RCC patients with CN is controversial. During the era of cytokine therapy for advanced RCC, two convincing prospective randomized studies demonstrated that CN followed by cytokine therapy provided a better outcome than interferon alone 24, 25. Upon entering the era of targeted therapy, the value of CN has become controversial. One retrospective study of IMDC demonstrated that patients treated with CN had improved IMDC prognostic profiles compared to those of patients who were not treated with CN 19. However, another surprising newly published prospective study concluded that sunitinib alone was not inferior to CN followed by sunitinib for patients with advanced RCC 26.
To explore how tumour burden and its dynamic changes shape the global immune status of RCC patients, and whether CN is critical for improving the immune status in advanced patients, we profiled the characteristics and dynamic changes of the TCRB repertoire and the activity of RNA‐seq–derived immune cell populations during tumour burden eradication or alteration by nephron‐sparing or radical nephrectomy and CN. We found that the TCRB diversity was much lower in advanced RCC patients than that in stage I RCC patients, who had borne low tumour burdens. In addition, this reduced TCRB diversity was restored after removal of the primary tumour by CN. We inferred that when the tumour burden reached a certain threshold, it might exert significant pressure on the TCR repertoire.
The systemic environment co‐evolves with tumour cells, and the two are mutually dysregulated 27. Tumours elicit a series of autologous tumour antigen–specific T‐cell clonotypes from their exposed neoantigens and immunogenic self‐antigens while blocking the cytotoxicity of these T cells using tumour‐derived PDL1 28. Because peripheral blood is a limited pool to store T cells, the expansion of one type of T‐cell clonotype may lead to suppression of the others 29, 30. That is to say that there is not enough residence for the diversified naïve T cells. In addition, the immune checkpoint molecules on expanded T cells in patients with advanced RCC before surgery were higher than that on unexpanded T cells before surgery (supplementary material, Figure S7A). The adaptive immune system, as the keeper to protect host, produced plentiful inefficient hyperexpanded T‐cell clonotypes, which occupied the space of naïve T cells. Consistently, bulk RNA‐seq–derived immune cell activity data revealed that exhausted T cell numbers were significantly reduced after CN. And the scRNA data also suggested the expression value of PD‐1 on expanded T cells decreased after CN (supplementary material, Figure S7B). Those findings indicated that although the immune system dispatched large numbers of tumour‐associated T cells with the same TCR, these immune cells not only failed to attack tumour cells but also disrupted the homeostasis of TCR diversity repertoire. The decreased TCRB diversity might be the tried‐but‐failed marker of the immune system to attack the tumour.
After the removal of the majority of the chronic and persistent immunogenic burden, which induced the immunosuppressive environment from the advanced RCC patients by CN, a part of invalid effector cells was eliminated and more naïve T cells appeared in their peripheral blood by peripheral proliferation 14 or homeostatic proliferation 31. Furthermore, there is an acute and transient process for the immune system to fight, that is, the newly exposed tumour‐associated antigens as the consequence of the destruction of tumour cells during surgery. Different from the chronic tumour‐bearing stimuli before CN, the immune response caused by the transient antigens released by surgery is more similar to that of acute infection, and would also re‐stimulate and mobilize the immune reaction. More clonotypes of naïve T cells appeared, and more opportunities to recognize the tumour‐associated antigens would emerge. After antigen clearance, most of the effector cells with high‐abundance clonotype were eliminated and a minor fraction of effector T cells survives to become memory T cells with low‐abundance clonotype. As a result, TCR diversity is also increased.
Based on our TCR repertoire and RNA‐seq–derived immune cell data, the global immune status improved after CN with respect to the increased TCR diversity by increased levels of naïve T cells. Similarly, another study illustrated that radiation therapy increases systemic responses to immune checkpoint inhibitors as a result of the radiation‐induced exposure of immunogenic mutations to the immune system in lung cancer 32. Thus, we cannot rule out the significance of CN for subsequent immune checkpoint inhibitors, especially because it decreases the tumour burden and improves the immune status.
As a whole, CN is effective in improving the diversity of the TCRB repertoire in patients with stage IV RCC. High‐throughput circulating TCR repertoire sequencing is a valid method that reflects the dynamic changes in the immune status and predicts prognosis. However, it is necessary to further confirm whether CN is necessary for subsequent immune checkpoint inhibitor therapy in synchronous metastatic RCC patients by a prospective clinical trial.
Author contributions statement
LF, KTZ, and JZS designed the study. XGB, YJL, LW, and WXJ collected the samples and clinical information. LPG performed the experiments. LPG and LF performed the statistical analysis. LPG wrote the first draft of the manuscript, and LF, XGB, KTZ, JZS WZ, and JM edited sections of the manuscript. All authors reviewed the manuscript and approved the submitted version.
Data availability statement
The raw TCRB repertoire and transcriptome sequencing data generated in this study are deposited in the Genome Sequence Archive at the BIG Data Center, Beijing Institute of Genomics (BIG), Chinese Academy of Sciences, under the accession number HRA000049 [http://bigd.big.ac.cn/gsa-human]. The bulk data and the raw single‐cell RNA sequencing data (not uploaded) are also available from the corresponding authors upon reasonable request.
Supporting information
Supplementary materials and methods
Figure S1. Quality control validation of the in‐house built SMART‐based UMI‐corrected TCRB sequencing platform
Figure S2. The TCRB diversity index across patients with different clinical stages and treatment statuses
Figure S3. Changes in the TCRB diversity estimated using (A) Chao1 and (B) Shannon–Wiener before and after surgery in benign samples and samples from patients with each clinical stage of cancer
Figure S4. Proportion of peripheral exhausted CD8 T cell was decreased after surgery
Figure S5. Identification of the subtype cell populations in PBMCs
Figure S6. Relationship between TCRB diversity and CD4 naïve T cells (estimated by the deconvolution of RNA‐seq data)
Figure S7. The dynamic changes of the expression of immune checkpoint molecules on expanded and nonexpanded T cells before and after CN
Table S1. Characteristics of the enrolled patients
Table S2. All the significant GO terms enriched by the genes that were significant positively correlated with TCRB diversity
Table S3. Sequences of primers used in the TCRB library construction
Acknowledgements
This work was supported by the Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (grant numbers 2016‐I2M‐1‐007 and 2016‐I2M‐3‐005). This study was also funded by the Beijing Hope Run Special Fund of the Cancer Foundation of China (grant number LC2018L02).
No conflicts of interest were declared.
Contributor Information
Lin Feng, Email: fenglin@cicams.ac.cn.
Kaitai Zhang, Email: zhangkt@cicams.ac.cn.
Jianzhong Shou, Email: shoujzh@126.com.
References
*Cited only in supplementary material.
- 1. Shurin MR. Cancer as an immune‐mediated disease. Immunotargets Ther 2012; 1 : 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jantzer P, Schendel DJ. Human renal cell carcinoma antigen‐specific CTLs: antigen‐driven selection and long‐term persistence in vivo. Cancer Res 1998; 58 : 3078–3086. [PubMed] [Google Scholar]
- 3. Davis MM, Bjorkman PJ. T‐cell antigen receptor genes and T‐cell recognition. Nature 1988; 335 : 744. [DOI] [PubMed] [Google Scholar]
- 4. Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol 2006; 126 : 25–31. [DOI] [PubMed] [Google Scholar]
- 5. Rosati E, Dowds CM, Liaskou E, et al Overview of methodologies for T‐cell receptor repertoire analysis. BMC Biotechnol 2017; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cui JH, Lin KR, Yuan SH, et al TCR repertoire as a novel indicator for immune monitoring and prognosis assessment of patients with cervical cancer. Front Immunol 2018; 9 : 2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hopkins AC, Yarchoan M, Durham JN, et al T cell receptor repertoire features associated with survival in immunotherapy‐treated pancreatic ductal adenocarcinoma. JCI Insight 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu YY, Yang QF, Yang JS, et al Characteristics and prognostic significance of profiling the peripheral blood T‐cell receptor repertoire in patients with advanced lung cancer. Int J Cancer 2019. [DOI] [PubMed] [Google Scholar]
- 9. Choudhury NJ, Kiyotani K, Yap KL, et al Low T‐cell receptor diversity, high somatic mutation burden, and high Neoantigen load as predictors of clinical outcome in muscle‐invasive bladder cancer. Eur Urol Focus 2016; 2 : 445–452. [DOI] [PubMed] [Google Scholar]
- 10. Gerlinger M, Quezada SA, Peggs KS, et al Ultra‐deep T cell receptor sequencing reveals the complexity and intratumour heterogeneity of T cell clones in renal cell carcinomas. J Pathol 2013; 231 : 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolotin DA, Mamedov IZ, Britanova OV, et al Next generation sequencing for TCR repertoire profiling: platform‐specific features and correction algorithms. Eur J Immunol 2012; 42 : 3073–3083. [DOI] [PubMed] [Google Scholar]
- 12. Shugay M, Britanova OV, Merzlyak EM, et al Towards error‐free profiling of immune repertoires. Nat Methods 2014; 11 : 653. [DOI] [PubMed] [Google Scholar]
- 13. Turchaninova MA, Davydov A, Britanova OV, et al High‐quality full‐length immunoglobulin profiling with unique molecular barcoding. Nat Protoc 2016; 11 : 1599–1616. [DOI] [PubMed] [Google Scholar]
- 14. den Braber I, Mugwagwa T, Vrisekoop N, et al Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 2012; 36 : 288–297. [DOI] [PubMed] [Google Scholar]
- 15. Wang Y, Song F, Zhu J, et al GSA: genome sequence archive. Genom Proteom Bioinf 2017; 15 : 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Members BIGDC. Database resources of the BIG data center in 2019. Nucleic Acids Res 2019; 47 : D8–D14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malta TM, Sokolov A, Gentles AJ, et al Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell 2018; 173 : 338–354 e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henrickson SE. Hurry up and wait, then activate and translate! Sci Immunol 2019; 4 : eaaw4888. [DOI] [PubMed] [Google Scholar]
- 19. Heng DY, Wells JC, Rini BI, et al Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol 2014; 66 : 704–710. [DOI] [PubMed] [Google Scholar]
- 20. Wen L, Guo L, Zhang W, et al Cooperation between the inflammation and coagulation systems promotes the survival of circulating tumor cells in renal cell carcinoma patients. Front Oncol 2019; 9 : 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu K, Lou L, Ye J, et al Prognostic role of the neutrophil‐lymphocyte ratio in renal cell carcinoma: a meta‐analysis. BMJ Open 2015; 5 : e006404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Radoja S, Frey AB. Cancer‐induced defective cytotoxic T lymphocyte effector function: another mechanism how antigenic tumors escape immune‐mediated killing. Mol Med 2000; 6 : 465–479. [PMC free article] [PubMed] [Google Scholar]
- 23. Yost KE, Satpathy AT, Wells DK, et al Clonal replacement of tumor‐specific T cells following PD‐1 blockade. Nat Med 2019; 25 : 1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flanigan RC, Salmon SE, Blumenstein BA, et al Nephrectomy followed by interferon alfa‐2b compared with interferon alfa‐2b alone for metastatic renal‐cell cancer. N Engl J Med 2001; 345 : 1655–1659. [DOI] [PubMed] [Google Scholar]
- 25. Mickisch GHJ, Garin A, van Poppel H, et al Radical nephrectomy plus interferon‐alfa‐based immunotherapy compared with interferon alfa alone in metastatic renal‐cell carcinoma: a randomised trial. Lancet 2001; 358 : 966–970. [DOI] [PubMed] [Google Scholar]
- 26. Mejean A, Ravaud A, Thezenas S, et al Sunitinib alone or after nephrectomy in metastatic renal‐cell carcinoma. N Engl J Med 2018; 379 : 417–427. [DOI] [PubMed] [Google Scholar]
- 27. Polyak K, Haviv I, Campbell IG. Co‐evolution of tumor cells and their microenvironment. Trends Genet 2009; 25 : 30–38. [DOI] [PubMed] [Google Scholar]
- 28. Jiang N, Schonnesen AA, Ma KY. Ushering in integrated T cell repertoire profiling in cancer. Trends Cancer 2019; 5 : 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lythe G, Callard RE, Hoare RL, et al How many TCR clonotypes does a body maintain? J Theor Biol 2016; 389 : 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grossman Z, Min B, Meier‐Schellersheim M, et al Concomitant regulation of T‐cell activation and homeostasis. Nat Rev Immunol 2004; 4 : 387–395. [DOI] [PubMed] [Google Scholar]
- 31. Boyman O, Letourneau S, Krieg C, et al Homeostatic proliferation and survival of naive and memory T cells. Eur J Immunol 2009; 39 : 2088–2094. [DOI] [PubMed] [Google Scholar]
- 32. Rudqvist NP, Pilones KA, Lhuillier C, et al Radiotherapy and CTLA‐4 blockade shape the TCR repertoire of tumor‐infiltrating T cells. Cancer Immunol Res 2018; 6 : 139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nazarov VI, Pogorelyy MV, Komech EA, et al tcR: an R package for T cell receptor repertoire advanced data analysis. BMC Bioinformatics 2015; 16 : 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Efron B, Thisted RJB. Estimating the number of unseen species: how many words did Shakespeare know? Biometrika 1976; 63 : 435–447. [Google Scholar]
- 35. Hughes JB, Hellmann JJ, Ricketts TH, et al Counting the uncountable: statistical approaches to estimating microbial diversity. Appl Environ Microbiol 2001; 67 : 4399–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shannon CE. A mathematical theory of communication. Bell Syst Tech J 1948; 27 : 379–423. [Google Scholar]
- 37. Martin M. Cutadapt removes adapter sequences from high‐throughput sequencing reads. EMBnetjournal 2011; 17 : 10–12. [Google Scholar]
- 38. Patro R, Duggal G, Love MI, et al Salmon provides fast and bias‐aware quantification of transcript expression. Nat Methods 2017; 14 : 417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Soneson C, Love MI, Robinson MD. Differential analyses for RNA‐seq: transcript‐level estimates improve gene‐level inferences. F1000Res 2015; 4 : 1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA‐Seq data. BMC Bioinformatics 2013: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Senbabaoglu Y, Gejman RS, Winer AG, et al Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol 2016; 17 : 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guo L, Chen G, Zhang W, et al A high‐risk luminal A dominant breast cancer subtype with increased mobility. Breast Cancer Res Treat 2019; 175 : 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liberzon A, Subramanian A, Pinchback R, et al Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011; 27 : 1739–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stuart T, Butler A, Hoffman P, et al Comprehensive integration of single‐cell data. Cell 2019; 177 : 1888–1902.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials and methods
Figure S1. Quality control validation of the in‐house built SMART‐based UMI‐corrected TCRB sequencing platform
Figure S2. The TCRB diversity index across patients with different clinical stages and treatment statuses
Figure S3. Changes in the TCRB diversity estimated using (A) Chao1 and (B) Shannon–Wiener before and after surgery in benign samples and samples from patients with each clinical stage of cancer
Figure S4. Proportion of peripheral exhausted CD8 T cell was decreased after surgery
Figure S5. Identification of the subtype cell populations in PBMCs
Figure S6. Relationship between TCRB diversity and CD4 naïve T cells (estimated by the deconvolution of RNA‐seq data)
Figure S7. The dynamic changes of the expression of immune checkpoint molecules on expanded and nonexpanded T cells before and after CN
Table S1. Characteristics of the enrolled patients
Table S2. All the significant GO terms enriched by the genes that were significant positively correlated with TCRB diversity
Table S3. Sequences of primers used in the TCRB library construction
Data Availability Statement
The raw TCRB repertoire and transcriptome sequencing data generated in this study are deposited in the Genome Sequence Archive at the BIG Data Center, Beijing Institute of Genomics (BIG), Chinese Academy of Sciences, under the accession number HRA000049 [http://bigd.big.ac.cn/gsa-human]. The bulk data and the raw single‐cell RNA sequencing data (not uploaded) are also available from the corresponding authors upon reasonable request.


