Abstract
Background
The objective of this study was to evaluate the outcomes of patients with high‐grade glioma who received treatment with particle radiotherapy.
Methods
Between June 2015 and October 2018, 50 consecutive and nonselected patients with glioblastoma multiforme (n = 34) or anaplastic glioma (n = 16) were treated at the Shanghai Proton and Heavy Ion Center. Twenty‐four patients received proton radiotherapy (at a dose of 60 gray‐equivalents in 30 daily fractions), and 26 patients received proton radiotherapy plus a carbon‐ion radiotherapy (CIRT) boost in various dose‐escalating schemes. All patients received temozolomide because of their age or their O‐6‐methylguanine‐DNA methyltransferase (MGMT) promoter methylation status. Progression‐free survival (PFS) and overall survival (OS) rates, as well as treatment‐induced toxicities, were analyzed.
Results
At a median follow‐up of 14.3 months (range, 4.8‐39.6 months), the 12‐month and 18‐month OS rates were 87.8% (95% CI, 77.6%‐98.0%) and 72.8% (95% CI, 56.7%‐88.9%), respectively, and the 12‐month and 18‐month PFS rates were 74.2% (95% CI, 60.9%‐87.5%) and 59.8% (95% CI, 43.1%‐76.5%), respectively. Univariate analyses revealed that age (>50 vs ≤50 years), World Health Organization grade (3 vs 4), and Karnofsky performance status (>80 vs ≤80) were significant prognosticators for OS, and IDH mutation and World Health Organization grade were significant for predicting PFS. Furthermore, MGMT promoter methylation, performance status, and age showed a trend toward predicting PFS. No significant predictive factors for PFS or OS were identified in multivariate analyses. Twenty‐nine patients experienced grade 1 treatment‐related acute adverse effects, and 11 developed grade 1 (n = 6) or grade 2 (n = 5) late adverse effect of radiation‐induced brain necrosis. No grade 3, 4, or 5 toxicities were observed.
Conclusions
Particle radiotherapy produced 18‐month OS and PFS rates of 72.8% and 59.8%, respectively, with acceptable adverse effects in patients with high‐grade glioma. Particle radiotherapy at a dose ≥60 gray‐equivalents appears to be safe and potentially effective.
Keywords: glioblastoma, high‐grade glioma, particle radiotherapy, survival, temozolomide
Short abstract
Particle radiotherapy with concurrent temozolomide could potentially produce better outcomes than conventional radiotherapy plus temozolomide. Particle radiotherapy to a dose of ≥60 gray‐equivalents with concurrent temozolomide is safe for patients with high‐grade glioma.
Introduction
Malignant glioma is the most commonly diagnosed primary tumor of the central nervous system, and is 1 of the most aggressive malignancies known to humans. High‐grade gliomas (HGGs), including glioblastoma multiforme (GBM) and anaplastic astrocytoma (AA), are characterized by rapid progression and diffuse infiltration, which make complete resection impossible. Recurrence is universal after aggressive surgical resection followed by high‐dose radiotherapy (RT). Investigations on the use of new RT technologies and strategies, such as dose escalation, altered fractionation, the addition of a stereotactic RT boost, and boron capture, have failed to become the standards of care. The addition of temozolomide has modestly improved overall survival (OS), especially in patients who have methylation of the O‐6‐methylguanine‐DNA methyltransferase (MGMT) promoter; however, the median survival time (MST) remains dismal at approximately 15 months.1 In addition, dose intensification of temozolomide failed to improve outcomes further.2 Moreover, polychemotherapy regimens, vaccinations, and targeted biologic agents have also failed to demonstrate significant OS benefits. Clearly, alternative approaches are needed to improve the outcomes of patients diagnosed with this dismal condition.
Charged‐particle (eg, proton and carbon‐ion) beams are characterized by a sharp lateral penumbra, minimal dose deposition at the beam path before a steep energy deposition (ie, the Bragg peak), as well as a sudden and nearly complete dose fall‐off thereafter. Results of a dosimetry study have demonstrated improved dose distributions in particle therapy for glioma compared with x‐ray (photon)‐based RT.3 In addition, particle RT potentially could improve efficacy and toxicity profiles.4, 5, 6
In addition to their superior physical characteristics, heavy‐ion particles such as carbon ions have higher linear energy transfer (LET), which inflicts more damage through direct DNA double‐strand breaks.7 The suggested relative biological effectiveness (RBE) of carbon‐ion beams ranges from 3 to 5 for GBM cells, and results from several in vitro studies have revealed greater cell‐kill efficiency in both GBM and glioma stem cell lines by carbon‐ion RT (CIRT) compared with low‐LET beams, such as proton or x‐ray beams.8, 9, 10, 11 CIRT is also potentially more effective in the killing of cells in a hypoxic condition, a proven feature of GBM tumors.12, 13
The results of a collaborative study from the Heidelberg Ion‐Beam Therapy Center and the National Institute of Radiological Sciences in Japan demonstrated that the addition of CIRT boost to conventional, photon‐based RT improved outcomes in terms of OS compared with photon RT alone.6 However, to our knowledge, the use of temozolomide in addition to any form of particle therapy has never been addressed.
At the Shanghai Proton and Heavy Ion Center (SPHIC), proton RT (PRT) with or without a CIRT boost has been used to treat patients who have newly diagnosed HGG with definitive intent. Temozolomide is routinely used for patients who have MGMT methylation or those aged <65 years. The objective of this study was to report the results from a group of patients with HGG who were treated at the SPHIC.
Materials and Methods
Pretreatment Evaluation
All cases were required to be presented and discussed in the multidisciplinary tumor clinic for their diagnosis, indication, and eligibility for the particle therapy protocol before registration at SPHIC. Required pretreatment evaluations included a complete history and physical examination, full blood count (FBC), serum electrolytes, liver and renal function tests, and magnetic resonance imaging (MRI) of the brain. Imaging studies with 11C methionine (MET)/18F‐fluoroethyltyrosine‐positron emission tomography (FET‐PET) were optional except for patients who were accrued in the latest dose‐escalation trial. All patients who were eligible for particle RT were registered in an institutional prospective database and were irradiated according to either the standard treatment protocol or the clinical trial of choice. Informed consent was obtained from each patient according to the research proposals approved by the Local Ethics Committee of SPHIC.
Particle Radiotherapy
All patients with intracranial tumor(s) were immobilized using VacLock (Merit Medical Systems) and an individualized thermoplastic mask in supine position. Computed tomography (CT) images for simulation from the vertex to the inferior margin of the second cervical vertebral body were obtained at 1.5‐mm slice thickness.
The high‐risk clinical target volume (CTV) was defined as the gross tumor volume (GTV) in patients who had residual disease observed on imaging studies and in the surgical bed plus a 0.5‐cm expansion, and the low‐risk CTV consisted of the GTV plus a 1.5‐cm margin. For patients who received a CIRT boost before or after PRT (based on clinical trials), the GTV was used for the CIRT boost without expansion to the CTV. An additional 3‐mm to 5‐mm margin was supplied to the CTVs (and to the GTV for CIRT boosts) to create the planning target volume (PTV) for uncertainty with regard to dose distribution and potential daily setup errors. The prescribed PRT dose to the high‐risk CTV and low‐risk CTV was 60 gray‐equivalents (GyE) and 50 GyE, respectively, using either a simultaneously integrated boost (SIB) or a sequential boost technique. Doses of particle RT were measured in GyE to account for the RBE differences compared with photons. Dose constraints of critical organs at risk were based on the TD5/5 tolerance dose (a 5% chance of injury showing up over the next 5 years) described by Emami et al for patients who receive PRT alone.14
PRT and CIRT boost were planned by using the Syngo treatment planning system (Siemens). Treatments typically consisted of 2 or 3 beams. PRT and CIRT were delivered with pencil‐beam scanning technology using the IONTRIS particle therapy system (Siemens). Treatment positions were confirmed with daily orthogonal x‐ray using bony landmarks as references before each treatment delivery by an on‐site radiation oncologist. Weekly verification CT scans were performed from the second week of therapy to assess for anatomic changes in the tumor. Additional MRI studies were ordered if indicated by the verification CT scan.
Chemotherapy
Chemotherapy with temozolomide was planned to be provided to patients aged <65 years and to those aged >65 years who had methylation of the MGMT promoter. Oral temozolomide was started on first day of particle RT therapy at 75 mg/m2, 7 days per week, followed by at least 6 cycles of adjuvant treatment at 150 to 200 mg/m2 for 5 days during each 28‐day cycle.
Treatment Response Assessments and Follow‐Up
All patients were admitted during their treatment, were examined daily, and were required to be followed according to the institutional follow‐up protocol of SPHIC after discharge. The first follow‐up is at 4 weeks after the completion of RT. Patients are then planned to be followed every 2 or 3 months in the first 3 years, every 4 to 6 months in the following 2 years, and annually thereafter. A complete history and physical examination with focus to neurologic studies, enhanced MRI studies of the brain, and laboratory tests are provided at each follow‐up. Target lesions are evaluated using the RANO (Response Assessment in Neuro‐Oncology) criteria15 with interpretation modifications.16
Data Analyses
OS was calculated from the date of pathologic diagnosis of HGG to the date of death from any cause. Progression‐free survival (PFS) was calculated from the date of diagnosis to the date of disease progression or recurrence. Both survival rates were calculated using the Kaplan‐Meier method. Log‐rank tests and a Cox regression model were used, respectively, for both univariate and multivariate analyses to compare differences in the survival probabilities and to define significant prognostic factors. All analyses were performed using the SPSS statistical software package (version 22.0; IBM Corporation).
Toxicities that occurred ≤3 months after the start of particle RT, and those that occurred >3 months or persisted for >3 months after the start of particle RT were defined as acute and late toxicities, respectively. The Common Terminology Criteria for Adverse Events (version 4.03; Cancer Therapy Evaluation Program, Division of Cancer Treatment & Diagnosis, National Cancer Institute) was used for the reporting of all adverse events.
Results
Characteristics of Patients and Treatment
Between May 2015 and October 2018, the first 50 consecutive and nonselected patients with histology‐confirmed HGG received either PRT or PRT with a CIRT boost at the SPHIC. No patient was excluded from this analysis. The characteristics of the patients, their condition, and particle RT are detailed in Table 1.
Table 1.
Characteristics of Patients, Their Condition, and Treatment
| Characteristic | No. of Patients |
|---|---|
| Sex | |
| Men | 30 |
| Women | 20 |
| Age, y | |
| Median (range) | 54.5 (22‐76) |
| <50 | 22 |
| ≥50 | 28 |
| Completeness of resection | |
| Partial/biopsy | 11 |
| Subtotal | 22 |
| Total | 17 |
| KPS | |
| >80 | 37 |
| ≤80 | 13 |
| Histology: WHO grade | |
| 4 | 34 |
| 3 | 16 |
| IDH mutation | |
| Wild type | 37 |
| Mutant | 13 |
| MGMT promoter | |
| Methylated | 22 |
| Unmethylated | 17 |
| NA | 11 |
| Dose of particle radiation | |
| Standard regimen: PRT 60 GyE/30 Fx | 24 |
| Dose escalation trial 1: PRT, 50 GyE/25 Fx + CIRT 10 GyE/5 Fx then 12 GyE/4 Fx a | 8 then 4 a |
| Dose escalation trial 2: PRT 60 GyE/30 Fx + CIRT boost to 9‐12 GyE/3 Fx | 12 |
| Dose escalation trial 3: PRT, 34 GyE/10 Fx + CIRT boost 9 GyE/3 Fx (aged >65 y only) | 2 |
Abbreviations: BED, biological equivalent dose; CIRT, carbon‐ion radiotherapy; GyE, gray equivalents; Fx, fractions; KPS, Karnofsky performance status; MGMT, O‐6‐methylguanine‐DNA methyltransferase; NA, not available; PRT, proton radiotherapy.
The first 8 patients included 4 who underwent total resection.
Particle Radiotherapy and Chemotherapy
PRT or PRT with a CIRT boost was received by in 24 and 26 patients, respectively. The standard treatment protocol for all patients (with or without gross tumor) was PRT to 60 GyE in 30 fractions. For patients who had gross tumor after surgery/biopsy, dose‐escalation trials using PRT at various doses and fractions followed by a CIRT escalating boost were encouraged (Table 1). All 50 patients completed planned particle RT without a break. Chemotherapy was planned for all patients who had MGMT promotor methylation and all those aged <65 years. Because all 50 patients met these criteria, they all received temozolomide.
Disease Control and Survival Outcomes
The median follow‐up for the entire cohort was 14.3 months (range, 4.8‐39.6 months). Eleven patients had died at the time of this analysis. The 12‐month and 18‐month OS rates were 87.8% (95% CI, 77.6%‐98.0%) and 72.8% (95% CI, 56.7%‐88.9%), respectively, for the entire cohort; and the corresponding PFS rates were 74.2% (95% CI, 60.9%‐87.5%) and 59.8% (95% CI, 43.1%‐76.5%), respectively (Fig. 1) In addition, for patients with GBM, the 12‐month and 18‐month OS rates were 77.4% and 61%, respectively, and the corresponding PFS rates were 61.3% and 42.7%. All 12‐month and 18‐month OS and PFS rates were 100% for patients who had World Health Organization (WHO) grade 3 disease.
Figure 1.
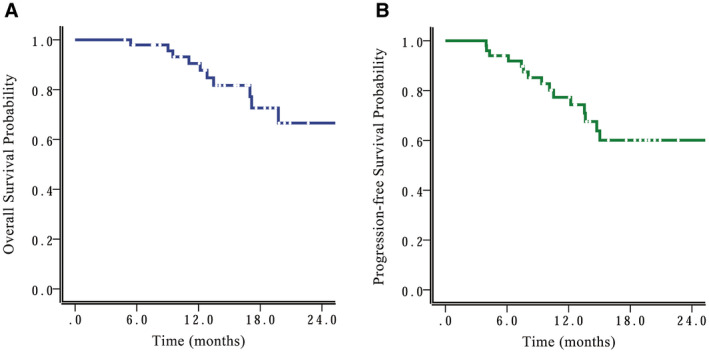
(A) Overall survival and (B) progression‐free survival curves are shown for the entire cohort.
Twelve patients developed radiologically evident local failure or progression during follow‐up. Three additional patients had a recurrence out of the RT field. None had a marginal recurrence. One patient who was diagnosed with “disease progression” on MRI at 6 months after particle RT underwent surgical resection and had a confirmed pathologic complete response (ie, pseudoprogression).
Acute and Late Toxicities
Twenty‐nine patients developed grade 1 dermatitis/alopecia during the course of particle RT. Seven patients developed pseudoprogression, which subsequently subsided or was confirmed by pathology either shortly or >3 months after the completion of particle RT. Furthermore, 11 patients developed grade 1 (n = 6) or grade 2 (n = 5) late adverse effect of radiation‐induced brain necrosis. No grade 3, 4, or 5 acute or late toxicities were observed.
Prognostic Factors
Univariate analyses using the log‐rank test indicated that age (>50 vs ≤50 years), WHO grade (3 vs 4), and Karnofsky performance status (>80 vs ≤80) were significant prognosticators for OS (Table 2 and Fig. 2A‐C). In addition, WHO grade and tumor IDH mutation status were significant prognostic factors for PFS (Table 2 and Fig. 3A,B). Furthermore, age, Karnofsky performance status, and MGMT promotor methylation showed a trend toward predicting PFS. No significant predictive factors for PFS or OS were identified in multivariate analyses. Interestingly, completeness of resection was not a significant factor for predicting OS or PFS in either univariate or multivariate analysis.
Table 2.
Overall Survival and Progression‐Free Survival Rates of All 50 Patients With High‐Grade Glioma
| Characteristics | No. of Patients | OS (95% CI), % | PFS (95% CI), % | ||||
|---|---|---|---|---|---|---|---|
| 12‐Month OS | 18‐Month OS | P | 12‐Month PFS | 18‐Month PFS | P | ||
| Sex | .705 | .786 | |||||
| Men | 30 | 86.2 (71.7‐100) | 77.2 (55.8‐98.6) | 79.2 (62.5‐95.7) | 54.8 (31.9‐77.7) | ||
| Women | 20 | 83.1 (65.7‐100) | 66.7 (40.6‐91.6) | 68.2 (45.5‐89.1) | 68.2 (45.5‐89.1) | ||
| Age, y | .019 | .060 | |||||
| <50 | 22 | 100 | 92.9 (79.4‐100) | 94.7 (84.7‐100) | 76.7 (52.8‐100) | ||
| ≥50 | 28 | 79.3 (63.2‐95.6) | 60.9 (38.6‐83.2) | 60.0 (40.4‐79.6) | 48.3 (26.7‐69.9) | ||
| Resection degree | .260 | .282 | |||||
| Partial/biopsy | 11 | 86.7 (62.4‐100) | 86.7 (62.4‐100) | 80.4 (56.1‐100) | 80.4 (56.1‐100) | ||
| Subtotal | 22 | 76.7 (56.3‐97.1) | 57.8 (29.8‐85.8) | 69.6 (49.0‐90.2) | 52.2 (26.3‐78.1) | ||
| Total | 17 | 92.0 (76.9‐100) | 79.7 (53.8‐100) | 76.4 (54.4‐98.4) | 57.3 (28.3‐86.3) | ||
| Symptom duration, mo | .531 | .547 | |||||
| >2 | 21 | 94.6 (84.2‐100) | 86.4 (68.4‐100) | 83.8 (66.6‐100) | 60.5 (29.7‐91.3) | ||
| ≤2 | 29 | 78.3 (61.2‐95.4) | 65.7 (44.3‐87.1) | 66.8 (47.8‐85.8) | 56.5 (35.7‐77.3) | ||
| KPS | .022 | .058 | |||||
| >80 | 37 | 85.8 (72.7‐98.9) | 79.5 (62.4‐96.6) | 81.6 (68.1‐95.1) | 72.3 (55.2‐89.4) | ||
| ≤80 | 13 | 81.8 (58.7‐100) | 53.7 (17.0‐90.4) | 64.1 (35.3‐92.9) | 30.5 (0.5‐60.5) | ||
| Histology: WHO grade | .023 | .002 | |||||
| 4 | 34 | 77.4 (61.3‐93.5) | 61.0 (40.0‐82.0) | 61.3 (42.9‐79.7) | 42.7 (22.7‐62.7) | ||
| 3 | 16 | 100 | 100 | 100 | 100 | ||
| IDH gene | .139 | .012 | |||||
| Wild type | 37 | 79.4 (64.5‐94.3) | 62.8 (42.0‐83.6) | 64.7 (47.5‐81.9) | 45.8 (26.0‐65.6) | ||
| Mutant | 13 | 100 | 100 | 100 | 100 | ||
| MGMT promoter | .337 | .058 | |||||
| Methylated | 22 | 94.1 (82.9‐100) | 87.1 (70.4‐100) | 90.8 (70.8‐100.0) | 75.6 (53.8‐97.4) | ||
| Unmethylated/NA | 28 | 82.1 (66.0‐98.2) | 57.6 (30.9‐84.3) | 59.4 (38.2‐80.6) | 45.2 (21.5‐68.9) | ||
| Radiation necrosis | .237 | .978 | |||||
| Yes | 11 | 90.9 (73.8‐100) | 67.8 (36.6‐99.0) | 72.7 (46.4‐99.0) | 60.6 (29.8‐91.4) | ||
| No | 39 | 86.5 (74.0‐99.0) | 75.3 (56.9‐93.7) | 74.5 (58.8‐90.2) | 59.5 (39.9‐79.1) | ||
| Pseudoprogression | .865 | .408 | |||||
| Yes | 7 | 100 | 100 | 100 | 50 (0.0‐100) a | ||
| No | 43 | 83.2 (70.8‐95.5) | 69.9 (52.4‐87.3) | 70.7 (55.8‐85.6) | 59.3 (42.2‐76.4) | ||
Abbreviations: KPS, Karnofsky performance status; MGMT, O‐6‐methylguanine‐DNA methyltransferase; NA, not available; OS, overall survival; PFS, progression‐free survival; WHO, World Health Organization.
Only 1 patient had 18‐month follow‐up.
Figure 2.
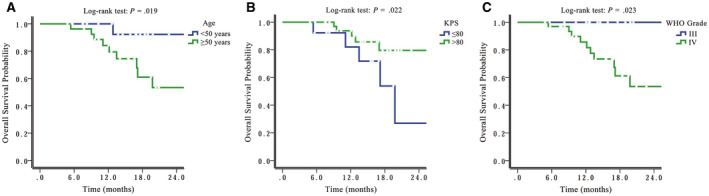
Overall survival is illustrated by (A) patient age (B) Karnofsky performance status (KPS), and (C) World Health Organization (WHO) grade.
Figure 3.
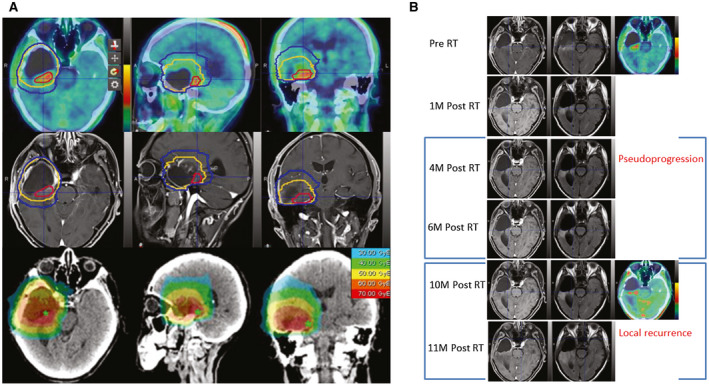
These are images from a typical patient with glioblastoma (a man aged 62 years with wild‐type IDH and unmethylated O‐6‐methylguanine‐DNA methyltransferase status) who underwent standard proton radiotherapy (RT) with carbon‐ion boost. (A) The radiation plan and dose distribution are illustrated. Red lines indicate a carbon‐ion boost to the clinical target volume (CTV) at 12 gray‐equivalents (GyE) in 3 fractions (12 GyE/3Fx); yellow lines, the CTV for high‐risk disease (proton RT at 60 GyE/30Fx); blue lines, the CTV for low‐risk disease (proton RT 50 at GyE/30Fx). (B) In a follow‐up series of magnetic resonance images, tumor residual is indicated with both T1‐contrast abnormal‐enhanced signal and high 18F‐fluoroethyltyrosine uptake (Pre‐RT); tumor residual shrank at 1 month after RT (1M post‐RT); an enhanced lesion with a surgical margin was present at 4 months post‐RT (4M Post RT), then shrank at 6 months post‐RT (6M Post RT) with continued temozolomide chemotherapy, and was considered pseudoprogression; aberrant enhanced signal with high up‐take of 18F‐fluoroethyltyrosine was documented as a local recurrence at 10 months post‐RT (10M Post RT).
Discussion
The current study is an analysis of 50 consecutive and nonselected patients with HGG who were treated prospectively with particle RT using our institutional protocol or dose‐escalation trials. Except for 2 elderly patients (aged >65 years), all patients received particle RT at a biological equivalent dose (BED) >60 GyE. All patients received temozolomide during PRT because of their MGMT promotor methylation status or age (<65 years). With a median follow‐up of 14.3 months, the 18‐month OS and PFS rates were highly acceptable at 72.8% and 59.8%, respectively. The OS and PFS rates for GBM at 18 months were 61% and 42.7%, respectively, and both OS and PFS rates for AA were 100%. No difference between OS or PFS were observed between patients received PRT only (for patients after total resection) and those who received PRT with a CIRT boost (mostly patients who had gross residual disease). Furthermore, no acute or late RT‐induced grade ≥3 toxicities were observed.
The results from the traditional trimodality strategy of management for HGG (maximal resection and low‐LET, photon‐based RT with concurrent temozolomide) remain discouraging. The addition of temozolomide increased MST from 12 months to 14.6 months for patients with GBM1 and was the only change in the war against GBM that led to a significant improvement in OS in previous decades. There have been no further breakthroughs in the decade since that trial was published, despite extensive efforts to build upon its progress. Clearly, a more radical and “outside‐the‐box” strategy is urgently needed.
Historical data have repeatedly demonstrated that alterations in RT dose or fractionation, as well as the addition of a stereotactic radiosurgery boost to conventional RT have failed to improve OS or disease control.17, 18, 19 Recently, the concurrent use of temozolomide and intensity‐modulated RT (IMRT) with an escalated dose was studied in phase 1 trials. Tsien et al reported that concurrent temozolomide with IMRT up to an escalated dose of 75 grays (Gy) in 30 fractions was safe for patients with HGG.20 Brain necrosis was observed in 3 of 16 patients who received 78 or 81 Gy but was not reported at doses ≤75 Gy. The safety of dose escalation using IMRT concurrent with temozolomide was further confirmed in another phase 1 trial.21 A moderately escalated IMRT dose up to 60 Gy in 22 fractions resulted in no severe acute or late toxicities. Despite the escalated doses and the more conformal RT technique, the median OS and PFS remained suboptimal at 20.1 and 9.0 months, respectively.
The biological effectiveness of proton RT is considered slightly greater than that of photon RT, with RBEs from 1.1 to 1.2. Therefore, when applied at the same dose, protons may provide biological efficacy similar to (or slightly improved) that of photons but at a reduced risk of long‐term toxicity because there is less integral dose to the normal brain tissue. In contrast, carbon‐ion beams have a high LET and may inflict double‐strand DNA breaks, which are difficult to repair.22, 23 RBEs from 3 to 5 have been reported in in vitro studies of GBM cells, depending on the cell lines and endpoints of the study,24, 25, 26 Furthermore, in vitro studies have repeatedly demonstrated that CIRT is more effective in either killing or inhibiting migration of GBM cells.27, 28, 29 CIRT appears to be suitable for the treatment of GBM, which is considered a radioresistant entity. However, the late effect of CIRT on the normal brain is largely unknown at this time. In a phase 1 and 2 study reported by Hasegawa and colleagues, 2 of 5 patients with low‐grade gliomas that were irradiated with CIRT alone (55.2 GyE in 24 fractions) developed severe late toxicities according to the Late Effects Normal Tissue/Subjective Objective Management Analytic (LENT/SOMA) grading system.30 Therefore, extreme caution should be applied in the use of high‐dose CIRT alone for central nervous system tumors. In our current study, low‐LET proton RT was used as the “baseline” treatment at a dose of 60 GyE using conventional fractionation. Furthermore, to prevention over‐irradiation to an excessive volume of normal tissue by the high‐LET carbon‐ion beam, the GTV without expansion to the CTV was used for patients who were accrued to the CIRT boost trial. The more focused particle RT dose to the diseased area might have helped to prevent severe adverse effects despite of the higher BED received by patients, although 11 of 50 patients experienced grade 1 or 2 radionecrosis.
In a phase 1 and 2 trial completed at Massachusetts General Hospital (MGH), 23 patients with HGG underwent surgery (residual lesion <60 mL) followed by photon RT plus an PRT boost or PRT up to 90 GyE.31 The 2‐year OS and MST were 34% and 20 months, respectively. In addition, tumor relapse occurred most commonly in the areas that received from 60 to 70 GyE, and only 1 occurred in an area that received 90 GyE. In a hypothesis‐generating study jointly reported by investigators from the Heidelberg Ion Therapy Center in Germany and the National Institute of Radiological Sciences in Japan, the OS of 48 patients with GBM (n = 32) or AA (n = 16) who received a CIRT boost after photon RT without chemotherapy was retrospectively compared with the OS of those who received photon RT only and photon RT with temozolomide (RCHT). The MST was 18 months after photon RT followed by CIRT compared with 9 months after RT alone and 14 months after RCHT.6 The difference in OS after photon RT with a CIRT boost and RCHT was not significant.
However, the receipt of particle RT, with either PRT and/or CIRT, in the management of HGG has not be sufficiently reported, especially in the temozolomide era.32 To our knowledge, this is the first study to report survival outcomes after particle RT and concurrent temozolomide. Our OS and PFS rates of 72.8% and 59.8%, respectively, at 18 months appear to be more favorable compared with the rates reported by Stupp et al1 (ie, 29.4% and 18.4% for OS and PFS, respectively, at 18 months) after conventional RT and temozolomide.
Recurrent HGG, and especially GBM, is inevitable even after aggressive surgery and high‐dose RT. Most of recurrences initiate within or at the margin of the CTV of photon‐based RT. Similarly, in the current study, 9 of 12 (75%) disease progressions occurred in the particle RT field for patients who failed. Although RT dose escalation does not preclude local recurrence, reducing the probability of an “in‐field” recurrence by RT with a higher BED would likely postpone the time to recurrence, as demonstrated in the MGH trial.32 In our study, most patients who had gross disease after surgery or biopsy received high‐dose PRT followed by a CIRT boost. Such a strategy may have produced the relatively more favorable survival outcomes in our patients. However, the doses we used for our patients were lower than those used in the MGH trial.32 The combined findings from the 2 studies indicates that dose escalation with particle RT might be necessary if the surrounding brain can be spared from high‐dose RT.
In our current cohort of patients, after complete tumor resection, no difference was demonstrated between patients who received particle RT and those who had gross residual disease. We consider the similar results in outcomes was caused, at least in part, by the higher biological effectiveness of the CIRT boost despite the limited number of patients. Nevertheless, the molecular features in the current cohort appeared to be more favorable in our patients who had IDH‐mutation and MGMT‐promoter methylation rates of 26% and 44%, respectively. Compared with the reported IDH‐mutation rate of 5% to 10% and the MGMT‐promoter methylation rate of 30% in a typical institutional cohort, the favorable molecular features certainly warrant further investigation in our future studies.
Several pitfalls need to be discussed. First, although all patients analyzed were treated prospectively on our institutional PRT protocol or dose‐escalating trials for CIRT boost, these 50 patients were accrued to several different protocols, including cases from a hypofractionated trial (PRT to 34 GyE in 10 fractions followed by a CIRT boost for elderly patients). The remaining 48 patients received a BED of 60 GyE in 2‐GyE fractions daily with or without boost to the residual disease foci; nevertheless, the included patients were relatively nonuniform. Second, among the 50 patients studied, 34 had GBM, and 16 had grade 3 glioma. The relatively small number of patients with either pathology, in addition to the heterogenic treatment regimens received, might be the reason why no significant factors (including known factors such as age, performance status, and surgical margin) were identified in the multivariate analysis. Third, the nature of this analysis is retrospective despite the trial protocols used for treatment. As such, potential selection bias was inevitable. The relatively high proportion of patients with IDH mutation (26% in our study vs 5%‐10% in a typical reported cohort) exemplifies such potential bias. In addition, the median follow‐up of 14.3 months is relatively short, and we were could only report 18‐month survival and disease control results with confidence. However, because of the dismal outcomes of patients with HGG and the MST of conventionally treated patients with GBM were only approximately 15 months,1 we believe our follow‐up was sufficient for an initial report.
Despite of our favorable outcomes in terms of OS and PFS at 18 months, further investigation is needed to understand the role of particle RT in the treatment of HGG. Currently, 2 randomized phase 2 trials are ongoing in the United States to compare the outcome of proton‐based versus photon‐based IMRT for patients with GBM. The NRG Oncology NRG‐BN001 study (Dose‐Escalated Photon IMRT or Proton Beam Radiation Therapy Versus Standard‐Dose Radiation Therapy and Temozolomide in Treating Patients With Newly Diagnosed Glioblastoma; clinical trials.gov identifier NCT02179086), sponsored by the US National Cancer Institute, intends to compare dose‐escalated, photon‐based IMRT or PRT versus standard‐dose 3‐dimensional, conformal RT or IMRT for OS.33 The primary endpoint of the trial at The University of Texas MD Anderson Cancer Center (Glioblastoma Multiforme Proton vs Intensity Modulated Radiotherapy; clinical trials.gov identifier NCT01854554) is to evaluate the time to cognitive failure between patients treated with intensity‐modulated PRT versus photon‐based IMRT on a standard dose‐fractionation schedule.34 The secondary outcome is local control after RT. In both trials, temozolomide is given concurrently. The investigators hypothesized that more precise or higher dose RT using proton beams may improve either disease control (and this survival) or the toxicity profile.
It is unlikely that particle RT with higher doses could completely prevent recurrences even in high‐dose regions. However, the accurate identification of subclinical disease foci that harbor high tumor burden and irradiation with higher or more biologically effective doses may delay recurrence. Therefore, we initiated a randomized trial to compare standard PRT (60 GyE in 30 fractions) with the same PRT regimen preceded by a CIRT boost (of which the dose will be determined in the phase 1 part of the trial) for patients who have GBM with gross residual disease after surgery.35 Several studies have reported that MET/FET‐PET uptake was associated with a higher probability of failure after concurrent temozolomide and RT for GBM36, 37 and could facilitate the delineation of target volumes of GBM in tumors with a suspected nonenhancing component.38, 39 Therefore, delineation of the GTV and CTV in our ongoing randomized trial is based on both enhanced MRI and 18F‐FET uptake in the PET/CT studies. We considered that any effort would be futile unless the targeting of the lesion is improved.
Conclusion
Particle RT at doses ≥60 GyE with concurrent temozolomide is safe and potentially effective for patients with HGG. The 18‐month OS and PFS rates were 72.8% and 59.8%, respectively. No acute or late severe toxicity was observed. Whether dose escalation using a carbon‐ion beam irradiation boost can further improve local control and delay local recurrence after PRT is the subject of our ongoing randomized trial.35
Funding Support
This project was supported by the National Key Research and Development Program of China (project 2017YFC0108603); the Shanghai Academic/Technology Research Leader Program (project 18XD1423000); Shanghai Hospital Development Center (Joint Breakthrough Project for New Frontier Technologies; project SHDC12016120); and the Science and Technology Development Fund of Shanghai Pudong New Area (projects PKJ2018‐Y51, PKJ2017‐Y50, and PKJ2017‐Y49).
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Lin Kong: Drafting or revising the article. Jinsong Wu: Drafting or revising the article. Jing Gao: Acquisition of data and analysis and interpretation of data. Xianxin Qiu: Acquisition of data and analysis and interpretation of data. Jing Yang: Acquisition of data. Jiyi Hu: Acquisition of data and analysis and interpretation of data. Weixu Hu: Acquisition of data. Ying Mao: Conception and design. Jiade J. Lu: Conception and design and drafting or revising the article.
The first two contributed equally to this work.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987‐996. [DOI] [PubMed] [Google Scholar]
- 2. Gilbert MR, Wang M, Aldape KD, et al. Dose‐dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31:4085‐4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dennis ER, Bussiere MR, Niemierko A, et al. A comparison of critical structure dose and toxicity risks in patients with low grade gliomas treated with IMRT versus proton radiation therapy. Technol Cancer Res Treat. 2013;12:1‐9. [DOI] [PubMed] [Google Scholar]
- 4. Schlaich F, Brons S, Haberer T, Debus J, Combs SE, Weber KJ. Comparison of the effects of photon versus carbon ion irradiation when combined with chemotherapy in vitro. Radiat Oncol. 2013;8:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Combs SE, Ellerbrock M, Haberer T, et al. Heidelberg Ion Therapy Center (HIT): initial clinical experience in the first 80 patients. Acta Oncol. 2010;49:1132‐1140. [DOI] [PubMed] [Google Scholar]
- 6. Combs SE, Bruckner T, Mizoe JE, et al. Comparison of carbon ion radiotherapy to photon radiation alone or in combination with temozolomide in patients with high‐grade gliomas: explorative hypothesis‐generating retrospective analysis. Radiother Oncol. 2013;108:132‐135. [DOI] [PubMed] [Google Scholar]
- 7. Huang YW, Pan CY, Hsiao YY, Chao TC, Lee CC, Tung CJ. Monte Carlo simulations of the relative biological effectiveness for DNA double strand breaks from 300 MeV u(‐1) carbon‐ion beams. Phys Med Biol. 2015;60:5995‐6012. [DOI] [PubMed] [Google Scholar]
- 8. Chiblak S, Tang Z, Campos B, et al. Radiosensitivity of patient‐derived glioma stem cell 3‐dimensional cultures to photon, proton, and carbon irradiation. Int J Radiat Oncol Biol Phys. 2016;95:112‐119. [DOI] [PubMed] [Google Scholar]
- 9. Isono M, Yoshida Y, Takahashi A, et al. Carbon‐ion beams effectively induce growth inhibition and apoptosis in human neural stem cells compared with glioblastoma A172 cells. J Radiat Res. 2015;56:856‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrandon S, Magne N, Battiston‐Montagne P, et al. Cellular and molecular portrait of eleven human glioblastoma cell lines under photon and carbon ion irradiation. Cancer Lett. 2015;360:10‐16. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi M, Hirakawa H, Yajima H, Izumi‐Nakajima N, Okayasu R, Fujimori A. Carbon ion beam is more effective to induce cell death in sphere‐type A172 human glioblastoma cells compared with x‐rays. Int J Radiat Biol. 2014;90:1125‐1132. [DOI] [PubMed] [Google Scholar]
- 12. Antonovic L, Lindblom E, Dasu A, Bassler N, Furusawa Y, Toma‐Dasu I. Clinical oxygen enhancement ratio of tumors in carbon ion radiotherapy: the influence of local oxygenation changes. J Radiat Res. 2014;55:902‐911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wenzl T, Wilkens JJ. Modelling of the oxygen enhancement ratio for ion beam radiation therapy. Phys Med Biol. 2011;56:3251‐3268. [DOI] [PubMed] [Google Scholar]
- 14. Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109‐122. [DOI] [PubMed] [Google Scholar]
- 15. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high‐grade gliomas: Response Assessment in Neuro‐Oncology Working Group. J Clin Oncol. 2010;28:1963‐1972. [DOI] [PubMed] [Google Scholar]
- 16. Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14:307‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan L, Soliman H, Sahgal A, Perry J, Xu W, Tsao MN. External beam radiation dose escalation for high grade glioma. Cochrane Database Syst Rev. 2016;8:CD011475. [DOI] [PubMed] [Google Scholar]
- 18. Cardinale R, Won M, Choucair A, et al. A phase II trial of accelerated radiotherapy using weekly stereotactic conformal boost for supratentorial glioblastoma multiforme: RTOG 0023. Int J Radiat Oncol Biol Phys. 2006;65:1422‐1428. [DOI] [PubMed] [Google Scholar]
- 19. Biswas T, Okunieff P, Schell MC, et al. Stereotactic radiosurgery for glioblastoma: retrospective analysis. Radiat Oncol. 2009;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsien CI, Brown D, Normolle D, et al. Concurrent temozolomide and dose‐escalated intensity‐modulated radiation therapy in newly diagnosed glioblastoma. Clin Cancer Res. 2012;18:273‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jastaniyah N, Murtha A, Pervez N, et al. Phase I study of hypofractionated intensity modulated radiation therapy with concurrent and adjuvant temozolomide in patients with glioblastoma multiforme. Radiat Oncol. 2013;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heilmann J, Taucher‐Scholz G, Haberer T, Scholz M, Kraft G. Measurement of intracellular DNA double‐strand break induction and rejoining along the track of carbon and neon particle beams in water. Int J Radiat Oncol Biol Phys. 1996;34:599‐608. [DOI] [PubMed] [Google Scholar]
- 23. Murakami M, Eguchi‐Kasai K, Sato K, Minohara S, Yatagai F, Kanai T. Differences in heavy‐ion‐induced DNA double‐strand breaks in a mouse DNA repair‐deficient mutant cell line (SL3‐147) before and after chromatin proteolysis. J Radiat Res. 1995;36:258‐264. [DOI] [PubMed] [Google Scholar]
- 24. Tsuboi K, Moritake T, Tsuchida Y, Tokuuye K, Matsumura A, Ando K. Cell cycle checkpoint and apoptosis induction in glioblastoma cells and fibroblasts irradiated with carbon beam. J Radiat Res. 2007;48:317‐325. [DOI] [PubMed] [Google Scholar]
- 25. Combs SE, Zipp L, Rieken S, et al. In vitro evaluation of photon and carbon ion radiotherapy in combination with chemotherapy in glioblastoma cells. Radiat Oncol. 2012;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Combs SE, Bohl J, Elsasser T, et al. Radiobiological evaluation and correlation with the local effect model (LEM) of carbon ion radiation therapy and temozolomide in glioblastoma cell lines. Int J Radiat Biol. 2009;85:126‐137. [DOI] [PubMed] [Google Scholar]
- 27. Iwadate Y, Mizoe J, Osaka Y, Yamaura A, Tsujii H. High linear energy transfer carbon radiation effectively kills cultured glioma cells with either mutant or wild‐type p53. Int J Radiat Oncol Biol Phys. 2001;50:803‐808. [DOI] [PubMed] [Google Scholar]
- 28. Tsuboi K, Tsuchida Y, Nose T, Ando K. Cytotoxic effect of accelerated carbon beams on glioblastoma cell lines with p53 mutation: clonogenic survival and cell‐cycle analysis. Int J Radiat Biol. 1998;74:71‐79. [DOI] [PubMed] [Google Scholar]
- 29. Rieken S, Habermehl D, Wuerth L, et al. Carbon ion irradiation inhibits glioma cell migration through downregulation of integrin expression. Int J Radiat Oncol Biol Phys. 2012;83:394‐399. [DOI] [PubMed] [Google Scholar]
- 30. Hasegawa A, Mizoe JE, Tsujii H, et al. Experience with carbon ion radiotherapy for WHO grade 2 diffuse astrocytomas. Int J Radiat Oncol Biol Phys. 2012;83:100‐106. [DOI] [PubMed] [Google Scholar]
- 31. Fitzek MM, Thornton AF, Rabinov JD, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg. 1999;91:251‐260. [DOI] [PubMed] [Google Scholar]
- 32. Rieken S, Habermehl D, Haberer T, Jaekel O, Debus J, Combs SE. Proton and carbon ion radiotherapy for primary brain tumors delivered with active raster scanning at the Heidelberg Ion Therapy Center (HIT): early treatment results and study concepts. Radiat Oncol. 2012;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. NRG Oncology . Dose‐Escalated Photon IMRT or Proton Beam Radiation Therapy Versus Standard‐Dose Radiation Therapy and Temozolomide in Treating Patients With Newly Diagnosed Glioblastoma. Accessed February 10, 2019. https://clinicaltrials.gov/ct2/show/NCT02179086.
- 34. The University of Texas MD Anderson Cancer Center . Glioblastoma Multiforme (GBM) Proton vs. Intensity Modulated Radiotherapy (IMRT). Accessed February 10, 2019. https://clinicaltrials.gov/ct2/show/NCT01854554.
- 35. Kong L, Gao J, Hu J, et al. Carbon ion radiotherapy boost in the treatment of glioblastoma: a randomized phase I/III clinical trial. Cancer Commun (Lond). 2019;39:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee IH, Piert M, Gomez‐Hassan D, et al. Association of 11C‐methionine PET uptake with site of failure after concurrent temozolomide and radiation for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2009;73:479‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iuchi T, Hatano K, Uchino Y, et al. Methionine uptake and required radiation dose to control glioblastoma. Int J Radiat Oncol Biol Phys. 2015;93:133‐140. [DOI] [PubMed] [Google Scholar]
- 38. Rieken S, Habermehl D, Giesel FL, et al. Analysis of FET‐PET imaging for target volume definition in patients with gliomas treated with conformal radiotherapy. Radiother Oncol. 2013;109:487‐492. [DOI] [PubMed] [Google Scholar]
- 39. Hayes AR, Jayamanne D, Hsiao E, et al. Utilizing 18F‐fluoroethyltyrosine (FET) positron emission tomography (PET) to define suspected nonenhancing tumor for radiation therapy planning of glioblastoma. Pract Radiat Oncol. 2018;8:230‐238. [DOI] [PubMed] [Google Scholar]


