Abstract
Objectives
WHO recommends HIV self‐testing (HIVST) as an additional approach to HIV testing services. The study describes the strategies used during phase‐in of HIVST under routine conditions in Eswatini (formerly Swaziland).
Methods
Between May 2017 and January 2018, assisted and unassisted oral HIVST was offered at HIV testing services (HTS) sites to people aged ≥ 16 years. Additional support tools were available, including a telephone hotline answered 24/7, HIVST demonstration videos and printed educational information about HIV prevention and care services. Demographic characteristics of HIVST users were described and compared with standard blood‐based HTS in the community. HIVST results were monitored with follow‐up phone calls and the hotline.
Results
During the 9‐month period, 1895 people accessed HIVST and 2415 HIVST kits were distributed. More people accessed HIVST kits in the community (n = 1365, 72.0%) than at health facilities (n = 530, 28.0%). The proportion of males and median age among those accessing HIVST and standard HTS in the community were similar (49.3%, 29 years HIVST vs. 48.7%, 27 years standard HTS). In total, 34 (3.9%) reactive results were reported from 938 people with known HIVST results; 32.4% were males, and median age was 30 years (interquartile range 25–36). Twenty‐one (62%) patients were known to have received confirmatory blood‐based HTS; of these, 20 (95%) had concordant reactive results and 19 (95%) were linked to HIV care at a clinic.
Conclusion
Integration of HIVST into existing HIV facility‐ and community‐based testing strategies in Eswatini was found to be feasible, and HIVST has been adopted by national testing bodies in Eswatini.
Keywords: HIV, Eswatini, HTS, undiagnosed
Abstract
Objectifs
L'OMS recommande l'autotest du VIH (HIVST) comme approche supplémentaire des services de dépistage du VIH. L'étude décrit les stratégies utilisées lors de l'introduction progressive du VIHST dans des conditions de routine à Eswatini (anciennement le Swaziland).
Méthodes
Entre mai 2017 et janvier 2018, des HIVST orales assistées et non assistées ont été proposés dans les sites des services de dépistage du VIH (HTS) aux personnes âgées de 16 ans et plus. Des outils de soutien supplémentaires étaient disponibles, notamment une permanence téléphonique répondue 24h/24 et 7j/7, des vidéos de démonstration sur le HIVST et des informations éducatives imprimées sur les services de prévention et de soins du VIH. Les caractéristiques démographiques des utilisateurs du VIHST ont été décrites et comparées aux tests sanguins standard des HTS dans la communauté. Les résultats des HIVST ont été contrôlés par des appels téléphoniques de suivi et la hotline.
Résultats
Au cours de la période de 9 mois, 1895 personnes ont eu accès au VIHST et 2415 kits VIHST ont été distribués. Plus de personnes ont eu accès aux kits VIHST dans la communauté (n = 1365, 72,0%) que dans les établissements de santé (n = 530, 28,0%). La proportion d'hommes et l'âge médian parmi ceux qui accèdent au VIHST et au HTS standard dans la communauté étaient similaires (49,3%, 29 ans VIHST vs 48,7%, 27 ans HTS standard). Au total, 34 (3,9%) résultats réactifs ont été signalés chez 938 personnes avec des résultats connus pour le VIHST; 32,4% étaient des hommes et l'âge médian était de 30 ans (intervalle interquartile 25–36). 21 patients (62%) ont reçu un test sanguin de confirmation HTS; parmi ceux‐ci, 20 (95%) avaient des résultats réactifs concordants et 19 (95%) ont été reliés aux soins du VIH dans une clinique.
Conclusion
L'intégration du HIVST dans les structures existantes de dépistage du VIH et les stratégies de dépistage à Eswatini s'est avérée réalisable, et le HIVST a été adopté par les organismes nationaux de dépistage à Eswatini.
Mots‐clés: VIH, Eswatini, HTS, non diagnostiqué
Introduction
HIV testing services (HTS) are the entry point to HIV prevention, treatment and care. Despite major improvements in the availability of HTS in Eastern and Southern Africa, where the vast majority of HIV cases are found, an estimated 24% of people living with HIV were unaware of their status in 2017 [1]. Certain population subgroups remain persistently hard to reach, including young people (15–24 years) and males [2, 3, 4]. Barriers to HTS have been documented (e.g. access and stigma), and initiatives to address these barriers have been implemented, including community‐based HTS through mobile clinics and door‐to‐door testing, as well as male and youth‐friendly clinics [5].
WHO recommends HIV self‐testing (HIVST) as an additional approach to HTS [6]. HIVST is a process whereby people collect and test their own blood or saliva samples, often in the privacy of their own homes. HIVST has the potential to overcome access and stigma barriers, give people the autonomy and privacy to test on their own, and may address persistent gaps in HTS coverage [7]. The feasibility and acceptability of oral HIVST have also been reported from various research settings throughout sub‐Saharan Africa [8, 9, 10, 11, 12, 13, 14, 15, 16]. However, documented experience of providing HIVST under routine conditions in public health facilities and communities in the context of a generalised epidemic is limited [17].
Médecins sans Frontières (MSF) and the Ministry of Health (MOH) have decentralised HIV and TB care in southern Eswatini (formerly Swaziland) [18]. Substantial efforts were made to bring HTS closer to a predominantly rural population through community‐based HIV testing models, such as mobile and home‐based testing [19]. However, these testing strategies continue to leave people behind in Eswatini – particularly males and young people, for whom estimates for the United Nations (UN) target to test 90% of all people living with HIV by 2020 are 77% and 66%, respectively, compared with 85% for the general population [20]. To reach the remaining undiagnosed HIV cases, the MOH introduced HIVST through pilot projects conducted by Non‐Governmental Organisations (NGOs) in different parts of the country. One of these pilot projects was conducted by MSF, who integrated oral HIVST into existing HIV testing services in the community and facilities in the Shiselweni Region. Here, we describe the strategies used during the phase‐in of HIVST under routine conditions and discuss challenges and successes with the aim of informing the provision of HIVST in other settings.
Methods
Context
Eswatini has a population of about 1.1 million [21] and faces a generalised HIV epidemic, with an HIV prevalence of 27% and an incidence of 1.48% per year among adults aged 15–49 years in 2016–17 [20]. The southern Shiselweni Region has a population of approximately 204 000 people [21] and is divided into three health zones, each of which has one secondary care facility and approximately eight HIV/TB care integrated primary care clinics. Community‐based HIV testing services are provided by MSF and include mobile and home‐based HTS [19]. A serial HIV testing algorithm that uses venous or capillary blood as specimen is used as per the national standard of care, starting with Determine HIV 1/2 and followed by Unigold HIV 1/2 for confirmation [22]. We refer to this testing approach as ‘standard HTS’ in the manuscript.
Implementation of oral HIVST
Between May 2017 and January 2018, oral HIVST was offered as an alternative test at 10 health facilities in Nhlangano health zone (facility model) and at mobile community‐based testing events in the entire region (community model) to people aged 16 years and above. In both models, people had a choice between standard HTS performed by a trained HIV testing counsellor or oral HIVST, which was either assisted or unassisted (see Table 1 for description of the models). Routine data collection, however, did not document the patient’s individual choice for the assisted or unassisted method. In assisted testing, the client performed the test and interpreted the results under the supervision of an HIV testing counsellor; in unassisted testing, people took the test and used it at a convenient time and place. After discussion with a counsellor, people could also obtain a second kit for a partner or peer.
Table 1.
Information and linkage strategies available in community and facility HIVST distribution models and assisted and unassisted testing methods
| Community model | Facility model | |||
|---|---|---|---|---|
| Assisted HIVST | Unassisted HIVST | Assisted HIVST | Unassisted HIVST | |
| Group health talk | ✓ | ✓ | (✓) | (✓) |
| Group pre‐test HIVST information | ✓ | ✓ | ||
| Group HIVST demonstration and video | ✓ | ✓ | ||
| Individual pre‐test HIVST information | ✓ | ✓ | ✓ | ✓ |
| Individual pre‐test HIVST video | ✓ | ✓ | ✓ | ✓ |
| Brochures and flyers distributed with HIVST kits | ✓ | ✓ | ✓ | ✓ |
| HCW interprets HIVST with client | ✓ | ✓ | ||
| Secondary distribution of HIVST for partner or peer | (✓) | (✓) | (✓) | (✓) |
| On‐site confirmatory blood‐based testing | ✓ | ✓ | ||
| Post‐test counselling | ✓ | ✓ | ||
| Referral card to facility for linkage to HIV care | ✓ | |||
| Self‐referral card to facility for confirmatory testing | ✓ | ✓ | ||
| Proactive follow‐up phone calls* | ✓ | ✓ | ||
| HIVST telephone hotline | ✓ | ✓ | ✓ | ✓ |
Community model implemented in three health zones and facility model in Nhlangano only. Parentheses indicate that these items were optionally available, that is group health talks were conducted according to facility scheduling, and secondary distribution of HIVST kits available on individual request.
HCW, healthcare worker; HIVST, HIV self‐testing.
Proactive follow‐up phone calls not exhaustive.
In both models, people received standard pre‐test information. The need for confirmatory testing for a reactive result was emphasised. Those opting for assisted self‐testing also received post‐test counselling and confirmatory standard HTS on the spot if the test was reactive. A national referral form was completed for those with a confirmatory positive test done at community level. A self‐referral card was used to encourage those who opted for unassisted HIVST to link to a facility of their choice in the region.
Mobile community events included larger events such as sports days and smaller events such as health talks at work places (e.g. garment factories). At the community events, group health talks were conducted to give general information about prevention and treatment of HIV and TB. Group pre‐test information on HIVST was provided. This included information on HIVST’s benefits (e.g. privacy) and risks (e.g. the need for re‐testing if exposure was less than 6 weeks before testing), as well as demonstrations on how to use and interpret the HIVST through videos. A private area for testing was established, such as a tent or a room at a workplace, where people could voluntarily go for standard HTS or oral testing in private.
Oral HIVST package and linkage strategies (assisted and unassisted)
At the time of the pilot, the oral fluid‐based OraQuick self‐test was packaged in two pouches, one with the testing device and another containing a test tube of testing fluid. The kits were placed inside small cardboard boxes with a stand to hold the test tube, and pre‐ and post‐test informational materials in English and siSwati. These included the following: 1) a pamphlet with general information on HIV prevention and care; 2) instructions on how to perform the HIV self‐test; 3) a post‐test information pamphlet addressing linkages to HIV prevention, care and treatment services; 4) a document with frequently asked questions about HIVST; and 5) a self‐referral card. Additional support tools were available, such as a telephone hotline answered 24/7 by trained HIV testing counsellors and a video HIVST demonstration. The video was filmed in English and siSwati, with male and female versions. The HIVST demonstration video was available on social media and could be shared on mobile phones. Previous studies have shown the need for community demonstrations of HIVST due to challenges of literacy [23, 24, 25].
At the request of the MOH, proactive community‐based follow‐up phone calls were made to provide post‐test counselling and encourage linkage to care. People choosing unassisted HIVST were asked to provide a mobile number and to give oral consent to being contacted 2 weeks after test distribution.
Data collection
Demographic characteristics of people accessing HIVST were collected, and test results were documented for people choosing the assisted method. Sex, age and primary reason for calling the hotline were recorded. Follow‐up phone calls were made to people choosing the unassisted method, and self‐reported HIVST results were documented. Overall, results for HIVST in this analysis came from three sources: assisted method, follow‐up phone calls made and hotline calls for those choosing the unassisted method. For linkage to care, HIV testing registers at all 10 facilities in the Nhlangano zone were periodically reviewed until June 2018 to identify whether HIVST users who had reported reactive results went for further confirmatory testing and enrolment into HIV care. We also planned to use facility data; however, these were not available at the time of analysis.
Statistical methods
Frequencies and proportions of people accessing HIVST kits were described by model and testing method (assisted or unassisted testing). People accessing standard HTS in the community during the same period were also described for reference. Comparisons were made using chi‐square tests for categorical variables (testing method, sex and youth) within distribution models (i.e. community and facility) and Kruskal–Wallis test for continuous variables (age). Data were analysed using Stata version 14.0.
Ethics
This analysis was approved by the Eswatini Health Research Board under an approval for analysis of routinely collected data for monitoring and evaluation of programmes. This analysis fulfilled the exemption criteria set by the MSF Ethics Review Board (ERB) for a posteriori analyses of routinely collected clinical data and thus did not require MSF ERB review. It was conducted with permission from the Medical Director, Operational Centre Geneva, MSF.
Results
Characteristics of people accessing HIVST
From May 2017 to January 2018, 1895 people accessed HIVST and 2415 HIVST kits were distributed (Table 2). More people accessed HIVST kits in the community (n = 1365, 72.0%) than at health facilities (n = 530,28.0%). Overall, 520 (27.4%) people took a second HIVST kit to distribute to a partner or peer. This proportion was twice as high in the community as at facilities (32.3% vs. 15.1%, P < 0.001).
Table 2.
Community and facility HIVST distribution (n = 1895) and standard HTS in community (n = 6892), May 2017–January 2018
| haracteristic | HIVST Models | P‐value* | HIV testing approaches | P‐value* | ||
|---|---|---|---|---|---|---|
| Facility HIVST | Community HIVST | Standard HTS in community | Community HIVST | |||
| n (%) | n (%) | n (%) | n (%) | |||
| Total | 530 | 1365 | 6892 | 1365 | ||
| Sex | ||||||
| Male | 184 (34.7) | 673 (49.3) | <0.001 | 3355 (48.7) | 673 (49.3) | 0.673 |
| Female | 346 (65.2) | 692 (50.7) | 3537 (51.3) | 692 (50.7) | ||
| Age† | ||||||
| Median (IQR) | 27 (22–32) | 29 (24–36) | <0.001 | 27 (20–42) | 29 (24–36) | <0.001 |
| Youth status | ||||||
| Youth‡ | 192 (36.2) | 383 (28.1) | 0.001 | 2943 (42.7) | 383 (28.1) | <0.001 |
| Non‐youth | 338 (63.8) | 982 (71.9) | 3949 (57.3) | 982 (71.9) | ||
| Second kit§ | 80 (15.1) | 440 (32.3) | <0.001 | n/a | 440 (32.3) | |
| HIVST result | ||||||
| Known¶ | 366 (69.0) | 423 (31.0) | 6892 (100) | 423 (31.0) | ||
| Reactive¶ | 17 (4.6) | 16 (3.8) | 77 (1.12) | 16 (3.8) | ||
No comparisons were made with standard HTS in facility due to lack of facility data. HIVST, HIV self‐testing; IQR, interquartile range; n, number; %, percentage.
P value comparing characteristics among facility HIVST and community HIVST then standard HTS in community and community HIVST. Chi‐square test for sex, youth and second kit.
Age is missing for 6 people.
Youth is defined as 16–24 years.
Restricted to people ≥ 16 years during same period.
Percentage is number reactive divided by number of known results.
At community events, the median age was 29 (interquartile range [IQR], 24–36) years, with more than a quarter (28.1%) aged 16–24 years. People were almost equally split by sex, with 673 (49.3%) males. The vast majority of people chose unassisted testing (n = 1297, 95.0%). While there was no difference by age between both groups, among people accessing unassisted HIVST, 51.7% were females and 27% were youth vs. 32% females and 42% youth under assisted HIVST.
In facilities, the mean age of people accessing HIVST was 27 (IQR 22–32) years, 192 (36.2%) were aged 16‐24 years, and 34.7% (n = 184) were males. There was an overall preference for unassisted testing (n = 340, 64.2%), with a higher proportion of assisted testing among females than males (70.1% vs. 28.9%, P = 0.037; Table 3).
Table 3.
Characteristics of HIVST users in community and facility by HIVST strategy (n = 1895) May 2017 to January 2018
| Characteristic | Facility HIVST, n = 530 | P‐value* | Community HIVST, n = 1365 | P‐value* | ||
|---|---|---|---|---|---|---|
| Assisted | Unassisted | Assisted | Unassisted | |||
| n (%) | n (%) | n (%) | n (%) | |||
| People accessed HIVST | 190 | 340 | 68 | 1297 | ||
| Sex | ||||||
| Male | 55 (28.9) | 129 (37.9) | 0.037 | 46 (67.6) | 627 (48.3) | 0.002 |
| Female | 135 (70.1) | 211 (62.1) | 22 (32.4) | 670 (51.7) | ||
| Age† | ||||||
| Median (IQR) | 26 (21–33) | 27 (23–32) | 0.28 | 27.5 (21–37) | 29 (24–36) | 0.522 |
| Youth Status | ||||||
| Youth‡ | 79 (41.6) | 113 (33.2) | 0.055 | 29 (42.6) | 354 (27.3) | 0.006 |
| Non‐youth | 111 (58.4) | 227 (66.8) | 39 (57.4) | 946 (72.9) | ||
| Secondary HIVST distribution§ | 12 (6.3) | 68 (20.0) | <0.001 | 9 (13.2) | 431 (33.2) | 0.001 |
| Known HIVST result | 187 (98.4) | 179 (52.6) | 66(97.0) | 357(27.5) | ||
| Reactive HIVST¶ | 11 (5.9) | 6 (3.4) | 2 (3.0) | 15 (4.2) | ||
HIVST, HIV self‐testing; IQR, interquartile range; n, number.
Age is missing for 6 people.
Youth is defined as 16–24 years.
Restricted to people ≥ 16 years during same period.
Percentage is number reactive divided by number of known results
Comparison between HIVST and standard HTS in community
During the 9‐month HIVST pilot period, community testing teams reached more than five times as many people with standard HTS (n = 6892) than with HIVST (n = 1365). The proportion of males was similar in both strategies (49.3% HIVST vs. 48.7% standard HTS, P = 0.673; Table 2). The median age among standard HTS users was lower (27 vs. 29, P < 0.001). The proportion of youth (16–24 years) was higher among people accessing standard HTS (42.7% vs. 28.1% HIVST, P < 0.001). The proportion of reactive results with HIVST among those who reported the result was 3.8% (n = 16) compared with 1.1% (n = 77) for standard HTS (Table 2).
Hotline, follow‐up phone calls and linkage to care
In total, 11.3% (215/1895) of people who accessed HIVST used the hotline, and 9.1% (149/1637) of those choosing the unassisted method self‐reported the results through the hotline (Table 4). The median age of callers was 30 (IQR 24–35) years (among 107 who disclosed age), and 54.0% of callers were male (of 198 callers with recorded sex). Most calls were to report HIVST results (149, 69.3%), and four people reported reactive HIVST results (4/149 = 2.7%).
Table 4.
Reasons for calling hotline and results of follow‐up phone calls to clients, n = 855
| Hotline phone calls (client to HTS) | Follow‐up phone calls (HTS to client) | ||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Reported results | 149 | 69.3 | Used test; results reported | 536 | 83.9 |
| How to perform test | 9 | 4.19 | Used test; results not disclosed | 1 | |
| General information | 18 | 8.37 | Did not use test | 42 | 6.6 |
| Requesting HIVST kit | 13 | 6.05 | Phone not reachable | 49 | 7.7 |
| Other | 26 | 12.1 | Other | 12 | 1.9 |
| Total | 215 | 100 | 640 | 100 | |
HIVST, HIV self‐testing; HTS, HIV testing services.
A further 640 people who took an HIVST kit for unassisted testing received at least one follow‐up call from the HTS counsellor (640/1460, 43.8%). In total, 537 (83.9%) people reported using the test, of whom 17 (3.2%) reported a positive result (Table 4). During the follow‐up calls, 5.8% (30/520) people reported results of a secondary test given to a partner, and three were reported to be reactive. A further seven people returned kits in‐person to the HTC, all of which were non‐reactive. No adverse events were reported via the hotline or the follow‐up calls among clients who provided feedback.
In total, 34 (3.9%) reactive results were reported from 938 people with known results; 32.4% were males, and the median age was 30 (IQR 25–36) years (Figure 1). Twenty‐one (61.8%) patients were known to have received confirmatory blood‐based HTS; of these, 20 (95.2%) had concordant reactive results and 19 (95.0%) were linked to HIV care at a clinic.
Figure 1.
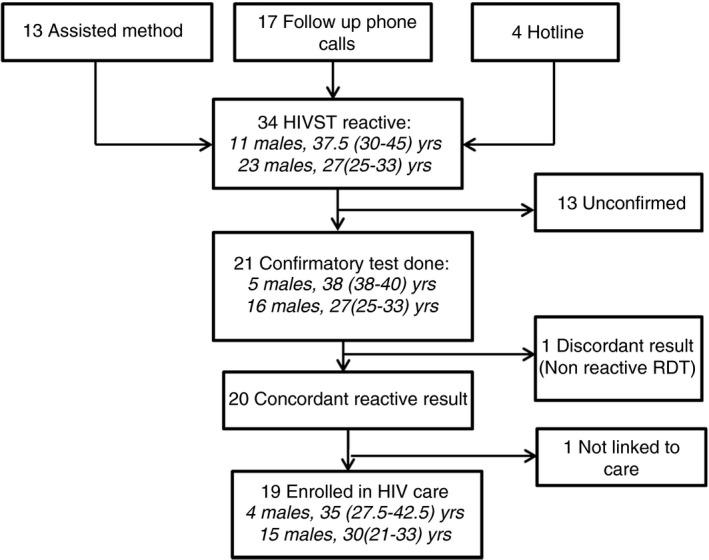
Linkage to care for reactive HIVST, n = 34. Note: Sex and median (interquartile range) age of clients are included in italics within each box. HIVST, HIV self‐testing; RDT, rapid diagnostic test.
Discussion
We have described the integration of HIVST into HTS strategies in the predominantly rural Shiselweni region and characteristics of HIVST users during a 9‐month phase‐in period. HIVST was considered acceptable given that 2415 test kits were distributed to 1895 people and no adverse events were reported among clients who provided feedback. Key lessons learned were that the community model reached twice as many people and distributed more HIVST kits than the facility model. In addition, more participants opted for the unassisted method, suggesting that people accessing HIVST in this setting appreciate the convenience, autonomy and privacy of this self‐screening test just like home pregnancy and blood glucose monitoring tests [25].
Implementation
The community model succeeded in reaching traditionally hard‐to‐reach groups, such as males and young people, with 49.5% of tests distributed to males and 28.1% to young people. However, standard HTS in the community showed similar success, with 48.7% males and 42.7% young people tested. This suggests that community testing activities were already reaching these groups and the addition of HIVST may not have had the anticipated impact. The yield of reactive results with HIVST in the community was higher than with standard HTS in the community, even when comparing assisted testing only (3.0% vs. 1.1%). Although these are small numbers, it is possible that HIVST may reach a higher‐risk population. Other methods to estimate the number of people diagnosed through use of HIVST results should be explored: for example, recording whether people coming for testing at a facility have previously tested with HIVST. This is currently implemented in Eswatini. Notably, other studies have also demonstrated that HIVST may be cost‐effective compared to community‐standard HTS, and targeted HIVST implementation can reach undiagnosed populations[26, 27].
The primary programmatic limitation of this pilot was the distribution of HIVST kits exclusively through trained HIV testing counsellors in the community and at health facilities. This strategy requires substantial human resource investment and limits the reach of HIVST, prioritising the provision of individual pre‐test information over the wide distribution of tests. As WHO recommends distribution of self‐tests through trained lay cadres, alternative distribution models include community health workers, peer educators or even traditional healers [28, 29]. For instance, in Zimbabwe the community preferred distribution of HIVST by locally based community distributors [30]. Although this study aimed to reach difficult‐to‐reach populations, additional standard HTS and HIVST strategies should be used to reach other underserved groups outside of routine testing sites.
HIVST offers an opportunity for integration of HIV services into sexual and reproductive health and other services, which continue to operate vertically in some settings despite policies and guidance promoting integration [31]. Offering HIVST during family planning consultations and antenatal or post‐natal care visits, at sexually transmitted infection clinics and within existing HIV prevention programmes, such as voluntary male medical circumcision and pre‐exposure prophylaxis (PrEP) programmes, takes advantage of contact already occurring with healthcare workers and can facilitate secondary distribution [32]. In Kenya, uptake was 98% among PrEP users offered HIVST, and users cited the confidentiality, ease of use and convenience as reasons why they would opt for this method of testing [12]. As regard to integrating HIVST more broadly into health systems, a randomised controlled trial in 15 health facilities in Malawi plans to evaluate offering oral HIVST to patients waiting for routine services in outpatient departments [33]. Our pilot offered HIVST as an alternative to standard HTS at several entry points (e.g. voluntary counselling and testing, and maternal health care) in outpatient facilities. However, limitations in data collection prevented a comparison of uptake by entry point, and future implementation should consider evaluating where HIVST distribution is most acceptable to clients and staff within facilities.
In this pilot, 27.4% of people opted to take a second test for a partner or peer. Secondary distribution occurred more frequently with unassisted than with assisted testing, and twice as often for community distribution as at the facilities. This is a promising way to provide HTS to people who do not readily access the health system themselves, and our results suggest that there is demand for secondary distribution of HIVST kits in Shiselweni. During follow‐up calls, 30 clients voluntarily reported results of the secondary test (5.8%) and three were reported to be positive (10%). This information was not systematically collected, and these results could not be confirmed. Key implementation questions remain, such as how best to encourage and support people to bring a test to a partner, how to monitor partners’ results and how to ensure that the partner links to care.
Linkage to care is a challenge for all HTS approaches and more especially with HIVST [25, 34]. Interventions to improve linkage after testing in the community are important [35], particularly for secondary distribution of HIV self‐tests[30]. Overall, 62% of people who reported reactive HIVST had confirmatory testing, which can be considered as a first linkage to care for those opting for unassisted testing, and 95% had a concordance positive result with the standard HTS testing. This is also similar to other studies, in which there is a higher rate of agreement between HIVST and standard HIV testing [24, 25]. Finally, 95% of confirmed HIV‐positive cases enrolled in HIV care, including pre‐antiretroviral therapy (ART) or ART at a facility. Although we reviewed registers at facilities for clients who had reported a reactive result up until 6 months following HIVST distribution, it is possible that some HIVST users were missed because they did not report a reactive result or sought care at facilities outside the intervention areas or time period. Qualitative research in this setting has shown that, in the context of ‘treat all’, patients sometimes felt pressured to start ART immediately following an HIV diagnosis, which can lead to later disengagement with HIV care [36]. The empowerment accompanying self‐testing could translate to patient autonomy in care‐seeking and treatment adherence as well, although this may lead to delayed engagement in HIV care and should be explored in future cohorts of HIVST users.
Limitations
An analytical limitation was the light data collection approach, which did not allow measurement of standard outcomes (e.g. HIVST uptake, linkage to HIV care and ART initiation). This precludes a comparison of these outcomes with standard HTS in the community and facilities. We describe community‐based standard HTS as a reference but do not make a formal statistical comparison.
Secondly, a large number of results and characteristics of HIVST users remain unknown. As a result, the interpretation of some of our findings should be cautioned. The available data did also not allow the full description of the continuum of HIV care from the performance of the HIVST to enrolment and ART initiation. Routine monitoring of HIVST is a key challenge during scale‐up. A key added benefit of HIVST is patient autonomy and privacy [6], so it may not be appropriate to contact all people who take the test for monitoring purposes, nor would this be feasible in a routine setting with constraints in human resources. Other less direct tools for monitoring and reporting have been suggested by WHO and were used in this pilot, such as monitoring the HIVST hotline [37].
Adverse events were passively rather than actively monitored, and participants were not systematically directly asked for adverse events during the phone calls. Although no reports of violence or self‐harm were recorded during any of the hotline calls or follow‐up phone calls, we cannot rule out underreporting. Despite substantial concern about the possibility of harm following self‐test distribution [38], studies of HIVST with rigorous monitoring have documented few cases of violence [13, 25]. In settings where the baseline level of violence is high, it was unclear whether the violence was a direct result of HIVST distribution [39]. This remains a topic of concern, and risk mitigation is an important component of HIVST programmes, as noted by WHO guideline [6].
Conclusion
This phase‐in and integration of HIVST into existing HIV facility‐ and community‐based testing strategies in rural Shiselweni region were found to be feasible, and HIVST has since been adopted by national testing bodies in Eswatini (e.g. HIVST integrated into HIV care guidelines, and several HIVST strategies scaled up). Future HIVST programmes should consider expanding the cadres distributing HIVST in communities and integrating HIVST into existing health services and HIV programmes. In addition, secondary distribution should be encouraged, as these approaches capitalise on the autonomy and privacy of the HIVST method, with the potential to reach the remaining undiagnosed HIV cases.
Acknowledgements
We thank all the health workers and the community of Shiselweni region for participating in the pilot.
Sustainable Development Goals (SDGs): SDG 3 (good health and well‐being), SDG 10 (reducedinequalities), SDG 17 (partnerships for the goals)
References
- 1. UNAIDS DATA 2017 UNAIDS [Internet]. (Available from: https://www.unaids.org/en/resources/documents/2017/2017_data_book). [5 Feb 2020]
- 2. Salazar‐Austin N, Chingono A, Chariyalertsak S et al Age‐Related Differences in Socio‐demographic and Behavioral Determinants of HIV Testing and Counseling in HPTN 043/NIMH Project Accept. AIDS Beha. 2018: 22: 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camlin CS, Ssemmondo E, Chamie G et al Men “missing” from population‐based HIV testing: insights from qualitative research. AIDS Care. 2016: 28(Suppl 3): 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson LF, Rehle TM, Jooste S, Bekker L‐G. Rates of HIV testing and diagnosis in South Africa: successes and challenges. AIDS Lond Engl 2015: 29: 1401–1409. [DOI] [PubMed] [Google Scholar]
- 5. Musheke M, Ntalasha H, Gari S et al A systematic review of qualitative findings on factors enabling and deterring uptake of HIV testing in Sub‐Saharan Africa. BMC Public Health 2013: 13: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Guidelines on HIV self‐testing and partner notification: supplement to Consolidated guidelines on HIV testing services. 2016. [PubMed]
- 7. Makusha T, Knight L, Taegtmeyer M et al Self‐testing could “revolutionize testing in South Africa, but it has got to be done properly”: perceptions of key stakeholders. PLoS ONE 2015: 10: e0122783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mugo PM, Micheni M, Shangala J et al Uptake and acceptability of oral HIV self‐testing among community pharmacy clients in Kenya: a feasibility study. PLoS ONE 2017: 12: e0170868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nangendo J, Obuku EA, Kawooya I et al Diagnostic accuracy and acceptability of rapid HIV oral testing among adults attending an urban public health facility in Kampala, Uganda. PLoS ONE 2017: 12: e0182050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chipungu J, Bosomprah S, Zanolini A et al Understanding linkage to care with HIV self‐test approach in Lusaka, Zambia ‐ A mixed method approach. PLoS ONE 2017: 12: e0187998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choko AT, MacPherson P, Webb EL et al Uptake, accuracy, safety, and linkage into care over two years of promoting annual self‐testing for hiv in blantyre, malawi: a community‐based prospective study. PLoS Med 2015: 12: e1001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ngure K, Heffron R, Mugo N et al Feasibility and acceptability of HIV self‐testing among pre‐exposure prophylaxis users in Kenya. J Int AIDS Soc 2017: 20: 21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thirumurthy H, Masters SH, Mavedzenge SN, Maman S, Omanga E, Agot K. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self‐tests by HIV‐negative female sex workers and women receiving antenatal and post‐partum care in Kenya: a cohort study. Lancet HIV 2016: 3: e266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masters SH, Agot K, Obonyo B, Napierala Mavedzenge S, Maman S, Thirumurthy H. Promoting Partner testing and couples testing through secondary distribution of HIV self‐tests: a randomized clinical trial. PLoS Medicine 2016: 13: e1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gichangi A, Bazant E, Mutwiwa S et alProvision of Oral HIV Self‐Test Kits Triples Uptake of HIV Testing among Male Partners of Antenatal Care Clients: Results of a Randomized Trial in Kenya. 1.
- 16. Ortblad K, Kibuuka Musoke D, Ngabirano T et al Direct provision versus facility collection of HIV self‐tests among female sex workers in Uganda: A cluster‐randomized controlled health systems trial. PLoS Medicine 2017: 14: e1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hatzold K, Gudukeya S, Mutseta MN et al HIV self‐testing: breaking the barriers to uptake of testing among men and adolescents in sub‐Saharan Africa, experiences from STAR demonstration projects in Malawi, Zambia and Zimbabwe. J Int AIDS Soc 2019: 22(Suppl 1): 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jobanputra K, Parker LA, Azih C et al Impact and programmatic implications of routine viral load monitoring in Swaziland. J Acquir Immune Defic Syndr 1999: 2014: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parker LA, Jobanputra K, Rusike L et al Feasibility and effectiveness of two community‐based HIV testing models in rural Swaziland. Trop Med Int Health 2015: 20: 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Government of the Kingdom of Eswatini . Swaziland HIV Incidence Measurement Survey (SHIMS2) 2016‐2017. Final Report. Mbabane: Government of the Kingdom of Eswatini; 2019. 256 p.
- 21. The 2017 population and housing census : preliminary results . Mbabane: Central Statistical Office; 2017. vi, 48 pages.
- 22. Swaziland integrated HIV management guidelines National HIV and AIDS Information and Training Centre [Internet]. [cited 2020 Feb 5]. (Available from: https://www.infocenter.nercha.org.sz/node/7340.)
- 23. Grésenguet G, de Longo JD, Tonen‐Wolyec S, Bouassa R‐SM, Belec L. Acceptability and usability evaluation of finger‐stick whole blood HIV self‐test as an HIV screening tool adapted to the general public in the Central African Republic. Open AIDS J 2017: 11: 101–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Figueroa C, Johnson C, Ford N et al Reliability of HIV rapid diagnostic tests for self‐testing compared with testing by health‐care workers: a systematic review and meta‐analysis. Lancet HIV 2018: 5: e277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wong V, Jenkins E, Ford N, Ingold H. To thine own test be true: HIV self‐testing and the global reach for the undiagnosed. J Int AIDS Soc 2019: 22(S1): e25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maheswaran H, Clarke A, MacPherson P et al Cost‐Effectiveness of community‐based human immunodeficiency virus self‐testing in Blantyre, Malawi. Clin Infect Dis 2018: 66: 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cambiano V, Johnson CC, Hatzold K et al The impact and cost‐effectiveness of community‐based HIV self‐testing in sub‐Saharan Africa: a health economic and modelling analysis. J Int AIDS Soc 2019: 22(Suppl 1): e25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mulubwa C, Hensen B, Phiri MM et al Community based distribution of oral HIV self‐testing kits in Zambia: a cluster‐randomised trial nested in four HPTN 071 (PopART) intervention communities. Lancet HIV 2019: 6: e81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choko AT, Nanfuka M, Birungi J, Taasi G, Kisembo P, Helleringer S. A pilot trial of the peer‐based distribution of HIV self‐test kits among fishermen in Bulisa, Uganda. PLoS ONE 2018: 13: e0208191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sibanda EL, d’Elbée M, Maringwa G et al Applying user preferences to optimize the contribution of HIV self‐testing to reaching the “first 90” target of UNAIDS Fast‐track strategy: results from discrete choice experiments in Zimbabwe. J Int AIDS Soc 2019: 22(S1): e25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hope R, Kendall T, Langer A, Bärnighausen T. Health systems integration of sexual and reproductive health and HIV services in sub‐Saharan Africa: a scoping study. J Acquir Immune Defic Syndr 1999: 67(Suppl 4): S259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choko AT, Corbett EL, Stallard N et al HIV self‐testing alone or with additional interventions, including financial incentives, and linkage to care or prevention among male partners of antenatal care clinic attendees in Malawi: An adaptive multi‐arm, multi‐stage cluster randomised trial. PLoS Med 2019: 16: e1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dovel K, Shaba F, Nyirenda M et al Evaluating the integration of HIV self‐testing into low‐resource health systems: study protocol for a cluster‐randomized control trial from EQUIP Innovations. Trials 2018: 19: 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neuman M, Taegtmeyer M, Hatzold K, Johnson CC, Weiss HA, Fielding K. Challenges in measurement of linkage following HIV self‐testing: examples from the STAR Project. J Int AIDS Soc. 2019: 22(S1): e25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MacKellar D, Williams D, Bhembe B et al Peer‐Delivered Linkage Case Management and Same‐Day ART Initiation for Men and Young Persons with HIV Infection ‐ Eswatini, 2015–2017. MMWR Morb Mortal Wkly Rep 2018: 67: 663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Horter S, Thabede Z, Dlamini V et al “Life is so easy on ART, once you accept it”: Acceptance, denial and linkage to HIV care in Shiselweni, Swaziland. Soc Sci Med 2017: 176: 52–59. [DOI] [PubMed] [Google Scholar]
- 37. WHO Consolidated guidelines on HIV testing services [Internet]. WHO. 2018 [8 May 2018]. (Available from: http://www.who.int/hiv/pub/guidelines/hiv‐testing‐services/en/.) [8 May 2018].
- 38. Scott PA. Unsupervised self‐testing as part public health screening for HIV in resource‐poor environments: some ethical considerations. AIDS Behav 2014: 18(S4): 438–444. [DOI] [PubMed] [Google Scholar]
- 39. Kumwenda MK, Johnson CC, Choko AT et al Exploring social harms during distribution of HIV self‐testing kits using mixed‐methods approaches in Malawi. J Int AIDS Soc 2019: 22(S1): e25251. [DOI] [PMC free article] [PubMed] [Google Scholar]


