Abstract
Problem
The effect of thyroid autoimmunity (TAI) on the prevalence of recurrent miscarriage (RM) is highly debatable. No meta‐analysis has been published in the past decade to investigate the impact of TAI on women with RM.
Method of Study
Systemic literature search was conducted on PubMed, Embase, Cochrane, and Web of Science databases. English language literatures published between 1993 and 2019 were selected. We assessed the relationship between the prevalence of RM and thyroid peroxidase antibodies (TPO‐Ab) or antithyroid antibodies (ATA) and evaluated the thyroid‐stimulating hormone (TSH) level in TPO‐Ab‐positive women with RM. We also observed the treatment effect with levothyroxine (LT4) for RM. Review Manager 5.3 software was used to obtain the pooled odds ratios (OR).
Results
Analysis of 22 eligible studies revealed significant association between TPO‐Ab and the prevalence of RM (OR = 1.85; 95% CI, 1.38 to 2.49; P < .001)(n ≥ 3), (OR = 1.82; 95% CI, 1.13 to 2.92; P = .01) (n ≥ 3). Women with ATA + had higher risk of RM (OR = 2.36; 95% CI, 1.71 to 3.25; P < .00001)(n ≥ 3), (OR = 2.34; 95% CI, 1.70 to 3.22; P < .00001)(n ≥ 2). RM women with TPO‐Ab had higher TSH level when compared with those negative for TPO‐Ab (random‐effect SMD = 0.60; 95% CI, 0.31 to 0.90; P < .0001). We also found beneficial effects of LT4 supplementation on the outcome of live birth rate (LBR) among pregnant women with TPO‐Ab (OR = 3.04; 95% CI, 0.69 to 13.36; P = .14).
Conclusion
The presence of serum antithyroid antibodies does harms to women and can even lead to recurrent miscarriage; LT4 treatment may have beneficial to RM women.
Keywords: autoimmunity, LT4 treatment, meta‐analysis, recurrent miscarriage, thyroid antibody
This study reveals the association between thyroid autoimmunity and TSH level, the prevalence of RM. And LT4 supplementation may effectively increase live birth rate.

1. INTRODUCTION
Thyroid disease is one of the most frequent endocrine conditions in women of childbearing age. 1 The most common cause of thyroid dysfunction is thyroid autoimmunity (TAI). 2 TAI is defined as the presence of antithyroid antibodies (ATA), specifically thyroid peroxidase antibodies (TPO‐Ab) and/or thyroglobulin antibodies (Tg‐Ab). 3 With a prevalence of 5%‐20%, TAI is the most common autoimmune condition in women of reproductive age. 4
Thyroid hormones play a role in menstrual cycle and in achieving fertility as they affect the actions of follicle‐stimulating hormone and luteinizing hormone on steroid biosynthesis by specific T3 sites on oocytes. 5 Thyroid autoimmunity has been found to be related to subclinical hypothyroidism (SCH), 6 which is defined as high levels of serum thyroid‐stimulating hormone (TSH) despite normal levels of serum free thyroxine (FT4). 7 Both thyroid dysfunction and thyroid autoimmunity are known to cause adverse pregnancy outcomes during all trimesters of pregnancy. 8 Nevertheless, recent evidence shows an association between euthyroid women with thyroid autoimmunity and poor obstetric outcomes. The presence of ATA, particularly TPO‐Ab, has been associated with miscarriage, preterm birth, and post‐partum thyroid disease. 9 , 10 , 11
Recurrent miscarriage (RM) has previously been defined as three or more pregnancy losses 12 which affects 1% of couples. However, in recent years, more guidelines have redefined it as two or more pregnancy losses 13 , 14 which affects < 5% of couples. 15 RM places a severe physical, emotional, and financial burden on many families and our communities. An effective management and treatment is necessary. A higher prevalence of TPO‐Ab in women with RM has been found in several studies, varying from 19% to 36%. 16 , 17 , 18
A majority of women with TAI have detectable antibodies such as TPO‐Ab and sometimes TG‐Ab, whereas few have TSH receptor antibodies. 19 The prevalence of TPO‐Ab ranges for 8% to 14% in women of reproductive age. 20 The prevalence of positive‐TPO‐Ab among pregnant women in countries with good iodine supply has been reported between 5.1 21 , 22 and 12.4%. 23 The effect of thyroid autoimmunity especially TPO‐Ab on the clinical outcome of RM is highly debated. In recent years, no new meta‐analysis has been published to reveal the relationship between RM and TPO‐Ab or ATA. 24 And the expanded definition of RM affects differential amounts of women of childbearing age. In the increasing affected women, the assay of TPO‐AB or ATA whether can effectively predict RM, it need more data.
In recent researches, TPO‐Ab is associated with unexplained RM and these women may benefit from treatment with levothyroxine (LT4) 25 , 26 with decreasing TPO‐Ab levels after 2‐3 months treatment. 26 But in other studies, euthyroid women with TPO‐Ab undergo a treatment with levothyroxine, compared with no levothyroxine treatment, did not reduce miscarriage rate or increase live birth rate. 27 , 28 It is therefore possible that patients with RM and TPO‐Ab benefit from the substitution of thyroid hormones in terms of a lower rate of miscarriage, but currently there are no data specifically on patients with RM.
Currently, the effect of thyroid autoimmunity by itself on the prevalence of RM has not been established yet. Moreover, there was only one guideline(reference) 15 with regard to the need of treatment for women with RM, but there is no management for thyroid autoimmune in the guideline, more information is needed for developing a new guideline. Combining data on this controversial issue might reveal useful information for the counseling and management of euthyroid TPO‐Ab + or ATA + women with RM. This study aims to conduct a systematic review and meta‐analysis to gain insight into the clinical significance of TAI in women with RM.
2. METHODS
2.1. Literature search strategy
The meta‐analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2009 guidelines. 22 This systematic review was restricted to published research articles that compared the prevalence of RM in women positive for TPO‐Ab or ATA with that observed in women negative for thyroid autoantibodies. We also compared the relationship between RM and TPO‐Ab or ATA in different definition of RM. A literature search was conducted on PubMed, Embase, Cochrane, and Web of Science using combinations of the medical subject heading terms: ('thyroid autoimmunity' or 'thyroid autoantibody' or 'thyroid gland' or 'thyroid') AND ('recurrent pregnancy loss' or 'recurrent spontaneous abortion' or 'recurrent miscarriage' or 'habitual abortion' or 'recurrent spontaneous miscarriage' or 'habitual abortion'). The main search was conducted from inception through to December 2019 and was restricted to English literature. All relevant articles were retrieved, and additional reports were chosen from their reference lists. Review articles published on thyroid autoimmunity were also consulted for further pertinent studies. Unpublished studies were not identified.
2.2. Study selection
Only English language literatures were included in our study. Published cohort or case‐control studies (retrospective or prospective) that described at least 10 patients were eligible for inclusion. Studies were excluded if only TAI‐positive women were reported without a control group of TAI‐negative women. Studies were excluded if women were known to have overt biochemical hypothyroidism or hyperthyroidism history or were receiving any treatment for thyroid dysfunction.
The quality of the included studies was assessed by the Newcastle–Ottawa scale, a validated modality for assessing observational and non‐randomized studies. 29 The scale uses a score system based on three major criteria: selection of participants, comparability of study groups, and completeness of follow‐up. A quantitative appraisal of overall quality of each observational study was obtained, and scores ranged from 6 to 8 (Table 1).
Table 1.
Characteristics of the studies included in the quantitative analysis
| First author | Year | Study type | Participants | Consecutive abortions | Hormone levels | Patients | Controls | Outcome measures | Quality features a | Intervention |
|---|---|---|---|---|---|---|---|---|---|---|
| Bagis | 2001 | Prospective study | 876 women | ≥3 or ≥ 2 | TPO‐Ab, Tg‐Ab: Chemiluminescent enzyme immunometric assay method. Positive: >35 IU/mL for TPO‐Ab and >40 IU/mL for Tg‐Ab. TSH: microparticle enzyme immunoassay method, normal range: 0.3‐4.0 μU/mL | 81 women positive for TPO‐Ab | 795 women negative for TPO‐Ab | RM | 8 | – |
| Bellver | 2008 | Prospective study | 30 women with RM, 32 healthy controls. | ≥2 | Anti‐TPO and anti‐TG were studied with a two‐site immunoluminometric assay. Normal range anti‐TPO: <25 UI/mL and anti‐TG: <100 UI/mL | 4 women positive for TPO‐Ab | 58 women negative for TPO‐Ab | RM | 6 | – |
| Bliddal | 2019 | Cohort study | 825 women with RM | ≥3 | TPO‐Ab was measured by the automated Kryptor immunofluorescence assay. TPO‐Ab positivity: ≥60 kIU/L | 139 women positive for TPO‐Ab | 686 women negative for TPO‐Ab | LBR | 7 | T4 treatment. Four of the women were treated with Euthyrox (Merck), one woman with Levaxin (Takeda) (75 μg/d), and the rest with Eltroxin (Aspen) |
| Bussen | 1995 | Case‐control study | 22 euthyroid non‐pregnant habitual aborters and 22 multigravidae without endocrine dysfunction served as controls. | ≥3 | TPO‐Ab and Tg‐Ab were assayed using enzyme‐linked immunosorbent assay kits. positive: TPO‐Ab, Tg‐Ab or both antibodies(>100 IU/mL). | 6 TPO‐Ab+, 9 ATA+ | 38 TPO‐Ab−, 35 ATA− | RM | 8 | – |
| Bussen | 1997 | Case‐control study | 28 non‐pregnant women with a history of RM and 28 multigravidae without endocrine dysfunctions | ≥3 | TPO‐Ab and TG‐Ab were assayed using ELISA kits. A positive result in both assays was defined as titers >100 IU/mL | 7 TPO‐Ab+, 13 ATA+ | 49 TPO‐Ab−, 43 ATA− | RM | 7 | – |
| Cueva | 2018 | Cohort study | 74 women with recurrent early pregnancy loss who were euthyroid or had subclinical hypothyroidism | ≥2 | Presence of maternal antithyroid antibodies was defined as anti‐TPO antibodies > 4 IU/mL or anti‐Tg antibodies > 9 IU/mL | 13 women positive for TPO‐Ab | 61 women negative for TPO‐Ab | TSH level | 8 | – |
| Dendrinos | 2000 | Case‐control study | 30 euthyroid women with RSM aged 25‐37 y were compared with 15 matched fertile controls | ≥3 | Thyroid peroxidase(TPO) and thyroglobulin antibodies were tested with a chemiluminescence immunoassay. The normal range for this assay was < 2 IU/mL | 13 ATA+ | 31 ATA− | RM | 6 | – |
| Dobson | 2018 | Retrospective cohort review | 242 patients with RM | ≥3 | TPO‐Ab not defined | 12 women positive for TPO‐Ab | 230 women negative for TPO‐Ab | LBR | 6 | thyroxine, Unknown dosage |
| Esplin | 1998 | Case‐control study | 74 RM and 75 healthy, fertile control | ≥3 | Levels of IgG anti‐Tg and IgG anti‐TPO were measured by means of radioimmunoassay kits. Normal range 0.36‐12 units/mL | 71 TPO‐Ab+, 82 ATA+ | 78 TPO‐Ab−, 67 ATA− | RSM | 8 | – |
| Iravani | 2008 | Case‐control study | A total of 641 RM patients and 269 healthy controls were included | ≥3 | TG‐Ab, TPO‐Ab were measured with ELISA method. Positive: Tg‐Ab >125 IU/mL, TPO‐Ab >40 IU/mL. TSH was tested by immunoradiometric assay, reference ranges of 0.4‐4 mIU/L | 145 women positive for TPO‐Ab | 765 women negative for TPO‐Ab | RSM, TSH level | 6 | – |
| Junhao Yan | 2012 | Cohort study | 496 women with unexplained RM and a control group of 220 women with a known cause for RM were included in the study | ≥3 | ELISA method. Positive: TPO‐Ab was > 50 U/mL | 34 women positive for TPO‐Ab | 330 women negative for TPO‐Ab | LBR | 8 | Some patients were given empirical thyroxine therapy with 50 mg of thyroxine, whereas others were given no treatment at all |
| KAIDER | 1999 | Case‐control study | 591 patients with recurrent pregnancy loss and 100 normal healthy individuals. | ≥3 | Serodia gel‐agglutination assays were used to test TPO‐Ab and Tg‐Ab. The positive threshold was | |||||
| established by manufacturer as greater than a titer of 1:300. | 61 TPO‐Ab+, 85 ATA+ | 337 TPO‐Ab−, 313 ATA− | RM | 7 | – | |||||
| Kutteh | 1999 | Retrospective, two‐centered study | 700 women with a history of RM and 200 healthy, reproductive‐aged female controls | ≥2 | TPO‐Ab and TG‐Ab were assayed using commercial ELISA test kits. Negative: ≤67 IU/mL for thyroglobulin and ≤ 40 IU/mL for thyroid peroxidase | 126 TPO‐Ab+, 187 ATA+ | 774 TPO‐Ab−, 713 ATA− | RM | 8 | – |
| Lata | 2013 | Case‐control study | 100 pregnant and 25 non‐pregnant women with a history of RM, 100 pregnant women without a history of RM as healthy controls | ≥2 | TSH and anti‐TPO is assessed by electro‐chemiluminescence immunoassay. The reference range for the above hormones are as follows: TSH, RR: 0.27‐4.2 μIU/mL and anti‐TPO, RR: <34 IU/mL | 49 women positive for TPO‐Ab | 151 women negative for TPO‐Ab | RM, TSH level | 8 | All patients with TPO‐Ab + were treated with 25 μg/L‐T4 and titrated according to TSH at the time of recruitment into the study |
| Mecacci | 2000 | Prospective study | 29 women with a history of early pregnancy loss and 69 healthy control | ≥2 | Serum levels of TSH were determined by RIA kit (ICN) (normal range 0.2‐4.0 μU/L).RIA kits (Biocode) were employed for the determination of anti‐TG (normal values ≤ 50 IU/mL) and anti‐TPO (normal values ≤ 10 IU/mL) antibodies | 21 ATA+ | 77 ATA‐ | RM, TSH level | 7 | – |
| Mosaddegh | 2012 | Cohort study | 900 women who had a history of recurrent pregnancy loss | ≥2 | Thyroid peroxidase (TPO) was tested with a chemiluminescence immunoassay, and women with anti‐TPO more than 40 UI/mL were treated with levothyroxine after signing inform consent | 45 TPO‐Ab+, 39 use LT4 | 6 TPO‐Ab + women never used Levothyroxine by their own decision | LBR | 6 | Levothyroxine doses were depended on the levels of anti‐TPO, which were decided by endocrinologist. It was 25‐100 μg every day. Treatment continued with levothyroxine and aspirin till pregnancy happened and these continued during pregnancy until delivery. |
| Motak‐Pochrzęst | 2013 | Retrospective study | 155 patients with primary RM and 50 control patients were analyzed | ≥3 | Tg‐Ab and TPO‐Ab were detected using immunoassay ELISA. Titers over 60 IU/mL for anti‐Tg and for anti‐TPO were considered to be positive | 42 ATA+ | 163 ATA− | RM | 6 | – |
| Mumusoglu | 2015 | Retrospective study | 515 women of reproductive age | ≥2 | Radioimmunoassay determined TPO‐Ab. Levels of 80 IU/mL were considered positive for TPO‐Ab | 67 women positive for TPO‐Ab | 448 women negative for TPO‐Ab | RM | 6 | – |
| Pratt | 1993 | Retrospective study | 45 RSM patients and 100 healthy controls | ≥3 | TPO‐Ab and Tg‐Ab were assayed with Kalibre radioimmunoassay kits. A positive result in both tests was defined as ≥0.3 U/mL | 25 TPO‐Ab+, 33 ATA+ | 120TPO‐Ab−, 112 ATA− | RM | 7 | – |
| Roberts | 1996 | Case‐control study | 53 pregnant or non‐pregnant women | ≥3 | TPO‐Ab, Tg‐Ab were tested by using ELISA kits. Positive: Tg‐Ab > 8 U/mL, TPO‐Ab > 1U/mL | 4 ATA+ | 18 ATA− | RM | 8 | 1 |
| Ticconi | 2011 | Case‐control study | 160 women with RM and 100 healthy women | 2 or ≥ 3 pregnancy losses | TG‐Ab and TPO‐Ab were detected using CLIA immunoassay. The sensitivity of the TPO‐Ab test was 25 IU⁄mL | 39 women positive for TPO‐Ab | 221 women negative for TPO‐Ab | RM | 7 | – |
| Vissenberg | 2015 | Retrospective cohort study | 344 euthyroid women with unexplained RM | ≥2 | TPO‐Ab was measured by a chemiluminescence immunoassay. TPO‐Ab− positivity was defined as TPO‐Ab > 60 kU/L | 28 TPO‐Ab− positive women | 174 TPO‐Ab− negative women | LBR, TSH level | 6 | levothyroxine, Unknown dosage |
Abbreviations: AI, thyroid autoimmunity; ATA, antithyroid antibody; LBR, live birth rate; RM, recurrent miscarriage; TPO‐Ab, thyroid peroxidase antibody.
Based on the Newcastle‐Ottawa scale. 29
2.3. Data extraction
Two authors independently evaluated all articles and abstracted data on standardized forms. Information regarding study characteristics (author name, year of publication, study design, sample size), methodology (definition of RM, thyroid autoantibodies and hormone measurement method, threshold and time of measurement, study quality) and characteristics of study groups, and outcome (prevalence of RM, serum TSH levels and live birth rate (LBR)) were extracted. Live birth rate was defined as the number of deliveries that resulted in at least one live born baby.
2.4. Statistical analysis
The overall effect of TPO‐Ab or ATA on a binary variable (RM‐prevalence, live birth rate) was assessed by a combined odds ratio (OR). The overall impact of TPO‐Ab on a continuous variable (TSH) was assessed by the difference in means for the two groups of patients; and by using the summary data published in the eligible studies, the results for outcomes were expressed as odds ratios (OR) with 95% confidence intervals (CI). 30
The inconsistency of studies’ results was measured using Cochrane Q and the I 2 statistics. 31 I 2 value of 0% indicates no observed heterogeneity, I 2 < 25% shows insignificant heterogeneity, I 2 values of 25%‐50% shows moderate heterogeneity and I 2 > 50% indicates significant heterogeneity. 31 The odds ratios (OR) were combined using a fixed‐effects model when heterogeneity observed among studies was absent to moderate; the DerSimonian & Laird method for a random‐effects model was employed when heterogeneity was high (I 2 > 50%). 32 , 33 All statistical analyses were performed using Review Manager 5.3 software (Cochrane Collaboration); P < .05 was considered statistically significant.
Funnel plots, which graph OR on a log scale (effect) against standard error of log‐OR (precision), were generated and visually inspected for asymmetry to determine if the included studies were non‐representative of the body of possible studies on the subject (as could result from small study effect or other biases, such as publication and poor‐quality bias).
3. RESULTS
3.1. Search results and study characteristics
The process of literature identification and selection of studies is summarized in Figure 1A total of 606 potentially relevant studies were identified through literature search. Out of these, 235 were double citations and 239 were rejected on the basis of title and abstract. The remaining 34 articles were reviewed in full. After evaluation of the full manuscripts, only 22 studies qualified for the final quantitative analysis. The twenty‐two studies included in the systematic review were published between 1993 and 2019. The data from the twenty‐two studies included 952 TPO‐Ab‐positive and 5315 TPO‐Ab‐negative women, 644 ATA‐positive and 1572 ATA‐negative women.
Figure 1.
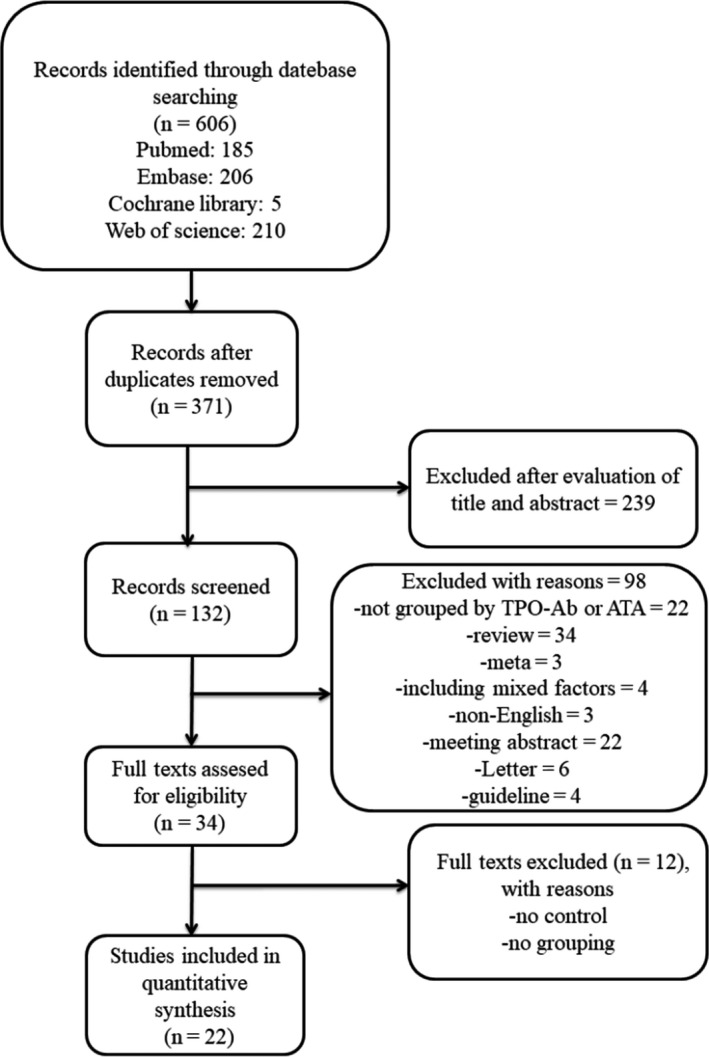
Eligibility of Studies for Inclusion in Meta‐analysis
The characteristics of the studies included in the quantitative analysis are summarized in Table 1. Six of these were retrospective cohort studies, three were prospective cohort studies, and four were cohort studies and nine were case‐control studies (Table 1).
3.2. Prevalence of RM
In the women with RM (n ≥ 3), comparing TPO‐Ab‐negative ones, TPO‐Ab‐positive women showed a higher prevalence of RM (random‐effects OR = 1.76; 95% CI, 1.00‐3.10; P = .05; Figure 2A). The heterogeneity test result was high (I 2 = 70%). However, on excluding the study by Esplin, 34 the degree of heterogeneity declined from high to low (I 2 = 43%, Figure 2B), indicating that this study was heterogeneous with other studies. Therefore, this study was not included in the following subgroup analysis.
Figure 2.
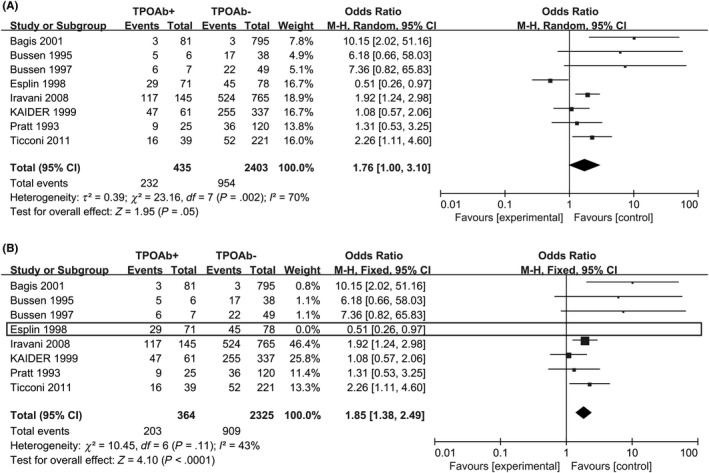
Forest plot of prevalence of RM (n ≥ 3) comparing TPO‐Ab + and TPO‐Ab− women. TPO‐Ab+ = positive for thyroid peroxidase; TPO‐Ab− = negative for thyroid peroxidase
When we defined RM as ≥ 2 consecutive abortions, in all, 366 TPO‐Ab‐positive pregnancies and 2447 TPO‐Ab‐negative pregnancies were followed up. The result was that 192 aborted in the TPO‐Ab‐positive group, while 950 aborted in the TPO‐Ab‐negative group. Positive‐TPO‐Ab women showed a higher prevalence of RM (random‐effects OR = 1.82; 95% CI, 1.13‐2.92; P = .01; I 2 = 58%; Figure 3).
Figure 3.
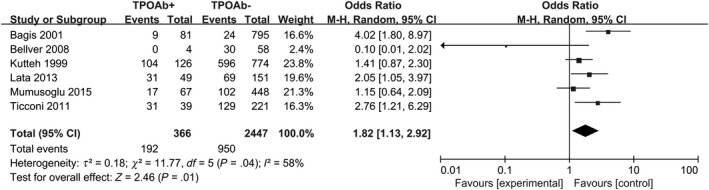
Forest plot of prevalence of RM (n ≥ 2) comparing TPO‐Ab+ and TPO‐Ab− women. TPO‐Ab+ = positive for thyroid peroxidase; TPO‐Ab− = negative for thyroid peroxidase
Diversity in the methodology applied to measure thyroid autoantibodies was also observed. In twelve studies, TPO‐Ab were detected. 17 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 Both TPO‐Ab and Tg‐Ab were determined in eleven studies. 18 , 35 , 36 , 37 , 38 , 39 , 43 , 44 , 45 , 46 , 47 When consecutive abortions ≥ 3, 12 studies showed an increased risk for RM in women with positive ATA compared with negative‐ATA controls (fixed‐effects OR = 2.36; 95% CI, 1.71‐3.25; P < .00001; Figure 4), with moderate heterogeneity (I 2 = 33%). Another 11 study, in 373 patients with ATA, 227 women suffered ≥ 2 consecutive abortions, data from these studies determine the risk for recurrent miscarriage rate in relation to ATA (fixed‐effects OR = 2.34; 95% CI, 1.70–3.22; P < .00001; Figure 5), with moderate heterogeneity (I 2 = 44%).
Figure 4.
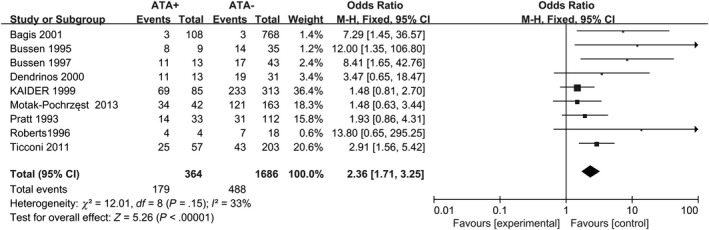
Forest plot of prevalence of RM (n ≥ 3) comparing ATA+ and ATA− women. ATA+ = positive for antithyroid antibodies; ATA− = negative for antithyroid antibodies
Figure 5.
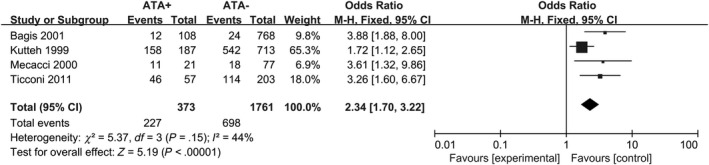
Forest plot of prevalence of RM (n ≥ 2) comparing ATA+ and ATA− women. ATA+ = positive for antithyroid antibodies; ATA− = negative for antithyroid antibodies
3.3. TSH level in RM patients
We examined the difference of mean basal serum TSH between the TPO‐Ab‐positive and TPO‐Ab‐negative group. Six studies compared it with and without TPO‐Ab. 2 , 17 , 41 , 47 , 48 , 49 From the meta‐analysis, it emerged that TPO‐Ab‐positive women had significantly higher serum TSH levels (random‐effect SMD = 0.60; 95% CI, 0.31 to 0.90; P < .0001; Figure 6A). The I 2 value was 80% indicating the present of heterogeneity. Serum TSH concentrations were significantly increased in a study 47 . However, the subgroup pooled result changed when the study by Federico Mecacci 47 was removed from the meta‐analysis (Fixed effect SMD = 0.60; 95% CI, 0.34 to 0.88; P < .00001; Figure 6B). When excluding the study by Mecacci, 47 the combined results of n ≥ 2 subgroup showed mild heterogeneity (I 2 = 12%).
Figure 6.

(A) Forest plot of TSH level in women with RM (n ≥ 3) or (n ≥ 2) comparing TPO‐Ab+ and TPO‐Ab− women; (B) Forest plot of TSH level in women with RM (n ≥ 2) comparing TPO‐Ab+ and TPO‐Ab− women, without the study of Mecacci. TPO‐Ab+ = positive for thyroid peroxidase; TPO‐Ab− = negative for thyroid peroxidase
3.4. Live birth rate
Five studies reported data on the association between LT4 supplementation and LBR in patients with TPO‐Ab. Three studies reported increased live birth rates among women receiving LT4 treatment, 2 , 26 , 50 whereas the other two did not. 48 , 51 The combined results of all five studies indicated significant effect of LT4 treatment on the live birth rate, with a pooled OR of 3.04 (95% CI: 0.69‐13.36, n = 207, I 2 = 64%) (Figure 7A). When excluding the study by Junhao Yan, 50 the subgroup combined results showed no significant effect of LT4 treatment on the live birth rate (OR = 0.70, 95% CI: 0.24‐2.05, n = 65, I 2 = 0%). But the I2 value was 0% indicating the absence of heterogeneity, a funnel plot showed no indication of asymmetry among studies.
Figure 7.
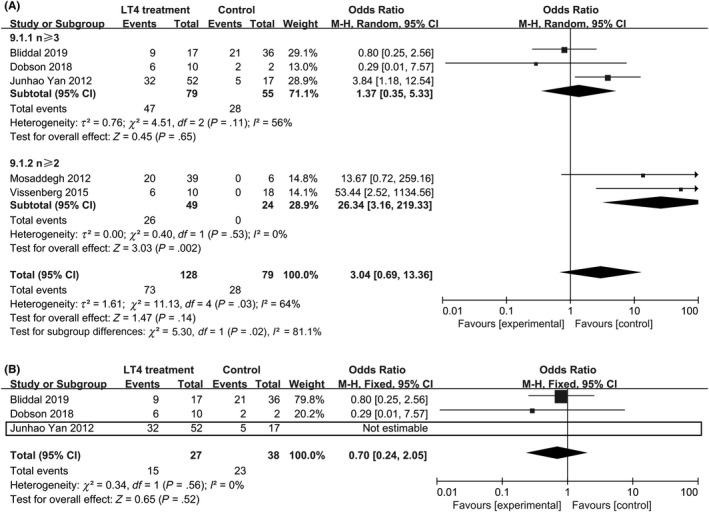
Forest plot of live birth rate, comparing TPO‐Ab+ and TPO‐Ab− women. TPO‐Ab+ = positive for thyroid peroxidase; TPO‐Ab− = negative for thyroid peroxidase
4. DISCUSSION
The present systematic review and meta‐analysis showed clear evidence for a relationship between the presence of TPO‐Ab or ATA and the prevalence of RM. RM women with TPO‐Ab had higher TSH level when compared with those negative for TPO‐Ab. We also found beneficial effects of LT4 supplementation on the outcome of live birth rate among pregnant women with TPO‐Ab. The subgroup analysis further indicated that when women suffered ≥ 2 consecutive abortions, LT4 supplementation can effectively increase live birth rate. As these subgroup analyses were based on a limited number of studies, further research is needed to draw firm conclusions.
Based on evidence regarding the dynamics of TPO‐Ab, ATA and TSH levels during pregnancy, 52 and the data (Figure 2) showed a significant heterogeneity from the study of Esplin. 34 So we excluded the study of Esplin, 34 from which samples were obtained ≥ 6 months after a pregnancy while the samples from other studies had been drawn no later than gestational week 8 or no pregnancy.
There has been found that women with TA has higher prevalence of recurrent miscarriage. 41 Our contrasting results on prevalence rate in women with thyroid autoimmunity can be explained by several reasons. Among the 22 studies included in our meta‐analysis, one showed lower prevalence rate in TPO‐Ab positive women. 40 However, the sample size of this study was relatively small. Additionally, the number of TPO‐Ab or ATA‐positive women enrolled in the studies was comparatively less than TPO‐Ab or ATA‐negative women in general.
Therefore, it has been suggested that thyroid auto antibodies may be employed as a marker for at‐risk pregnancies. 53 Although the mechanism is not completely understood, it is postulated that TAI results in early pregnancy loss because of activation of immune system 54 or act as an infertility factor and results in delayed conception. 55
In this meta‐analysis, subjects with thyroid dysfunction or undergoing treatment were excluded in all the studies included. Although the TSH values in TPO‐Ab positive women were significantly higher than that in TPO‐Ab negative women. In particular, mean TSH was significantly higher by 0.60 mIU/L (95% CI 0.31‐0.90; P < .0001) in the TPO‐Ab‐positive group compared with TPO‐Ab‐negative group. The mean TSH of the participants in half studies was within the normal range (<2.5 mIU/L). Iravani 17 excluded some participants who had serum TSH levels outside the reference range. In the other studies (Mecacci 2000, 47 Lata 2013, 41 Curve 49 ), the TSH level was significantly higher than normal (>2.5 mIU/L). The data from Curve 49 included some subclinical hypothyroidism patients.
Many institutions define RM as “the loss of three or more consecutive pregnancies”, 12 , 56 and biochemical pregnancies are included by The UK Royal College of Obstetricians and Gynaecologists (RCOG). While the American Society for Reproductive Medicine (ASRM) defines RM as “two or more failed clinical pregnancies” 13 and exclude biochemical pregnancies. This rationale is supported by a large study with >1000 participants, which found that the likelihood of detecting an abnormality after two losses was similar to that after three or four or more losses. 57 In our data, no matter how the definition changes, women with TPO‐Ab or ATA have a higher risk of RM than that in TPO‐Ab or ATA‐negative women.
Van den Boogard 24 et al did a meta‐analysis in 2011, in which they found a clear evidence for a relationship between the presence of thyroid antibodies or subclinical hypothyroidism on unexplained subfertility, miscarriage, recurrent miscarriage, preterm birth, and post‐partum thyroid disease. But they paid no attention to treatment. Several meta‐analyses of studies of ART patients with higher levels of TPO‐Ab showed that substitution of thyroid hormones decreased the rate of miscarriages, 10 , 58 , 59 and increased the delivery, clinical pregnancy, and fertilization rates. 60 However, other studies such as the study by Wang published in 2017 27 were unable to demonstrate the effect.
Live birth rate is an effective indicator for assessing pregnancy outcome. In the present meta‐analysis, we included more recently published studies and found a relative increment in live birth rate by LT4 supplementation among pregnant women with TPO‐Ab compared with that by no treatment/placebo. Subjects with thyroid dysfunction or undergoing treatment were excluded or analyzed separately in all the studies included.
Two studies 48 , 51 reported no significant increment in the LBR by LT4 supplementation, which is in contrast to the results 2 , 26 , 50 for naturally conceiving women with TPO‐Ab who were likely to exhibit reduced PBR due to LT4 supplementation. But all of these studies never explain the type of conception. And Junhao Yan 50 had different result from Dobson 51 and Bliddal, 48 considering its weight, we should sight the pooled result of three studies (OR = 1.37, 95% CI: 0.35‐5.33, n = 134, P = .65, I 2 = 56%). And the combined results of all five studies indicated significant effect of LT4 treatment on the live birth rate, with a pooled OR of 3.04 (95% CI: 0.69‐13.36, n = 207, I 2 = 64%). But in Lata's study, 41 31 patients with TPO‐Ab + were treated with 25 μg LT4, after that there was no difference in prevalence of miscarriage between hypothyroid and euthyroid individuals in TPO‐Ab + women.
Generally speaking, treatment for hypothyroidism usually begins with thyroxine (T4) replacement initiated at 25‐50 μg per day for 4 weeks and then verified according to biochemical and clinical analyses, this takes on average 6‐8 weeks. The dose is approximately 1.5 μg/body weight in pounds. 61 In Mosaddegh's study, 26 levothyroxine was used to TPO‐Ab + women, researchers vary considerably in TPO‐Ab level in different people. It was 25‐100 μg every day. This study showed that levothyroxine reduces the incidence of spontaneous abortions in women with high TPO‐Ab. It also decreased TPO‐Ab levels after 2‐3 months treatment. Therefore, further research is needed to draw firm conclusions, especially sighting the adjustment of medication orders on the basis of TPO‐Ab level.
Endometrial volume is an important parameter to evaluate endometrial receptivity and therefore a possible predictor for successful implantation. 62 , 63 Zhong et al reported lower implantation rates in women with TAI, but the authors did not report on thyroid function. 64 The study of Merhan Dorostghoal 65 demonstrate that the endometrial ER‐α expression may lead to defects in uterine receptivity and contribute to unexplained infertility. Furthermore, Zhangbi Wu 66 indicated that TPO‐Ab induces a non‐receptive endometrial milieu in the euthyroid state, which may underlie the detrimental effects of Hashimoto's thyroiditis (HT) itself on embryo implantation. More research is needed to identify the role of thyroid autoantibodies on implantation.
Several studies have assessed the effect of different treatments of TPO‐Ab + women on pregnancy procedures. Thangaratinam et al pooled the results of two studies 67 , 68 and observed a significant reduction in risk of miscarriage in women treated with levothyroxine. 10 Revelli et al retrospectively analyzed the effect of adjuvant treatments on IVF results in TAI + patients and they found that treating TAI + women with a combination of levothyroxine, acetylsalicylic acid and prednisolone resulted in higher ovarian responsiveness to gonadotropins and higher pregnancy rates but did not decrease the miscarriage rate. 4 As TAI is hypothesized to be a marker of an underlying generalized autoimmune imbalance, Litwicka et al tested the efficacy of glucocorticoids administered alone and observed significantly higher clinical pregnancy and live birth rates in the treated group. 69 But their sample size was small and all patients were treated in the same center. 69 Vaquero et al compared intravenous immunoglobulin (IVIg) therapy and levothyroxine (LT4) replacement therapy for TAI during pregnancy and concluded that the abortion rate in the LT4 replacement group was significantly lower than in the IVIg group. 70 In contrast, Sher et al compared the effect of heparin/aspirin therapy alone versus heparin/aspirin in combination with intravenous IVIg immunotherapy on IVF outcomes of patients with positive ATA. 71 They found that IVIg was linked to increased live birth rate, but had no effect on miscarriage rate. 71 In the meta‐analysis by Velkeniers et al, the authors concluded that LT4 treatment significantly improves delivery rate and reduces miscarriage rate in women undergoing ART, especially if the serum TSH level is ≥ 2.5 mIU/L with TAI or ≥4.0 mIU/L in general. 72 In any case, all these studies followed different protocols and their results cannot be generalized. Further large scale studies regarding the cost effectiveness and safety profile of any treatment strategies are essential before we can establish the need for thyroid autoantibodies screening tests and treatments in patients with RM.
Our systematic review and meta‐analysis elucidates the association between thyroid autoimmunity and the prevalence of RM. The presence of thyroid autoantibodies may increase the TSH level in RM patients. This may recommend women to test for TPO‐Ab and TSH level after two pregnancy losses.
Antithyroid antibodies are known to occur in normal, healthy populations, and these autoantibodies are five times more common in women than in men. 73 The clear association between TAI and RM suggests that women with RM need to be aggressively tested or treated for antithyroid antibodies. The tests and treatments are not only expensive but also involve potential health risks for the mother as well as the offspring. A recent study evaluated the association of maternal thyroid function during early pregnancy with offspring intelligence quotient and brain morphology in childhood and raised some concerns on possible overtreatment of patients with thyroxine, because offspring of patients with suppressed TSH may have worse neuropsychological outcomes. 44 So we must concern proper screening crowd.
The statement from the American Society for Reproductive Medicine mentioned that the available data support the routine measurement of TSH in infertile women attempting pregnancy, but not that of TPO‐abs, unless TSH levels are ≥2.5 mIU/L. 74 Nonetheless, TAI should not be neglected in women of childbearing age as it is a risk factor for thyroid dysfunction and hypothyroidism during pregnancy, and can affect fetal growth and neuropsychological outcome. 21 , 75 TAI can affect the pregnancy outcome in ART if elevated TSH levels are recorded simultaneously. 76 Recently, the American Thyroid Association issued clinical practice guidelines after evaluating the evidence on thyroid function during ovarian stimulation. 77 According to their guidelines, LT4 administration is recommended in women with TAI and TSH concentrations higher than the pregnancy‐specific reference range, whereas it may be considered in women without TAI and TSH concentrations higher than the pregnancy‐ specific reference range, but below 10 mIU/L. 78 We propose that TSH levels of TAI women are monitored stringently for women with RM before pregnancy and further large scale prospective, randomized, placebo controlled trials are carried out to evaluate the effect of treatment for antithyroid antibodies in euthyroid women with RM.
It should be noted that our meta‐analysis had certain limitations. Due to the small number of studies included to analyze certain outcomes, we cannot rule out the existence of publication bias in our analysis. 79 Furthermore, the included studies used different threshold values for TAI positivity (Table 1). 18 studies only measured TPO‐Ab as opposed to the remaining 11 studies which measured both TPO‐Ab and Tg‐Ab. It has been shown that women with TPO‐Ab + women had higher mean serum TSH levels than women without TPO‐Ab. Therefore, if we cannot use TPO‐Ab effectively evaluating the thyroid function of women with RM, we can test for TSH first.
Women with TAI are prone to develop subclinical hypothyroidism (SCH) during pregnancy, even though they can be euthyroid during the first trimester of pregnancy. 80 Consequently, in order to decipher the effect of TSH on pregnancy outcome in women with RM, it is imperative to conduct further studies.
Our analysis opens the way for more fundamental studies in order to gain deeper insights into the pathophysiological mechanisms of thyroid autoimmunity. The supposed association between TAI and endometrium deserves attention in particular. The process of fertilization in TAI‐positive women also needs to be studied in details. Finally, we require further evidence regarding the factors involved in implantation of the embryo, including studies on endometrial receptivity, embryo quality and immunological factors.
5. CONCLUSION
This study reveals the association between thyroid autoimmunity per se and the prevalence of RM. Euthyroid women positive for antithyroid antibodies have higher risk of RM. TSH level in positive‐TPO‐Ab women with RM is higher than negative‐TPO‐Ab women. And LT4 supplementation may effectively increase live birth rate. More trials involving endometrial receptivity in TAI women should be carried out to decode the effect of TPO‐Ab or ATA. More RCTs are necessary to clarify the efficacy of LT4 in the treatment of RM. Advanced research is also necessary to elucidate the pathophysiological mechanisms of thyroid autoimmunity and its involvement in RM and subsequent pregnancy.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this article.
ACKNOWLEDGMENT
We would like to thank Dr Dan‐Qing Yu for her expert guidance and continuous encouragement throughout this research project. This project was supported by the National Natural Science Foundation of China (NSFC) (81671487).
Xie J, Jiang L, Sadhukhan A, et al. Effect of antithyroid antibodies on women with recurrent miscarriage: A meta‐analysis. Am J Reprod Immunol. 2020;83:e13238 10.1111/aji.13238
Xie and Jiang equally contribute to the work.
REFERENCES
- 1. Ramprasad M, Bhattacharyya SS, Bhattacharyya A. Thyroid disorders in pregnancy. Indian J Endocrinol Metab. 2012;16(Suppl 2):S167‐S170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vissenberg R, Manders VD, Mastenbroek S, et al. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum Reprod Update. 2015;21(3):378‐387. [DOI] [PubMed] [Google Scholar]
- 3. He H, Jing S, Gong F, Tan YQ, Lu GX, Lin G. Effect of thyroid autoimmunity per se on assisted reproduction treatment outcomes: A meta‐analysis. Taiwan J Obstet Gynecol. 2016;55(2):159‐165. [DOI] [PubMed] [Google Scholar]
- 4. Revelli A, Casano S, Piane LD, et al. A retrospective study on IVF outcome in euthyroid patients with anti‐thyroid antibodies: effects of levothyroxine, acetyl‐salicylic acid and prednisolone adjuvant treatments. Reprod Biol Endocrinol. 2009;7:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medenica S, Nedeljkovic O, Radojevic N, Stojkovic M, Trbojevic B, Pajovic B. Thyroid dysfunction and thyroid autoimmunity in euthyroid women in achieving fertility. Eur Rev Med Pharmacol Sci. 2015;19(6):977‐987. [PubMed] [Google Scholar]
- 6. Johnson N, Chatrani V, Taylor‐Christmas A‐K, et al. Population reference values and prevalence rates following universal screening for subclinical hypothyroidism during pregnancy of an Afro‐Caribbean Cohort. Eur Thyroid J. 2014;3(4):234‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291(2):228‐238. [DOI] [PubMed] [Google Scholar]
- 8. Abalovich M, Amino N, Barbour LA, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92(8 Suppl):S1‐S47. [DOI] [PubMed] [Google Scholar]
- 9. Glinoer D. Miscarriage in women with positive anti‐TPO antibodies: is thyroxine the answer? J Clin Endocrinol Metab. 2006;91(7):2500‐2502. [DOI] [PubMed] [Google Scholar]
- 10. Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta‐analysis of evidence. BMJ. 2011;342: d2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sen A, Kushnir VA, Barad DH, Gleicher N. Endocrine autoimmune diseases and female infertility. Nat Rev Endocrinol. 2014;10(1):37‐50. [DOI] [PubMed] [Google Scholar]
- 12. Gynaecologists RCoOa . The Investigation and Treatment of Couples with Recurrent First Trimester and Second Trimester Miscarriage. London: RCOG; 2011. [Google Scholar]
- 13. Practice Committee of the American Society for Reproductive M . Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103‐1111. [DOI] [PubMed] [Google Scholar]
- 14. Little AB. There's many a slip 'twixt implantation and the crib. N Engl J Med. 1988;319(4):241‐242. [DOI] [PubMed] [Google Scholar]
- 15. Hong Li Y, Marren A. Recurrent pregnancy loss: A summary of international evidence‐based guidelines and practice. Aust J Gen Pract. 2018;47(7):432‐436. [DOI] [PubMed] [Google Scholar]
- 16. Rushworth FH, Backos M, Rai R, Chilcott IT, Baxter N, Regan L. Prospective pregnancy outcome in untreated recurrent miscarriers with thyroid autoantibodies. Hum Reprod. 2000;15(7):1637‐1639. [DOI] [PubMed] [Google Scholar]
- 17. Iravani AT, Saeedi MM, Pakravesh J, Hamidi S, Abbasi M. Thyroid autoimmunity and recurrent spontaneous abortion in Iran: a case‐control study. Endoc Pract. 2008;14(4):458‐464. [DOI] [PubMed] [Google Scholar]
- 18. Roberts J, Jenkins C, Wilson R, et al. Recurrent miscarriage is associated with increased numbers of CD5/20 positive lymphocytes and an increased incidence of thyroid antibodies. Eur J Endocrinol. 1996;134(1):84‐86. [DOI] [PubMed] [Google Scholar]
- 19. Poppe K, Autin C, Veltri F, et al. Thyroid autoimmunity and intracytoplasmic sperm injection outcome: a systematic review and meta‐analysis. J Clin Endocrinol Metab. 2018;103(5):1755‐1766. [DOI] [PubMed] [Google Scholar]
- 20. Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31(5):702‐755. [DOI] [PubMed] [Google Scholar]
- 21. Medici M, de Rijke YB, Peeters RP, et al. Maternal early pregnancy and newborn thyroid hormone parameters: the Generation R study. J Clin Endocrinol Metab. 2012;97(2):646‐652. [DOI] [PubMed] [Google Scholar]
- 22. Shields BM, Knight BA, Hill AV, Hattersley AT, Vaidya B. Five‐year follow‐up for women with subclinical hypothyroidism in pregnancy. J Clin Endocrinol Metabol. 2013;98(12):E1941‐E1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Medici M, Porcu E, Pistis G, et al. Identification of novel genetic loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet. 2014;10(2):e1004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van den Boogaard E, Vissenberg R, Land JA, et al. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2011;17(5):605‐619. [DOI] [PubMed] [Google Scholar]
- 25. Vissenberg R, Fliers E, van der Post JA, van Wely M, Bisschop PH, Goddijn M. Live‐birth rate in euthyroid women with recurrent miscarriage and thyroid peroxidase antibodies. Gynecol Endocrinol. 2016;32(2):132‐135. [DOI] [PubMed] [Google Scholar]
- 26. Mosaddegh MH, Ghasemi N, Jahaninejad T, Mohsenifar F, Aflatoonian A. Treatment of recurrent pregnancy loss by Levothyroxine in women with high Anti‐TPO antibody. Iran J Reprod Med. 2012;10(4):373‐376. [PMC free article] [PubMed] [Google Scholar]
- 27. Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318(22):2190‐2198. [DOI] [PubMed] [Google Scholar]
- 28. Dhillon‐Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in Women with Thyroid Peroxidase Antibodies before Conception. N Engl J Med. 2019;380(14):1316‐1325. [DOI] [PubMed] [Google Scholar]
- 29. Wells G, Shea B, Oconnell D, Peterson J, Welch V, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses: 3rd Symposium on Systematic Reviews: Beyond the Basics. Oxford, UK; 2000.
- 30. Egger MDS, Altman DG. Systematic Reviews in Health Care: Meta‐Analysis in Context. Part IV, Chapters 15–16, 2nd edn London: BMJ Publishing Group; 2001:285‐312. [Google Scholar]
- 31. Higgins JP, Thompson SG, Fau‐Deeks JJ, Deeks JJ, Fau‐Altman DG, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DerSimonian R, Laird N. Meta‐analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105‐114. [DOI] [PubMed] [Google Scholar]
- 34. Esplin MS, Branch DW, Silver R, Stagnaro‐Green A. Thyroid autoantibodies are not associated with recurrent pregnancy loss. Am J Obstet Gynecol. 1998;179(6 Pt 1):1583‐1586. [DOI] [PubMed] [Google Scholar]
- 35. Kaider AS, Kaider BD, Janowicz PB, Roussev RG. Immunodiagnostic evaluation in women with reproductive failure. Am J Reprod Immunol. 1999;42(6):335‐346. [DOI] [PubMed] [Google Scholar]
- 36. Bussen SS, Steck T. Thyroid antibodies and their relation to antithrombin antibodies, anticardiolipin antibodies and lupus anticoagulant in women with recurrent spontaneous abortions (antithyroid, anticardiolipin and antithrombin autoantibodies and lupus anticoagulant in habitual aborters). Eur J Obstet Gynecol Reprod Biol. 1997;74(2):139‐143. [DOI] [PubMed] [Google Scholar]
- 37. Ticconi C, Giuliani E, Veglia M, Pietropolli A, Piccione E, Di Simone N. Thyroid autoimmunity and recurrent miscarriage. Am J Reprod Immunol. 2011;66(6):452–459. [DOI] [PubMed] [Google Scholar]
- 38. Pratt D, Novotny M, Kaberlein G, Dudkiewicz A, Gleicher N. Antithyroid antibodies and the association with non‐organ‐specific antibodies in recurrent pregnancy loss. Am J Obstet Gynecol. 1993;168(3):837‐841. [DOI] [PubMed] [Google Scholar]
- 39. Bussen S, Steck T. Thyroid autoantibodies in euthyroid non‐pregnant women with recurrent spontaneous abortions. Hum Reprod. 1995;10(11):2938‐2940. [DOI] [PubMed] [Google Scholar]
- 40. Bellver J, Soares SR, Alvarez C, et al. The role of thrombophilia and thyroid autoimmunity in unexplained infertility, implantation failure and recurrent spontaneous abortion. Hum Reprod. 2008;23(2):278‐284. [DOI] [PubMed] [Google Scholar]
- 41. Lata K, Dutta P, Sridhar S, et al. Thyroid autoimmunity and obstetric outcomes in women with recurrent miscarriage: a case‐control study. Endocr Connect. 2013;2(2):118‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mumusoglu S, Beksac MS, Ekiz A, Ozdemir P, Hascelik G. Does the presence of autoantibodies without autoimmune diseases and hereditary thrombophilia have an effect on recurrent pregnancy loss? J Maternal‐Fetal Neonatal Med. 2016;29(14):2352‐2357. [DOI] [PubMed] [Google Scholar]
- 43. Bagis T, Gokcel A, Saygili ES. Autoimmune thyroid disease in pregnancy and the postpartum period: relationship to spontaneous abortion. Thyroid. 2001;11(11):1049‐1053. [DOI] [PubMed] [Google Scholar]
- 44. Kutteh WH, Yetman DL, Carr AC, Beck LA, Scott RT Jr. Increased prevalence of antithyroid antibodies identified in women with recurrent pregnancy loss but not in women undergoing assisted reproduction. Fertil Steril. 1999;71(5):843‐848. [DOI] [PubMed] [Google Scholar]
- 45. Dendrinos S, Papasteriades C, Tarassi K, Christodoulakos G, Prasinos G, Creatsas G. Thyroid autoimmunity in patients with recurrent spontaneous miscarriages. Gynecol Endocrinol. 2000;14(4):270‐274. [DOI] [PubMed] [Google Scholar]
- 46. Motak‐Pochrzest H, Malinowski A. The occurrence of immunological disturbances in patients with recurrent miscarriage (RM) of unknown etiology. Neuro Endocrinol Lett. 2013;34(7):701‐707. [PubMed] [Google Scholar]
- 47. Mecacci F, Parretti E, Cioni R, et al. Thyroid autoimmunity and its association with non‐organ‐specific antibodies and subclinical alterations of thyroid function in women with a history of pregnancy loss or preeclampsia. J Reprod Immunol. 2000;46(1):39‐50. [DOI] [PubMed] [Google Scholar]
- 48. Bliddal S, Feldt‐Rasmussen U, Rasmussen AK, et al. Thyroid peroxidase antibodies and prospective live birth rate: a cohort study of women with recurrent pregnancy loss. Thyroid. 2019;29(10):1465‐1474. [DOI] [PubMed] [Google Scholar]
- 49. Cueva S, Burks C, McQueen D, Barkoff MS, Stephenson MD. Maternal antithyroid antibodies and euploid miscarriage in women with recurrent early pregnancy loss. Fertil Steril. 2018;110(3):452‐458. [DOI] [PubMed] [Google Scholar]
- 50. Yan J, Sripada S, Saravelos SH, Chen ZJ, Egner W, Li TC. Thyroid peroxidase antibody in women with unexplained recurrent miscarriage: prevalence, prognostic value, and response to empirical thyroxine therapy. Fertil Steril. 2012;98(2):378‐382. [DOI] [PubMed] [Google Scholar]
- 51. Dobson SJA, Jayaprakasan KM. Aetiology of recurrent miscarriage and the role of adjuvant treatment in its management: a retrospective cohort review. J Obstet Gynaecol. 2018;38(7):967‐974. [DOI] [PubMed] [Google Scholar]
- 52. Andersen SL, Andersen S, Carlé A, et al. Pregnancy week‐specific reference ranges for thyrotropin and free thyroxine in the north denmark region pregnancy cohort. Thyroid. 2019;29(3):430‐438. [DOI] [PubMed] [Google Scholar]
- 53. Marai I, Carp H, Shai S, Shabo R, Fishman G, Shoenfeld Y. Autoantibody panel screening in recurrent miscarriages. Am J Reprod Immunol. 2004;51(3):235‐240. [DOI] [PubMed] [Google Scholar]
- 54. Stagnaro‐Green A, Roman SH, Cobin RH, El‐Harazy E, Alvarez‐Marfany M, Davies TF . Detection of at‐risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA. 1990;264(11):1422‐1425. [PubMed] [Google Scholar]
- 55. Lejeune B, Grun JP, de Nayer P, Servais G, Glinoer D. Antithyroid antibodies underlying thyroid abnormalities and miscarriage or pregnancy induced hypertension. Br J Obstet Gynaecol. 1993;100(7):669‐672. [DOI] [PubMed] [Google Scholar]
- 56. Toth B, Würfel W, Bohlmann M, et al. Recurrent miscarriage: Diagnostic and therapeutic procedures. Guideline of the DGGG, OEGGG and SGGG (S2k‐Level, AWMF Registry Number 015/050). Geburtshilfe Frauenheilkd. 2018;78(4):364‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jaslow CR, Carney JL, Kutteh WH. Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil Steril. 2010;93(4):1234‐1243. [DOI] [PubMed] [Google Scholar]
- 58. Rao M, Zeng Z, Zhou F, et al. Effect of levothyroxine supplementation on pregnancy loss and preterm birth in women with subclinical hypothyroidism and thyroid autoimmunity: a systematic review and meta‐analysis. Hum Reprod Update. 2019;25(3):344‐361. [DOI] [PubMed] [Google Scholar]
- 59. Rao M, Zeng Z, Zhao S, Tang L. Effect of levothyroxine supplementation on pregnancy outcomes in women with subclinical hypothyroidism and thyroid autoimmuneity undergoing in vitro fertilization/intracytoplasmic sperm injection: an updated meta‐analysis of randomized controlled trials. Reprod Biol Endocrinol. 2018;16(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li J, Shen J, Qin L. Effects of levothyroxine on pregnancy outcomes in women with thyroid dysfunction: a meta‐analysis of randomized controlled trials. Altern Ther Health Med. 2017;23(2):49‐58. [PubMed] [Google Scholar]
- 61. Roberts CP, Murphy AA. Endocrinopathies associated with recurrent pregnancy loss. Semin Reprod Med. 2000;18(4):357‐362. [DOI] [PubMed] [Google Scholar]
- 62. Noyes N, Liu HC, Sultan K, Schattman G, Rosenwaks Z. Endometrial thickness appears to be a significant factor in embryo implantation in in‐vitro fertilization. Hum Reprod. 1995;10(4):919‐922. [DOI] [PubMed] [Google Scholar]
- 63. Kovachev E, Ganchev Z, Cherneva S, Zokhav E, Shperberg A. Measurement of endometrial volume and endometrial thickness for assessment of endometrial receptivity in assisted reproductive techniques. Akush Ginekol (Sofiia). 2005;44(Suppl 2):27‐33. [PubMed] [Google Scholar]
- 64. Zhong Y‐P, Ying Y, Wu H‐T, et al. Relationship between antithyroid antibody and pregnancy outcome following in vitro fertilization and embryo transfer. Int J Med Sci. 2012;9(2):121‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dorostghoal M, Ghaffari H‐OA, Marmazi F, Keikhah N. Overexpression of endometrial estrogen receptor‐alpha in the window of implantation in women with unexplained infertility. Int J Fertil Steril. 2018;12(1):37‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wu Z, Cai Y, Xia Q, et al. Hashimoto's thyroiditis impairs embryo implantation by compromising endometrial morphology and receptivity markers in euthyroid mice. Reprod Biol Endocrinol. 2019;17(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Negro R, Mangieri T, Coppola L, et al. Levothyroxine treatment in thyroid peroxidase antibody‐positive women undergoing assisted reproduction technologies: a prospective study. Hum Reprod. 2005;20(6):1529‐1533. [DOI] [PubMed] [Google Scholar]
- 68. Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab. 2006;91(7):2587‐2591. [DOI] [PubMed] [Google Scholar]
- 69. Litwicka K, Arrivi C, Varricchio MT, Mencacci C, Greco E. In women with thyroid autoimmunity, does low‐dose prednisolone administration, compared with no adjuvant therapy, improve in vitro fertilization clinical results? J Obstet Gynaecol Res. 2015;41(5):722‐728. [DOI] [PubMed] [Google Scholar]
- 70. Vaquero E, Lazzarin N, De Carolis C, Valensise H, Moretti C, Ramanini C. Mild thyroid abnormalities and recurrent spontaneous abortion: diagnostic and therapeutical approach. Am J Reprod Immunol. 2000;43(4):204‐208. [DOI] [PubMed] [Google Scholar]
- 71. Sher G, Maassarani G, Zouves C, et al. The use of combined heparin/aspirin and immunoglobulin G therapy in the treatment of in vitro fertilization patients with antithyroid antibodies. Am J Reprod Immunol. 1998;39(4):223‐225. [DOI] [PubMed] [Google Scholar]
- 72. Velkeniers B, Van Meerhaeghe A, Poppe K, Unuane D, Tournaye H, Haentjens P. Levothyroxine treatment and pregnancy outcome in women with subclinical hypothyroidism undergoing assisted reproduction technologies: systematic review and meta‐analysis of RCTs. Hum Reprod Update. 2013;19(3):251‐258. [DOI] [PubMed] [Google Scholar]
- 73. Chiovato L, Lapi P, Fiore E, Tonacchera M, Pinchera A. Thyroid autoimmunity and female gender. J Endocrinol Invest. 1993;16(5):384‐391. [DOI] [PubMed] [Google Scholar]
- 74. Practice Committee of the American Society for Reproductive M . Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545‐553. [DOI] [PubMed] [Google Scholar]
- 75. Shields BM, Knight BA, Hill A, Hattersley AT, Vaidya B. Fetal thyroid hormone level at birth is associated with fetal growth. J Clin Endocrinol Metab. 2011;96(6):E934‐E938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Poppe K, Glinoer D, Tournaye H, et al. Assisted reproduction and thyroid autoimmunity: an unfortunate combination? J Clin Endocrinol Metab. 2003;88(9):4149‐4152. [DOI] [PubMed] [Google Scholar]
- 77. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27(3):315‐389. [DOI] [PubMed] [Google Scholar]
- 78. Mintziori G, Goulis DG. In vitro fertilization/intracytoplasmic insemination and thyroid function: reviewing the evidence. Metabolism. 2018;86:44‐48. [DOI] [PubMed] [Google Scholar]
- 79. Polyzos NP, Valachis A, Patavoukas E, et al. Publication bias in reproductive medicine: from the European Society of Human Reproduction and Embryology annual meeting to publication. Hum Reprod. 2011;26(6):1371‐1376. [DOI] [PubMed] [Google Scholar]
- 80. Glinoer D, Riahi M, Grün JP, Kinthaert J. Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. J Clin Endocrinol Metab. 1994;79(1):197‐204. [DOI] [PubMed] [Google Scholar]


