Abstract
Atypical EEG patterns not consistent with standard sleep staging criteria have been observed in medical intensive care unit (ICU) patients. Our aim was to examine the relationship between sleep architecture and sedation in critically ill mechanically ventilated patients pre‐ and post‐extubation. We performed a prospective observational repeated measures study where 50 mechanically ventilated patients with 31 paired analyses were examined at an academic medical centre. The sleep efficiency was 58.3 ± 25.4% for intubated patients and 45.6 ± 25.4% for extubated patients (p = .02). Intubated patients spent 76.33 ± 3.34% of time in non‐rapid eye movement (NREM) sleep compared to 64.66 ± 4.06% of time for extubated patients (p = .02). REM sleep constituted 1.36 ± 0.67% of total sleep time in intubated patients and 2.06 ± 1.09% in extubated patients (p = .58). Relative sleep atypia was higher in intubated patients compared to extubated patients (3.38 ± 0.87 versus 2.79 ± 0.42; p < .001). Eleven patients were sedated with propofol only, 18 patients with fentanyl only, 11 patients with fentanyl and propofol, and 10 patients had no sedation. The mean sleep times on “propofol”, “fentanyl”, “propofol and fentanyl,” and “no sedation” were 6.54 ± 0.64, 4.88 ± 0.75, 6.20 ± 0.75 and 4.02 ± 0.62 hr, respectively. The sigma/alpha values for patients on “propofol”, “fentanyl”, “propofol and fentanyl” and “no sedation” were 0.69 ± 0.04, 0.54 ± 0.01, 0.62 ± 0.02 and 0.57 ± 0.02, respectively. Sedated patients on mechanical ventilation had higher sleep efficiency and more atypia compared to the same patients following extubation. Propofol was associated with higher sleep duration and less disrupted sleep architecture compared to fentanyl, propofol and fentanyl, or no sedation.
Keywords: intensive care unit, mechanical ventilation, sedation, sleep
1. INTRODUCTION
Restorative sleep is vital to adequate human functioning, yet sleep disturbances are common among patients in the intensive care unit (ICU) (Milbrandt et al., 2004; Tembo, Parker, & Higgins, 2013). Environmental stimuli (Freedman, Gazendam, Levan, Pack, & Schwab, 2001; Meyer et al., 1994), medication effects (Weinhouse & Watson, 2011) and severe illness (Cooper et al., 2000) contribute to sleep deprivation. Several studies have shown that critically ill patients have increased N1 sleep, as well as a paucity of rapid eye movement (REM) sleep and slow‐wave sleep (non‐rapid eye movement [NREM] sleep stages III and IV) (De Jong et al., 2005; Freedman et al., 2001; Meyer et al., 1994). This has been shown to promote wakefulness, sleep fragmentation and non‐restorative sleep.
Atypical sleep electroencephalogram (EEG) patterns that do not meet standard sleep‐staging criteria have been inconsistently described in this patient population. This largely stems from limited data characterizing sleep architecture in ICU patients. Conventional polysomnography (PSG) has thus far proven difficult in the critically ill. The majority of PSG studies to date have been conducted in non‐sedated critical care patients with and without invasive mechanical ventilation (IMV) (Cabello et al., 2008; Roche‐Campo et al., 2013). Finally, the data on whether IMV contributes to sleep deprivation are controversial (Cooper et al., 2000; Parthasarathy & Tobin, 2002).
Sleep deprivation may be a risk factor for delirium and prolonged mechanical ventilation, both of which have been independently linked to ICU mortality and length of ICU stay (Lin et al., 2004; Milbrandt et al., 2004; Ouimet, Kavanagh, Gottfried, & Skrobik, 2007). However, the potential effect of dysregulated sleep on health outcomes in this patient population has yet to be elucidated. In this prospective cohort study with paired analysis, we sought to compare sleep architecture in critically ill medical patients during and after mechanical ventilation, using individual patients as their own controls. Our aim is to examine the relationship between sleep architecture, sedation and mortality in the ICU.
2. MATERIALS AND METHODS
2.1. Study participants
We enrolled 50 patients requiring IMV who were admitted to the Medical Intensive Care Unit (MICU) at Mount Sinai St. Luke's and Mount Sinai West hospitals, in whom reliable overnight EEG recording could be obtained. Exclusion criteria included patients younger than 18 years old, patients with an anticipated ICU stay <48 hr, patients with underlying neurological or known sleep disorders (by medical history), patients demonstrating haemodynamic instability, and patients with a Glasgow Coma Scale (GCS) score of <10. The Institutional Review Board of Mount Sinai St. Luke's and Mount Sinai West hospitals reviewed and approved the study protocols. Written informed consent to participate in the study was obtained from all patients or their authorized substitute decision maker.
2.2. Design
For each patient, two overnight EEGs were acquired, while intubated and post‐extubation. In this way patients served as their own controls. The decision to initiate sedation or analgesia was based on the recommendations of the ICU staff in accordance with standard protocols (e.g., Richmond Agitation‐Sedation Scale) to guide the level of sedation. Patients received propofol or fentanyl in isolation or concurrently, while others received no sedation.
2.3. Data acquisition
Overnight EEG recordings were made with the Sleep Profiler™ EEG Sleep Monitor (Advanced Brain Monitoring, Carlsbad, CA) (Levendowski et al., 2017). Snoring and environmental noise were measured with an acoustic microphone, whereas head movement and head position were derived from a triaxial accelerometer. The EEG monitor was applied to the forehead around 20:00 hours and the device was removed in the morning with the time selection chosen for convenience. The recordings were transmitted to the portal and software was used to review the signals for quality and visual confirmation of auto‐staging. Signals acquired from the forehead provided total time and percentage sleep, rapid eye movement (REM) and slow wave sleep (SWS), sleep efficiency, and total and average number of cortical, sympathetic and behavioural arousals. The Sleep Efficiency Index in this study was the ratio of total sleep time (based on EEG recordings) to time in bed (period between 20:00 hours and 08:00 hours).
The Sleep Profiler auto‐staging algorithms, developed to be consistent with the AASM scoring rules (Levendowski, Popovic, Berka, & Westbrook, 2012; Pisani et al., 2015; Stepnowsky, Levendowski, Popovic, Ayappa, & Rapoport, 2013), have been previously described in detail and validated in previous studies (Cooper et al., 2000; Finan et al., 2016; Kamdar et al., 2013; Weinhouse & Schwab, 2006). The system captured 30‐s epochs and used predefined frequency bins to automatically detect REM sleep, cortical and micro‐arousals, and stages of NREM sleep. Additionally, the power spectra bands used to stage sleep were normalized to derive ratios of delta/theta, sigma/alpha and sigma/beta (Figure 1).
Figure 1.
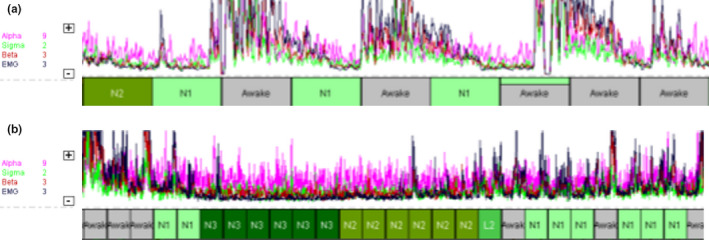
Relative changes in the alpha, sigma, beta and electromyography (EMG) power spectra identify repetitive disruptions of unknown origin that affected the patient's ability to fall or remain asleep, as shown on a 10‐min timescale (a) and a 30‐min timescale (b)
To facilitate comparison between groups, frequencies were converted to normalized ratios of delta/theta, sigma/alpha and sigma/beta. Epochs that were auto‐staged as NREM stage N3 as a result of large polymorphic delta activity, but with an electromyography (EMG) power that exceeded 75% of the presentation window, were reclassified as awake (Figure 2). Periods with burst suppression (Figure 3), unusual spikes and/or possible non‐convulsive seizure activity were allocated to the stage “sleep not‐otherwise‐specified”. Epochs that were auto‐staged as NREM stage N1 were treated as transitory because of the multitude of factors that influenced the EEG in the ICU, and excluded from the computation of sleep time.
Figure 2.
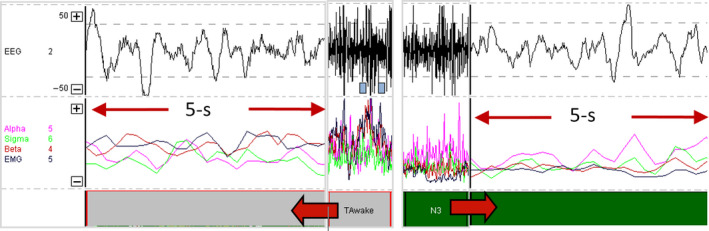
Polymorphic delta activity auto‐staged as N3 that was (a) manually edited to awake based on elevated gamma/electromyography (EMG) power or (b) unchanged
Figure 3.
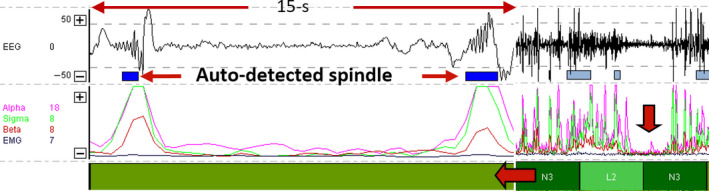
Patterns of (a) frontal burst suppression characterized as spindle activity based on the alpha (pink) and sigma (green) power spectra and auto‐staged N2, and (b) flat lining of the power spectra in a 10‐min timescale used to detect burst suppression activity
2.4. Data analysis
Across patients, aggregate statistics on sleep architecture and waveforms were used to investigate the role of sedation in sleep in intubated patients. Patients who died prior to extubation or were unable to participate after extubation were excluded from the paired analysis, but their readings were analysed to investigate the impact of critical illness and sedation on sleep in intubated patients. Significant differences in sleep duration were compared using a paired t test. The comparisons of no sedation, fentanyl only, propofol only or both fentanyl and propofol were carried out using the ANOVA test. Statistical significance was defined as an alpha value <.05. All statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC).
3. RESULTS
A total of 50 intubated patients were enrolled in the study, of whom 31 patients had completed both the intubation and post‐extubation studies. Among the remaining 19 patients, 16 patients died while intubated and three patients withdrew from the study after they were extubated. Of the 50 intubated patients enrolled in the study, 40 patients (80.0%) were chemically sedated during their sleep study. Of the 31 patients eligible for complete analysis, 48.4% were male (n = 15) and 51.6% were female (n = 16). The mean age of these patients was 67.6 years and the mean APACHE II score was 20.5. Eight patients (25.8%) died during their hospital stay (Table 1).
Table 1.
Baseline characteristics
| Variables | Participants (n = 31) |
|---|---|
| Male | 15 |
| Female | 16 |
| Age in years (mean) | |
| Male | 69.58 |
| Female | 65.65 |
| Primary diagnosis | |
| Sepsis/septic shock | 15 |
| Congestive heart failure/cardiogenic shock | 5 |
| Respiratory failure | 5 |
| Angioedema | 2 |
| Diabetic ketoacidosis | 1 |
| Upper gastrointestinal bleed | 1 |
| Apache (mean) | 20.58 |
| Male | 23.80 |
| Female | 17.56 |
| Pressors | |
| Yes | 11 |
| No | 20 |
| Febrile during study | |
| Yes | 5 |
| No | 26 |
| Mortality | |
| Died | 8 |
| Survived | 23 |
| Mean FiO2 | 44.19% |
| #Days following intubation (mean) | 2.83 |
| #Days following extubation (mean) | 2.35 |
3.1. Comparison of sleep architecture
Among the 31 patients analysed, the average total sleep times were 5.4 ± 2.5 hr for intubated patients and 4.2 ± 2.4 hr for extubated patients (p = .03). Sleep efficiency was 58.3 ± 25.4% for intubated patients and 45.6 ± 25.4% for extubated patients (p = .02) (Table 2). The ‘wake after sleep onset’ (WASO) time for intubated patients was 259 ± 122 min compared to 203 ± 126 min for extubated patients (p = .04). Intubated patients spent 76.63 ± 3.34% of their time in NREM sleep, whereas extubated patients spent 64.66 ± 4.06% of the time in NREM sleep (p = .02). Only a small percentage of time was spent in REM sleep in both subsets of patients: 1.36 ± 0.67% in intubated patients and 2.06 ± 1.09% in extubated patients (p = .58). Atypical sleep, defined as polymorphic delta/theta ratio, was also higher in intubated patients (3.38 ± 0.87, compared to extubated patients 2.79 ± 0.42) and was statistically significant (p < .001). The differences in sigma/alpha and sigma/beta values between intubated and extubated cohorts were not significantly different (sigma/alpha p = .89 and sigma/beta p = .86).
Table 2.
Sleep quantity and quality in mechanically ventilated patients in the intensive care unit
| Variables | Intubated | Extubated | p value |
|---|---|---|---|
| Sleep duration (hr) | 5.4 ± 2.5 | 4.2 ± 2.4 | .036 |
| Sleep efficiency (%) | 58.3 ± 25.4 | 45.6 ± 24.4 | .025 |
| Wake time (hr) | 3.8 ± 2.3 | 4.9 ± 2.3 | .023 |
| Wake time after sleep onset (min) | 203 ± 126 | 259 ± 122 | .04 |
| Arousal index | 4.5 ± 2.5 | 6.6 ± 3.8 | .008 |
| Delta/theta wave ratio | 3.38 ± 0.87 | 2.79 ± 0.42 | <.001 |
3.2. Effect of sedation on sleep architecture
The effect of sedation on sleep architecture in intubated patients was studied in 50 intubated patients. For this analysis, we compared four groups: no sedation (n = 10), fentanyl only (n = 18), propofol only (n = 11) and both fentanyl and propofol (n = 11). The mean sleep time between patients without sedation, with fentanyl only, with propofol only and with both fentanyl and propofol was 4.02 ± 0.62, 4.88 ± 0.75, 6.54 ± 0.64 and 6.20 ± 0.75, respectively. The percentages of time spent in REM sleep for patients on no sedation, fentanyl only, propofol only and both fentanyl and propofol were 0.17 ± 0.11, 0.31 ± 0.03%, 1.77 ± 1.63% and 0.31 ± 0.03%, respectively. The structure of non‐REM (NREM) sleep spectra was less disrupted in the subjects receiving only propofol and most disrupted in the group receiving only fentanyl (sigma/alpha values in patients with propofol only 0.69 ± 0.04 versus fentanyl only 0.54 ± 0.01, p = .003). Moreover, subjects receiving no sedation, as well as those receiving both fentanyl and propofol, had more atypical sleep architecture compared to those receiving propofol alone (delta/theta values in patients with no sedation 2.82 ± 0.19 versus fentanyl/propofol 3.67 ± 0.37 versus propofol only 3.22 ± 0.23, p = .003).
4. DISCUSSION
The utilization of conventional PSG in the ICU has demonstrated that critically ill patients experience severe sleep fragmentation and distorted sleep architecture characterized by a reduction in stage 3 NREM sleep and REM sleep with a concomitant abundance of stage 1 or light sleep (Pisani et al., 2015; Weinhouse & Schwab, 2006). These changes have been shown to adversely affect patient outcomes; thus, there is a heightened interest in various quality improvement measures regarding the ICU environment to prevent sleep disruption (Kamdar et al., 2013). In spite of a growing body of literature examining the correlation between environmental stimuli and sleep disturbances, there is a paucity of data reflecting the effect of mechanical ventilation (MV) on sleep parameters. Cooper et al. (2000) established that patients on mechanical ventilation exhibit distorted sleep architecture akin to other critically ill populations, including an abundance of “atypical sleep”. There are suggestions that these atypical polysomnographic findings necessitate a revised sleep scoring scheme in critically ill mechanically ventilated patients (Ambrogio, Koebnick, Quan, Ranieri, & Parthasarathy, 2008; Watson et al., 2013). For instance, benzodiazepines and propofol, both gamma‐Aminobutyric acid agonists, have been found to increase stage 1 sleep while decreasing slow wave sleep and REM sleep (Pandharipande & Ely, 2006; Weinhouse & Watson, 2009). Conversely, appropriate sedation and analgesia can also mitigate arousals and awakenings attributable to the ICU environment and patient‐care activities, although the impact of environmental stimuli on sleep disturbance in the ICU has been shown to be overstated (Gabor et al., 2003). Furthermore, the cessation of sedatives in patients following MV can predispose them to poor sleep quality by precipitating an acute withdrawal syndrome (Cammarano, Pittet, Weitz, Schlobohm, & Marks, 1998).
Our understanding of sleep alterations in critically ill patients has grown considerably over the past two decades. In this study, we sought to further investigate the impact of mechanical ventilation on sleep in the ICU, and examine the effects of sedation on sleep quality. Based on EEGs, patients slept significantly more while sedated on mechanical ventilation than following extubation and had higher sleep efficiency in this critically ill cohort. This is contrary to the notion that sedation during IMV dampens sleep efficiency and IMV is associated with sleep deprivation in the ICU (Rotondi et al., 2002). Our results are consistent with the findings of Fanfulla et al., who found that sleep duration and quality were not appreciably different in patients on mechanical ventilation compared to those spontaneously breathing in a step‐down unit (Fanfulla et al., 2011). Moreover, Roche‐Campo et al. (2013) documented that mechanical ventilation was associated with increased total sleep time in a group of tracheostomy patients in a randomized crossover trial, although that cohort was off sedation.
Although sleep architecture revealed marked atypia in both intubated and extubated patients consistent with previous studies (Ambrogio et al., 2008; Cooper et al., 2000; Watson et al., 2013), the delta/theta ratio was higher in intubated patients. One possible explanation for the augmented sleep quality seen during MV could stem from a reduced workload of breathing and more efficient gas exchange compared to spontaneous ventilation (SV) (Roche‐Campo et al., 2013). Improved patient–ventilator synchrony with modern modes of mechanical ventilation may also encourage sleep by reducing the frequency of awakenings (Bosma et al., 2007; Rittayamai et al., 2016). Another plausible explanation is that patients recovering from critical illness shortly following extubation are prone to experiencing anxiety and stress, resulting in dyspnoea, which may contribute to a reduced quality of sleep (Rittayamai et al., 2016; Schmidt et al., 2011). Additionally, IMV can mask baseline sleep‐related breathing disorders common to the general population, including obstructive and central sleep apnea. In a trial performed by Parthasarathy et al., 11 patients underwent PSG using assist control ventilation (ACV), pressure support ventilation (PSV) and PSV with dead space (Parthasarathy & Tobin, 2002). Sleep fragmentation was more pronounced during PSV compared to ACV, which was attributed to an increase in central apneas. During ACV no apneas were documented and the introduction of dead space to PSV decreased the incidence of central apneas. All patients included in our study were ventilated via pressure‐related volume control (PRVC), which is analogous to assist‐control ventilation; however, inspiratory time and flow are regulated to minimize plateau pressure. If this postulation holds, practitioners should be vigilant in evaluating extubated patients for baseline sleep‐disordered breathing and utilize non‐invasive ventilation if necessary (Rittayamai et al., 2016). Mechanically ventilated patients on propofol displayed less sleep distortion compared to those receiving fentanyl or no sedation. Although various studies have compared benzodiazepines to other sedatives, we are among the first to compare sleep quality in critically ill patients sedated using propofol and fentanyl. The tendency for less sleep fragmentation with propofol in comparison to fentanyl could be attributed to its favourable pharmacokinetics. With a shorter half‐life, higher clearance rate and smaller volume of distribution, it might be easier to titrate propofol and establish an effective level of sedation to promote sleep (Wagner & O'Hara, 1997). This discrepancy may also be due to differences in mechanisms and sites of action in the brain. Furthermore, in studies conducted on rats, Tung et al. demonstrated that continuous sedation with propofol was comparable to naturally occurring sleep and may even be regulated in a similar fashion (Tung, Bergmann, Herrera, Cao, & Mendelson, 2004; Tung, Lynch, & Mendelson, 2001). Conversely, a randomized crossover study conducted by Kondili, Alexopoulou, Xirouchaki, & Georgopoulos (2012) found that patients on MV sedated with propofol exhibited suppressed REM sleep and worsening sleep quality compared to MV patients without sedation. The design and patient population of this study may explain the contrasting findings.
In a previous evaluation of sleep patterns in the ICU, Watson et al. (19) described six visual scored patterns of atypical ICU sleep which could not be staged according to the conventional standards (i.e., American Academy of Sleep Medicine (AASM) sleep staging criteria).(34) Many of the atypical ICU sleep patterns described by Watson, however, were recognized in the frontopolar EEG signals when the autostaging was combined with visual inspection (19). In our study, when patients were sedated and asleep, the non‐REM epochs were typically assigned stage light N2. The characterization of light N2, that is, conventionally staged N2 with K‐complex, or dominant theta activity with relatively elevated levels of alpha or EMG power, and absence of spindle activity, is similar to Watson's description of atypical stage A1. Burst‐suppression activity, labelled by Watson as atypical stage A4, was auto‐staged N2 due to the bursts in alpha and sigma power being misclassified as sleep spindles. When the signals were viewed on a timescale of 10 m or greater, burst suppression was recognized by the marked reduction when the alpha, sigma, beta and EMG power was interspersed between the sleep spindles (Figure 3). Uni‐ and bilateral, non‐convulsive epileptiform activity of various amplitudes recognized during visual inspection was typically auto‐staged as invalid when the large amplitude spikes were rejected, or staged awake.
Our study has several strengths, including that it explores, through a prospective study design with patient self‐controls, a clinical topic that has not been extensively studied. To our knowledge, this study is among the first to objectively analyse sleep architecture using continuous sleep monitoring in sedated and non‐sedated patients on mechanical ventilation, as well as following extubation. Prolonged sedation in the ICU has been linked to the development of delirium and sedatives have previously been shown to have detrimental effects on sleep architecture. However, our findings suggest that hypnotic sedatives, such as propofol, may have a more favourable effect on sleep efficiency than opioid analgesics alone. Although a causal relationship cannot be clearly elucidated and confounding environmental factors cannot be excluded, these data may prompt the clinician to not use opioid analgesics as a single agent for ventilated patients. In this study, we demonstrated that the type of sedation that patients received while on MV affected various sleep parameters. Clinicians should be cognisant of the impact of sedatives on sleep and consider minimizing the use of sedatives whenever possible. A sedation vacation offers an objective approach to constantly reevaluate the need for continuous sedation. The finding of less sleep distortion with propofol compared to fentanyl suggests an easily applicable intervention at the bedside if the finding of this pilot study is replicated in larger follow‐up studies. Further research using other sedatives, including amnestic agents (i.e., ketamine) as well as non‐amnestic agents (i.e., dexmedetomidine), will be useful in identifying modifiable factors that portend to atypical sleep.
It should be noted, however, that this study has several limitations. First, the small sample size hinders the external validity and generalizability of our data. Further studies conducted on a larger sample are warranted to further control for confounders, including baseline comorbidities and severity of illness. Second, although patients with severe underlying neurological disorders, including massive CVA, active seizures or CNS tumours, were excluded from our study, the administration of medications known to disrupt sleep architecture such as antiarrhythmics and psychotropics was not part of the exclusion criteria. Third, sleep studies on extubated patients were occasionally performed several days after liberation from the ventilator, as a result of medical care responsibilities and patient preference. Because severity of illness has been linked to sleep disturbances and may be decreased in the latter stages of the ICU course, this could conceivably affect our data. However, our results indicate that patients exhibited better sleep quality while on IMV shortly after admission to the ICU, mitigating this potential bias. Finally, sedation and analgesia in our patients was achieved using propofol and fentanyl, which would limit the generalizability of our results, with newer agents such as dexmedetomidine becoming more ubiquitous in the ICU.
5. CONCLUSION
In conclusion, sedated patients on IMV had higher sleep efficiency and more atypical polymorphic delta activity as compared to the same patient cohort following extubation. Given that sleep deprivation has been associated with the onset of delirium and possibly increased length of stay in the ICU, further research evaluating sleep disturbances and safe approaches to sedation among ICU patients is warranted.
CONFLICTS OF INTEREST
All authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
CDG, RJ and PO were involved in the conception, hypothesis delineation and design of the study. EY, PS, FG, KG, AB, IV and MM were involved in the acquisition of the data. DL performed the data analysis. All authors contributed to the clinical content of the manuscript. CDG drafted the submitted article and revised it critically for important clinical content. RJ had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Jean R, Shah P, Yudelevich E, et al. Effects of deep sedation on sleep in critically ill medical patients on mechanical ventilation. J Sleep Res. 2020;29:e12894 10.1111/jsr.12894
Funding information: We would like to thank Dr Hassan Khouli and the Department of Pulmonary and Critical Care Medicine for the financial support of the proposed research.
REFERENCES
- Ambrogio, C. , Koebnick, J. , Quan, S. F. , Ranieri, M. , & Parthasarathy, S. (2008). Assessment of sleep in ventilator‐supported critically III patients. Sleep, 31(11), 1559–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma, K. , Ferreyra, G. , Ambrogio, C. , Pasero, D. , Mirabella, L. , Braghiroli, A. , … Ranieri, V. M. (2007). Patient‐ventilator interaction and sleep in mechanically ventilated patients: Pressure support versus proportional assist ventilation. Critical Care Medicine, 35(4), 1048–1054. [DOI] [PubMed] [Google Scholar]
- Cabello, B. , Thille, A. W. , Drouot, X. , Galia, F. , Mancebo, J. , d'Ortho, M. P. , Brochard, L. (2008). Sleep quality in mechanically ventilated patients: Comparison of three ventilatory modes. [Comparative Study Research Support, Non‐U.S. Gov't.] Critical Care Medicine, 36(6), 1749–1755. [DOI] [PubMed] [Google Scholar]
- Cammarano, W. B. , Pittet, J. F. , Weitz, S. , Schlobohm, R. M. , & Marks, J. D. (1998). Acute withdrawal syndrome related to the administration of analgesic and sedative medications in adult intensive care unit patients. Critical Care Medicine, 26(4), 676–684. [DOI] [PubMed] [Google Scholar]
- Cooper, A. B. , Thornley, K. S. , Young, G. B. , Slutsky, A. S. , Stewart, T. E. , & Hanly, P. J. (2000). Sleep in critically ill patients requiring mechanical ventilation. Chest, 117(3), 809–818. [DOI] [PubMed] [Google Scholar]
- De Jong, M. M. , Burns, S. M. , Campbell, M. L. , Chulay, M. , Grap, M. J. , Pierce, L. N. , Simpson, T. (2005). Development of the American association of critical‐care nurses’ sedation assessment scale for critically ill patients. American Journal of Critical Care, 14(6), 531–544. [PubMed] [Google Scholar]
- Fanfulla, F. , Ceriana, P. , D'Artavilla Lupo, N. , Trentin, R. , Frigerio, F. , & Nava, S. (2011). Sleep disturbances in patients admitted to a step‐down unit after ICU discharge: The role of mechanical ventilation. Sleep, 34(3), 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan, P. H. , Richards, J. M. , Gamaldo, C. E. , Han, D. , Leoutsakos, J. M. , Salas, R. , Smith, M. T. (2016). Validation of a wireless, self‐application, ambulatory electroencephalographic sleep monitoring device in healthy volunteers. Journal of Clinical Sleep Medicine, 12(11), 1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman, N. S. , Gazendam, J. , Levan, L. , Pack, A. I. , & Schwab, R. J. (2001). Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. [Research Support, U.S. Gov't, P.H.S.] American Journal of Respiratory and Critical Care Medicine, 163(2), 451–457. [DOI] [PubMed] [Google Scholar]
- Gabor, J. Y. , Cooper, A. B. , Crombach, S. A. , Lee, B. , Kadikar, N. , Bettger, H. E. , Hanly, P. J. (2003). Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. American Journal of Respiratory and Critical Care Medicine, 167(5), 708–715. [DOI] [PubMed] [Google Scholar]
- Kamdar, B. B. , King, L. M. , Collop, N. A. , Sakamuri, S. , Colantuoni, E. , Neufeld, K. J. , … Needham, D. (2013). The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Critical Care Medicine, 41(3), 800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondili, E. , Alexopoulou, C. , Xirouchaki, N. , & Georgopoulos, D. (2012). Effects of propofol on sleep quality in mechanically ventilated critically ill patients: A physiological study. Intensive Care Medicine, 38(10), 1640–1646. [DOI] [PubMed] [Google Scholar]
- Levendowski, D. J. , Ferini‐Strambi, L. , Gamaldo, C. , Cetel, M. , Rosenberg, R. , & Westbrook, P. R. (2017). The accuracy, night‐to‐night variability, and stability of frontopolar sleep electroencephalography biomarkers. Journal of Clinical Sleep Medicine, 13(6), 791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levendowski, D. J. , Popovic, D. , Berka, C. , & Westbrook, P. R. (2012). Retrospective cross‐validation of automated sleep staging using electroocular recording in patients with and without sleep disordered breathing. International Archives of Medicine, 5(1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, S. M. , Liu, C. Y. , Wang, C. H. , Lin, H. C. , Huang, C. D. , Huang, P. Y. , Kuo, H. P. (2004). The impact of delirium on the survival of mechanically ventilated patients. [Research Support, Non‐U.S. Gov't Validation Studies.] Critical Care Medicine, 32(11), 2254–2259. [DOI] [PubMed] [Google Scholar]
- Meyer, T. J. , Eveloff, S. E. , Bauer, M. S. , Schwartz, W. A. , Hill, N. S. , & Millman, R. P. (1994). Adverse environmental conditions in the respiratory and medical ICU settings. [Research Support, Non‐U.S. Gov't.] Chest, 105(4), 1211–1216. [DOI] [PubMed] [Google Scholar]
- Milbrandt, E. B. , Deppen, S. , Harrison, P. L. , Shintani, A. K. , Speroff, T. , Stiles, R. A. , … Ely, E. W. (2004). Costs associated with delirium in mechanically ventilated patients. [Research Support, Non‐U.S. Gov't Research Support, U.S. Gov't, P.H.S.] Critical Care Medicine, 32(4), 955–962. [DOI] [PubMed] [Google Scholar]
- Ouimet, S. , Kavanagh, B. P. , Gottfried, S. B. , & Skrobik, Y. (2007). Incidence, risk factors and consequences of ICU delirium. Intensive Care Medicine, 33(1), 66–73. [DOI] [PubMed] [Google Scholar]
- Pandharipande, P. , & Ely, E. W. (2006). Sedative and analgesic medications: Risk factors for delirium and sleep disturbances in the critically ill. Critical Care Clinics, 22(2), 313–327, vii. [DOI] [PubMed] [Google Scholar]
- Parthasarathy, S. , & Tobin, M. J. (2002). Effect of ventilator mode on sleep quality in critically ill patients. American Journal of Respiratory and Critical Care Medicine, 166(11), 1423–1429. [DOI] [PubMed] [Google Scholar]
- Pisani, M. A. , Friese, R. S. , Gehlbach, B. K. , Schwab, R. J. , Weinhouse, G. L. , & Jones, S. F. (2015). Sleep in the intensive care unit. American Journal of Respiratory and Critical Care Medicine, 191(7), 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittayamai, N. , Wilcox, E. , Drouot, X. , Mehta, S. , Goffi, A. , & Brochard, L. (2016). Positive and negative effects of mechanical ventilation on sleep in the ICU: A review with clinical recommendations. Intensive Care Medicine, 42(4), 531–541. [DOI] [PubMed] [Google Scholar]
- Roche‐Campo, F. , Thille, A. W. , Drouot, X. , Galia, F. , Margarit, L. , Cordoba‐Izquierdo, A. , Brochard, L. (2013). Comparison of sleep quality with mechanical versus spontaneous ventilation during weaning of critically III tracheostomized patients. Critical Care Medicine, 41(7), 1637–1644. [DOI] [PubMed] [Google Scholar]
- Rotondi, A. J. , Chelluri, L. , Sirio, C. , Mendelsohn, A. , Schulz, R. , Belle, S. , … Pinsky, M. R. (2002). Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Critical Care Medicine, 30(4), 746–752. [DOI] [PubMed] [Google Scholar]
- Schmidt, M. , Demoule, A. , Polito, A. , Porchet, R. , Aboab, J. , Siami, S. , Sharshar, T. (2011). Dyspnea in mechanically ventilated critically ill patients. Critical Care Medicine, 39(9), 2059–2065. [DOI] [PubMed] [Google Scholar]
- Stepnowsky, C. , Levendowski, D. , Popovic, D. , Ayappa, I. , & Rapoport, D. M. (2013). Scoring accuracy of automated sleep staging from a bipolar electroocular recording compared to manual scoring by multiple raters. Sleep Medicine, 14(11), 1199–1207. [DOI] [PubMed] [Google Scholar]
- Tembo, A. C. , Parker, V. , & Higgins, I. (2013). The experience of sleep deprivation in intensive care patients: Findings from a larger hermeneutic phenomenological study. Intensive & Critical Care Nursing, 29(6), 310–316. [DOI] [PubMed] [Google Scholar]
- Tung, A. , Bergmann, B. M. , Herrera, S. , Cao, D. , & Mendelson, W. B. (2004). Recovery from sleep deprivation occurs during propofol anesthesia. Anesthesiology, 100(6), 1419–1426. [DOI] [PubMed] [Google Scholar]
- Tung, A. , Lynch, J. P. , & Mendelson, W. B. (2001). Prolonged sedation with propofol in the rat does not result in sleep deprivation. Anesthesia and Analgesia, 92(5), 1232–1236. [DOI] [PubMed] [Google Scholar]
- Wagner, B. K. , & O'Hara, D. A. (1997). Pharmacokinetics and pharmacodynamics of sedatives and analgesics in the treatment of agitated critically ill patients. Clinical Pharmacokinetics, 33(6), 426–453. [DOI] [PubMed] [Google Scholar]
- Watson, P. L. , Pandharipande, P. , Gehlbach, B. K. , Thompson, J. L. , Shintani, A. K. , Dittus, B. S. , … Ely, E. W. (2013). Atypical sleep in ventilated patients: Empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Critical Care Medicine, 41(8), 1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhouse, G. L. , & Schwab, R. J. (2006). Sleep in the critically ill patient. Sleep, 29(5), 707–716. [DOI] [PubMed] [Google Scholar]
- Weinhouse, G. L. , & Watson, P. L. (2009). Sedation and sleep disturbances in the ICU. Critical Care Clinics, 25(3), 539–549, ix. [DOI] [PubMed] [Google Scholar]
- Weinhouse, G. L. , & Watson, P. L. (2011). Sedation and sleep disturbances in the ICU. Anesthesiology Clinics, 29(4), 675–685. [DOI] [PubMed] [Google Scholar]


