Summary
Incorporating male sterility into hybrid seed production reduces its cost and ensures high varietal purity. Despite these advantages, male‐sterile lines have not been widely used to produce tomato (Solanum lycopersicum) hybrid seeds. We describe the development of a biotechnology‐based breeding platform that utilized genic male sterility to produce hybrid seeds. In this platform, we generated a novel male‐sterile tomato line by clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR‐associated protein 9 (Cas9)‐mediated mutagenesis of a stamen‐specific gene SlSTR1 and devised a transgenic maintainer by transforming male‐sterile plants with a fertility‐restoration gene linked to a seedling‐colour gene. Offspring of crosses between a hemizygous maintainer and the homozygous male‐sterile plant segregated into 50% non‐transgenic male‐sterile plants and 50% male‐fertile maintainer plants, which could be easily distinguished by seedling colour. This system has great practical potential for hybrid seed breeding and production as it overcomes the problems intrinsic to other male‐sterility systems and can be easily adapted for a range of tomato cultivars and diverse vegetable crops.
Keywords: Solanum lycopersicum, CRISPR/Cas9, male sterility, colour‐maintainer, hybrid seed production, technical advance
Significance Statement
This study describes a biotechnology‐based male sterility system, including a novel male‐sterile tomato line by CRISPR/Cas9‐mediated mutagenesis of a stamen‐specific gene, and a transgenic maintainer by transforming male‐sterile plants with a fertility‐restoration gene linked to a seedling‐colour gene. This system has great practical potential for hybrid seed breeding and can be easily adapted for a range of tomato cultivars and diverse vegetable crops.

Introduction
Most commercial varieties of vegetable and field crops are F1 hybrids that perform stably over a wide range of environments. Incorporating male sterility reduces the labour required for hybrid seed production and ensures high varietal purity (Chen and Liu, 2014; Kim and Zhang, 2018). Male sterility of tomato has been paid great attention by plant breeders since the first mutant was described in 1915, and the use of male sterility for producing tomato hybrid seeds has been widely discussed (Crane, 1915; Gorman and McCormick, 1997; Sawhney, 2004). To this purpose, different genes controlling male sterility including male‐sterile‐10 (ms‐10), ms‐15, ms‐32, positional sterile‐2 (ps‐2), exserted stigma (ex) and 7B‐1 have been studied, and several ideas and systems for their efficacious application have been developed and tested (Crane, 1915; Gorman and McCormick, 1997; Atanassova and Georgiev, 2002; Sawhney, 2004; Cheema and Dhaliwal, 2005; Gorguet et al., 2009; Jeong et al., 2014; Quinet et al., 2014; Zhang et al., 2016; Pucci et al., 2017; Cao et al., 2019; Liu et al., 2019). However, until present, male‐sterile lines have not been used on a large scale in tomato hybrid seed production.
Although many recessive genic male‐sterile tomato mutations are useful for producing hybrid seeds (Atanassova and Georgiev, 2002; Gorguet et al., 2009; Jeong et al., 2014; Quinet et al., 2014; Zhang et al., 2016; Pucci et al., 2017; Cao et al., 2019; Liu et al., 2019), two major factors have limited their commercial application: the difficulty of propagating large quantities of pure male‐sterile seeds (Perez‐Prat and van Lookeren Campagne, 2002; Chang et al., 2016; Wu et al., 2016); and the fact that traditional backcrossing requires a long time and many generations to transfer recessive male sterility into other genetic backgrounds. The classic approach to obtain seeds of homozygous male‐sterile plants is to cross‐pollinate homozygous male‐sterile plants (ms/ms) with heterozygote male‐fertile (Ms/ms) plants. Progeny from such a cross will segregate 50% male‐sterile (ms/ms) and 50% male‐fertile plants (Ms/ms). Use of linked genetic markers expressing at the seedling stage can help identify the male‐sterile plants from the segregating population prior to flowering. This was demonstrated effectively by linkage of ms‐10 and ms‐15 with ‘anthocyanin absent (aa)’ and ‘anthocyanin without (aw)’, which are responsible for the absence of anthocyanin pigment in seedlings (Jeong et al., 2014; Zhang et al., 2016; Cao et al., 2019). This approach, however, neither guarantees an accuracy rate of 100% for identifying the male‐sterile plants nor provides a practical way for other male‐sterile genes.
We have devised a biotechnology‐based male‐sterility platform for producing hybrid tomato seed. In this platform, we generated a novel male‐sterile tomato line by CRISPR/Cas9‐mediated mutagenesis (Chen et al., 2019; Mao et al., 2019) of a stamen‐specific gene, and devised a transgenic maintainer by transforming male‐sterile plants with a fertility‐restoration gene linked to a seedling‐colour gene. This system overcomes the problems innate to traditional male‐sterility systems, and can be easily transferred into new cultivars and other vegetable crops. It thus has great practical potential for hybrid seed breeding and production.
Results
Identification of tomato genes specifically expressed in stamens
To identify genes required for stamen development and male sterility in tomato, we used public RNA sequencing data (The Tomato Genome Consortium, 2012) from root, leaf, unopened flower buds, open flowers and fruit (six stages) to identify genes specifically expressed in unopened flower buds in which stamens are forming (Figure 1a). We devised the Bud Expression Index (BEI) to indicate quantitatively the specific expression of a gene in unopened flower buds. The BEI was defined as the ratio of the normalized‐RPKM (reads per kilobase million) in unopened flower buds to the sum of normalized‐RPKM in all tissues tested (Figure 1a). BEI ranged from 0 (not expressed in flower buds) to 1 (exclusively expressed in flower buds). Using BEI ≥ 0.5 as the threshold value, we identified 2044 genes that were predominantly expressed in flower buds compared with other tissues; that is, the RPKM in flower buds equalled or was higher than the sum of RPKM in other tissues. These 2044 genes were subjected to K‐means clustering analysis. After grouping these genes into different numbers of clusters, we found that grouping them into five clusters conveyed the major characteristic spatial expression patterns (Figure 1b). The most interesting cluster was cluster 3 (154 genes), because most genes in this cluster were highly and predominantly, if not exclusively, expressed in the flower bud (Figure 1c). As genes in cluster 3 were preferentially expressed in unopened flower buds but not in open flowers, it was reasonable to predict that they were specifically expressed in stamens. We randomly selected 21 genes from cluster 3, and measured their expression in different parts (sepal, petal, stamen and pistil) of 5‐mm flower buds using reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR). Most (20 genes) showed stamen‐specific expression (Figure S1), strongly supporting the conclusion that cluster 3 contained genes involved in stamen development and/or male sterility in tomato.
Figure 1.
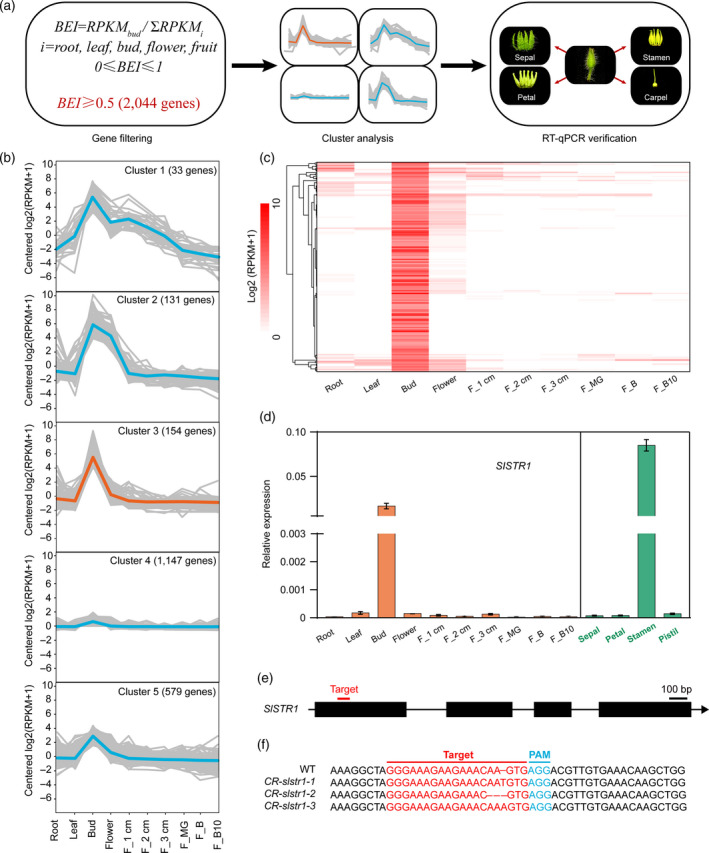
Identification of tomato genes specifically expressed in stamens.
(a) Methodology for identifying stamen‐specific genes. BEI, Bud Expression Index; RPKM, reads per kilobase million.
(b) Expression patterns of 2044 genes grouped in five clusters.
(c) Heatmap of expression of 154 genes from cluster 3 in different tissues.
(d) Expression of SlSTR1 in different tissues. F_1 cm, fruit 1 cm in diameter; F_2 cm, fruit 2 cm in diameter; F_3 cm, fruit 3 cm in diameter; F_MG, fruit at mature green stage; F_B, fruit at break stage; F_B10, fruit at 10 days post‐break stage.
(e) Schematic illustrating sgRNA (red line) targeting the SlSTR1 coding sequence.
(f) Representative mutant alleles of CR‐slstr1 identified from three T0 plants. Red, sgRNA target; blue, protospacer‐adjacent motif (PAM) sequences.
Generation of SlSTR1‐edited plants using CRISPR/Cas9 system
As a new strategy for generating tomato male‐sterile lines, we used the CRISPR/Cas9 system to disrupt a putative strictosidine synthase gene, SlSTR1 (Solyc03g053130), which was grouped into cluster 3 and showed a stamen‐specific expression pattern (Figure 1d). The tomato genome contains 14 putative strictosidine synthases, but a search of the public expression databases (Matas et al., 2011; The Tomato Genome Consortium, 2012) did not find any other SlSTRs showing a stamen‐specific expression pattern. The Cas9/sgRNA construct was designed to target a sequence in the first exon of SlSTR1 (positions 72–92 in the coding region; Figure 1e) that had no homology with other SlSTR genes. An elite tomato inbred line, TB0993, was transformed with the Cas9/sgRNA construct, and multiple chimeric T0 plants were obtained that contained various out‐of‐frame insertion and deletion alleles. Two T0 plants, CR‐slstr1‐2 and CR‐slstr1‐3, were self‐pollinated and allowed to produce T1 offspring. The segregation ratio of the Cas9 transgene was approximately 3:1 in the T1 offspring of CR‐slstr1‐3 (Figure S2), indicating a single transgenic locus. PCR, followed by sequencing of PCR products, was used to genotype 20 non‐transgenic T1 offspring of CR‐slstr1‐3. This identified four homozygous plants with an adenine insertion in the target sequence region of SlSTR1, including CR‐slstr1‐3‐6 (Figure S2). The same mutation was obtained by transforming the other two elite inbred lines TB0240 and TB0249 with the Cas9/sgRNA construct (Figure S2). This insertion was predicted to introduce a premature stop codon at amino acid position 49, supporting the idea that SlSTR1 was knocked‐out in these plants. CR‐slstr1‐3‐6 was selected for further analysis.
CR‐slstr1‐3‐6 plants are defective in pollen development and male fertility
There were no differences in growth and development between wild‐type (WT; TB0993) and CR‐slstr1‐3‐6 plants until the flowering stage. At flowering, the whole‐flower, sepal, petal and pistil phenotypes of CR‐slstr1‐3‐6 resembled those of WT plants (Figure 2a); however, CR‐slstr1‐3‐6 plants had much thinner anther cones (Figure 2b) with an average width about 70% that of WT cones (Figure 2c). CR‐slstr1‐3‐6 anthers produced visible pollen grains. When compared with WT pollen, CR‐slstr1‐3‐6 pollen grains were small, shrunken and had an abnormal shape, displaying a structurally weakened exine that led to easy collapse of the pollen grain (Figure 2d,e). To determine the viability of these abnormal pollen grains, a fluorescein diacetate (FDA) staining assay (Li, 2011), which measures cell viability, was performed with mature pollen grains released from anthers at the dehiscence stage. Pollen from WT flowers produced green fluorescence, whereas no signals were detected in CR‐slstr1‐3‐6 pollen grains (Figure 2f), indicating that they were not viable; consistent with this observation, CR‐slstr1‐3‐6 plants did not set fruit following self‐pollination (Figure 2g). Sterility did not result from defective female structures as CR‐slstr1‐3‐6 pistils pollinated with WT pollen produced normal, seed‐bearing fruit (Figure 2h,i). Defective pollen development and male fertility was also observed in CR‐slstr1‐1 and CR‐slstr1‐2 plants (Figures S3 and S4).
Figure 2.
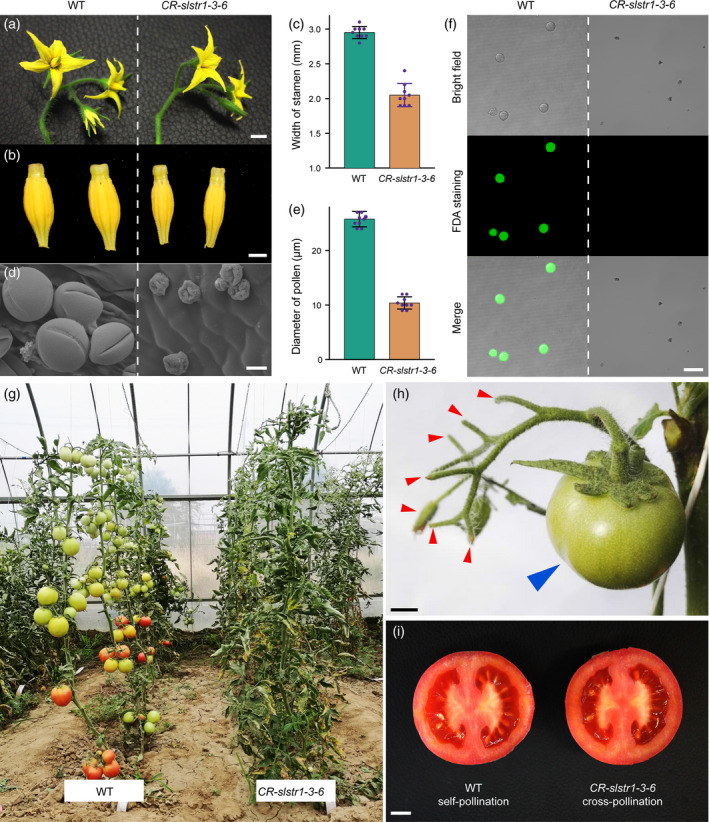
CR‐slstr1‐3‐6 plants are defective in pollen development and male fertility.
(a) Morphological comparison of wild‐type (WT) and CR‐slstr1‐3‐6 flowers.
(b) Morphology of detached WT and CR‐slstr1‐3‐6 anther cones; scale bar: 2 mm.
(c) Width of WT and CR‐slstr1‐3‐6 anther cones (mean ± SD; *significant difference from WT at P < 0.01).
(d) Scanning electron micrographs of WT and CR‐slstr1‐3‐6 pollen grains; scale bar: 10 μm.
(e) Diameter of WT and CR‐slstr1‐3‐6 pollen grains (mean ± SD; *significant difference from WT at P < 0.01).
(f) Viability of WT and CR‐slstr1‐3‐6 pollen grains at the dehiscence stage; scale bar: 50 μm.
(g) Defective fruitset in field‐grown CR‐slstr1‐3‐6 plants indicating male sterility.
(h) CR‐slstr1‐3‐6 plants do not set fruit following self‐pollination (red arrows); scale bar: 1 cm.
(i) CR‐slstr1‐3‐6 plants produce seed‐bearing fruit after cross‐pollination with WT pollen (blue arrow in h); scale bar: 1 cm.
F1 plants produced from backcrossing CR‐slstr1‐3‐6 plants with WT pollen produced normal pollen grains and were capable of self‐fertilization. In the F2 generation, male‐fertile and male‐sterile phenotypes segregated 216:68, a ratio of approximately 3:1, indicating that male sterility was caused by a single recessive mutation. To confirm that this was the CRISPR/Cas9‐mediated mutation in SlSTR1, we developed a Kompetitive Allele‐Specific PCR (KASP; Semagn et al., 2014) marker based on the mutation in the target sequence region, and used it to genotype the WT, CR‐slstr1‐3‐6 and F1 plants, as well as 142 individuals from the F2 population. The F2 plants were clearly grouped into the three genotypic classes defined by the WT, CR‐slstr1‐3‐6 and F1 plants (Figure S5). The mutant allele, genotyped using the KASP marker, co‐segregated with male sterility in the F2 population, supporting the conclusion that male sterility resulted from the CRISPR/Cas9‐mediated mutation in SlSTR1.
Generation of colour‐restorer lines of CR‐slstr1‐3‐6
CR‐slstr1‐3‐6 displayed several highly desirable traits of male‐sterile lines used in hybrid seed production. These included normal vegetative growth and female fertility but complete male sterility; recessive male sterility that could easily be restored in F1 hybrids; and suitability for emasculation during anthesis, which produced more seeds and required less time than emasculation of male‐fertile floral buds (Atanassova, 1999). Most importantly, although the yield performance of CR‐slstr1‐3‐6‐derived F1 hybrids was comparable to WT‐derived F1 hybrids, their seed purity was much higher (Figure S6). We therefore decided to develop a system for producing hybrid seeds using CR‐slstr1‐3‐6.
We first generated colour‐restorer lines of male‐sterile plants by transforming CR‐slstr1‐3‐6 with the T‐DNA binary vector pANT1‐COMP, which contained a fertility‐restoration gene linked to a seedling‐colour gene (Figure 3a). The T‐DNA contained three functional modules: NPTII under the CaMV 35S promoter for transformation selection; SlSTR1 under its native promoter to restore male fertility; and the tomato R2R3 MYB transcription factor gene ANT1 under the CaMV 35S promoter to mark transgenic plants at the seedling stage (Mathews et al., 2003). We generated nine T0 transgenic plants homozygous for the CR‐slstr1‐3‐6 mutation. All produced normal pollen grains and were allowed to self‐pollinate. Two independent transgenic lines, Comp 1# and Comp 2#, each harbouring a single T‐DNA copy, were selected for further analysis. Both transgenic lines showed enhanced anthocyanin synthesis, resulting in abundant anthocyanin pigmentation of cotyledons, leaves and anther cones of flowers (Figures 3b–d and S7), consistent with previous reports that ANT1 is an important regulator of anthocyanin synthesis. Weak anthocyanin pigmentation was also observed in fruits of the transgenic lines (Figure 3g). Unlike the male‐sterile CR‐slstr1‐3‐6 plants, both transgenic lines showed WT‐like anther cones, pollen shape and pollen viability (Figures 3e,f and S8). Furthermore, following self‐pollination, transgenic plants produced a similar number of seeds to WT plants, suggesting male fertility was fully restored (Figure 3g,h), and further confirming that it resulted from the SlSTR1 mutation.
Figure 3.
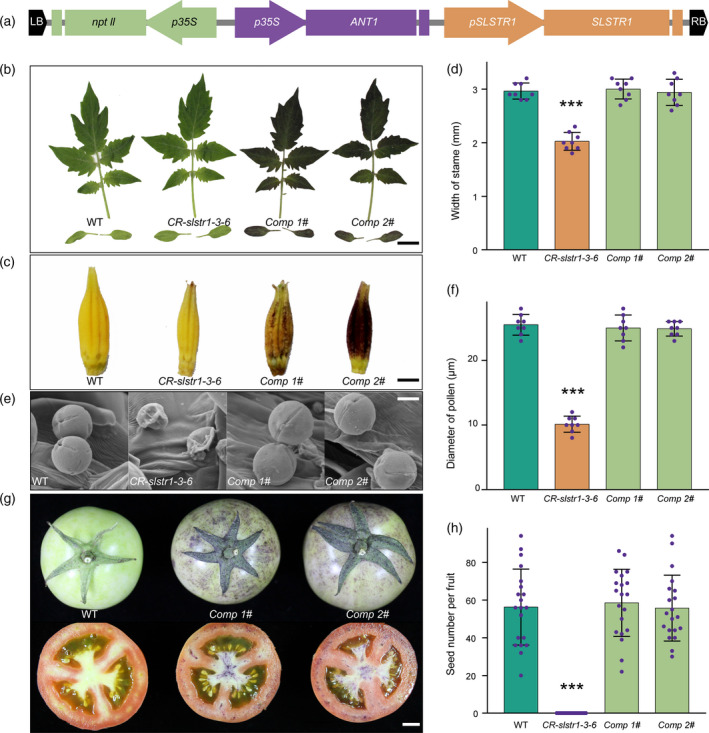
Generation of colour‐restorer lines of CR‐slstr1‐3‐6.
(a) Structure of the T‐DNA binary vector pANT1‐COMP used for plant transformation.
(b) Leaves and cotyledons from wild‐type (WT), CR‐slstr1‐3‐6, and Comp 1# and Comp 2# colour‐restorer lines; scale bar: 3 cm.
(c) Anther cones from WT, CR‐slstr1‐3‐6, and Comp 1# and Comp 2# colour‐restorer lines; scale bar: 2 mm.
(d) Width of anther cones from WT, CR‐slstr1‐3‐6 and colour‐restorer lines (mean ± SD; *significant difference from WT at P < 0.01).
(e) Scanning electron micrographs of pollen grains from WT, CR‐slstr1‐3‐6 and colour‐restorer lines; scale bar: 10 μm.
(f) Diameter of pollen grains from WT, CR‐slstr1‐3‐6 and colour‐restorer lines (mean ± SD; *significant difference from WT at P < 0.01).
(g) Fruits from WT and colour‐restorer lines; scale bar: 1 cm.
(h) Seed number per fruit in WT, CR‐slstr1‐3‐6 and colour‐restorer lines (mean ± SD; *significant difference from WT at P < 0.01).
A biotechnology‐based male‐sterility system for hybrid seed production
To propagate and identify male‐sterile plants for hybrid seed production, homozygous male‐sterile CR‐slstr1‐3‐6 plants (hereafter named TB0993‐S) were cross‐pollinated with pollen from the homozygous colour‐restorer lines Comp 1# and Comp 2# to generate a hemizygous colour‐maintainer line (hereafter named TB0993‐M). The progeny of a cross between TB0993‐M and TB0993‐S was predicted to segregate into 50% non‐transgenic male‐sterile plants and 50% transgenic male‐fertile plants, the two groups being easily distinguishable by seedling colour. One hemizygous colour‐maintainer plant generated by Comp 2# was selected and tested to determine if it could act as a maintainer. Cross‐pollination of TB0993‐S plants by TB0993‐M pollen produced 541 seedlings; 264 of these had purple cotyledons and 277 green (Figure 4a), a 1:1 ratio as predicted. Subsequent analysis revealed that seedlings with purple cotyledons were male‐fertile and genetically identical to TB0993‐M, whilst seedlings with green cotyledons were male‐sterile and genetically identical to TB0993‐S, confirming that TB0993‐M acted as a maintainer of TB0993‐S. The TB0993‐S and TB0993‐M seedlings could be easily distinguished 2–3 days post‐germination (Figure 4a). Visible anthocyanin pigmentation in TB0993‐M anthers provided another marker for distinguishing seedlings at the flowering stage. The produced TB0993‐S seedlings could be used for producing hybrid seeds at a commercial scale.
Figure 4.
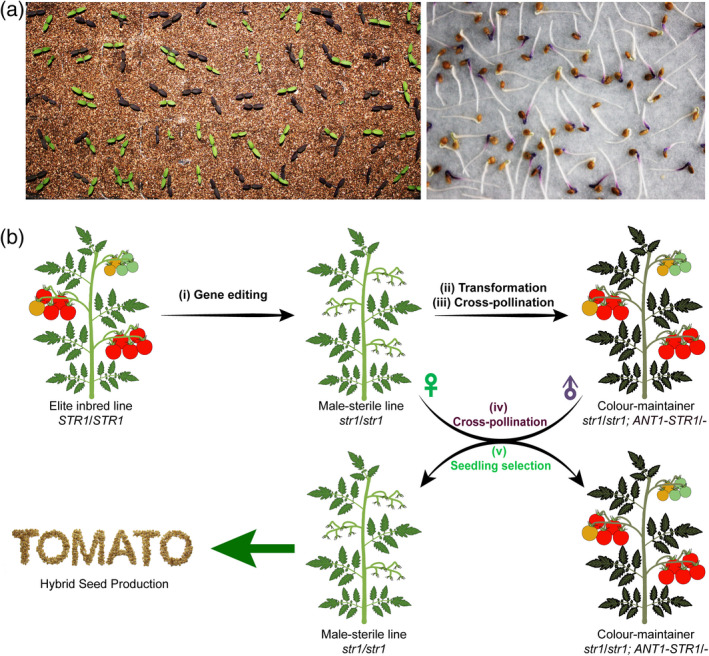
A biotechnology‐based male‐sterility system for hybrid seed production in tomato.
(a) Representative example for distinguishing male‐sterile plants (green cotyledons) and colour‐maintainers (purple cotyledons) at seedling stages. Left: seedlings 10 days post‐germination. Right: seedlings 3 days post‐germination.
(b) Schematic diagram of the nuclear gene male‐sterility system for hybrid seed production. (i) Male‐sterile lines were generated by CRISPR/Cas9‐mediated mutagenesis of SlSTR1 in an elite tomato inbred line; (ii) colour‐restorer lines were generated by transforming the fertility‐restoration gene linked with a seedling‐colour gene ANT1 into the male‐sterile plant; (iii) colour‐maintainer lines were generated by cross‐pollination of male‐sterile line with the pollen grains of colour‐restorer; (iv) propagation of male‐sterile plants by cross‐pollination of male‐sterile line with the pollen grains of colour‐maintainer; (v) the non‐transgenic male‐sterile plants can be easily identified and selected for hybrid seed production.
Discussion
One of the most prominent tools of plant breeding is the production of F1 hybrid seeds, which give rise to offspring with better characteristics in terms of yield, environmental fitness and disease resistance. For many crops, like rice, maize and tomato, emasculation of the female line by hand is required, raising costs and labour expenses of seed production. The utilization of male sterility is an efficient approach to reduce the cost of hybrid seeds and also ensure high varietal purity (Perez‐Prat and van Lookeren Campagne, 2002; Chen and Liu, 2014; Kim and Zhang, 2018). Tomato, with its wealth of spontaneous male‐sterile mutants, is a good system to study plant male reproduction and incorporate male sterility for hybrid seeds production (Gorman and McCormick, 1997; Atanassova, 2000; Atanassova and Georgiev, 2002; Sawhney, 2004). However, until present, male sterility has not been widely used in tomato hybrid seed production, due to the disadvantages of most natural male‐sterile mutants.
Traditional methods for exploitation of spontaneous genic male sterility, following a forward genetic approach, require a closely linked marker to assist people with identifying and backcrossing the male sterility into other cultivars. This requirement largely limits the application of most natural male‐sterile mutants in hybrid seed production. As significant progress has been made in the understanding of pollen development (Wilson and Zhang, 2009; Gomez et al., 2015; Shi et al., 2015) and targeted mutagenesis tools have been broadly applied in plant genetics and crop improvement (Chen et al., 2019; Mao et al., 2019), we proposed a reversed genetic approach to incorporate male sterility in hybrid seeds production. By this approach, a novel genic male‐sterile line can be easily generated by targeted mutagenesis of genes that are required for pollen development. As a case study, we disrupted a stamen‐expressed gene Solyc03g053130 in an elite tomato inbred line using the CRISPR/Cas9 genome editing system. Expectedly, the CRISPR/Cas9‐mediated mutation conferred tomato with severe defects in pollen development and male fertility. Based on the fact that pollen formation in plants is highly conserved at the biochemical and genetic level (Shi et al., 2015), it is reasonable to speculate that targeted mutagenesis of other stamen‐specific genes conserved across plants could potentially confer more desirable male sterility suitable for hybrid seeds production.
SlSTR1 is one of 14 tomato proteins that have been annotated as strictosidine synthase family proteins. Strictosidine synthase catalyses the Pictet–Spengler condensation of tryptamine and secologanin; in higher plants this reaction is central for the biosynthesis of a large number of alkaloids of the monoterpenoid indole family (Stockigt et al., 2008). However, whether SlSTR1 acts as a strictosidine synthase in pollen development is uncertain. Less Adherent Pollen 3 (LAP3), the Arabidopsis orthologue of SlSTR1, was previously reported to be involved in pollen development and proper exine formation (Dobritsa et al., 2009). lap3‐2 mutation leads to a broad range of metabolic changes, including levels of a straight‐chain hydrocarbon nonacosane and naringenin chalcone, an obligate compound in the flavonoid biosynthesis pathway. However, LAP3 lacks the key residues for substrate‐binding and the conserved catalytic residues found in all functionally active strictosidine synthases, casting substantial doubt on its role as a strictosidine synthase (Dobritsa et al., 2009). Similarly, the role of SlSTR1 as a strictosidine synthase is also doubtful. Besides LAP3, the SlSTR1 orthologue from maize, male‐sterile 45 (Ms45), is also required for pollen development and male fertility (Cigan et al., 2001). Both LAP3 and Ms45 are predominantly expressed in young anthers. The strong evolutionary conservation of protein structure and expression pattern of SlSTR1 orthologues make it a good candidate gene to be targeted to generate male sterility in other crop species (Dobritsa et al., 2009).
Commercial application of recessive genic male sterility is limited because of the difficulty to propagate a large quantity of pure male‐sterile seeds. The classic approach to obtain seeds of homozygous male‐sterile plants is to cross‐pollinate homozygous male‐sterile plants (ms/ms) with heterozygote male‐fertile (Ms/ms) plants. Progeny from such a cross will segregate 50% male‐sterile (ms/ms) and 50% male‐fertile plants (Ms/ms). To produce F1 hybrid seeds, the 50% of female Ms/ms plants must be rogued from the field as soon as their fertility can be identified, which is resource‐consuming and labour‐intensive. Use of linked genetic markers expressing at the seedling stage can help rogue fertile plants from female plants. This approach, however, does not provide a practical way for most male‐sterile mutants. In 2002, Perez‐Prat and van Lookeren Campagne proposed two strategies to obtain transgenic maintainers for propagating male‐sterile plants: one to transform the fertility restoration gene linked with a pollen‐lethality gene into the male‐sterile plant; and the other to transform the fertility‐restoration gene linked with a seed‐colour gene into the male‐sterile plant (Perez‐Prat and van Lookeren Campagne, 2002). Cross‐pollination of a hemizygous maintainer (ms/ms, Maintainer/−) to the male‐sterile line (ms/ms, −/−) would generate either a pure male‐sterile line (pollen‐lethality maintainer) or 50% of male‐sterile seeds and 50% of maintainer seeds (seed‐colour maintainers). Such transgenic maintainers have been recently constructed for utilizing nuclear male sterility to produce hybrids in maize and rice (Chang et al., 2016; Wu et al., 2016; Kim and Zhang, 2018; Zhang et al., 2018; Wan et al., 2019). Based on these ideas, we devised a tomato seedling‐colour maintainer by transforming the fertility‐restoration gene linked with the tomato ANT1 gene into the male‐sterile plant (Mathews et al., 2003). Our proof‐of‐concept experiments demonstrated that the transgenic maintainer completely restored the male fertility of the male‐sterile plants. Offspring of crosses between a hemizygous maintainer and the homozygous male‐sterile plant segregated into 50% non‐transgenic male‐sterile plants and 50% male‐fertile maintainer plants, which could be easily distinguished by seedling colour. These results indicated that this maintainer system can efficiently propagate the male‐sterile plants and is promising in application. Furthermore, the seedling‐colour maintainer system provides a practical way for the use of genic male‐sterile mutants in tomato, including the cloned ms‐10, ms‐15 and those generated by targeted mutagenesis (Jeong et al., 2014; Cao et al., 2019).
Collectively, we report the development of a biotechnology‐based genetic male‐sterility system involving the generation of a novel male‐sterile tomato line by CRISPR/Cas9‐mediated mutagenesis of a stamen‐specific gene and a seedling‐colour maintainer, constructed to propagate and identify male‐sterile plants at the seedling stage (Figure 4b). We describe the key steps in the development of this system, the utilization of the technology for tomato hybrid seed production, and the potential use for other vegetable crops. This system is advantageous in several aspects for hybrid tomato seed production. First, the control of male sterility by a single recessive nuclear gene means that any tomato germplasm containing the dominant male‐fertile allele could serve as a restorer line. This approach provides hybrid breeding programmes with a broad choice of germplasm as paternal lines. Second, male sterility was induced by CRISPR/Cas9‐mediated mutagenesis, enabling its rapid introduction (1–2 years) into elite breeding lines, removing linkage drags and decreasing the time required compared with traditional breeding methods. Third, our seedling‐colour maintainer system allowed propagation of male‐sterile seeds on a production scale and enabled easy identification of homozygous male‐sterile plants at the seedling stage, greatly reducing the resources and labour required. Furthermore, although the technology involved transgenic plants, neither the male‐sterile line nor the hybrid seeds were themselves transgenic: only the maintainer line carried the transgene and thus required transgenic oversight. Our genetic manipulation of SlSTR1 in tomato provides a good example of how this male‐sterility system may be deployed. This biotechnology‐based system could be extended to other vegetable crops by manipulating stamen‐specific genes that are conserved across species.
Experimental procedures
Plant materials and growth conditions
Tomato (Solanum lycopersicum) inbred lines TB0993, TB0240 and TB0249, used in this study, were originally developed by Beijing Vegetable Research Center (BVRC), Beijing Academy of Agriculture and Forestry Sciences (Beijing, China). TB0993 was used as a WT in this study. Tomato seeds were germinated for 48 h on moistened filter paper. Seedlings were grown in 50‐plug trays containing sterilized soil under controlled light and temperature conditions consisting of 16 h light (200 μE m−2 sec−1) at 25°C and 8 h dark at 18°C at constant 60% relative humidity. Seedlings were transplanted at the six‐leaf stage to a greenhouse at the farm of the Beijing Vegetable Research Center (BVRC), Beijing Academy of Agriculture and Forestry Sciences (Beijing, China).
Bioinformatics analysis
Published RNA sequencing data of root, leaf, unopened flower bud (~5 mm), fully open flower, and fruit (six stages; The Tomato Genome Consortium, 2012) were used to identify stamen‐specific genes. The BEI was introduced to quantify the level of expression of a specific gene in the flower bud. The BEI was defined as the ratio of the normalized RPKM in flower buds to the sum of normalized RPKM in all tissues. The BEI values ranged from 0 (not expressed in the flower bud) to 1 (exclusively expressed in the flower bud). A threshold BEI value of ≥ 0.5 identified 2044 genes for further analysis. These genes were subjected to K‐means clustering using a bioinformatics analysis system BMKCloud (Biomarker Technologies, Beijing, China, http://www.biomarker.com.cn). The genes were initially grouped into different numbers of clusters until it was determined that grouping them into five clusters conveyed the major characteristic spatial expression patterns. The heat map shows log2 (RPKM + 1) values for each gene (Figure 1c).
Gene expression analysis
Samples of roots, leaves, unopened flower buds (~5 mm), fully open flowers, different stages of fruits, and different parts (sepal, petal, stamen and pistil) of 5 mm unopened flower buds were harvested and immediately frozen in liquid nitrogen. Total RNA was extracted from each sample using a TRIzol kit (Invitrogen, USA, https://www.thermofisher.com) according to the manufacturer's instructions, treated with DNase, and purified with RNA Clean and Concentration‐25 (Zymo Research, USA, https://www.zymoresearch.com). The quality of the total RNA was determined using a NanoDrop spectrophotometer (Thermo Fisher, USA, https://www.thermofisher.com). Each 2 μg sample of total RNA was subjected to RT‐qPCR analysis, as previously described (Du et al., 2017). Expression levels of target genes were normalized against those of tomato ACTIN2 (Du et al., 2017). Primers used to quantify gene expression levels are listed in Table S1.
CRISPR/Cas9 construct
The CRISPR/Cas9‐targeted genome editing tool was used to create mutant alleles of SlSTR1 in tomato elite inbred lines. A pCAMBIA‐based Cas9/sgRNA binary vector pKSE401, which harbours a maize‐codon optimized Cas9 and a single‐guide RNA (sgRNA) scaffold, was used to insert the target sequence (Xing et al., 2014), located in the first exon of SlSTR1. The annealed oligonucleotides containing the target sequence were cloned into the Bsa I site of the pKSE401 vector to generate the final CRISPR/Cas9 construct. The CRISPR/Cas9 construct was introduced into Agrobacterium tumefaciens LBA4404 and used to transform TB0993 and other inbred lines, generating T0 transgenic plants. Agrobacterium tumefaciens‐mediated transformations were performed as described previously (Brooks et al., 2014).
DNA extraction, PCR genotyping, and sequencing
Leaflets were collected from each T0 plant, and genomic DNA was extracted using a standard cetyl‐trimethyl‐ammonium bromide protocol (Porebski et al., 1997). Each plant was genotyped with respect to the presence of transgene using PCR primers designed to amplify a fragment of Cas9. To evaluate the types of mutations generated, T0 transgenic plants were analysed by PCR with primers that flanked the sgRNA target. All PCR products were purified and cloned into the pMD18‐T vector, and the resulting Escherichia coli colonies were sequenced using the M13F and M13R primers. A KASP marker was developed to identify the CR‐slstr1‐3‐6 mutation using the KASP genotyping platform (LGC Genomics, UK, https://www.lgcgroup.com; Semagn et al., 2014).
Microscopy
For scanning electron microscopy studies, anthers at the dehiscence stage were collected and examined using an S‐3000N scanning electronic microscope (Hitachi, Japan) with an acceleration voltage of 15 kV. FDA staining was performed as described (Li, 2011). Fresh samples of pollen grains, collected from mature flowers at the dehiscence stage, were stained with FDA (2 μg ml−1) on a glass slide. Microscopic observations were performed using an LSM 710 confocal laser‐scanning microscope (Zeiss, Germany, https://www.zeiss.com) under blue light (wavelength = 495 nm) conditions.
Fruit yield and seed purity
Data on total fruit yield were collected for F1 hybrids derived from WT or CR‐slstr1‐3‐6 plants grown in the greenhouse of Beijing Vegetable Research Center (Beijing, 2018) according to the commercial cultivation protocols. Seedlings were grown in 50‐cell flats for 30 days and transplanted to the greenhouse at the end of March 2018. Plants were grown under drip irrigation and standard fertilizer regimes under natural light supplemented with high‐pressure sodium bulbs (50 μmol m−2 sec−1; 16‐h light/8‐h dark cycle). Each genotype was represented by 50 replicates (plants). Total fruit yield was represented by the sum of the weights of 24 fruits (six inflorescences, each bearing four fruits) from each plant. Mean values were compared using the Student's t‐test.
Seed purity of F1 hybrids derived from WT or CR‐slstr1‐3‐6 plants was tested using a co‐dominant molecular marker tightly linked to the tomato yellow leaf curl virus (TYLCV) resistant gene Ty1 (Verlaan et al., 2013). The female parents, TB0993 and CR‐slstr1‐3‐6, contained the resistant allele of Ty1, but all the male parents (TB0994, TB098 and TB0182) lacked this resistant allele. Approximately 1000 seeds of each F1 hybrid were tested. Seed purity of CR‐slstr1‐3‐6‐derived F1 hybrids was also confirmed using the KASP marker.
Generation of colour‐restorer lines
To develop a transgenic seedling‐colour maintainer, we constructed a pCAMBIA2300‐35S‐based T‐DNA binary vector that contained three expression units: NPT II under the CaMV 35S promoter for transformation selection; SlSTR1 under its native promoter for restoration of male fertility; and ANT1 under the CaMV 35S promoter to mark transgenic plants. The full‐length coding sequence of ANT1 was amplified and inserted into the binary vector pCAMBIA2300‐35S‐OCS between the Pst I and Kpn I sites to generate pCAMBIA2300‐35S‐ANT1‐OCS. Next, a 4.1‐kb genomic DNA fragment of Solyc03g053130, containing the coding region, a 1.5‐kb fragment of upstream region and a 1.0‐kb fragment of downstream region, was amplified and cloned in the Hind III site of pCAMBIA2300‐35S‐ANT1‐OCS by seamless cloning to produce the final T‐DNA binary vector pANT1‐COMP.
The pANT1‐COMP construct was introduced into A. tumefaciens LBA4404, and used to transform an F2 population derived from a cross between CR‐slstr1‐3‐6 plants and WT pollen grains. To identify the homozygous CR‐slstr1‐3‐6 mutation in T0 transgenic plants, a forward primer was designed that specifically recognized the genomic sequence of SlSTR1 but not the SlSTR1 transgene; this primer was used in PCR to amplify the genomic sequence of SlSTR1 prior to sequencing. Transgenic T0 plants that were homozygous for the CR‐slstr1‐3‐6 mutation and whose T1 offspring showed a 3:1 segregation ratio of transgene were selected for further analysis.
Accession numbers
Sequence data from this article can be found in the Sol Genomics Network data library under the following accession numbers: SlSTR1: Solyc03g053130; ANT1: Solyc07g063410; and ACTIN2: Solyc11g005330.
Conflict of interest
The authors declare no conflicts of interest.
Author contributions
MD, CYL and C‐BL designed the research strategy. MD, KZ, YL and LD performed most of the experiments. XZ performed the microscopy observations. LL performed the bioinformatics analysis and prepared the figures. MZ helped generate the constructs and transgenic plants. WZ helped identify the transgenics. CW helped develop the KASP marker. MZ and JX helped grow the plants. MD, KZ, YL, C‐BL and CYL analysed the data. C‐BL and CYL contributed reagents, materials and analysis tools. MD and CYL wrote the article.
Supporting information
Figure S1. Expression patterns in different floral organs of 20 genes randomly selected from cluster 3.
Figure S2. Generation of SlSTR1‐edited plants using the CRISPR/Cas9 system.
Figure S3. CR‐slstr1‐1 and CR‐slstr1‐2 plants are defective in pollen development.
Figure S4. Fluorescein diacetate (FDA) assay of pollen viability of wild‐type (WT), CR‐slstr1‐1 and CR‐slstr1‐2 plants.
Figure S5. Development of a KASP marker to detect the presence of the CR‐slstr1‐3‐6 mutation.
Figure S6. Statistical analysis of fruit yield and seed purity in F1 hybrids derived from wild‐type (WT) and CR‐slstr1 plants.
Figure S7. Morphological analysis of wild‐type (WT), CR‐slstr1‐3‐6 and colour‐restorer lines at different stages.
Figure S8. Fluorescein diacetate (FDA) assay of pollen viability of wild‐type (WT), CR‐slstr1‐3‐6 and colour‐restorer lines.
Table S1. Sequences of PCR primers used in this study.
Dataset S1. Stamen‐specific genes identified in this study.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFD0101703), the National Natural Science Foundation of China (31672157 and 31730010), a Tomato Breeding Programme from Guangdong Province (2018B020202006), and Beijing Talents Program (2017A33).
Contributor Information
Minmin Du, Email: duminmin7@hotmail.com.
Chang‐Bao Li, Email: lichangbao@nercv.org.
Chuanyou Li, Email: cyli@genetics.ac.cn.
References
- Atanassova, B. (1999) Functional male sterility (ps‐2) in tomato (Lycopesicon esculentum Mill.) and its application in breeding and hybrid seed production. Euphytica, 107, 13–21. [Google Scholar]
- Atanassova, B. (2000) Functional male sterility in tomato (Lycopersicon esculentum Mill.) and its application in hybrid seed production. Acta Physiol. Plant, 22, 221–225. [Google Scholar]
- Atanassova, B. and Georgiev, H. (2002) Using genic male sterility in improving hybrid seed production in tomato (Lycopersicon esculentum Mill.). Acta Hortic. 579, 185–188. [Google Scholar]
- Brooks, C. , Nekrasov, V. , Lippman, Z.B. and Van Eck, J. (2014) Efficient gene editing in tomato in the first generation using the clustered regularly interspaced short palindromic repeats/CRISPR‐associated9 system. Plant Physiol. 166, 1292–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X. , Liu, X.Y. , Wang, X.T. et al . (2019) B‐class MADS‐box TM6 is a candidate gene for tomato male sterile‐15 26 . Theor. Appl. Genet. 132, 2125–2135. [DOI] [PubMed] [Google Scholar]
- Chang, Z.Y. , Chen, Z.F. , Wang, N. , Xie, G. , Lu, J.W. , Yan, W. , Zhou, J.L. , Tang, X.Y. and Deng, X.W. (2016) Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proc. Natl Acad. Sci. USA, 113, 14 145–14 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema, D.S. and Dhaliwal, M.S. (2005) Hybrid tomato breeding. J. New Seeds, 6, 1–14. [Google Scholar]
- Chen, L. and Liu, Y.G. (2014) Male sterility and fertility restoration in crops. Annu. Rev. Plant Biol. 65, 579–606. [DOI] [PubMed] [Google Scholar]
- Chen, K.L. , Wang, Y.P. , Zhang, R. , Zhang, H.W. and Gao, C.X. (2019) CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 70, 667–697. [DOI] [PubMed] [Google Scholar]
- Cigan, A.M. , Unger, E. , Xu, R.J. , Kendall, T. and Fox, T.W. (2001) Phenotypic complementation of ms45 maize requires tapetal expression of MS45 . Sex. Plant Reprod. 14, 135–142. [Google Scholar]
- Crane, M.B. (1915) Heredity of types of inforescence and fruits in tomato. J. Genet. 5, 1–11. [Google Scholar]
- Dobritsa, A.A. , Nishikawa, S.I. , Preuss, D. , Urbanczyk‐Wochniak, E. , Sumner, L.W. , Hammond, A. , Carlson, A.L. and Swanson, R.J. (2009) LAP3, a novel plant protein required for pollen development, is essential for proper exine formation. Sex. Plant Reprod. 22, 167–177. [DOI] [PubMed] [Google Scholar]
- Du, M.M. , Zhao, J.H. , Tzeng, D.T.W. et al . (2017) MYC2 orchestrates a hierarchical transcriptional cascade that regulates jasmonate‐mediated plant immunity in tomato. Plant Cell, 29, 1883–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, J.F. , Talle, B. and Wilson, Z.A. (2015) Anther and pollen development: a conserved developmental pathway. J. Integr. Plant Biol. 57, 876–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorguet, B. , Schipper, D. , van Lammeren, A. , Visser, R.G.F. and van Heusden, A.W. (2009) ps‐2, the gene responsible for functional sterility in tomato, due to non‐dehiscent anthers, is the result of a mutation in a novel polygalacturonase gene. Theor. Appl. Genet. 118, 1199–1209. [DOI] [PubMed] [Google Scholar]
- Gorman, S.W. and McCormick, S. (1997) Male sterility in tomato. Crit. Rev. Plant Sci. 16, 31–53. [Google Scholar]
- Jeong, H.J. , Kang, J.H. , Zhao, M.A. , Kwon, J.K. , Choi, H.S. , Bae, J.H. , Lee, H.A. , Joung, Y.H. , Choi, D. and Kang, B.C. (2014) Tomato male sterile 10(35) is essential for pollen development and meiosis in anthers. J. Exp. Bot. 65, 6693–6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.J. and Zhang, D.B. (2018) Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci. 23, 53–65. [DOI] [PubMed] [Google Scholar]
- Li, X. (2011) Pollen fertility/viability assay using FDA staining. Bio Protoc. 1, e75. [Google Scholar]
- Liu, X.Y. , Yang, M.X. , Liu, X.L. et al . (2019) A putative bHLH transcription factor is a candidate gene for male sterile 32, a locus affecting pollen and tapetum development in tomato. Hortic. Res. 6, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, Y.F. , Botella, J.R. , Liu, Y.G. and Zhu, J.K. (2019) Gene editing in plants: progress and challenges. Natl Sci. Rev. 6, 421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matas, A.J. , Yeats, T.H. , Buda, G.J. et al . (2011) Tissue‐ and cell‐type specific transcriptome profiling of expanding tomato fruit provides insights into metabolic and regulatory specialization and cuticle formation. Plant Cell, 23, 3893–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews, H. , Clendennen, S.K. , Caldwell, C.G. et al . (2003) Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. Plant Cell, 15, 1689–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Prat, E. and van Lookeren Campagne, M.M. (2002) Hybrid seed production and the challenge of propagating male‐sterile plants. Trends Plant Sci. 7, 199–203. [DOI] [PubMed] [Google Scholar]
- Porebski, S. , Bailey, L.G. and Baum, B.R. (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 15, 8–15. [Google Scholar]
- Pucci, A. , Picarella, M.E. and Mazzucato, A. (2017) Phenotypic, genetic and molecular characterization of 7B ‐1, a conditional male‐sterile mutant in tomato. Theor. Appl. Genet. 130, 2361–2374. [DOI] [PubMed] [Google Scholar]
- Quinet, M. , Bataille, G. , Dobrev, P.I. , Capel, C. , Gomez, P. , Capel, J. , Lutts, S. , Motyka, V. , Angosto, T. and Lozano, R. (2014) Transcriptional and hormonal regulation of petal and stamen development by STAMENLESS, the tomato (Solanum lycopersicum L.) orthologue to the B‐class APETALA3 gene. J. Exp. Bot. 65, 2243–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney, V.K. (2004) Photoperiod‐sensitive male‐sterile mutant in tomato and its potential use in hybrid seed production. J. Hortic. Sci. Biotechnol. 79, 138–141. [Google Scholar]
- Semagn, K. , Babu, R. , Hearne, S. and Olsen, M. (2014) Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): overview of the technology and its application in crop improvement. Mol. Breed. 33, 1–14. [Google Scholar]
- Shi, J.X. , Cui, M.H. , Yang, L. , Kim, Y.J. and Zhang, D.B. (2015) Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci. 20, 741–753. [DOI] [PubMed] [Google Scholar]
- Stockigt, J. , Barleben, L. , Panjikar, S. and Loris, E.A. (2008) 3D‐Structure and function of strictosidine synthase – the key enzyme of monoterpenoid indole alkaloid biosynthesis. Plant Physiol. Biochem. 46, 615. [DOI] [PubMed] [Google Scholar]
- The Tomato Genome Consortium (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature, 485, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlaan, M.G. , Hutton, S.F. , Ibrahem, R.M. , Kormelink, R. , Visser, R.G.F. , Scott, J.W. , Edwards, J.D. and Bai, Y.L. (2013) The tomato yellow leaf curl virus resistance genes Ty‐1 and Ty‐3 are allelic and code for DFDGD‐class RNA‐dependent RNA polymerases. PLoS Genet. 9, e1003399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, X.Y. , Wu, S.W. , Li, Z.W. , Dong, Z.Y. , An, X.L. , Ma, B. , Tian, Y.H. and Li, J.P. (2019) Maize genic male‐sterility genes and their applications in hybrid breeding: progress and perspectives. Mol. Plant, 12, 321–342. [DOI] [PubMed] [Google Scholar]
- Wilson, Z.A. and Zhang, D.B. (2009) From Arabidopsis to rice: pathways in pollen development. J. Exp. Bot. 60, 1479–1492. [DOI] [PubMed] [Google Scholar]
- Wu, Y.Z. , Fox, T.W. , Trimnell, M.R. , Wang, L.J. , Xu, R.J. , Cigan, A.M. , Huffman, G.A. , Garnaat, C.W. , Hershey, H. and Albertsen, M.C. (2016) Development of a novel recessive genetic male sterility system for hybrid seed production in maize and other cross‐pollinating crops. Plant Biotechnol. J. 14, 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, H.L. , Dong, L. , Wang, Z.P. , Zhang, H.Y. , Han, C.Y. , Liu, B. , Wang, X.C. and Chen, Q.J. (2014) A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 14, 327 10.1186/s12870-014-0327-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L.Y. , Huang, Z.J. , Wang, X.X. , Gao, J.C. , Guo, Y.M. , Du, Y.C. and Hu, H. (2016) Fine mapping and molecular marker development of anthocyanin absent, a seedling morphological marker for the selection of male sterile 10 in tomato. Mol. Breed. 36, 107. [Google Scholar]
- Zhang, D.F. , Wu, S.W. , An, X.L. et al . (2018) Construction of a multicontrol sterility system for a maize male‐sterile line and hybrid seed production based on the ZmMs7 gene encoding a PHD‐finger transcription factor. Plant Biotechnol. J. 16, 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression patterns in different floral organs of 20 genes randomly selected from cluster 3.
Figure S2. Generation of SlSTR1‐edited plants using the CRISPR/Cas9 system.
Figure S3. CR‐slstr1‐1 and CR‐slstr1‐2 plants are defective in pollen development.
Figure S4. Fluorescein diacetate (FDA) assay of pollen viability of wild‐type (WT), CR‐slstr1‐1 and CR‐slstr1‐2 plants.
Figure S5. Development of a KASP marker to detect the presence of the CR‐slstr1‐3‐6 mutation.
Figure S6. Statistical analysis of fruit yield and seed purity in F1 hybrids derived from wild‐type (WT) and CR‐slstr1 plants.
Figure S7. Morphological analysis of wild‐type (WT), CR‐slstr1‐3‐6 and colour‐restorer lines at different stages.
Figure S8. Fluorescein diacetate (FDA) assay of pollen viability of wild‐type (WT), CR‐slstr1‐3‐6 and colour‐restorer lines.
Table S1. Sequences of PCR primers used in this study.
Dataset S1. Stamen‐specific genes identified in this study.


