Abstract
Obesity is a risk factor for breast cancer in postmenopausal and high‐risk premenopausal women. Changes within the obese breast microenvironment may increase breast cancer risk. Transforming growth factor beta‐1 (TGFβ1) is a major regulator of mammary epithelial stem/progenitor cells, and its activity is dysregulated under conditions of obesity. Using a high‐fat diet model of obesity in mice and breast tissue from women, we observed that TGFβ1 activity is reduced in breast epithelial cells in obesity. Breast ducts and lobules demonstrated increased decorin in the extracellular matrix (ECM) surrounding epithelial cells, and we observed that decorin and latent TGFβ1 complexed together. Under conditions of obesity, macrophages expressed higher levels of decorin and were significantly increased in number surrounding breast epithelial cells. To investigate the relationship between macrophages and decorin expression, we treated obese mice with either IgG control or anti‐F4/80 antibodies to deplete macrophages. Mice treated with anti‐F4/80 antibodies demonstrated reduced decorin surrounding mammary ducts and enhanced TGFβ1 activity within mammary epithelial cells. Given the role of TGFβ1 as a tumor suppressor, reduced epithelial TGFβ1 activity and enhanced TGFβ1 within the ECM of obese mammary tissue may enhance breast cancer risk.
Keywords: decorin, macrophages, mammary gland, obesity, transforming growth factor beta
Abbreviations
- ASCs
adipose‐derived stromal cells
- BMI
body mass index
- CD
control diet
- ECM
extracellular matrix
- ERα
estrogen receptor alpha
- H&E
hematoxylin and eosin
- HFD
high‐fat diet
- HPGRT
hypoxanthine phosphoribosyltransferase
- IRB
institutional review board
- MEC
mammary epithelial cell
- pSMAD2
phosphorylated SMAD2
- SMA
smooth muscle actin
- TGFβ1
transforming growth factor beta 1
- TGFβR
transforming growth factor beta receptor
- TSP1
thrombospondin‐1
1. INTRODUCTION
Obesity is a risk factor for developing breast cancer after menopause, as well as for high‐risk premenopausal women. 1 , 2 , 3 Alterations within breast tissue prior to tumor formation may contribute to the risk of breast cancer development and malignant progression. Within the breast, the epithelium is composed of luminal cells, which express estrogen receptor alpha (ERα), and basal/myoepithelial (basal) cells. Epithelial cells in both the luminal and basal cell lineages are thought to be derived from lineage‐restricted stem/progenitor cells. 4 , 5 , 6 Increased numbers of specific types of stem/progenitor cells may lead to enhanced risk for the development of distinct types of breast cancer. 7 We previously observed that obesity increased epithelial cell expression of ERα and elevated stem/progenitor activity in mammary tissue of both mice and women. 8 In addition to the effects on breast epithelial cells, we and others have observed changes within the surrounding breast stroma, including elevated fibrosis within breast fat, activation of adipose‐derived stromal cells (ASCs), and recruitment of macrophages into breast adipose tissue. 9 , 10 , 11 , 12 The underlying cause of these changes within breast tissue under conditions of obesity are not well understood.
In the mammary gland, transforming growth factor beta (TGFβ1) is a major regulator of mammary gland development and has been strongly implicated in breast cancer progression. 13 , 14 In adult mice, epithelial cells are the main source of TGFβ1, 15 , 16 and TGFβ1 has been shown to reduce mammary epithelial stem/progenitor activity. 17 , 18 , 19 , 20 TGFβ1 is produced as an inactive, latent form which must undergo extracellular activation prior to receptor binding. 14 Once activated, TGFβ1 ligand binds type II TGFβ1 receptor (TGFβRII), which complexes with TGFβRI, and transmits its signals through transcription factors called SMADs. 21 There are many proteins that can modulate the activity of TGFβ1 at the extracellular, cytoplasmic, and nuclear level. 22 Within the extracellular matrix (ECM), latent TGFβ1 complexes both with latent TGFβ1 binding proteins as well as multiple matrix components including matricellular protein, decorin, which sequester inactive TGFβ1 until activated through proteases, integrin activity or changes in matrix elasticity. 23 This level of regulation provides stringent control of TGFβ1 function and ensures targeted TGFβ1 signaling. In obese adipose tissue, TGFβ1 concentrations are enhanced, 24 , 25 , 26 and these changes in TGFβ1 signaling may impact the breast microenvironment.
Here, we investigated how obesity alters TGFβ1 activity within the mammary epithelium of both mice and humans. We observed decreased TGFβ1 activity in mammary epithelial cells under conditions of obesity and increased decorin in the stroma surrounding mammary ducts. Decorin and TGFβ1 were enriched within isolated mammary ECM, and we identified complexes of decorin with latent TGFβ1. Within obese mammary tissue, macrophages demonstrated elevated expression of decorin and were increased in number surrounding mammary epithelium. Depletion of macrophages in obese mice resulted in reduction of decorin surrounding mammary epithelial cells and enhanced TGFβ1 activity within mammary epithelial cells. Reduction in TGFβ1 activity within breast epithelium and enhanced concentrations of latent TGFβ1 within the mammary stroma may contribute to increased breast cancer risk under conditions of obesity.
2. MATERIALS AND METHODS
2.1. Primary human tissue
All human breast tissue procurement for these experiments was in compliance with the laws and institutional guidelines, as approved by the Institutional Review Board (IRB) committee from the University of Wisconsin‐Madison. Disease‐free, de‐identified breast tissues were obtained from patients undergoing elective reduction mammoplasty with informed consent through the Translational Science BioBank of the Carbone Cancer Center at the University of Wisconsin‐Madison. This research study was approved by the IRB as “Not Human Subject Research” with a limited patient dataset including patient age, date of service, and body mass index (BMI). Tissue samples from patients aged 18‐45 years were included in these studies. BMI > 30 kg/m2 was defined as obese, and BMI < 25 kg/m2 was defined as normal range.
2.2. Mouse studies
Animal procedures were conducted in accordance with a protocol approved by the University of Wisconsin‐Madison Institutional Animal Care and Use Committee. FVB/N (001800) and C57BL/6 (000664) female mice were purchased from Jackson Laboratories (Ellsworth, MA, USA) and housed and handled in accordance with the guidelines from the National Institutes of Health Guide for Care and Use of Laboratory Animals. All mice were given food and water ad libitum. For obesity studies, 3‐week‐old FVB/N or 8‐week‐old C57BL/6 mice were fed either low‐fat maintenance chow (CD, 18% kcal from fat; Teklad Global #2018, Envigo) or a high‐fat diet (HFD, 60% kcal from fat; #58126, Test Diet) for 16 weeks and weighed weekly. We have previously shown 8‐week‐old FVB/N mice are more resistant to weight gain when fed a HFD. 8 Purified diets contained equal amounts of vitamins and micronutrients. Thoracic and inguinal mammary glands were collected from diestrus‐staged mice for analysis. Inguinal glands were utilized for histological assessment, and the remaining glands were collagenase‐digested to isolate cells or snap frozen in liquid nitrogen for molecular analysis.
2.3. Mammary epithelial cell isolation
Mouse mammary glands were digested in DMEM/Ham's F‐12 (10‐090‐CV, Corning Inc, Corning, New York, USA) supplemented with 5 ng/mL of human epidermal growth factor (EGF, E9644, MilliporeSigma), 10 μg/mL of insulin (I0516, MilliporeSigma), 0.5 μg/mL of hydrocortisone (H0888, MilliporeSigma), 5% of calf serum, and 1% of antibiotic/antimycotic solution (30‐004‐CI, Corning Inc) with 3 mg/mL of collagenase A (11088793001, MilliporeSigma) and 100 U/mL of hyaluronidase (H3506, MilliporeSigma) at 37°C for 1 hour. Clusters of mammary epithelial cells were allowed to settle at room temperature for 20 minutes, followed by centrifugation for 5 minutes at 8× g relative centrifugal force. Following centrifugation, the lipid‐rich adipose fraction was removed, and the stromal vascular fraction supernatant was filtered through a 70 µm filter for isolation of macrophages. Following macrophage isolation, the remaining stromal vascular fraction was plated in DMEM containing 10% of FBS (10437‐28, Gibco, Thermo Fisher Scientific) and 1% of antibiotic/antimycotic solution (30‐004‐CI, Mediatech, Thermo Fisher Scientific) and incubated at 37°C with 5% of CO2 until adipose‐derived stromal cells (ASCs) grew to confluency. The epithelial cells were centrifuged in PBS for 5 minutes at 8× g. The supernatant was removed, red blood cells were lysed (ACK Lysing Buffer, 10‐548E, Lonza), and the epithelial cells were washed with 5% of calf serum in PBS. Epithelial cells were dissociated to single cells as described. 8 Cell pellets were stored at −80°C for further analysis.
2.4. Mammary macrophage isolation
To isolate macrophages, Sheep Anti‐Rat IgG beads (Dynabeads, 11035, Thermo Fisher Scientific) were washed, then, conjugated to anti‐F4/80 antibodies (0.1 µg/mL; BM8, 12302, BioLegend, San Diego, CA, USA) at 4°C with rocking for 30 minutes per manufacturer's instructions. The antibody‐conjugated beads were washed, then, incubated with the stromal vascular fraction with rocking for 30 minutes at 4°C. The cells bound to antibodies were isolated using a magnet and washed three times. F4/80+ cells were examined on a hemocytometer to quantify the percentage of cells bound to beads. Isolates with >95% of cells bound to beads were snap frozen in liquid nitrogen prior to RNA extraction.
2.5. Immunohistochemistry and immunofluorescence
Mammary glands were fixed in 10% neutral‐buffered formalin for 48 hours, embedded in paraffin and sectioned at 5 µm. Tissue sections were stained for immunohistochemistry and immunofluorescence using SMAD4 (1:100, PA5‐34806, Thermo Fisher Scientific), phosphorylated SMAD2 (pSMAD2, 1:250, 44‐244G, Thermo Fisher Scientific), decorin (1:25, sc‐22613, Santa Cruz Biotechnologies, Dallas, TX, USA), F4/80 (1:250, BM8, 12302, BioLegend, San Diego, CA, USA), CD68 (1:200, 50‐112‐2648, Thermo Fisher Scientific), alpha‐smooth muscle actin (SMA, 1:5000, 5528, MilliporeSigma), and TGFβ1 (1:250, LS‐B14345, LSBio, Seattle, WA, USA) as described. 8 Sections were imaged using Nikon Eclipse 80 t microscope with NIS Elements BR 3.2 software. The percentage of labeled cells was quantified by manual counting by dividing the total number of positive cells by the total number of epithelial cells within the ducts and multiplying by 100. Five 400× magnification images from each gland and at least 500 cells were analyzed. F4/80+ cells were quantified by dividing the total number of F4/80+ cells within a 50 µm radius around the mammary ducts by the area of the duct. Total CD68+ cells were quantified on each image, and an average was generated from five images/tissue section. Percent decorin was quantified by quantifying the area of decorin expression divided by the total area of the stroma and multiplying by 100. All cell counting and image alignment was performed using Image J software (National Institutes of Health, Bethesda, MD, USA).
2.6. Quantitative RT‐PCR
RNA was isolated from cell pellets and tissue with TRIzol (15596026, Thermo Fisher Scientific) and purified using Qiagen RNeasy Mini Kit (74104, Qiagen) or RNeasy Micro Kit (74004, Qiagen). The RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (4368814, Applied Biosciences, Beverly Hills, CA, USA) or Ovation RNA Amplification System V2 (3100‐12, Tecan, Mannedorf, CH) and Techne Thermal Cycler (Bio‐Techne, Minneapolis, MN, USA). Quantitative PCR was performed using iTaq SYBR Green Supermix (1725121, Bio‐Rad, Hercules, CA, USA) with a Bio‐Rad CFX Connect Real‐Time PCR Detection System (Bio‐Rad). The following primers were used: decorin F: GATTTTCCACCCGACACAAC, R: TTCTTGAAGGCCCCTTCTTT; TGFβ1 F: ATACGTCAGACATTCGGGAAGCAGTG, R: AATAGTTGGTATCCAGGGCTCTCCG; TGFβRII F: CCAAGTCGGATGTGGAAATGG, R: TGTCGCAAGTGGACAGTCTC; cyclophilin F: TGTGCCAGGGTGGTGACTT, R: TCAAATTTCTCTCCGTAGATGGACTT; hypoxanthine phosphoribosyltransferase (HPGRT) F: TGCTGACCTGCTGGATTACA, R: TTTATGTCCCCCGTTGACTGA. Data were analyzed using the ∆∆Cq method, and transcripts were normalized to cyclophilin (ASCs and macrophages) or HPGRT (mammary epithelial cells).
2.7. ECM isolation and culture of breast ASCs
ECM was isolated from human breast tissue as described. 27 ECM protein was quantified using the BCA Protein Assay (23223, Thermo Fisher Scientific). ECM was stored at −80°C until used for molecular analysis. Breast ASCs were previously isolated and immortalized using human telomerase as described. 28 Breast ASCs were grown in DMEM supplemented with 10% of calf serum and 1% of antibiotic/mycotic, and fibroblasts were cultured in serum‐free DMEM for 24 hours prior to growth on ECM. Rat tail collagen (Corning Rat Tail Type I collagen, 354263, Corning, NY, USA) was diluted to 1 mg/mL and used alone or mixed with 200 µg/mL of isolated ECM to coat the surface of 8‐well chamber culture slides (354108, Thermo Fisher Scientific). Collagen polymerized for 8 hours, then, 5000 breast ASCs were diluted in 500 µL of serum‐free media supplemented with vehicle (DMSO), 5 µg/mL of recombinant human TGFβ1 (10804‐HNAC, Sino Biological, Beijing, China), or 5 µg/mL of recombinant human TGFβ1 and 10 µM of TGFβ inhibitor SB431542 (A10826A, Adooq Biosciences, Irvine, CA, USA) and plated on the ECM. ASCs were cultured for 48 h, then, fixed in methanol and stored at −80°C until analyzed.
2.8. Immunoprecipitation and western blotting
Protein was extracted from whole mammary glands and breast tissue using RIPA buffer including protease and phosphatase inhibitors. Decorin protein was captured using SureBeads Magnetic Beads and SureBeads Protein A Beads (161‐4011, Bio‐Rad) with decorin antibody (0.1 ng/µL, PA5‐13538, Thermo Fisher Scientific) from 50 to 100 µL of total protein. The elution buffer (4x Laemmli Sample Buffer; 161‐0747, Bio‐Rad) was used to remove beads from decorin protein. Isolated protein was mixed with 2‐mercaptoethanol and boiled for 10 minutes, then, loaded on a 4%‐20% of tris/glycine gel (456‐8093, Bio‐Rad) with Tris/Glycine/SDS buffer (161‐0772, Bio‐Rad) and electrophoresis was performed at 150 V. Proteins were transferred to nitrocellulose membrane (162‐0232, Bio‐Rad) at 100 V for 1 hour using tris/glycine buffer (161‐0771, Bio‐Rad). Ponceau S stain (BP103‐10, Thermo Fisher Scientific) was applied to membranes and imaged with a UVP imager. Membranes were then washed with 0.05% of TBST until the stain was cleared, followed by blocking using 5% of milk and 1% of BSA (A4503, MilliporeSigma) in 0.05% of TBST for one hour at room temperature. Membranes were probed for TGFβ1 (1:1000, LS‐B14345, LSBio), decorin (1:500, PA513538, Thermo Fisher Scientific) or GAPDH (1:1000, RI237368, Thermo Fisher Scientific) overnight at 4°C. Membranes were then probed against secondary antibodies conjugated to HRP goat anti‐rabbit (1:10 000, 31460, Thermo Fisher Scientific) or goat anti‐mouse (1:10 000, 31430, Thermo Fisher Scientific) for 1 hour at room temperature. Probed membranes were treated with Clarity Western ECL Substrate (170‐5060, Bio‐Rad), and then, developed on film using the All‐Pro Imaging Corp 100 Plus Automatic X‐Ray Film Processor. Total protein was quantified from images of Ponceau S stain and film by measuring pixel density using Image J.
2.9. Macrophage depletion
FVB/N female mice were fed HFD for 16 weeks, then, were randomized to receive intraperitoneal injections of 400 µg of either rat IgG2b isotype control (BE0090, BioXCell, West Lebanon, NH, USA) or InVivoMAb anti‐mouse F4/80 (BE0206, BioXCell). Following the loading dose, mice received 200 µg injections every 48 hours for 2 weeks.
2.10. Statistical analyses
Results were expressed as the mean ± standard error of the mean (SEM), unless stated otherwise. Statistical differences were determined using Student's t test. P values of .05 or less were considered to denote significance. Statistical analyses were conducted using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA).
3. RESULTS
3.1. Obesity decreases epithelial cell expression of downstream targets of the TGFβ1 pathway
To examine how obesity impacts normal mammary tissue, we utilized a high‐fat diet (HFD) model of obesity in mice. To identify strain‐specific differences within this model, 3‐week‐old FVB/N and 8‐week‐old C57BL/6 female mice were randomized into groups and fed either control diet (CD) or HFD for 16 weeks to induce obesity. 8 The HFD‐fed mice of both strains gained significantly more weight than those in the CD group within 6 weeks after starting the diets (Figure 1A). We have previously shown that weight gain after 16 weeks results in the formation of crown‐like structures of macrophages surrounding dying adipocytes in obese fat, 8 , 28 similar to those observed in breast adipose tissue of obese women, defined as a BMI > 30 kg/m2. 10 , 28
FIGURE 1.
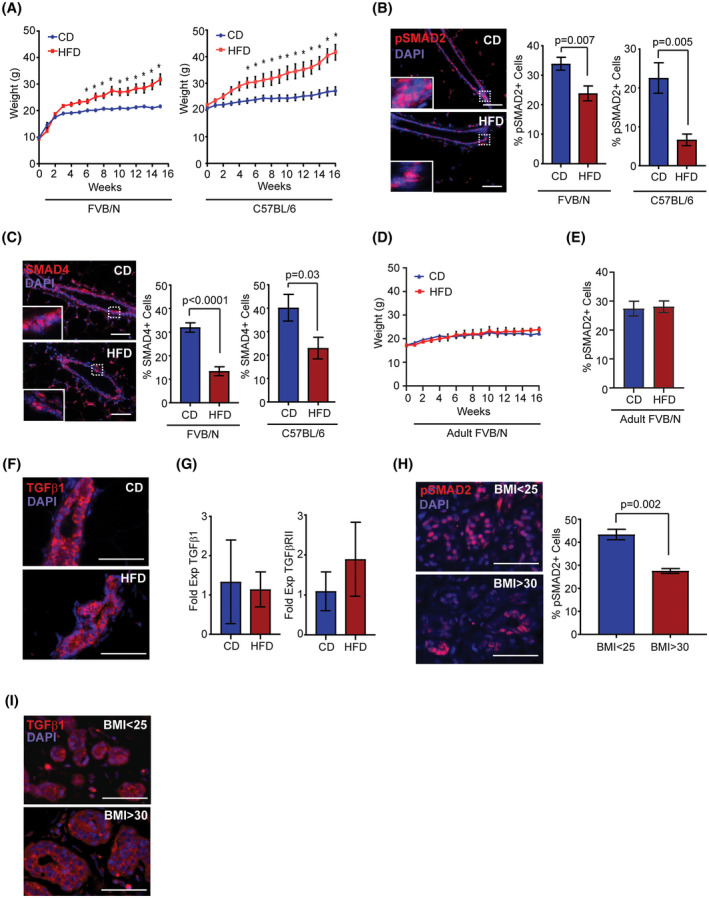
Obesity decreases downstream targets of the TGFβ1 pathway in mammary epithelial cells. A, Weight of 3‐week‐old FVB/N or 8‐week‐old C57BL/6 female mice fed a control diet (CD, n = 6) or high‐fat diet (HFD, n = 6; *P < .05; repeated measures ANOVA with Sidak's multiple comparison test). B, Percent pSMAD2+ cells in mouse mammary ducts (n = 5 mice/group). C, Percent SMAD4+ cells in mouse mammary ducts (n = 5 mice/group). D, Weight of 8‐week‐old (adult) FVB/N female mice fed CD or HFD for 16 weeks (n = 5 mice/group). E, Percent pSMAD2+ cells in mammary ducts of adult FVB/N mice (n = 5 mice/group). F, TGFβ1 expression in mouse mammary ducts (n = 5 mice/group). G, Fold change of expression of TGFβ1 and TGFβRII relative to HPGRT in epithelial cells isolated from mouse mammary glands (n = 3 mice/group). H, Percent pSMAD2+ cells in breast ducts of women (n = 6 tissue samples/group). I, TGFβ1 expression in human breast ducts (n = 5 tissue samples/group). Data analyzed with Student's t test, mean ± SEM. Magnification bar = 50 µm
TGFβ1 is secreted in a latent complex, thus, expression of TGFβ1 protein is not directly indicative of the activity of the TGFβ pathway. In canonical TGFβ1 signaling, activated TGFβRI phosphorylates either SMAD2 or SMAD3, which form complexes with SMAD4 and translocate to the nucleus to regulate transcription. 13 , 14 , 29 To quantify changes in TGFβ1 activity in mammary epithelial cells, we examined nuclear expression of phosphorylated SMAD2 (pSMAD2) and binding partner, SMAD4 in mammary tissue from CD and HFD‐fed mice. Compared to CD‐fed mice, epithelial cells from HFD‐fed mice had decreased nuclear expression of pSMAD2 (Figure 1B) and SMAD4 (Figure 1C) within mammary epithelial cells. These data suggest that TGFβ1 signaling is reduced in epithelial cells of HFD‐fed mice.
We have previously shown that feeding a HFD after puberty in FVB/N female mice leads to greater weight gain resistance than feeding the same diet at weaning. 8 Consistent with this observation, feeding 8‐week‐old FVB/N female mice HFD for 16 weeks resulted in limited weight gain compared to CD‐fed mice (Figure 1D). To examine diet‐specific effects on TGFβ1 activity, we quantified nuclear expression of pSMAD2 within mammary epithelial cells of CD and weight gain resistant HFD‐fed mice. In contrast to FVB/N mice that gained significant weight when fed the HFD, weight gain resistant FVB/N females fed the HFD expressed pSMAD2 in a similar percentage of epithelial cells compared to those fed CD (Figure 1E). Together, these results suggest that the observed reduction in TGFβ1 activity in mammary epithelial cells occurs as a consequence of obesity rather than HFD diet‐specific effects.
We hypothesized that changes in local or systemic growth factors associated with obesity could lead to altered expression of either TGFβ1 ligand or its receptor. Within mammary tissue, we observed no differences in either the expression levels or pattern of expression of TGFβ1 protein within epithelial cells of CD or HFD‐fed mice (Figure 1F). To more closely examine expression levels, we isolated RNA from primary epithelial cells from the mammary glands of CD or HFD‐fed mice, and quantified expression of TGFβ1 and its receptor, TGFβRII. No significant differences in expression levels were observed in either TGFβ1 or TGFβRII transcripts from epithelial cells isolated from CD or HFD‐fed mice (Figure 1G). These results suggest obesity does not directly regulate expression of TGFβ1 ligand or its receptor within mammary epithelial cells.
To examine whether TGFβ1 signaling is also reduced in breast tissue from obese women, breast tissue was collected from women with a known BMI undergoing reduction mammoplasty surgery. Epithelial cells within breast lobules of obese women (BMI > 30 kg/m2) demonstrated decreased expression of pSMAD2 compared to those from lean women (BMI < 25 kg/m2; P = .002, Figure 1H). Although some variability was present in individual breast tissue samples, no significant differences were observed in the expression levels or pattern of expression of TGFβ1 in breast tissue of obese women compared that of lean women (Figure 1I). Together, these results suggest that TGFβ1 activity, but not TGFβ1 protein levels, are decreased within epithelial cells from both obese mice and women.
3.2. Obesity increases decorin around mouse mammary ducts and in lobules of women
Within adipose tissue, obesity has been shown to significantly alter the composition of the ECM. 30 The proteoglycan decorin is expressed in the ECM of visceral and subcutaneous adipose tissue and increases with obesity. 31 To investigate how obesity impacts decorin within the mammary gland, we isolated total protein from the mammary glands of CD and HFD‐fed mice and quantified decorin levels. Decorin protein was significantly increased in the mammary glands of HFD‐fed mice compared to the controls (P = .03, Figure 2A). Consistent with previous studies, 31 we observed elevated decorin expression in the ECM surrounding adipocytes (Figure 2B), as well as in the connective tissue surrounding the mammary gland. In addition, decorin expression was elevated in the ECM surrounding the mammary ducts of HFD‐fed mice compared to controls (P = .003, Figure 2C). Together these data suggest that decorin protein is increased directly surrounding epithelial cells as well as distally within the mammary stroma.
FIGURE 2.
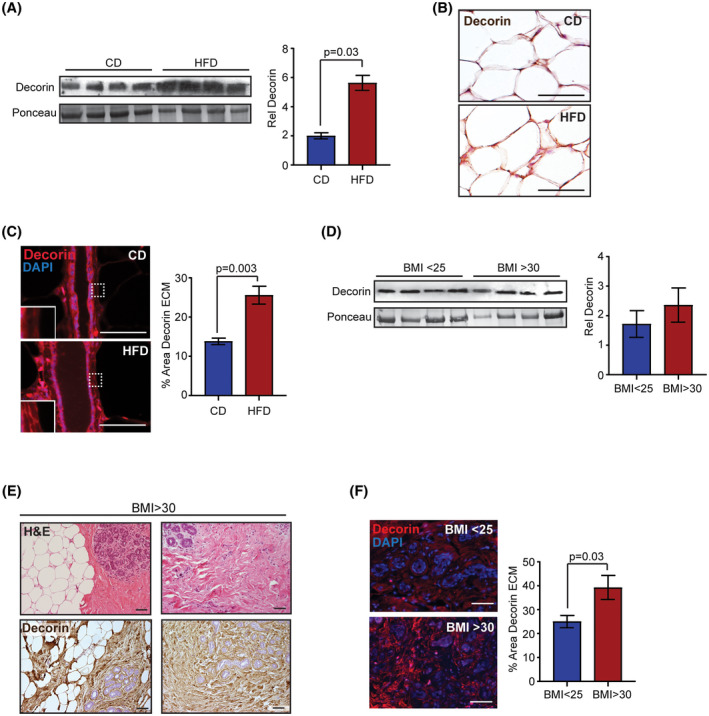
Obesity enhances decorin around mouse mammary ducts and in lobules of women. A, Western analysis and quantification of decorin from FVB/N mouse mammary glands (n = 4 mice/group). B, Decorin expression in ECM surrounding adipocytes within FVB/N mouse mammary glands (n = 5 mice/group). C, Percent area of decorin in ECM surrounding mouse mammary ducts (n = 5 mice/group). D, Western analysis and quantification of decorin from breast tissue of women (n = 4 samples/group). E, Representative images of breast tissue from obese women (BMI > 30) stained for Hematoxylin and Eosin (H&E) and decorin. F, Percent area of decorin in ECM within breast lobules of women (n = 6 samples/group). Data analyzed with Student's t test, mean ± SEM. Magnification bar = 50 µm
No significant differences in decorin protein were detected in protein extracted from whole breast tissue from obese and lean women (Figure 2D). In contrast to mouse mammary tissue, human breast tissue is comprised of breast lobules surrounded by variable amounts of fibrous tissue and adipose tissue. Within the breast tissue of both obese and lean women, we observed variability in the amount of fibrous tissue in between the lobules that was not dependent upon obesity (Figure 2E). This is consistent with studies examining breast density, which demonstrated that obese women can have variably dense breast tissue. 32 Decorin was abundantly expressed in the ECM within the breast lobules, as well as in the stromal tissue between the lobules (Figure 2E), which is consistent with a previous study. 33 To quantify decorin expression specifically in the ECM surrounding the breast lobules, we stained breast tissue with antibodies to detect decorin protein using immunofluorescence. Decorin was significantly increased within the ECM surrounding the breast lobules of obese women compared to those from lean women (P = .03, Figure 2F). This suggests that obesity enhanced accumulation of decorin surrounding the mammary epithelium.
3.3. TGFβ1 complexes with decorin in ECM of breast tissue
Decorin has been implicated in inhibiting TGFβ1 activity by sequestering latent TGFβ1 in the ECM. 34 , 35 In this manner, the ECM serves as a critical regulator of TGFβ1 bioavailability. 36 To examine decorin and TGFβ1 within the ECM, we isolated ECM from whole breast tissue. Both decorin and latent TGFβ1 proteins were enhanced within the isolated ECM protein fraction compared to protein from whole breast tissue (Figure 3A). To assess the interaction between decorin and TGFβ1, we collected total protein from whole mammary glands of mice and breast tissue of women and isolated decorin using immunoprecipitation. In both mice and women, latent TGFβ1 was detected complexed with decorin protein (Figure 3B). We also observed dimers of the active form of TGFβ1 complexed with decorin protein isolated from murine mammary glands (Figure 3B). These results suggest that higher concentrations of decorin protein surrounding epithelial cells under conditions of obesity may increase concentrations of TGFβ1 sequestered within the ECM.
FIGURE 3.
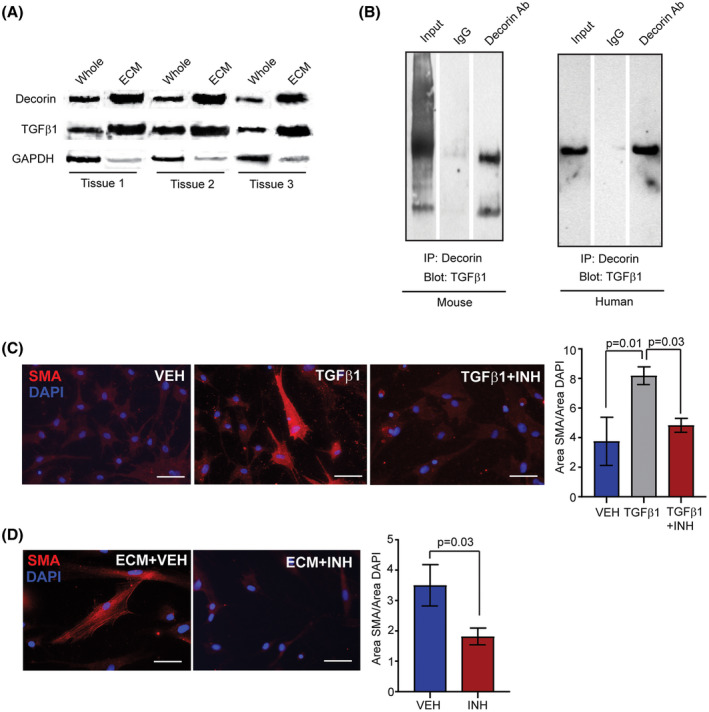
TGFβ1 associated with decorin in ECM enhances SMA expression in breast ASCs. A, Western analysis of whole breast tissue and isolated ECM protein fraction for decorin (35 kDa), latent TGFβ1 (47 kDa), and GAPDH (36 kDa) (n = 3 breast samples). B, Immunoprecipitation of decorin and Western analysis to detect latent TGFβ1 (47 kDa) in protein isolated from whole mammary glands of FVB/N HFD‐fed mice and breast tissue from obese women (BMI > 30). Dimers of the active form of TGFβ1 (25 kDa) were also observed in the mouse samples. Representative blots from three experiments. C, SMA expression in breast ASCs cultured on collagen I and treated with vehicle, recombinant TGFβ1 or TGFβ1 and TGFβ1 inhibitor, SB431542 (n = 3 experiments; one way ANOVA analysis with Tukey's multiple comparison posttest). D, SMA expression in breast ASCs cultured on isolated ECM with vehicle or SB431542 (n = 3 experiments; Student's t test). Bars represent mean ± SEM. Magnification bar = 50 µm
To test for the presence of TGFβ1 within the ECM isolated from breast tissue, we utilized breast adipose‐derived stromal cells (ASC) that we have previously isolated from reduction mammoplasty tissue. 28 ASCs are a mixed population of cells, which are enriched for fibroblasts and adipose stem cells. When treated in culture with TGFβ1, ASCs develop a myofibroblastic phenotype with increased expression of α‐smooth muscle actin (SMA), a myofibroblast marker. 37 When grown on type I collagen gels and treated with vehicle, breast ASCs expressed low levels of SMA (Figure 3C). In contrast, treatment with recombinant TGFβ1 protein resulted in increased numbers of breast ASCs that expressed SMA (P = .01; Figure 3C). This expression of SMA was blocked in the presence of TGFβ1 inhibitor, SB431542 (P = .03; Figure 3C). To examine the presence of TGFβ1 within the isolated ECM, breast ASCs were grown on gels composed of type I collagen and isolated breast ECM. In the presence of breast‐derived ECM and vehicle, increased numbers of breast ASCs expressed SMA after 48 hours (Figure 3D). Expression of SMA was inhibited in the presence of SB431542 (P = .03; Figure 3D). Together these results suggest that decorin elevates sequestered TGFβ1 in the ECM of breast tissue under conditions of obesity.
3.4. Macrophages from mammary glands of obese mice express increased transcripts for decorin
Since we observed increased expression of decorin within the mammary tissue of obese mice, we hypothesized that specific cell types within the mammary gland may increase decorin expression under conditions of obesity. To address this question, we dissociated mammary gland tissue from CD and HFD‐fed mice and isolated primary ASCs, mammary epithelial cells (MECs), and macrophages. Decorin expression was detected in in all cell types examined (Figure 4A). However, decorin expression was only significantly increased in macrophages isolated from mammary glands of HFD‐fed mice compared to those from CD‐fed mice (P = .05, Figure 4A), suggesting that macrophages enhance decorin expression under conditions of obesity.
FIGURE 4.
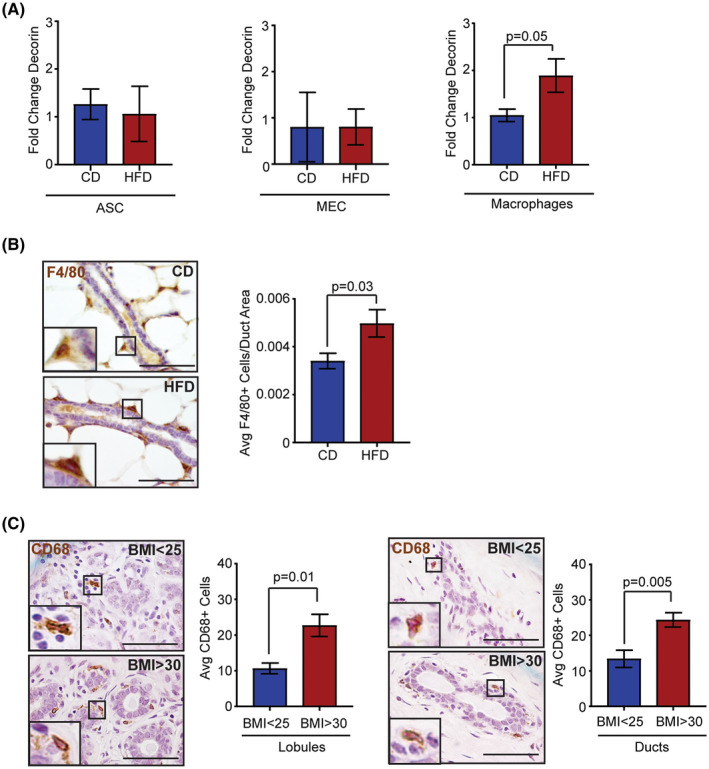
Obesity increases macrophages expressing decorin around mammary ducts and within breast lobules. A, Fold change of decorin expression relative to cyclophilin or HPGRT of ASCs, mammary epithelial cells (MEC) and macrophages isolated from mammary glands of CD or HFD‐fed FVB/N mice (n = 6 mice/group). B, Number of F4/80+ macrophages surrounding mouse mammary ducts divided by the ductal area (n = 6 FVB/N mice/group). C, Number of CD68+ macrophages within the lobular and ductal area in human breast tissue (n = 6 breast samples/group). Data analyzed with Student's t test, mean ± SEM. Magnification bar = 50 µm
As a sequela of obesity, macrophages are observed in greater numbers within adipose tissue of the mammary gland. 9 , 10 , 28 We hypothesized that macrophages may also be increased surrounding the epithelium of the mammary ducts. To test this hypothesis, we quantified F4/80+ macrophages surrounding mammary ducts in tissue from CD and HFD‐fed mice. Elevated numbers of F4/80+ macrophages were observed surrounding and interdigitating between epithelial cells of ducts in mammary glands of HFD‐fed mice compared to those from controls (P = .03, Figure 4B). Similar to mice, breast tissue from obese women had increased numbers of CD68+ macrophages surrounding and interdigitating between epithelial cells of breast lobules and ducts (Figure 4C). This suggests that enhanced decorin surrounding breast ducts is likely due to both increased secretion and elevated numbers of macrophages within the obese breast microenvironment.
3.5. Macrophage depletion in obesity decreases decorin and increases downstream targets of the TGFβ1 pathway
Since we observed that macrophages increased decorin expression in obesity, we hypothesized that depletion of macrophages in obese mice may reduce decorin within the ECM surrounding mammary ducts. To address this question, mice were fed HFD to induce obesity for 16 weeks, then, randomized into two groups that received either IgG control or anti‐F4/80 antibodies for an additional 2 weeks (Figure 5A). Treatment with anti‐F4/80 antibodies did not alter weight gain in the HFD‐fed mice (Figure 5B). To confirm macrophage depletion within the mammary glands, we detected F4/80+ macrophages using antibodies that detected a different epitope of the protein. Mice treated with anti‐F4/80 antibodies had significantly decreased numbers of macrophages surrounding mammary ducts compared to those treated with IgG controls (P = .04, Figure 5C). Macrophage depletion also resulted in significantly decreased decorin protein within the ECM surrounding the mammary ducts compared to IgG‐treated mice (P = .007, Figure 5D). These results suggest that macrophages significantly enhance decorin expression surrounding mammary ducts in obese mice.
FIGURE 5.
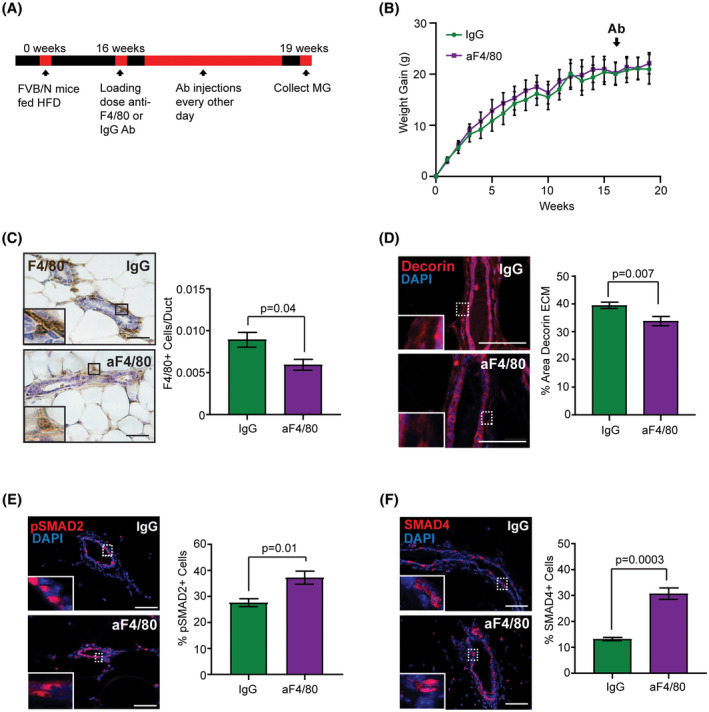
Macrophage depletion in obese mice decreases decorin expression in ECM and increases downstream targets of the TGFβ1 pathway within the epithelium. A, Schematic of macrophage depletion experiment in HFD‐fed mice. B, Weight gain of female FVB/N mice fed HFD starting at 3 weeks of age. Mice received treatment with either IgG or anti‐F4/80 antibodies (n = 4 mice/group, repeated measures ANOVA with Sidak's multiple comparison test). C, Number of F4/80+ macrophages surrounding mammary ducts divided by the ductal area (n = 4 mice/group; Student's t test). D, Percent area decorin in ECM surrounding mammary ducts (n = 4 mice/group; Student's t test). E, Percent pSMAD2+ cells in mammary ducts (n = 4 mice/group; Student's t test). F, Percent SMAD4+ cells in mammary ducts (n = 4 mice/group; Student's t test). Bars represent mean ± SEM. Magnification bar = 50 µm
With reduced decorin within the ECM, we hypothesized that reduced amounts of TGFβ1 may be sequestered in the ECM. To assess TGFβ1 activity, we quantified changes in pSMAD2 and SMAD4 within the mammary glands of obese mice treated with anti‐F4/80 and IgG antibodies. In macrophage‐depleted mice, the percentage of epithelial cells expressing nuclear pSMAD2 was significantly increased compared to mice treated with IgG (P = .01, Figure 5E). Similarly, nuclear expression of SMAD4 was significantly increased in epithelial cells of mice treated with anti‐F4/80 antibodies compared to controls (P = .0003, Figure 5F). Together these findings suggest that macrophages indirectly regulate TGFβ1 activity within the mammary gland in obesity through elevated expression of decorin within the ECM.
4. DISCUSSION
To better understand how obesity alters, the microenvironment of normal breast tissue, potentially leading to increased breast cancer risk, we examined how obesity alters TGFβ1 activity using a well‐characterized HFD mouse model and human breast tissue from reduction mammoplasty surgeries. Under conditions of obesity, mammary epithelial cells demonstrated reduced TGFβ1 activity, measured by diminished nuclear expression of TGFβ1 downstream target, pSMAD2, and partner SMAD4. We also observed increased accumulation of decorin within the ECM complexed with latent TGFβ1. Decorin has been shown to sequester the latent form of TGFβ1 in the ECM of other organs. 34 , 38 , 39 Within breast tissue of mice and women, obesity results in the emergence of myofibroblasts, increased collagen deposition, and elevated ECM proteins within adipose tissue, 9 , 11 , 12 , 40 which are all processes associated with increased TGFβ1 activity. Enhanced TGFβ1 expression has also been observed in other adipose tissue depots during obesity. 24 , 25 , 26 Our results suggest that elevated concentrations of decorin, which forms complexes with latent TGFβ1, may result in enhanced TGFβ1 activity within stromal cells and reduced epithelial TGFβ1 bioavailability.
Obesity is known to induce a pro‐inflammatory state due to an influx of macrophages into adipose tissue. 41 Here, we show that macrophages are also increased surrounding ducts and lobules in obese breast tissue. While the secretion of inflammatory cytokines by obesity‐activated macrophages has been characterized, 42 the ability of macrophages to secrete collagen and matricellular proteins has only recently been described in the context of cancer and fibrosis. 43 , 44 In humans, obesity results in a significant increase in decorin expression within adipose tissue depots. 31 , 45 The source of decorin expression was identified within the adipose tissue stromal vascular fraction, which is enriched in immune cells and ASCs. 31 Through isolation of macrophages and ASCs, we observed that macrophages isolated from HFD‐fed mice expressed elevated decorin transcripts compared to those from CD‐fed mice. With macrophage depletion in HFD‐fed mice, decorin was significantly reduced within the ECM surrounding mammary ducts. In separate preclinical models of tendon repair and cholangiopathy, depletion of macrophages resulted in significantly reduced collagen and ECM deposition within two weeks, 46 , 47 similar to the time point that we examined. Further studies are necessary to identify how obesity promotes a fibrotic response in macrophages, leading to increased decorin expression.
Latent TGFβ1 is activated through multiple mechanisms, which may enhance adipose tissue fibrosis under conditions of obesity. Thrombospondin‐1 (TSP1), which is a major regulator of TGFβ1 activity, 48 , 49 is secreted by adipocytes and has elevated expression under conditions of obesity. 50 , 51 , 52 TGFβ1 can also be activated within the ECM by localized concentrations of reactive oxygen species, which may be elevated in obese adipose tissue by macrophages and adipocytes. 53 , 54 While we have shown that macrophages enhance secretion of decorin, macrophages can also express latent TGFβ1. 55 In a model of lung fibrosis, direct interactions between macrophages secreting latent TGFβ1 and myofibroblasts resulted in elevated levels of activated TGFβ1, 56 suggesting that close proximity among stromal cells in the mammary ECM may enhance local activation of TGFβ1. While enhanced TGFβ1 activity within obese adipose tissue has been linked with adipose tissue fibrosis and insulin resistance, 57 increased TGFβ1 within the ECM may also promote breast tumor progression through rapid formation of cancer associated fibroblasts surrounding developing tumors. Interestingly, loss of decorin within the ECM of ductal carcinoma in situ due to ECM remodeling is a marker for tumor progression and correlates with more aggressive disease. 33 , 58 Loss of decorin during tumor progression may also enhance local TGFβ1 bioavailability to tumor cells potentially leading to elevated numbers of invasive cells within the developing tumor.
In early stages of breast cancer progression, TGFβ1 acts as tumor suppressor, 14 , 59 suggesting that decreased TGFβ1 activity in epithelial cells could contribute to the increased risk for breast cancer in obesity. When activated, TGFβ1 reduces mammary epithelial cell proliferation. 60 TGFβ1 has also been implicated in the negative regulation of stem/progenitor cells within the mammary epithelium. 17 , 61 Recently, ERα+ progenitor cells have been described within the mammary gland, 62 , 63 and active TGFβ1 has been shown to play a key role in their regulation through restriction of proliferation of ERα+ epithelial cells. 64 Proliferating ERα+ cells have been identified within the human breast after menopause, 65 when women are at enhanced risk for the formation of ERα+ breast tumors. 66 , 67 , 68 This observation has led to the hypothesis that these ERα+ progenitor cells may be the cells of origin for ERα+ breast cancers. 69 We have observed that obesity elevates the number of ERα+ epithelial cells in both premenopausal and postmenopausal breast tissue and increases the population of ERα+ proliferating cells, 8 and reduced TGFβ1 activity in the mammary gland may play a role the expansion of these cells. Although TGFβ1 has been well‐characterized in regulating cellular proliferation, TGFβ1 also acts to maintain genomic stability through its participation in the DNA damage response. 70 Inhibition of TGFβ1 activity with small molecule inhibitors significantly enhanced centrosome aberration frequency, tetraploidy, and aneuploidy in cultured human mammary epithelial cells. 71 Thus, reduced TGFβ1 activity within the mammary epithelium under conditions of obesity could lead to enhanced risk for breast cancer development through both expansion of stem/progenitor cells as well as increased genomic instability. Future studies will uncover how this mechanism may impact mammary tumor formation.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
T. Chamberlin, V. Thompson, and L.M. Arendt designed the research; T. Chamberlin, V. Thompson, and L.M. Arendt analyzed data; T. Chamberlin, V. Thompson, L.E. Hillers‐Ziemer, and B. Walton performed research; T. Chamberlin and L.M. Arendt drafted the manuscript; L.M. Arendt oversaw the research.
ACKNOWLEDGMENTS
The authors would like to thank Caylee Silver for technical assistance. This work was supported by the University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520, National Institutes of Health (NIH) Grants R01 CA227542 (to LMA) and T32 OD010423 (to TC) and Susan G. Komen Career Catalyst Grant CCR1532611 (to LMA).
Chamberlin T, Thompson V, Hillers‐Ziemer LE, Walton BN, Arendt LM. Obesity reduces mammary epithelial cell TGFβ1 activity through macrophage‐mediated extracellular matrix remodeling. The FASEB Journal. 2020;34:8611–8624. 10.1096/fj.202000228RR
REFERENCES
- 1. Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625‐1638. [DOI] [PubMed] [Google Scholar]
- 3. Cecchini RS, Costantino JP, Cauley JA, et al. Body mass index and the risk for developing invasive breast cancer among high‐risk women in NSABP P‐1 and STAR breast cancer prevention trials. Cancer Prev Res. 2012;5:583‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Keymeulen A, Rocha AS, Ousset M, et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189‐193. [DOI] [PubMed] [Google Scholar]
- 5. Boras‐Granic K, Dann P, Wysolmerski JJ. Embryonic cells contribute directly to the quiescent stem cell population in the adult mouse mammary gland. Breast Cancer Res. 2014;16:487‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prater MD, Petit V, Alasdair Russell I, et al. Mammary stem cells have myoepithelial cell properties. Nat Cell Biol. 2014;16:942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ginestier C, Wicha MS. Mammary stem cell number as a determinate of breast cancer risk. Breast Cancer Res. 2007;9:109‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chamberlin T, D'Amato JV, Arendt LM. Obesity reversibly depletes the basal cell population and enhances mammary epithelial cell estrogen receptor alpha expression and progenitor activity. Breast Cancer Res. 2017;19:128‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Subbaramaiah K, Howe LR, Bhardwaj P, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res. 2011;4:329‐346. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Subbaramaiah K, Morris PG, Zhou XK, et al. Increased levels of COX‐2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356‐365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Seo BR, Bhardwaj P, Choi S, et al. Obesity‐dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:301ra130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hillers LE, D'Amato JV, Chamberlin T, Paderta G, Arendt LM. Obesity‐activated adipose‐derived stromal cells promote breast cancer growth and invasion. Neoplasia. 2018;20:1161‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y, Alexander PB, Wang XF. TGF‐β family signaling in the control of cell proliferation and survival. Cold Spring Harb Perspect Biol. 2017;9:a022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moses H, Barcellos‐Hoff MH. TGF‐beta biology in mammary development and breast cancer. Cold Spring Harb Perspect Biol. 2011;3:a003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ewan KB, Shyamala G, Ravani SA, et al. Latent transforming growth factor‐beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am J Pathol. 2002;160:2081‐2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robinson SD, Silberstein GB, Roberts AB, Flanders KC, Daniel CW. Regulated expression and growth inhibitory effects of transforming growth factor‐beta isoforms in mouse mammary gland development. Development. 1991;113:867‐878. [DOI] [PubMed] [Google Scholar]
- 17. Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH. Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol. 1995;168:47‐61. [DOI] [PubMed] [Google Scholar]
- 18. Boulanger CA, Smith GH. Reducing mammary cancer risk through premature stem cell senescence. Oncogene. 2001;20:2264‐2272. [DOI] [PubMed] [Google Scholar]
- 19. Booth D, Haley JD, Bruskin AM, Potten CS. Transforming growth factor‐B3 protects murine small intestinal crypt stem cells and animal survival after irradiation, possibly by reducing stem‐cell cycling. Int J Cancer. 2000;86:53‐59. [DOI] [PubMed] [Google Scholar]
- 20. van't Land B, Meijer HP, Frerichs J, et al. Transforming Growth Factor‐beta2 protects the small intestine during methotrexate treatment in rats possibly by reducing stem cell cycling. Br J Cancer. 2002;87:113‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feng X‐H, Derynck R. Specificity and versatility in TGF‐β signaling through SMADS. Annu Rev Cell Dev Biol. 2005;21:659‐693. [DOI] [PubMed] [Google Scholar]
- 22. Miyazono K. Positive and negative regulation of TGF‐beta signaling. J Cell Sci. 2000;113(Pt 7):1101‐1109. [DOI] [PubMed] [Google Scholar]
- 23. Horiguchi M, Ota M, Rifkin DB. Matrix control of transforming growth factor‐β function. J Biochem. 2012;152:321‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor‐beta in adipose tissue from obese mice. Mol Med. 1997;3:37‐48. [PMC free article] [PubMed] [Google Scholar]
- 25. Alessi MC, Bastelica D, Morange P, et al. Plasminogen activator inhibitor 1, transforming growth factor‐beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes. 2000;49:1374‐1380. [DOI] [PubMed] [Google Scholar]
- 26. Fain JN, Tichansky DS, Madan AK. Transforming growth factor beta1 release by human adipose tissue is enhanced in obesity. Metabolism. 2005;54:1546‐1551. [DOI] [PubMed] [Google Scholar]
- 27. O'Brien J, Fornetti J, Schedin P. Isolation of mammary‐specific extracellular matrix to assess acute cell‐ECM interactions in 3D culture. J Mammary Gland Biol Neoplasia. 2010;15:353‐364. [DOI] [PubMed] [Google Scholar]
- 28. Arendt LM, McCready J, Keller PJ, et al. Obesity promotes breast cancer by CCL2‐mediated macrophage recruitment and angiogenesis. Cancer Res. 2013;73:6080‐6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neuzillet C, Tijeras‐Raballand A, Cohen R, et al. Targeting the TGFβ pathway for cancer therapy. Pharmacol Ther. 2015;147:22‐31. [DOI] [PubMed] [Google Scholar]
- 30. Lin DE, Chun T‐H, Kang LI. Adipose extracellular matrix remodelling in obesity and insulin resistance. Biochem Pharmacol. 2016;119:8‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bolton K, Segal D, McMillan J, et al. Decorin is a secreted protein associated with obesity and type 2 diabetes. Int J Obes. 2008;32:1113‐1121. [DOI] [PubMed] [Google Scholar]
- 32. Shieh Y, Scott CG, Jensen MR, et al. Body mass index, mammographic density, and breast cancer risk by estrogen receptor subtype. Breast Cancer Res. 2019;21:48‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oda G, Sato T, Ishikawa T, et al. Significance of stromal decorin expression during the progression of breast cancer. Oncol Rep. 2012;28:2003‐2008. [DOI] [PubMed] [Google Scholar]
- 34. Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor‐beta by the proteoglycan decorin. Nature. 1990;346:281‐284. [DOI] [PubMed] [Google Scholar]
- 35. Zhang Z, Garron TM, Li XJ, et al. Recombinant human decorin inhibits TGF‐beta1‐induced contraction of collagen lattice by hypertrophic scar fibroblasts. Burns. 2009;35:527‐537. [DOI] [PubMed] [Google Scholar]
- 36. Rifkin DB. Latent transforming growth factor‐beta (TGF‐beta) binding proteins: orchestrators of TGF‐beta availability. J Biol Chem. 2005;280:7409‐7412. [DOI] [PubMed] [Google Scholar]
- 37. Desai VD, Hsia HC, Schwarzbauer JE. Reversible modulation of myofibroblast differentiation in adipose‐derived mesenchymal stem cells. PLoS ONE. 2014;9:e86865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang W, Ge Y, Cheng Q, Zhang Q, Fang L, Zheng J. Decorin is a pivotal effector in the extracellular matrix and tumour microenvironment. Oncotarget. 2018;9:5480‐5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Costanza B, Umelo IA, Bellier J, Castronovo V, Turtoi A. Stromal modulators of TGF‐beta in cancer. J Clin Med. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iyengar NM, Brown KA, Zhou XK, et al. Metabolic obesity, adipose inflammation and elevated breast aromatase in women with normal body mass index. Cancer Prev Res. 2017;10:235‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Afik R, Zigmond E, Vugman M, et al. Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med. 2016;213:2315‐2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang YY, Jiang H, Pan J, et al. Macrophage‐to‐myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J Am Soc Nephrol. 2017;28:2053‐2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fang L, Kojima K, Zhou L, Crossman DK, Mobley JA, Grams J. Analysis of the human proteome in subcutaneous and visceral fat depots in diabetic and non‐diabetic patients with morbid obesity. J Proteomics Bioinformatics. 2015;8:133‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Best J, Verhulst S, Syn WK, et al. Macrophage depletion attenuates extracellular matrix deposition and ductular reaction in a mouse model of chronic cholangiopathies. PLoS ONE. 2016;11:e0162286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de la Durantaye M, Piette AB, van Rooijen N, Frenette J. Macrophage depletion reduces cell proliferation and extracellular matrix accumulation but increases the ultimate tensile strength of injured Achilles tendons. J Orthop Res. 2014;32:279‐285. [DOI] [PubMed] [Google Scholar]
- 48. Murphy‐Ullrich JE, Poczatek M. Activation of latent TGF‐beta by thrombospondin‐1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59‐69. [DOI] [PubMed] [Google Scholar]
- 49. Crawford SE, Stellmach V, Murphy‐Ullrich JE, et al. Thrombospondin‐1 is a major activator of TGF‐beta1 in vivo. Cell. 1998;93:1159‐1170. [DOI] [PubMed] [Google Scholar]
- 50. Varma V, Yao‐Borengasser A, Bodles AM, et al. Thrombospondin‐1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes. 2008;57:432‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hida K, Wada J, Zhang H, et al. Identification of genes specifically expressed in the accumulated visceral adipose tissue of OLETF rats. J Lipid Res. 2000;41:1615‐1622. [PubMed] [Google Scholar]
- 52. Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology. 2005;146:4545‐4554. [DOI] [PubMed] [Google Scholar]
- 53. Gao CL, Zhu JG, Zhao YP, et al. Mitochondrial dysfunction is induced by the overexpression of UCP4 in 3T3‐L1 adipocytes. Int J Mol Med. 2010;25:71‐80. [PubMed] [Google Scholar]
- 54. Rendra E, Riabov V, Mossel DM, Sevastyanova T, Harmsen MC, Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;224:242‐253. [DOI] [PubMed] [Google Scholar]
- 55. Assoian RK, Fleurdelys BE, Stevenson HC, et al. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci. 1987;84:6020‐6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lodyga M, Cambridge E, Karvonen HM, et al. Cadherin‐11‐mediated adhesion of macrophages to myofibroblasts establishes a profibrotic niche of active TGF‐beta. Sci Signal. 2019;12:1‐19. [DOI] [PubMed] [Google Scholar]
- 57. Lee MJ. Transforming growth factor beta superfamily regulation of adipose tissue biology in obesity. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1160‐1171. [DOI] [PubMed] [Google Scholar]
- 58. Van Bockstal M, Lambein K, Gevaert O, et al. Stromal architecture and periductal decorin are potential prognostic markers for ipsilateral locoregional recurrence in ductal carcinoma in situ of the breast. Histopathology. 2013;63:520‐533. [DOI] [PubMed] [Google Scholar]
- 59. Zarzynska JM. Two faces of TGF‐beta1 in breast cancer. Mediators Inflamm. 2014;2014:141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Daniel CW, Silberstein GB, Van Horn K, Strickland P, Robinson S. TGF‐beta 1‐induced inhibition of mouse mammary ductal growth: developmental specificity and characterization. Dev Biol. 1989;135:20‐30. [DOI] [PubMed] [Google Scholar]
- 61. Boulanger CA, Wagner KU, Smith GH. Parity‐induced mouse mammary epithelial cells are pluripotent, self‐renewing and sensitive to TGF‐beta1 expression. Oncogene. 2005;24:552‐560. [DOI] [PubMed] [Google Scholar]
- 62. Van Keymeulen A, Fioramonti M, Centonze A, Bouvencourt G, Achouri Y, Blanpain C. Lineage‐restricted mammary stem cells sustain the development, homeostasis, and regeneration of the estrogen receptor positive lineage. Cell Rep. 2017;20:1525‐1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang C, Christin JR, Oktay MH, Guo W. Lineage‐biased stem cells maintain estrogen‐receptor‐positive and ‐negative mouse mammary luminal lineages. Cell Rep. 2017;18:2825‐2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ewan KB, Oketch‐Rabah HA, Ravani SA, Shyamala G, Moses HL, Barcellos‐Hoff MH. Proliferation of estrogen receptor‐alpha‐positive mammary epithelial cells is restrained by transforming growth factor‐beta1 in adult mice. Am J Pathol. 2005;167:409‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shoker BS, Jarvis C, Clarke RB, et al. Estrogen receptor‐positive proliferating cells in the normal and precancerous breast. Am. J. Pathol. 1999;155:1811‐1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Phipps AI, Buist DS, Malone KE, et al. Breast density, body mass index, and risk of tumor marker‐defined subtypes of breast cancer. Ann Epidemiol. 2012;22:340‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Body size and risk of luminal, HER2‐overexpressing, and triple‐negative breast cancer in postmenopausal women. Cancer Epidemiol. Biomarkers Prev. 2008;17:2078‐2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Biglia N, Peano E, Sgandurra P, et al. Body mass index (BMI) and breast cancer: impact on tumor histopathologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecol Endocrinol. 2013;29:263‐267. [DOI] [PubMed] [Google Scholar]
- 69. Walker RA, Martin CV. The aged breast. J Pathol. 2007;211:232‐240. [DOI] [PubMed] [Google Scholar]
- 70. Barcellos‐Hoff MH, Cucinotta FA. New tricks for an old fox: impact of TGFbeta on the DNA damage response and genomic stability. Sci Signal. 2014;7:re5. [DOI] [PubMed] [Google Scholar]
- 71. Maxwell CA, Fleisch MC, Costes SV, et al. Targeted and nontargeted effects of ionizing radiation that impact genomic instability. Cancer Res. 2008;68:8304‐8311. [DOI] [PubMed] [Google Scholar]


