Abstract
Aims
To evaluate the efficacy and safety of dapagliflozin (DAPA) + saxagliptin (SAXA) compared with glimepiride (GLIM) in patients with type 2 diabetes who were inadequately controlled [glycated haemoglobin (HbA1c) 7.5–10.5% (58–91 mmol/mol)] on metformin monotherapy.
Materials and methods
This 52‐week, multicentre, double‐blind, active‐controlled study (NCT02419612) randomized (1:1) patients on metformin to add‐on DAPA 10 mg + SAXA 5 mg (n = 227) or GLIM 1–6 mg (titrated; n = 217). The primary efficacy endpoint was change in HbA1c from baseline to week 52.
Results
Baseline mean ± standard deviation of age, duration of diabetes and HbA1c were 56.1 ± 9.7 years, 7.8 ± 6.4 years and 8.5% ± 0.8% (69 ± 9.0 mmol/mol), respectively. Adjusted mean change from baseline in HbA1c was −1.35% (−14.8 mmol/mol) with DAPA + SAXA versus −0.98% (−10.7 mmol/mol) with GLIM (P <0.001). Changes from baseline in body weight and systolic blood pressure were −3.1 kg and −2.6 mmHg with DAPA + SAXA versus +1.0 kg (P <0.001) and +1.0 mmHg (P = 0.007) with GLIM. More patients achieved HbA1c <7.0% (53 mmol/mol) (44.3% vs. 34.3%; P = 0.044), and fewer patients required treatment intensification (1.3% vs. 8.8%; P = 0.002) with DAPA + SAXA than with GLIM.
Conclusions
Compared with GLIM, concurrent addition of DAPA + SAXA significantly improved glycaemic control, body weight and other metabolic parameters in patients inadequately controlled on metformin.
Trial: NCT02419612, ClinicalTrials.gov.
Keywords: dapagliflozin, glimepiride, glycaemic control, intensification, metformin, saxagliptin
1. INTRODUCTION
Type 2 diabetes is a complex progressive disease that makes achieving and maintaining glycaemic control a challenge.1 The typical treatment paradigm for type 2 diabetes comprises initial metformin monotherapy, with stepwise addition of further antidiabetes agents as glycaemic control worsens.2 Most patients eventually require treatment with two or more agents to achieve or maintain their glycaemic targets.3, 4
Sulphonylureas are widely used as second‐line therapy for type 2 diabetes, owing to their favourable efficacy as antihyperglycaemic agents and low cost. However, their disadvantages include an increased risk of hypoglycaemia, weight gain and poor durability of treatment efficacy.5 Newer classes of oral antidiabetes drugs, including sodium‐glucose cotransporter‐2 (SGLT‐2) inhibitors and dipeptidyl peptidase‐4 (DPP‐4) inhibitors, are promising alternative second‐line therapies to sulphonylureas. Results from clinical and observational studies suggest that the addition of these agents to metformin provides similar or superior efficacy and an improved side effect profile compared with the addition of sulphonylureas to metformin.6, 7, 8, 9, 10, 11, 12, 13, 14
The combination of dapagliflozin (DAPA), an SGLT‐2 inhibitor, and saxagliptin (SAXA), a DPP‐4 inhibitor, has shown superior efficacy when added to metformin compared with add‐on of either monotherapy alone.15, 16, 17, 18, 19 Moreover, results from a recent German study demonstrated that the combination of DAPA, SAXA and metformin was associated with greater improvements in glycaemic control than glimepiride (GLIM; a sulphonylurea) + metformin.20 However, there remains a need for further head‐to‐head studies to compare this triple therapy regimen with the combination of a sulphonylurea and metformin.
The aim of this study was to evaluate changes in glycaemic control with concurrent addition of DAPA and SAXA, compared with GLIM, in patients with type 2 diabetes and inadequate glycaemic control on a maximum tolerated dose of metformin background therapy.
2. MATERIALS AND METHODS
2.1. Study design
This was a 52‐week, multicentre, randomized, parallel‐group, double‐blind, active‐controlled, phase 3b study (NCT02419612), conducted at 87 centres in Germany, the Czech Republic, Hungary, Mexico, Poland, Romania, Russia, Sweden, the UK and the United States. The study design also included a blinded 104‐week extension period, which has recently been completed and the results of which will be published separately. Local regulatory authorities and the responsible ethics committees/institutional review boards of the participating centres approved the study protocol, and all participants provided written informed consent. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and Good Clinical Practice guidelines of the International Conference on Harmonisation.
2.2. Patients
Men and women aged ≥18 years were eligible for inclusion in the study if they fulfilled the following criteria: diagnosis of type 2 diabetes; currently treated with metformin, and on a stable dose (≥1500 mg/day) for ≥8 weeks before enrolment; body mass index (BMI) 20–45 kg/m2; fasting plasma glucose (FPG) ≤270 mg/dL (≤15 mmol/L) at the time of randomization; and glycated haemoglobin (HbA1c) 7.5%–10.5% (58–91 mmol/mol). Major exclusion criteria included a cardiovascular event in the 3 months before enrolment; an estimated glomerular filtration rate (GFR) <60 mL/min; and the presence or history of unstable, acute or severe congestive heart failure (New York Heart Association Functional Classification III and IV) and/or left ventricular ejection fraction ≤40%, obtained from medical records.
2.3. Treatments
The study comprised a 2‐week screening period and 2‐week lead‐in period, after which patients completed a randomization visit, followed by a 52‐week, double‐blind treatment phase (Figure S1A; see Supporting Information). At the randomization visit, patients were randomized (1:1) to one of two treatment arms by an interactive voice response system: DAPA 10 mg, plus SAXA 5 mg, plus GLIM placebo; or GLIM (1, 2, 3, 4 or 6 mg, titrated), plus SAXA and DAPA matching placebos. DAPA and SAXA were taken orally, once daily, at fixed doses throughout the treatment period, whereas GLIM treatment was initiated at 1 mg/day and could be up‐titrated in increments of 1–2 mg at 3‐week intervals during the first 12 weeks of the study to a maximum of 6 mg/day. Up‐titration, permitted only during the first 12 weeks, was performed based on FPG levels [target of ≤110 mg/dL (6.1 mmol/L)] or to the highest tolerable dose; down‐titration, permitted throughout the study, was allowed in patients who experienced hypoglycaemic episodes. Although guidance was provided, the decision to titrate was at the investigator's discretion. Patients were eligible for initiation of open‐label rescue with insulin from week 9 of the study if their FPG levels met the following criteria: week 9, FPG >270 mg/dL (15.0 mmol/L); weeks 10–16, FPG >240 mg/dL (13.3 mmol/L); weeks 17–28, FPG >220 mg/dL (12.2 mmol/L); and weeks 29–52, FPG >200 mg/dL (11.1 mmol/L).
2.4. Endpoints and assessments
The primary efficacy endpoint was the mean change in HbA1c from baseline to the end of the 52‐week treatment period. Secondary endpoints at 52 weeks included: change from baseline in total body weight; proportion of patients achieving a therapeutic response, defined as HbA1c <7.0% (53 mmol/mol); change from baseline in systolic blood pressure (SBP); and time to treatment intensification (addition of insulin or other glucose‐lowering agent for rescue therapy or discontinuation for lack of glycaemic control) during the 52‐week treatment period. Exploratory endpoints included the proportion of patients achieving HbA1c <7.0% (53 mmol/mol) either without any hypoglycaemia or without both any hypoglycaemia or weight gain at week 52, and the change from baseline in FPG at week 52. In addition, the change from baseline in glycaemic variability [defined by the mean amplitude of glycaemic excursions (MAGE)], 24‐h mean glucose, time spent in the euglycaemic range [71–180 mg/dL (3.9–10.0 mmol/L)] and time spent in low range [≤70 mg/dL (≤3.9 mmol/L)] were assessed in a subgroup of patients, using blinded iPro®2 (Medtronic, Minnesota) professional continuous glucose monitoring (CGM) between weeks −2 and −1 and weeks 51 and 52 of the study. To be eligible for inclusion in the CGM substudy, patients needed to have a successful baseline CGM reading, BMI of 20.0–40.0 kg/m2 at the enrolment visit and to have provided informed consent.
Safety endpoints included the proportion of patients experiencing adverse events (AEs) and hypoglycaemia, as well as findings from physical examinations, electrocardiograms (ECGs) and clinical laboratory evaluations. Hypoglycaemic events were classified, according to the 2013 American Diabetes Association recommendations,21 as follows: severe hypoglycaemia (an event requiring assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions); documented symptomatic hypoglycaemia [typical symptoms of hypoglycaemia accompanied by a measured plasma glucose concentration of ≤70 mg/dL (3.9 mmol/L)]; and asymptomatic hypoglycaemia [an event not accompanied by typical symptoms of hypoglycaemia but with a measured plasma glucose concentration of ≤70 mg/dL (3.9 mmol/L). Events could fall within more than one category. We also classified hypoglycaemic events as either “major”, which was defined as a symptomatic episode that required third‐party assistance due to severe impairment in consciousness or behaviour, with a plasma glucose level of <54 mg/dL (<3 mmol/L) and a prompt recovery after glucose or glucagon administration, or “minor”, which was defined as either a symptomatic or an asymptomatic episode, with a plasma glucose level of <63 mg/dL (<3.5 mmol/L), which did not qualify as a major episode.
2.5. Statistical methods
Sample size was determined assuming a 0.35% difference in the mean change from baseline in HbA1c for DAPA + SAXA + metformin versus GLIM + metformin. Calculations used a common standard deviation (SD) of 1.1%, and a two‐sided significance level of 0.05 for the comparison, assuming that 5% of the data would not be evaluable. In total, 220 randomized patients per treatment arm were required to provide approximately 90% power for the comparison between the two treatment groups.
Efficacy analyses were conducted on the randomized analysis data set, which comprised all randomized patients who received at least one dose of study medication during the double‐blind treatment period. Unless specified, analyses included only values collected before rescue or treatment discontinuation. The primary efficacy endpoint was analysed using a longitudinal, repeated‐measures, mixed‐effects model that included the fixed categorical effects of treatment, week and treatment‐by‐week interaction. Several sensitivity analyses using different assumptions concerning missing data were conducted for the primary endpoint, including one using all available values regardless of rescue or treatment discontinuation.
Analyses of change from baseline in total body weight and SBP were performed using the same longitudinal model as for the primary endpoint. The proportion of patients achieving a therapeutic response was compared between treatment groups using logistic regression, and time to treatment intensification was analysed using a Cox proportional hazards model. To protect the overall type I error rate, secondary endpoints were assessed for significance using a stepwise testing procedure. The order of testing was: mean change from baseline in total body weight at week 52; proportion of patients achieving a therapeutic response at week 52; mean change in SBP at week 52; and time to treatment intensification during the double‐blind treatment period. No exploratory endpoints were included in the stepwise testing procedure; however, nominal P‐values are reported. Data for the CGM substudy were centrally collected, reviewed and analysed by Phase V Technologies, Inc. (Wellesley Hills, Massachusetts).
All safety analyses were performed on the data set of treated patients, which consisted of patients who received at least one dose of study medication (all randomized patients in this study). Safety data were summarized using descriptive statistics.
3. RESULTS
3.1. Patient characteristics
The first patient was enrolled on August 14, 2015 and the last patient was enrolled on August 3, 2016. The participant flow diagram for this study is shown in Figure S1B (see Supporting Information). In total, 823 patients were enrolled in the study and 444 were randomized, of whom 385 (86.9%) completed the study. In the CGM substudy, 118 patients were randomized (61 patients to the DAPA + SAXA arm and 57 patients to the GLIM arm), of whom 98 (83.1%) completed the study.
Baseline demographic and diabetes characteristics were balanced across treatment groups (Table 1). The mean ± SD of patient age was 56.1 ± 9.7 years; 49.2% of patients were men and most (90.1%) were white. The mean ± SD of duration of type 2 diabetes, HbA1c, FPG, BMI and SBP were 7.8 ± 6.4 years, 8.45 ± 0.82% (69 ± 9.0 mmol/mol), 174.7 ± 41.9 mg/dL (9.7 ± 2.3 mmol/L), 32.3 ± 5.2 kg/m2 and 131.2 ± 14.0 mmHg, respectively. The mean ± SD of estimated GFR was 93.3 ± 22.1 mL/min/1.73 m2. Baseline characteristics for the patients enrolled in the CGM substudy were broadly comparable with the overall population, although, notably, the duration of diabetes was numerically greater in the CGM subset (9.5 vs. 7.7 years in the DAPA + SAXA group, and 11.2 vs. 7.9 years in the GLIM group) (Table S1; see Supporting Information).
Table 1.
Participant demographics and baseline characteristics (randomized analysis set)a
| Variable | DAPA + SAXA + MET (N = 227) | GLIM + MET (N = 216) | Total (N = 443) |
|---|---|---|---|
| Age (years) | 56.1 (10.1) | 56.1 (9.2) | 56.1 (9.7) |
| Sex (n) (%) | |||
| Women | 110 (48.5) | 115 (53.2) | 225 (50.8) |
| Men | 117 (51.5) | 101 (46.8) | 218 (49.2) |
| Race (n) (%) | |||
| White | 204 (89.9) | 195 (90.3) | 399 (90.1) |
| Black/African American | 4 (1.8) | 5 (2.3) | 9 (2.0) |
| American Indian/Alaska Native | 11 (4.8) | 10 (4.6) | 21 (4.7) |
| Other | 8 (3.5) | 6 (2.8) | 14 (3.2) |
| Ethnic group (n) (%)b | |||
| Hispanic or Latino | 36 (15.9) | 35 (16.2) | 71 (16.0) |
| Duration of type 2 diabetes (years) | 7.7 (6.4) | 7.9 (6.5) | 7.8 (6.4) |
| HbA1c (%) | 8.41 (0.82) | 8.50 (0.82) | 8.45 (0.82) |
| HbA1c (mmol/mol) | 68 (9.0) | 69 (9.0) | 69 (9.0) |
| FPG (mg/dL) | 172.9 (41.5) | 176.5 (42.4) | 174.7 (41.9) |
| Weight (kg) | 91.0 (19.8) | 88.4 (17.1) | 89.7 (18.5) |
| BMI (kg/m2) | 32.4 (5.3) | 32.2 (5.1) | 32.3 (5.2) |
| eGFR (MDRD) (mL/min/1.73 m2) | 93.7 (23.0) | 93.0 (21.1) | 93.3 (22.1) |
| SBP (mmHg) | 129.9 (13.9) | 132.5 (14.2) | 131.2 (14.0) |
| Vascular history | 163 (71.8) | 160 (74.1) | 323 (72.9) |
| Hypertension | 160 (70.5) | 158 (73.1) | 318 (71.8) |
| Carotid artery disease | 1 (0.4) | 0 | 1 (0.2) |
| Coronary artery disease | 21 (9.3) | 16 (7.4) | 37 (8.4) |
| Peripheral vascular disease | 13 (5.7) | 5 (2.3) | 18 (4.1) |
| Stable angina | 17 (7.5) | 11 (5.1) | 28 (6.3) |
| Otherc | 22 (9.7) | 16 (7.4) | 38 (8.6) |
Abbreviations: BMI, body mass index; CABG, coronary artery bypass graft; DAPA, dapagliflozin; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; GLIM, glimepiride; HbA1c, glycated haemoglobin; MDRD, Modification of Diet in Renal Disease; MET, metformin; MI, myocardial infarction; PCI, percutaneous coronary intervention; SAXA, saxagliptin; SBP, systolic blood pressure; SD, standard deviation; TIA, transient ischaemic attack.
Data are presented as mean ± SD or number (%).
For participants from the United States only (n = 57 for DAPA + SAXA + MET, n = 55 for GLIM + MET, n = 112 in total).
Includes CABG, carotid endarterectomy or stenting, cerebrovascular accident, congestive heart failure, hospitalization for unstable angina, PCI, peripheral vascular surgery, previous MI and TIA.
3.2. Study drug treatment
The mean ± SD of GLIM dose at week 52 was 3.8 ± 2.0 mg. During the 12‐week titration period, 188 patients (87%) up‐titrated their dose of GLIM, of which 80 patients (37%) up‐titrated to the maximum dose of 6 mg. During the 52‐week study period, 43.5% of patients down‐titrated their dose of GLIM. Doses of DAPA (10 mg/day) and SAXA (5 mg/day) were fixed throughout the treatment period.
3.3. Efficacy
The adjusted mean change from baseline in HbA1c at 52 weeks was significantly greater with DAPA + SAXA [n = 193; −1.35% (−14.8 mmol/mol)] than with GLIM [n = 171; −0.98% (−10.7 mmol/mol); P < 0.001 vs. GLIM] (Figure 1A; Table 2). The change in HbA1c over time from baseline to week 52 is shown in Figure 1(B). Results of the sensitivity analysis of change from baseline in HbA1c at 52 weeks that included all patients, regardless of rescue or discontinuation, were essentially identical to those from the primary analysis [DAPA + SAXA: −1.37% (−15.0 mmol/mol); GLIM: −1.05% (−11.5 mmol/mol); P < 0.001]. The proportion of patients who achieved HbA1c <7.0% (53 mmol/mol) at 52 weeks was significantly greater with DAPA + SAXA than with GLIM (P = 0.044) (Table 2; Figure 2A).
Figure 1.
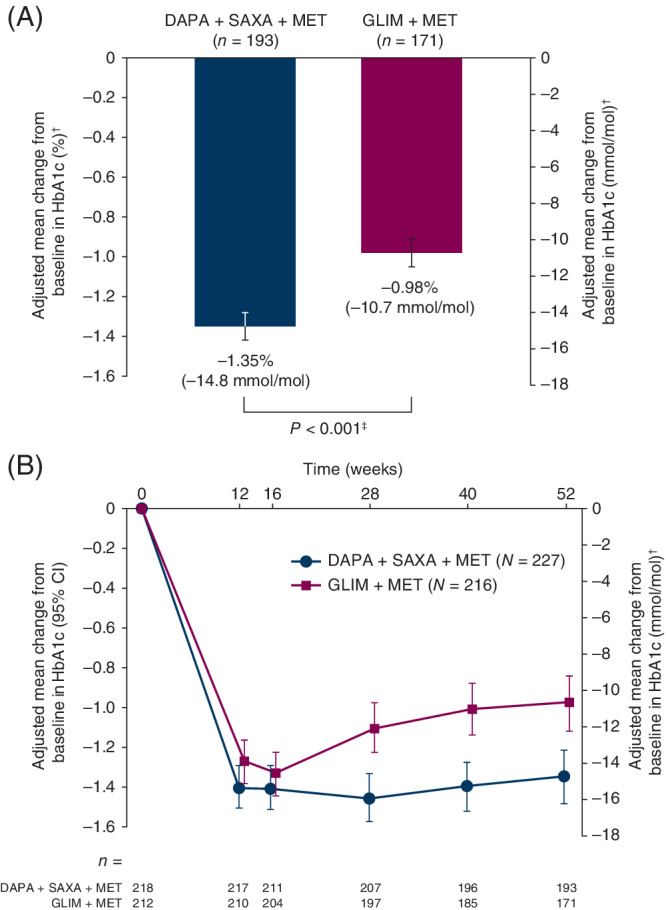
A, Adjusted mean change from baseline to week 52 in HbA1c. B, Adjusted mean change from baseline in HbA1c during the 52‐week, double‐blind treatment period (randomized analysis set). N refers to the number of patients in each group who were randomized and received at least one dose of treatment with the study drug; n denotes the number of patients with available measurements at baseline and week 52. †All values are least‐squares mean ± standard error. ‡ P‐value obtained using a mixed model of repeated measures with terms for treatment, baseline HbA1c, week, treatment‐by‐week interaction and baseline HbA1c‐by‐week interaction. Abbreviations: CI, confidence interval; DAPA, dapagliflozin; GLIM, glimepiride; HbA1c, glycated haemoglobin; MET, metformin; SAXA, saxagliptin
Table 2.
Primary, secondary and exploratory endpoints at 52 weeks, before rescue (randomized analysis set)a
| DAPA + SAXA + MET (N = 227) | GLIM + MET (N = 216) | |||
|---|---|---|---|---|
| Efficacy endpoint (week 52) | Baseline | Week 52 | Baseline | Week 52 |
| HbA1c (%, mmol/mol)b | ||||
| Mean (SD) | n = 218 | n = 193 | n = 212 | n = 171 |
| 8.4 (68.0) [0.8 (8.7)] | 7.0 (53.0) [1.0 (10.8)] | 8.5 (69.0) [0.8 (9.0)] | 7.3 (56.0) [1.0 (11.2)] | |
| Change from baseline, adjusted LS mean (SE) | – | −1.35% (−14.8) [0.07 (0.8)] | – | −0.98% (−10.7) [0.07 (0.8)] |
| Difference from GLIM + MET, adjusted LS mean (95% CI) | – | −0.37 (−0.57, −0.18) | – | – |
| P‐value vs. GLIM + MET | – | <0.001 | – | – |
| Weight (kg)b | ||||
| Mean ± SD | n = 224 | n = 194 | n = 214 | n = 172 |
| 90.8 ± 19.7 | 88.4 ± 18.1 | 88.4 ± 17.0 | 90.6 ± 17.4 | |
| Change from baseline, adjusted LS mean (SE) | – | −3.1 (0.3) | – | 1.0 (0.3) |
| Difference from GLIM + MET, adjusted LS mean (95% CI) | – | −4.1 (−4.8, −3.3) | – | – |
| P‐value vs. GLIM + MET | – | <0.001 | – | – |
| Patients achieving HbA1c <7.0% (53 mmol/mol) | ||||
| Number of responders (% adjusted for baseline HbA1cc) | – | 105 (44.3) | – | 77 (34.3) |
| 95% CI for % adjusted | – | 37.5, 51.3 | – | 27.9, 41.3 |
| Odds ratio (95% CI) vs. GLIM + MET | – | 1.5 (1.01, 2.29) | – | – |
| P‐value vs. GLIM + MET | – | 0.044 | – | – |
| SBP (mmHg)b | ||||
| Mean (SD) | n = 224 | n = 191 | n = 214 | n = 171 |
| 129.9 (13.9) | 128.3 (14.8) | 132.5 (14.2) | 133.7 (15.1) | |
| Change from baseline, adjusted LS mean ± SE | – | −2.6 ± 0.9 | – | 1.0 ± 1.0 |
| Difference from GLIM + MET, adjusted LS mean (95% CI) | – | −3.6 (−6.3, −1.0) | – | – |
| P‐value vs. GLIM + MET | – | 0.007 | – | – |
| Patients requiring treatment intensificationd | ||||
| Number of patients receiving treatment intensification (%) | – | 3 (1.3) | – | 19 (8.8) |
| Hazard ratio (95% CI) vs. GLIM + MET | – | 0.15 (0.04, 0.50) | – | – |
| P‐value vs. GLIM + MET | – | 0.002 | – | – |
| Patients achieving HbA1c <7.0% (53 mmol/mol) without any hypoglycaemia | ||||
| Number of responders as % adjusted for baseline HbA1cc (95% CI) | – | 34.8 (28.9, 41.2) | – | 14.8 (10.7, 20.2) |
| Odds ratio (95% CI) vs. GLIM + MET | – | 3.1 (1.9, 4.9) | – | – |
| Nominal P‐value vs. GLIM + MET | – | <0.001 | – | – |
| Patients achieving HbA1c <7.0% (53 mmol/mol), without any hypoglycaemia or weight gain | ||||
| Number of responders as % adjusted for baseline HbA1cc (95% CI) | – | 30.0 (24.4, 36.2) | 6.5 (3.9, 10.6) | |
| Odds ratio (95% CI) vs. GLIM + MET | – | 6.2 (3.4, 11.4) | – | – |
| Nominal P‐value vs. GLIM + MET | – | <0.001 | – | – |
| FPG (mg/dL)b | ||||
| Mean ± SD | n = 224 | n = 194 | n = 214 | n = 170 |
| 172.7 ± 41.6 | 136.1 ± 28.4 | 176.6 ± 42.5 | 152.3 ± 34.1 | |
| Change from baseline, adjusted LS mean (SE) | – | −35.8 (2.0) | – | −17.0 (2.2) |
| Difference from GLIM + MET, adjusted LS mean (95% CI) | – | −18.8 (−24.6, −12.9) | – | – |
| Nominal P‐value vs. GLIM + MET | – | <0.001 | – | – |
| CGM substudy | DAPA + SAXA + MET ( N = 61) | GLIM + MET ( N = 57) | ||
| Baseline | Week 52 | Baseline | Week 52 | |
| MAGE (mg/dL) | ||||
| Mean ± SD | n = 45 | n = 45 | n = 32 | n = 32 |
| 92.2 ± 28.1 | 75.6 ± 29.1 | 94.7 ± 19.5 | 100.4 ± 28.4 | |
| Change from baseline, adjusted LS mean (SE)†† | – | −16.0 (3.9) | – | 7.2 (4.6) |
| Difference from GLIM + MET, adjusted LS mean (95% CI) | – | −23.2 (−35.3, −11.1) | – | – |
| Nominal P‐value vs. GLIM + MET | – | 0.0003 | – | – |
| 24‐hour glucose (mg/dL) | ||||
| Mean ± SD | 194.6 ± 38.4 | 148.3 ± 27.6 | 182.1 ± 28.3 | 162.6 ± 38.4 |
| Change from baseline, adjusted LS mean (SE)†† | – | −41.9 (4.6) | – | −25.4 (5.5) |
| Difference from GLIM + MET, adjusted LS mean (95% CI) | – | −16.5 (−30.8, −2.2) | – | – |
| Nominal P‐value vs. GLIM + MET | – | 0.024 | – | – |
| Percentage of time spent in euglycaemic range [71–180 mg/dL (3.9–10.0 mmol/L)] | ||||
| Mean ± SD | 45.6 ± 29.4 | 78.6 ± 19.5 | 53.8 ± 24.5 | 66.8 ± 25.5 |
| Change from baseline, adjusted LS mean (SE)†† | – | 30.3 (3.1) | – | 17.0 (3.6) |
| Difference from GLIM + MET, adjusted LS mean (95% CI) | – | 13.3 (3.8, 22.7) | – | – |
| Nominal P‐value vs. GLIM + MET | – | 0.0067 | – | – |
| Percentage of time spent in low range [≤70 mg/dL (≤3.9 mmol/L)] | ||||
| Mean ± SD | 0.24 ± 0.83 | 0.60 ± 1.57 | 0.15 ± 0.44 | 0.97 ± 2.16 |
| Change from baseline, adjusted LS mean (SE)†† | – | 0.41 (0.28) | – | 0.79 (0.33) |
| Difference from GLIM + MET, adjusted LS mean (95% CI) | – | −0.38 (−1.24, −0.49) | – | – |
| Nominal P‐value vs. GLIM + MET | – | 0.3887 | – | – |
Abbreviations: ANCOVA, analysis of covariance; CGM, continuous glucose monitoring; CI, confidence interval; DAPA, dapagliflozin; FPG, fasting plasma glucose; GLIM, glimepiride; HbA1c, glycated haemoglobin; LS, least‐squares; MAGE, mean amplitude of glycaemic excursions; MET, metformin; SAXA, saxagliptin; SBP, systolic blood pressure; SD, standard deviation; SE, standard error.
LS mean, LS mean treatment difference, SE, CI and P‐value were obtained from an ANCOVA model for change from baseline; baseline measurement was a covariate and treatment group was a fixed effect.
N refers to the number of patients in each group who were randomized and received at least one dose of treatment with the study drug; n denotes the number of patients with available measurements at baseline and week 52.
Mixed model of repeated measures with terms for treatment, baseline (HbA1c, weight, FPG or SBP), week, treatment‐by‐week interaction and baseline (HbA1c, weight, FPG, or SBP)‐by‐week interaction.
Logistic regression method with adjustment for baseline HbA1c.
Defined as addition of insulin or other glucose‐lowering agent for rescue therapy, or discontinuation for lack of glycaemic control.
Figure 2.
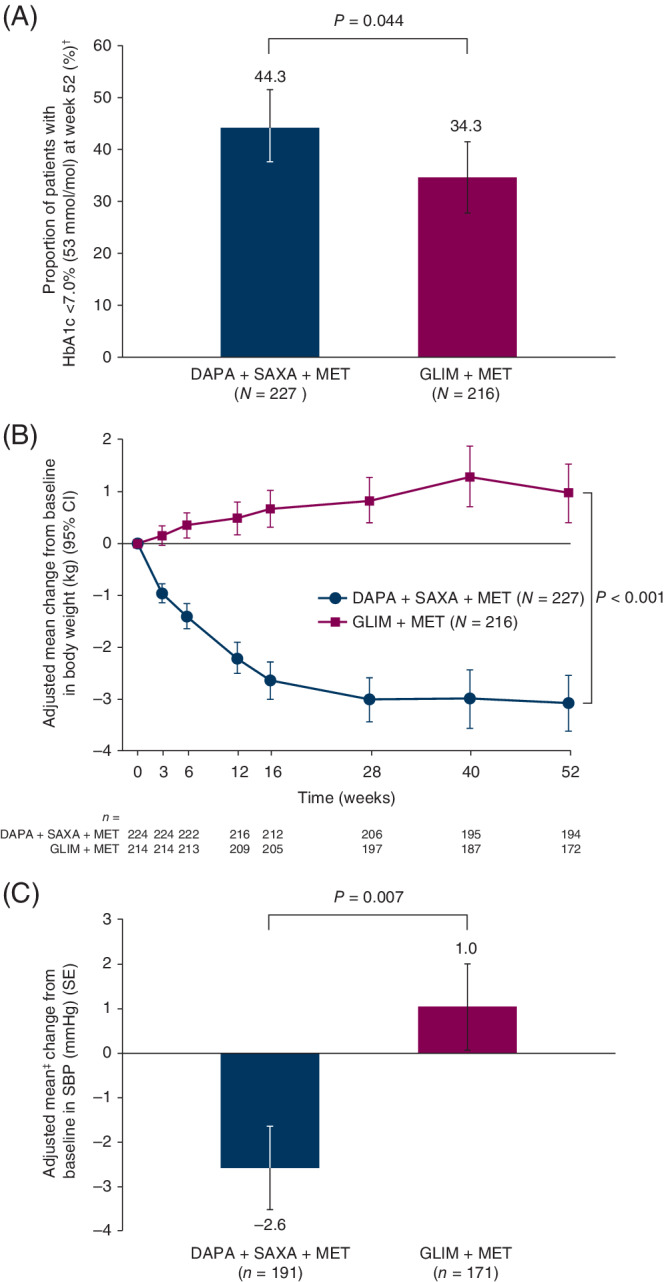
A, Proportion of patients with HbA1c <7.0% (53 mmol/mol) at week 52. B, Adjusted mean change from baseline in total body weight during the 52‐week, double‐blind treatment period. C, Adjusted mean change from baseline in SBP at week 52. N refers to the number of patients in each group who were randomized and received at least one dose of treatment with the study drug; n denotes the number of patients with available measurements at baseline and week 52. In the analysis of patients with HbA1c <7.0% (53 mmol/mol) at week 52, patients with an unknown status at week 52 and patients rescued before week 52 were treated as non‐responders. †MMRM model with terms for treatment, baseline body weight/SBP, week, treatment‐by‐week interaction and baseline body weight/SBP‐by‐week interaction. ‡Logistic regression method with adjustment for baseline HbA1c. Abbreviations: CI, confidence interval; DAPA, dapagliflozin; GLIM, glimepiride; HbA1c, glycated haemoglobin; MET, metformin; MMRM, mixed model of repeated measures; SAXA, saxagliptin; SBP, systolic blood pressure; SE, standard error
Total body weight decreased from baseline to week 52 with DAPA + SAXA, whereas it increased with GLIM (P < 0.001) (Table 2; Figure 2B). Similarly, SBP decreased from baseline to week 52 with DAPA + SAXA and increased with GLIM (P = 0.007) (Table 2; Figure 2C). Significantly fewer patients required treatment intensification with DAPA + SAXA than with GLIM (P = 0.002) (Table 2); however, these results were not included in sequential testing, because there were <10 patients in each treatment group.
In terms of exploratory efficacy variables, significantly more patients in the DAPA + SAXA group achieved HbA1c <7.0% (53 mmol/mol) without any hypoglycaemia or HbA1c <7.0% without any hypoglycaemia and without weight gain than in the GLIM group (nominal P <0.001 for both comparisons) (Table 2). There were also greater reductions in FPG from baseline with DAPA + SAXA than with GLIM alone (nominal P <0.001) (Table 2).
For patients in the CGM substudy, MAGE decreased from baseline to week 52 with DAPA + SAXA, whereas it increased with GLIM (Table 2). The 24‐h glucose levels decreased from baseline in both groups; however, the decrease was greater with DAPA + SAXA than with GLIM. Time spent in the euglycaemic range [71–180 mg/dL (3.9–10.0 mmol/L)] at week 52 was greater with DAPA + SAXA than with GLIM (78.6% vs. 66.8%), whereas time spent in the low glucose range [≤70 mg/dL (≤3.9 mmol/L)] was not significantly different between treatment groups (Table 2). Between‐treatment differences in MAGE, 24‐h glucose and time spent in the euglycaemic range were larger in the sensitivity analysis that included all patients without exclusion from analysis because of rescue or treatment discontinuation. For these patients, mean changes from baseline ± standard error were: MAGE: −15.9 ± 3.7 mg/dL (DAPA + SAXA, n = 47) vs. 9.7 ± 4.0 mg/dL (GLIM, n = 42), P < 0.0001; 24‐h glucose: −43.8 ± 4.7 mg/dL (DAPA + SAXA) vs. −18.8 ± 5.0 mg/dL (GLIM), P = 0.0005; percentage of time spent in euglycaemic range: 31.9% ± 3.3% (DAPA + SAXA) vs. 12.2 ± 3.5% (GLIM), P < 0.0001.
3.4. Safety and tolerability
The proportions of patients experiencing at least one hypoglycaemic event [≤70 mg/dL (3.9 mmol/L)] were 18.5% (148 events) and 44.0% (570 events) for the DAPA + SAXA and GLIM groups, respectively (Table 3). The proportion of patients experiencing documented symptomatic hypoglycaemia for the DAPA + SAXA and GLIM groups was 6.2% (29 events) and 20.8% (184 events), respectively; the proportion of patients experiencing asymptomatic hypoglycaemia was 13.7% (101 events) and 32.9% (363 events), respectively (Table 3). Using a lower plasma glucose cut‐off level of 63 mg/dL (3.5 mmol/L), minor hypoglycaemia was present in 8.4% (52 events) and 27.3% (219 events) of patients (Table 3). There were no episodes of severe hypoglycaemia in the DAPA + SAXA group, whereas three severe hypoglycaemic events occurred in two patients in the GLIM group (Table 3). One of these patients was receiving the maximum dose (6 mg) of GLIM before experiencing one of the reported severe episodes of hypoglycaemia, while the other patient was receiving 1 mg of GLIM at the time of experiencing the other two reported events. Major hypoglycaemia (requiring third‐party assistance and with a plasma glucose level measurement of <54 mg/dL (<3.0 mmol/L)] was not reported in either group. One patient in the GLIM group experienced a hypoglycaemic event that led to discontinuation of the study drug, whereas no patients discontinued the study drug because of hypoglycaemia in the DAPA + SAXA group.
Table 3.
Treatment‐emergent AEs (treated patient data set; data regardless of rescue)
| Number of patients (%) | ||
|---|---|---|
| AE category | DAPA + SAXA + MET (N = 227)a | GLIM + MET (N = 216)a |
| Hypoglycaemia | ||
| Severe hypoglycaemiab | 0 (0.0) | 2 (0.9) |
| Overall hypoglycaemia | ||
| ≤70 mg/dL (3.9 mmol/L) | 42 (18.5) | 95 (44.0) |
| <63 mg/dL (3.5 mmol/L) | 19 (8.4) | 59 (27.3) |
| Documented symptomatic hypoglycaemiac | 14 (6.2) | 45 (20.8) |
| Asymptomatic hypoglycaemia | 31 (13.7) | 71 (32.9) |
| Adverse events | ||
| ≥1 AE | 144 (63.4) | 137 (63.4) |
| ≥1 treatment‐related AE | 29 (12.8) | 13 (6.0) |
| AE leading to discontinuation of study medication | 8 (3.5) | 2 (0.9) |
| ≥1 SAE | 12 (5.3) | 10 (4.6) |
| ≥1 treatment‐related SAE | 2 (0.9) | 1 (0.5) |
| Death | 1 (0.4) | 2 (0.9) |
| Most common AEs by preferred term (frequency ≥2% of patients) | ||
| Upper respiratory tract infection | 17 (7.5) | 12 (5.6) |
| Urinary tract infection | 11 (4.8) | 8 (3.7) |
| Headache | 10 (4.4) | 11 (5.1) |
| Hypertension | 3 (1.3) | 11 (5.1) |
| AEs of special interestd | ||
| Urinary tract infection | 14 (6.2) | 9 (4.2) |
| Genital infections | ||
| Overall | 12 (5.3) | 4 (1.9) |
| Males | 6 (5.1) | 1 (1.0) |
| Females | 6 (5.5) | 3 (2.6) |
| Renal impairment or failure | 9 (4.0) | 3 (1.4) |
Abbreviations: AE, adverse event; DAPA, dapagliflozin; GLIM, glimepiride; MET, metformin; SAE, serious adverse event; SAXA, saxagliptin.
N refers to the number of patients in each group who were randomized and received at least one dose of treatment with the study drug.
Severe hypoglycaemia (an event requiring assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions). Three events were reported in two patients in the GLIM + MET group.
Documented symptomatic hypoglycaemia [typical symptoms of hypoglycaemia accompanied by a measured plasma glucose concentration of ≤70 mg/dL (3.9 mmol/L)].
Based on a group of terms, rather than one preferred term, for urinary tract infections, this included urinary tract infections and cystitis.
The proportion of patients reporting an AE was the same (63.4%) for both treatment groups (Table 3). The majority of these AEs were mild to moderate in intensity and resolvable. In total, 10 AEs led to discontinuation of study treatment: eight events in the DAPA + SAXA group and two in the GLIM group. The most commonly reported AEs with DAPA + SAXA were upper respiratory tract infection, urinary tract infection (UTI) and headache. The most commonly reported AEs with GLIM were upper respiratory tract infection, headache and hypertension (Table 3). The AE of special interest, UTI, occurred in more patients receiving DAPA + SAXA than in those receiving GLIM [14 (6.2%) and nine (4.2%), respectively)] (Table 3). Most of these events were mild to moderate in intensity, occurred once per patient and did not lead to discontinuation of the study drug. Genital infections, another AE of special interest, were reported in 12 patients (5.3%) and four patients (1.9%) in the DAPA + SAXA and GLIM groups, respectively (Table 3).
AEs of renal impairment or failure were reported in nine patients (4.0%) and three patients (1.4%) in the DAPA + SAXA and GLIM groups, respectively. None of these events was reported as a serious AE (SAE). AEs in this category that were considered causally related to the study drug comprised: increased blood creatinine, decreased GFR and renal impairment in one patient in the DAPA + SAXA group; and decreased creatinine clearance in two patients taking DAPA + SAXA and in one patient taking GLIM.
No patients in either treatment group reported AEs of diabetic ketoacidosis, and there were no confirmed adjudicated hospitalizations for heart failure. No AEs of hypotension, hypovolaemia or dehydration were reported with DAPA + SAXA.
The proportions of patients experiencing SAEs were balanced between treatment groups (Table 3); three patients died during the study period [one patient in the DAPA + SAXA group (pneumonia) and two in the GLIM group (road traffic accident in one patient, ischaemic stroke in the other patient)], but these deaths were considered by the investigator to be unrelated to the study treatment. Overall, there were no clinically meaningful drug effects on haematological or clinical chemistry parameters in either treatment group. Likewise, no clinically meaningful changes from baseline were observed for vital signs or ECG variables in either group during the treatment period.
4. DISCUSSION
This study compared the efficacy and safety of concurrent add‐on therapy with DAPA + SAXA versus GLIM in patients with type 2 diabetes whose blood glucose was inadequately controlled with metformin. For the primary endpoint of change from baseline in HbA1c at week 52, the combination of DAPA, SAXA and metformin was superior to GLIM + metformin. The addition of DAPA and SAXA to metformin was also associated with the benefits of weight loss and decreased SBP, a greater proportion of patients achieving a therapeutic glycaemic response [HbA1c <7.0% (53 mmol/mol)] and fewer patients requiring treatment intensification than with GLIM + metformin. The combination of DAPA, SAXA and metformin was well tolerated, and the safety profile was similar to those of the individual drugs. These findings are consistent with those of previous studies both of triple therapy with DAPA, SAXA and metformin,15, 16, 17, 18, 19 and of comparisons between other SGLT‐2 or DPP‐4 inhibitors and sulphonylureas.6, 7, 8, 9, 10, 11, 12, 13, 14
The CGM 24‐h mean glucose decreased for both groups, but this decrease was significantly greater for DAPA + SAXA than for GLIM. Additionally, treatment with DAPA + SAXA resulted in significantly more time in the euglycaemic range of 71–180 mg/dL (3.9–10.0 mmol/L) and a comparable time in the low range ≤70 mg/dL (≤3.9 mmol/L) compared with GLIM treatment. A recent large‐scale cross‐sectional study suggests that “time in range” is an independent predictor of retinopathy in patients with type 2 diabetes.22 Paradoxically, the reduction in CGM 24‐h glucose levels with GLIM was associated with an increase in glycaemic variability as measured using MAGE. As such, glycaemic variability decreased over the 52‐week study period with DAPA + SAXA and increased in the GLIM group. Glycaemic variability is increasingly recognized as an integral component of overall glycaemic control, and evidence suggests that frequent blood glucose fluctuations contribute independently to diabetes complications and hypoglycaemic episodes.23, 24
Consistent with previous reports, patients receiving DAPA + SAXA lost weight, whereas patients in the GLIM group gained weight.15, 16, 17 There was a clinically meaningful and statistically significant between‐treatment difference of 4.1 kg, which represents a substantial benefit of the triple therapy regimen over GLIM + metformin. Weight loss is frequently a desired outcome when treating type 2 diabetes, because patients are often overweight and at risk of cardiometabolic complications.2, 25 The superior reduction in SBP in patients who received DAPA + SAXA, compared with those who received GLIM, is similarly advantageous given that hypertension is also a key risk factor for the development of diabetes complications.2
In this study, both treatment regimens were generally well tolerated. The proportion of patients experiencing genital infections, a known side effect of DAPA treatment, was comparable to rates from earlier studies of SGLT‐2 inhibitors.15, 19 As seen previously, most infections were of mild to moderate intensity, were easily treated and did not result in treatment discontinuation. Other AEs of special interest were generally balanced between the two treatment groups. As seen in other studies, there was a substantial difference (not tested for statistical significance) in the proportion of patients who had hypoglycaemic events with DAPA + SAXA compared with GLIM.10 Hypoglycaemia, like weight gain, is a well‐documented side effect of sulphonylurea therapy and has been associated with poor adherence to antidiabetes treatments.26 This disparity between the two treatment groups was seen, even though fewer than half of patients in the GLIM group up‐titrated to the maximum allowed dose of the study drug. Two patients in the GLIM group also experienced episodes of severe hypoglycaemia, whereas no episodes of severe hypoglycaemia occurred with DAPA + SAXA. In the CGM substudy, glucose lowering in the GLIM group was associated with an increase in glycaemic excursions, a finding that might partially explain the difference in the rate of hypoglycaemia with GLIM compared with DAPA + SAXA, as suggested by results from previous studies.23, 24
Cardiovascular outcomes were not evaluated in this study. However, the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients – Removing Excess Glucose (EMPA REG OUTCOME), Canagliflozin Cardiovascular Assessment Study (CANVAS) and DAPA Effect on Cardiovascular Events (DECLARE‐TIMI 58) trials have shown beneficial cardiovascular effects with the SGLT‐2 inhibitors empagliflozin, canagliflozin and DAPA, respectively.27, 28, 29 Furthermore, preliminary findings from the CARdiovascular Outcome study of LINAgliptin versus GLIM in patients with type 2 diabetes (CAROLINA) showed similar cardiovascular safety profiles between a DPP‐4 inhibitor (linagliptin) and GLIM, with a lower risk for hypoglycaemia and weight gain with the DPP‐4 inhibitor.30 Thus, the potential cardiovascular benefits of DAPA and the consistently reported reduction in hypoglycaemia risk and weight gain with DPP‐4 inhibitors highlight several advantages of using these agents for treatment intensification over the use of GLIM alone with metformin.
The practical benefits of add‐on therapy with DAPA + SAXA, compared with GLIM, are also worthy of mention. Importantly, this combination regimen does not require the careful dose titration and frequent glucose monitoring associated with sulphonylurea therapy, so may increase adherence to treatment. Nevertheless, a dual‐therapy regimen with sulphonylurea might still be an appropriate treatment choice because of its relatively lower costs compared with other glucose‐lowering agents.
The findings from this study add to the body of evidence for the benefits of early combination therapy in patients with type 2 diabetes who have insufficient glycaemic control on metformin monotherapy.31 Administration of multiple antidiabetes agents early on in the disease may enable more rapid attainment of glycaemic control than with the traditional stepwise treatment approach and could possibly delay the deterioration of β‐cell function that characterizes type 2 diabetes.32
This study only reports results from 52 weeks of treatment, thus not allowing an assessment of the long‐term efficacy of the triple therapy regimen versus GLIM + metformin. However, this will be addressed in the long‐term extension phase of the study. It is notable that a previous study demonstrated sustained glycaemic efficacy of the combination of DAPA and metformin, compared with the sulphonylurea drug glipizide, over a 4‐year treatment period.33 Although the study population was 90% white, in terms of ethnicity, approximately 15% of the study population was of Hispanic origin. Even so, assessments in a more diverse ethnic mix as well as other populations (e.g. elderly patients or patients who are more vulnerable to hypoglycaemia) merit further exploration. Finally, although some patients might have benefited from further up‐titration of their GLIM dose during the study, GLIM up‐titration was only allowed during the initial 12 weeks of the study to achieve a stable and maximum dose of GLIM.
In conclusion, addition of DAPA and SAXA to metformin significantly improved glycaemic control relative to the addition of GLIM to metformin in patients with type 2 diabetes and inadequate glycaemic control on metformin monotherapy. Patients who received DAPA + SAXA also benefited from weight loss, whereas patients receiving GLIM gained weight. The triple therapy regimen was well tolerated. These results suggest that a treatment regimen comprising concurrent addition of DAPA and SAXA to metformin could be a promising alternative to add‐on therapy with GLIM in patients who have inadequate glycaemic control on metformin monotherapy.
CONFLICT OF INTEREST
J.F. has received research support from Allergan, AstraZeneca, Boehringer Ingelheim, BMS, Eli Lilly, Genentech, IONIS, Janssen, Lexicon, Ligand, Merck, Novartis, Novo Nordisk, Pfizer, Sanofi and Theracos. He has participated in advisory boards and received consulting fees from Eli Lilly, Johnson & Johnson, Merck, Novo Nordisk and Sanofi. G.G. is an advisory board member and speaker for Amgen, AstraZeneca, Janssen, MSD, Novo Nordisk, Sanofi, Stendhal and Takeda. A.P. has participated as an advisor for Abbott Diabetes Care, Boehringer Ingelheim, Eli Lilly and Company, Medscape, Mannkind, Novo Nordisk, and Sanofi. She has also received research support from Dexcom and vTv. She has stock options from Mellitus Health, Omada Health, Stability Health, Pendulum Therapeutics, and Livongo. M.T. is a board member of, and a stockholder in, Phase V Technologies, Inc. D.S. is a board member of Phase V Technologies, Inc. and a stockholder in GI Windows. J.M. and E.J. are employees of and stockholders in AstraZeneca. R.G. was an employee of AstraZeneca at the time of the study but as of publication is an employee of Bristol‐Myers Squibb; R.G. is a stockholder in AstraZeneca. N.D. is an employee of AstraZeneca.
AUTHOR CONTRIBUTIONS
E.J., M.T., D.S. and N.D. contributed to the study concept and design. E.J., M.T., D.S., J.F., N.D. and J.M. contributed to data analysis and interpretation. All authors wrote, reviewed and edited the manuscript. J.F. is the guarantor of this work and, as such, had full access to all data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Supporting information
Supplemental Figure S1 (A) Study design and (B) patient disposition. N refers to the number of patients enrolled in the study; n denotes the number of patients included at each stage. Abbreviations: DAPA, dapagliflozin; GLIM, glimepiride; HbA1c, glycated haemoglobin; IR, instant release; MET, metformin; R, randomization; SAXA, saxagliptin; XR, extended release.
Supplemental Table S1Participant demographics and baseline characteristics in the CGM subset†
ACKNOWLEDGMENTS
This study was funded by AstraZeneca. Medical writing support was provided by Lucy Ambrose, D.Phil., of Oxford PharmaGenesis (Oxford, UK), with funding from AstraZeneca. The findings from this study were communicated in oral presentation form at the 78th Scientific Sessions of the American Diabetes Association, 2018.
Frias JP, Gonzalez‐Galvez G, Johnsson E, et al. Efficacy and safety of dual add‐on therapy with dapagliflozin plus saxagliptin versus glimepiride in patients with poorly controlled type 2 diabetes on a stable dose of metformin: Results from a 52‐week, randomized, active‐controlled trial. Diabetes Obes Metab. 2020;22:1083–1093. 10.1111/dom.13997
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13997.
Funding information American Diabetes Association; AstraZeneca
REFERENCES
- 1. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151‐S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(Suppl 1):S1‐S132. [DOI] [PubMed] [Google Scholar]
- 3. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: A patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429‐442. [DOI] [PubMed] [Google Scholar]
- 4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm ‐ 2017 executive summary. Endocr Pract. 2017;23:207‐238. [DOI] [PubMed] [Google Scholar]
- 5. Sola D, Rossi L, Schianca GP, et al. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11:840‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goring S, Hawkins N, Wygant G, et al. Dapagliflozin compared with other oral anti‐diabetes treatments when added to metformin monotherapy: A systematic review and network meta‐analysis. Diabetes Obes Metab. 2014;16:433‐442. [DOI] [PubMed] [Google Scholar]
- 7. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: A randomized, double‐blind, phase 3 study. Diabetes Care. 2015;38:355‐364. [DOI] [PubMed] [Google Scholar]
- 8. Mishriky BM, Cummings DM, Tanenberg RJ. The efficacy and safety of dpp4 inhibitors compared to sulfonylureas as add‐on therapy to metformin in patients with type 2 diabetes: A systematic review and meta‐analysis. Diabetes Res Clin Pract. 2015;109:378‐388. [DOI] [PubMed] [Google Scholar]
- 9. Cefalu WT, Leiter LA, Yoon K‐H, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (cantata‐su): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet. 2013;382:941‐950. [DOI] [PubMed] [Google Scholar]
- 10. Nauck MA, Del Prato S, Duran‐Garcia S, et al. Durability of glycaemic efficacy over 2 years with dapagliflozin versus glipizide as add‐on therapies in patients whose type 2 diabetes mellitus is inadequately controlled with metformin. Diabetes Obes Metab. 2014;16:1111‐1120. [DOI] [PubMed] [Google Scholar]
- 11. Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add‐on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: A randomized, 52‐week, double‐blind, active‐controlled noninferiority trial. Diabetes Care. 2011;34:2015‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gallwitz B, Rosenstock J, Emser A, von Eynatten M, Woerle HJ. Linagliptin is more effective than glimepiride at achieving a composite outcome of target hba(1)c < 7% with no hypoglycaemia and no weight gain over 2 years. Int J Clin Pract. 2013;67:317‐321. [DOI] [PubMed] [Google Scholar]
- 13. Gitt AK, Bramlage P, Binz C, Krekler M, Deeg E, Tschope D. Prognostic implications of dpp‐4 inhibitor vs. sulfonylurea use on top of metformin in a real world setting ‐ results of the 1 year follow‐up of the prospective diaregis registry. Int J Clin Pract. 2013;67:1005‐1014. [DOI] [PubMed] [Google Scholar]
- 14. Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP, Sitagliptin SG. Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: A randomized, double‐blind, non‐inferiority trial. Diabetes Obes Metab. 2007;9:194‐205. [DOI] [PubMed] [Google Scholar]
- 15. Rosenstock J, Hansen L, Zee P, et al. Dual add‐on therapy in type 2 diabetes poorly controlled with metformin monotherapy: A randomized double‐blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38:376‐383. [DOI] [PubMed] [Google Scholar]
- 16. Mathieu C, Herrera Marmolejo M, Gonzalez Gonzalez JG, et al. Efficacy and safety of triple therapy with dapagliflozin add‐on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18:1134‐1137. [DOI] [PubMed] [Google Scholar]
- 17. Mathieu C, Ranetti AE, Li D, et al. Randomized, double‐blind, phase 3 trial of triple therapy with dapagliflozin add‐on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38:2009‐2017. [DOI] [PubMed] [Google Scholar]
- 18. Matthaei S, Aggarwal N, Garcia‐Hernandez P, et al. One‐year efficacy and safety of saxagliptin add‐on in patients receiving dapagliflozin and metformin. Diabetes Obes Metab. 2016;18:1128‐1133. [DOI] [PubMed] [Google Scholar]
- 19. Matthaei S, Catrinoiu D, Celinski A, et al. Randomized, double‐blind trial of triple therapy with saxagliptin add‐on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care. 2015;38:2018‐2024. [DOI] [PubMed] [Google Scholar]
- 20. Muller‐Wieland D, Kellerer M, Cypryk K, et al. Efficacy and safety of dapagliflozin or dapagliflozin plus saxagliptin versus glimepiride as add‐on to metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:2598‐2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: A report of a workgroup of the american diabetes association and the endocrine society. Diabetes Care. 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41:dc181131. [DOI] [PubMed] [Google Scholar]
- 23. Uemura F, Okada Y, Torimoto K, Tanaka Y. Relation between hypoglycemia and glycemic variability in type 2 diabetes patients with insulin therapy: A study based on continuous glucose monitoring. Diabetes Technol Ther. 2018;20:140‐146. [DOI] [PubMed] [Google Scholar]
- 24. Torimoto K, Okada Y, Hajime M, Tanaka K, Tanaka Y. Risk factors of hypoglycemia in patients with type 2 diabetes mellitus: A study based on continuous glucose monitoring. Diabetes Technol Ther. 2018;20:603‐612. [DOI] [PubMed] [Google Scholar]
- 25. Wilding JP. The importance of weight management in type 2 diabetes mellitus. Int J Clin Pract. 2014;68:682‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pollack MF, Purayidathil FW, Bolge SC, Williams SA. Patient‐reported tolerability issues with oral antidiabetic agents: Associations with adherence; treatment satisfaction and health‐related quality of life. Diabetes Res Clin Pract. 2010;87:204‐210. [DOI] [PubMed] [Google Scholar]
- 27. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 28. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 29. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380:1881‐1882. [DOI] [PubMed] [Google Scholar]
- 30. Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: The carolina randomized clinical trial. JAMA. 2019;322:1155‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bianchi C, Daniele G, Dardano A, Miccoli R, Del Prato S. Early combination therapy with oral glucose‐lowering agents in type 2 diabetes. Drugs. 2017;77:247‐264. [DOI] [PubMed] [Google Scholar]
- 32. Cahn A, Cefalu WT. Clinical considerations for use of initial combination therapy in type 2 diabetes. Diabetes Care. 2016;39(Suppl 2):S137‐S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Del Prato S, Nauck M, Duran‐Garcia S, et al. Long‐term glycaemic response and tolerability of dapagliflozin versus a sulphonylurea as add‐on therapy to metformin in patients with type 2 diabetes: 4‐year data. Diabetes Obes Metab. 2015;17:581‐590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1 (A) Study design and (B) patient disposition. N refers to the number of patients enrolled in the study; n denotes the number of patients included at each stage. Abbreviations: DAPA, dapagliflozin; GLIM, glimepiride; HbA1c, glycated haemoglobin; IR, instant release; MET, metformin; R, randomization; SAXA, saxagliptin; XR, extended release.
Supplemental Table S1Participant demographics and baseline characteristics in the CGM subset†


