Abstract
Aim
Six‐month recipient mortality after adult‐to‐adult living‐donor liver transplantation (LDLT) remains high. Early and accurate prediction of recipient outcome and continuous monitoring of recipient severity after surgery are both essential for guiding appropriate care. This study was designed to identify early post‐transplant parameters associated with 6‐month mortality, and thereby to construct a discriminatory prognostic index (PI).
Methods
We retrospectively analyzed 400 consecutive primary adult‐to‐adult LDLTs in our center (2006–2017). Perioperative variables were comprehensively analyzed for their accuracy in predicting recipient mortality by comparing the area under the receiver operating characteristic (AUROC) of each factor.
Results
The AUROCs of preoperative predictive factors, for example, Model for End‐stage Liver Disease (MELD) score and donor age, were 0.56 and 0.64, respectively, whereas those of post‐transplant platelet count (PLT), total bilirubin (T‐BIL), and prothrombin time – international normalized ratio (INR) on postoperative day (POD)‐7−14 were 0.71/0.84, 0.68/0.82, and 0.71/0.78, respectively. Logistic regression analysis provided a formula: PIPOD‐14 = 3.39 + 0.12 × PLTPOD‐14 − 0.09 × T‐BILPOD‐14 − 1.23 × INRPOD‐14, indicating a high AUROC of 0.87. Recipient 6‐month survival with PIPOD‐14 < 2.38 (n = 173) was 71.7%, whereas that with PIPOD‐14 ≥ 2.38 (n = 222) was 97.7% (P < 0.001). The AUROCs of PIPOD‐7 were as high as 0.8 in the subgroups with younger donors (<50 years of age), right lobe grafts, ABO‐identical/compatible combinations, or low MELD score (<20), indicating usefulness of PI to identify unexpectedly complicated cases within the first week.
Conclusions
A novel, post‐transplant survival estimator, PI, accurately predicts recipient 6‐month mortality within 1–2 weeks after adult LDLT. Daily monitoring of PI could facilitate early interventions including retransplantation in critically ill patients.
Keywords: bilirubin, liver transplantation, mortality, prothrombin time, ROC curve, thrombocytopenia
Introduction
Living‐donor liver transplantation (LDLT) has been recognized as a feasible alternative to deceased‐donor liver transplantation (DDLT) in the era of critical donor shortage, along with the remarkable progress of surgical techniques and patient care.1, 2 Living‐donor liver transplantation provides comparable long‐term outcome to DDLT;2, 3, 4 however, compared with DDLT, partial liver grafts in LDLT always increase the risk of postoperative liver failure until they regenerate sufficiently.5, 6 In fact, the high mortality and morbidity rates during the early postoperative period, especially within the first 6 months after adult LDLT, has long and widely been a common obstacle that needs to be addressed.2, 7, 8
In general, postoperative parameters reflect both recipient and graft post‐transplant conditions more accurately than preoperative variables. Therefore, many risk estimators based on postoperative factors, such as various binary definitions of early allograft dysfunction (EAD),9 Model for Early Allograft Function (MEAF) score,10 and Liver Graft Assessment Following Transplantation risk score,11 have been advocated thus far in DDLT. Although several binary EAD definitions based on DDLT data were validated in some LDLT cohorts,12, 13 no risk estimators have been created from postoperative variables in a large‐scale LDLT database. Minimal evidence has been reported in LDLT regarding the early postoperative parameters that are associated with recipient outcomes.14, 15
Although dichotomous risk criteria, such as most of the EAD definitions, are simple, the binary categorization discards variance that can be related to recipient outcomes. The predictive accuracy of binary models is, therefore, inferior to that of comparable continuous risk scores. Hence, we hypothesized that more accurate prediction by a continuous risk score certainly provides more practical information than by binary models to screen high‐risk or critically ill recipients, and to facilitate targeted interventions, including plasmapheresis, infection control, or retransplantation (Re‐LTx).
This study was thus designed first to identify early post‐transplant variables within 2 weeks after surgery that are associated with recipient 6‐month mortality, and thereby to construct a discriminatory prognostic index (PI) that precisely and timely quantifies recipient severity after adult LDLT, based on accurate prediction of probability of recipient survival.
Methods
Patients
We undertook 680 cases of liver transplantation (LTx) in our single center between January 2006 and October 2017 (Fig. 1). Of these, DDLTs, pediatric cases, and retransplants or re‐retransplants were excluded from this study. We also excluded six recipients who died within 14 days after LDLT. A total of 400 cases of primary adult‐to‐adult LDLT (recipient age ≥ 18 years) were enrolled in this retrospective cohort study. The median follow‐up period was 60.8 months (range, 0.5–141.1 months). All patients provided written informed consent. All study protocols were approved by the Ethics Committee of Kyoto University (approval no. R1473). The study was carried in accordance with the institutional guidelines, as well as with the Declaration of Helsinki principles (2000) for medical research involving human subjects.
Figure 1.
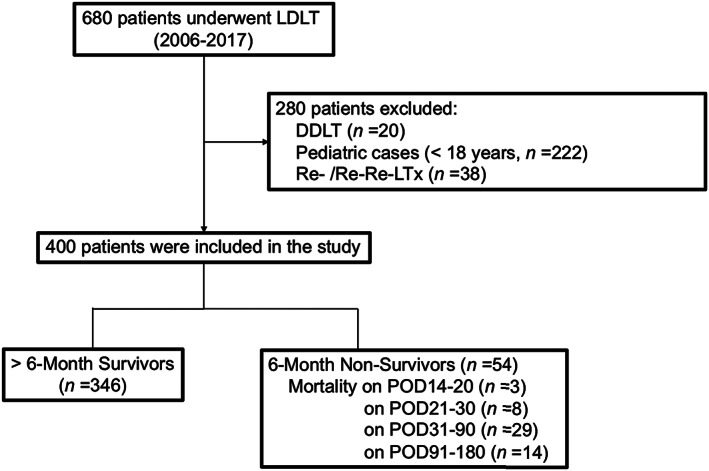
Flow chart of this study showing patient inclusion and exclusion. DDLT, deceased‐donor liver transplantation; LDLT, living‐donor liver transplantation; POD, postoperative day; Re‐LTx, retransplantation.
Perioperative management of donors and recipients
The selection criteria for donors and recipients and the surgical procedures are described in detail elsewhere.13, 16, 17, 18 The criteria for the lower limit of graft‐versus‐recipient weight ratio (GRWR) had been updated to minimize donor risks, as follows: ≥0.8% until November 2007, ≥0.7% from December 2007 until March 2009, and ≥0.6% from April 2009, as detailed elsewhere.19, 20 For biliary reconstruction, duct‐to‐duct anastomosis was our priority in adult‐to‐adult LDLT. Portal venous pressure was intentionally controlled at 15 mmHg or lower after reflow,20 with splenectomy as the first intervention of choice for reducing portal pressure.19
Postoperatively, recipients were managed in the intensive care unit (ICU) during the first week, and enteral nutrition started on postoperative day (POD)‐1. Blood tests, including complete blood cell counts, biochemical, and coagulation tests, were routinely carried out twice a day, until POD‐14. Doppler ultrasonography was also undertaken daily until POD‐14, to examine blood flow in the portal vein, hepatic artery, and hepatic vein and assess intrahepatic bile duct dilatation or intra‐abdominal/‐thoracic fluid collection. Administration of fresh frozen plasma for prothrombin time – international normalized ratio (INR) >2.0, red blood cell transfusion for hemoglobin concentration <8.0 g/dL, and platelets for platelet count (PLT) <20 000/μL were considered, if indicated.13 The standard immunosuppression protocol with tacrolimus, mycophenolate mofetil, and steroids was used.21, 22 In ABO blood‐type incompatible cases, the recipients were preoperatively treated with anti‐CD20 antibody (rituximab 375 mg/m2) and plasma exchange to prevent antibody‐mediated/humoral rejection.23
Variables
The demographic and preoperative clinical variables of interest included recipient gender and age, underlying liver etiologies, malignant or benign diseases, preoperative recipient status (at home, hospitalized, or ICU‐bound), ABO blood‐type compatibility, Child–Pugh–Turcotte and Model for End‐stage Liver Disease (MELD) scores, donor age, graft type (right‐ or left‐side), and GRWR. The operative variables included operation time, intraoperative blood loss, cold ischemic time, warm ischemic time, with or without splenectomy, and the portal venous pressure at the end of the operation. The postoperative variables were laboratory findings on POD‐3, ‐7, and ‐14, including hemoglobin concentration, PLT, INR, total bilirubin (T‐Bil), aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, and creatinine levels.
Outcome parameters
We identified the perioperative risk factors for 6‐month mortality using the following steps. First, the prognostic factors among pre‐ and intraoperative variables were identified. Second, the postoperative objective parameters associated with recipient 6‐month mortality on POD‐3, ‐7, and ‐14 were identified by univariate analyses. Third, these factors were investigated in a multivariate analysis, together with the pre‐ and intraoperative significant factors that were identified in the first step. Thereafter, we compared the discriminatory power of the prognostic factors for recipient 6‐month mortality using receiver operating characteristic (ROC) curves. Finally, we separately evaluated the predictive efficacy of each prognostic factor in each subgroup with and without widely recognized risk factors.5, 8, 24, 25, 26 Using the coefficients in the logistic regression model, we also created a PI consisting of the significant postoperative factors that had high accuracies for predicting recipient 6‐month mortality. The overall survival rates after adult LDLT were investigated according to the postoperative prognostic factors and the PI.
Statistical analysis
Data are presented as medians with interquartile ranges (IQRs) for continuous variables, unless otherwise indicated. Risk factors for 6‐month mortality were identified using uni‐ and multivariate logistic regression analyses. Interactions of variables were examined using Spearman's rank correlation coefficient. Any variable that was identified as significant (P < 0.05) in the univariate analysis was considered to be a candidate for the multivariate analysis. The ROC curves were plotted for 6‐month mortality, and the area under the ROC (AUROC) curve was calculated. To evaluate the discriminatory power of the predictors for post‐transplant mortality, we compared AUROC of each factor, using the DeLong test.27 The optimal cut‐off values were defined in each ROC curve, with the sensitivity set to approximately 90%, if clinically relevant. Regarding the PI, we assessed calibration using plots that compared the estimated probabilities of the PI with the observed event rates. The Hosmer–Lemeshow test was applied to evaluate calibration (P > 0.05 is considered favorable).28 Bootstrapping was used to assess the internal validation of the model.29 We used 1000 bootstrap resamples to evaluate the reliability of the regression coefficients and the AUROC. Overall survival was determined using the Kaplan–Meier method, followed by a log–rank test. All analyses were two‐sided, and P < 0.05 was considered statistically significant. All statistical analyses were undertaken using JMP Pro13 (SAS Institute, Cary, NC, USA) and R 3.5.0 (https://cran.r‐project.org/).
Results
Recipient characteristics
The baseline recipient characteristics are summarized in Table 1. The median MELD score was 18 (IQR, 14–24). The Child–Pugh classifications were A (24 cases [6%]), B (100 [25%]), and C (276 [69%]). Regarding blood‐type combinations, 99 cases (25%) were ABO incompatible, and the remaining 301 (75%) were identical or compatible. Left‐side grafts were chosen in 154 cases (39%) and right‐side grafts in 246 (61%). The median GRWR was 0.90% (0.76–1.05).
Table 1.
Perioperative characteristics of 400 living‐donor liver transplantation (LDLT) cases
| Patients (n = 400) | |
|---|---|
| Recipient age, years | 55 (46–60) |
| Sex, male/female | 198 (49)/202 (51) |
| Indication for LDLT | |
| Hepatocellular carcinoma | 138 (35) |
| HBV/HCV‐associated liver cirrhosis | 81 (20) |
| PBC/PSC | 67 (17) |
| Others | 114 (28) |
| HCV/not | 136 (34) /264 (66) |
| ALF/not | 27 (7)/373 (93) |
| Malignant/benign | 131 (33)/269 (67) |
| ABO compatibility, incompatible/not | 99 (25)/301 (75) |
| Preoperative medical condition, at home/hospitalized/ICU | 300(75)/71(18)/29(7) |
| CPT score, A/B/C | 24 (6)/100 (25)/276 (69) |
| MELD score | 18 (14–24) |
| Donor age, years | 45 (33–55) |
| Graft type, left−/right‐side | 153 (38)/246 (62) |
| GRWR, % | 0.90 (0.76–1.05) |
| Splenectomy/not | 222 (58)/162 (42) |
| Operation time, h | 13.6 (12.1–15.6) |
| Blood loss, g | 6140 (3580–10 768) |
| Cold ischemic time, min | 90 (59–146) |
| Warm ischemic time, min | 42 (36–52) |
| Final PVP, mmHg | 13 (11–15) |
Data are presented as median (interquartile range) for continuous variables and n (%) for categorical variables.
ALF, acute liver failure; CPT, Child–Pugh–Turcotte; GRWR, graft‐versus‐recipient weight ratio; HBV, hepatitis B virus; HCV, hepatitis C virus; ICU, intensive care unit; MELD, Model for End‐stage Liver Disease; PBC, primary biliary cholangitis/cirrhosis; PSC, primary sclerosing cholangitis; PVP, portal venous pressure.
Mortality after LDLT
As shown in Figure S1, the overall 6‐month and 1‐, 3‐, and 5‐year recipient survival rates were 86.5%, 83.2%, 78.5%, and 76.7%, respectively. The majority of recipient deaths occurred within the first 6 months after LDLT, and a total of 54 patients died within 6 months after LDLT. The leading cause of death was sepsis (n = 25), followed by graft failure (n = 16), pulmonary complications (n = 7), cerebral bleeding (n = 5), and recurrence of hepatocellular carcinoma (n = 1).
Analysis of perioperative factors affecting recipient survival after LDLT
As we recently reported,8 the multivariate analysis among pre‐ and intraoperative factors revealed that older donor age (P < 0.001) and the use of left‐side graft (P =0.006) were significant risk factors for 6‐month mortality in adult LDLT recipients (Table S1). No recipient factors, including MELD, Child–Pugh–Turcotte scores, nor even their own age, were related to recipient mortality. Although warm ischemic time was significantly longer in the survivor group by the univariate analysis (P < 0.05), the multivariate analysis revealed that it was not significant as an independent risk factor. Among early blood data within 2 weeks after transplant, PLT, T‐Bil, and INR were associated with recipient 6‐month mortality. Aspartate aminotransferase was significant on POD‐3, however, it was not significant on POD‐7 or ‐14 (Table 2).
Table 2.
Analysis of factors affecting recipient 6‐month mortality after living‐donor liver transplantation on postoperative day (POD)‐3, ‐7, and ‐14.
| POD‐3 | POD‐7 | POD‐14 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||
| P‐value | OR per SD (95% CI) | P‐value | P‐value | OR per SD (95% CI) | P‐value | P‐value | OR per SD (95% CI) | P‐value | |
| Donor age, years | 1.74 (1.23–2.46) | 0.002 | 1.66 (1.16–2.36) | 0.005 | 1.59 (1.06–2.39) | 0.026 | |||
| Left lobe graft | 2.20 (1.12–4.35) | 0.023 | 1.70 (0.30–3.30) | 0.110 | 1.49 (0.71–3.14) | 0.290 | |||
| HB | 0.980 | 0.470 | 0.230 | ||||||
| PLT | 0.640 | <0.001 | 0.52 (0.31–0.86) | 0.011 | <0.001 | 0.23 (0.09–0.59) | <0.001 | ||
| PT‐INR | <0.001 | 1.28 (0.93–1.75) | 0.130 | <0.001 | 1.60 (1.21–2.12) | <0.001 | <0.001 | 2.18 (1.19–4.01) | 0.007 |
| AST | 0.011 | 1.33 (1.02–1.72) | 0.033 | 0.059 | <0.001 | 1.32 (0.46–3.79) | 0.600 | ||
| ALT | 0.500 | 0.093 | 0.003 | 1.03 (0.40–2.66) | 0.960 | ||||
| T‐Bil | 0.019 | 1.22 (0.87–1.70) | 0.250 | <0.001 | 1.31 (0.96–1.77) | 0.085 | <0.001 | 1.71 (1.21–2.42) | 0.003 |
| ALB | 0.250 | 0.350 | 0.380 | ||||||
| CRE | 0.190 | 0.530 | 0.710 | ||||||
Postoperative prognostic factors were identified each day by univariate analysis then incorporated into the multivariate analysis together with pre‐ and intraoperative prognostic factors identified in Table SS1.
P‐values highlighted in bold are significant. For multivariate analysis, the interaction of each factor was assessed using Spearman's rank correlation coefficient.
ALB, albumin; ALT, alanine transaminase; AST, aspartate aminotransferase; CI, confidence interval; CRE, creatinine; HB, hemoglobin; OR, odds ratio; PLT, platelets; PT‐INR, prothrombin time – international normalized ratio; SD, standard deviation; T‐Bil, total bilirubin.
Discriminatory power of pre‐, intra‐, and postoperative factors for predicting recipient mortality after LDLT
The AUROCs of MELD score, donor age, and D‐MELD score, incorporating donor age and MELD score,25 were 0.56 (95% confidence interval [CI], 0.48–0.64), 0.64 (0.57–0.70), and 0.67 (0.59–0.74), respectively. The AUROCs of preoperative PLT, INR, AST, ALT, T‐Bil, albumin concentration, and creatinine were 0.53 (95% CI, 0.45–0.61), 0.49 (0.40–0.57), 0.52 (0.44–0.61), 0.49 (0.40–0.58), 0.55 (0.46–0.63), 0.60 (0.52–0.67), and 0.50 (0.41–0.60), respectively.
Figure 2 shows the chronological alterations of the postoperative variables during the first 4 weeks after surgery, in both the 6‐month survivor and non‐survivor cohorts. As seen, the intergroup difference became significant in PLT, T‐Bil, and INR on POD‐14. Among the other postoperative variables, the intergroup difference was significant only in albumin on POD‐28, and AST on POD‐14 and ‐21. Although the AUROCs of PLT, T‐Bil, and INR on POD‐3 were 0.44 (95% CI, 0.35–0.54), 0.61 (0.52–0.68), and 0.62 (0.53–0.71), respectively, all of these variables became accurate predictors for 6‐month mortality on POD‐14 (0.84 [0.78–0.89], 0.82 [0.76–0.87], and 0.78 [0.71–0.84], respectively). All of these variables on POD‐14 were superior to those on POD‐7, but similar to those on POD‐21 (Fig. 3). The AUROCs of the other blood data were all lower than 0.7 on POD‐14 (hemoglobin concentration, 0.56; AST, 0.65; ALT, 0.53; albumin, 0.54; and creatinine, 0.53). No significant difference was found in AUROCs among these three factors on POD‐14 (PLT vs. T‐Bil, P = 0.58; T‐Bil vs. INR, P = 0.28; PLT vs. INR, P = 0.10).
Figure 2.
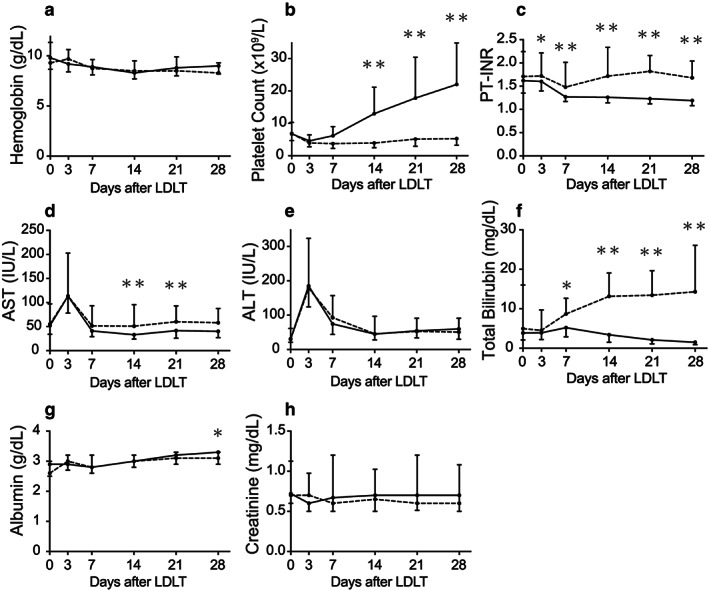
Chronological alterations of postoperative variables in survivors and non‐survivors of adult living‐donor liver transplantation (LDLT). Chronological alterations of (a) hemoglobin concentration, (b) platelet count, (c) prothrombin time – international normalized ratio (PT‐INR), (d) aspartate aminotransferase (AST), (e) alanine aminotransferase (ALT), (f) total bilirubin (T‐Bil), (g) serum albumin concentration, and (h) serum creatinine, between survivors (≥6 months, n = 346; solid line) and non‐survivors (<6 months, n = 54; dotted line). The intergroup difference reached statistical significance only in (b), (c), and (f). The values are presented as medians with interquartile ranges. All differences between the groups were assessed by two‐way repeated‐measures anova. Time‐point assessments were carried out using Bonferroni's post test (*P < 0.05, **P < 0.001).
Figure 3.
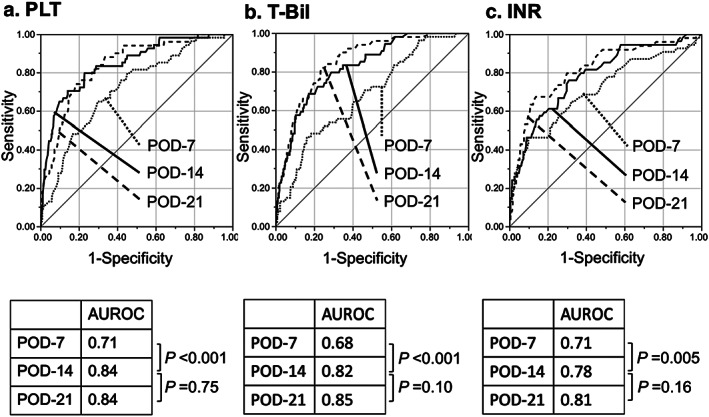
Receiver operating characteristic (ROC) curves of platelet count (PLT), total bilirubin (T‐Bil), and prothrombin time – international normalized ratio (PT‐INR) in relation to recipient 6‐month survival following adult living‐donor liver transplantation. Predictive power analyses using area under the ROC curves (AUROCs) of (a) PLT, (b) T‐Bil, and (c) PT‐INR for recipient 6‐month survival. AUROCs were 0.84 (95% confidence interval [CI], 0.78–0.89), 0.82 (0.76–0.87), and 0.78 (0.71–0.84), respectively, all of which were significantly higher than those on postoperative day (POD)‐7, but not significantly different from those on POD‐21 by DeLong tests. P‐values <0.05 were regarded as statistically significant.
The logistic regression analysis provided the following formula:
The distribution of PI POD‐14 between 6‐month survivors and non‐survivors is illustrated in Figure S2. The AUROC of PI POD‐14 for predicting 6‐month mortality was as high as 0.87 (Fig. 4a). Using the ROC curve analysis, the cut‐off value of the PI POD‐14 was determined to be 2.38, based on a sensitivity of approximately 90%. As shown in Figure 4b, the 6‐month survival rate in the high‐risk group (PI POD‐14 < 2.38, n = 173) was 71.7%, while that in the low‐risk group (PI POD‐14 ≥ 2.38, n = 222) was as high as 97.7% (P < 0.001, log–rank test).
Figure 4.
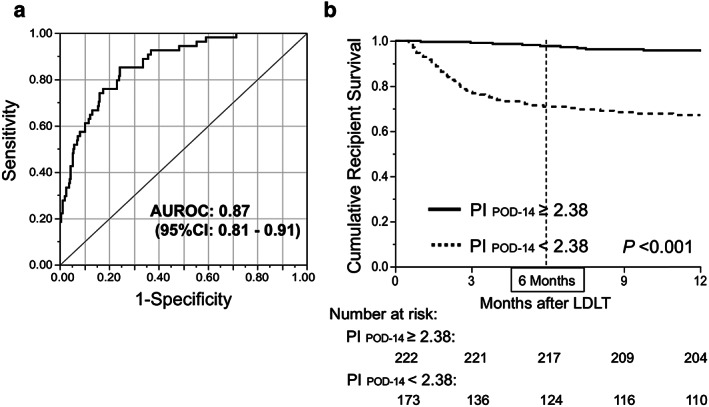
Prognostic index (PI) by platelet count (PLT), total bilirubin (T‐Bil), and prothrombin time – international normalized ratio (PT‐INR) on postoperative day (POD)‐14 following adult living‐donor liver transplantation (LDLT). (a) Logistic regression analysis provided the following formula: PI POD‐14 = 3.39 + 0.12 × (PLT POD‐14) – 0.09 × (T‐Bil POD‐14) − 1.23 × (INR POD‐14). Area under the receiver operating characteristic (ROC) curve (AUROC) of this formula for predicting recipient 6‐month mortality was 0.87 (95% confidence interval, 0.81–0.91). (b) Cut‐off value of PI, 2.38, was determined based on sensitivity of 90.7% (specificity, 63.9%) in the ROC curve. Recipient survival proportion within 6 months in the low‐risk group (≥2.38, n = 222; solid line) was significantly higher than that in the high‐risk group (<2.38, n = 17; dotted line). Survival gap between the curves widened during the first 6 months after LDLT, and became parallel with each other (almost plateau) thereafter. Survival rates were estimated with the Kaplan–Meier method followed by the log–rank test. P‐values <0.05 were regarded as statistically significant.
The logistic regression analysis also provided the following formula:
The sigmoid curve in Figure 5a was obtained by plotting the estimated probability of 6‐month survival according to the PI POD‐14. Figure 5b shows the Kaplan–Meier curves for overall recipient survival according to the PI POD‐14 quartile. Although the PI POD‐14 was designed for predicting recipient 6‐month mortality, it was also significantly associated with 5‐year recipient survival after LDLT (P < 0.001, log–rank test). The whole population (n = 400) was divided into 10 groups by percentile of PI POD‐14, and model‐predicted versus actual 6‐month mortalities were plotted according to each subgroup (Fig. 6). The PI POD‐14 was well calibrated, and the Hosmer–Lemeshow test showed no significance (P =0.94). Using bootstrap validation, the optimism‐corrected ROC area was 0.87 (95% CI, 0.83–0.91), which represents the predictive ability of the model for future patients. We compared the predictive accuracy of PI on the three major causes of recipient mortality in our cohort: sepsis (n =25); graft failure (n =16); and pulmonary complications (n =7). Interestingly, the respective AUROCs of PI POD‐14 were 0.88 (95% CI, 0.80–0.93), 0.84 (0.71–0.92), and 0.88 (0.76–0.94), indicating that the predictive power of PI was similarly high, regardless of the underlying etiology. As for 6‐month graft survival, the AUROC of the three factors on POD‐14 was 0.88 (95% CI, 0.83–0.92) that was almost the same as that for predicting 6‐month recipient survival.
Figure 5.
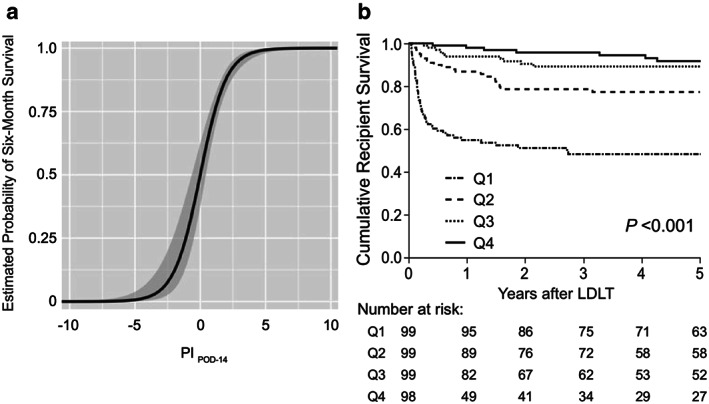
Estimated 6‐month survival rate by prognostic index (PI) on postoperative day (POD)‐14 following adult living‐donor liver transplantation (LDLT). (a) A sigmoidal curve represents the estimated probability of 6‐month recipient survival according to PI POD‐14. The logistic regression analysis provided the following formula: Estimated 6‐month survival rate = 1/1 + e0.029 – 1.02 × PI POD‐14. Shaded area indicates 95% confidence interval. (b) Kaplan–Meier estimates of 5‐year recipient survival for each PI POD‐14 quartile. Although PI POD‐14 was originally designed for predicting recipient 6‐month mortality, overall recipient survival was also clearly stratified by PI POD‐14 quartiles (Q1–Q4) (P < 0.001, log–rank test).
Figure 6.
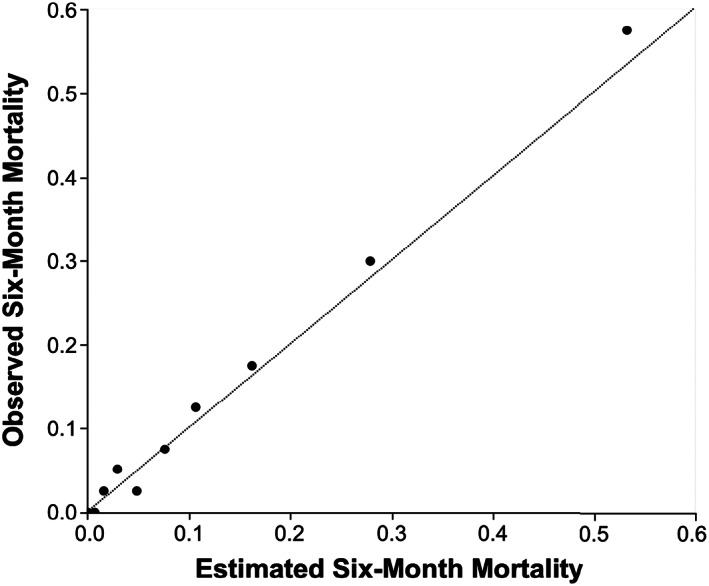
Calibration plot of observed to estimated 6‐month mortality following adult living‐donor liver transplantation. Calibration plot of the prognostic index on postoperative day 14 (PI POD‐14), in the overall dataset (n = 400). The whole cohort was divided into 10 groups by percentile, and model‐predicted versus actual 6‐month mortalities were plotted according to each subgroup. PI POD‐14 was well calibrated, and the diagonal line represents a perfect calibration.
Subgroup analysis for the discriminatory power of PLT, T‐Bil, and PT‐INR on POD‐7 and ‐14
As summarized in Table 3, the AUROCs of the three factors on POD‐7 and ‐14 for 6‐month mortality were higher in the recipients with younger donors (<50 years of age), right lobe grafts, ABO‐identical/compatible combinations, and low MELD score (<20) than those in the groups with elderly donors (≥50 years of age), left lobe grafts, ABO‐incompatible matching, and high MELD score (≥20), respectively. However, the AUROCs were similar between the large (≥0.8%) and small (<0.8%) graft groups. Notably, in most of the subgroups with two of the four conditions including younger donors, right‐lobe use, ABO‐compatible/identical combinations, and low‐MELD score, all the AUORCs on POD‐7, and ‐14 were as high as 0.76–0.86 and 0.90–0.94, respectively.
Table 3.
Subgroup analyses of the discriminatory power of platelets (PLT), total bilirubin (T‐Bil), and prothrombin time – international normalized ratio (PT‐INR) on postoperative day (POD)‐7 and ‐14 for recipient 6‐month survival after living‐donor liver transplantation
| AUROC (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| PLT | T‐Bil | INR | Three factors combined | |||||
| POD‐7 | POD‐14 | POD‐7 | POD‐14 | POD‐7 | POD‐14 | POD‐7 | POD‐14 | |
| Donor age | ||||||||
| ≥50 years (n = 165) | 0.72 (0.60–0.81) | 0.81 (0.70–0.89) | 0.63 (0.50–0.74) | 0.76 (0.65–0.84) | 0.67 (0.52–0.79) | 0.74 (0.61–0.84) | 0.73 (0.61–0.83) | 0.82 (0.73–0.89) |
| <50 years (n = 235) | 0.71 (0.60–0.80) | 0.87 (0.77–0.93) | 0.71 (0.60–0.79) | 0.87 (0.79–0.92) | 0.74 (0.62–0.83) | 0.82 (0.72–0.89) | 0.78 (0.67–0.86) | 0.89 (0.82–0.94) |
| Graft type | ||||||||
| Right lobe (n = 246) | 0.76 (0.64–0.86) | 0.88 (0.77–0.94) | 0.67 (0.56–0.77) | 0.84 (0.75–0.90) | 0.71 (0.57–0.81) | 0.83 (0.73–0.90) | 0.77 (0.65–0.86) | 0.90 (0.82–0.95) |
| Left lobe (n = 153) | 0.64 (0.53–0.73) | 0.79 (0.68–0.86) | 0.67 (0.54–0.77) | 0.79 (0.68–0.87) | 0.68 (0.55–0.79) | 0.71 (0.58–0.81) | 0.73 (0.61–0.82) | 0.80 (0.70–0.88) |
| ABO compatibility | ||||||||
| Incompatible (n = 99) | 0.63 (0.48–0.75) | 0.73 (0.59–0.84) | 0.58 (0.44–0.71) | 0.78 (0.66–0.87) | 0.65 (0.47–0.79) | 0.70 (0.55–0.82) | 0.67 (0.51–0.79) | 0.80 (0.68–0.88) |
| Not (n = 301) | 0.76 (0.67–0.83) | 0.88 (0.80–0.93) | 0.73 (0.63–0.80) | 0.83 (0.75–0.89) | 0.75 (0.65–0.83) | 0.83 (0.76–0.89) | 0.81 (0.72–0.88) | 0.89 (0.82–0.94) |
| MELD score | ||||||||
| ≥20 (n = 155) | 0.69 (0.57–0.79) | 0.79 (0.66–0.87) | 0.60 (0.46–0.72) | 0.76 (0.64–0.85) | 0.61 (0.46–0.74) | 0.72 (0.59–0.83) | 0.68 (0.55–0.79) | 0.80 (0.68–0.87) |
| <20 (n = 245) | 0.73 (0.62–0.82) | 0.88 (0.80–0.93) | 0.73 (0.63–0.81) | 0.86 (0.79–0.92) | 0.78 (0.68–0.86) | 0.82 (0.73–0.88) | 0.81 (0.70–0.88) | 0.91 (0.84–0.95) |
| GRWR | ||||||||
| ≥0.8% (n = 282) | 0.72 (0.63–0.80) | 0.84 (0.76–0.90) | 0.68 (0.59–0.76) | 0.83 (0.76–0.88) | 0.72 (0.62–0.81) | 0.81 (0.73–0.87) | 0.78 (0.69–0.85) | 0.88 (0.81–0.92) |
| <0.8% (n = 118) | 0.71 (0.55–0.83) | 0.86 (0.72–0.94) | 0.68 (0.51–0.81) | 0.86 (0.76–0.92) | 0.68 (0.49–0.82) | 0.71 (0.52–0.85) | 0.73 (0.54–0.86) | 0.87 (0.78–0.93) |
| Risk factors combined | ||||||||
| Donor age <50 years/Right lobe (n = 129) | 0.78 (0.58–0.90) | 0.89 (0.74–0.96) | 0.72 (0.56–0.84) | 0.87 (0.75–0.94) | 0.68 (0.47–0.83) | 0.84 (0.65–0.93) | 0.76 (0.58–0.88) | 0.93 (0.81–0.98) |
| Donor age <50 years/Not incompatible (n = 178) | 0.76 (0.64–0.86) | 0.93 (0.82–0.97) | 0.78 (0.64–0.88) | 0.90 (0.81–0.96) | 0.76 (0.61–0.87) | 0.85 (0.73–0.92) | 0.84 (0.69–0.92) | 0.93 (0.82–0.98) |
| Donor age <50 years/MELD <20 (n = 141) | 0.66 (0.51–0.78) | 0.86 (0.74–0.93) | 0.73 (0.60–0.84) | 0.88 (0.77–0.94) | 0.76 (0.60–0.87) | 0.82 (0.69–0.90) | 0.77 (0.62–0.87) | 0.90 (0.79–0.95) |
| Right lobe/Not incompatible (n = 194) | 0.83 (0.70–0.91) | 0.91 (0.76–0.97) | 0.77 (0.64–0.86) | 0.88 (0.78–0.94) | 0.76 (0.60–0.86) | 0.86 (0.77–0.92) | 0.86 (0.74–0.93) | 0.93 (0.81–0.97) |
| Right lobe/MELD <20 (n = 160) | 0.76 (0.60–0.87) | 0.92 (0.80–0.97) | 0.72 (0.58–0.83) | 0.87 (0.77–0.93) | 0.78 (0.62–0.89) | 0.87 (0.73–0.94) | 0.81 (0.65–0.90) | 0.94 (0.85–0.98) |
| Not incompatible/MELD <20 (n = 183) | 0.78 (0.64–0.87) | 0.94 (0.85–0.98) | 0.78 (0.64–0.87) | 0.88 (0.79–0.94) | 0.81 (0.67–0.90) | 0.85 (0.74–0.91) | 0.85 (0.71–0.93) | 0.94 (0.84–0.98) |
Area under the receiver operating characteristic curve (AUROCs) were calculated by the combination of the three factors on POD‐7 and ‐14, and the results are highlighted in bold.
CI, confidence interval; GRWR, graft‐versus‐recipient weight ratio; MELD, Model for End‐stage Liver Disease.
Discussion
In contrast to the relatively steady rate of decline in graft/recipient survival after DDLT, the drastic decline of recipient survival during the first 6 months after surgery is a characteristic burden in adult‐to‐adult LDLT.2, 8, 30 To overcome such rapid deterioration in recipient survival after adult LDLT, it is important to identify the associated risk/prognostic factors therein. Our study analyzed the prognostic impact of post‐transplant blood tests on recipient survival, using the common parameters that are routinely measured within a few weeks after LDLT. To our knowledge, this is the first report of a continuous risk score consisting of early postoperative variables with high accuracy for predicting recipient prognosis following adult LDLT.
Interestingly, a recent retrospective cohort study proposed 60‐5 criterion and postulated that postoperative PLT <60 × 109/L on POD‐5 was associated with worse patient and graft survival after DDLT.31 In LDLT, PLT on POD‐3 was also reported to be an independent predictor for grade IIIb/IV complications within 90 days after surgery.14 Consistent with these reports, the present study showed that PLT, a marker of not only hemostatic ability but also liver regeneration,32 became a prognostic factor within 1 week after LDLT. Furthermore, T‐Bil and INR, parameters for graft synthetic and metabolic functions, are incorporated in several definitions of EAD, which is recognized as an early indicator for high morbidity and mortality after LTx.9, 10 Olthoff et al. defined EAD by T‐Bil ≥10 mg/dL or INR ≥1.6 on POD‐7 or aminotransferase >2000 IU/L within the first week.9 Toshima et al. reported that MELD score, consisting of INR, T‐Bil, and creatinine, above 19 on POD‐2 or later, was associated with recipient 6‐month mortality after LDLT.15 Our results also support that T‐Bil and INR are both significant prognostic factors for 6‐month mortality.
In terms of accuracy and timing for predicting recipient 6‐month mortality, the AUROCs of PLT, T‐Bil, and INR on POD‐7 remained around 0.7. This finding is consistent with a large‐scale retrospective analysis that compared different predictive models for graft failure in DDLT.33 The AUROCs for predicting 6‐month graft failure by Olthoff's EAD and MEAF score were just 0.628 and 0.693, respectively, both of which are inferior to PI proposed in the current study. This observation might be explained in part by the fact that these variables earlier than POD‐7 were influenced by pre‐ and intraoperative conditions, such as pretransplant bilirubin levels of recipients, blood loss, and amounts of transfusion during surgery. Another plausible reason is the population heterogeneity. In fact, the present study showed that the predictive power of the three factors was high even on POD‐7 in the subgroups without preoperative risk factors. From this perspective, early judgements during the first week might not be feasible in all cases, and the indices for transplanted liver function later than POD‐7 could be appropriate as surrogate markers for recipient/graft survival after LDLT. Thus, PI, consisting of PLT, T‐Bil, and INR on POD‐7 to ‐14, was a powerful predictor in our study for recipient 6‐month mortality, with an AUROC of almost 0.9. Daily monitoring/quantification of recipient severity or improving tendency with PI will facilitate to identify high‐risk patients, thereby allowing up‐front investigations for insidious complications in critically ill recipients.
In the subgroups with younger donors (<50 years), right lobe grafts, ABO‐compatible/identical matching, and low MELD score (<20), the predictive power of PI POD‐14 was excellent, with AUROCs ≥0.90. Furthermore, most of the AUROCs were ≥0.80 in these subgroups as early as on POD‐7. Elderly donors, left lobe use, ABO‐incompatible combination, and high MELD status are well‐known pretransplant risk factors in adult LDLT4, 5, 8, 24, 25, 26 These facts suggest that the three factors are useful on POD‐7 in these subgroups in identifying the unexpectedly complicated cases who were expected to be at low risk preoperatively.
It is also noteworthy that PI POD‐7 to ‐14 provides an indication for Re‐LTx, which is the last therapeutic option for the patients with graft failure.34, 35, 36 In fact, 1‐year recipient survival after Re‐LTx is just 60–75%, whereas that in primary LTx it reaches 80–90%.4, 37, 38 The timing and the indication for Re‐LTx is sometimes difficult; however, there have been no universally accepted criteria or guidelines thus far. A large‐scale retrospective study reported that the severity of patient conditions, such as requirement for ventilator support, hypoalbuminemia (<2.5 g/dL), or MELD score >27, were independent predictors for graft failure after Re‐LTx.37 This observation suggests that early judgement for re‐LTx before patient conditions become progressively severe is of great importance. The PI proposed here would certainly facilitate appropriate decision‐making for Re‐LTx before the patient is too sick, by quantitating the probability of recipient survival with high accuracy.
It is also important to note the simplicity and feasibility of PI that we proposed. The three constituting parameters, PLT, T‐Bil, and INR, are all widely used, objective, and routinely measured blood tests after LDLT. Therefore, we can easily screen high‐risk patients, and continuously monitor time‐dependent PI alterations in such cases, regardless of the causes of patient/graft deterioration. Several studies have used ROC curve analysis and the Youden index, which gives equal weight to sensitivity and specificity in defining cut‐off values for prognostic factors after LDLT.39 However, we determined the cut‐off value for the PI according to a sensitivity value of approximately 90%, to maximize clinical relevance.
This study has several limitations. First, this is a retrospective cohort study undertaken in a single institution and is thus prone to associated risks of unintended bias. Further large‐scale, multicenter studies are warranted to validate PI. However, the high mortality rate that occurs early after adult‐to‐adult LDLT is universal, and the selection of PLT, T‐Bil, and INR as postoperative prognostic variables should be valid worldwide. Second, we did not analyze all other possible parameters, such as vital signs or drain output (ascites volume), which might affect recipient mortality. Accuracy of the model is certainly improved by incorporating many more such parameters; however, we intended to include only objective, quantitative, and daily measurable blood tests that are carried out in most transplant centers worldwide, to avoid information bias by subjective factors. Finally, our study did not extensively search other variables or methods that would enable us to accurately predict recipient prognosis within 7 days after LDLT. Of course, earlier estimation of patient outcome would be favorable; however, early judgement does not seem feasible because of remaining influences of pre‐ and intraoperative conditions and the population heterogeneity.
In conclusion, we showed that an integrated formula, PI, consisting of PLT, T‐Bil, and INR early after LDLT, shows high accuracy for predicting recipient outcome. Thus, PI could facilitate screening and identification of high‐risk cases early after LDLT, and provide a guide for appropriate interventions including Re‐LTx before patients are too sick.
Supporting information
Figure S1 Overall recipient survival in adult‐to‐adult living‐donor liver transplantation
Figure S2 Distribution of prognostic index at postoperative day 14 (PI POD‐14) between 6‐month survivors and non‐survivors following living‐donor liver transplantation.
Table S1 Pre‐ and intraoperative risk factors associated with recipient 6‐month mortality after living‐donor liver transplantation
Acknowledgments
This work was supported by the Medical Research and Development Programs Focused on Technology Transfer, Development of Advanced Measurement and Analysis Systems (SENTAN) from the Japan Agency for Medical Research and Development, AMED (no. 18hm0102063h0001).
Kusakabe, J. , Hata, K. , Tanaka, S. , Omae, K. , Okamura, Y. , Tajima, T. , Tamaki, I. , Miyauchi, H. , Kubota, T. , Tanaka, H. , and Uemoto, S. (2020) Prognostic index consisting of early post‐transplant variables <2 weeks in adult living‐donor liver transplantation. Hepatol Res, 50: 741–753. 10.1111/hepr.13489.
Conflict of interest: The authors have no conflict of interest.
Financial support: This work was supported by the Medical Research and Development Programs Focused on Technology Transfer, Development of Advanced Measurement and Analysis Systems (SENTAN) from the Japan Agency for Medical Research and Development, AMED (No. 18hm0102063h0001).
References
- 1. Lee S‐G. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant 2015; 15: 17–38. [DOI] [PubMed] [Google Scholar]
- 2. Olthoff KM, Smith AR, Abecassis M et al Defining long‐term outcomes with living donor liver transplantation in North America. Ann Surg 2015; 262: 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wan P, Yu X, Xia Q. Operative outcomes of adult living donor liver transplantation and deceased donor liver transplantation: a systematic review and meta‐analysis. Liver Transpl 2014; 20: 425–436. [DOI] [PubMed] [Google Scholar]
- 4. Umeshita K, Eguchi S, Egawa H et al Liver transplantation in Japan: registry by the Japanese Liver Transplantation Society. Hepatol Res 2019; 49: 964–980. [DOI] [PubMed] [Google Scholar]
- 5. Kiuchi T. Small‐for‐size graft in living donor liver transplantation: how far should we go? Liver Transpl 2003; 9: S29–S35. [DOI] [PubMed] [Google Scholar]
- 6. Dahm F, Georgiev P, Clavien P‐A. Small‐for‐size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant 2005; 5: 2605–2610. [DOI] [PubMed] [Google Scholar]
- 7. Freise CE, Gillespie BW, Koffron AJ et al Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL retrospective cohort study. Am J Transplant 2008; 8: 2569–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kubota T, Hata K, Sozu T et al Impact of donor age on recipient survival in adult‐to‐adult living‐donor liver transplantation. Ann Surg 2018; 267: 1126–1133. [DOI] [PubMed] [Google Scholar]
- 9. Olthoff KM, Kulik L, Samstein B et al Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl 2010; 16: 943–949. [DOI] [PubMed] [Google Scholar]
- 10. Pareja E, Cortes M, Hervás D et al A score model for the continuous grading of early allograft dysfunction severity. Liver Transpl 2015; 21: 38–46. [DOI] [PubMed] [Google Scholar]
- 11. Agopian VG, Harlander‐Locke MP, Markovic D et al Evaluation of early allograft function using the liver graft assessment following transplantation risk score model. JAMA Surg 2018; 153: 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pomposelli JJ, Goodrich NP, Emond JC et al Patterns of early allograft dysfunction in adult live donor liver transplantation. Transplantation 2016; 100: 1490–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okamura Y, Yagi S, Sato T et al Coexistence of bilirubin ≥10 mg/dl and prothrombin time‐international normalized ratio ≥1.6 on day 7: a strong predictor of early graft loss after living donor liver transplantation. Transplantation 2018; 102: 440–447. [DOI] [PubMed] [Google Scholar]
- 14. Akamatsu N, Sugawara Y, Kanako J et al Low platelet counts and prolonged prothrombin time early after operation predict the 90 days morbidity and mortality in living‐donor liver transplantation. Ann Surg 2017; 265: 166–172. [DOI] [PubMed] [Google Scholar]
- 15. Toshima T, Ikegami T, Kimura K et al Application of postoperative model for end‐stage liver disease scoring system for evaluating liver graft function after living donor liver transplantation. Transplant Proc 2014; 46(1): 81–86. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka K, Kiuchi T, Kaihara S. Living related liver donor transplantation: techniques and caution. Surg Clin North Am 2004; 84(2): 481–493. [DOI] [PubMed] [Google Scholar]
- 17. Morioka D, Egawa H, Kasahara M et al Outcomes of adult‐to‐adult living donor liver transplantation. Ann Surg 2007; 245: 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mori A, Kaido T, Ogura Y et al Standard hepatic vein reconstruction with patch plasty using the native portal vein in adult living donor liver transplantation. Liver Transpl 2012; 18: 602–607. [DOI] [PubMed] [Google Scholar]
- 19. Uemura T, Wada S, Kaido T et al How far can we lower graft‐to‐recipient weight ratio for living donor liver transplantation under modulation of portal venous pressure? Surgery 2016; 159: 1623–1630. [DOI] [PubMed] [Google Scholar]
- 20. Ogura Y, Hori T, El Moghazy WM et al Portal pressure <15mmHg is a key for the successful adult living donor liver transplantation utilizing smaller grafts than before. Liver Transpl 2010; 16: 718–728. [DOI] [PubMed] [Google Scholar]
- 21. Raut V, Uemoto S. Management of ABO‐incompatible living‐donor liver transplantation: Past and present trends. Surg Today 2011; 41(3): 317–322. [DOI] [PubMed] [Google Scholar]
- 22. Yoshizawa A, Sakamoto S, Ogawa K et al New protocol of immunosuppression for liver transplantation across ABO barrier: the use of rituximab, hepatic arterial infusion, and preservation of spleen. Transplant Proc 2005; 37(4): 1718–1719. [DOI] [PubMed] [Google Scholar]
- 23. Egawa H, Ohmori K, Haga H et al B‐cell surface marker analysis for improvement of rituximab prophylaxis in ABO‐incompatible adult living donor liver transplantation. Liver Transpl 2007; 13: 579–588. [DOI] [PubMed] [Google Scholar]
- 24. Ikegami T, Yoshizumi T, Sakata K et al Left lobe living donor liver transplantation in adults: what is the safety limit? Liver Transpl 2016; 22: 1666–1675. [DOI] [PubMed] [Google Scholar]
- 25. Ikegami T, Imai D, Wang H et al D‐MELD as a predictor of early graft mortality in adult‐to‐adult living‐donor liver transplantation. Transplantation 2014; 97(4): 457–462. [DOI] [PubMed] [Google Scholar]
- 26. Egawa H, Teramukai S, Haga H et al. Impact of rituximab desensitization on blood‐type‐incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant 2014; 14 1: 102–114. [DOI] [PubMed] [Google Scholar]
- 27. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 28. Lemeshow S, Hosmer DW. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol 1982; 115: 92–106. [DOI] [PubMed] [Google Scholar]
- 29. Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 30. Iwamura S, Kaido T, Morita S et al Risk–benefit point of the Model for End‐stage Liver Disease score in patients waiting for deceased‐donor liver transplantation: a single‐center experience. Hepatol Res 2019; 49: 687–694. [DOI] [PubMed] [Google Scholar]
- 31. Lesurtel M, Raptis DA, Melloul E et al Low platelet counts after liver transplantation predict early posttransplant survival: the 60‐5 criterion. Liver Transpl 2014; 20: 147–155. [DOI] [PubMed] [Google Scholar]
- 32. Alkozai EM, Nijsten MW, de Jong KP et al Immediate postoperative low platelet count is associated with delayed liver function recovery after partial liver resection. Ann Surg 2010; 251: 300–306. [DOI] [PubMed] [Google Scholar]
- 33. Jochmans I, Fieuws S, Monbaliu D, Pirenne J. “Model for early allograft function” outperforms “early allograft dysfunction” as a predictor of transplant survival. Transplantation 2017; 101: e258–e264. [DOI] [PubMed] [Google Scholar]
- 34. Zimmerman MA, Ghobrial RM. When shouldn't we retransplant? Liver Transpl 2005; 11: S14–S20. [DOI] [PubMed] [Google Scholar]
- 35. Uemura T, Randall HB, Sanchez EQ et al Liver retransplantation for primary nonfunction: analysis of a 20‐year single‐center experience. Liver Transpl 2007; 13: 227–233. [DOI] [PubMed] [Google Scholar]
- 36. Rana A, Petrowsky H, Kaplan B et al Early liver retransplantation in adults. Transpl Int 2014; 27: 141–151. [DOI] [PubMed] [Google Scholar]
- 37. Hong JC, Kaldas FM, Kositamongkol P et al Predictive index for long‐term survival after retransplantation of the liver in adult recipients. Ann Surg 2011; 254: 444–449. [DOI] [PubMed] [Google Scholar]
- 38. Azoulay D, Linhares MM, Huguet E et al Decision for retransplantation of the liver: an experience‐ and cost‐based analysis. Ann Surg 2002; 236: 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fluss R, Faraggi D, Reiser B. Estimation of the Youden index and its associated cutoff point. Biom J 2005; 47: 458–472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Overall recipient survival in adult‐to‐adult living‐donor liver transplantation
Figure S2 Distribution of prognostic index at postoperative day 14 (PI POD‐14) between 6‐month survivors and non‐survivors following living‐donor liver transplantation.
Table S1 Pre‐ and intraoperative risk factors associated with recipient 6‐month mortality after living‐donor liver transplantation


