Abstract
Delayed time to chemotherapy (TTC) is associated with decreased outcomes of breast cancer patients. Recently, studies suggested that the association might be subtype‐dependent and that TTC within 30 days should be warranted in patients with triple‐negative breast cancer (TNBC). The aim of the current study is to determine if TTC beyond 30 days is associated with reduced 10‐year overall survival in TNBC patients. We identified all TNBC patients diagnosed between 2006 and 2014 who received adjuvant chemotherapy in the Netherlands. We distinguished between breast‐conserving surgery (BCS) vs. mastectomy given the difference in preoperative characteristics and outcomes. The association was estimated with hazard ratios (HRs) using propensity‐score matched Cox proportional hazard analyses. In total, 3,016 patients were included. In matched patients who underwent BCS (n = 904), 10‐year overall survival was favorable for patients with TTC within 30 days (84.4% vs. 76.9%, p = 0.001). Patients with TTC beyond 30 days were more likely than those with TTC within 30 days to die within 10 years after surgery (HR 1.69 (95% CI 1.22–2.34), p = 0.002). In matched patients who underwent mastectomy (n = 1,568), there was no difference in 10 years overall survival between those with TTC within or beyond 30 days (74.5% vs. 74.7%, p = 0.716), nor an increased risk of death for those with TTC beyond 30 days (HR 1.04 (95% CI 0.84–1.28), p = 0.716). Initiation of adjuvant chemotherapy beyond 30 days is associated with decreased 10 years overall survival in TNBC patients who underwent BCS. Therefore, timelier initiation of chemotherapy in TNBC patients undergoing BCS seems warranted.
Keywords: breast cancer, triple‐negative, overall survival, delayed chemotherapy
Short abstract
What's new?
Current breast cancer treatment guidelines recommend that chemotherapy is initiated between 6‐12 weeks after surgery. Delayed treatment can lead to poorer outcome, but there's no precise definition of the optimal window. Recent work suggests that for patients with triple negative breast cancer (TNBC), it's best to initiate chemotherapy within 30 days. Here, the authors evaluated outcomes for TNBC patients correlated with time to chemotherapy. They found that in patients undergoing breast‐conserving surgery, an interval of more than 30 days before chemotherapy was associated with decreased survival. If the patient had undergone a mastectomy, a longer delay before chemotherapy did not impact survival.
Abbreviations
- BCS
breast‐conserving surgery
- CIs
confidence intervals
- HER
human epidermal growth factor Receptor
- HRs
hazard ratios
- NCR
Netherlands cancer registry
- PALGA
pathology archive
- SES
socioeconomic status
- TNBC
triple‐negative breast cancer
- TTC
time to chemotherapy
Introduction
Breast cancer is the most commonly diagnosed cancer and has the second‐highest mortality rate among women.1 Patients diagnosed with high‐risk tumors such as triple‐negative breast cancer (TNBC) have an adverse prognosis compared to patients with other subtypes.2 For these patients, locoregional treatment consists of breast‐conserving surgery (BCS) with radiation therapy or mastectomy with or without radiation therapy. Adjuvant chemotherapy is standard of care for patients with TNBC.3, 4 as the treatment reduces the risk on a distant recurrence and improves overall survival.5, 6
Although an optimal interval from surgery to adjuvant chemotherapy (Time to Chemotherapy, TTC) is not precisely defined, guidelines recommend to initiate chemotherapy within 6–12 weeks after surgery.7, 8 Several studies showed an association between delayed initiation of chemotherapy and worse breast cancer outcomes, though with different cut‐off points between 6 and 12 weeks.9, 10, 11, 12 Although patients with TNBC are less likely to have delayed TTC compared to other subtypes,13, 14, 15, 16 between 35% and 74% of patients with TNBC receive adjuvant chemotherapy beyond 30 days after surgery.9, 10, 16, 17, 18
In the recent years, several studies have suggested that the impact of TTC on survival might be subtype‐dependent and that initiation of adjuvant chemotherapy within 30 days could be warranted particularly in patients with high‐risk breast cancer such as TNBC,9, 10, 17, 18 as this type has a more aggressive biology and rapid proliferation rate compared to other subtypes.19 However, the current evidence that a TTC within 30 days is warranted in patients with TNBC is based on single‐center studies with weak methodology.11 None of the previous studies stratified analyses for the type of surgery or adjusted for confounding by indication by matching patients on the likelihood to receive adjuvant chemotherapy within 30 days. This latter is crucial as patients with certain baseline characteristics, such as old age, use of breast reconstruction or hospital transfer, do not have the same chance of TTC within 30 days so that it is not clear whether it is the TTC or the underlying indication causing the reduced survival.9, 15, 20 Furthermore, it is not always clear if previous studies excluded patients who received adjuvant radiation therapy before chemotherapy.
In the current study, we conducted a propensity score‐matched analysis in a prospective, population‐based cohort to assess the extent to which TTC beyond 30 days is associated with survival among patients diagnosed with TNBC. To further limit confounding by indication, we focused only on patients who underwent surgery followed by chemotherapy and stratified the analyses by type of surgery.
Methods
Data were anonymously obtained from the Netherlands Cancer Registry (NCR). The NCR is a prospective nationwide register for all malignancies diagnosed in all hospitals in the Netherlands. Based on notification from the Pathology Archive (PALGA) it includes patient, tumor and treatment characteristics, which are registered by trained data managers. Vital status is regularly obtained in the NCR database through linkages with the municipality register. Our study has been approved by the privacy committee of the NCR.
All women diagnosed with Stage I–III TNBC between 2006 and 2014 who underwent breast‐conserving surgery (BCS) or a mastectomy were selected. The hormonal receptors were categorized as negative when <10% of tumor cells were positively stained following the Dutch Breast Cancer guidelines. Human Epidermal growth factor Receptor (HER) 2 was defined negative in case of protein overexpression in an immunohistochemistry test or gene amplification in a fluorescence in situ hybridization test. TNBC was defined when estrogen‐negative, progesterone‐negative and HER2‐negative. For the current study, only TNBC patients who received adjuvant chemotherapy were selected. We excluded patients who were treated with radiotherapy before chemotherapy, as the current study focused on impact of delayed TTC and part of the delay could otherwise be due to delay in or recovery from radiotherapy. Furthermore, we excluded patients diagnosed with metachronous primary breast cancers, metastatic disease, treatment with neoadjuvant chemotherapy, unknown date of operation or start of chemotherapy as this makes calculation of TTC impossible. Patients with extreme TTCs beyond 6 months were also excluded as these were most likely data entry errors.
All patients received adjuvant chemotherapy according to the Dutch Breast Cancer Guidelines applicable at that point in time. Alterations to the guidelines were made in 2008 and 2012 which is shown in Supporting Information Table S1. Every treatment schedule contained an Anthracycline. Since 2008 additionally a taxane was included for high‐risk patients.
Since baseline characteristics and breast cancer outcomes differ between the different types of surgery, patients were categorized into (i) patients who underwent BCS and (ii) patients who underwent mastectomy. The type of surgery was defined by the definitive surgery performed. TTC was defined as the number of days between definitive breast surgery and initiation of adjuvant chemotherapy. Type or moment of axillary surgery was not taken into account. Within each patient group, patients were categorized into two‐time interval groups; TTC ≤30 days and TTC >30 days. The primary outcome of our study was 10‐year overall survival, which was defined as time from definitive surgery until last contact, being the date of death or last linkage of the NCR with the municipality register. The last linkage for the current study was on February 1, 2018.
To limit confounding by indication, a propensity score was created for having TTC beyond 30 days using a logistic regression model.21 The following covariates were included in the propensity score; year of diagnosis, age, socioeconomic status (SES), histological tumor type, differentiation grade, stage, re‐excision and change of hospital after surgery. Patients were matched on having the same propensity for TTC > 30 days using a matching ratio of 1:1.22 The caliper width used in our analyses was 0.2 times the standard deviation of the logit of the propensity score. We checked for possible imbalance in baseline characteristics before and after matching using standardized differences. A standardized difference of a variable of ≥10% indicates an imbalance in baseline characteristics between the time interval groups.23 Median follow‐up was determined using the reverse Kaplan–Meier method.24 The 10‐year overall survival was estimated using the Kaplan–Meier method and compared between the matched time interval groups using the log‐rank test. The hazard ratios (HR) with 95% confidence intervals (CIs) for occurrence of death were determined using a Cox regression model in matched patients. We tested the proportionality assumption using log–log plots and Schoenfeld residuals which were all satisfied. To minimize the impact of radiotherapy after chemotherapy, subsequent analyses were conducted with patients who did and did not receive radiation therapy. All analyses were performed in STATA® version 14.2 (StataCorp LLC, College Station, TX).
Results
The analyses included 3,016 patients, of whom 1,079 (35.8%) underwent BCS and 1,937 (64.2%) underwent mastectomy. The mean (standard deviation) age was 51.1 (10.7) and 50.9 (12.4) years at diagnosis for patients who underwent BCS and mastectomy, respectively.
Of the 1,079 patients who underwent BCS before matching, 485 (45.0%) patients received adjuvant chemotherapy ≤30 days and 594 (55.1%) patients >30 days. Before matching, the absolute standardized difference was more than 10% in seven categories of baseline characteristics, suggesting an inadequate balance (Table 1). In total, 452 (50.0%) patients with TTC >30 days were successfully matched to 452 (50.0%) patients with TTC ≤30 days. In these patients, absolute standardized differences for the covariates, except the year of inclusion 2010, were <10%, suggesting an overall adequate balance across the two TTC groups. For matched patients who underwent BCS within and beyond 30 days, median (interquartile range) TTC was 26 (22–28) days and 43 (35–72) days, respectively.
Table 1.
Comparison of baseline characteristics of patients who underwent breast‐conserving surgery according to time to chemotherapy ≤30 days and >30 days before and after matching
| Before matching (n = 1,079) | After matching (n = 904) | |||||
|---|---|---|---|---|---|---|
| ≤30 days (n = 485) | >30 days (n = 594) | Standardized difference | ≤30 days (n = 452) | >30 days (n = 452) | Standardized difference | |
| Year of inclusion | ||||||
| 2006 | 32 (6.6) | 51 (8.6) | 0.075 | 31 (6.9) | 27 (6.0) | 0.036 |
| 2007 | 31 (6.4) | 42 (7.1) | 0.027 | 31 (6.9) | 36 (8.0) | 0.042 |
| 2008 | 55 (11.3) | 69 (11.6) | 0.009 | 54 (11.9) | 59 (13.1) | 0.033 |
| 2009 | 51 (10.5) | 71 (12.0) | 0.046 | 51 (11.3) | 54 (11.9) | 0.021 |
| 2010 | 57 (11.8) | 106 (17.9) | 0.172 | 57 (12.6) | 37 (8.2) | 0.145 |
| 2011 | 68 (14.0) | 74 (12.5) | 0.046 | 67 (14.8) | 71 (15.7) | 0.025 |
| 2012 | 70 (14.4) | 69 (11.6) | 0.084 | 63 (13.9) | 67 (14.8) | 0.025 |
| 2013 | 48 (9.9) | 64 (10.8) | 0.029 | 48 (10.6) | 54 (11.9) | 0.042 |
| 2014 | 73 (15.1) | 48 (8.1) | 0.219 | 50 (11.1) | 47 (10.4) | 0.021 |
| Age (years) | ||||||
| <40 | 79 (16.3) | 93 (15.7) | 0.017 | 77 (17.0) | 78 (17.3) | 0.006 |
| 40–49 | 157 (32.4) | 147 (24.8) | 0.169 | 138 (30.5) | 141 (31.2) | 0.014 |
| 50–59 | 134 (27.6) | 198 (33.3) | 0.124 | 131 (29.0) | 125 (27.7) | 0.029 |
| 60–69 | 101 (20.8) | 140 (23.6) | 0.066 | 93 (20.6) | 94 (20.8) | 0.005 |
| ≥70 | 14 (2.9) | 16 (2.7) | 0.012 | 13 (2.9) | 14 (3.1) | 0.013 |
| SES | ||||||
| Low | 149 (30.7) | 183 (30.8) | 0.002 | 139 (30.8) | 134 (29.6) | 0.024 |
| Medium | 167 (34.4) | 199 (33.5) | 0.020 | 156 (34.5) | 157 (34.7) | 0.005 |
| High | 169 (34.9) | 212 (35.7) | 0.018 | 157 (34.7) | 161 (35.6) | 0.029 |
| Histological tumor type | ||||||
| Ductal | 436 (89.9) | 544 (91.6) | 0.058 | 410 (90.7) | 407 (90.0) | 0.023 |
| Lobular | 6 (1.2) | 6 (0.8) | 0.039 | 3 (0.7) | 4 (0.9) | 0.025 |
| Other | 43 (8.9) | 45 (7.6) | 0.047 | 39 (8.6) | 41 (9.1) | 0.016 |
| Differentiation grade | ||||||
| Well | 3 (0.6) | 6 (1.0) | 0.044 | 3 (0.7) | 2 (0.4) | 0.030 |
| Intermediate | 58 (12.0) | 65 (10.9) | 0.032 | 48 (10.6) | 53 (11.7) | 0.035 |
| Poor | 419 (86.4) | 505 (85.0) | 0.039 | 396 (87.6) | 395 (87.4) | 0.007 |
| Unknown | 5 (1.0) | 18 (3.0) | 0.142 | 5 (1.1) | 2 (0.4) | 0.076 |
| Stage | ||||||
| I | 136 (28.0) | 193 (32.5) | 0.096 | 134 (29.6) | 124 (27.4) | 0.049 |
| II | 286 (59.0) | 312 (52.5) | 0.130 | 259 (57.3) | 266 (58.8) | 0.031 |
| III | 63 (13.0) | 89 (15.0) | 0.058 | 59 (13.1) | 62 (13.7) | 0.019 |
| Re‐excision | ||||||
| Yes | 33 (6.8) | 27 (4.6) | 0.098 | 22 (4.9) | 24 (5.3) | 0.020 |
| Change in hospital | ||||||
| Yes | 154 (31.8) | 217 (36.5) | 0.101 | 146 (32.3) | 136 (30.1) | 0.048 |
Note: Values are numbers (percentages) unless stated otherwise. Percentages may not add up to exactly 100% as a result of rounding.
A standardized difference of a variable of ≥10% is presented in bold. This indicates an imbalance in baseline characteristics between the time interval groups.
Abbreviations: SD, standard deviation; SES, socioeconomic status.
Of the 1937 patients who underwent mastectomy, 806 (41.6%) patients received adjuvant chemotherapy ≤30 days and 1,131 (58.4%) patients >30 days after mastectomy. Before matching, patients showed a significant imbalance in four categories of baseline characteristics (Table 2). In total, 784 (50.0%) patients with TTC >30 days were successfully matched to 784 (50.0%) patients with TTC ≤30 days. After matching, imbalance in two groups remained (year of inclusion 2008 and age beyond 70). For matched patients who underwent mastectomy within and beyond 30 days, median (interquartile range) TTC was 26 (22–28) days and 38 (34–47) days, respectively. All subsequent analyses were performed in the matched patient populations.
Table 2.
Comparison of baseline characteristics of patients who underwent mastectomy according to time from surgery to chemotherapy ≤30 days and >30 days before and after matching
| Before matching (n = 1937) | After matching (n = 1,568) | |||||
|---|---|---|---|---|---|---|
| ≤30 days (n = 806) | >30 days (n = 1,131) | Standardized difference | ≤30 days (n = 784) | >30 days (n = 784) | Standardized difference | |
| Year of inclusion | ||||||
| 2006 | 60 (7.4) | 83 (7.3) | 0.004 | 60 (7.7) | 68 (8.7) | 0.037 |
| 2007 | 71 (8.8) | 101 (8.9) | 0.004 | 69 (8.8) | 77 (9.8) | 0.035 |
| 2008 | 66 (8.2) | 129 (11.4) | 0.108 | 66 (8.4) | 33 (4.2) | 0.174 |
| 2009 | 89 (11.0) | 144 (12.7) | 0.052 | 89 (11.4) | 91 (11.6) | 0.008 |
| 2010 | 98 (12.2) | 160 (14.1) | 0.059 | 96 (12.2) | 87 (11.1) | 0.036 |
| 2011 | 118 (14.6) | 151 (13.4) | 0.037 | 116 (14.8) | 120 (15.3) | 0.014 |
| 2012 | 111 (13.8) | 148 (13.1) | 0.020 | 109 (13.9) | 123 (15.7) | 0.050 |
| 2013 | 112 (13.9) | 113 (10.0) | 0.121 | 104 (13.3) | 104 (13.3) | 0.000 |
| 2014 | 81 (10.0) | 102 (9.0) | 0.035 | 75 (9.6) | 81 (10.3) | 0.026 |
| Age (years) | ||||||
| <40 | 216 (26.8) | 214 (18.9) | 0.188 | 199 (25.4) | 206 (26.3) | 0.020 |
| 40–49 | 195 (24.2) | 277 (24.5) | 0.007 | 193 (24.6) | 206 (26.3) | 0.038 |
| 50–59 | 196 (24.3) | 280 (24.8) | 0.010 | 193 (24.6) | 215 (27.4) | 0.064 |
| 60–69 | 172 (21.3) | 283 (25.0) | 0.087 | 172 (21.9) | 153 (19.5) | 0.060 |
| ≥70 | 27 (3.3) | 77 (6.8) | 0.158 | 27 (3.4) | 4 (0.5) | 0.212 |
| SES | ||||||
| Low | 263 (32.6) | 256 (31.5) | 0.025 | 255 (32.5) | 262 (33.4) | 0.019 |
| Medium | 276 (34.2) | 378 (33.4) | 0.017 | 271 (34.6) | 272 (34.7) | 0.003 |
| High | 267 (33.1) | 397 (35.1) | 0.042 | 258 (32.9) | 250 (31.9) | 0.022 |
| Histological tumor type | ||||||
| Ductal | 722 (89.6) | 1,034 (91.4) | 0.063 | 705 (89.9) | 702 (89.5) | 0.013 |
| Lobular | 20 (2.5) | 20 (1.8) | 0.049 | 20 (2.6) | 18 (2.3) | 0.017 |
| Other | 64 (7.9) | 77 (6.8) | 0.043 | 59 (7.5) | 64 (8.2) | 0.024 |
| Differentiation grade | ||||||
| Well | 6 (0.7) | 10 (0.9) | 0.016 | 6 (0.8) | 4 (0.5) | 0.032 |
| Intermediate | 113 (14.0) | 143 (12.6) | 0.040 | 109 (13.9) | 118 (15.1) | 0.033 |
| Poor | 672 (83.4) | 960 (84.9) | 0.041 | 656 (83.7) | 649 (82.8) | 0.024 |
| Unknown | 15 (1.9) | 18 (1.6) | 0.021 | 13 (1.7) | 13 (1.7) | 0.000 |
| Stage | ||||||
| I | 185 (23.0) | 307 (27.1) | 0.097 | 182 (23.2) | 173 (22.1) | 0.027 |
| II | 467 (57.9) | 616 (54.5) | 0.070 | 453 (57.8) | 466 (59.4) | 0.034 |
| III | 154 (19.1) | 208 (18.4) | 0.018 | 149 (19.0) | 145 (18.5) | 0.013 |
| Re‐excision | ||||||
| Yes | 69 (8.6) | 73 (6.5) | 0.080 | 63 (8.0) | 67 (8.5) | 0.019 |
| Change of hospital | ||||||
| Yes | 269 (33.4) | 413 (36.5) | 0.066 | 267 (34.1) | 261 (33.3) | 0.016 |
| IBR | ||||||
| Yes | 93 (11.5) | 146 (12.9) | 0.042 | 93 (11.9) | 90 (11.5) | 0.012 |
Note: Values are numbers (percentages) unless stated otherwise. Percentages may not add up to exactly 100% as a result of rounding.
A standardized difference of a variable of ≥10% is presented in bold. This indicates an imbalance in baseline characteristics between the time interval groups.
Abbreviations: IBR, immediate breast reconstruction; SES, socioeconomic status.
Median follow‐up was 82.9 (95% CI: 80.5–86.5) and 81.4 (95% CI: 79.5–83.9) months for patients who underwent BCS and mastectomy, respectively. During the study period, 157 (17.2%) of the BCS matched patients died. In these matched patients undergoing BCS, 10‐year overall survival was significantly better in patients with TTC ≤30 days compared to patients with TTC > 30 days (84.4% [95% CI 79.7–88.1] vs. 76.9% [95% CI 72.2–81.0], p = 0.001) as shown in Figure 1. Patients with TTC >30 days were more likely than those with TTC ≤30 days to die within 10 years after surgery (HR 1.69 (95% CI 1.22–2.34), p = 0.002). During the study period, 349 (22.3%) of the mastectomy matched patients died. In matched patients undergoing mastectomy, 10‐year overall survival was similar between patients with TTC ≤30 days and patients with TTC > 30 days (74.5% [95% CI 70.6–77.9] vs. 74.7% [95% CI 70.9–78.1], p = 0.716) as shown in Figure 2. Patients with TTC >30 days had the same likelihood as patients with TTC ≤30 days to die within 10 years after surgery (HR 1.04 [95% CI 0.84–1.28], p = 0.716).
Figure 1.
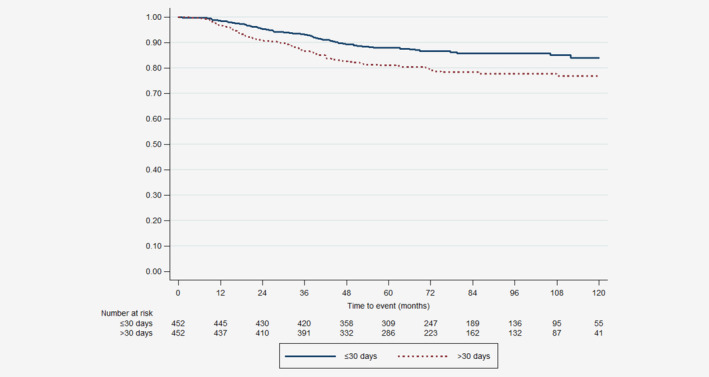
Ten‐years overall survival for matched patients who underwent breast‐conserving surgery with time from surgery to chemotherapy ≤30 days and >30 days. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.
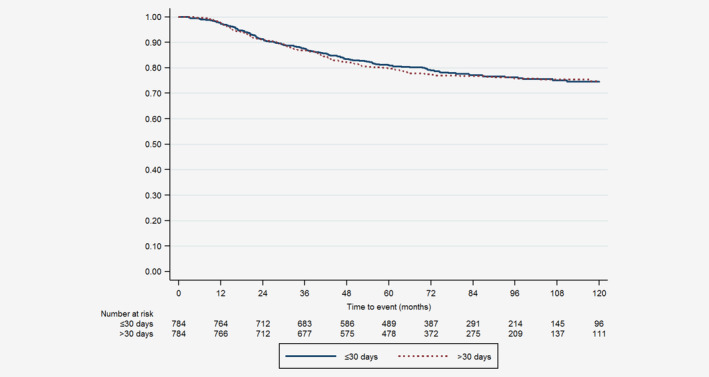
Ten‐years overall survival for matched patients who underwent mastectomy with time from surgery to chemotherapy ≤30 days and >30 days. [Color figure can be viewed at wileyonlinelibrary.com]
A small number of patients who underwent BCS did not receive radiotherapy after chemotherapy (n = 53). To limit the potential impact of the use of radiotherapy after chemotherapy on overall survival, subsequent analyses were performed in BCS matched patients who received radiotherapy (n = 880). In this subgroup, the increased likelihood to die within 10 years associated with TTC > 30 days remained (HR 1.58 [95% CI 1.13–2.22], p = 0.008). In patients who underwent mastectomy, 1,461 (75.4%) patients did not receive radiotherapy after chemotherapy. In the subgroup analyses of patients who did not receive radiotherapy (n = 1,128), patients with TTC > 30 days had a similar 10‐year overall survival compared to patients with TTC ≤30 days (HR 1.10 [95% CI 0.83–1.47], p = 0.500). The same was found in matched patients who did receive radiotherapy after chemotherapy (n = 342, HR 0.98 [95% CI 0.70–1.39], p = 0.930).
Discussion
In this population‐based cohort study, we demonstrated that in propensity‐score matched patients diagnosed with TNBC who underwent BCS with TTC beyond 30 days was associated with a significantly increased risk of death compared to those with a TTC within 30 days, while a TTC beyond 30 days had no impact on survival for patients undergoing a mastectomy. Furthermore, we demonstrated that this association was independent of the use of adjuvant radiotherapy after chemotherapy. These results suggest timelier initiation of chemotherapy in TNBC patients is warranted after BCS, which is relevant because previous studies showed delay beyond 30 days to occur in a substantial proportion of these patients.
Even though current literature does not clearly state the optimal timing for adjuvant chemotherapy for all patients, consensus exists on a more aggressive treatment for TNBC as it has a more aggressive biology and rapid proliferation rate compared to other subtypes.4, 8, 19 Therefore, it makes sense that timely adjuvant chemotherapy is particularly relevant for these patients. Adjuvant chemotherapy improves breast cancer outcomes especially by the cytotoxic effects on micrometastases. Possible explanations for the decrease in overall survival in patients with delayed TTC include increased angiogenesis in the tumor and growth of distant micrometastases, given that TNBC is characterized by rapid growth which is now given more time due to delay in TTC.19 The current study adds to the current evidence that the association is only present in patients who underwent BCS and not in those who underwent mastectomy, as previous studies did not stratify analyses for type of surgery.11 It is possible that the impact on survival after BCS is due to patient selection. Patients preference for BCS and less significant surgery can be related to comorbidity or frailty as well as be the reason for delayed TTC, which independently may impact patients’ survival.25 This might also explain a smaller impact on survival in patients who underwent mastectomy as these patients are known to have more comorbidities compared to those who underwent BCS.25 Unfortunately, comorbidities and frailty measures are not registered in the NCR‐database and could therefore not be included in the current study.
Another explanation for the different association of TTC and overall survival between the two surgical procedures may be found in the different range of TTC among patients who underwent BCS with TTC beyond 30 days (median 43 days, interquartile range 35–72 days) compared to those who underwent mastectomy with TTC beyond 30 days (median 38 days, interquartile range 34–47 days). However, subsequent multivariable analyses categorizing patients into three‐time interval groups showed that both patients who underwent mastectomy with TTC between 31 and 60 days and beyond 60 days had a similar survival compared to those with TTC within 30 days (data not shown), despite the small number of patients with TTC beyond 60 days (n = 121).
Most previous studies reported an association between delayed TTC in TNBC patients and adverse outcomes.11 Two studies reported an average 26% significantly increased risk of death in patients with TNBC who have TTC between 31 and 60 days compared to those with TCC within 30 days.9, 10 More recently, Yu et al. reported in a subgroup analyses (n = 270) that TNBC patients who had TTC beyond 8 weeks have a worse OS (HR 2.55, 95% CI 1.25–5.18).18 Unfortunately, these three studies did not stratify analyses for the type of definitive surgery nor performed subgroup analyses for use of radiotherapy. The observed association in these three studies might thus be biased due to difference in prognosis between provided treatment or due to insufficiently adjusting for unbalanced baseline characteristics. In the current study, an imbalance in baseline characteristics was seen before matching between patients who received adjuvant chemotherapy within and beyond 30 days. These characteristics could also be underlying indications for timelier initiation of chemotherapy. Poor prognostic characteristics such as older age, higher differentiation grade, triple‐negative receptor status and lymph node involvement are associated with reduced TTC in many previous studies, despite using different time thresholds. Since these poor prognostic characteristics influence both the indication for timelier initiation as well as the outcome, estimating a reliable effect of TTC on survival as performed in our study demanded adjustment for this confounding by indication. Still, we cannot rule out residual confounding by unmeasured factors (i.e., comorbidity of dose‐intensity of chemotherapy).
In contrast with the previously mentioned studies, a recent propensity score‐matched single‐center study in 724 TNBC patients observed no difference in disease‐free survival and overall survival between patients who had TTC within 30, 32–42, 43–56 or beyond 56 days.26 The difference in results with the current study might be due to the smaller sample size or absence of stratified analyses for type of surgery. Moreover, the single‐center setting decreases the generalizability of the former results due to local clinical practice.
Most previous studies did not specify if adjuvant chemotherapy was initiated before or after radiotherapy.11 The exclusion of patients who received radiotherapy before chemotherapy in the current study is essential to obtain reliable results, as there is a significant difference in baseline characteristics and breast cancer outcomes between those who receive chemotherapy before or after radiotherapy.27
In high‐income countries, today's tendency is to give chemotherapy in the neoadjuvant setting, thus before surgery, specifically for patients with locally advanced breast cancer aged <70 years.28 For our study, the number of neoadjuvant treated patients was too low and follow‐up since introduction in the Netherlands was too short to make reliable conclusions. In future research, it would be interesting to evaluate the impact of time to treatment on survival in patients with TNBC.
Our study has several limitations. First, we could only adjust for confounding by indication of measured and known variables, but several unknown as well as unmeasured confounders could influence the outcomes. For instance, the absence of information regarding the reason for the delayed TTC limits the interpretation of the association. There are several valid reasons that could delay TTC rather than being poor quality of delivered care which are associated with worse overall survival, such as complications due to the surgery, poor physical health, ECOG performance status or comorbidities that do not allow timelier initiation of chemotherapy. This information is not registered in the NCR database and could therefore not be included in our analyses, but might influence results if distributed differently between patient groups or time intervals. Comorbidity was only registered for a small percentage of the current population that this factor, unfortunately, could not be analyzed. Second, the results of the current study need to be evaluated in a large cohort including additional information regarding the chemotherapy type, dose, number of cycles and rate of completion as these are known to influence survival. This information was not included in the current overall analyses, as the type of chemotherapy is considered incomplete in the NCR before 2011. Nonetheless, analysis of a subgroup of patients treated between 2011 and 2014 while adjusting for type of chemotherapy (anthracycline, taxanes or a combination of both; data not shown), both for patients who underwent BCS and mastectomy, revealed similar results even despite the short follow‐up. A strength of the present study is both its sample size, stratified analyses by type of surgery and strong methodology to reduce confounding by indication given the impossibility to randomize patients by TTC.
Conclusions
The current results suggest that the initiation of chemotherapy beyond 30 days is associated with decreased overall survival in TNBC patients who underwent BCS. However, no association was observed for those who underwent mastectomy. These results suggest timelier initiation of chemotherapy in TNBC patients is warranted after BCS.
Supporting information
Table S1 Dose and intensity schedule of chemotherapy for patients diagnosed with triple‐negative breast cancer according to the Dutch Breast Cancer Treatment guidelines between 2004 and 2014
Acknowledgements
We thank The Netherlands Cancer Registry for providing the data, as well as the registration clerks for their effort in gathering the data in the Netherlands Cancer Registry.
Conflict of interest: The authors declare no conflicts of interest.
Data availability
Data can be made available upon reasonable request to the NCR (data application number K18.145).
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Li X, Yang J, Peng L, et al. Triple‐negative breast cancer has worse overall survival and cause‐specific survival than non‐triple‐negative breast cancer. Breast Cancer Res Treat 2017;161:279–87. [DOI] [PubMed] [Google Scholar]
- 3. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA 2019;321:288–300. [DOI] [PubMed] [Google Scholar]
- 4. Curigliano G, Burstein HJ, Winer EP, et al. De‐escalating and escalating treatments for early‐stage breast cancer: the St. Gallen international expert consensus conference on the primary therapy of early breast cancer 2017. Ann Oncol 2017;28:1700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta‐analyses of long‐term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. (EBCTCG) EBCTCG . Long‐term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta‐analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mureau MAM, Group BRGW . Dutch breast reconstruction guideline. J Plast Reconstr Aesthet Surg 2018;71:290–304. [DOI] [PubMed] [Google Scholar]
- 8. Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2015;26(Suppl 5):v8–30. [DOI] [PubMed] [Google Scholar]
- 9. Chavez‐MacGregor M, Clarke CA, Lichtensztajn DY, et al. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol 2016;2:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gagliato DM, Gonzalez‐Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 2014;32:735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhan QH, Fu JQ, Fu FM, et al. Survival and time to initiation of adjuvant chemotherapy among breast cancer patients: a systematic review and meta‐analysis. Oncotarget 2018;9:2739–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raphael MJ, Biagi JJ, Kong W, et al. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta‐analysis. Breast Cancer Res Treat 2016;160:17–28. [DOI] [PubMed] [Google Scholar]
- 13. DICA . Jaarrapportage 2017, vol. 2018 Leiden: Dutch Institute for Clinical Auditing, 2018. [Google Scholar]
- 14. Liederbach E, Sisco M, Wang C, et al. Wait times for breast surgical operations, 2003‐2011: a report from the National Cancer Data Base. Ann Surg Oncol 2015;22:899–907. [DOI] [PubMed] [Google Scholar]
- 15. Downing A, Twelves C, Forman D, et al. Time to begin adjuvant chemotherapy and survival in breast cancer patients: a retrospective observational study using latent class analysis. Breast J 2014;20:29–36. [DOI] [PubMed] [Google Scholar]
- 16. Losk K, Vaz‐Luis I, Camuso K, et al. Factors associated with delays in chemotherapy initiation among patients with breast cancer at a Comprehensive Cancer Center. J Natl Compr Canc Netw 2016;14:1519–26. [DOI] [PubMed] [Google Scholar]
- 17. Li S, Ma D, Shi HH, et al. The effect of delayed adjuvant chemotherapy on relapse of triple‐negative breast cancer. J Thorac Dis 2018;10:2837–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu KD, Fan L, Qiu LX, et al. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype‐dependent. Oncotarget 2017;8:46549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foulkes WD, Smith IE, Reis‐Filho JS. Triple‐negative breast cancer. N Engl J Med 2010;363:1938–48. [DOI] [PubMed] [Google Scholar]
- 20. Bleicher RJ, Chang C, Wang CE, et al. Treatment delays from transfers of care and their impact on breast cancer quality measures. Breast Cancer Res Treat 2018;173:603–17. [DOI] [PubMed] [Google Scholar]
- 21. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Austin PC. Comparing paired vs non‐paired statistical methods of analyses when making inferences about absolute risk reductions in propensity‐score matched samples. Stat Med 2011;30:1292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med 2009;28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shuster JJ. Median follow‐up in clinical trials. J Clin Oncol 1991;9:191–2. [DOI] [PubMed] [Google Scholar]
- 25. Christiansen P, Carstensen SL, Ejlertsen B, et al. Breast conserving surgery versus mastectomy: overall and relative survival‐a population based study by the Danish breast cancer cooperative group (DBCG). Acta Oncol 2018;57:19–25. [DOI] [PubMed] [Google Scholar]
- 26. Pomponio MK, Keele LJ, Fox KR, et al. Does time to adjuvant chemotherapy (TTC) affect outcomes in patients with triple‐negative breast cancer? Breast Cancer Res Treat 2019;177:137–43. [DOI] [PubMed] [Google Scholar]
- 27. van Maaren MC, Bretveld RW, Jobsen JJ, et al. The influence of timing of radiation therapy following breast‐conserving surgery on 10‐year disease‐free survival. Br J Cancer 2017;117:179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. nabon . Breast Cancer Guideline, vol. 2019, 2nd edn. Zwanenburg, Netherlands: Compression Control Corporation, 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Dose and intensity schedule of chemotherapy for patients diagnosed with triple‐negative breast cancer according to the Dutch Breast Cancer Treatment guidelines between 2004 and 2014
Data Availability Statement
Data can be made available upon reasonable request to the NCR (data application number K18.145).


