Summary
Background
Dupilumab [a monoclonal antibody blocking the shared receptor subunit for interleukin (IL)‐4 and IL‐13] is approved for patients aged ≥ 12 years with inadequately controlled, moderate‐to‐severe atopic dermatitis (AD). Dupilumab trials of up to 52 weeks demonstrated efficacy and a favourable safety profile in patients with moderate‐to‐severe AD inadequately controlled with topical medications.
Objectives
To further characterize the safety of dupilumab by evaluating clinical laboratory findings from three randomized, double‐blinded, placebo‐controlled phase III trials (LIBERTY AD SOLO 1 & 2 and LIBERTY AD CHRONOS).
Methods
Patients were randomized 1 : 1 : 1 (SOLO 1 & 2) or 3 : 1 : 3 (CHRONOS) for 16 and 52 weeks, respectively, to dupilumab weekly, every 2 weeks or placebo. CHRONOS patients received a standardized concomitant topical corticosteroid regimen. Laboratory outcomes were summarized descriptively in 1376 patients from SOLO 1 & 2 and 740 from CHRONOS.
Results
Treatment groups had similar results in baseline laboratory parameters. Platelets and neutrophils showed mild decreases from baseline in dupilumab vs. placebo groups. Some dupilumab‐treated patients had small transient increases in eosinophils. Grade 3 eosinophilia was reported in < 1% of dupilumab‐treated and placebo‐treated patients; no adverse events were associated with eosinophilia. Lactate dehydrogenase levels decreased from baseline during dupilumab treatment in all trials. No clinically meaningful changes were observed between treatment groups in other haematology, chemistry or urinalysis parameters.
Conclusions
There were no clinically important changes in routine laboratory parameters that could be attributed to dupilumab. This study supports the use of dupilumab as a systemic treatment for moderate‐to‐severe AD that does not require laboratory monitoring.
What's already known about this topic?
Long‐term treatment of atopic dermatitis (AD) with conventional immunosuppressive agents is limited by the risk of significant side‐effects and a need for repeated tests to monitor haematological and/or organ (e.g. liver, kidney) toxicities.
Dupilumab [a monoclonal antibody blocking the shared receptor subunit for interleukin (IL)‐4 and IL‐13] is approved for the treatment of patients with inadequately controlled, moderate‐to‐severe AD.
In 16‐week and 52‐week studies, dupilumab demonstrated a positive risk/benefit profile in moderate‐to‐severe AD.
What does this study add?
This study is the first comprehensive analysis of dupilumab laboratory safety data of the 16‐week SOLO 1 & 2 (pooled N = 1376) and 52‐week CHRONOS (N = 740) trials, demonstrating an absence of clinically important changes in haematology, serum chemistry and urinalysis parameters in patients with moderate‐to‐severe AD treated with dupilumab.
Our data support the use of dupilumab as a systemic treatment for the long‐term management of moderate‐to‐severe AD without routine laboratory monitoring in clinical practice.
Short abstract
Atopic dermatitis (AD) is a common, chronic and relapsing inflammatory skin disorder characterized by intense pruritus and eczematous lesions.1 AD is associated with disruption of the skin barrier and immune‐mediated abnormalities with skewing towards type 2 immune responses2, 3 and increased susceptibility of patients to cutaneous infections, including Staphylococcus aureus colonization, eczema herpeticum4 and noncutaneous or systemic infections.5, 6 Topical corticosteroids (TCS) and calcineurin inhibitors (TCI) remain the mainstay of AD therapy;7, 8 however, moderate‐to‐severe AD often cannot be adequately controlled with topical treatments and requires the use of systemic agents.9 Currently, oral corticosteroids (e.g. prednisolone) are approved by the U.S. Food and Drug Administration (FDA) for the treatment of inflammatory skin diseases, but they are only recommended in short courses for AD, and their use should be limited to specific circumstances such as a lack of other adequate treatment options or during episodes of acute flares where immediate relief is required.10 Among conventional immunosuppressants, only ciclosporin has approval, limited to short‐term treatment of severe AD in most European countries and Japan. Other off‐label systemic medications are also used in clinical practice, including azathioprine, mycophenolate mofetil and methotrexate.8, 11, 12, 13, 14, 15 Long‐term treatment of AD with systemic immunosuppressive agents is limited by safety concerns and a need for repeated tests to monitor clinical laboratory abnormalities and/or organ (e.g. liver and kidney) toxicities.8, 11, 12, 13, 14, 15
Dupilumab, a fully human VelocImmune®‐derived16, 17 monoclonal antibody, blocks the shared receptor subunit for interleukin (IL)‐4 and IL‐13, thus inhibiting signalling of both IL‐4 and IL‐13. These cytokines are key drivers of type 2 inflammatory diseases such as AD as well as asthma, allergic rhinitis and food allergies, which are common AD comorbidities.18 Dupilumab is approved for subcutaneous (SC) administration at 300 mg every 2 weeks (q2w) for the treatment of patients aged 12 years and older in the U.S.A. with moderate‐to‐severe AD inadequately controlled with topical prescription therapies or when those therapies are not advisable,19 for the treatment of adult patients with AD not adequately controlled with existing therapies in Japan and for use in patients aged 12 years and older with moderate‐to‐severe AD who are candidates for systemic therapy in the European Union (EU).20 Dupilumab is also approved by the FDA as an add‐on maintenance treatment in patients with moderate‐to‐severe asthma aged 12 years and older with an eosinophilic phenotype or with oral corticosteroid‐dependent asthma and as an add‐on maintenance treatment in adult patients with inadequately controlled chronic rhinosinusitis with nasal polyps.19 Three phase III clinical trials of dupilumab [LIBERTY AD SOLO 1 (NCT02277743), LIBERTY AD SOLO 2 (NCT02277769), LIBERTY AD CHRONOS (NCT02260986)] have demonstrated efficacy in improving AD signs, symptoms and quality of life and showed a favourable safety profile for treatment durations of 16 weeks (SOLO 1 & 2) and 52 weeks (CHRONOS) in patients with moderate‐to‐severe AD with inadequate response to topical medications.21, 22, 23 Other randomized, placebo‐controlled trials in patients with AD, as well as randomized, placebo‐controlled trials in other diseases mediated by type 2 inflammation, including asthma, chronic sinusitis with nasal polyps and eosinophilic oesophagitis, demonstrated similar results.24, 25, 26, 27, 28, 29, 30, 31, 32, 33 Due to its selective targeting of type 2 inflammation, dupilumab has not been associated with significant infection‐related adverse events (AEs) often seen with monoclonal antibodies that affect type 1‐mediated immune responses, such as antitumour necrosis factor‐α therapies.21, 22, 26, 34, 35 However, in most dupilumab AD trials, higher incidences of conjunctivitis were observed in dupilumab vs. placebo groups; most cases were mild‐to‐moderate, and most recovered/resolved during the treatment period.36 Conjunctivitis incidence was very low and similar for dupilumab and placebo in other type 2 diseases.36
To further characterize the safety profile of dupilumab, we evaluated clinical laboratory data from three large, randomized, double‐blinded, placebo‐controlled phase III trials in adult patients with moderate‐to‐severe AD.
Patients and methods
Study design
SOLO 1, SOLO 2 and CHRONOS were randomized, double‐blinded, placebo‐controlled phase III trials conducted in adherence with the Declaration of Helsinki principles. Institutional review boards and independent ethics committees reviewed and approved the protocols, informed consent forms and patient information prior to study initiation. All patients provided signed informed consent prior to any study procedures being performed.
Study designs and patient populations have been described previously.21, 22 Briefly, SOLO 1 & 2 had identical study designs. Patients were randomized 1 : 1 : 1 to monotherapy with SC dupilumab 300 mg once weekly (qw), dupilumab 300 mg q2w or placebo qw for 16 weeks. Key inclusion criteria were: AD inadequately controlled with or inadvisable for topical medications; age ≥ 18 years; moderate‐to‐severe AD for ≥ 3 years prior to screening; Investigator's Global Assessment (IGA) score ≥ 3 (moderate‐to‐severe; scale 0–4) and Eczema Area and Severity Index (EASI) ≥ 16 (scale 0–72).21 In CHRONOS, patients were randomized 3 : 1 : 3 to receive SC dupilumab 300 mg qw, dupilumab 300 mg q2w or placebo qw for 52 weeks. All patients in CHRONOS received concomitant medium‐potency TCS, or low‐potency TCS in thin or sensitive skin areas (e.g. face or neck); TCI could be used in certain areas such as the face and intertriginous areas, if not used concomitantly with TCS to treat the same areas. Once lesions were clear/almost clear, TCS ± TCI could be tapered, then stopped. Key inclusion criteria were: documented history of inadequate response to medium‐to‐high‐potency TCS and/or documented systemic treatment use within the last 6 months; AD inadequately controlled with or inadvisable for topical medications; age ≥ 18 years; moderate‐to‐severe AD for ≥ 3 years prior to screening; IGA ≥ 3 (moderate‐to‐severe; scale 0–4) and EASI ≥ 16 (scale 0–72).22
Safety analysis
The safety analysis set (SAF) included all randomized patients who received any study drug; it was based on the treatment received (as treated); for CHRONOS, this includes patients who had completed treatment as of the cut‐off date for FDA submission of the dupilumab biologics license application,22 as well as those whose treatment was still ongoing after the FDA submission. If a patient received a dose regimen different to that assigned during the study, this patient was considered as treated by the lowest active dose. SOLO 1 & 2 data were pooled. Safety assessments included clinical laboratory evaluations (haematology, clinical chemistry and urinalysis) and reported AEs. For patients who discontinued study treatment, laboratory data were collected during subsequent monitoring visits, but no laboratory data were collected after withdrawal from the study. Blood samples for testing of haematology and chemistry parameters were collected at baseline and prespecified postbaseline time points, including at the end of the treatment period. Blood samples were collected after a 6–8‐h fast, if possible; fasting was not mandatory. Haematology, serum chemistry and urinalysis samples were analysed by a central laboratory (SOLO 1 & 2: PPD Laboratories; Highland Heights, KY, U.S.A.; CHRONOS: Covance Laboratories; Indianapolis, IN, U.S.A.). All untoward medical occurrences, including medically relevant laboratory abnormalities, were considered AEs according to the protocol definition. The number and proportion of patients reporting treatment‐emergent adverse events (TEAEs) were listed by Medical Dictionary for Regulatory Activities (MedDRA) system Preferred Terms (PTs). All safety outcomes were assessed using descriptive statistics.
Laboratory variables
Haematology analyses covered haematocrit, haemoglobin, red blood cells, white blood cells, red cell indices and platelet count. Further differential outcomes included neutrophils, lymphocytes, monocytes, basophils and eosinophils.
Serum chemistry analyses covered metabolic function parameters, including albumin, creatine phosphokinase [CPK; CPK isoenzymes were measured when CPK was > 5 times the upper limit of normal (ULN)], glucose, haemoglobin A1C and total serum protein; electrolyte parameters, including bicarbonate, calcium, chloride, potassium and sodium; renal function parameters, including blood urea nitrogen, creatinine and uric acid; liver function parameters, including alkaline phosphatase, alanine transaminase, aspartate transaminase, total bilirubin (direct and indirect bilirubin were measured when the total bilirubin was above the ULN) and lactate dehydrogenase (LDH) as well as lipid profile parameters, including total cholesterol, low‐density lipoprotein, high‐density lipoprotein and triglycerides. Urinalysis included testing of pH and specific gravity.
Statistical methods
All laboratory data analyses were descriptive; there were no statistical comparisons of treatment groups for any laboratory variables. All data were summarized as observed, and no imputations were used for missing data.
Results
Baseline
Clinical laboratory data were assessed in 1376 patients from SOLO 1 & 2 (SAF for pooled data: placebo, n = 456; dupilumab q2w, n = 465; dupilumab qw, n = 455) and 740 patients from CHRONOS (SAF: placebo + TCS, n = 315; dupilumab q2w + TCS, n = 110; dupilumab qw + TCS, n = 315). Baseline haematology, serum chemistry and urinalysis characteristics were balanced across treatment groups (Tables S1a, b, S2a–e, S3; see Supporting Information). Baseline serum levels of LDH, a marker of tissue damage that correlates with AD disease activity and severity, were elevated in a large proportion of patients (Table S2d; see Supporting Information); details are provided below. In SOLO 1 & 2, 372 (81·6%; placebo), 435 (93·5%; dupilumab q2w) and 413 (90·8%; dupilumab qw) patients completed the 16‐week study treatment period; in CHRONOS, 225 (71·4%; placebo + TCS), 95 (86·4%; dupilumab q2w + TCS) and 276 (87·6%; dupilumab qw + TCS) patients completed the 52‐week study treatment period.
Clinical laboratory parameters during the treatment period
Platelets
Platelet values for the dupilumab and placebo groups were within normal ranges, with greater decreases from baseline in the dupilumab groups than the placebo groups (Fig. 1a, b; Table S1a; see Supporting Information). In SOLO 1 & 2, the mean change from baseline to week 16 for platelets (× 109 L−1) was −3·1, −5·7 and −11·2 for placebo, dupilumab q2w and dupilumab qw, respectively; for baseline to week 52 in CHRONOS, these values were −2·2, −12·9 and −13·1, for placebo + TCS, dupilumab q2w + TCS and dupilumab qw + TCS, respectively. These changes in platelet counts were of no particular clinical concern and did not necessitate additional testing or changes to treatment. Moreover, no notable differences in platelet counts were observed between treatment groups in shifts from normal values at baseline to high or low values during the study period (Table S4a; see Supporting Information). For patients with grade 2 (platelet count: 100–124 × 109 L−1; moderate)37 and grade 3 (25–99 × 109 L−1; severe)37 thrombocytopenia, magnitudes of change in platelet counts from baseline were similar across the dupilumab and placebo groups (Fig. 2a). One patient in the placebo group in SOLO 1 had grade 4 thrombocytopenia (platelet count < 25 × 109 L−1; potentially life‐threatening)37 at week 8 (Table 1). Grade 3 thrombocytopenia incidence rates were < 1% across all treatment groups (Fig. 1c; Table 1).
Figure 1.
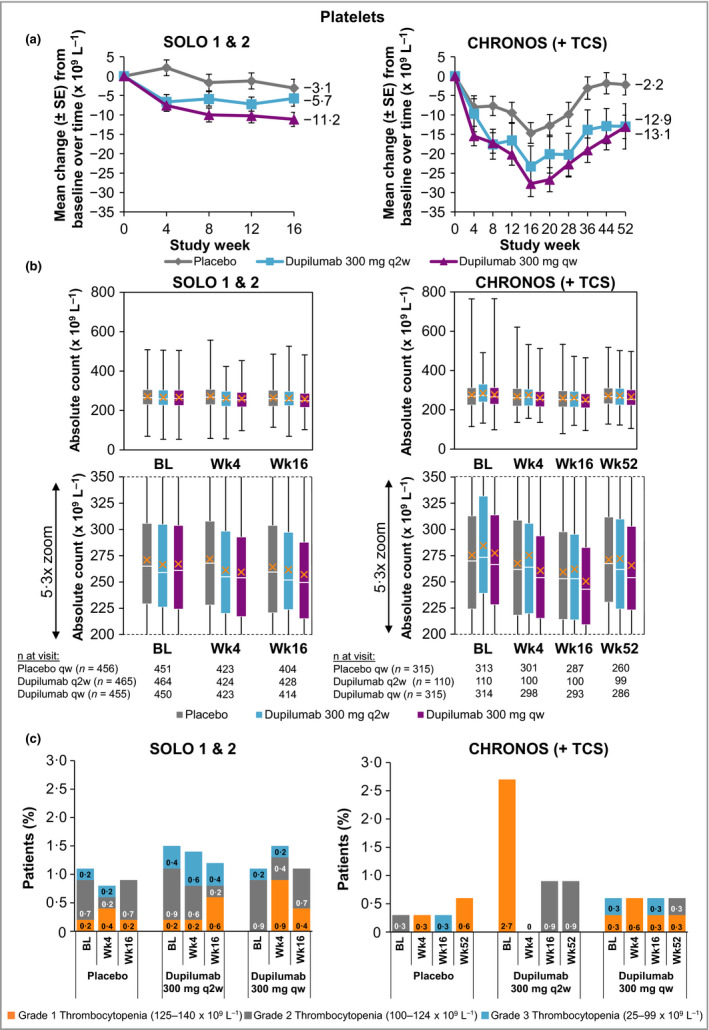
(a) Mean change in platelet count from baseline to week 16 (SOLO 1 & 2) and week 52 (CHRONOS). (b) Absolute platelet count. A close‐up view of the box‐and‐whisker plots is depicted below. White horizontal lines indicate medians. X depicts mean values. Top and bottom of each box represent Q3 and Q2, respectively. Upper and lower vertical bars represent Q4 and Q1, respectively; horizontal segments on each end of the vertical bars represent minimum and maximum values. (c) Proportion of patients with thrombocytopenia grades 1–3; patient numbers are provided in Table 1. Thrombocytopenia grade scale follows the guidance provided by the U.S. Food and Drug Administration.37 BL, baseline; Q, quartile; qw, once weekly; q2w, every 2 weeks; TCS, topical corticosteroids; Wk, week.
Figure 2.
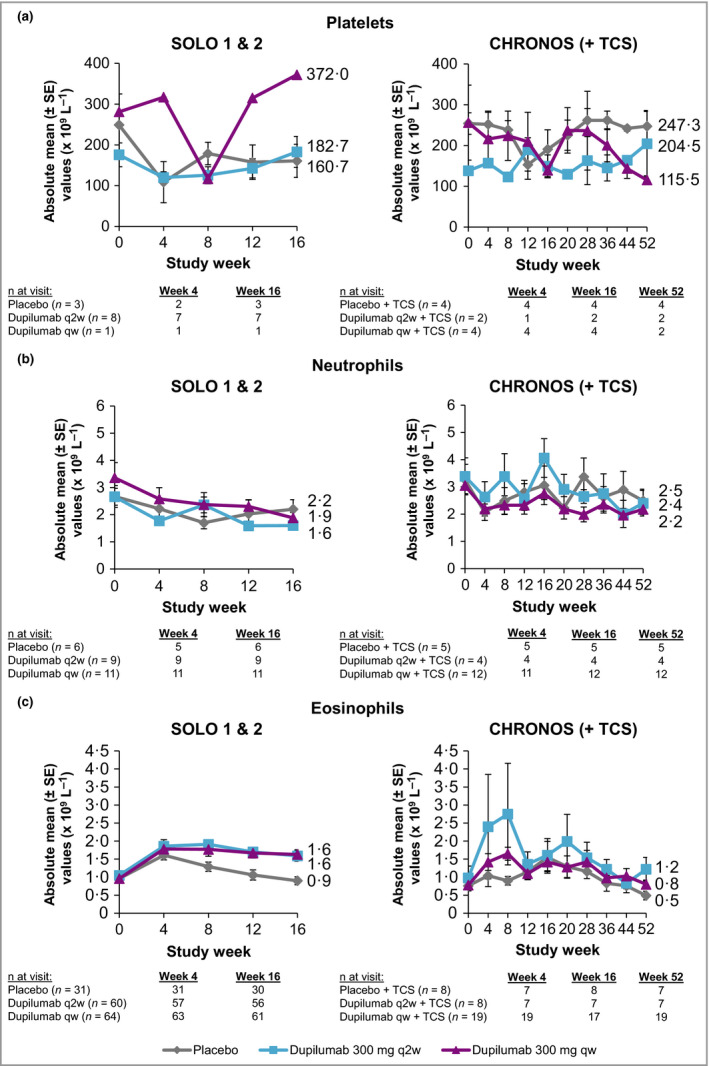
(a) Absolute mean platelet counts from baseline in the subset of patients with thrombocytopenia grades 2 and 3 through week 16 (SOLO 1 & 2) and week 52 (CHRONOS). (b) Absolute mean neutrophil counts from baseline in the subset of patients with neutropenia grades 2 and 3 through week 16 (SOLO 1 & 2) and week 52 (CHRONOS). (c) Absolute mean eosinophil counts from baseline in the subset of patients with eosinophilia grades 2 and 3 through week 16 (SOLO 1 & 2) and week 52 (CHRONOS). Toxicity grade scales follow the guidance provided by the U.S. Food and Drug Administration.37 qw, once weekly; q2w, every 2 weeks; TCS, topical corticosteroids.
Table 1.
| Study week | Thrombocytopenia grade | SOLO 1 & 2, n (%)b | CHRONOS, n (%) | ||||
|---|---|---|---|---|---|---|---|
| Placebo qw (n = 456) | Dupilumab 300 mg q2w (n = 465) | Dupilumab 300 mg qw (n = 455) | Placebo qw + TCS (n = 315) | Dupilumab 300 mg q2w + TCS (n = 110) | Dupilumab 300 mg qw + TCS (n = 315) | ||
| 0 | 1 | 1 (0·2) | 1 (0·2) | 0 | 0 | 3 (2·7) | 1 (0·3) |
| 2 | 3 (0·7) | 4 (0·9) | 4 (0·9) | 1 (0·3) | 0 | 0 | |
| 3 | 1 (0·2) | 2 (0·4) | 1 (0·2) | 0 | 0 | 1 (0·3) | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4 | 1 | 2 (0·4) | 1 (0·2) | 4 (0·9) | 1 (0·3) | 0 | 2 (0·6) |
| 2 | 1 (0·2) | 3 (0·6) | 2 (0·4) | 0 | 0 | 0 | |
| 3 | 1 (0·2) | 3 (0·6) | 1 (0·2) | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 8 | 1 | 4 (0·9) | 1 (0·2) | 1 (0·2) | 0 | 1 (0·9) | 3 (1·0) |
| 2 | 1 (0·2) | 2 (0·4) | 1 (0·2) | 0 | 1 (0·9) | 0 | |
| 3 | 0 | 3 (0·6) | 0 | 0 | 0 | 0 | |
| 4 | 1 (0·2) | 0 | 0 | 0 | 0 | 0 | |
| 12 | 1 | 3 (0·7) | 3 (0·6) | 0 | 3 (1·0) | 0 | 5 (1·6) |
| 2 | 1 (0·2) | 5 (1·1) | 2 (0·4) | 1 (0·3) | 0 | 0 | |
| 3 | 0 | 1 (0·2) | 1 (0·2) | 1 (0·3) | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 16 | 1 | 1 (0·2) | 3 (0·6) | 2 (0·4) | 0 | 0 | 1 (0·3) |
| 2 | 3 (0·7) | 1 (0·2) | 3 (0·7) | 0 | 1 (0·9) | 0 | |
| 3 | 0 | 2 (0·4) | 0 | 1 (0·3) | 0 | 1 (0·3) | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 20 | 1 | – | – | – | 1 (0·3) | 0 | 1 (0·3) |
| 2 | – | – | – | 1 (0·3) | 1 (0·9) | 0 | |
| 3 | – | – | – | 0 | 0 | 0 | |
| 4 | – | – | – | 0 | 0 | 0 | |
| 28 | 1 | – | – | – | 1 (0·3) | 0 | 3 (1·0) |
| 2 | – | – | – | 0 | 1 (0·9) | 1 (0·3) | |
| 3 | – | – | – | 0 | 0 | 0 | |
| 4 | – | – | – | 0 | 0 | 0 | |
| 36 | 1 | – | – | – | 0 | 0 | 1 (0·3) |
| 2 | – | – | – | 0 | 1 (0·9) | 0 | |
| 3 | – | – | – | 0 | 0 | 0 | |
| 4 | – | – | – | 0 | 0 | 0 | |
| 44 | 1 | – | – | – | 0 | 0 | 0 |
| 2 | – | – | – | 0 | 0 | 2 (0·6) | |
| 3 | – | – | – | 0 | 0 | 0 | |
| 4 | – | – | – | 0 | 0 | 0 | |
| 52 | 1 | – | – | – | 2 (0·6) | 0 | 1 (0·3) |
| 2 | – | – | – | 0 | 1 (0·9) | 1 (0·3) | |
| 3 | – | – | – | 0 | 0 | 0 | |
| 4 | – | – | – | 0 | 0 | 0 | |
Thrombocytopenia grade scale follows the guidance provided by the U.S. Food and Drug Administration:37 platelet counts of 125–140 × 109 L−1 are defined as grade 1, 100–124 × 109 L−1 as grade 2, 25–99 × 109 L−1 as grade 3 and < 25 × 109 L−1 as grade 4.
Post‐16‐week data were not collected for SOLO. qw, once weekly; q2w, every 2 weeks; TCS, topical corticosteroids.
Neutrophils
Neutrophil values were within normal ranges across all treatment groups, with greater decreases from baseline in the dupilumab groups vs. placebo groups in SOLO 1 & 2 [mean change from baseline to week 16: −0·1, −0·4 and −0·5 (× 109 L−1) for placebo, dupilumab q2w and dupilumab qw, respectively] and CHRONOS [mean change from baseline to week 52: −0·2, −0·6 and −0·6 (× 109 L−1) for placebo + TCS, dupilumab q2w + TCS and dupilumab qw + TCS, respectively] (Fig. 3a, b; Table S1b; see Supporting Information). These changes in neutrophil counts were of no particular clinical concern and did not necessitate additional testing or changes to treatment. Moreover, no notable differences in neutrophil counts were observed between treatment groups in shifts from normal values at baseline to high or low values during the study period (Table S4b; see Supporting Information). Patients with grade 2 [neutrophil count: 1·0–1·499 × 109 L−1 (moderate)]37 and grade 3 [0·5–0·999 × 109 L−1 (severe)]37 neutropenia had lower neutrophil counts at baseline than the overall study populations (Fig. 2b; Table S1b; see Supporting Information), and the magnitudes of change in neutrophil counts from baseline in this subset of patients were similar across dupilumab and placebo groups (Fig. 2b). No patient had neutropenia grade 4 [neutrophil count < 0·5 × 109 L−1 (potentially life‐threatening)]. Grade 3 neutropenia incidence rates were < 1% across all treatment groups (Fig. 3c; Table 2).
Figure 3.
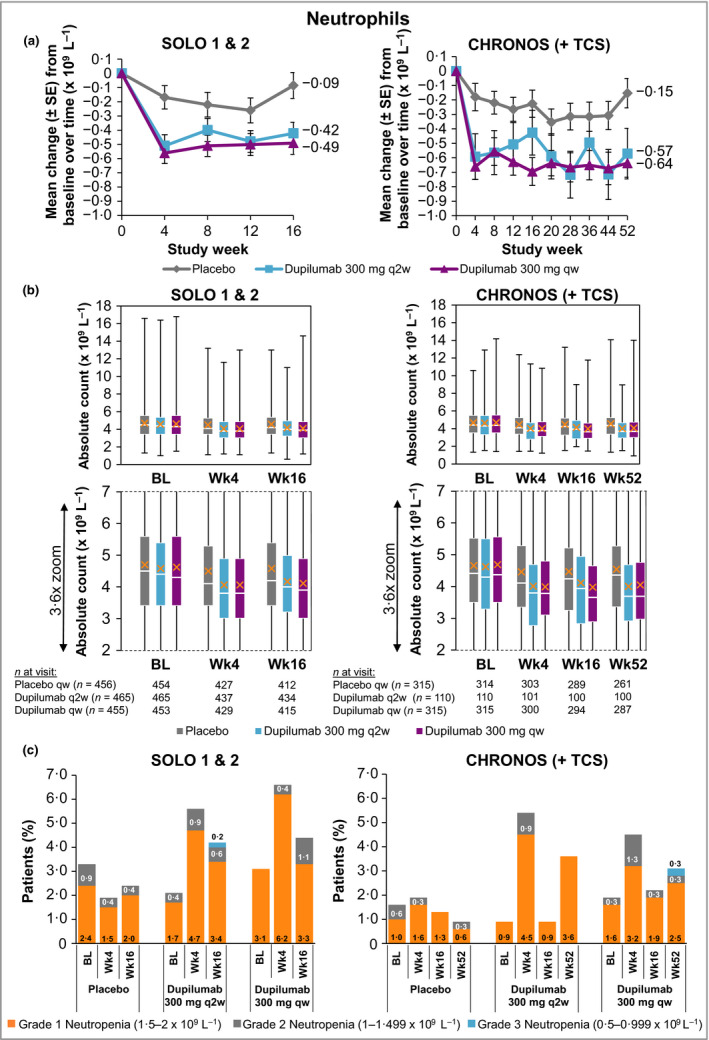
(a) Mean change in neutrophil count from baseline to week 16 (SOLO 1 & 2) and week 52 (CHRONOS). (b) Absolute neutrophil count. A close‐up view of the box‐and‐whisker plots is depicted below. White horizontal lines indicate medians. X depicts mean values. Top and bottom of each box represent Q3 and Q2, respectively. Upper and lower vertical bars represent Q4 and Q1, respectively; horizontal segments on each end of the vertical bars represent minimum and maximum values. (c) Proportion of patients with neutropenia grades 1–3; patient numbers are provided in Table 2. The neutropenia grade scale follows the guidance provided by the U.S. Food and Drug Administration.37 BL, baseline; Q, quartile; qw, once weekly; q2w, every 2 weeks; TCS, topical corticosteroids; Wk, week.
Table 2.
| Study week | Neutropenia grade | SOLO 1 & 2, n (%)c | CHRONOS, n (%) | ||||
|---|---|---|---|---|---|---|---|
| Placebo qw (n = 456) | Dupilumab 300 mg q2w (n = 465) | Dupilumab 300 mg qw (n = 455) | Placebo qw + TCS (n = 315) | Dupilumab 300 mg q2w + TCS (n = 110) | Dupilumab 300 mg qw + TCS (n = 315) | ||
| 0 | 1 | 11 (2·4) | 8 (1·7) | 14 (3·1) | 3 (1·0) | 1 (0·9) | 5 (1·6) |
| 2 | 4 (0·9) | 2 (0·4) | 0 | 2 (0·6) | 0 | 1 (0·3) | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4 | 1 | 7 (1·5) | 22 (4·7) | 28 (6·2) | 5 (1·6) | 5 (4·5) | 10 (3·2) |
| 2 | 2 (0·4) | 4 (0·9) | 2 (0·4) | 1 (0·3) | 1 (0·9) | 4 (1·3) | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 8 | 1 | 12 (2·6) | 22 (4·7) | 23 (5·1) | 4 (1·3) | 3 (2·7) | 11 (3·5) |
| 2 | 4 (0·9) | 1 (0·2) | 2 (0·4) | 1 (0·3) | 0 | 1 (0·3) | |
| 3 | 0 | 0 | 1 (0·2) | 0 | 0 | 0 | |
| 12 | 1 | 11 (2·4) | 22 (4·7) | 24 (5·3) | 2 (0·6) | 1 (0·9) | 11 (3·5) |
| 2 | 0 | 4 (0·9) | 1 (0·2) | 1 (0·3) | 1 (0·9) | 1 (0·3) | |
| 3 | 1 (0·2) | 0 | 0 | 0 | 0 | 1 (0·3) | |
| 16 | 1 | 9 (2·0) | 16 (3·4) | 15 (3·3) | 4 (1·3) | 1 (0·9) | 6 (1·9) |
| 2 | 2 (0·4) | 3 (0·6) | 5 (1·1) | 0 | 0 | 1 (0·3) | |
| 3 | 0 | 1 (0·2) | 0 | 0 | 0 | 0 | |
| 20 | 1 | – | – | – | 9 (2·9) | 2 (1·8) | 3 (1·0) |
| 2 | – | – | – | 1 (0·3) | 0 | 1 (0·3) | |
| 3 | – | – | – | 0 | 0 | 0 | |
| 28 | 1 | – | – | – | 4 (1·3) | 3 (2·7) | 11 (3·5) |
| 2 | – | – | – | 0 | 0 | 4 (1·3) | |
| 3 | – | – | – | 0 | 0 | 0 | |
| 36 | 1 | – | – | – | 5 (1·6) | 8 (7·3) | 8 (2·5) |
| 2 | – | – | – | 0 | 0 | 3 (1·0) | |
| 3 | – | – | – | 0 | 0 | 0 | |
| 44 | 1 | – | – | – | 8 (2·5) | 3 (2·7) | 8 (2·5) |
| 2 | – | – | – | 0 | 2 (1·8) | 3 (1·0) | |
| 3 | – | – | – | 0 | 0 | 0 | |
| 52 | 1 | – | – | – | 2 (0·6) | 4 (3·6) | 8 (2·5) |
| 2 | – | – | – | 1 (0·3) | 0 | 1 (0·3) | |
| 3 | – | – | – | 0 | 0 | 1 (0·3) | |
Neutropenia grade scale follows the guidance provided by the U.S. Food and Drug Administration37 grade 1: neutrophil counts of 1·5–2·0 × 109 L−1; grade 2: 1·0–1·499 × 109 L−1; grade 3: 0·5–0·999 × 109 L−1; grade 4: < 0·5 × 109 L−1. bNo patients in SOLO 1 & 2 and CHRONOS reported grade 4 neutropenia.
Post‐16‐week data were not collected for SOLO; qw, once weekly; q2w, every 2 weeks; TCS, topical corticosteroids.
Eosinophils
In SOLO 1 & 2, the dupilumab groups had greater mean and median initial increases from baseline in eosinophil counts than the placebo groups, with the highest increase observed at week 4 (Fig. 4a, b; Table S1b; see Supporting Information). Eosinophils returned to near‐baseline levels by week 16 [mean change (× 109 L−1), baseline to week 16: −0·2, −0·0 and −0·0, for placebo, dupilumab q2w and dupilumab qw, respectively]. By contrast, there was no such eosinophil increase in the overall study population in CHRONOS [mean change (× 109 L−1) baseline to week 52: −0·2, −0·2 and −0·2, for placebo + TCS, dupilumab q2w + TCS and dupilumab qw + TCS, respectively] (Fig. 4a, b; Table S1b; see Supporting Information). A higher proportion of patients in the dupilumab groups vs. placebo had shifts from normal eosinophil counts at baseline to high (> ULN) values throughout the treatment period in SOLO 1 & 2 (for dupilumab q2w and qw, respectively vs. placebo; week 4, 12·6% and 13·2% vs. 9·0%; week 16, 9·5% and 14·8% vs. 6·2%) and CHRONOS (for dupilumab q2w + TCS and qw + TCS, respectively vs. placebo + TCS; week 4, 9·1% and 9·6% vs. 7·8%; week 52, 14·5% and 9·1% vs. 6·7%) (Table S4c; see Supporting Information). In the subset of patients with grade 2 [eosinophil count: 1·501–5·0 × 109 L−1 (moderate)]37 and grade 3 [eosinophil count: > 5·0 × 109 L−1 (severe)]37 eosinophilia, small initial increases in absolute mean counts were observed in all treatment groups in SOLO 1 & 2 and CHRONOS, with a trend to return to near‐baseline levels; this was particularly evident in CHRONOS (Fig. 2c). Incidence rates of treatment‐emergent eosinophilia were numerically higher in the dupilumab groups in all three trials (Fig. 4c; Table 3). Grade 3 treatment‐emergent eosinophilia was reported in < 1% of dupilumab‐treated and placebo‐treated patients (Fig. 4c; Table 3). The transient increases in eosinophils had no apparent clinical consequences, as dupilumab‐treated patients with high eosinophil counts did not show any clinical manifestations or high frequency of AEs (Table S5; see Supporting Information).
Figure 4.
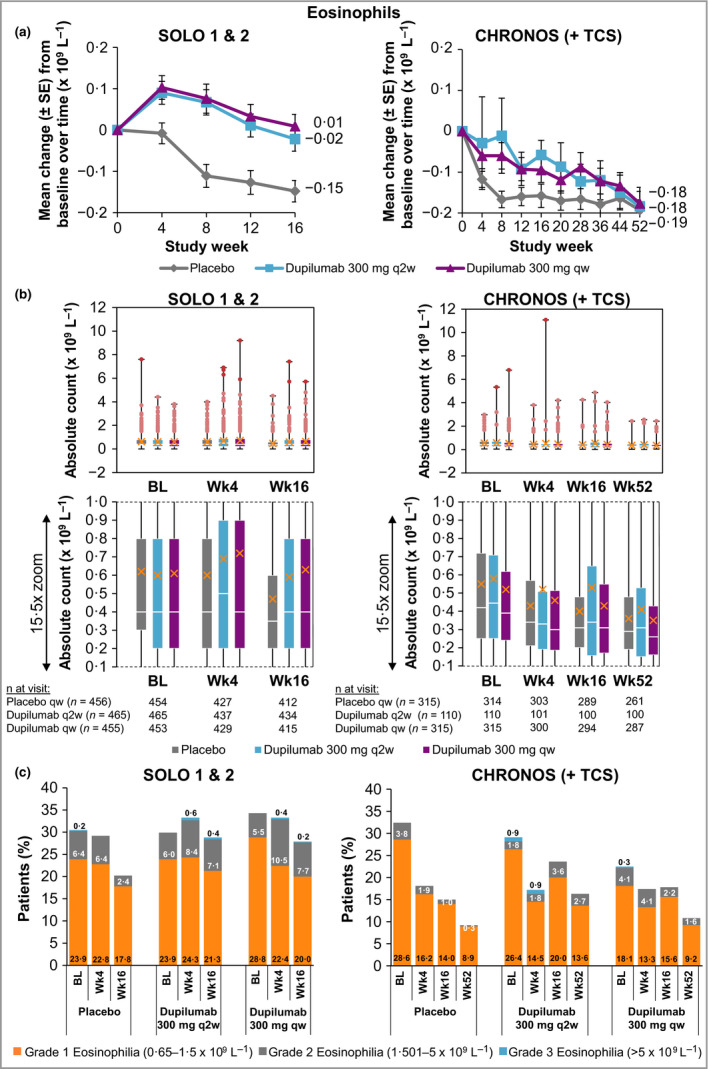
(a) Mean change in eosinophil count from baseline to week 16 (SOLO 1 & 2) and week 52 (CHRONOS). (b) Absolute eosinophil count. A close‐up view of the box‐and‐whisker plots is depicted below. White horizontal lines indicate medians. X depicts mean values. Top and bottom of each box represent Q3 and Q2, respectively. Upper and lower vertical bars represent Q4 and Q1, respectively; horizontal segments on each end of the vertical bars represent minimum and maximum values. Outliers for eosinophil counts 1·501–5·0 × 109 L−1 and > 5·0 × 109 L−1 are presented as light red and dark red dots, respectively. (c) Proportion of patients with eosinophilia grades 1–3; patient numbers are provided in Table 3. Eosinophilia grade scale follows the guidance provided by the U.S. Food and Drug Administration.37 BL, baseline; Q, quartile; qw, once weekly; q2w, every 2 weeks; TCS, topical corticosteroids; Wk, week.
Table 3.
| Study week | Eosinophilia grade | SOLO 1 & 2, n (%)b | CHRONOS, n (%) | ||||
|---|---|---|---|---|---|---|---|
| Placebo qw (n = 456) | Dupilumab 300 mg q2w (n = 465) | Dupilumab 300 mg qw (n = 455) | Placebo qw + TCS (n = 315) | Dupilumab 300 mg q2w + TCS (n = 110) | Dupilumab 300 mg qw + TCS (n = 315) | ||
| 0 | 1 | 109 (23·9) | 111 (23·9) | 131 (28·8) | 90 (28·6) | 29 (26·4) | 57 (18·1) |
| 2 | 29 (6·4) | 28 (6·0) | 25 (5·5) | 12 (3·8) | 2 (1·8) | 13 (4·1) | |
| 3 | 1 (0·2) | 0 | 0 | 0 | 1 (0·9) | 1 (0·3) | |
| 4 | 1 | 104 (22·8) | 113 (24·3) | 102 (22·4) | 51 (16·2) | 16 (14·5) | 42 (13·3) |
| 2 | 29 (6·4) | 39 (8·4) | 48 (10·5) | 6 (1·9) | 2 (1·8) | 13 (4·1) | |
| 3 | 0 | 3 (0·6) | 2 (0·4) | 0 | 1 (0·9) | 0 | |
| 8 | 1 | 79 (17·3) | 92 (19·8) | 105 (23·1) | 47 (14·9) | 21 (19·1) | 45 (14·3) |
| 2 | 19 (4·2) | 45 (9·7) | 39 (8·6) | 1 (0·3) | 3 (2·7) | 14 (4·4) | |
| 3 | 0 | 1 (0·2) | 3 (0·7) | 0 | 1 (0·9) | 0 | |
| 12 | 1 | 84 (18·4) | 86 (18·5) | 95 (20·9) | 34 (10·8) | 19 (17·3) | 53 (16·8) |
| 2 | 14 (3·1) | 43 (9·2) | 40 (8·8) | 3 (1·0) | 4 (3·6) | 7 (2·2) | |
| 3 | 0 | 0 | 1 (0·2) | 0 | 0 | 0 | |
| 16 | 1 | 81 (17·8) | 99 (21·3) | 91 (20·0) | 44 (14·0) | 22 (20·0) | 49 (15·6) |
| 2 | 11 (2·4) | 33 (7·1) | 35 (7·7) | 3 (1·0) | 4 (3·6) | 7 (2·2) | |
| 3 | 0 | 2 (0·4) | 1 (0·2) | 0 | 0 | 0 | |
| 20 | 1 | – | – | – | 27 (8·6) | 20 (18·2) | 37 (11·7) |
| 2 | – | – | – | 3 (1·0) | 3 (2·7) | 5 (1·6) | |
| 3 | – | – | – | 0 | 1 (0·9) | 1 (0·3) | |
| 28 | 1 | – | – | – | 32 (10·2) | 14 (12·7) | 37 (11·7) |
| 2 | – | – | – | 3 (1·0) | 4 (3·6) | 8 (2·5) | |
| 3 | – | – | – | 0 | 0 | 1 (0·3) | |
| 36 | 1 | – | – | – | 29 (9·2) | 18 (16·4) | 42 (13·3) |
| 2 | – | – | – | 2 (0·6) | 3 (2·7) | 2 (0·6) | |
| 3 | – | – | – | 0 | 0 | 0 | |
| 44 | 1 | – | – | – | 29 (9·2) | 17 (15·5) | 33 (10·5) |
| 2 | – | – | – | 1 (0·3) | 1 (0·9) | 5 (1·6) | |
| 3 | – | – | – | 0 | 0 | 0 | |
| 52 | 1 | – | – | – | 28 (8·9) | 15 (13·6) | 29 (9·2) |
| 2 | – | – | – | 1 (0·3) | 3 (2·7) | 5 (1·6) | |
| 3 | – | – | – | 0 | 0 | 0 | |
The eosinophilia grade scale follows the guidance provided by the U.S. Food and Drug Administration:37 grade 1: eosinophil counts of 0·65–1·5 × 109 L−1; grade 2: 1·501–5·0 × 109 L−1; grade 3: > 5·0 × 109 L−1.
Post‐16 week data were not collected for SOLO; qw, once weekly; q2w, every 2 weeks; TCS, topical corticosteroids.
Lactate dehydrogenase
LDH levels were elevated at baseline (median, 231·0–247·0; Table S2d; see Supporting Information). At baseline, 25·2–27·0% of patients in SOLO 1 & 2 and 50·5–57·3% of patients in CHRONOS had levels above the ULN [per central laboratory determination; normal ranges: SOLO 1 & 2, males: 135–281 IU L−1, females: 135–330 IU L−1; CHRONOS, 53–234 IU L−1 (both sexes)]. Serum LDH decreased over time in SOLO 1 & 2 (mean change from baseline to week 16: −26·2, −69·7 and −76·0 IU L−1 for placebo, dupilumab q2w and dupilumab qw, respectively) and CHRONOS (mean change, baseline to week 52: −49·7, −88·2 and −85·1 IU L−1 for placebo + TCS, dupilumab q2w + TCS and dupilumab qw + TCS, respectively) (Fig. 5a, b; Table S2d; see Supporting Information). A higher proportion of patients in the dupilumab groups vs. placebo had shifts from high LDH at baseline to normal values throughout the treatment period (Fig. 5c; Table S4d; see Supporting Information).
Figure 5.
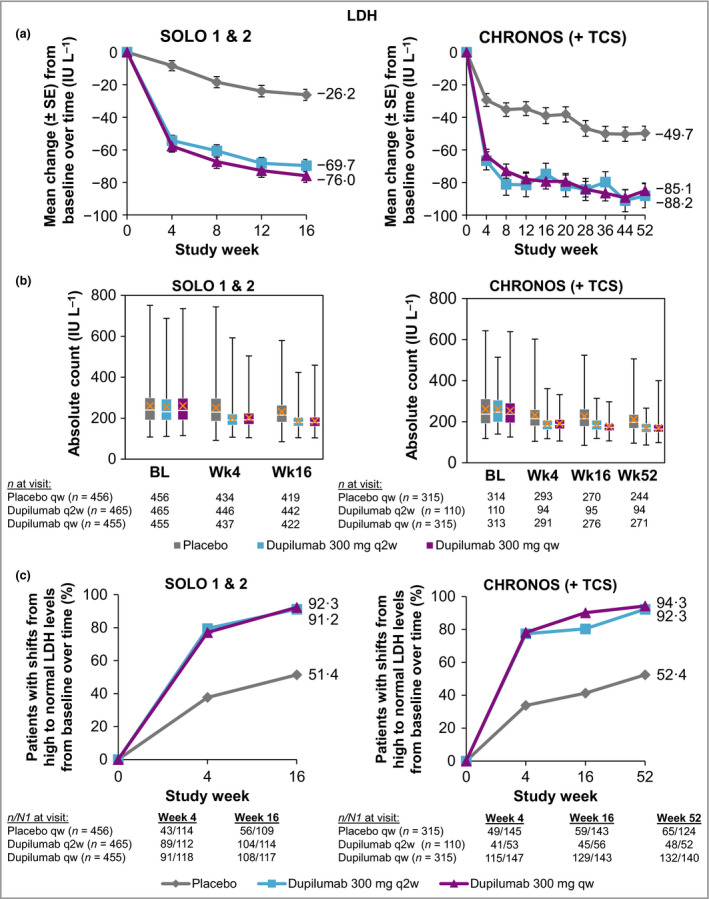
(a) Mean change in LDH from baseline to week 16 (SOLO 1 & 2) and week 52 (CHRONOS). (b) Absolute LDH levels. White horizontal lines indicate medians. X depicts mean values. Top and bottom of each box represent Q3 and Q2, respectively. Upper and lower vertical bars represent Q4 and Q1, respectively; horizontal segments on each end of the vertical bars represent minimum and maximum values. (c) Proportion of patients with shifts from high to normal values from baseline to week 16 (SOLO 1 & 2) and week 52 (CHRONOS). Normal range: 135–330 IU L−1 (female) and 135–281 IU L−1 (male) in SOLO 1 & 2, respectively, and 53–234 IU L−1 in CHRONOS. BL, baseline; LDH, lactate dehydrogenase; n, number of patients; N1, total number of patients with at least one value at visit; Q, quartile; qw, once weekly; q2w, every 2 weeks; TCS, topical corticosteroids; Wk, week.
Other laboratory parameters
No changes in other haematology (Table S1a, b; see Supporting Information), serum chemistry and urinalysis (Table S2a–e; Table S3; see Supporting Information) parameters in any of the treatment groups were of clinical concern or necessitated additional testing or changes to treatment.
Treatment withdrawal
Three patients discontinued study treatment due to abnormal laboratory values (Fig. S1a–c; see Supporting Information). These included one patient in the dupilumab qw group in SOLO 1 with a TEAE of lymphocytosis, which was not considered by the investigator to be related to dupilumab; and one patient each in the dupilumab q2w group in SOLO 2 and the placebo + TCS group in CHRONOS with TEAEs of neutropenia, which were considered by the investigators to be related to the study drug. The patients in SOLO 1 & 2 continued to be monitored to week 16, whereas the CHRONOS patient withdrew from the study and was not monitored after week 8. No other laboratory test abnormalities led to treatment discontinuation in these studies. There were no notable differences in the laboratory results for the patients who withdrew due to haematological changes or patients who discontinued due to any TEAEs compared with the general patient population, other than those that would be expected for those particular patients (Tables S6a, b; see Supporting Information).
Adverse events
AEs reported for the overall study populations in SOLO 1 & 2 and CHRONOS were published previously.21, 22 Overall, similar rates of TEAEs were reported across treatment groups during these studies. The overall incidence of infections was similar in patients treated with dupilumab and placebo.21, 22, 34 Conjunctivitis (both MedDRA PT ‘conjunctivitis’ and a compiled term for any PT with the word ‘conjunctivitis’) and injection‐site reactions (MedDRA high‐level term) were more frequent in patients treated with dupilumab.21, 22, 36 Higher rates of AD exacerbations (MedDRA PT) and nonherpetic skin infections (adjudicated) were reported in the placebo groups. Laboratory outcomes that were serious adverse events (SAEs) were rare: one patient (0·5%) in SOLO 1 had an SAE of anaemia; one (0·4%) in SOLO 2 had an SAE of thrombocytopenia and one (0·3%) in CHRONOS had an SAE of an abnormal liver function test (MedDRA PTs); all were in the placebo group in their respective studies.22, 23, 24
Discussion
This analysis of more than 2000 patients from three large phase III placebo‐controlled studies did not reveal any adverse changes in laboratory parameters that could be attributed to dupilumab. None of the reported changes necessitated additional testing or changes to treatment. Mean and median haematology and serum chemistry laboratory values were generally consistent with baseline values or showed small changes from baseline. Small decreases from baseline were noted for platelets and neutrophils within the dupilumab vs. placebo groups, findings that may reflect a decrease in systemic inflammation and AD severity.38, 39, 40, 41, 42 Overall, platelet and neutrophil counts remained within the normal range at any visit, and the changes were not clinically meaningful for any treatment group.
Transient and usually mild‐to‐moderate increases in eosinophils were observed in a small number of patients treated with dupilumab in these studies; no AEs were attributed to eosinophilia. Of note, eosinophilia was less marked in patients treated with dupilumab + TCS in CHRONOS, suggesting that concomitant use of TCS may blunt the effect on eosinophils seen in the monotherapy studies.43 Blood and tissue eosinophilia are characteristic of AD and other atopic diseases and correlate with disease activity.44, 45, 46, 47, 48, 49 Type 2 inflammation mediated by IL‐4, IL‐13 and IL‐5 promotes eosinophil growth, differentiation and trafficking to sites of inflammation.50 Moreover, IL‐4 and IL‐13 regulate the expression of eotaxin‐1 [C–C motif chemokine 11 (CCL11)], eotaxin‐3 (CCL26), RANTES (CCL5) and MCP‐4 (CCL13), potent chemoattractants for eosinophils.51, 52, 53, 54 IL‐4 and IL‐13 also induce vascular cell adhesion molecule 1 (VCAM‐1) on endothelial cells, which is an adhesin involved in eosinophil transmigration.51, 52, 53, 54 IL‐4 and IL‐13 promote eosinophil recruitment to sites of inflammation by increasing production of these chemokines and VCAM‐1.47, 50, 51, 52, 54 In lesional skin of patients with AD, dupilumab decreased eotaxin‐3 and MCP‐4.27, 55 In mouse models of asthma, dupilumab blockage of IL‐4 and IL‐13 signalling prevents eosinophils from entering tissue, and thus, eosinophils accumulate in the bloodstream.56, 57 Hence, the transient increases in circulating eosinophils observed in a subset of patients could be a result of dupilumab inhibiting IL‐4/IL‐13‐induced migration of eosinophils from the bloodstream into tissues.50, 58 The fact that this phenomenon is not observed in every dupilumab‐treated patient with AD suggests that net effects on eosinophil counts depend on the magnitude of chemokine suppression and the rate of eosinophil production in a given patient. Of note, higher baseline levels of circulating eosinophils have been associated with increased incidence of conjunctivitis in both placebo‐treated and dupilumab‐treated patients in clinical trials in AD.36 In monotherapy studies, patients with high baseline eosinophil levels reported 0·03–0·15 events of conjunctivitis per 100 patient‐years (PYs) for placebo and dupilumab combined, respectively, whereas patients with low baseline levels reported 0·02–0·03 events per 100 PYs.36 Similarly, in CHRONOS, patients with high eosinophils at baseline had 0·04–0·13 conjunctivitis events per 100 PYs, respectively for placebo + TCS and dupilumab qw + TCS vs. 0·03–0·05 events per 100 PYs for patients with low baseline eosinophil levels;36 similar associations were also observed for baseline levels of the biomarkers thymus and activation‐regulated chemokine (TARC; CCL17) and IgE.36 AD severity is associated with biomarker levels as well as with incidence of conjunctivitis, suggesting that the association of these biomarkers with conjunctivitis may result from their relationship to AD severity.36
A decrease in LDH levels from baseline was seen during dupilumab treatment regardless of dose regimen in all trials. More dupilumab‐treated patients achieved normalization of LDH during the treatment period compared with placebo‐treated patients. LDH is an intracellular enzyme that is widely expressed in tissues such as the skin.59, 60, 61 It is a biomarker of tissue damage, and serum LDH concentrations have been reported to correlate with AD disease activity and severity.60, 61, 62, 63
No clinically meaningful changes were observed between treatment groups for other haematology, chemistry or urinalysis parameters. Overall, dupilumab was well tolerated in patients with moderate‐to‐severe AD with and without concomitant TCS use; both dupilumab dose regimens had acceptable safety profiles, which were generally comparable with that of placebo, apart from conjunctivitis and injection‐site reactions.21, 22, 23 SAEs of laboratory outcomes were rare and were reported only in placebo‐treated patients.
This analysis had limitations. These analyses included a total of 2116 patients; most (1376 in SOLO 1 & 2) received dupilumab for up to 16 weeks, and the remainder (740 in CHRONOS) for up to 52 weeks. These data showed that mean changes in laboratory parameters were transient and returned to near‐baseline levels during study treatment and were not associated with any clinical AEs, supporting the current guidance in the prescribing information that there is no requirement for routine laboratory monitoring. However, greater numbers of patients exposed to dupilumab for longer periods of time and in real‐world situations will provide the sample sizes and more varied patient populations needed to discern rare events. Eligibility criteria for these trials excluded patients with serious concomitant health conditions; thus, further data are needed to determine laboratory monitoring needs for patients with the variety of conditions that may be encountered in a real‐world setting.
In conclusion, although transient reductions in neutrophils and platelets as well as transient increases in blood eosinophils were observed in a small number of dupilumab‐treated patients, they were not associated with any clinically significant AEs. The laboratory data described here, along with the efficacy and safety data provided in the previous reports of these studies, demonstrate that dupilumab results in a favourable risk/benefit ratio profile, further supporting the use of dupilumab as a systemic treatment for long‐term management of moderate‐to‐severe AD.21, 22, 23 Consistent with clinical trial data, EU20 and U.S.19 prescribing information do not require routine laboratory monitoring in clinical practice before initiation or during treatment with dupilumab. Because patient exclusion criteria in the dupilumab trials may have limited the variability of the study population, real‐world evidence, including long‐term data beyond 52 weeks of treatment, will provide additional valuable information on the role of laboratory monitoring in dupilumab‐treated patients with AD.
Supporting information
Table S1 Summary statistics for haematology laboratory parameters: (a) red blood cells and platelets; (b) white blood cells.
Table S2 Summary statistics for serum chemistry laboratory parameters: (a) metabolic function; (b) electrolytes; (c) renal function; (d) liver function; (e) lipid panel.
Table S3 Summary statistics for urinalysis laboratory parameters.
Table S4 Shift from baseline: (a) platelets (× 109 L−1); (b) neutrophils (× 109 L−1); (c) eosinophils (× 109 L−1); (d) lactate dehydrogenase (IU L−1).
Table S5 Adverse events (based on MedDRA‐Preferred Term) in dupilumab‐treated patientsa with an eosinophil count > 5·0 × 109 L−1 at any visit in SOLO 1 & 2 and CHRONOS.
Table S6 Summary statistics for haematology laboratory parameters in patients who discontinued study treatment due to haematological TEAEs or any TEAE: (a) red blood cells and platelets; (b) white blood cells.
Fig S1. Haematological data over time in patients who withdrew due to haematological adverse events.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.
Acknowledgments
The research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov Identifiers: NCT02277743 (SOLO 1), NCT02277769 (SOLO 2) and NCT02260986 (CHRONOS). Medical writing/editorial assistance was provided by Manuela Pigors, PhD, and Vicki Schwartz, PhD, of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc. The authors thank the patients and their families for their participation in the studies; their colleagues for their support; and Jennifer D. Hamilton, Linda Williams (Regeneron Pharmaceuticals, Inc.) and El‐Bdaoui Haddad (Sanofi Genzyme) for their contributions.
Funding sources Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. The study sponsors participated in the study design; collection, analysis and interpretation of data; writing of the manuscript; and the decision to submit the manuscript for publication. Medical writing and editorial support were funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Conflicts of interest A.W. has been an advisor, speaker or investigator for ALK Abelló, Almirall, Anacor, Astellas, Beiersdorf, Bencard, Bioderma, Chugai, Galderma, GlaxoSmithKline, Hans Karrer, LEO Pharma, Eli Lilly, L'Oreal, Maruho, MedImmune, Novartis, Pfizer, Pierre Fabre, Sanofi and Regeneron Pharmaceuticals, Inc. L.A.B. has received honoraria as a consultant for AbbVie, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Novan, Novartis, Realm Therapeutics, Regeneron Pharmaceuticals, Inc., Sanofi and UCB; has received grants for clinical trials from AbbVie, Pfizer, Realm Therapeutics and Regeneron Pharmaceuticals, Inc.; and has stock in Pfizer and Medtronics. A.B. has been a scientific advisor and clinical study investigator for AbbVie, Aclaris Therapeutics, Akros, Allergan, Almirall, Amgen, Arena, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Eli Lilly, Galderma, Genentech/Roche, GlaxoSmithKline, Janssen, LEO Pharma, Meiji, Merck, Novartis, Pfizer, Purdue Pharma, Regeneron Pharmaceuticals, Inc., Revance, Sandoz, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, UCB, Valeant and Vidac; and has been a paid speaker for Janssen, Regeneron Pharmaceuticals, Inc. and Sanofi Genzyme. E.L.S. has received honoraria for consulting services from AbbVie, Anacor Pharma, Celgene, Dermira, Eli Lilly, Galderma, Genentech, GlaxoSmithKline, LEO Pharma, Menlo Therapeutics, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme and Valeant; and has provided study support for Anacor, Eli Lilly, GlaxoSmithKline, MedImmune, Novartis, Regeneron Pharmaceuticals, Inc., Roivant Sciences, Tioga and Vanda. Z.C., Q.C., B.S., F.A.K., N.M.H.G., Y.L. and M.A. are employees and shareholders of Regeneron Pharmaceuticals, Inc. T.H., E.R., A.B.R. and G.P. are employees of Sanofi and may hold stock and/or stock options in the company.
References
- 1. Wollenberg A, Oranje A, Deleuran M et al ETFAD/EADV eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol 2016; 30:729–47. [DOI] [PubMed] [Google Scholar]
- 2. Eichenfield LF, Tom WL, Chamlin SL et al Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014; 70:338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weidinger S, Beck LA, Bieber T et al Atopic dermatitis. Nat Rev Dis Primers 2018; 4:1. [DOI] [PubMed] [Google Scholar]
- 4. Ong PY, Leung DY. Bacterial and viral infections in atopic dermatitis: a comprehensive review. Clin Rev Allergy Immunol 2016; 51:329–37. [DOI] [PubMed] [Google Scholar]
- 5. Langan SM, Abuabara K, Henrickson SE et al Increased risk of cutaneous and systemic infections in atopic dermatitis – a cohort study. J Invest Dermatol 2017; 137:1375–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Narla S, Silverberg JI. Association between atopic dermatitis and serious cutaneous, multiorgan and systemic infections in US adults. Ann Allergy Asthma Immunol 2018; 120:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wollenberg A, Barbarot S, Bieber T et al Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J Eur Acad Dermatol Venereol 2018; 32:657–82. [DOI] [PubMed] [Google Scholar]
- 8. Boguniewicz M, Fonacier L, Guttman‐Yassky E et al Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol 2018; 120:10–22. [DOI] [PubMed] [Google Scholar]
- 9. Arkwright PD, Motala C, Subramanian H et al Atopic Dermatitis Working Group of the Allergic Skin Diseases Committee of the AAAAI. Management of difficult‐to‐treat atopic dermatitis. J Allergy Clin Immunol Pract 2013; 1:142–51. [DOI] [PubMed] [Google Scholar]
- 10. Drucker AM, Eyerich K, de Bruin‐Weller MS et al Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br J Dermatol 2018; 178:768–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roekevisch E, Spuls PI, Kuester D et al Efficacy and safety of systemic treatments for moderate‐to‐severe atopic dermatitis: a systematic review. J Allergy Clin Immunol 2014; 133:429–38. [DOI] [PubMed] [Google Scholar]
- 12. Megna M, Napolitano M, Patruno C et al Systemic treatment of adult atopic dermatitis: a review. Dermatol Ther (Heidelb) 2017; 7:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ring J, Alomar A, Bieber T et al Guidelines for treatment of atopic eczema (atopic dermatitis). Part II. J Eur Acad Dermatol Venereol 2012; 26:1176–93. [DOI] [PubMed] [Google Scholar]
- 14. Sidbury R, Davis DM, Cohen DE et al Guidelines of care for the management of atopic dermatitis: Section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014; 71:327–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wollenberg A, Barbarot S, Bieber T et al Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part II. J Eur Acad Dermatol Venereol 2018; 32:850–78. [DOI] [PubMed] [Google Scholar]
- 16. Macdonald LE, Karow M, Stevens S et al Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci USA 2014; 111:5147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murphy AJ, Macdonald LE, Stevens S et al Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci USA 2014; 111:5153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL‐4/IL‐13 pathway in atopic diseases. Expert Rev Clin Immunol 2017; 13:425–37. [DOI] [PubMed] [Google Scholar]
- 19. DUPIXENT® (dupilumab) . Highlights of prescribing information. US Food and Drug Administration 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761055s014lbl.pdf (last accessed 5 August 2019).
- 20. Dupixent (dupilumab) . Summary of Product Characteristics. European Medicines Agency 2017. Available from: https://www.ema.europa.eu/documents/product-information/dupixent-epar-product-information_en.pdf (last accessed 16 April 2019).
- 21. Simpson EL, Bieber T, Guttman‐Yassky E et al Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375:2335–48. [DOI] [PubMed] [Google Scholar]
- 22. Blauvelt A, de Bruin‐Weller M, Gooderham M et al Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomised, double‐blinded, placebo‐controlled, phase 3 trial. Lancet 2017; 389:2287–303. [DOI] [PubMed] [Google Scholar]
- 23. Thaçi D, Simpson EL, Deleuran M et al Efficacy and safety of dupilumab monotherapy in adults with moderate‐to‐severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J Dermatol Sci 2019; 94:266–75. [DOI] [PubMed] [Google Scholar]
- 24. Beck LA, Thaçi D, Hamilton JD et al Dupilumab treatment in adults with moderate‐to‐severe atopic dermatitis. N Engl J Med 2014; 371:130–9. [DOI] [PubMed] [Google Scholar]
- 25. Thaçi D, Simpson EL, Beck LA et al Efficacy and safety of dupilumab in adults with moderate‐to‐severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo‐controlled, dose‐ranging phase 2b trial. Lancet 2016; 387:40–52. [DOI] [PubMed] [Google Scholar]
- 26. de Bruin‐Weller M, Thaçi D, Smith CH et al Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo‐controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol 2018; 178:1083–101. [DOI] [PubMed] [Google Scholar]
- 27. Guttman‐Yassky E, Bissonnette R, Ungar B et al Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol 2019; 143:155–172. [DOI] [PubMed] [Google Scholar]
- 28. Wenzel S, Ford L, Pearlman D et al Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med 2013; 368:2455–66. [DOI] [PubMed] [Google Scholar]
- 29. Bachert C, Mannent L, Naclerio RM et al Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA 2016; 315:469–79. [DOI] [PubMed] [Google Scholar]
- 30. Wenzel S, Castro M, Corren J et al Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium‐to‐high‐dose inhaled corticosteroids plus a long‐acting β2 agonist: a randomised double‐blind placebo‐controlled pivotal phase 2b dose‐ranging trial. Lancet 2016; 388:31–44. [DOI] [PubMed] [Google Scholar]
- 31. Hirano I, Dellon ES, Hamilton JD et al Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology 2019; DOI: 10.1053/j.gastro.2019.09.042. [DOI] [PubMed] [Google Scholar]
- 32. Castro M, Corren J, Pavord I et al Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med 2018; 378:2486–96. [DOI] [PubMed] [Google Scholar]
- 33. Rabe K, Nair P, Brusselle G et al Efficacy and safety of dupilumab in glucocorticoid‐dependent severe asthma. N Engl J Med 2018; 378:2475–85. [DOI] [PubMed] [Google Scholar]
- 34. Eichenfield L, Bieber T, Beck LA et al Infections in dupilumab clinical trials in atopic dermatitis: a comprehensive pooled analysis. Am J Clin Dermatol 2019; 20:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ali T, Kaitha S, Mahmood S et al Clinical use of anti‐TNF therapy and increased risk of infections. Drug Healthc Patient Saf 2013; 5:79–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akinlade B, Guttman‐Yassky E, de Bruin‐Weller M et al Conjunctivitis in dupilumab clinical trials. Br J Dermatol 2019; 181:459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. 2007. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical (last accessed 5 October 2019).
- 38. Tamagawa‐Mineoka R, Katoh N, Ueda E et al The role of platelets in leukocyte recruitment in chronic contact hypersensitivity induced by repeated elicitation. Am J Pathol 2007; 170:2019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Katoh N. Platelets as versatile regulators of cutaneous inflammation. J Dermatol Sci 2009; 53:89–95. [DOI] [PubMed] [Google Scholar]
- 40. Choy DF, Hsu DK, Seshasayee D et al Comparative transcriptomic analyses of atopic dermatitis and psoriasis reveal shared neutrophilic inflammation. J Allergy Clin Immunol 2012; 130:1335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hon KL, Wang SS, Pong NH et al Circulating immunoglobulins, leucocytes and complements in childhood‐onset atopic eczema. Indian J Pediatr 2013; 80:128–31. [DOI] [PubMed] [Google Scholar]
- 42. Tamagawa‐Mineoka R. Important roles of platelets as immune cells in the skin. J Dermatol Sci 2015; 77:93–101. [DOI] [PubMed] [Google Scholar]
- 43. Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol 2009; 101:81–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uehara M, Izukura R, Sawai T. Blood eosinophilia in atopic dermatitis. Clin Exp Dermatol 1990; 15:264–6. [DOI] [PubMed] [Google Scholar]
- 45. Kägi MK, Joller‐Jemelka H, Wüthrich B. Correlation of eosinophils, eosinophil cationic protein and soluble interleukin‐2 receptor with the clinical activity of atopic dermatitis. Dermatology 1992; 185:88–92. [DOI] [PubMed] [Google Scholar]
- 46. Kiehl P, Falkenberg K, Vogelbruch M et al Tissue eosinophilia in acute and chronic atopic dermatitis: a morphometric approach using quantitative image analysis of immunostaining. Br J Dermatol 2001; 145:720–9. [DOI] [PubMed] [Google Scholar]
- 47. Liu FT, Goodarzi H, Chen HY. IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol 2011; 41:298–310. [DOI] [PubMed] [Google Scholar]
- 48. de Graauw E, Beltraminelli H, Simon HU et al Eosinophilia in dermatologic disorders. Immunol Allergy Clin North Am 2015; 35:545–60. [DOI] [PubMed] [Google Scholar]
- 49. Werfel T, Allam JP, Biedermann T et al Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol 2016; 138:336–49. [DOI] [PubMed] [Google Scholar]
- 50. Gandhi NA, Bennett BL, Graham NM et al Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov 2016; 15:35–50. [DOI] [PubMed] [Google Scholar]
- 51. Yamamoto H, Nagata M, Sakamoto Y. CC chemokines and transmigration of eosinophils in the presence of vascular cell adhesion molecule 1. Ann Allergy Asthma Immunol 2005; 94:292–300. [DOI] [PubMed] [Google Scholar]
- 52. Schleimer RP, Sterbinsky SA, Kaiser J et al IL‐4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM‐1. J Immunol 1992; 148:1086–92. [PubMed] [Google Scholar]
- 53. Esche C, Stellato C, Beck LA. Chemokines: key players in innate and adaptive immunity. J Invest Dermatol 2005; 125:615–28. [DOI] [PubMed] [Google Scholar]
- 54. Doran E, Cai F, Holweg CTJ et al Interleukin‐13 in asthma and other eosinophilic disorders. Front Med (Lausanne) 2017; 4:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hamilton JD, Suárez‐Fariñas M, Dhingra N et al Dupilumab improves the molecular signature in skin of patients with moderate‐to‐severe atopic dermatitis. J Allergy Clin Immunol 2014; 134:1293–300. [DOI] [PubMed] [Google Scholar]
- 56. Allinne J, Scott G, Birchard D et al Broader impact of IL‐4Rα blockade than IL‐5 blockade on mediators of type 2 inflammation and lung pathology in a house dust mite‐induced asthma mouse model. Am J Respir Crit Care Med 2019; 199:A5555. [Google Scholar]
- 57. Orengo JM, Allinne J, Le Floc'h A et al Blocking IL‐4Rα with dupilumab prevents lung inflammation in a mouse asthma model. Eur Respir J 2018; 52 (Suppl. 62):PA977. [Google Scholar]
- 58. Webb DC, McKenzie ANJ, Koskinen AML et al Integrated signals between IL‐13, IL‐4, and IL‐5 regulate airways hyperreactivity. J Immunol 2000; 165:108–13. [DOI] [PubMed] [Google Scholar]
- 59. Morishima Y, Kawashima H, Takekuma K et al Changes in serum lactate dehydrogenase activity in children with atopic dermatitis. Pediatr Int 2010; 52:171–4. [DOI] [PubMed] [Google Scholar]
- 60. Thijs J, Krastev T, Weidinger S et al Biomarkers for atopic dermatitis: a systematic review and meta‐analysis. Curr Opin Allergy Clin Immunol 2015; 15:453–60. [DOI] [PubMed] [Google Scholar]
- 61. Simpson EL, Villarreal M, Jepson B et al Patients with atopic dermatitis colonized with Staphylococcus aureus have a distinct phenotype and endotype. J Invest Dermatol 2018; 138:2224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mukai H, Noguchi T, Kamimura K et al Significance of elevated serum LDH (lactate dehydrogenase) activity in atopic dermatitis. J Dermatol 1990; 17:477–81. [DOI] [PubMed] [Google Scholar]
- 63. Kou K, Aihara M, Matsunaga T et al Association of serum interleukin‐18 and other biomarkers with disease severity in adults with atopic dermatitis. Arch Dermatol Res 2012; 304:305–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Summary statistics for haematology laboratory parameters: (a) red blood cells and platelets; (b) white blood cells.
Table S2 Summary statistics for serum chemistry laboratory parameters: (a) metabolic function; (b) electrolytes; (c) renal function; (d) liver function; (e) lipid panel.
Table S3 Summary statistics for urinalysis laboratory parameters.
Table S4 Shift from baseline: (a) platelets (× 109 L−1); (b) neutrophils (× 109 L−1); (c) eosinophils (× 109 L−1); (d) lactate dehydrogenase (IU L−1).
Table S5 Adverse events (based on MedDRA‐Preferred Term) in dupilumab‐treated patientsa with an eosinophil count > 5·0 × 109 L−1 at any visit in SOLO 1 & 2 and CHRONOS.
Table S6 Summary statistics for haematology laboratory parameters in patients who discontinued study treatment due to haematological TEAEs or any TEAE: (a) red blood cells and platelets; (b) white blood cells.
Fig S1. Haematological data over time in patients who withdrew due to haematological adverse events.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.


