Abstract
Background: Polycystic ovarian syndrome (PCOS) is a kind of common gynecological endocrine disorder. And the mutations of melatonin receptor (MTNR) genes are related to the occurrence of PCOS. But previous researches have shown opposite results. So, the object of our systematic review and meta-analysis is to investigate the relationship between MTNR 1A/B polymorphisms and PCOS.
Methods: PubMed, Embase, Ovid, the Cochrane Library, Web of Science and three Chinese databases (VIP, CNKI and Wanfang) were used to retrieve eligible articles published between January 1980 and February 2020. And we used the odds ratio (OR) and its 95% confidence interval (CI) to investigate the strength of the association by six genetic models, allelic, codominant (homozygous and heterozygous), dominant, recessive and superdominant models. Review Manager 5.3, IBM SPSS statistics 25 and Stata MP 16.0 software were used to do this meta-analysis.
Results: Our meta-analysis involved 2553 PCOS patients and 3152 controls, for two single nucleotide polymorphisms (rs10830963 C> G in MTNR1B and rs2119882 T> C in MTNR1A) and significant associations were found in some genetic models of these single nucleotide polymorphisms (SNPs). For rs10830963, strongly significant was found in the heterozygote model (GC vs. CC, P=0.02). Additionally, a slight trend was detected in the allelic (G vs. C), homozygote (GG vs. CC) and dominant (GG+GC vs. CC) model of rs10830963 (P=0.05). And after further sensitivity analysis, a study with high heterogeneity was removed. In the allelic (P=0.000), homozygote (P=0.001), dominant (P=0.000) and recessive (GG vs. GC+CC, P=0.001) model, strong associations between rs10830963 and PCOS were found. Moreover, for rs2119882, five genetic models, allelic (C vs. T, P=0.000), codominant (the homozygote (CC vs. TT, P=0.000) and heterozygote model (CT vs. TT, P=0.02), dominant (CC + CT vs. TT, P=0.03) and recessive model (CC vs. CT + TT, P=0.000) showed significant statistical associations with PCOS.
Conclusion: MTNR1B rs10830963 and MTNR1B rs2119882 polymorphisms are associated with PCOS risk. However, the above conclusions still require being confirmed by much larger multi-ethnic studies.
Keywords: melatonin receptor, meta analysis, polycystic ovarian syndrome, single nucleotide polymorphisms
Background
Melatonin secreted by the pineal gland during the dark phase of the sleep/wake cycle, is a kind of hormone which periodically regulates several physiological functions [1], including glucose homeostasis [2] and insulin secretion [3]. When the melatonin secretion or the corresponding Melatonin Receptor (MTNR) is abnormal, the metabolism in humans may be seriously damaged [4]. And MTNR exists in ovarian granulosa cells membranes [5,6], therefore, melatonin as a pleiotropic molecule has a direct effect on ovarian function [7,8]. Low melatonin levels in follicular fluid affect the quality and number of oocytes, and ultimately affect the outcome of in vitro fertilization (IVF) [9]. Moreover, melatonin treatment can also be used as a combination therapy to help control the blood glucose level [2], slow the progress or improve type 2 diabetes mellitus (T2DM) [10], increase pregnancy rates and improve endocrine levels eventually [11].
Additionally, multiple studies reveal that melatonin secretion is increased in polycystic ovarian syndrome (PCOS) patients [12–14]. PCOS is a kind of common metabolic and endocrine disorder in reproductive women [15], of which global prevalence is approximately 6–10% [16,17]. It has a variety of heterogeneous clinical manifestations, and approximately 20–30% of patients with PCOS suffer from various complications [18], like metabolic syndrome [19] and insulin resistance (IR) [20], which makes PCOS hard to be explained by a single factor. PCOS is a highly clinical as well as genetic heterogeneity syndrome [21]. For example, oocytes of PCOS patients also have abnormal gene transcription and expression, which will affect individual reproductive capacity [22]. As metabolic disorders have become much more prevalent recently [23], more genome-wide association and cohort studies were conducted and more variant genes and novel single nucleotide polymorphisms (SNPs) are found in large Chinese PCOS population [24,25], which have been confirmed later by multi-ethnic studies [26,27], including thyroid associated protein gene [28], Fat Mass and Obesity (FTO) gene [29,30], Follicle-stimulating Hormone Receptor (FSHR) gene [31,32], DENN/MADD domain containing 1A gene [33], Vitamin D Receptor (VDR) gene [34–36] and so on.
Additionally, the SNPs of MTNR 1A/B gene (MTNR1A/B) are found to have associations with many kinds of metabolic disorders. For example, MTNR1B rs10830963 polymorphism is not only associated with fasting blood glucose levels, but also with IR and T2DM risk [37]. And MTNR1A rs2119882 is also associated with gestational diabetes mellitus and IR [38]. However, whether MTNR polymorphisms relate to another metabolic disorder, PCOS, is still inconclusive because previous genetic research has shown conflicting conclusions [39]. So, the object of our meta-analysis is to ascertain the association between MTNR1A/B polymorphisms and PCOS.
Methods
Our study was conducted with the requirements of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [40]. All data were available from currently published studies, so no informed consent and Ethics Committee approval were required.
Study protocol
Five English databases (PubMed, Embase, Ovid, the Cochrane Library and Web of Science) and three Chinese databases (VIP, CNKI and Wanfang) were searched in our study. And we used three subject heading terms, ‘Polycystic ovarian syndrome’, ‘melatonin’ and ‘melatonin receptor’, as well as the synonyms of these terms confirmed by Medical Subject Headings (MeSH) referred to in the Supplementary Material S1 (Search strategies) [41], where other free words were registered. The latest search results were updated on 14 February 2020. And there were no language restrictions in these search strategies. Finally, all references were exported to EndNote X9 software for further research.
Definitions and results
As the definition of PCOS used in the present study, all the eligible studies adopted the 2003 Rotterdam diagnostic inclusion criteria of PCOS [42]: (1) thin ovulation or anovulation. (2) Clinical manifestations of hyperandrogen or hyperandrogenemia. (3) Polycystic ovarian changes, that is, ≥12 follicles with a diameter of 2–9 millimeters on ovary, or ovarian volume > 10 ml. As long as two of the above criteria are met and other diseases are excluded, PCOS can be diagnosed. The results were the genotype distribution in MTNR gene and the odds ratio (OR) of PCOS.
Eligibility criteria
Included references needed to meet the following criteria:
Assess the association between SNP in MTNR and PCOS risk,
Contain particular data, such as the frequency of MTNR genotypes.
Excluded studies should meet the following criteria:
Abstracts and reviews,
Repeated dissertation,
No research on genotype distribution,
Non-human research.
Among them, Newcastle–Ottawa Scale (NOS), available from URL (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm) [43], was performed to evaluate the quality of all included studies.
Study selection
All studies retrieved from all the databases were assessed and reviewed by the first and second authors (Shiqi Yi and Hao Shi) based on eligibility criteria. The data extraction results were agreed upon discussion with another first author (Jiawei Xu). Study and descriptors such as author, publication time, country, ethnicity, study period, study type, NOS scores, the number of participants and methods of study were extracted and recorded from the included studies.
The third and fourth authors (Wenbo Li and Qian Li) independently assessed the quality of eligible studies using NOS which consisted of three parts, namely selection, comparability and exposure, and their corresponding eight scoring items. Except for comparability which could score up to two stars, each item could only score up to one star. Possible maximum number of stars in each study was 9. When the score of eligible study in NOS was ≥7, the present study was regarded as high-quality research [44]. When scoring divergences occurred, the sixth investigator (Ying-pu Sun) was consulted.
Statistical analysis
Review Manager 5.3 software (available from the Cochrane Community, https://community.cochrane.org/help/tools-and-software/revman-5) was performed for data analysis [45]. Each study was evaluated by the Hardy–Weinberg equilibrium (H–W equilibrium, HWE) with a chi-square test (χ2 test) by IBM SPSS statistics 25 software [46]. Odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were utilized to test the association between each SNPs, rs2119882 and rs10830963 of MTNR1A/B and PCOS with six genetic models used to test the influence of genotype, that is, for rs10830963 (C> G): allelic (G vs. C), codominant (homozygote (GG vs. CC) and heterozygote (GC vs. CC) models), dominant (GG + GC vs. CC), recessive (GG vs. GC + CC) and superdominant (GG + CC vs. GC) models were calculated; and for rs2119882 (T> C): allelic (C vs. T), codominant (homozygote (CC vs. TT) and heterozygote (CT vs. TT) models), dominant (CC + CT vs. TT), recessive (CC vs. CT + TT) and superdominant (CC + TT vs. CT) models were calculated [47,48]. Our study used Cochrane Q-test for heterogeneity testing and the I2, a test statistic, for quantification [49]. In the Q-test, when P≥0.1 or I2 ≤ 50%, the fixed-effects model could be established to analyze. Otherwise, the random-effects model could be used. Next, a Z-test was performed to evaluate the statistical significance of combined ORs in quantitative synthesis. And all analysis will use 0.05 as significantly statistical level. Finally, by eliminating the heterogeneous merger studies one by one, the effect of a single heterogeneous study on the entire model was further discussed through a sensitivity analysis. And Stata MP 16.0 software was used to analyze the publication bias by Egger’s and Begg’s tests.
Results
Study selection
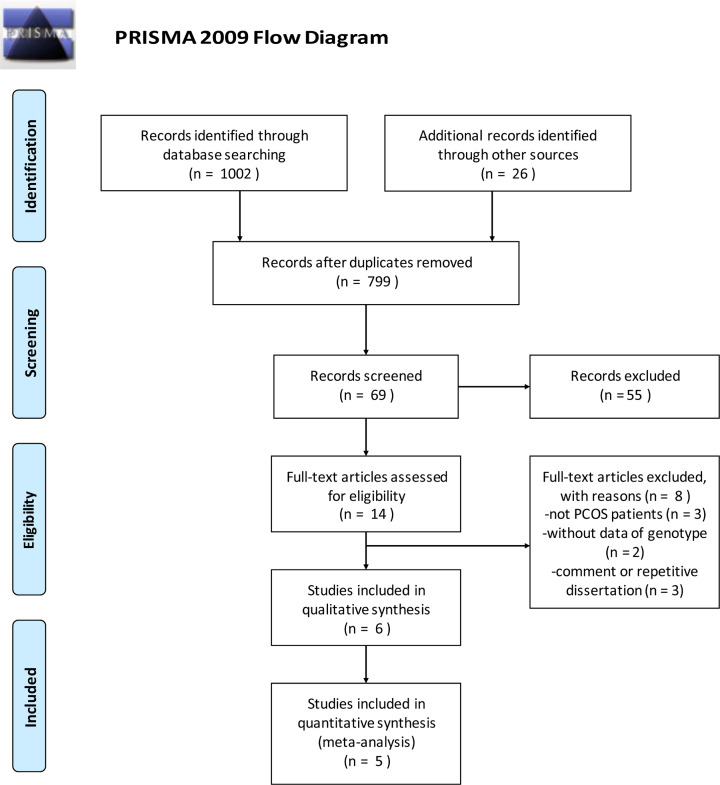
In total, 1028 references were identified after searching from databases (refer to Supplementary Material S1, Search strategies) and their recommended links. Then, all searched reference lists were imported into EndNote X9, which helps to remove duplicate studies. The majority of articles were excluded for unrelated titles and abstracts and other eight articles were excluded because of which studied populations were not PCOS patients, genotype data were not valid, or references were unrelated. Additionally, Song et al. (2015) study [50] was removed because it was a family-based transmission disequilibrium test without control group. Finally, five articles met the quality requirements of meta-analysis and the flow diagram of our study selection is shown in Figure 1.
Figure 1. Flow diagram of selection process on genetic studies of MTNR polymorphisms with PCOS.
Study characteristics
The eligible references in the present study involved two genes and six polymorphisms, MTNR1B gene (rs10830963, rs10830962, rs4753426, rs1562444, rs12792653) and MTNR1A (rs2119882). Four references, including Xu et al. (2019) [51], Yang et al. (2016) [52], Li et al. (2010) [53] and Wang et al. (2010) [39], reported the associations between rs10830963 and PCOS, including 1145 patients and 1408 controls. And two references, Xu et al. (2019) [51] and Li et al. (2011) [54], reported rs2119882, including 1680 patients and 1472 controls. Other SNPs had not been included in subsequent analysis due to insufficient data. All included studies with an NOS score more than 7, indicated a good level of quality (Table 1). The majority populations of the eligible references were Chinese and the methods used to detect gene polymorphisms were polymerase chain reaction (PCR) and sequencing. The studies characteristics are described in Table 2. Allele frequencies and genotypes of each study, and the outcome of HWE was listed in Table 3.
Table 1. The NOS.
| Study | Selection | Comparability | Exposure | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Definition adequate | Representativeness | Selection of controls | Definition of controls | Ascertainment | Same method | Non-response rate | |||
| Xu et al. (2019) | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Yang et al. (2016) | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Song et al. | ★ | ★ | ★★ | ★ | ★ | ★ | 7 | ||
| Li et al. (2011) | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Li et al. (2010) | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Wang et al. (2010) | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
Table 2. Characteristics of the eligible studies in the meta-analysis.
| Study | Year | Ethnicity | Patients | MTNR | Study period | Stydy type | NOS | Case/control | Genotyping methods |
|---|---|---|---|---|---|---|---|---|---|
| Xu et al. | 01/2019 | Chinese | PCOS | MTNR1A, MTNR1B | March 2013–May 2015 | Case–control | 8 | 191+168/215 | PCR- sequencing |
| Yang et al. | 03/2016 | Chinese | PCOS | MTNR1B | January 2012–May 2013 | Case–control | 8 | 182/196 | PCR- sequencing |
| Song et al. | 09/2015 | Chinese, Han, Shandong | PCOS | MTNR1A, MTNR1B | July 2007–April 2014 | Family trios | 7 | 263(789) | PCR- sequencing |
| Li et al. | 04/2011 | Chinese, Han, Shandong | PCOS | MTNR1A | September 2006–February 2008 | Case–control | 8 | 481/522 | PCR Tm-shift |
| Li et al. | 10/2010 | Chinese, Han, Shandong | PCOS | MTNR1B | February 2005–January 2007 | Case–control | 8 | 526/547 | PCR Tm-shift |
| Wang et al. | 11/2010 | Chinese | PCOS | MTNR1B | / | Case–control | 8 | 364/687 | TaqMan-PCR |
Table 3. Genotype and allele frequency of rs10830963 and rs2119882 in PCOS patients and controls.
| rs10830963 | Group | Genotype (n) | χ2 | P-value | Allele (n) | χ2 | P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| CC | CG | GG | C | G | ||||||
| Xu et al. (2019) | PCOS | 125 | 169 | 65 | 30.002 | 0.000 | 419 | 299 | 29.351 | 0.000 |
| Control | 114 | 91 | 10 | 319 | 111 | |||||
| Yang et al. (2016) | PCOS | 50 | 89 | 43 | 6.426 | 0.040 | 189 | 175 | 5.934 | 0.015 |
| Control | 70 | 98 | 28 | 238 | 154 | |||||
| Li et al. (2010) | PCOS | 143 | 258 | 125 | 15.352 | 0.000 | 544 | 508 | 13.207 | 0.000 |
| Control | 185 | 281 | 81 | 651 | 443 | |||||
| Wang et al. (2010) | PCOS | 126 | 185 | 53 | 1.201 | 0.549 | 437 | 291 | 0.746 | 0.388 |
| Control | 229 | 340 | 118 | 798 | 576 | |||||
| rs2119882 | Group | Genotype (n) | χ2 | P-value | Allele (n) | χ2 | P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| TT | TC | CC | T | C | ||||||
| Xu et al. (2019) | PCOS | 68 | 165 | 126 | 16.446 | 0.000 | 301 | 417 | 17.507 | 0.000 |
| Control | 69 | 97 | 49 | 235 | 195 | |||||
| Li et al. (2011) | PCOS | 167 | 215 | 99 | 9.962 | 0.007 | 549 | 413 | 9.824 | 0.002 |
| Control | 215 | 236 | 71 | 666 | 376 | |||||
Quantitative synthesis
The results of the association between the MTNR polymorphisms and the PCOS risk are shown in Table 4. The forest plots of each genetic model are described in Figures 2 and 3.
Table 4. Six genetic models of MTNR genes.
| Allele | Homozygote | Heterozygote | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | n | OR | 95% CI | P | I2 | OR | 95% CI | P | I2 | OR | 95% CI | P | I2 |
| rs10830963 | 4 | 1.38 | [1.00, 1.89] | 0.05 | 88% | 2.05 | [0.99, 4.21] | 0.05 | 89% | 1.21 | [1.03, 1.42] | 0.02 | 44% |
| rs2129882 | 2 | 1.44 | [1.25, 1.67] | 0.00 | 54% | 2.07 | [1.55, 2.76] | 0.00 | 34% | 1.32 | [1.05, 1.65] | 0.02 | 56% |
| Dominant | Recessive | Superdominant | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | n | OR | 95% CI | P | I2 | OR | 95% CI | P | I2 | OR | 95% CI | P | I2 |
| rs10830963 | 4 | 1.40 | [1.00, 1.95] | 0.05 | 78% | 1.79 | [0.98, 3.27] | 0.06 | 87% | 0.99 | [0.85, 1.14] | 0.87 | 0% |
| rs2129882 | 2 | 1.59 | [1.05, 2.41] | 0.03 | 69% | 1.72 | [1.34, 2.22] | 0.00 | 0% | 1.00 | [0.82, 1.22] | 0.99 | 0% |
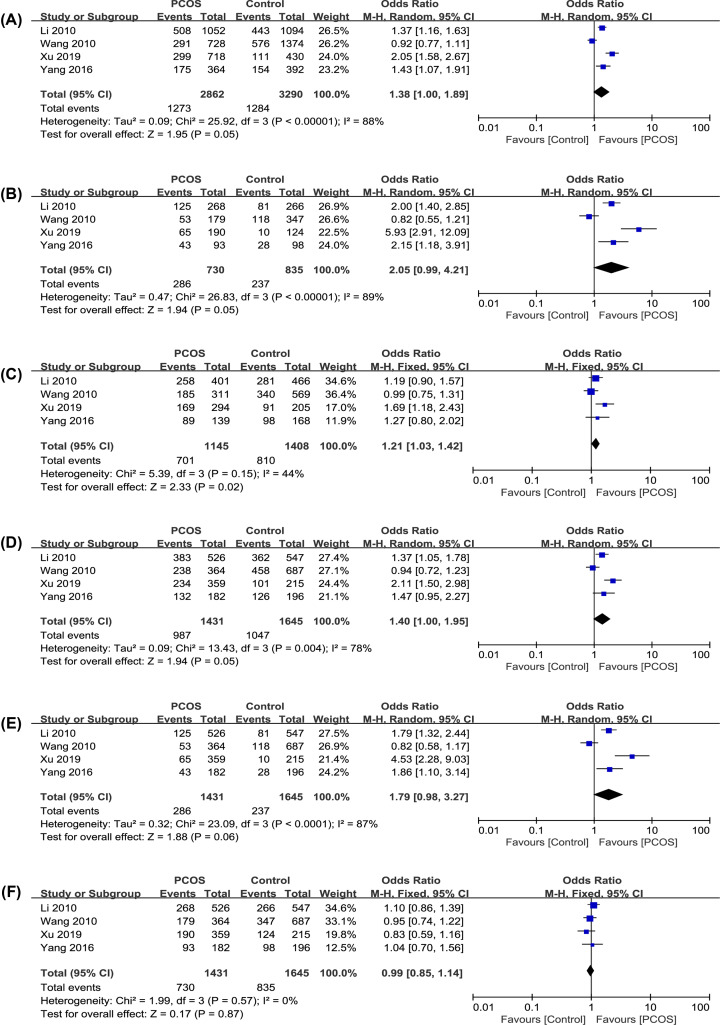
Figure 2. Forest plots of PCOS risk and the polymorphism of rs10830963 C>G in MTNR1B.
(A) G vs. C in allele. (B) GG vs. CC in homozygote. (C) GC vs. CC in heterozygote. (D) GG+GC vs. CC in dominant. (E) GG vs. GC+CC in recessive. (F) GG+CC vs. GC in superdominant.
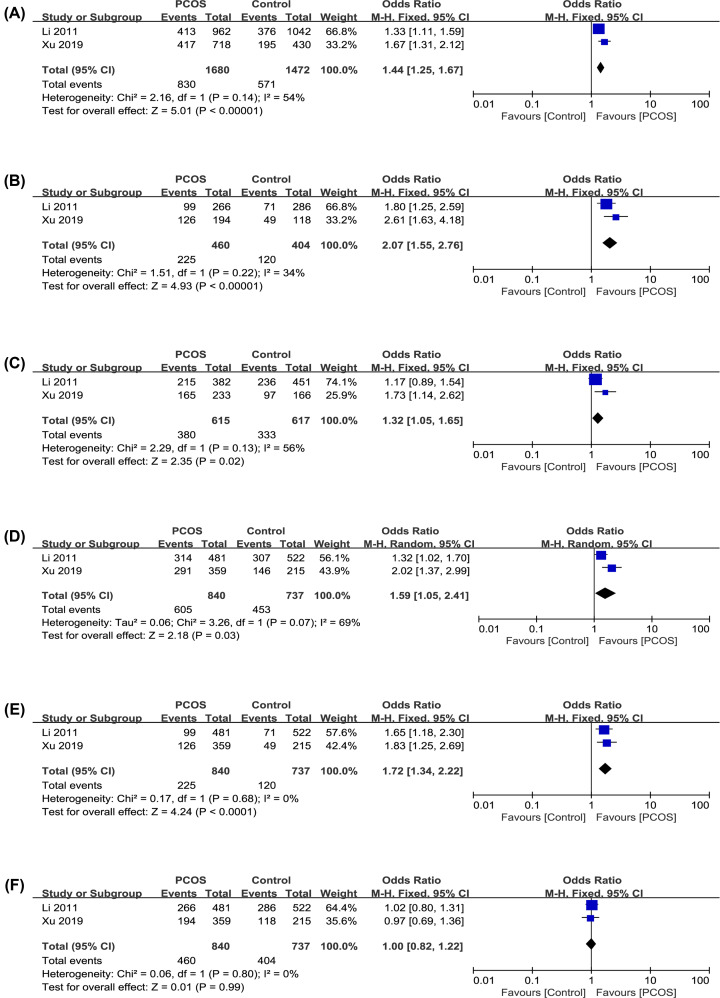
Figure 3. Forest plots of PCOS risk and the polymorphism of rs2119882 T>C in MTNR1A.
(A) C vs. T in allele. (B) CC vs. TT in homozygote. (C) CT vs. TT in heterozygote. (D) CC+CT vs. TT in dominant. (E) CC vs. CT+TT in recessive. (F) CC+TT vs. CT in superdominant.
Analysis of rs10830963 polymorphisms in MTNR1B
To study the relationship between rs10830963 (C>G) and PCOS risk, we included four case–control studies (Table 3) and analyzed them with six genetic models [55] (Table 4, Figure 2). Among them, in the heterozygote model, the variation was significantly associated with PCOS occurrence (OR: 1.21, 95% CI: 1.03–1.42, P=0.02, I2 = 44%). In the allelic, homozygote and dominant model, the variations were weakly associated to PCOS risk (OR: 1.38, 95% CI: 1.00–1.89, P=0.05, I2 = 88%; OR: 2.05, 95% CI: 0.99–4.21, P=0.05, I2 = 89%; OR: 1.40, 95% CI: 1.00–1.95, P=0.05, I2 = 78%). In the recessive model, this mutation had a weaker association (OR: 1.79, 95% CI: 0.98–3.27, P=0.06, I2 = 87%). And in last model, the superdominant model, no statistically significant association were found (OR: 0.99, 95% CI: 0.85–1.14, P=0.87, I2 = 0%). All studies were evenly distributed on both sides of the center line, and no obvious publication bias was found.
rs2119882 polymorphisms analysis in MTNR1A
To study the association between rs2119882 and PCOS risk, we included two case–control studies (Table 3) and used six genetic models for analysis (Table 4, Figure 3). In five genetic models, allelic, homozygote, heterozygote, dominant, recessive and superdominant, significant statistical associations were found (OR: 1.44, 95% CI: 1.25–1.67, P<0.001, I2 = 54%; OR: 2.07, 95% CI: 1.55–2.76, P<0.001, I2 = 34%; OR: 1.32, 95% CI: 1.05–1.65, P=0.02, I2 = 56%; OR: 1.59, 95% CI: 1.05–2.41, P=0.03, I2 = 69%; OR: 1.72, 95% CI: 1.34–2.22, P<0.001, I2 = 0%). However, in the superdominant model, no significant statistical association was found (OR: 1.00, 95% CI: 0.82–1.22, P=0.99, I2 = 0%). All models had low or moderate heterogeneity, so the fixed-effect models were used to analyze them.
Sensitivity analysis
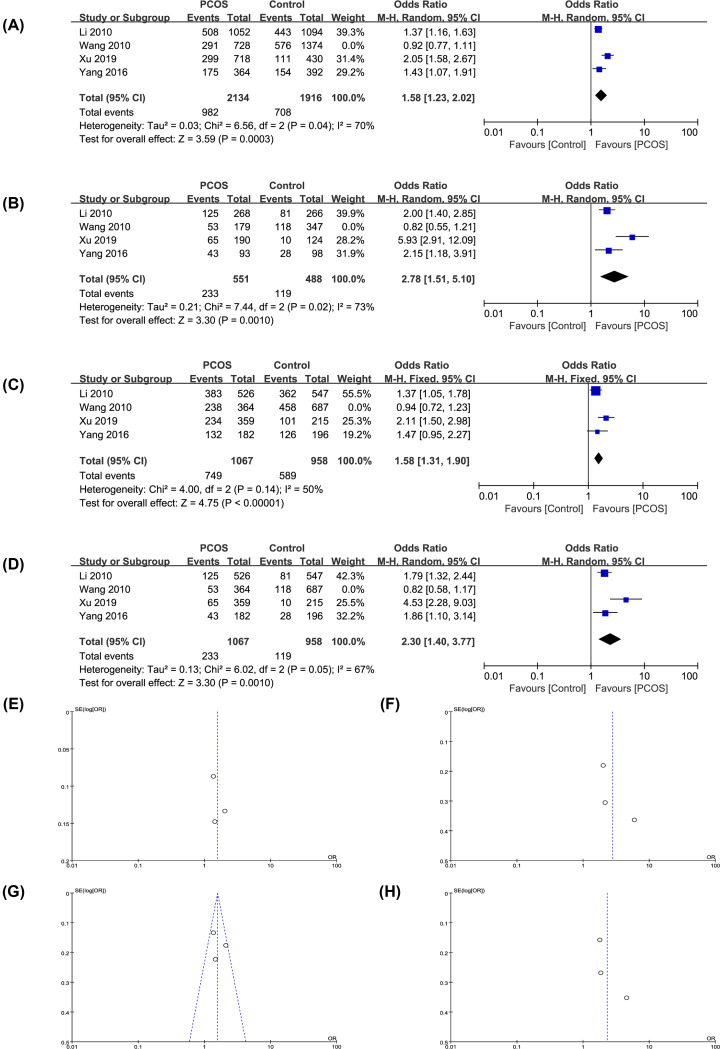
For genetic models with high heterogeneity (I2 and P≤0.01), a sensitivity analysis was conducted by removing all the eligible references one by one in each model to assess the influence of each reference heterogeneity on the whole model analysis [56]. More details in forest plots and funnel plots are shown in Figure 4 and Table 5. After excluding the study of Wang et al. (2010) [39], the allelic, homozygote, dominant and recessive models in rs10830963 had significant statistical correlation with PCOS risk (OR: 1.58, 95% CI: 1.23–2.02, P=0.000, I2 = 70%; OR: 2.78, 95% CI: 1.51–5.10, P=0.001, I2 = 73%; OR: 1.58, 95% CI: 1.31–1.90, P=0.000, I2 = 50%; OR: 2.30, 95% CI: 1.40–3.77, P=0.001, I2 = 67%).
Figure 4. Forest plots and funnel plots of rs10830963 when Wang et al.’s study was removed.
(A,E) G vs. C in allele. (B,F) GG vs. CC in homozygote. (C,G) GG+GC vs. CC in dominant. (D,H) GG vs. GC+CC in recessive.
Table 5. Sensitivity analysis of rs10830963 C> G.
| Model | OR | 95% CI | P | I2 |
|---|---|---|---|---|
| Allele | 1.58 | [1.34, 2.02] | 0.000 | 70% |
| Homozygote | 2.78 | [1.51, 5.10] | 0.001 | 73% |
| Dominant | 1.58 | [1.31, 1.90] | 0.000 | 50% |
| Recessive | 2.30 | [1.40, 3.77] | 0.001 | 67% |
Publication bias
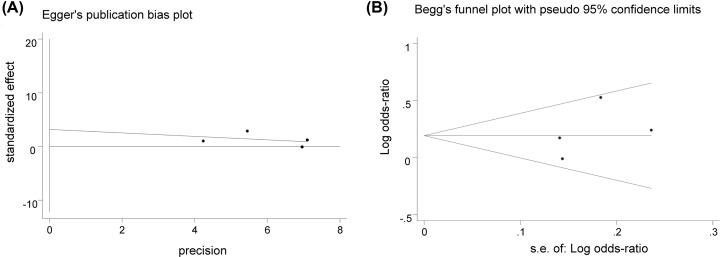
Due to the limited number of available references [44], publication bias was investigated only for the relationship between rs10830963 and PCOS risk by Stata MP 16.0 software. The result of heterozygote model (GC vs. CC) is shown in Figure 5. In Egger’s test, P was 0.469, while similarly in Begg’s test P was 0.497. Because both the P-values were more than 0.1, so no publication bias was found. Relationship between rs10830963 and PCOS risk was relatively stable and convincing.
Figure 5. Egger’s test and Begg’s funnel plot of rs10830963 for heterozygote model.
(A) Egger’s regression test. (B) Begg’s funnel plot.
Discussion
As one among common endocrine disorders in gynecological reproduction, the PCOS pathogenesis is still unclear. Family studies revealed aggregation in PCOS [57], but no precise genetic mechanism can explain the etiology. Because these patients have hyperinsulinemia and IR, genes like MTNR1A/B related to insulin secretion possibly play a crucial role in PCOS progression. At the same time, recently several researches have been conducted or been registered [58] to determine the relationship between MTNR genes and PCOS susceptibility. But these researches have shown opposite results, so our study aims to further elaborate the relationship between MTNR1A/B gene and the risk of PCOS.
Melatonin works by activating two important G protein-coupled receptors, MT1 and MT2 [59]. By querying this National Center for Biotechnology Information (NCBI) database, it is known that the MTNR1A gene that encodes MT1 is located on the chromosome 4q35.2, and the MTNR1B gene of MT2 is located on chromosome 11q14.3. Rs10830963 is located in the intron region between the two exons encoding MTNR1B, while rs2119882 is located in the MTNR1A promoter region. Although these relative positions of SNPs in MTNR are different, the results for all genetic models in this two SNPs are still similar. However, the limitation of our study is that we only discussed from the perspective of MTNR gene while other SNPs are ignored. Because the pathogenesis of PCOS is complex and different genome-wide association studies (GWASs) conclusions are more heterogeneous [60], multiple SNPs mutations may affect metabolic and endocrine profiles in the same PCOS patient, such as insulin receptor gene (INSR) and luteinizing hormone/choriogonadotropin receptor (LHCGR) [61]. Therefore, further research is expected to combining multi-racial GWAS results of arrays by bioinformatics [62,63] and take other gene receptor into consideration to analysis-related molecular pathways [64].
In our meta-analysis, we find that in the heterozygote model, MTNR1B rs10830963 polymorphism is significantly associated with the PCOS occurrence. Through the sensitivity analysis, after excluding Wang et al. study [39], multiple model analyses showed that rs10830963 also has a significant correlation (the allelic, homozygote, dominant and recessive models). This may be due to the large heterogeneity of the present study. First, the study was conducted 10 years ago and its population was not in H–W equilibrium, possibly due to the bias in collecting participants in a limited geographical region. Second, the article does not report the period for recruiting the participants. Therefore, the study cannot rule out the effect of selection bias on the population. Finally, the SNPs are in a strong linkage disequilibrium and there may be a certain association between linkage markers. In addition, due to the large heterogeneity in different ethnicity, our study still needs large sample, multi-center and multi-ethnic studies to confirm.
Moreover, obese women in PCOS patients can be complicated with T2DM [65]. At the same time, their IR level is higher than normal, which can effectively predict impaired glucose tolerance and the occurrence of metabolic syndrome [66]. MTNR is also a candidate gene for IR and gestational diabetes mellitus [38]. So, further studies can take the clinical heterogeneity of PCOS into consideration and divide patients into different groups, such as obese and non-obese groups by body mass index [67], in following subgroup and phenotype analysis of SNPs.
As a result of weak endocrine regulation of melatonin through mutation receptor, to some extent, our meta-analysis supports the feasibility and acceptability of appropriate melatonin supplementation for PCOS [68,69]. At the same time, some studies have suggested that inositol can also be used to an novel and additional treatment of PCOS to improve symptoms [70–72], because as an insulin sensitizer it can decrease the levels of androgen, improve glucose metabolism [73], and restore spontaneous ovulation [74,75]. But due to the limited study of melatonin and inositol combination therapy [76,77], the clinical outcomes of IVF are still uncertain [78,79]. Therefore, the follow-up systematic review and meta-analysis is recommended to further confirm the combination therapy effect of melatonin and other drugs for PCOS.
Conclusion
The SNPs rs10830963 and rs2119882 of MTNR1B and MTNR1A, are associated with a much higher PCOS risk in Chinese population. And above conclusions still require confirmation by future study including much larger multi-ethnic studies and other SNPs instead of the above conclusions.
Acknowledgements
We thank all participants for their contributions to this meta-analysis.
Abbreviations
- CI
confidence interval
- H–W equilibrium/HWE
Hardy–Weinberg equilibrium
- GWAS
genome-wide association study
- IR
insulin resistance
- IVF
in vitro fertilization
- MTNR
melatonin receptor
- NOS
Newcastle–Ottawa Scale
- OR
odds ratio
- PCOS
polycystic ovarian syndrome
- SNP
single nucleotide polymorphism
- T2DM
type 2 diabetes mellitus
Contributor Information
Jiawei Xu, Email: jiawxu@foxmail.com.
Ying-pu Sun, Email: syp2008@vip.sina.com.
Data Availability
The analytical data are all included in the published articles and the final supplementary materials.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Key R&D Program of China [grant number 2019YFA0110900 (to Ying-pu Sun and Jiawei Xu), 2019YFA0802200 (to Jiawei Xu)]; the Scientific and Technological Innovation Talent Project of Universities of Henan Province [grant number 20HASTIT045 (to Jiawei Xu)].
Author Contribution
Shiqi Yi and Jiawei Xu designed the present study. Shiqi Yi and Hao Shi conducted literature searches, statistical analysis, and charting. Jiawei Xu provided guidance on the genetics section of this article. Wenbo Li and Qian Li finished quality evaluations. Shiqi Yi wrote the draft and completed the present paper with the assistance of Ying-pu Sun. All authors agree with the conclusions of our study. Data sorting: Shiqi Yi, Hao Shi, Wenbo Li and Qian Li. Methods: Shiqi Yi and Jiawei Xu. Writing-draft: Shiqi Yi; Writing-reviewing and modifying: Ying-pu Sun.
References
- 1.Dubocovich M.L., Delagrange P., Krause D.N., Sugden D., Cardinali D.P. and Olcese J. (2010) International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 62, 343–380 10.1124/pr.110.002832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doosti-Irani A., Ostadmohammadi V., Mirhosseini N. et al. (2018) The effects of melatonin supplementation on glycemic control: a systematic review and meta-analysis of randomized controlled trials. Horm. Metab. Res. 50, 783–790 [DOI] [PubMed] [Google Scholar]
- 3.Shabani A., Foroozanfard F., Kavossian E. et al. (2019) Effects of melatonin administration on mental health parameters, metabolic and genetic profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J. Affect. Disord. 250, 51–56 10.1016/j.jad.2019.02.066 [DOI] [PubMed] [Google Scholar]
- 4.Lyssenko V., Nagorny C.L.F., Erdos M.R. et al. (2009) Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 41, 82–88 10.1038/ng.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo M.M., Tai C.J., Kang S.K., Nathwani P.S., Pang S.F. and Leung P.C. (2001) Direct action of melatonin in human granulosa-luteal cells. J. Clin. Endocrinol. Metab. 86, 4789–4797 10.1210/jcem.86.10.7912 [DOI] [PubMed] [Google Scholar]
- 6.Yie S.M., Niles L.P. and Younglai E.V. (1995) Melatonin receptors on human granulosa cell membranes. J. Clin. Endocrinol. Metab. 80, 1747–1749 [DOI] [PubMed] [Google Scholar]
- 7.Li Y., Fang L., Yu Y. et al. (2019) Higher melatonin in the follicle fluid and MT2 expression in the granulosa cells contribute to the OHSS occurrence. Reprod. Biol. Endocrinol. 17, 37 10.1186/s12958-019-0479-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarinci E., Tropea A., Notaristefano G. et al. (2019) “Hormone of darkness” and human reproductive process: direct regulatory role of melatonin in human corpus luteum. J. Endocrinol. Invest. 42, 1191–1197 10.1007/s40618-019-01036-3 [DOI] [PubMed] [Google Scholar]
- 9.Tong J., Sheng S., Sun Y. et al. (2017) Melatonin levels in follicular fluid as markers for IVF outcomes and predicting ovarian reserve. Reproduction 153, 443–451 10.1530/REP-16-0641 [DOI] [PubMed] [Google Scholar]
- 10.Dantas-Ferreira R.F., Raingard H., Dumont S. et al. (2018) Melatonin potentiates the effects of metformin on glucose metabolism and food intake in high-fat-fed rats. Endocrinol. Diabetes Metab. 1, e00039 10.1002/edm2.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mokhtari F., Akbari Asbagh F., Azmoodeh O., Bakhtiyari M. and Almasi-Hashiani A. (2019) Effects of melatonin administration on chemical pregnancy rates of polycystic ovary syndrome patients undergoing intrauterine insemination: a randomized clinical trial. Int. J. Fertil. Steril. 13, 225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarquini R., Bruni V., Perfetto F., Bigozzi L., Tapparini L. and Tarquini B. (1996) Hypermelatoninemia in women with polycystic ovarian syndrome. Eur. J. Contracept. Reprod. Health Care 1, 349–350 [DOI] [PubMed] [Google Scholar]
- 13.Luboshitzky R., Qupti G., Ishay A., Shen-Orr Z., Futerman B. and Linn S. (2001) Increased 6-sulfatoxymelatonin excretion in women with polycystic ovary syndrome. Fertil. Steril. 76, 506–510 10.1016/S0015-0282(01)01930-6 [DOI] [PubMed] [Google Scholar]
- 14.Luboshitzky R., Herer P. and Shen-Orr Z. (2004) Urinary 6-sulfatoxymelatonin excretion in hyperandrogenic women: the effect of cyproterone acetate-ethinyl estradiol treatment. Exp. Clin. Endocrinol. Diab. 112, 102–107 10.1055/s-2004-815765 [DOI] [PubMed] [Google Scholar]
- 15.Goodman N.F., Cobin R.H., Futterweit W. et al. (2015) American association of clinical endocrinologists, American college of Endocrinology, and Androgen Excess and PCOS Society Disease State Clinical Review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome–part 1. Endocr. Pract. 21, 1291–1300 10.4158/EP15748.DSC [DOI] [PubMed] [Google Scholar]
- 16.Bozdag G., Mumusoglu S., Zengin D., Karabulut E. and Yildiz B.O. (2016) The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 31, 2841–2855 10.1093/humrep/dew218 [DOI] [PubMed] [Google Scholar]
- 17.Wolf W.M., Wattick R.A., Kinkade O.N. and Olfert M.D. (2018) Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int. J. Environ. Res. Public Health 15, 2589 10.3390/ijerph15112589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li R., Zhang Q., Yang D. et al. (2013) Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum. Reprod. 28, 2562–2569 10.1093/humrep/det262 [DOI] [PubMed] [Google Scholar]
- 19.Otaghi M., Azami M., Khorshidi A., Borji M. and Tardeh Z. (2019) The association between metabolic syndrome and polycystic ovary syndrome: a systematic review and meta-analysis. Diabetes Metab. Syndr. 13, 1481–1489 [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz B., Vellanki P., Ata B. and Yildiz B.O. (2018) Diabetes mellitus and insulin resistance in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil. Steril. 110, 523.e14–533.e14 10.1016/j.fertnstert.2018.04.024 [DOI] [PubMed] [Google Scholar]
- 21.Chen Z.-J., Zhao H., He L. et al. (2011) Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat. Genet. 43, 55–59 10.1038/ng.732 [DOI] [PubMed] [Google Scholar]
- 22.Wood J.R., Dumesic D.A., Abbott D.H. and Strauss J.F. (2007) Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J. Clin. Endocrinol. Metab. 92, 705–713 10.1210/jc.2006-2123 [DOI] [PubMed] [Google Scholar]
- 23.Aguilar M., Bhuket T., Torres S., Liu B. and Wong R.J. (2015) Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA 313, 1973–1974 10.1001/jama.2015.4260 [DOI] [PubMed] [Google Scholar]
- 24.Shi Y., Zhao H., Shi Y. et al. (2012) Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat. Genet. 44, 1020–1025 10.1038/ng.2384 [DOI] [PubMed] [Google Scholar]
- 25.Chen Z.J., Zhao H., He L. et al. (2011) Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat. Genet. 43, 55–59 10.1038/ng.732 [DOI] [PubMed] [Google Scholar]
- 26.Brower M.A., Jones M.R., Rotter J.I. et al. (2015) Further investigation in europeans of susceptibility variants for polycystic ovary syndrome discovered in genome-wide association studies of Chinese individuals. J. Clin. Endocrinol. Metab. 100, E182–E186 10.1210/jc.2014-2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louwers Y.V., Stolk L., Uitterlinden A.G. and Laven J.S. (2013) Cross-ethnic meta-analysis of genetic variants for polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 98, E2006–E2012 10.1210/jc.2013-2495 [DOI] [PubMed] [Google Scholar]
- 28.Goodarzi M.O., Jones M.R., Li X. et al. (2012) Replication of association of DENND1A and THADA variants with polycystic ovary syndrome in European cohorts. J. Med. Genet. 49, 90–95 10.1136/jmedgenet-2011-100427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu A.L., Xie H.J., Xie H.Y. et al. (2017) Association between fat mass and obesity associated (FTO) gene rs9939609 A/T polymorphism and polycystic ovary syndrome: a systematic review and meta-analysis. BMC Med. Genet. 18, 89 10.1186/s12881-017-0452-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wojciechowski P., Lipowska A., Rys P. et al. (2012) Impact of FTO genotypes on BMI and weight in polycystic ovary syndrome: a systematic review and meta-analysis. Diabetologia 55, 2636–2645 10.1007/s00125-012-2638-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laven J.S.E. (2019) Follicle stimulating hormone receptor (FSHR) polymorphisms and polycystic ovary syndrome (PCOS). Front. Endocrinol. (Lausanne) 10, 23 10.3389/fendo.2019.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutharasan P., Galdones E., Peñalver Bernabé B. et al. (2013) Evidence for chromosome 2p16.3 polycystic ovary syndrome susceptibility locus in affected women of European ancestry. J. Clin. Endocrinol. Metab. 98, E185–E190 10.1210/jc.2012-2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welt C.K., Styrkarsdottir U., Ehrmann D.A. et al. (2012) Variants in DENND1A are associated with polycystic ovary syndrome in women of European ancestry. J. Clin. Endocrinol. Metab. 97, E1342–E1347 10.1210/jc.2011-3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddamalla S., Reddy T.V., Govatati S. et al. (2018) Vitamin D receptor gene polymorphisms and risk of polycystic ovary syndrome in South Indian women. Gynecol. Endocrinol. 34, 161–165 10.1080/09513590.2017.1371128 [DOI] [PubMed] [Google Scholar]
- 35.Shi X.Y., Huang A.P., Xie D.W. and Yu X.L. (2019) Association of vitamin D receptor gene variants with polycystic ovary syndrome: a meta-analysis. BMC Med. Genet. 20, 32 10.1186/s12881-019-0763-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deswal R., Nanda S. and Dang A.S. (2017) Unveiling the association between vitamin D receptor and poly cystic ovary syndrome - a systematic review and meta-analysis. Int. J. Vitam. Nutr. Res. 87, 207–218 10.1024/0300-9831/a000298 [DOI] [PubMed] [Google Scholar]
- 37.de Luis Román D.A., Primo D., Aller R. and Izaola O. (2019) Association of the rs10830963 polymorphism in MTNR1B with fasting glucose, serum adipokine levels and components of metabolic syndrome in adult obese subjects. Nutr. Hosp. 36, 60–65 [DOI] [PubMed] [Google Scholar]
- 38.Li C., Qiao B., Zhan Y. et al. (2013) Association between genetic variations in MTNR1A and MTNR1B genes and gestational diabetes mellitus in Han Chinese women. Gynecol. Obstet. Invest. 76, 221–227 10.1159/000355521 [DOI] [PubMed] [Google Scholar]
- 39.Wang L., Wang Y., Zhang X. et al. (2010) Common genetic variation in MTNR1B is associated with serum testosterone, glucose tolerance, and insulin secretion in polycystic ovary syndrome patients. Fertil. Steril. 94, 2486.e24892–2489.e24892 10.1016/j.fertnstert.2010.01.059 [DOI] [PubMed] [Google Scholar]
- 40.Moher D., Liberati A., Tetzlaff J., Altman D.G. and PRISMA.Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y.T., Li X., Liu Z.L. et al. (2017) Hepatitis B virus reactivation and antiviral prophylaxis during lung cancer chemotherapy: A systematic review and meta-analysis. PLoS ONE 12, e0179680 10.1371/journal.pone.0179680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rotterdam EA-SPcwg (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 19, 41–47 10.1093/humrep/deh098 [DOI] [PubMed] [Google Scholar]
- 43.Stang A. (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 44.Wu H., Yu K. and Yang Z. (2015) Associations between TNF-α and interleukin gene polymorphisms with polycystic ovary syndrome risk: a systematic review and meta-analysis. J. Assist. Reprod. Genet. 32, 625–634 10.1007/s10815-015-0449-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chandler J. and Hopewell S. (2013) Cochrane methods–twenty years experience in developing systematic review methods. Syst. Rev. 2, 76 10.1186/2046-4053-2-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan A., Cai G., Fu N. et al. (2016) Relevance study on cerebral infarction and resistin gene polymorphism in Chinese Han population. Aging Dis. 7, 593–603 10.14336/AD.2016.0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minelli C., Thompson J.R., Abrams K.R., Thakkinstian A. and Attia J. (2005) The choice of a genetic model in the meta-analysis of molecular association studies. Int. J. Epidemiol. 34, 1319–1328 10.1093/ije/dyi169 [DOI] [PubMed] [Google Scholar]
- 48.Lewis C.M. and Knight J. (2012) Introduction to genetic association studies. Cold Spring Harb. Protoc. 2012, 297–306 10.1101/pdb.top068163 [DOI] [PubMed] [Google Scholar]
- 49.Huedo-Medina T.B., Sanchez-Meca J., Marin-Martinez F. and Botella J. (2006) Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 11, 193–206 10.1037/1082-989X.11.2.193 [DOI] [PubMed] [Google Scholar]
- 50.Song X., Sun X., Ma G. et al. (2015) Family association study between melatonin receptor gene polymorphisms and polycystic ovary syndrome in Han Chinese. Eur. J. Obstet. Gynecol. Reprod. Biol. 195, 108–112 10.1016/j.ejogrb.2015.09.043 [DOI] [PubMed] [Google Scholar]
- 51.Xu X.H., Kou L.C., Wang H.M., Bo C.M. and Song X.C. (2019) Genetic polymorphisms of melatonin receptors 1A and 1B may result in disordered lipid metabolism in obese patients with polycystic ovary syndrome. Mol. Med. Rep. 19, 2220–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J.F., Yang H.M. and C Z.J. (2016) Association of rs10830963 SNPs in the melatonin receptor (MTNR IB) gene with polycystic ovary syndrome. J. Pract. Gynecol. Endocrinol. 3, 145–148 [Google Scholar]
- 53.Li C., Shi Y.H., You L., Wang L.C. and Chen Z.J. (2011) Association of rs10830963 and rs10830962 SNPs in the melatonin receptor (MTNR1B) gene among Han Chinese women with polycystic ovary syndrome. Mol. Hum. Reprod. 17, 193–198 10.1093/molehr/gaq087 [DOI] [PubMed] [Google Scholar]
- 54.Li C., Shi Y., You L., Wang L. and Chen Z.-J. (2011) Melatonin receptor 1A gene polymorphism associated with polycystic ovary syndrome. Gynecol. Obstet. Invest. 72, 130–134 10.1159/000323542 [DOI] [PubMed] [Google Scholar]
- 55.Thakkinstian A., McElduff P., D’Este C., Duffy D. and Attia J. (2005) A method for meta-analysis of molecular association studies. Stat. Med. 24, 1291–1306 10.1002/sim.2010 [DOI] [PubMed] [Google Scholar]
- 56.Champagne N., Eadie L., Regan L. and Wilson P. (2019) The effectiveness of ultrasound in the detection of fractures in adults with suspected upper or lower limb injury: a systematic review and subgroup meta-analysis. BMC Emerg. Med. 19, 17 10.1186/s12873-019-0226-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song X., Sun X., Ma G. et al. (2015) Family association study between melatonin receptor gene polymorphisms and polycystic ovary syndrome in Han Chinese. Eur. J. Obstet. Gynecol. Reprod. Biol. 195, 108–112 10.1016/j.ejogrb.2015.09.043 [DOI] [PubMed] [Google Scholar]
- 58.Iwata M., Carvalho K., Maciel G., Neto J., Baracat E. and Maria J. (2016) MTNR1B melatonin receptor gene polymorphisms and carbohydrate metabolism in young women with polycystic ovary syndrome. Gynecol. Endocrinol. 32, 84 [Google Scholar]
- 59.Jockers R., Delagrange P., Dubocovich M.L. et al. (2016) Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 173, 2702–2725 10.1111/bph.13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Y., Yuan Y., Yang H. et al. (2016) Association between common genetic variants and polycystic ovary syndrome risk in a Chinese Han population. J. Clin. Res. Pediatr. Endocrinol. 8, 405–410 10.4274/jcrpe.2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui L., Li G., Zhong W. et al. (2015) Polycystic ovary syndrome susceptibility single nucleotide polymorphisms in women with a single PCOS clinical feature. Hum. Reprod. 30, 732–736 10.1093/humrep/deu361 [DOI] [PubMed] [Google Scholar]
- 62.Lee H., Oh J.Y., Sung Y.A. and Chung H.W. (2016) A genetic risk score is associated with polycystic ovary syndrome-related traits. Hum. Reprod. 31, 209–215 10.1093/humrep/dev282 [DOI] [PubMed] [Google Scholar]
- 63.Shen H., Liang Z., Zheng S. and Li X. (2017) Pathway and network-based analysis of genome-wide association studies and RT-PCR validation in polycystic ovary syndrome. Int. J. Mol. Med. 40, 1385–1396 10.3892/ijmm.2017.3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vitale S.G., Lagana A.S., Nigro A. et al. (2016) Peroxisome proliferator-activated receptor modulation during metabolic diseases and cancers: master and minions. PPAR Res. 2016, 6517313 10.1155/2016/6517313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kakoly N.S., Khomami M.B., Joham A.E. et al. (2018) Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum. Reprod. Update 24, 455–467 10.1093/humupd/dmy007 [DOI] [PubMed] [Google Scholar]
- 66.Liang S.J., Liou T.H., Lin H.W., Hsu C.S., Tzeng C.R. and Hsu M.I. (2012) Obesity is the predominant predictor of impaired glucose tolerance and metabolic disturbance in polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 91, 1167–1172 10.1111/j.1600-0412.2012.01417.x [DOI] [PubMed] [Google Scholar]
- 67.Jones M.R., Brower M.A., Xu N. et al. (2015) Systems genetics reveals the functional context of PCOS loci and identifies genetic and molecular mechanisms of disease heterogeneity. PLoS Genet. 11, e1005455 10.1371/journal.pgen.1005455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mokhtari F., Akbari Asbagh F., Azmoodeh O., Bakhtiyari M. and Almasi-Hashiani A. (2019) Effects of melatonin administration on chemical pregnancy rates of polycystic ovary syndrome patients undergoing intrauterine insemination: a randomized clinical trial. Int. J. Fertil. Steril. 13, 225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu K.L., Ye X., Wang S. and Zhang D. (2020) Melatonin application in assisted reproductive technology: a systematic review and meta-analysis of randomized trials. Front. Endocrinol. (Lausanne) 11, 160 10.3389/fendo.2020.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lagana A.S., Rossetti P., Sapia F. et al. (2017) Evidence-based and patient-oriented inositol treatment in polycystic ovary syndrome: changing the perspective of the disease. Int. J. Endocrinol. Metab. 15, e43695 10.5812/ijem.43695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Facchinetti F., Appetecchia M., Aragona C. et al. (2020) Experts’ opinion on inositols in treating polycystic ovary syndrome and non-insulin dependent diabetes mellitus: a further help for human reproduction and beyond. Expert Opin. Drug Metab. Toxicol. 16, 255–274 10.1080/17425255.2020.1737675 [DOI] [PubMed] [Google Scholar]
- 72.Paul C., Lagana A.S., Maniglio P., Triolo O. and Brady D.M. (2016) Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: state-of-the-art and future perspectives. Gynecol. Endocrinol. 32, 431–438 10.3109/09513590.2016.1144741 [DOI] [PubMed] [Google Scholar]
- 73.Unfer V., Facchinetti F., Orru B., Giordani B. and Nestler J. (2017) Myo-inositol effects in women with PCOS: a meta-analysis of randomized controlled trials. Endocr. Connect. 6, 647–658 10.1530/EC-17-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papaleo E., Unfer V., Baillargeon J.P. et al. (2007) Myo-inositol in patients with polycystic ovary syndrome: a novel method for ovulation induction. Gynecol. Endocrinol. 23, 700–703 10.1080/09513590701672405 [DOI] [PubMed] [Google Scholar]
- 75.Lagana A.S., Garzon S., Casarin J., Franchi M. and Ghezzi F. (2018) Inositol in polycystic ovary syndrome: restoring fertility through a pathophysiology-based approach. Trends Endocrinol. Metab. 29, 768–780 10.1016/j.tem.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 76.Pacchiarotti A., Carlomagno G., Antonini G. and Pacchiarotti A. (2016) Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol. Endocrinol. 32, 69–73 10.3109/09513590.2015.1101444 [DOI] [PubMed] [Google Scholar]
- 77.Unfer V., Raffone E., Rizzo P. and Buffo S. (2011) Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol. Endocrinol. 27, 857–861 10.3109/09513590.2011.564687 [DOI] [PubMed] [Google Scholar]
- 78.Banaszewska B., Pawelczyk L. and Spaczynski R. (2019) Current and future aspects of several adjunctive treatment strategies in polycystic ovary syndrome. Reprod. Biol. 19, 309–315 10.1016/j.repbio.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 79.Showell M.G., Mackenzie-Proctor R., Jordan V., Hodgson R. and Farquhar C. (2018) Inositol for subfertile women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 12, CD012378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analytical data are all included in the published articles and the final supplementary materials.