ABSTRACT
The Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: A Global Perspective by the World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR) represents the most comprehensive, detailed, and objective analysis of the accumulated research in the discipline. The report provides a framework for public health efforts around the globe by governments and other organizations with the goal of significantly reducing the burden of cancer, enhancing health, and improving quality of life for cancer survivors. Coupled with the WCRF/AICR Continuous Update Panel reports on specific cancers, these efforts also provide guidance to healthcare practitioners engaged in counseling individuals who may benefit from diet and lifestyle changes. Most critically, this report defines priorities for future research efforts that will improve the evidence base of future recommendations both for population-based public health efforts and increasingly for more personalized strategies targeting individuals who are cancer survivors or at risk due to genetic predisposition or carcinogenic exposures.
Keywords: diet, nutrition, physical activity, cancer, public health
Introduction
The World Cancer Research Fund (WCRF) and its affiliates, including the American Institute for Cancer Research (AICR), have completed a decade-long effort to objectively review and interpret the rapidly expanding scientific literature on diet, nutrition, physical activity, and cancer (1). The third report builds upon the foundation established by the previous 2 iterations of the report in 1998 and 2007 and will have significant impacts on the scientific direction of the field while providing guidance to individuals for their personal goals to improve health, as well as educating healthcare practitioners in evidence-based interventions for their patients. Most importantly, the summary recommendations provide guidance for governments and a vast array of public health organizations around the globe, including the WHO, in their quest to optimize strategies and polices regarding agriculture and food production with the goal to ensure universal access to health-promoting dietary patterns and thereby reduce the burden of cancer and other disease processes throughout the life cycle (2, 3). Scientists, policy makers, clinicians, and individuals are indebted to the WCRF and AICR for their remarkable forethought, perseverance, and investment of philanthropic resources over 3 decades to lead the effort to impartially address the complexity of the diet and its constituents and their effects on multiple cancer subtypes while also integrating the varied environmental and genetic factors acting over a lifetime to impact cancer risk. The combination of efforts to control tobacco product addiction, optimize dietary patterns and physical activity, and target the obesity epidemic represents the most compelling opportunity to reduce the global incidence of and mortality from cancer, while diminishing the spiraling costs devoted to cancer care.
The Process
The First Expert Report, published in 1997 (4), was a comprehensive review of the published scientific literature, but it was produced at a time when large prospective cohort studies were few and of modest duration, and pooling studies and meta-analyses were in their infancy. Thus, epidemiologic evidence was dominated by ecologic and case-control studies that are often significantly confounded by systemic biases; yet, the accumulated evidence was supported by laboratory investigations in a limited but rapidly growing array of experimental cancer models. Most critically, the need to develop and apply improved dietary assessment tools, including biomarkers, for individuals over time in prospective studies stimulated research activity and funding. These initial efforts also highlighted the challenges of defining causal relationships for nutrients, foods, or their components, such as fiber and phytochemicals, in the carcinogenesis process in a discipline where randomized controlled clinical trials of cancer outcomes are unlikely to be a feasible and practical undertaking. Yet, history provided us with a framework, elegantly delivered by Bradford Hill (5) for assessing the likelihood that a given exposure, such as many dietary variables, may be causally associated with a cancer risk. In retrospect, a major achievement of the 1997 report was to bring a discipline that was viewed as a fringe area of investigation into mainstream cancer research.
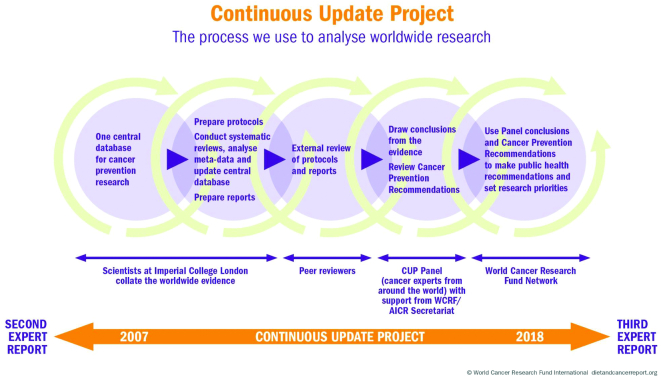
The second report, published in 2007 (6), benefited greatly from an organized and structured approach for collecting and systematically reviewing the published literature on human cancer risk (6). In addition, the evidence was rigorously reviewed by an international team of recognized experts providing diverse perspectives and the wisdom to develop accurate conclusions and recommendations based upon the Bradford Hill principles (5). The 2007 report (6), like the previous effort, provided guidance for research activity but was also a landmark achievement in defining a clear set of public health recommendations to be employed with confidence by policy makers around the globe (6). The rapid growth in published literature coupled with improved quality of the evidence following the 2007 report made it unfeasible to convene a new team every decade to expediently assess the findings and generate new recommendations. Thus, the WCRF/AICR was compelled to establish a “real-time,” ongoing interrogation of the scientific evidence, known as the Continuous Update Project (CUP), as illustrated in Figure 1.
FIGURE 1.
The CUP process established by the WCRF/AICR to evaluate the research on diet, nutrition, physical activity, and cancer. Reproduced with permission from the World Cancer Research Fund/American Institute for Cancer Research (1). CUP, Continuous Update Project; WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research.
The CUP effort began with experts providing guidance and establishing a nonbiased approach with meticulous methods for collecting, organizing, and systematically reviewing the evidence for each of the major cancer types. The process has been implemented through the leadership and efforts of scientists at the Imperial College London under the direction of Dr. Teresa Norat (1). The CUP program began to systematically and continuously update a database of relevant publications for specific cancers. This database is currently available to researchers upon request to the WCRF/AICR. An international and multidisciplinary panel of experts, known as the CUP panel, evaluates the evidence and systematic reviews provided by the Imperial College for each cancer. Through a rigorous review process coupled with annual summits, conclusions are formalized, recommendations for cancer prevention are defined, and priority areas for future research are proposed. Most critically, for each type of cancer examined, a summary table is prepared based upon the evidence, and this summary provides a foundation for future research and actionable public health recommendations (Tables 1 and 2). The criteria for grading the evidence include the number and types of quality studies, precision of exposure and outcomes assessments, heterogeneity within and between studies, exclusion of chance as well as bias and confounding, demonstration of a biological gradient, size of the effect, and support from mechanistic research relevant to biological plausibility.
TABLE 1.
WCRF/AICR summary of recommended behaviors to promote health and that when implemented for a population will promote the prevention of cancer and multiple chronic disease conditions, including those associated with obesity1
| Recommendation | Commentary |
|---|---|
| Be at a healthy weight. |
|
| Be physically active. |
|
| Eat a diet rich in whole grains, vegetables, fruit, and beans. |
|
| Limit consumption of “fast foods” and other processed foods high in fat, starches, or sugars. |
|
| Limit consumption of red and processed meat. |
|
| Limit consumption of sugar-sweetened drinks. |
|
| Limit alcohol consumption. |
|
| Do not use supplements for cancer prevention. |
|
| For mothers: breastfeed your baby, if you can. |
|
| After a cancer diagnosis: follow the recommendations, if you can. |
|
Adapted with permission from (1). AOAC, Association of Official Analytical Chemists.
TABLE 2.
WCRF/AICR summary of exposures that affect the risk of developing cancer, where evidence to make cancer prevention recommendations is strong, or where evidence appears strong but key aspects of evidence remains inadequate1
| Issue of public health significance | Commentary and recommendations |
|---|---|
| Height and birth weight |
|
| Arsenic in drinking water |
|
| Aflatoxins |
|
| Maté |
|
| Foods preserved by salting |
|
| Coffee |
|
| Mediterranean type dietary pattern |
|
| Dairy products and calcium |
|
Adapted with permission from (1). IARC, International Agency for Research on Cancer; WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research.
From 2008 to 2018, a total of 17 cancer organ site–specific CUP reports for cancer risk and prevention as well as a single CUP report for breast cancer survivors have been completed and are available online (7). Perhaps most importantly, the systematic reviews of the literature for each cancer type reviewed by the CUP, representing over 12,000 pages of data and analysis, are available at the WCRF/AICR website (7). An example of the evidence table for colorectal cancer is shown in Table 3.
TABLE 3.
WCRF/AICR evidence table for diet, nutrition, physical activity, and colon cancer risk1
| Evidence grade | Relationship | Decreases risk | Increases risk |
|---|---|---|---|
| Strong evidence | Convincing | Physical activity1,2 | Processed meat3 Alcoholic drinks4 Body fatness5 Adult attained height6 |
| Probable | Whole grains Foods containing dietary fiber7 Dairy products8 Calcium supplements9 | Red meat10 | |
| Limited evidence | Limited–suggestive | Foods containing vitamin C11 Fish Vitamin D12 Multivitamin supplements13 | Low intake of nonstarchy vegetables14 Low intake of fruits Foods containing haem iron15 |
| Limited–no conclusion | Cereals (grains) and their products, potatoes, animal fat, poultry, shellfish and other seafood, fatty acid composition, cholesterol, dietary n-3 fatty acid from fish, legumes, garlic, nondairy sources of calcium, foods containing added sugars, sugar (sucrose), coffee, tea, caffeine, carbohydrate, total fat, starch, glycemic load, glycemic index, folate, vitamin A, vitamin B6, vitamin E, selenium, low fat, methionine, β-carotene, α-carotene, lycopene, retinol, energy intake, meal frequency, dietary pattern | ||
| Strong evidence | Substantial effect on risk unlikely | ||
| 1Physical activity of all types: occupational, household, transport and recreational. 2The panel judges that the evidence for colon cancer is convincing. No conclusion was drawn for rectal cancer. 3The term “processed meat” refers to meats preserved by smoking, curing, or salting, or addition of chemical preservatives. 4Based on the evidence for alcohol intake >∼30 g/d (∼2 drinks/d). 5Body fatness marked by BMI (kg/m2), waist circumference, or waist–hip ratio. 6Adult attained height is unlikely to directly influence the risk of cancer. It is a marker for genetic, environmental, hormonal, and nutritional growth factors affecting growth during the period from preconception to completion of linear growth. 7Includes both foods naturally containing the constituent and foods that have the constituent added. Dietary fiber is contained in plant foods. 8Includes evidence from total dairy, milk, cheese, and dietary calcium intakes. 9The evidence is derived from supplements at a dose of >200 mg/d. 10The term “red meat” refers to beef, pork, lamb, and goat from domesticated animals. 11The panel judges that the evidence for colon cancer is limited. No conclusion was drawn for rectal cancer. 12Includes evidence from foods containing vitamin D, serum vitamin D, and supplemental vitamin D. 13Definitions and categorization of multivitamin supplements are not standardized. 14Increased risk observed at low intakes (>100 g/d). 15Foods include red and processed meat, fish, and poultry. | |||
Adapted with permission from (8).
In the months immediately prior to publication of the Third Report, the evidence for various cancer types was again updated, and the CUP panel focused on defining the overall global recommendations for cancer prevention that are relevant to public health and policy action (Table 1). As part of the synthesis of evidence and development of recommendations, the CUP panel also focused on a review of biological pathways and mechanisms that may support the observed links between the various dietary components, anthropometrics, and exercise with risk of human cancer, such as those defined by Hanahan and Weinberg in 2011 (9). In addition, findings of the CUP that are not appropriate for inclusion in the global recommendations but have strong evidence for regional or special circumstances were defined and are summarized in Table 2.
A Decade of Progress: Similarities and Changes
Upon initial evaluation, the 2018 conclusions and global public health recommendations appear very similar to those included in the 2007 report. However, there is a tangible evolution of the conceptual underpinnings for the Third Expert Report and its recommendations (1). The CUP recognized, as data for each specific cancer was reviewed, that the current evidence warranted a shift in emphasis toward a more integrative approach, perhaps best labeled as a more holistic focus. Over several decades of research, investigations in the field of diet and cancer have been dominated by a reductionist strategy with a goal to identify specific components such as fiber or individual nutrients and, more recently, specific phytochemicals that enhance or reduce the risk of individual cancers. As our knowledge increases, it appears that single nutrients or phytochemicals over a range of usual intake will modestly impact the overall burden of cancer. Rather, a significant impact will result from an integrated pattern of diet and exercise that combines to create a healthy internal host environment or metabolic state that over time makes cells of an array of tissues less susceptible to the accumulation of DNA alterations that are fundamental to the carcinogenesis cascade. Internal homeostasis is maintained through complex communication between neurologic/behavioral, endocrine, immunologic, and other integrative processes as the host is subject to variations in diet and environment. Diet and nutrition are environmental variables, yet host digestion and metabolism are highly adaptive and, with the exception of frank nutrient deficiency or toxicity, homeostasis is remarkably well maintained with a diet that varies day-to-day and over time. Modern agriculture, food preservation techniques, fortification, and processing/preservation, coupled with efficient and low-cost transportation of food, provides a remarkably diverse and potentially healthy food supply for many nations. Nevertheless, disparities and economic barriers persist within countries, coupled with a reluctance of many governments to use their regulatory powers to promote healthy diets. These barriers and a lack of knowledge prevent many individuals from having access to or choosing foods which contribute to a healthy dietary pattern. Unfortunately, large segments of the global population are choosing a dietary pattern that includes highly manufactured foods, which are often rich in refined carbohydrates and fats but relatively low in nutrient-dense fruits, vegetables, legumes, and whole grains. This dietary pattern combined with sedentary behavior is contributing to an obesity epidemic. It is likely that over a lifetime such a dietary pattern stresses the homeostatic mechanisms and compromises host resiliency and the ability to withstand internal and external procarcinogenic processes.
In recent years, epidemiologic tools and statistical approaches have evolved to allow a clearer assessment of various dietary patterns and associated health risks. For example, studies examining the impact of adherence to the 2007 WCRF/AICR cancer prevention recommendations indicate a benefit in regard to specific cancers, overall cancer risk, and death from any cause (10–12). More recently, empirically defined dietary patterns based on specific biomarker or metabolomic patterns, such as the inflammatory dietary index or the insulinemic dietary index, have been developed and shown to be associated with reduced cancer risk and longer survival (13–18). Although the CUP panel members continue to endorse adherence to each of the individual global recommendations, there is an increasing emphasis on the potential benefit to be obtained by treating recommendations as an integrated pattern of health behaviors that together will have a major impact on cancer risk.
The overall global public health recommendations found in the Third Report are consistent with those in the previous report and represent an evolutionary process that is supported by a far larger body of research, enhancing our confidence in the process established to assess the published literature. The Third Report benefits from a dramatic growth in the number of large and high-quality cohort studies with longer follow-up. For example, obesity was strongly associated with cancer risk in the Second Report, and the strength of the relationship has been enhanced in the Third Report through studies showing a significant association with risk for an increasing number of cancer types. Perhaps even more impressive is the evidence derived from pooling studies, which allows deeper investigation of the impact of diet and exercise on subgroups or subtypes of specific cancers. For example, the accumulating data allow more precise evaluation of squamous cell carcinoma of the upper esophagus as a distinct entity from adenocarcinoma of the distal esophagus. Indeed, histopathologic subgroup analysis has elucidated how different dietary and physical activity factors coupled with other environmental factors, such as tobacco exposure, impact upper and lower esophageal cancer in unique ways. Improvement in statistical tools and more detailed evaluation of participants in cohort studies of greater power have allowed stratified analyses to provide more clarity and certainty when considering how exposure to certain substances such as tobacco combines with diet, exercise, and body composition to impact risk. As such, the current recommendations in the Third Expert Report provide even stronger guidance to policymakers, health care professionals, and the public while directing our future research agenda.
Mechanisms and Biological Plausibility
Defining causality for dietary patterns or specific components in cancer etiology and prevention is a challenge that the WCRF/AICR has accepted, and defined criteria are employed in the CUP to establish actionable relationships. Unfortunately, randomized controlled intervention trials investigating associations of diet and nutrition with cancer outcomes are rarely feasible due to the cost, difficulty in ensuring compliance among control and intervention groups, and long-term exposures necessary to impact a carcinogenesis process that may be decades long. One key criterion that can enhance confidence when causal relationships are considered is establishing mechanisms of action that are biologically plausible based on current knowledge of nutrition and the carcinogenesis process in various tissues. Indeed, these principles are integrated into the classic Hill criteria (5). Although the panel of expert reviewers involved in the CUP and the WCRF/AICR staff recognize that informative mechanistic data complements strong epidemiologic data, the robust methodology which has been developed to systematically examine the published human epidemiologic literature was lacking for a thorough evaluation of mechanistic studies. Establishing a strategy to objectively collect and evaluate the mechanistic data is an enormous challenge. In vitro cell and organ culture, as well as in vivo experimental models of carcinogenesis and human biomarker studies, are particularly diverse in methodology and relevance to human cancer. Key challenges exist in determining the relevant mechanisms for a particular dietary variable and cancer outcomes, including coping with the enormous volume of publications, particularly of cell-based studies; defining criteria of quality for cell and animal model studies; assessing the extent of publication bias; and developing methodology to integrate the evidence (19). In an attempt to develop new tools and strategies for the assessment of dietary mechanisms in carcinogenesis, the WCRF commissioned several research efforts in this area that have recently been published (19, 20).
The effort at the University of Bristol by Lewis et al. (19) to address these issues was applied as a case study to the exposure–cancer relationship for milk insulin-like growth factor 1 and prostate cancer. A multidisciplinary team providing expertise in nutrition, cancer biology, statistics, informatics, and systematic review methodology collaborated to establish an approach for conducting literature searches. The team developed a 2-stage strategy, beginning with an effort to identify mechanisms underpinning a specific exposure–cancer relationship followed by a second stage to conduct a targeted systematic review of high-quality peer-reviewed publications regarding a specific mechanism. As part of this strategy, the University of Bristol developed, specifically for use by investigators, an online tool using TeMMPo (Text Mining for Mechanism Prioritization) and a new graphic approach for displaying heterogeneous data from human studies (the Albatross plot) (19).
In parallel, the WCRF commissioned a collaboration between the University of Maastricht in the Netherlands (20) and the German Cancer Research Center to independently apply the University of Bristol framework without direct contact with each other, as a validation study. The topic was the mechanistic relationship between body fatness and breast cancer. The 2 teams independently evaluated the feasibility and reproducibility of the novel University of Bristol 2-stage framework. Interestingly, while the 2 teams followed the same steps defined in the framework, they reported dissimilar rankings of potential mechanisms during stage I because of different approaches employed, likely resulting from the variation in expertise and research backgrounds of the team members, the search terms (MeSH and free text) employed for database searches, and the definition of inclusion and exclusion criteria (20). However, both teams chose to conduct a systematic review during stage II examining the role of the insulin-like growth factor-1 receptor (IGF-IR) as a mediator of the obesity impact on breast cancer. Despite including panels of studies that were varied, both teams concluded that the evidence was inconclusive for IGF-1R mediating the impact of body fatness on breast cancer risk (20). These novel and laborious efforts are simply the beginning of a scientific process to devise meaningful methodology that can objectively define the evidence base used to make conclusions regarding mechanistic relationships between diet, body composition, exercise, and risk of specific cancers. It is a starting point for future efforts and builds upon the formalization process for systematic review of data with the ultimate goal of an unbiased assessment and synthesis of the available mechanisms literature. Among the challenges is a critical need for investigators to consistently document and report experimental design and methodological procedures so as to allow comparison among studies and subsequent systematic evaluation. To address poor standards of reporting, the ARRIVE (Animals in Research: Reporting In Vivo Experiments) guidelines were first published in 2010 (21–25) in hopes of reducing bias in the scientific literature, improving study reproducibility, reducing financial waste, and limiting unnecessary animal experimentation. This effort has also been addressed and supported by the International Council for Laboratory Animal Science (26) and by others seeking to improve reporting standards for in vitro cell-based research (27). We encourage investigators to support these efforts.
Public Health and Policy Implications
Individuals can benefit by making well-informed and healthy choices consistent with the current WCRF/AICR recommendations as well as those from other organizations providing rigorous reviews of scientific evidence, such as Dietary Guidelines for America (2). Still, we recognize there are many challenges for individuals, including awareness and access to educational materials, lack of skills in implementing behavioral changes, and social and economic barriers to making and sustaining changes in diet and physical activity. As has been proven for many successful public health efforts in the past, such as tobacco control, industrial health and safety, and prevention of communicable disease, our ability to secure public health requires the systematized efforts of society as a whole. Thus, it is clear that societies and governments must be committed to public health through creation of an environment where healthy dietary and physical activity behaviors are supported and implemented for a wider proportion of the population; this is an investment that provides substantial savings in downstream healthcare costs to society. Governments and global organizations, such as the United Nations and the WHO, are also positioned to provide guidance among nations for cooperative efforts that ensure a healthy food supply is consistent with policies for global sustainable development while protecting the environment (28). The global cancer prevention recommendations of the Third Expert Report provide a blueprint for public health efforts by governments and many other organizations. Soon after the 2007 update (29), the WCRF/AICR provided a public policy report with great impact, influencing public health guidelines and food and agriculture policy in hundreds of nations around the world in the last decade. In 2018, the new policy framework was updated (Figure 2) with 3 overarching policy domains: health-enhancing environments, systems changes, and behavior change communication. These are detailed in Appendix 2 of the Third Expert Report.
FIGURE 2.
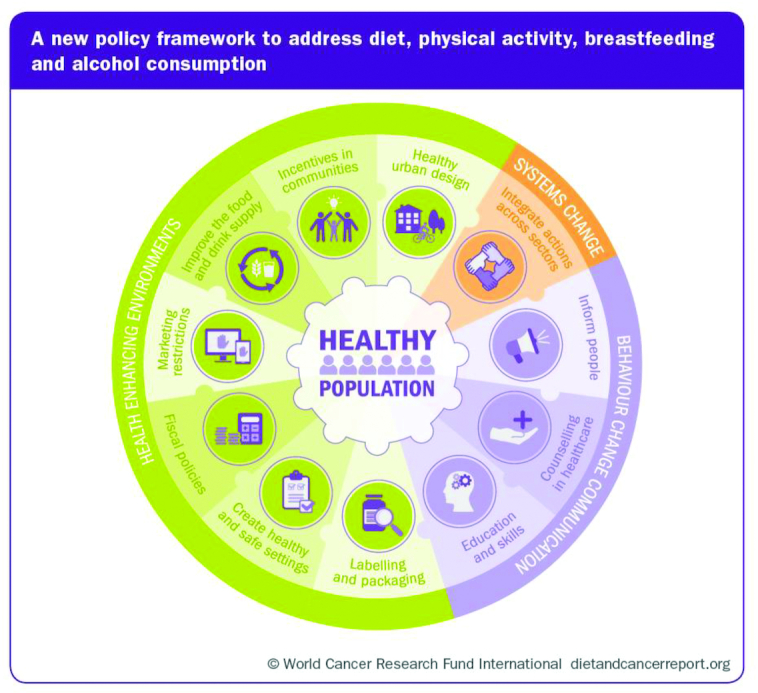
The WCRF/AICR framework for public policy regarding food, nutrition, physical activity, and cancer prevention. Reproduced with permission from the World Cancer Research Fund/American Institute for Cancer Research (1). CUP, Continuous Update Project; WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research.
It is appreciated that there are multiple processes through which change can be implemented, including: multinational and regional cooperative efforts, professional medical and health organizations, governments, the private sector, and civil society, and that these act via educational institutions, the workplace, public institutions, cities, towns and rural communities, social media and networks, and the home and family environment (1). Each of these components must contribute and indeed take responsibility for promoting public health, but taking action for long-term goals like cancer prevention is likely only possible when the highest level of government is motivated to mandate and empower such efforts with resources. History has demonstrated that success in the public health arena requires government actions supported by society and professional organizations working in synergy with a coherent strategy. Unfortunately, many of these efforts are opposed by segments of the food industry or agencies involved in trade and agricultural policy, an unfortunate conundrum that requires strong advocacy for safeguards against financial conflicts of interest that are increasingly pervasive in development of government policy.
The Future
The CUP panel has recognized a number of areas where future research should be prioritized. As a whole, biological mechanisms whereby diet, nutrition, and physical activity impact cancer processes remain relatively obscure and warrant additional efforts, including standardization of research methods and development of novel strategies for systematic review of the published data. Emerging areas of impactful research in “-omics” with a focus on integrative studies of genomics, transcriptomics, proteomics, and metabolomics along with parallel technological and bioinformatic advances will provide insights into mechanisms of action that can revolutionize our understanding of exposures and cancer risk for individuals and populations. The rapidly emerging appreciation for the critical role that the host microbiome plays in carcinogenesis and the dynamic changes resulting from diet, physical activity, or other environmental variables is a particularly stimulating theme for future impactful investigation. Although mechanistic data is often viewed primarily in vitro and in rodents, much more effort should be devoted to well-designed and statistically powered human studies with relevant biomarkers that define biological exposure of diet and physical activity variables but also examine impact on cancer-relevant biological possess. Nutritional epidemiology will benefit from new and more precise and objective tools for measuring dietary exposures and their impact on the host. This effort requires not only improved tools for individual dietary assessment, but also databases that capture the large range of nutrients and bioactive phytochemicals in foods. Much more will be learned from epidemiological cohort studies with improved measures of energy intake and expenditure, as well as more precise definitions of body composition relative to body fatness and muscle mass. Research that includes the goal of defining dose–response relationships with relevant cancer-related outcomes are critically needed. It is likely that diet and physical activity do not impact carcinogenic processes in a linear relationship, and details regarding threshold and maximally beneficial exposures are needed in order to guide public recommendations. Carcinogenesis in humans is often a prolonged process, and we lack critical evidence regarding the impact of diet and physical activity throughout the life course and its effect on subsequent cancer risk, which of course may vary by the cancer subtype. We are in an era where better characterization of cancer outcomes will provide new tools for integration into epidemiologic and mechanism-based human studies. In the future, simple categorization of cancer based on the tissue of origin will be insufficient for cohort studies, and subtypes of cancer based on specific biomarkers (such as estrogen receptor–positive or –negative breast cancer or triple-negative breast cancer) that define etiologic exposures and biological progression will improve our understanding of how diet, physical activity, and body composition impact the risk of relevant subtypes of cancer. In due course, subtypes of cancer based on the genomic and epigenetic landscape (30) will be integrated into our epidemiologic efforts. In addition to diet, body composition, and physical activity, we appreciate the enormous impact of host genetics in defining how an individual responds to these dietary variables. Evidence is clear that nutrient and phytochemical digestion, absorption, metabolism, clearance, and bioactivity are all impacted by genetic variation in a complex dynamic that is rarely integrated into our human epidemiologic efforts, yet may greatly improve the understanding of exposure–cancer associations.
We are also in an era where individuals diagnosed with cancer have a far greater chance of long-term survival than in decades past. Cancer therapy is increasingly complex, and the role of diet, nutrition, and physical activity as an adjunct to therapy, with the goal of improving the efficacy of treatment while reducing acute and long-term toxicity from surgery, chemotherapy, radiation, and the rapidly growing array of biological therapies is critical for success. Upon completion of curative cancer therapy, individuals often experience a “teachable moment” and are motivated to make changes in diet and lifestyle to enhance health and fitness, quality of life, and survival. We must seamlessly integrate diet and lifestyle interventions into healthcare and cancer survivorship programs so as to provide survivors with effective evidence-based guidelines and monitoring strategies. Thus, continued research efforts in behavior and implementation science are necessary to define the most effective and impactful strategies to change diet and exercise patterns to promote health and reduce chronic disease risk. Accordingly, there is a critical need for reimbursement of such providers, particularly registered dietitians/registered dietitian nutritionists and subspecialty-trained physicians, who provide this specialized care.
We also recognize that the vast majority of human epidemiological studies of diet, exercise, and other risk factors are undertaken in higher-income nations, in populations primarily of European descent and more recently in Asian populations. There is a great need for research in lower- and middle-income nations and in geographic areas with more diverse ethnicity and genetic ancestry. In parallel, research on optimization of strategies for implementation of the evidence-based guidelines on diet, nutrition, physical activity, and cancer prevention in diverse populations must be a priority.
Finally, we are increasingly characterizing cohorts of individuals at greater risk of cancer due to the presence of premalignant conditions and past exposures to carcinogenic risk factors, such as tobacco and UV irradiation. In parallel, the swift pace of research regarding germ-line inheritance and cancer risk has led to the rapid expansion of genetic testing in clinics, both for patients with cancers and for their siblings and children. It is critical that we complement efforts for screening and early detection in affected individuals with cancer prevention strategies based on diet and lifestyle. Consequently, research to define effective strategies to implement and maintain a healthy lifestyle in these groups is critically needed.
In Summary
The publication of the WCRF/AICR Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer is another landmark for this philanthropic organization. This effort is the most comprehensive review of the published literature with the application of meticulous methodology and an emphasis on objectivity and impartiality. Like the previous reports, this document will serve governments, organizations, industry, healthcare providers, and individuals in a variety of capacities with the goal of improving health and reducing the global cancer burden through diet, nutrition, and physical activity. The need for increased support for cancer prevention efforts has never been greater, and the report clearly defines key areas for immediate investment in research that will have global impact.
Acknowledgments
All authors read and approved the manuscript.
Notes
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: AICR, American Institute for Cancer Research; CUP, Continuous Update Project; IGF-1R, insulin like growth factor-1 receptor; MeSH, Medical Subject Headings; WCRF, World Cancer Research Fund.
References
- 1. World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: a global perspective. Continuous Update Project Expert Report2018. [Google Scholar]
- 2. Dietary Guidelines Advisory Committee. Scientific report of the 2015 Dietary Guidelines Advisory Committee: Advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Washington (DC): United States Department of Health and Human Services/United States Department of Agriculture, 2015. [Google Scholar]
- 3. Time to deliver: report of the WHO Independent High-Level Commission on Noncommunicable Diseases. Geneva: World Health Organization, 2018. [Google Scholar]
- 4. World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: a global perspective. Washington (DC): American Institute for Cancer Research, 1997. [Google Scholar]
- 5. Hill AB. The environment and disease: association or causation?. Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 6. World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: a global perspective. Continuous Update Project Expert Report Washington (DC): American Institute for Cancer Research, 2007. [Google Scholar]
- 7. World Cancer Research Fund/American Institute for Cancer Research. Resources and toolkits [homepage on the Internet]. 2018; [cited 2019 Sep 24]. Available from: https://www.wcrf.org/dietandcancer/resources-and-toolkit. [Google Scholar]
- 8. World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and colorectal cancer: Continuous Update Project Expert Report. American Institute for Cancer Research; 2018. [Google Scholar]
- 9. Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 10. Vergnaud AC, Romaguera D, Peeters PH, van Gils CH, Chan DS, Romieu I, Freisling H, Ferrari P, Clavel-Chapelon F, Fagherazzi G et al.. Adherence to the World Cancer Research Fund/American Institute for Cancer Research guidelines and risk of death in Europe: results from the European Prospective Investigation into Nutrition and Cancer cohort study 1,4. Am J Clin Nutr. 2013;97:1107–20. [DOI] [PubMed] [Google Scholar]
- 11. Romaguera D, Vergnaud AC, Peeters PH, van Gils CH, Chan DS, Ferrari P, Romieu I, Jenab M, Slimani N, Clavel-Chapelon F et al.. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am J Clin Nutr. 2012;96:150–63. [DOI] [PubMed] [Google Scholar]
- 12. Kohler LN, Garcia DO, Harris RB, Oren E, Roe DJ, Jacobs ET. Adherence to diet and physical activity cancer prevention guidelines and cancer outcomes: a systematic review. Cancer Epidemiol Biomarkers Prev. 2016;25:1018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keum N, Yuan C, Nishihara R, Zoltick E, Hamada T, Martinez Fernandez A, Zhang X, Hanyuda A, Liu L, Kosumi K et al.. Dietary glycemic and insulin scores and colorectal cancer survival by tumor molecular biomarkers. Int J Cancer. 2017;140:2648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petimar J, Smith-Warner SA, Fung TT, Rosner B, Chan AT, Hu FB, Giovannucci EL, Tabung FK. Recommendation-based dietary indexes and risk of colorectal cancer in the Nurses' Health Study and Health Professionals Follow-up Study. Am J Clin Nutr. 2018;108:1092–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petimar J, Tabung FK, Valeri L, Rosner B, Chan AT, Smith-Warner SA, Giovannucci EL. Mediation of associations between adiposity and colorectal cancer risk by inflammatory and metabolic biomarkers. Int J Cancer. 2019;144:2945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tabung FK, Liu L, Wang W, Fung TT, Wu K, Smith-Warner SA, Cao Y, Hu FB, Ogino S, Fuchs CS et al.. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncol. 2018;4:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tabung FK, Wang W, Fung TT, Smith-Warner SA, Keum N, Wu K, Fuchs CS, Hu FB, Giovannucci EL. Association of dietary insulinemic potential and colorectal cancer risk in men and women. Am J Clin Nutr. 2018;108:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tabung FK, Satija A, Fung TT, Clinton SK, Giovannucci EL. Long-term change in both dietary insulinemic and inflammatory potential is associated with weight gain in adult women and men. J Nutr. 2019;149:804–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewis SJ, Gardner M, Higgins J, Holly JMP, Gaunt TR, Perks CM, Turner SD, Rinaldi S, Thomas S, Harrison S et al.. Developing the WCRF International/University of Bristol methodology for identifying and carrying out systematic reviews of mechanisms of exposure-cancer associations. Cancer Epidemiol Biomarkers Prev. 2017;26:1667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ertaylan G, Le Cornet C, van Roekel EH, Jung AY, Bours MJL, Damms-Machado A, van den Brandt PA, Schock H, de Kok TM, Theys J et al.. A comparative study on the WCRF International/University of Bristol methodology for systematic reviews of mechanisms underpinning exposure-cancer associations. Cancer Epidemiol Biomarkers Prev. 2017;26:1583–94. [DOI] [PubMed] [Google Scholar]
- 21. Kilkenny C, Parsons N, Kadyszewski E, Festing MF, Cuthill IC, Fry D, Hutton J, Altman DG. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One. 2009;4:e7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG; National Centre for the Replacement, Refinement and Reduction of Amimals in Research. Animal research: reporting in vivo experiments—the ARRIVE guidelines. J Cereb Blood Flow Metab. 2011;31:991–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H et al.. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Macleod MR, Lawson McLean A, Kyriakopoulou A, Serghiou S, de Wilde A, Sherratt N, Hirst T, Hemblade R, Bahor Z, Nunes-Fonseca C et al.. Risk of bias in reports of in vivo research: a focus for improvement. PLoS Biol. 2015;13:e1002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Osborne N, Avey MT, Anestidou L, Ritskes-Hoitinga M, Griffin G. Improving animal research reporting standards: HARRP, the first step of a unified approach by ICLAS to improve animal research reporting standards worldwide. EMBO Rep. 2018;19(5):e46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hartung T, De Vries R, Hoffmann S, Hogberg HT, Smirnova L, Tsaioun K, Whaley P, Leist M. Toward good in vitro reporting standards. ALTEX. 2019;36:3–17. [DOI] [PubMed] [Google Scholar]
- 28. Swinburn BA, Kraak VI, Allender S, Atkins VJ, Baker PI, Bogard JR, Brinsden H, Calvillo A, De Schutter O, Devarajan R et al.. The global syndemic of obesity, undernutrition, and climate change: the Lancet Commission report. Lancet. 2019;393:791–846. [DOI] [PubMed] [Google Scholar]
- 29. World Cancer Research Fund/American Institute for Cancer Research.Policy and action for cancer prevention. Food, nutrition, and physical activity: a global perspective. Washington (DC): 2009. [Google Scholar]
- 30. Hutter C, Zenklusen JC.. The cancer genome atlas: creating lasting value beyond its data. Cell. 2018;173:283–5. [DOI] [PubMed] [Google Scholar]