Highlights
-
•
Acute management of SLEs differs from usual therapy in classic stroke patients.
-
•
IV L-Arginine should be administered urgently in the setting of a SLE.
-
•
If mental status is altered, an EEG should be performed to rule out a non-convulsive status.
Dear Editor,
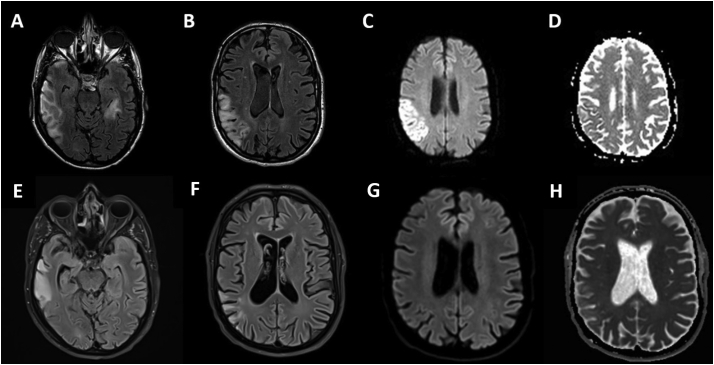
A 38-year-old man was admitted to the emergency department for acute onset of speech impairment, behavioral change and irritability in the past 2 weeks and moderate headache for the last 4 days. Neurological examination revealed an expressive language disorder, left homonymous hemianopsia, astereognosis and left distal arm weakness. He had Mitochondrial Encephalomyopathy, Lactic Acidosis and Stroke-like episodes (MELAS) syndrome diagnosed 8 years before (2011) when he consulted for hearing loss, fatigue and persistent elevation of serum creatine kinase (>9000 U/l). A recent diagnosis of MELAS in his maternal uncle gave the clue to perform genetic testing, which confirmed a mitochondrial DNA mutation (3243A > G). He had a multidisciplinary follow up including Ophthalmology, Cardiology, Endocrinology and Otorhinolaryngology, with a normal brain magnetic resonance imaging (MRI) in 2014 and no stroke-like episodes (SLEs) or other neurological symptoms registered before. When he consulted the emergency department, he was on oral levocarnitine, coenzyme Q10 and L-arginine 7 g three times a day, which he had been taking since the diagnosis was made. Hematological, biochemical, and routine coagulation tests were normal except abnormal high lactate levels (2,2 mmol/l [reference range, 0,55–1,98 mmol/l]). A computed tomography (CT) scan of the head and a CT angiography of cerebral and carotid arteries were performed. There was no arterial occlusion, but the CT demonstrated right temporoparietooccipital and left temporal hypodensities which did not fit vascular territories. A lumbar puncture was normal including negative results of PCR for herpesvirus. Based on his medical history, a SLE was suspected. Therefore, he was admitted to the Stroke Unit where intravenous (I.V.) arginine (bolus of 30 g + 30 g as continuous infusion over 24 h for 3 days) and continuous perfusion of dextrose 10% (1500 ml over 24 h) were initiated. I.V. Levetiracetam was added with the suspicion of a non-convulsive focal status epilepticus, which was confirmed by electroencephalogram (EEG). The status was resolved after adding I.V. Lacosamide. A brain MRI revealed a temporoparietooccipital right lobe lesion and a left temporal lesion showing restriction in the axial diffusion weighted images (DWI) not confirmed in the apparent diffusion coefficient (ADC) maps (Fig. 1A-D). MR spectroscopy was normal. Within 3 days, his treatment was tapered to oral L-arginine 7 g three times a day and he continued with levocarnitine and coenzyme Q10 at the same dosage as before admission. He was discharged in premorbid status 7 days after admission. A second MRI performed one month after clinical onset, demonstrated a partial resolution of the lesions (Fig. 1E-H).
Fig. 1.
(A) and (B): axial fluid attenuation inversion recovery (FLAIR) images reveal increased signal in cortical and subcortical white matter of the right temporoparietal lobe as well as a smaller focus in the left temporal lobe. (C) axial diffusion weighted images demonstrated restriction in the parietal lobe. (D) apparent diffusion coefficient maps do not confirm this restriction. (E) and (F): axial FLAIR images show partial resolution of the lesions, confirmed also in the axial diffusion images (G) and ADC maps (H).
1. Discussion
MELAS syndrome is one of the most common mitochondrial diseases, with an estimated prevalence of 1 in 100,000 people [1], commonly affecting the pediatric population. A multisystem involvement has been described, but SLEs are the cardinal feature and the primary reason for its diagnosis. Nevertheless, these episodes are infrequent in emergency departments, so few neurologists have faced them in clinical practice. Rapid recognition and treatment of them are essential for a better prognosis.
We present a case of the first SLE in an adult patient with MELAS syndrome with good outcome after acute neurological management. The etiopathogenesis of SLEs remains controversial and several hypotheses have been proposed. There is now mounting evidence that the stroke-like episodes in MELAS result, at least in part, from impaired perfusion in cerebral microvasculature due to different mechanisms. On the one hand, cells respond to energy deficiency with abnormal mitochondrial proliferation which leads to angiopathy. On the other hand, impaired perfusion can also result from the deficit of nitric oxide (NO), usually formed from arginine and citrulline [2]. Evidence shows that these precursors may have a therapeutic role in the acute phase of a SLE, reverting the deficient cerebral blood flow caused by NO deficit [3,4]. In clinical practice, acute management of SLEs differs from usual therapy in classic stroke, although time is brain even for these patients.
Despite limited published data, in a patient with MELAS syndrome and acute neurological symptoms urgent administration of intravenous nitric oxide (NO) precursors like L-arginine at high dosage bolus (0,5 g/kg) should be considered. After the initial arginine bolus, an additional dosage of 0.5 g/kg/day should be administered as continuous infusion during the next 3 to 5 days. Afterwards, oral L-arginine (0,15–0,30 g/kg) administered three times a day is recommended as secondary prevention to avoid another SLE [5]. In addition, fluid therapy should be reinforced during the acute management of a SLE, trying to maintain cerebral perfusion with normal saline boluses and avoiding cellular catabolism with dextrose containing fluids. Epilepsy in individuals with MELAS syndrome is heterogeneous. Seizures may trigger and/or perpetuate (by increasing cellular energy demands in a situation of already limited mitochondrial function) SLEs. Therefore, if the clinical presentation is altered mental status, patients should immediately undergo electroencephalography to assess for non-convulsive status epilepticus [5]. However, clinician must be careful when administrating antiepileptic drugs (AEDs), because some medications like valproic acid and phenytoin have deleterious effects on mitochondrial function, worsening patient's clinical outcome. Other AEDs such as carbamazepine, oxcarbazepine, gabapentin or topiramate may also trigger mitochondrial adverse reactions and should be managed carefully [6]. There is no evidence of the usefulness of recombinant tissue plasminogen activator (rtPA) or antiplatelets in clinical management of these episodes.
These key points update the main considerations that a neurologist should bear in mind during the acute management of characteristic SLEs in MELAS. To our knowledge, this is the first MELAS adult case where good clinical outcome after a quick implementation of this approach is reported, as the previously reported cases with good outcome are pediatric. This case might contribute to the currently expanding evidence of the benefits of administering I.V. Arginine during the acute treatment for SLEs also in adults. However, it should be stated that the good outcome refers to the SLE, and not to the disease as a whole.
This SLE treatment update might be paramount considering the current era of stroke care and would be of value to many neurologists and to their patients.
References
- 1.El-Hattab A.W., Adesina A.M., Jones Scaglia F. MELAS syndrome: clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab. 2015;116:4–12. doi: 10.1016/j.ymgme.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Sproule D.M., Kaufmann P. Mitochondrial encephalopathy, lactic acidosis, and strokelike episodes. basic concepts, clinical phenotype, and therapeutic management of MELAS syndrome Ann N Y Acad Sci. 2008;1142:133–158. doi: 10.1196/annals.1444.011. [DOI] [PubMed] [Google Scholar]
- 3.Koga Y., Akita Y., Nishioka J. L-arginine improves the symptoms of strokelike episodes in MELAS. Neurology. 2005;64:710–712. doi: 10.1212/01.WNL.0000151976.60624.01. [DOI] [PubMed] [Google Scholar]
- 4.Ganetzky R.D., Falk M.J. 8-year retrospective analysis of intravenous arginine therapy for acute metabolic strokes in pediatric mitochondrial disease. Mol Genet Metab. 2018;123(3):301–308. doi: 10.1016/j.ymgme.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koenig M.K., Emrick L., Karaa A. Recommendations for the Management of Strokelike Episodes in patients with mitochondrial Encephalomyopathy, lactic acidosis, and Strokelike epis odes. JAMA Neurol. 2016;73:591–594. doi: 10.1001/jamaneurol.2015.5072. [DOI] [PubMed] [Google Scholar]
- 6.Finsterer J., Zarrouk Mahjoub S. Mitochondrial toxicity of antiepileptic drugs and their tolerability in mitochondrial disorders. Drug Metab Toxicol. 2012;8(1):71–79. doi: 10.1517/17425255.2012.644535. [DOI] [PubMed] [Google Scholar]