Graphical abstract
Keywords: TSNA, Nitrosamines, NNN, NNK, Smokeless tobacco products
Highlights
-
•
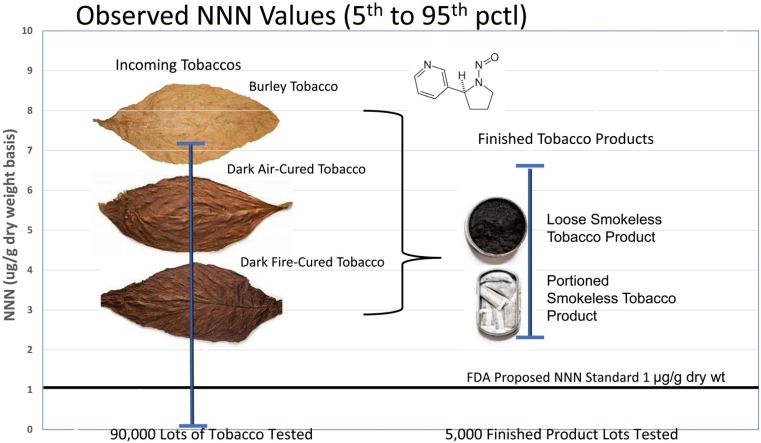
Incoming tobaccos have extremely variable nitrosamine content.
-
•
Blending reduces the variation in tobacco products, but it is still considerable.
-
•
Few, if any, U.S. moist smokeless tobacco products meet the proposed FDA standard.
-
•
Products must be targeted well below the proposed standard to consistently meet it.
Abstract
Tobacco-specific nitrosamines (TSNAs) have been of concern to the public health community for decades and their reduction through agricultural practices, plant breeding, and tobacco processing has also been a decades-long industry effort. Despite those efforts, TSNAs, though lower, continue to be constituents of concern in tobacco products. This paper examines the TSNA levels of dark air-cured, dark fire-cured, and burley tobaccos purchased in the United States by U.S. Smokeless Tobacco Company LLC (USSTC) and of nine finished USSTC moist smokeless tobacco products. TSNA values of the incoming purchased tobaccos and the finished products showed considerable variability. For the incoming tobaccos, the coefficient of variation was generally more than 100 % for each tobacco type and for each of the measured TSNAs. The relative TSNA variability of the finished tobacco products was also considerable, averaging approximately 25 %. It was also found that the measured values for the finished products averaged well above the proposed FDA NNN proposed product standard of 1.0 μg/g dry weight. Because of the large variability in NNN values, products would have to average well below FDA’s proposed product standard to be consistently compliant.
1. Introduction
Tobacco-specific nitrosamines (TSNAs) are nitrosated alkaloids found in tobacco, cigarettes, cigars and smokeless tobacco products [1,2,3]. Two compounds in this class, N’-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), have been classified as human carcinogens (IARC Group 1) by the International Agency for Research on Cancer [4]. Two additional TSNAs, nitrosoanatabine (NAT) and nitrosoanabasine (NAB) have also been identified but have not been classified as known human carcinogens (IARC Group 3 – not classifiable; [4]). Numerous factors affect the TSNA content of tobacco and tobacco products. A combination of genetics, agronomic practices, climatic conditions, and leaf curing methods and storage conditions determine the chemical composition of the tobacco leaf and finished tobacco products and its potential to form TSNA [5]. Extensive efforts by the industry have resulted in significant TSNA reductions. See for example [1] that showed an average reduction of individual TSNAs of 83 % in two U.S. smokeless tobacco products from 1980 to 1992. Fisher et al. [6] showed that finished product nitrosamines declined significantly from the late 1990s until 2005 or so. Nonetheless, despite significant advances in plant breeding, agronomics, and downstream tobacco processing methods to lower TSNAs, they continue to be constituents of concern in tobacco products.
Moist smokeless tobacco (MST) products are the most widely used smokeless tobacco products in the U.S. [7]. Snus products were not included as MST products in this study. MST products are principally comprised of blends of dark air-cured, dark fire-cured and burley tobaccos, additives and water [4]. It has long been known that the TSNA levels in these cured tobaccos are extremely variable, often with coefficients of variation in excess of 100 % of the mean (see for example, [8,9], 1994, [10,11]). Substantial variability in NNN, NNK, NAT, and NAB levels within different portions of dark air-cured tobacco leaves have also been reported [12]. Researchers have published several surveys of TSNA levels in a wide variety of smokeless tobacco products showing broad variation in the marketplace [[13], [14], [15], [16], [17], [18]]. Kaluba et al. [19] examined nitrosamines in alternative smokeless tobacco products outside the United States.
The U.S. Food and Drug Administration (FDA) recently proposed a limit on NNN levels in finished smokeless tobacco products [20]. The proposal would require products to maintain mean levels of NNN not exceeding 1.0 μg per gram of tobacco on a dry weight basis1 through the product’s labeled expiration date. The FDA estimated that 30 % of the marketed MST products were compliant with the proposed product standard ([21], Table 3) based upon industry reports to the FDA in 2012 under section 904 of the Tobacco Control Act. However, based upon the levels previously reported in published data [[13], [14], [15], [16], [17], [18]] very few if any conventional MST products would meet the proposed product standard. One explanation of the difference in estimates of MST products that would meet the proposed product standard was provided by Morton et al. [22]. It was shown that, since none of the USSTC MST products reported in 2012 met the proposed product standard on a dry weight basis, an implausibly high percentage (∼60 %) of the non-USSTC MST products would have to meet the proposed product standard for the FDA estimates to be accurate. Such a high percentage below 1.0 μg/g dry weight is not consistent with the values reported in the literature in which virtually none of the tested MST products meet the proposed product standard. However, if one assumes that the FDA misinterpreted values reported on a wet weight basis as already being dry weight, approximately 40 % of the reported USSTC values would have appeared to meet the proposed product standard, which would be consistent with the FDA estimates. Based on those calculations, and the knowledge that USSTC submitted values to the FDA on a wet weight basis, it was hypothesized that, though many or most of the values reported to the FDA under Section 904 of the Tobacco Control Act (Reporting Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke) were reported on a wet weight basis, the FDA assumed that all values were reported on a dry weight basis. This argument is given in more detail in Morton et al. [22]. Values expressed on a dry weight basis for MST products are generally a little more than two times the wet weight values, so the hypothesized error has a large effect and closely explains the FDA’s conclusions with regards to proportion of products meeting the proposed product standard.
Limited data have been published on the variability of TSNA levels over time in tobacco crops used to make smokeless tobacco products sold in the U.S. Even less data have been published demonstrating how variability in TSNA levels in source tobaccos are reflected in the variability of TSNA levels in smokeless tobacco products made from those tobaccos. The two objectives of this work were to examine the variability in NNN, NNK and NAT levels not only in tobacco crops but also commercial smokeless tobacco products made by USSTC from those tobacco crops and to assess their compliance with the FDA’s proposed product standard of 1.0 μg/g dry weight.
2. Materials and methods
2.1. Tobacco sample collection
All of the tobaccos discussed herein were sourced from the United States and grown between 2005 and 2017. The sampling plans each year were intended to include samples from every grower selling tobacco to USSTC (though a small number were inadvertently missed), except for the 2012 and 2013 samples from dark air-cured tobaccos when sampling was from every 30th bale purchased by USSTC, not from every grower.
For dark fired-cured tobacco, Fisher et al. [6] described how samples were collected and processed from 2005−2010. Similar collection and processing techniques were used for dark fired-cured tobacco collected in 2011–2013 and 2016 and 2017. Specifically, for 2005–2011, three random leaf-grade bales were selected from each curing barn and two core samples were collected from each bale and combined to comprise an analytical sample. Core samples were 3–4 inches in diameter and approximately 34 in. in length. Length varied due to the size of the bale (typically between 600–800 lbs.) and compressibility of the tobacco. In 2012 and 2013, the same sampling protocol from 2005 to 2011 was used except that the three random leaf-grade bale samples were combined into a single analytical sample and blended together prior to analysis. In 2016, two samples from each farmer’s delivery were obtained. A composite taken from the first three bales was taken for the first sample and a composite from the last three bales was taken as the second sample. In 2017 the sampling protocol from 2012 and 2013 was used.
Sampling of dark air-cured tobacco was similar to dark fire-cured tobacco. From 2005 to 2011, three random leaf-grade bales were selected from each farmer’s delivery and two core samples were collected from each bale and combined to comprise an analytical sample. In 2012 and 2013, one sample was taken from every 30th bale. In 2016 and 2017, two composite samples were obtained, one from the first three bales and the second from the last three bales of a farmer’s delivery.
Sampling of burley tobacco was conducted from 2005 to 2011 and in 2017 and the protocol mirrored the protocol used for dark air-cured tobacco in the years burley was sampled.
Sample preparation procedures for all tobacco types were largely consistent with CORESTA Guide No. 13. Cored bale samples of all tobacco types were frozen in liquid nitrogen and ground prior to TSNA analysis but were not stored in a freezer after grinding due to the short time duration between grinding and analysis.
2.2. Smokeless tobacco product
NNN, NNK and NAT levels in nine different commercial smokeless tobacco products from 2008 to 2017 were measured at the end of the product stability testing period. These products represent seven different blends of dark fire-cured, dark air-cured and burley tobacco. The products are all MST products and represent a mix of fine cut and long cut products. From 2008 to 2010, NNN, NNK and NAT levels were measured from a maximum of 26 batches/year per product. From 2011 to 2017, NNN, NNK and NAT levels were measured from a maximum of 26 batches of each product per year per packaging configuration (some products are made in more than one packaging configuration). Sample cans of each product were stored in a controlled environment (73.0 ± 3.0 °F and 51 % ± 10 % relative humidity) for the duration of the product stability testing periods listed in Table 1. Typically, products are kept as 5-can logs wrapped in plastic shrink wrap for the first 2 weeks of storage and then unwrapped for the remainder of the study. These storage conditions are intended to reflect average retail conditions as product is shipped to retail in shrink wrapped logs where it may then be sold in logs of five cans or unwrapped for single can sales.
Table 1.
Product Stability Testing Periods.
| Product Number |
Product Stability Testing Period (weeks) |
|---|---|
| 1, 7, & 8 | 8–12 |
| 2, 3, 4, 5, 6 & 9 | 20 |
2.3. TSNA analysis
The analytical methodology employed to determine NNN, NNK and NAT levels varied over the course of the data collected for this work. From 2005 to 2011, dark air-cured, dark fire-cured, and burley tobacco and retain samples of smokeless tobacco product were analyzed using a high-throughput GC-TEA or GC-NCD method ([23], Morgan et al., [24]). The focus of these methods was the identification of high TSNA results and for that reason, the peak integration was not optimized for low-level values. Beginning in 2012, NNN, NNK and NAT levels were measured using an LC–MS/MS method [25] in a laboratory accredited to the ISO 17025 standard and was within the scope of accreditation. To allow all of the results to be quantitatively combined, low values were extrapolated beyond the calibration curve. All values are reported on a dry weight basis. In this paper, all TSNA values should be understood as being on a dry weight basis unless explicitly stated otherwise. Reporting on a dry weight basis adjusts for differences in water or moisture content.
3. Results
3.1. TSNA levels in tobaccos
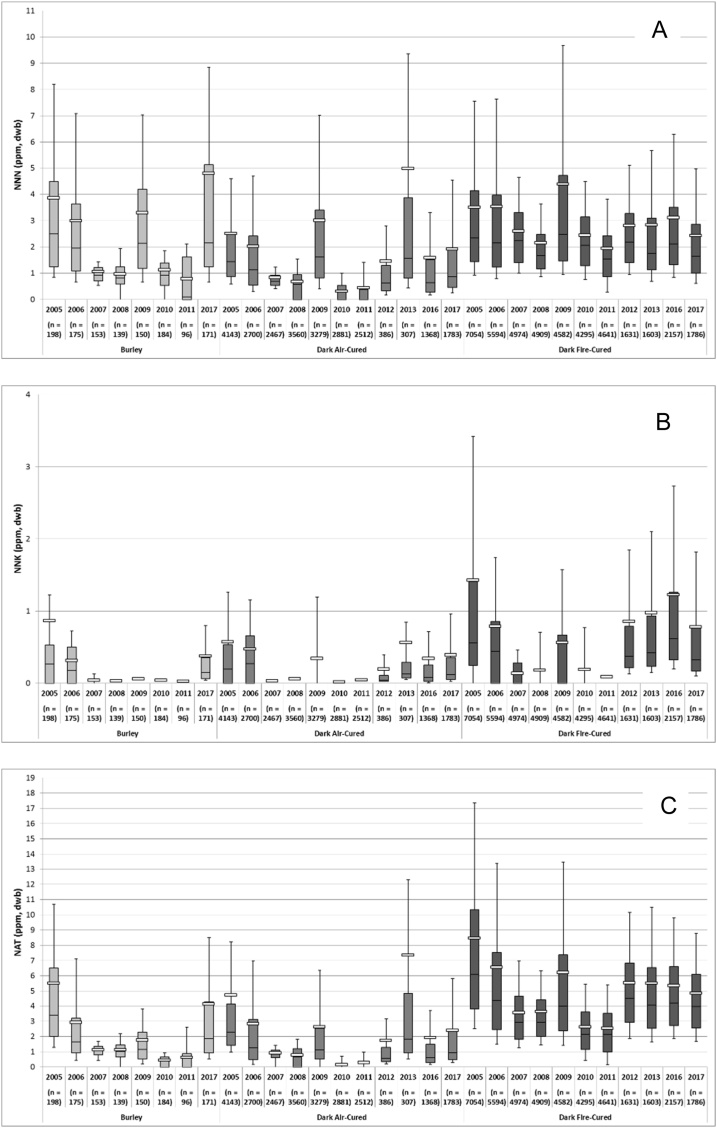
Significant variability was observed in NNN, NNK and NAT levels for each of the three types of tobacco within single crop years as well as from year-to-year (Fig. 1; see supplemental data for tabulated values). The within year relative variability in measured NNN levels (standard deviation divided by the mean value expressed as a percentage; also called the coefficient of variation) averaged 104 % in dark fire-cured, 115 % in burley, and 193 % in dark air-cured tobacco. The range of observed values was generally quite large. For example, in 2017 for dark air-cured tobaccos, the 10th percentile NNN result was 0.26 μg/g and the 90th percentile result was 4.54 μg/g, a ratio of 17.5. There was also substantial year-to-year variability in the NNN levels. For example, the average NNN values were 0.31 μg/g in 2010 and 4.99 in 2013. The relative variability in measured NNK levels was even larger than for NNN, averaging 232 % for dark fire-cured, 444 % for dark air-cured, and 381 % for burley. The relative NAT variability was similar to the variability seen with NNN, 89 % for dark fire-cured, 242 % for dark air-cured, and 128 % for burley.
Fig. 1.
NNN (A), NNK (B) and NAT (C) levels (μg/g or ppm, dry weight basis) in burley, dark fire-cured and dark air-cured tobaccos harvested in crop years 2005 through 2017. Boxes represent the 25th and 75th percentile value and whiskers indicate the 10th and 90th percentile. The median and mean are represented as a black line and a white bar, respectively. Numbers of samples analyzed are in parenthesis.
No sustained trend of increasing or decreasing NNN, NNK or NAT levels or variability over this time period was identified.
3.2. TSNA levels in smokeless tobacco products
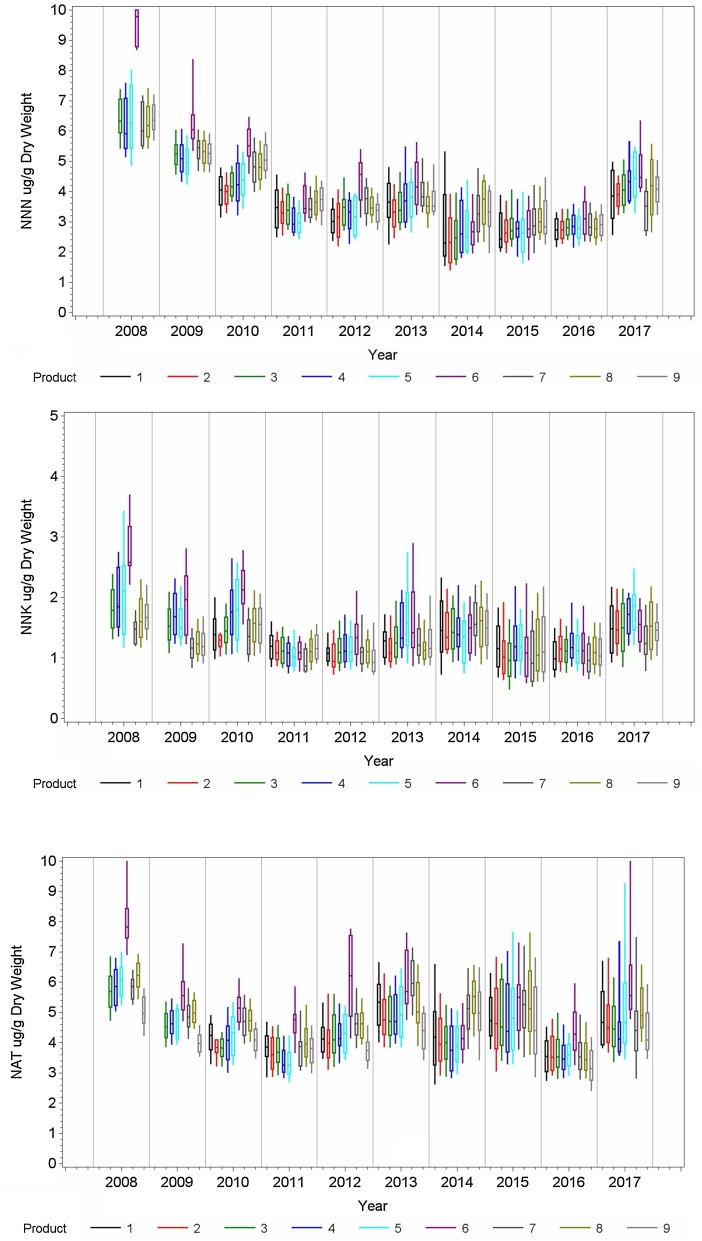
Measured NNN, NNK and NAT levels at the end of the product stability testing period (Table 1) for the nine smokeless tobacco products manufactured from 2008 to 2017 are shown in Fig. 2 (data shown on a dry weight basis) and demonstrated substantial variability. The average within year relative variability of NNN, NNK, and NAT across all of the products were 21.6 %, 31.2 %, and 21.9 %, respectively. Though this is less variation than was seen in tobacco leaf samples, it still represents considerable product variation. For example, for Product 1 in 2017, the 10th percentile NNN value was 2.57 μg/g and the 90th percentile value was 4.99 μg/g, almost a factor of two difference.
Fig. 2.
NNN, NNK and NAT levels on a dry weight basis in smokeless tobacco products at the end of product stability testing period from 2008 to 2017. The top panel is NNN, middle panel is NNK and bottom panel is NAT. Boxes represent the 25th and 75th percentile value and the whiskers indicate the 10th and 90th percentile. The median is the horizontal bar within the box. The number of lots measured per product per year is given in the supplemental information.
Though significant reductions were noted by [1] and Fisher et al. [6] in prior years, the tobacco nitrosamine levels show some year-to-year differences, but do not show any clear year-to-year trends over this time period. It is possible that any year-to-year tobacco trends could be obscured by the extreme variability of the data, changes in sampling protocol, and changes in the analytical methodology over time. The NNN levels measured at the end of product stability testing periods (Fig. 2) dropped from 2008 to 2010. From 2010 to 2017, the NNN levels measured at the end of product stability testing periods do not show any clear year-to-year trends. NNK and NAT levels do not show clear year-to-year trends from 2008 to 2017 for most of the tested products. A possible exception is Product 6 that shows higher NNK and NAT values in 2008 than in the remaining years.
4. Discussion
Our data showed that burley, dark air-cured and dark fire-cured tobaccos purchased by USSTC from 2005 to 2017 consistently exhibited high variability in NNN, NNK and NAT levels. Variability in levels of NNN, NNK, and NAT was observed within crop years and year-to-year with no clear temporal trend toward increased or decreased levels or variability. Relative to the source tobaccos, significantly lower, although still substantial, variability in NNN, NNK, and NAT levels was observed in the nine smokeless tobacco products manufactured from 2008 to 2017. The reduction in variability in finished product is likely due to the smoothing effect coming from blending tobaccos together to make the finished product.
The variability of NNN, NNK, and NAT levels observed in tobaccos examined in the current work are consistent with natural variation expected in an agricultural crop grown and cured on independent farms. Differences in farming practices, processes and technologies can impact levels of NNN, NNK and NAT in tobacco. For example, Chamberlain and Chortyk [26] showed a large increase in TSNAs in burley and flue-cured tobaccos with increasing levels of nitrogen fertilizer. Less dramatic but statistically significant differences in TSNA levels were found to be correlated with fertilizer use within normal production ranges in census data taken from contracted dark fire-cured tobacco farmers [6]. Fisher et al. [6] also investigated effects of rainfall on total NNN, NNK and NAT in dark fire-cured tobacco crops. For samples collected from dark fire-cured tobacco crops produced from 2005 to 2010, intra and inter-year NNN, NNK and NAT levels varied up to 2-fold, while lower levels and less variability were generally correlated with dryer environmental conditions during curing. In a field experiment using two varieties of dark air-cured tobacco (one low converter seed variety), as summarized in a CORESTA grant final report [27], statistically significant differences in total TSNAs (NNN, NNK, NAT, and NAB) were measured in tobacco from different areas within a single curing barn and at two different geographic locations.
The variability in TSNA levels in different types of tobacco has been previously reported by several authors ([8,9], 1992, 1994, [11,8,9]) and found substantial variability (up to 11 fold) in measured levels of NNN, NNK and NAT (wet weight basis) in a single crop year of burley tobacco post-harvest using controlled curing conditions (temperature and relative humidity) which is consistent with variability of measured levels of NNN, NNK and NAT in burley tobacco seen in the current study. Burton et al. [12] also found substantial variability in measured levels (wet weight basis) of NNN (up to 7 fold), NNK (≥ 20 fold) and NAT (≥ 4 fold) in 41 different segments of dark air-cured tobacco leaves collected from the top third of the tobacco plant. Burton et al. [28] found substantial variation in NNN (up to 3 fold), NNK (up to 6 fold) and NAT (up to 3 fold) levels between leaves from the top, middle and bottom of air-cured and burley tobacco plants. Over the three crop years of tobacco studied, NNN levels varied up to 3 fold, NNK levels varied up to 8 fold and NAT levels varied up to 2 fold. Substantial variability (3–6 fold) in levels of TSNA was also noted by de Roton et al. [11] who analyzed burley tobacco samples from a small number of farms in France over 3 crop years (N = 5 for 2001; N = 9 for 2002; and N = 13 in 2003).
TSNA levels and their variability in U.S.-sourced smokeless tobacco products have been published by several authors [[13], [14], [15], [16], [17], [18]] using different analytical methods (GC–MS/MS, GC-TEA, LC–MS/MS) and reported results either on a dry weight or wet weight basis. The averages and standard deviations of the NNN values from the MST products in those studies are shown in Table 2. Richter et al. [15] measured levels of NNN, NNK, NAT and NAB in 40 brands of smokeless tobacco products purchased in Atlanta in 2004 and expressed results on a wet weight basis. The NNN values reported by Richter (after conversion to dry weight) averaged 14.5 μg/g and were quite variable from product-to-product and notably higher than the NNN values reported in the other studies in Table 2. Similar to the findings in this study, all of the results shown in Table 2 were quite variable and none of the measured MST values were below the product standard proposed by the FDA.
Table 2.
Summary of NNN from moist smokeless tobacco products in referenced studies.
| Borgerding | Richter | Stepanov 2008 | Ammann | Stepanov 2013 | Song | |
|---|---|---|---|---|---|---|
| Mean (μg/g, dry weight) | 5.1 | 14.5 | 4.4 | 3.4 | 4.9 | 4.5 |
| Stdev (μg/g, dry weight) | 2.3 | 14.3 | 1.9 | 2.0 | 1.9 | 1.0 |
| Min (μg/g, dry weight) | 3.1 | 4.4 | 1.7 | 1.03 | 2.8 | NA |
| Max (μg/g, dry weight) | 12.7 | 91.1 | 6.9 | 9.5 | 8.9 | NA |
| Number of MST products | 19 | 39 | 5 | 18 | 14 | 7 |
Only 2 (0.04 %) of 5008 NNN measurements for the nine smokeless tobacco products included in this paper were below the FDA’s proposed product standard of 1.0 μg/g NNN on a dry weight basis. Similarly, as noted above, none of the MST NNN results in the references above [[13], [14], [15], [16], [17], [18]] were below the proposed product standard. Given the variability observed in this paper and other published results, in order to consistently meet any proposed NNN product standard, the average NNN value of any given product would need to be well below the proposed NNN product standard NNN level. Considering the mean NNN results published here plus the other published results, it is likely that few, if any, MST products in the U.S. market meet the proposed NNN product standard. However, the FDA concluded that 30 % of the currently marketed MST products meet the proposed NNN product standard, contrary to the results of this paper and contrary to published results. Their conclusion also did not consider the inherent NNN variability of MST products when establishing the proposed NNN product standard. That is, products must average well below the proposed NNN product standard to be consistently below the proposed product standard given the variability of NNN. More importantly, despite the fact that many or most of the values reported under Section 904 of the Tobacco Control Act (Reporting Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke) were reported on a wet weight basis, the FDA evaluation appears to have assumed that all values were reported on a dry weight basis (discussed above and in more detail in [22]). For MST products the difference between dry weight and wet weight NNN is a little more than a factor of two; the calculations given in Morton et al. [22], assuming the FDA ignored the conversion between wet weight and dry weight, are consistent with the FDA’s findings.
There are several limitations in the current work which include changes in the sampling plan over time used both for the different tobacco types and smokeless tobacco products. Changes in the tobacco sampling plans and analytical methodology and limitations in the analytical methodology make direct year-to-year comparisons difficult. For the years 2011–2016, NNN, NNK, and NAT were not measured in burley tobacco. Another limitation is the use of GC/TEA and GC/NCD analytical methods which are much less sensitive than LC/MS/MS. Further, the GC/TEA and GC/NCD methods for tobacco samples were focused on identifying high TSNA values. Because of the emphasis on the high end of the method range, the results near the limit of detection received less attention and the sample chromatogram integration was not optimized when results were low. The GC/NCD was validated in 2012 and re-validated in 2017. Analytical method validation, which specifies the method sensitivity, appears to have corrected this limitation after 2011. Because of the year-to-year sampling and testing differences, one should not over-interpret the precise quantitation of the TSNA values. The primary conclusion that the variation in TSNAs observed in tobaccos and MST products are considerable is not affected by these limitations.
5. Conclusion
High variability in NNN, NNK and NAT levels was observed in U.S. purchased burley, dark air-cured and dark fire-cured tobaccos from 2005 to 2017. Less, but still considerable variability, was observed in measured NNN, NNK and NAT levels between 2008–2017 for nine smokeless tobacco products that represented seven different tobacco blends. With an approximate 25 % relative variability in NNN levels noted in the nine smokeless tobacco products, a target level well below the proposed NNN product standard, perhaps 0.5 μg/g or less, would be required to assure that most batches of products would meet the FDA proposed NNN product standard of 1.0 μg/g dry weight in finished product. This is not possible considering the NNN levels currently present in burley, dark air-cured and dark fire-cured tobaccos as received from tobacco growers. Tobacco is an agricultural crop where variations in farming practices, processes, technologies and naturally occurring weather patterns can impact levels of NNN, NNK and NAT in tobaccos and in smokeless tobacco products made using these tobaccos. In the 11 years of data for the nine smokeless tobacco products, only 2 (0.04 %) of 5181 NNN measurements over this period were below the NNN product standard proposed by the FDA of 1.0 μg/g dry weight. Based on the results reported here plus those reported elsewhere in the literature, it is likely that few, if any, MST products currently sold in the U. S. would meet FDA’s proposed NNN product standard.
CRediT authorship contribution statement
M.J. Oldham: Conceptualization, Writing - original draft, Project administration. K.E. Lion: Conceptualization, Writing - original draft, Project administration. D.J. Phillips: Formal analysis, Data curation, Methodology. M.J. Morton: Formal analysis, Writing - review & editing. M.F. Lusso: Data curation, Methodology. E.A. Harris: Data curation, Methodology. J.L. Jordan: Conceptualization. J.E. Franke: Conceptualization, Resources, Writing - review & editing. J.A. Strickland: Conceptualization, Resources.
Declaration of Competing Interest
All authors were employees of Altria Client Services LLC or U.S. Smokeless Tobacco Company at the time the research was done, and those companies funded the work.
Acknowledgments
The authors would like to acknowledge the assistance of Dr. James A. Fishback in preparation of this manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.05.008.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Djordjevic M.V., Gay S.L., Bush L.P., Chaplin J.F. Tobacco-specific nitrosamine accumulation and distribution in flue-cured tobacco alkaloid isolines. J. Agric. Food Chem. 1989;37:752–756. [Google Scholar]
- 2.Hoffmann D., Djordjevic M.V., Brunnemann K.D. On the control of toxic substances in smokeless tobacco. J. Smoking Rel. Dis. 1991;2:165–172. [Google Scholar]
- 3.Hoffmann D., Brunnemann K.D., Prokopczyk B., Djordjevic M.V. Tobacco-specific N-nitrosamines and Areca-derived N-nitrosamine chemistry, biochemistry, carcinogenicity, and relevance to humans. J. Toxic Environ. Hlth. 1994;41:1–52. doi: 10.1080/15287399409531825. [DOI] [PubMed] [Google Scholar]
- 4.IARC . Vol. 89. International Agency for Research on Cancer; Lyon, France: 2007. IARC monographs on the evaluation of carcinogenic risks to humans. (Smokeless Tobacco and Some Tobacco-Specific N-Nitrosamines). [PMC free article] [PubMed] [Google Scholar]
- 5.Tso T.C. Ideals Inc.; Beltsville, MD. USA: 1990. Production, Physiology and Biochemistry of Tobacco Plant. [Google Scholar]
- 6.Fisher M.T., Bennett C.B., Hayes A., Kargalioglu Y., Knox B.L., Xu D., Muhammad-Kah R., Gaworski C.L. Sources of and technical approaches for the abatement of tobacco specific nitrosamine formation in moist smokeless tobacco products. Food Chem. Toxicol. 2012;50(3-4):942–948. doi: 10.1016/j.fct.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 7.NCI . National Cancer Institute, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2014. Smokeless Tobacco and Public Health: A Global Perspective. pp 75-114. [Google Scholar]
- 8.Burton H.R., Childs G.H., Jr., Andersen R.A., Fleming P.D. Changes in chemical composition of burley tobacco during senescence and curing. 3. Tobacco-specific nitrosamines. J. Agric. Food Chem. 1989;37:426–430. [Google Scholar]
- 9.Burton H.R., Bush L.P., Djordjevic M.V. Influence of temperature and humidity on the accumulation of tobacco-specific nitrosamines in stored burley tobacco. J. Agric. Food Chem. 1989;37:1372–1377. [Google Scholar]
- 10.Wahlberg I., Ringberger T. Smokeless tobacco in tobacco production. In: Davis D.L., Nielsen M.T., editors. Chemistry and Technology. Blackwell Science; Malden, MA: 1999. pp. 455–456. [Google Scholar]
- 11.de Roton C., Wiernik A., Wahlberg I., Vidal B. Factors influencing the formation of tobacco-specific nitrosamines in French air-cured tobaccos in trials and at the farm level. Beitrage Zur Tabakforschung Int. Contrib. Tobacco Res. 2005;21:305–320. doi: 10.2478/cctr-2013-0797. [DOI] [Google Scholar]
- 12.Burton H.R., Dye N.K., Bush L.P. Distribution of tobacco constituents in tobacco leaf tissue. 1. Tobacco-specific nitrosamines, nitrate, nitrite, and alkaloids. J. Agric. Food Chem. 1992;40:1050–1055. [Google Scholar]
- 13.Ammann J.R., Lovejoy K.S., Walters M.J., Holman M.R. A survey of N’-Nitrosonornicotine (NNN) and total water content in select smokeless tobacco products purchased in the United States in 2015. J. Agric. Food Chem. 2016;64(21):4400–4406. doi: 10.1021/acs.jafc.6b00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borgerding M.F., Bodnar J.A., Curtin G.M., Swauger J.E. The chemical composition of smokeless tobacco: a survey of products sold in the United States in 2006 and 2007. Regul. Toxicol. Pharmacol. 2012;64(3):367–387. doi: 10.1016/j.yrtph.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Richter P., Hodge K., Stanfill S., Zhang L., Watson C. Surveillance of moist snuff: total nicotine, moisture, pH, un-ionized nicotine, and tobacco-specific nitrosamines. Nicotine Tob. Res. 2008;10:1645–1652. doi: 10.1080/14622200802412937. [DOI] [PubMed] [Google Scholar]
- 16.Song M.A., Marian C., Brasky T.M., Reisinger S., Djordjevic M., Shields P.G. Chemical and toxicological characteristics of conventional and low-tsna moist snuff tobacco Products. Toxicol. Lett. 2016;245:68–77. doi: 10.1016/j.toxlet.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stepanov I., Jensen J., Hatsukami D., Hecht S.S. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tob. Res. 2008;10:1773–1782. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stepanov I., Yershova K., Carmella S., Upadhyaya P., Hecht S.S. Levels of (S)-N’-Nitrosonornicotine in U.S. Tobacco products. Nicotine Tob. Res. 2013;15:1305–1310. doi: 10.1093/ntr/nts249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalubula M., Shen H., Khanam T. Assessment of carcinogenic and toxic substances in ‘Insunko’ herb. Toxicol. Rep. 2020;7(2020):468–474. doi: 10.1016/j.toxrep.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FDA Proposed tobacco product standard for N-nitrosnornicotine level in finished smokeless tobacco products. Fed. Reg. Jan. 2017;23:8004–8053. [Google Scholar]
- 21.FDA . Preliminary Regulatory Impact Analysis; 2017. Tobacco Product Standard for N-Nitrosnornicotine Level in Finished Smokeless Tobacco Products. Docket No. FDA-2016-N-2527. [Google Scholar]
- 22.Morton M.J., Phillips D.J., Jordan J.L., Oldham M.J., Lion III K.E., Lusso M.F., Franke J.E., Strickland J.A. 2018. Variability of NNN in Tobacco and NNN Levels in Smokeless Tobacco Products, CORESTA - Smoke Science & Product Technology Meeting.https://www.coresta.org/sites/default/files/abstracts/2017_IG01_Morton.pdf [Google Scholar]
- 23.Bennett C.B., Midgett C.H., Johnston K.S., Owen J.K., Welden J.C., Shanmugan S.M., Franke J.E., Balasubramanian S. Smoke Science and Product Technology Presentation No. 33 at 2002. CORESTA Congress; New Orleans, LA: 2002. An automated analytical method for determination of tobacco specific nitrosamines in tobacco; pp. 22–27. September. [Google Scholar]
- 24.Harris W.E., Gibson S.M., Finch J.N., Hart F., Heltsley D., Ray C., Thorburn T. Productivity improvements through chromatography and automation for GC/TEA TSNA analysis of tobacco leaf. Presentation No. 29 at the 62nd Tobacco Science Research Conference; September 9, Nashville, TN; 2009. [Google Scholar]
- 25.CORESTA . Cooperation Centre for Scientific Research Relative to Tobacco; 2011. Determination of Tobacco-specific Nitrosamines in Tobacco and Tobacco Products by LC-MS/MS - Version 1, CORESTA Recommended Method No. 72. June. [Google Scholar]
- 26.Chamberlain W.J., Chortyk O.T. Effects of curing and fertilization on nitrosamine formation in bright and burley tobacco. Beitrage Zur Tabakforschung Int. Contrib. Tobacco Res. 1992;15:87–92. doi: 10.2478/cttr-2013-0625. [DOI] [Google Scholar]
- 27.CORESTA . Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA); Paris, France: 2015. Study Grant Final Report, Analysis of Variability in Curing Conditions and TSNA within Barns of Dark Air-cured Tobacco. May May. [Google Scholar]
- 28.Burton H.R., Dye N.K., Bush L.P. Relationship between tobacco-specific nitrosamines and nitrite from different air-cured tobacco varieties. J. Agric. Food Chem. 1994;42(9):2007–2011. doi: 10.1021/jf00045a033. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.