Abstract
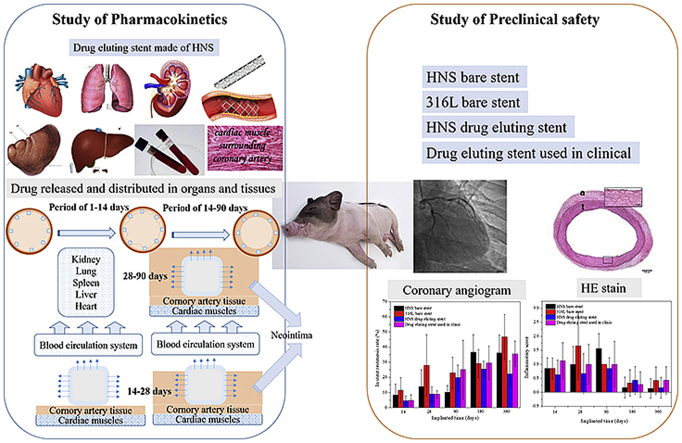
Pharmacokinetic analyses were performed using 20 pigs for 120-days implantation, while one sirolimus-eluting stent was implanted into one of their coronary artery. At different time points, the residual sirolimus on the stent, delivered locally (to artery wall), regionally (to adjacent and downstream muscle) and systemically (to plasma and visceral organs), was detected throughout 120 days. Preclinical safety evaluation was performed using 32 pigs for 180-days implantation to study the safety of metal platform material and the effectiveness of sirolimus eluting coating on the HNS stent. The neointima area, restenosis rate and inflammatory grade for HNS and control group stents were detected and analyzed. Approximately 80% sirolimus was eluted from the sirolimus-eluting stents after 30-days implantation in vivo. Additionally, there was sustained sirolimus in the artery wall, cardiac muscle and heart throughout 120-days implantation, and sirolimus accumulated to the peak at 90-days implantation. It was inferred that the sirolimus eluting stent in this study was covered by neointima before 90-days implantation, indicating that the sirolimus eluting coating on the HNS stent was safe and effective. Very little sirolimus was distributed in visceral organs after 14-days implantation. HNS sirolimus-eluting stent exhibited lower restenosis rate and lower inflammatory grade than control group, which verified that the sirolimus-eluting coating design in this study was reasonable and practical. In addition, there were no significant difference in restenosis rate and inflammatory score between HNS bare-metal stent and drug-eluting stents, illustrating that HNS has good bio-compatibility and is suitable to use as coronary artery stent material.
Keywords: Sirolimus-eluting stent, Pharmacokinetics, Preclinical safety, Tissue response
Graphical abstract
Highlights
-
•
First time to investigate the pharmacokinetics of drug eluting stents for 120 days, found the relationship between the pharmacokinetics and tissue response, which has been rarely reported.
-
•
Verified that the drug-eluting stent made of high nitrogen stainless steel endothelialization finished after 90 days implantation, without endothelialization delay.
-
•
HNS has been proved that it is a better biocompatibility and bio-safe metal platform material, owing better property to be used in clinic.
1. Introduction
High nitrogen stainless steel (HNS) is a novel metal material with better mechanical properties and biocompatibility, which has great potential to be used as a metal platform material for artery stents [1]. In terms of mechanical properties, the tensile strength of HNS is higher than L605 cobalt-base alloy and twice of the 316L stainless steel, which are the two widely used platform materials for clinical stents [2]. Therefore, the size of HNS support wire is expected to be smaller than that of L605 cobalt-based alloy but with higher support strength. In terms of biocompatibility, HNS is a kind of austenitic stainless steel, but in its novel composition design the austenitizing element nickel has been replaced by nitrogen to avoid the allergy, infection and teratogenicity problems caused by the nickel-based austenitic stainless steels. Fujiu et al. [3] found significantly less neointima formation and inflammation in arteries implanted with Ni-free stents compared to SUS316L stents. While notably, Ni2+ was eluted into the medium from SUS316L but not from Ni-free stainless steel. Ren et al. [4] compared the platelet adhesion characteristics of nickel-free and conventional 316L stainless steel and found that nickel-free stainless steel has better anti-platelet adhesion performance than 316L stainless steel, which can reduce the probability of thrombosis to some extent. In terms of corrosion resistance properties, the pitting corrosion has a great impact on the safety of implants. Studies have found that cold deformation reduces the pitting corrosion resistance of stainless steel materials and increases the risk of fracture failure [5]. However, for HNS with low nitrogen content, cold deformation significantly reduced the pitting corrosion resistance of nickel-free stainless steel [6]. But with the increase of nitrogen content, the influence of cold deformation on pitting corrosion resistance becomes less obvious [7,8], thus implying that HNS stents can effectively reduce the risk of fracture failure. Hence, HNS stent has good clinical application prospect due to its many advantages.
Generally, being a bioinert metal device, stent implantation could lead to the foreign body reaction. In order to reduce such side-effect, the drug-eluting stents have been developed and used in clinic as a kind of targeted drug delivery medical devices. Since, the targeting place for a drug-eluting stent is the coronary artery. The active drug released from the external surface of the stent will directly act on the contact artery wall, and the drug released from the internal surface of the stent will mix into the blood to participate in blood circulation. Pharmacokinetic analysis is a standard method to evaluate the drug release behavior, drug distribution, and metabolism in vivo. There are many reports about the pharmacokinetic analyses of coronary artery stents with drug-release coating. Paclitaxel, sirolimus, and derivative of sirolimus have been verified to be clinically useful to prevent in-stent restenosis (ISR) and have been used worldwide [[9], [10], [11], [12]]. One study defined the initial in vivo systemic pharmacokinetics of sirolimus-eluting stent in a porcine coronary modal for the implantation period of 30 days [13]. It was concluded that the peak sirolimus level in the tissue occurred in one day while the peak level in blood occurd at 60 min. Besides, arteries and myocardial tissue adjacent to or distant from the implanted stent contained much less sirolimus than the stented vessel. Sirolimus-eluting stents can achieve effective local drug delivery to the target lesion with minimum drug concentration in the surrounding tissue. Preclinical safety and pharmacokinetics of the NEVO™ stent were also evaluated on single stent implant for 60 days [14]. It was shown that more than 90% sirolimus eluted from the stent and sirolimus levels in blood, myocardium, and peripheral organs declined below the quantification limit at 60-day implantation. To evaluate the pharmacokinetics and safety of the Zilver PTX drug-eluting stent (Cook Medical, Bloomington, Indiana) in an average porcine artery model, 18 pigs were implanted [15]. Drug residuals on the stent, artery wall, adjacent and downstream muscle, and plasma were detected during 56-day implantation. It was found that almost 95% of the total paclitaxel was delivered within 24 h after deployment because of the drug coating without polymer. However, there was sustained drug in the artery walls after 56-day implantation, and very little paclitaxel was distributed regionally or systemically, while it became undetectable in plasma within 10 h. Ma et al. studied the pharmacokinetic behavior of the stent with paclitaxel and sirolimus double drug coating during 21-day implantation [16]. The data suggests that paclitaxel and sirolimus can be combined in a DES pharmacokinetically for the treatment of coronary arterial diseases. It is noteworthy that the pharmacokinetics study is essential before pre-clinical study for drug eluting stent, but all the previous studies on the time of pharmacokinetics are shorter than 60 days.
The properties of the drug-eluting coated stent are important determinant to control the drug release behaviors in vivo. In our previous study, it was shown that the polymer/drug ratio could affect the drug released profile of the coating [17]. Based on the above studies, a sirolimus-eluting coating was prepared on a HNS stent in this study. Standard analyses were adopted to evaluate the pharmacokinetics of sirolimus-eluting stent in porcine arteries like the previous reports in literature, i.e., the drug concentration in plasma, artery wall, myocardium and primary organs. It is worth mentioning that an extended research period, i.e., for 120 days was set in order to obtain more reliable results. Furthermore, both restenosis rate and inflammatory grade of sirolimus-eluting stent made of HNS in vivo were studied to assess the preclinical safety of the drug-eluting stent in this study.
2. Materials and methods
2.1. Materials
In this study, the metal stent platform was made of a novel metal- HNS mini-tube by using a laser cutting machine (Model micro T15F-300, Swisstec Corporation, Switzerland). The metal stent platform was coated with a drug-eluting coating which was composed of sirolimus and poly lactic-co-glycolic acid (PLGA, MW = 100,000, D,l-lactide:glycolide = 75:25) and served as the drug carrier. Sirolimus was purchased from North China Pharmaceutical Co., Ltd., while PLGA was purchased from LACTEL Absorbable Polymers. All the coating materials were permitted for clinical use. Both sirolimus and PLGA were dissolved in 1% acetone solvent (purchased from Sinopharm Group Co., Ltd.) where the ratio of PLGA over sirolimus was 1.5:1. The sirolimus-eluting coating was prepared by using a MediCoat DES 1000 Benchtop Stent Coating System (Sono-Tek Corp., Milton, NY) under a clean hood environment. The drug loaded on each stent was about 100 μg/cm. All stents were sterilized with ethylene oxide before implantation.
2.2. Characterization and drug release behavior in vitro
The surface morphology and the coating thickness of the prepared drug-eluting stent were observed by scanning electron microscope (Model S–3400 N, Hitachi Limited, Tokyo, Japan). To detect the coating thickness of drug-eluting stent, it was embedded in epoxy resin after applying a gold spray and then was grinded along the cross section of the stent wire.
The purpose to study drug release behavior in vitro was to support the drug release data in vitro and set up the incidence relation between drug release behaviors in vivo and in vitro. During the in vitro drug release experiment, drug coated stents were immersed in PBS solution with 3% FBS (fetal bovine serum) for 2 h and 1, 3, 5, 7, 14, 28, 60, 90, 120, and 180 days, respectively. At each set point, three stents were taken out to detect the residual sirolimus on the stent, and calculated their drug release rate according to the following formula. The test method was the same as reported in our previous work [8].
2.3. Pharmacokinetics study in vivo
The purpose of pharmacokinetics study in vivo was to evaluate release, distribution, and metabolism of the drug during the stent implantation in porcine coronary arteries for 120 days. In this study, there were eight set of time points (1, 3, 7, 14, 30, 60, 90 and 120 days) after implantation while six stents were implanted in 2–3 porcine at each time point. After 0.5, 1, 2, 3, 6, 12 h and 1, 3, 7, 14, 30, 60, 90, 120 days implantation, the venous blood was drawn to detect the sirolimus plasma concentration by high-performance liquid chromatography-mass spectrometry (HPLC-MS, Shimadzu, Shimadezu LC-30AD). Moreover, the porcines with stent implantation were sacrificed at 1, 3, 14, 30, 60, 90, 120 days to detect the sirolimus concentration in the stented vessel, stent, myocardium and vital organs (lung, liver, spleen and kidney) to learn about the drug distribution and metabolism of sirolimus-eluting stent on organs. During this test, one standard curve was built in each batch analyses, which contained no less than six standard concentration points and the accompanied quality control (QC) samples. Among them, the accuracy of more than 3/4 concentration points in the standard curve was within 85–115%. Whereafter, the standard curve was used to calculate the drug concentration of determinants. The research plan was approved by IACUC of Huizhi yinghua medical technology research and development (Shanghai) Co., LTD.
According to the individual concentration-time data, using the actual sampling times, the following pharmacokinetic parameters were derived from the bioanalytical results of sirolimus: Tmax, the time peak concentration (day); Cmax, the peak blood concentration (nanogram per milliliter); AUC, the area under the concentration-time curve (nanogram hour per milliliter).
2.4. Tissue response study in vivo
In this part of the study, the preclinical safety of HNS made sirolimus-eluting stent was studied in vivo. The research plan was approved by the animal ethics committee of Shanghai zhongshan hospital affiliated to fudan university. Besides, a bare HNS stent, a bare 316L stent (SUN 316L stainless steel bare stent system fabricated by Sun Technology Inc) and a commercial sirolimus-eluting stent (Helios drug-eluting stent system fabricated by Kinhely biotechnology Co., Ltd.) were used as control group. Stents from two different groups were implanted in one porcine for contrast. There were five time points (14, 30, 90, 180 and 360 days) and six stents were implanted in three porcine at each time point. The stented coronary arteries for each group were fixed in 4% formaldehyde, which were then embedded in methyl-methacrylate (Technovit 7200, Kulzer, Germany). Cross-section slices of 50 μm in thickness were cut at 10 mm below the greater trochanter by using a cutting-grinding system (Exakt, Norderstedt, Germany). The sections were stained by hematoxylin-eosin staining to analyze the restenosis rate and inflammation score of the stents in each group.
The microimage analyzer (leica DM 2500) was used to detect the following indicators: residual lumen area, internal area of internal elastic plate, internal area of external elastic plate, area of neointima (internal area of internal elastic plate - area of residual lumen), area stenosis percentage (area of internal intima/internal area of internal elastic plate). Besides, Inflammation score is the sum of inflammation scores at each scaffold/the number of scaffolds.
2.5. Statistical analysis
All values were expressed as mean ± standard deviation. Paired T test was used for each group. SPSS 20.0 statistical software was used to analyze the data. P < 0.05 was considered statistically significant.
3. Results
The drug-eluting stent is a leading way to treat coronary heart disease. As an ideal local drug delivery platform, the drug on stent could release controllably to inhibit the vascular smooth muscle proliferation and reduce the restenosis risk after stent implantation. It was reported that the vascular smooth muscle proliferation began after drug-eluting stent was implanted for 24–48 h and reached the highest proliferation rate at 10–14 days [18]. To obtain the best curative effect, the concentration of released drug should be kept at an optimal level. As an immunosuppressant, sirolimus is used to cure the rejection reaction after organ transplantation. In one report, a 6 mg single-dose oral sirolimus was used for the first time with a subsequent dose of 2 mg/day, which kept the blood concentration at the range of 10–20 ng/ml [19]. The side effect of sirolimus is dose-dependent as shown in many clinical tests. In this study, the drug concentration of 100 μg/cm was used on each stent, which means that, the longest duration drug eluting stent has a total drug load quantity of 340 μg. Therefore, owing to the lower amount of drug release each day, the drug elution of HNS drug eluting stent is even less than the oral dose. Therefore, HNS sirolimus-eluting stent should be safe since the total body exposure of sirolimus was remarkably lower than the oral dose. For the sirolimus eluting stent, delay of endothelialization is a serious side effect after implantation in lots of clinical applications [20]. In this study, the pharmacokinetic and the tissue response are studied to evaluate the bio-safety and effectiveness of HNS sirolimus-eluting stent, especially endothelialization.
3.1. Drug eluting coating properties
3.1.1. Surface morphology and coating thickness
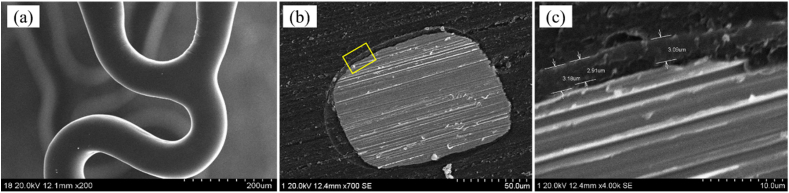
Fig. 1 shows the surface morphologies and the thickness of sirolimus eluting coating on the HNS stent. As shown in Fig. 1(a), the surface morphologies are smooth, uniform and with out any adhesion at the corners. The cross-section of coating is shown in Fig. 1(b) and (c), which shows the coating thickness is about 2.91–3.18 μm and is relatively uniform.
Fig. 1.
SEM images showing the surface morphologies and coating thickness of stent.
3.1.2. Drug release in vitro
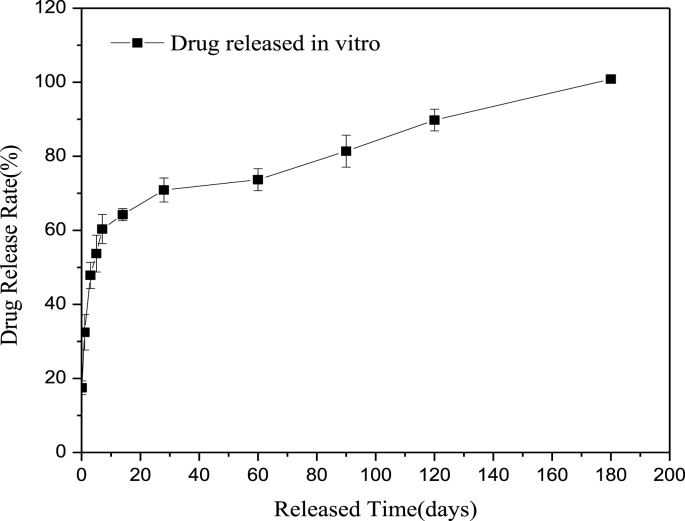
Fig. 2 shows the drug release profile of the HNS sirolimus-eluting stent after immersion in PBS for a set period. The result displayed that the drug release rate was faster and the cumulative drug release was approximately up to 50% after 3-days immersion. Whereafter, the drug release rate decreased gradually, releasing about 90% at 120-days immersion and all at 180 days.
Fig. 2.
In vitro release profile of sirolimus from PLGA coated HNS stents in PBS.
3.2. Pharmacokinetics in vivo
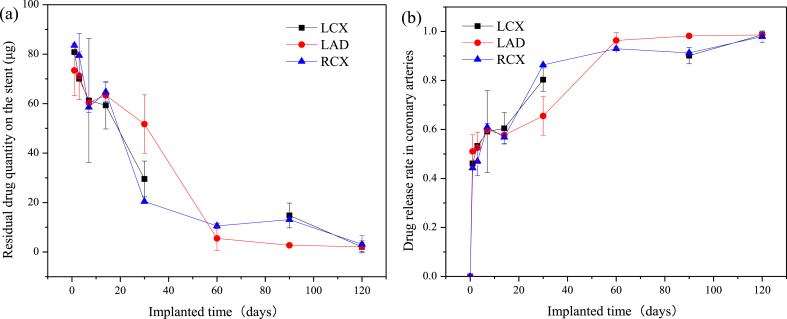
The residual sirolimus on the HNS sirolimus-eluting stent was detected after implantation, the drug residual quantity profile is shown in Fig. 3 (a). It can be seen that there are three drug residual quantity profiles, respectively, representing the stents implanted in LCX, LAD and RCA. Moreover, there is no significant difference in the residual drug quantity among the stents implanted in different coronary arteries. According to the total sirolimus on the stent and the calculated formula of drug release rate, the drug release rate profiles of the stents implanted in LCX, LAD and RCA arteries were obtained, as shown in Fig. 3 (b). After 1 day implantation, the drug release rate almost reached 50% (70–80 μg remaining). Whereafter, the drug release rate decreased gradually, releasing about 90% (5–15 μg remaining) at 60-days implantation and all at 120-days implantation. The pharmacokinetic parameters of HNS sirolimus eluting stents implanted in different arteries were calculated and are list in Table 1. The Tmax of sirolimus released from the stent was 1-day after implantation in different arteries. The Cmax values of implanted stents were 80840.00, 73410.00 and 83560.00 μg/L; the mean AUC values were 3562470.00, 3157885.00 and 2537040.00 μg·d/L, respectively, in LCX, LAD and RCX. Terminal half-life values varied with the ability to quantify sirolimus residual on the stents were 40.76, 21.60 and 25.19 days, respectively, in LCX, LAD and RCX.
Fig. 3.
Drug release profiles, (a) residual drug quantity on the stent, (b) drug release rate in different coronary arteries.
Table 1.
Pharmacokinetic parameters of sirolimus rested on the stents after implantation.
| Parameter (Unit) | LCX | LAD | RCA |
|---|---|---|---|
| AUC (ug·d/L) | 3562470.00 | 3157885.00 | 2537040.00 |
| Tmax (d) | 1.00 | 1.00 | 1.00 |
| Cmax (ug/L) | 80840.00 | 73410.00 | 83560.00 |
| t1/2 (d) | 40.76 | 21.60 | 25.19 |
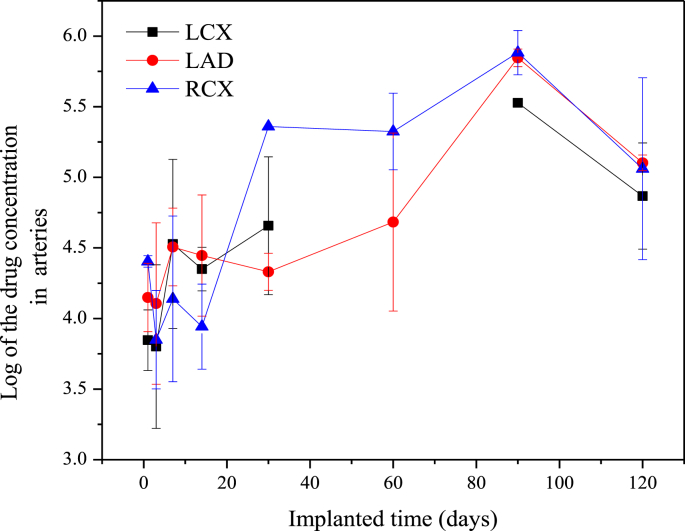
After implantation of the HNS sirolimus-eluting stent, the sirolimus concentration in different adjacent tissue was detected. The first detected position was coronary artery, which was the nearest tissue and the target position for a drug-eluting stent. The Log of the sirolimus concentration of the stented coronary arteries (LCX, LAD and RCX) are shown in Fig. 4, and the pharmacokinetics data are list in Table 2. There was a high drug concentration in the coronary arteries after 1-day implantation that kept the same level for 14 days. Then the drug concentration in the coronary arteries increased gradually and peaked at 90 days implantation. So the Tmax was 90 days and the corresponding Cmax were 187705.50, 211933.73 and 249354.57, respectively, in LCX, LAD and RCX. Whereafter, the drug concentration in the coronary arteries declined rapidly, however, there was also a large amount of residual drug in the arteries at 120-days implantation. Furthermore, there was no significant difference in drug release rate among the stents implanted into LCX, LAD and RCA.
Fig. 4.
Drug concentration in the stented coronary arteries.
Table 2.
Pharmacokinetic parameters of sirolimus accumulated in different coronary arteries after implantation.
| Parameter (Unit) | LCX | LAD | RCA |
|---|---|---|---|
| AUC (ug·d/L) | 9804359.44 | 10018602.25 | 12327649.70 |
| Tmax (d) | 90.00 | 90.00 | 90.00 |
| Cmax (ug/L) | 187705.50 | 211933.73 | 249354.57 |
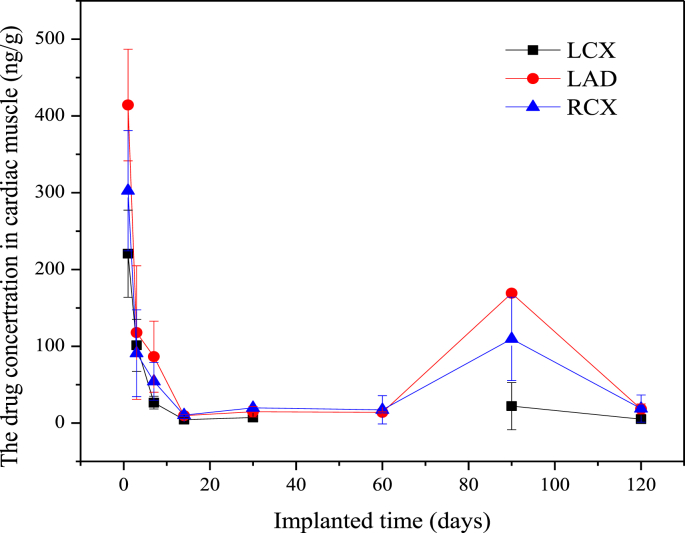
As shown in Fig. 5, the drug concentration in cardiac muscles near the stented coronary arteries reached to the highest level after 1-day implantation. After that, the drug concentration declined gradually during the first 14-days implantation and remained at a lower level until 60 days. Then the drug concentration in cardiac muscles quickly rose up to another peak value at 90-days implantation, and the drug in cardiac muscles metabolized gradually soon afterward. There was no significant difference in drug concentration among different coronary arteries. According to the above pharmacokinetics data and the trend curve, the pharmacokinetic parameters were calculated and are list in Table 3. As listed, the Tmax was 1-day in cardiac muscle, but was 90-days in heart. The AUC in cardiac muscle was higher than the heart. The Cmax was 205.07 (LCX), 218.20 (LAD) and 235.85 (RCA), respectively, which was higher than the Cmax in heart. The t1/2 was 39.82, 22.61 and 17.19, in LCX, LAD and RCA cardiac muscle, respectively.
Fig. 5.
Drug concentration in the cardiac muscles near the stented coronary arteries.
Table 3.
Pharmacokinetic parameters of sirolimus accumulated in cardiac muscles and heart tissue after implantation.
| Parameter (Unit) | LCX cardiac muscles | LAD cardiac muscles | RCA cardiac muscles | Heart |
|---|---|---|---|---|
| AUC (ug·d/L) | 2732.74 | 4157.90 | 7159.28 | 2183.60 |
| Tmax (d) | 1.00 | 1.00 | 1.00 | 90.00 |
| Cmax (ug/L) | 205.07 | 218.20 | 235.85 | 56.50 |
| t1/2 (d) | 39.82 | 22.61 | 17.19 | 26.46 |
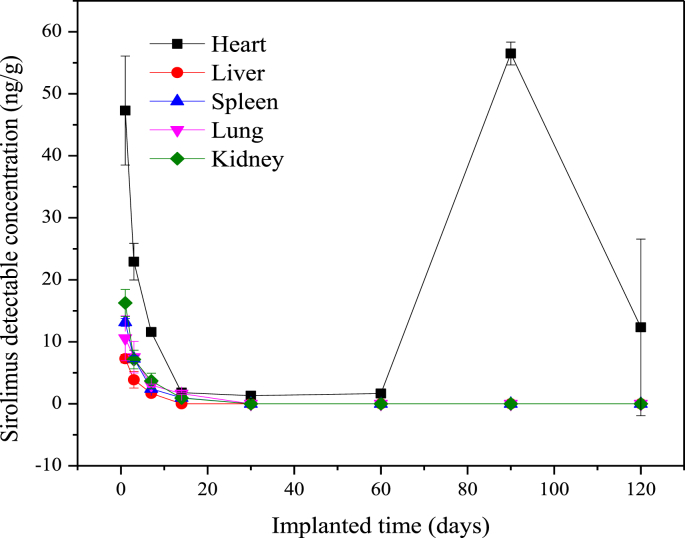
The distribution tendencies of the drug concentration in visceral organs after drug-eluting stents implantation are shown in Fig. 6. It can be seen that the drug concentration in each visceral organ rose up to the highest level after 1-day implantation, followed by a declination of drug concentration in each organ. There was only a trace drug concentration residue in the organs after 14-days implantation, and the drug concentration was below the detectable limit (BDL, 0.2 ng/ml) after stents implantation of 30 days. However, the drug concentration in the heart rose up to another peak value after 90-days implantation and then metabolized gradually. The distribution tendency of the drug concentration in the heart was same as those in coronary arteries and cardiac muscles with different drug concentration levels.
Fig. 6.
Sirolimus concentration in visceral organs after drug-eluting stent implantation.
The average drug concentration in plasma was detected after implantation of the HNS sirolimus-eluting stents, is shown in Table 4. It is demonstrated that the sirolimus concentration in plasma was lower than the detectable limit 0.2 ng/ml, denoted as BLLOQ.
Table 4.
Average drug concentration in plasma after sirolimus-eluting stents implantation.
| Implantation time | Average drug concentration in plasma (ng/g) |
|---|---|
| Pre-implantation | BLLOQ |
| 0.5h | BLLOQ |
| 1h | BLLOQ |
| 2h | BLLOQ |
| 3h | BLLOQ |
| 6h | BLLOQ |
| 12h | BLLOQ |
| 1d | BLLOQ |
| 3d | BLLOQ |
| 7d | BLLOQ |
| 14d | BLLOQ |
| 30d | BLLOQ |
| 60d | BLLOQ |
| 90d | BLLOQ |
| 120d | BLLOQ |
3.3. Histological morphology and quantitative analysis
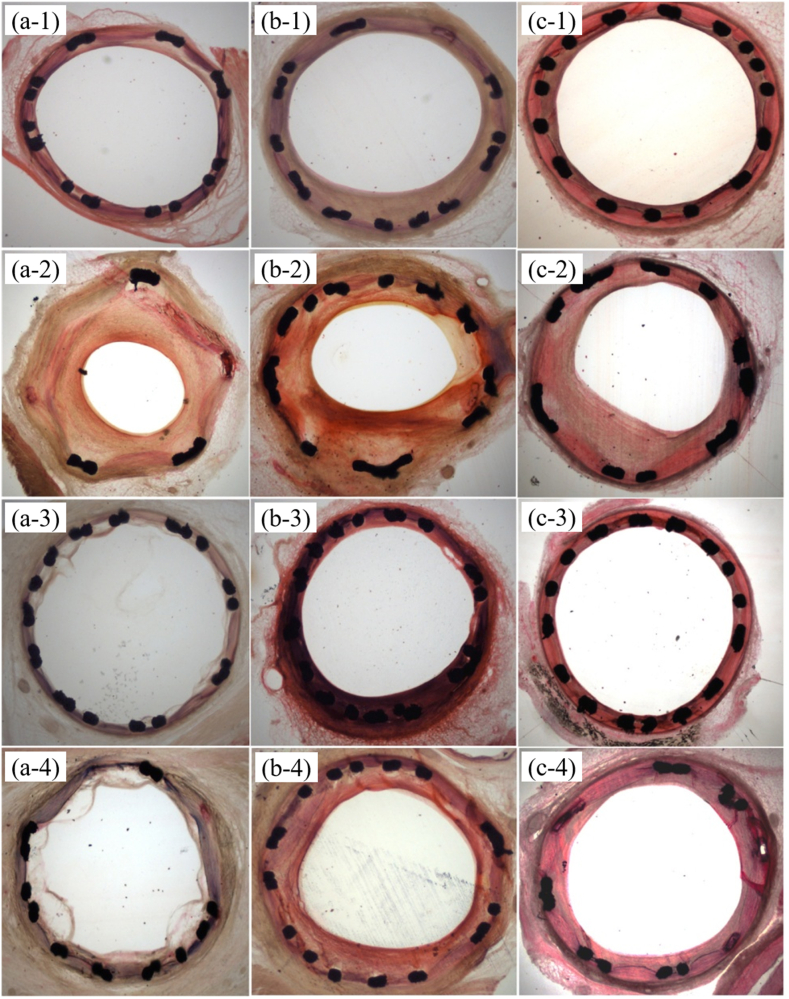
Fig. 7 shows the HE stain morphologies of each stent group, where Figs. 7(a-1- a-4) is the HE stain morphologies of four stent groups after 14-days implantation. It can observe that the Lumen stenosis occurred in the coronary arteries implanted with 316L bare stent. Two group of drug eluting stents were all exposed to the blood but only HNS bare stent was covered partly by neointima. Figs. 7(b-1- b-4), shows the HE stain morphologies of four stent groups after 30-days implantation. It can found that the lumen stenosis phenomenon for the 316L bare stent implanted coronary arteries continued up to 90 days (Fig. 7(c-1-c-4)). The two groups of drug eluting stents inhibited intimal hypertrophy, in addition, the neointima thickness was moderate after HNS bare stent implantation for 30 and 90 days.
Fig. 7.
HE stain morphologies of each stent group after different implantation times; (a-1 to a-4) after 14-days implantation, (b-1 to b-4) after 30-days implantation, (c-1 to c-4) after 90-days implantation.
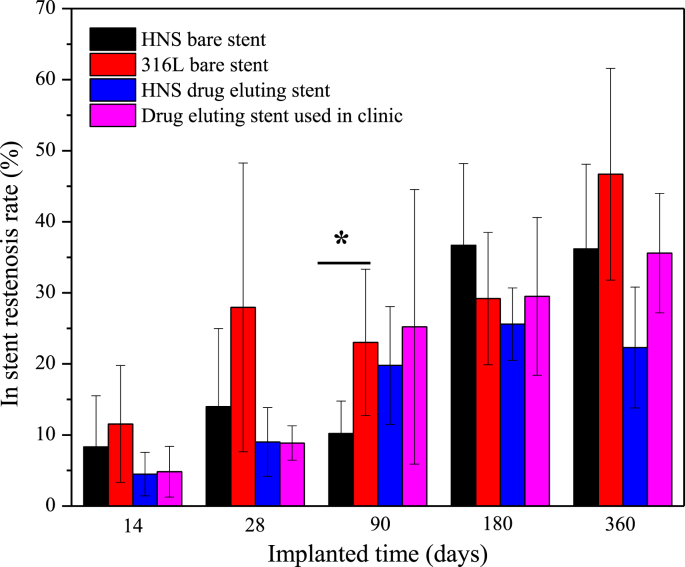
Fig. 8 shows the results of the restenosis rate of each stent group after different implantation times. Except the implantation for 90 days, the mean restenosis rates of both drug-eluting stent groups were lower than those of both bare stent groups, i.e., the mean restenosis rate of the HNS drug-eluting stent group was slightly lower than that of the control drug-eluting stent group, and the mean restenosis rate of the HNS bare stent group was slightly lower than that of the control bare stent group. The variation trend in restenosis rate of each group increased with prolonging the implantation time. However, there was no significant difference among the four groups at each implantation time.
Fig. 8.
Restenosis rate of each stent group after different implantation times, * indicates a significant difference.
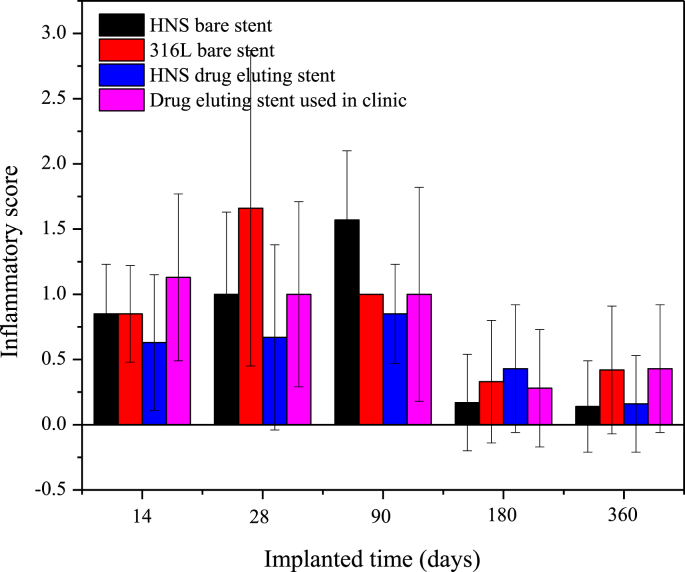
Fig. 9 shows the results of the inflammatory score of each stent group after different implantation times. Except for the 180-days implantation point, the mean inflammatory score of the HNS drug eluting stent group was lower than those of the other three control groups. After 360-days implantation, the mean inflammatory scores of the HNS (bare and drug eluting) stent groups were lower than those of the control groups. However, there was no significant difference among the three groups at each implantation time.
Fig. 9.
Inflammatory score of each stent group after different implantation time.
4. Discussion
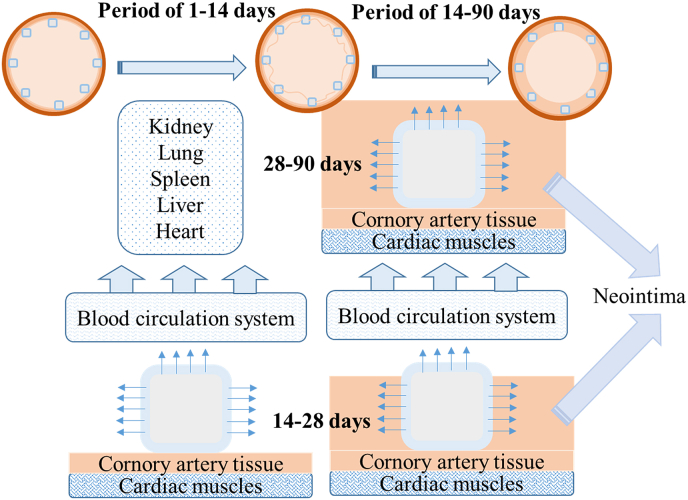
This study obtained many results, revealing the drug release behavior and the drug delivery path in different organs and tissues after implantation of the HNS sirolimus-eluting stent in the coronary artery. Combined with the coronary artery tissue response to this drug-eluting stent, a schematic of the drug delivery path was proposed and is shown in Fig. 10. It can be inferred from this study that there were two stages for the drug release, and the first one was from 1 to 14 days of implantation period. After the HNS sirolimus-eluting stents were implanted into the coronary arteries, a large mass of sirolimus diffused into the blood circulation system and a fraction of sirolimus spread to the surrounding coronary artery tissues. It was revealed that the drug release quantity in plasma was lower than the detection limit of high-performance liquid chromatography-mass spectrometry (HPLC-MS), as shown in Table 1, the reason for which is discussed below. It has been reported that only 6% sirolimus was distributed in plasma, while most of it was distributed in the red blood cells [21]. Since the detectable limit of HPLC-MS is 0.2 ng/ml, the sirolimus concentration in the whole blood may be lower than 3.33 ng/mL after conversion from sirolimus concentration in plasma. Fig. 5 shows the sirolimus distribution in heart, liver, lung, spleen, and kidney after 1-day implantation, which shows that the sirolimus concentration is at its peak. This result illustrates that the maximum amount of sirolimus was diffused into the blood at the initial implantation stage and was deposited into organs through blood circulatory system. The comparison of drug concentration in cardiac muscles with other organs shows the same tendency in the initial implantation stage (1–14 days) before the stent was covered by neointima. Another delivery path for sirolimus was spreading to coronary arteries by interorganal diffusion. The sirolimus concentrations in the coronary arteries (LCX, LAD, and RCX) with stenting are shown in Fig. 4. It is revealed that the sirolimus concentrations in the coronary arteries remained at the same level during the first 14-days implantation. The restenosis rate of the HNS sirolimus eluting stent was at the same level as the drug eluting control group, but lower than the bare metal control groups. This indicates that the HNS sirolimus-eluting stent could improve the inhibition of restenosis at the initial implantation stage.
Fig. 10.
Schematic of the drug delivery path after the implantation of HNS sirolimus-eluting stent.
The 14–90 days implantation period was regarded as second stage. Fig. 6 shows that no sirolimus was detected in the organs after 28-days implantation, in other words, only a small amount of sirolimus diffused into the blood circulation system, and sirolimus in organs was completely metabolized. It was observed that the stent was partly covered by neointima till 28-days implantation which means slightly released drug diffused into the blood. Besides, Fig. 4 shows that the sirolimus concentration in the surrounding coronary arteries increased gradually and peaked at 90 days, indicating that the drug accumulated in the stented coronary arteries after they were covered by neointima. There was the same sirolimus concentration tendency in coronary arteries, cardiac muscles and heart owing to the drug's gradient distribution and tissue exchange. In many previous studies, the drug eluting stent implantation periods were lasted for 30 days or 60 days to assess the pharmacokinetics of drug-eluting stent. It was indicated that the peak sirolimus in tissue occurred in one day [13]. However, the sirolimus level in other tissues peaked at 90 days in this study, revealing that the sirolimus-eluting stents were covered by the neointima after stent implantation. This is a highlight of this investigation on the pharmacokinetics of drug-eluting stent, indicating that the sirolimus eluting stent in this study did not induce delay in endothelialization. After 90-days implantation, almost entire drug released from stents, and the accumulated drug in the surrounding tissues diffused into the blood gradually showing that the drug concentration decreased in coronary arteries, cardiac muscles and heart. Compared with the results obtained from the tissue response test, the mean restenosis rate of HNS sirolimus-eluting stent group was lower than other three groups during implantation which indicates that sirolimus enriched in surrounding coronary artery tissue was high enough to inhibit the restenosis. Furthermore, the HNS bare-metal stent exhibited excellent performance than the other groups at 90-days implantation. Compared with 316L stainless steel and CoCr Alloy, HNS is a kind of austenitic stainless steel where nitrogen is used as the austenite stabilizing element instead of nickel. Hence, for HNS stents, due to absence of nickel, the restenosis rate induced by nickel element could be lower. In addition, the blood compatibility of HNS is better than conventional metal stent platform thus has lower thrombosis risk. Therefore, the novel HNS has great potential to be used as an artery stent platform.
In this study, HNS sirolimus-eluting stents achieved effective local drug delivery to target lesion with minimum drug concentration in surrounding tissue, demonstrating that the drug release tendency and drug distribution of the sirolimus-eluting stent in this study could meet the expected curative effect.
5. Conclusion
The drug elution and distribution behavior of HNS drug eluting stents were studied in the pharmacokinetics assessment. According to the data, a large amount of drug eluted into blood circulation system and distributed in to vital organs through blood circulation at the initial (1–14 days) implantation stage. Increasing amount of drug was distributed in the coronary artery tissues and neighboring cardiac muscle tissues as the drug eluting stent covered by neointima gradually at the 14–90 days implantation. Prolonging the implantation period was a highlight of this study, where the other drug peak was obtained to illustrate the endothelialization after implantation of drug-eluting stents. In the preclinical safety evaluation, we conclude that HNS sirolimus-eluting stent showed non-inferiority statistical to the drug eluting stent used in clinic (control group). All the data indicate that the HNS sirolimus-eluting stent is bio-safe and effective for clinical application.
CRediT authorship contribution statement
Shanshan Chen: Conceptualization, Writing - original draft, Writing - review & editing. Zhifeng Yao: Investigation, Writing - original draft. Yongbiao Guan: Project administration. Hui Yang: Investigation. M. Babar Shahzad: Writing - review & editing, Funding acquisition. Yizhe Wu: Investigation. Bingchun Zhang: Project administration, Funding acquisition. Li Shen: Project administration, Funding acquisition. Ke Yang: Conceptualization, Project administration.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgments
This work was financially supported by National Key Research and Development Program of China (NO.2016YFC1102404 and NO.2016YFC1102405), Chinese Academy of Sciences President's International Fellowship Initiative (Grant No. 2018FYE0005) and National Natural Science Fund of China (81670319, 81521001).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Yongbiao Guan, Email: guanyb@hotmail.com.
Li Shen, Email: shen.li1@zs-hospital.sh.cn.
Ke Yang, Email: kyang@imr.ac.cn.
References
- 1.Ren Y.B. Nickel free alloy for cardiovascular stents application against restenosis associated with nickel: a review. Sci. Adv. Mater. 2020;12:44–55. doi: 10.1166/sam.2020.3725. [DOI] [Google Scholar]
- 2.Misawa T., Tanabe H. In-situ observation of dynamic reacting species at pit precursors of nitrogen-bearing austenitic stainless steels. ISIJ Int. 1996;36:787. [Google Scholar]
- 3.Fujiu Katsuhito, Manabe Ichiro, Sasaki Makoto, Inoue Motoki, Iwata Hiroshi, Hasumi Eriko, Komuro Issei, Katada Yasuyuki, Taguchi Tetsushi, Nagai Ryozo. Nickel-free stainless steel avoids neointima formation following coronary stent implantation. Sci. Technol. Adv. Mater. 2012;13 doi: 10.1088/1468-6996/13/6/064218. 10pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren Yibin, Yang Ke, Zhang Bingchun. In vitro study of platelet adhesion on medical nickel-free stainless steel surface. Mater. Lett. 2005;59:1785–1789. doi: 10.1016/j.matlet.2005.01.067. [DOI] [Google Scholar]
- 5.Ravi K.B., Mahato B., Singh R. Influence of cold-worked structure on electrochemical properties of austenitic stainless steels. Metall. Mater. Trans. A. 2007;38:2085. [Google Scholar]
- 6.Fu Yao, Wu Xinqiang, Han Enhou, Ke Wei, Yang Ke, Jiang Zhouhua. Influence of cold work on pitting corrosion behavior of a high nitrogen stainless steel. J. Electrochem. Soc. 2008;155:C455–C463. doi: 10.1149/1.2939213. [DOI] [Google Scholar]
- 7.Ren Yibin, Zhao Haochuan, Liu Wenpeng, Yang Ke. Effect of cold deformation on pitting corrosion of 00Cr18Mn15Mo2N0.86 stainless steel for coronary stent application. Mater. Sci. Eng. C. 2016;60:293–297. doi: 10.1016/j.msec.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 8.Wang Qingchuan, Zhang Bingchun, Ren Yibin, Yang Ke. Eliminating detrimental effect of cold working on pitting corrosion resistance in high nitrogen austenitic stainless steels. Corrosion Sci. 2017;123:351–355. doi: 10.1016/j.corsci.2017.04.006. [DOI] [Google Scholar]
- 9.Ma X.D., Oyamada S.Z., Gao F., Wu T., Robich M.P., Wu H., Wang X.W., Buchholz B., McCarthy S., Gu Z.Y., Bianchi C.F., Sellke F.W., Laham R. Paclitaxel/sirolimus combination coated drug-eluting stent: in vitro and in vivo drug release studies. J. Pharmaceut. Biomed. Anal. 2011;54:807–811. doi: 10.1016/j.jpba.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirtane Ajay J., Leon Martin B. Clinical use of sirolimus-eluting stents. Cardiovasc. Drug Rev. 2007;25:316–332. doi: 10.1111/j.1527-3466.2007.00024.x. [DOI] [PubMed] [Google Scholar]
- 11.Saia F., Lemos P.A., Hoye A., Sianos G., Arampatzis C.A., de Feyter P.J., van der Giessen W.J., Smits P.C., van Domburg R.T., Serruys P.W. Clinical outcomes for sirolimus-eluting stent implantation and vascular brachytherapy for the treatment of in-stent restenosis, Catheter. Cardiovasc. Interv. 2004;62:283–288. doi: 10.1002/ccd.20068. [DOI] [PubMed] [Google Scholar]
- 12.Cipollari Stefano, HiroyoshiYokoi TakaoOhki, KimihikoKichikawa MasatoNakamura, KimihiroKomori ShinsukeNanto, O'Leary Erin E., Lottes Aaron E., Saunders Alan T., Dake Michael D. Long-term effectiveness of the zilver PTX drug-eluting stent for femoropopliteal peripheral artery disease in patients with No patent tibial runoff vessels—results from the zilver PTX Japan post-market surveillance study. J. Vasc. Intervent. Radiol. 2018;29:9–17. doi: 10.1016/j.jvir.2017.08.014. e1. [DOI] [PubMed] [Google Scholar]
- 13.Ikeno Fumiaki. JACC; 2004. Sirolimus-Eluting Stents: Pharmacokinetics in Blood, Vessel, and Myocardium in a Porcine Coronary Model; pp. 1139–1149. [DOI] [Google Scholar]
- 14.Tzafriri R., Sun Y.P., Price S., Rogers C. Systemic and local tissue pharmacokinetics of single and overlapping NEVO™ sirolimus-eluting stents in the porcine coronary artery model. JACC (J. Am. Coll. Cardiol.) 2011;58:7–11. doi: 10.1016/j.jacc.2011.10.453. [DOI] [Google Scholar]
- 15.Dake M.D., Van Alstine W.G., Zhou Q., Ragheb A.O. Polymer-free paclitaxel-coated zilver PTX stents-evaluation of pharmacokinetics and comparative safety in porcine arteries. J. Vasc. Intervent. Radiol. 2011;22:603–610. doi: 10.1016/j.jvir.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Ma Xiaodong, Oyamada Shizu, Gao Fan, Wu Tim, Robich Michael P., Wu Hao, Wang Xingwei, Buchholz Bryan, McCarthy Stephen, Gu Zhiyong, Bianchi Cesario F., Sellke Frank W., Laham Roger. Paclitaxel/sirolimus combination coated drug-eluting stent: in vitro and in vivo drug release studies. J. Pharmaceut. Biomed. Anal. 2011;54:807–811. doi: 10.1016/j.jpba.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S.S., Tan L.L., Teng Y.X., Zhang B.C., Yang K. Study of drug-eluting coating on metal coronary stent. Mater. Sci. Eng. C. 2013;33:1476–1480. doi: 10.1016/j.msec.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 18.Lu Li, Lin Zhibin. A novel immunosuppressor-sirolimus. Chin. Pharmaceut. J. 2001;36:643–645. [Google Scholar]
- 19.Kahan B.D., Podbielski J., Napoli K.L., Katz S.M., Meier-Kriesche H.-U., Van Buren C.T. Immunosuppressive effects and safety of a Sirolimus/cyclosporine combination regimen for renal transplantation. J. Urol. 1999;161:2030. doi: 10.1016/S0022-5347(05)68892-1. [DOI] [PubMed] [Google Scholar]
- 20.Camenzind E., Wijns W., Mauri L., Kurowski V., Parikh K., Gao R., Bode C., Greenwood J.P., Boersma E., Vranckx P., McFadden E., Serruys P.W., O'Neil W.W., Jorissen B., Leeuwen F.V., Steg P.G. Stent thrombosis and major clinical events at 3 years after zotarolimus-eluting or sirolimus-eluting coronary stent implantation: a randomised, multicentre, open-label, controlled trial. Lancet. 2012;380:1396–1405. doi: 10.1016/S0140-6736(12)61336-1. [DOI] [PubMed] [Google Scholar]
- 21.Trepanier Daniel J., Gallant Heather, Legatt Donald F., Yatscoff Randall W. Rapamycin: distribution, pharmacokinetics and therapeutic range investigations: an update. Clin. Biochem. 1998;31:345–351. doi: 10.1016/S0009-9120(98)00048-4. [DOI] [PubMed] [Google Scholar]