Abstract
This article emphasizes the role of the technological progress in changing the landscape of epilepsy surgery and provides a critical appraisal of robotic applications, laser interstitial thermal therapy, intraoperative imaging, wireless recording, new neuromodulation techniques, and high‐intensity focused ultrasound. Specifically, (a) it relativizes the current hype in using robots for stereo‐electroencephalography (SEEG) to increase the accuracy of depth electrode placement and save operating time; (b) discusses the drawback of laser interstitial thermal therapy (LITT) when it comes to the need for adequate histopathologic specimen and the fact that the concept of stereotactic disconnection is not new; (c) addresses the ratio between the benefits and expenditure of using intraoperative magnetic resonance imaging (MRI), that is, the high technical and personnel expertise needed that might restrict its use to centers with a high case load, including those unrelated to epilepsy; (d) soberly reviews the advantages, disadvantages, and future potentials of neuromodulation techniques with special emphasis on the differences between closed and open‐loop systems; and (e) provides a critical outlook on the clinical implications of focused ultrasound, wireless recording, and multipurpose electrodes that are already on the horizon. This outlook shows that although current ultrasonic systems do have some limitations in delivering the acoustic energy, further advance of this technique may lead to novel treatment paradigms. Furthermore, it highlights that new data streams from multipurpose electrodes and wireless transmission of intracranial recordings will become available soon once some critical developments will be achieved such as electrode fidelity, data processing and storage, heat conduction as well as rechargeable technology. A better understanding of modern epilepsy surgery will help to demystify epilepsy surgery for the patients and the treating physicians and thereby reduce the surgical treatment gap.
Keywords: epilepsy surgery, high‐intensity focused ultrasound, intraoperative MRI, laser ablation, neuromodulation, robots
Key Points.
Robots are used increasingly for depth electrode implantation. They are drivers in the transition from SDEs (subdural electrodes) to SEEG (stereo‐electroencephalography) seen in many centers
Laser ablation has become an accepted minimally invasive alternative to hypothalamic hamartomas and mesial temporal lobe epilepsy
Intraoperative magnetic resonance imaging (MRI) guidance proved particularly useful for focal cortical dysplasia (FCD), long‐term epilepsy‐associated tumors (LEATS), and cavernomas
The advantages and disadvantages of closed and open‐loop neuromodulation systems need to be further investigated
High‐intensity focused ultrasound is on the horizon to allow for novel treatment paradigms
1. INTRODUCTION
The landscape of epilepsy surgery has changed considerably since the early days and since the first randomized clinical trial (RCT) demonstrated superiority of temporal lobectomy over medical treatment for refractory temporal lobe epilepsy. 1 Resective surgery remains the main type of procedure for refractory epilepsy, although a trend to move away from large, open, resective procedures, to minimally invasive, neuromodulatory, targeted disconnective, or ablative procedures has been noted over time. This change was driven mainly by recent technological advances made possible by a multiprofessional effort involving neurologists, neurophysiologists, neurosurgeons, engineers, and basic scientists. The modern epilepsy surgeon became increasingly armed with technical adjuncts that not only influence the surgical decision tree, but also the way the epileptogenic zone is targeted surgically. Robotic applications, lasers, wireless recording, intraoperative imaging, new neuromodulation techniques, and high‐intensity focused ultrasound are only a few examples highlighting why epilepsy surgery is becoming increasingly attractive. These developments, extensively discussed at the biannual “Epilepsy Surgery Techniques Meetings (ESTM), have the potential to further demystify epilepsy surgery for the patient and the treating physician. Ideally, this would contribute to decrease the time for patients' referral to a dedicated epilepsy program and to potentially reduce the surgical treatment gap. This review aims at giving an overview of these developments and at critically discussing their future potential.
1.1. Robots influence on selecting intracranial electroencephalography (EEG) applications
Subdural electrodes (SDE) placement through a craniotomy or burr hole has been the main approach for invasive electroencephalography (EEG) recordings in North America, the United Kingdom, Germany, and Austria for the past 2‐3 decades. 2 , 3 In contrast, evolving from Talairach's stereo EEG (SEEG) approach, the Montreal, French, and Italian schools advocated insertion of depth electrodes into the brain through twist drill holes. 4 , 5 Both schools coexisted for almost half a century. Recently, and quite abruptly, the approach using depth electrodes took over in many centers. This development has been driven largely by the emergence and the availability of stereotactic robots and three‐dimensional navigational platforms. This switch from SDE to depth electrode placement often occurred without regard to the fact that SDE and depth electrode techniques have specific advantages and disadvantages.
The perceived success of this transition could be explained by several factors. First, there was evidence that morbidity and complication rates for depth electrode procedures are lower than in SDE implantations. The reported complication rates after SDE implantations ranged from 5% to 17% per procedure, whereas the complication rate per SEEG procedure was less than 1%. 6 , 7 Second, the introduction of new radiological and computational innovations and a wide range of navigational image‐guided applications, in addition to new stereotactic methods, allowed for a more convenient and faster depth electrode insertion. 5 , 6 , 8 , 9 Numerous targets can now be reached in a simplified, more efficient and accurate fashion. Automated trajectory planning was developed to follow these needs. 9 Third, these advantages were translated to robotic applications aimed at further increasing the accuracy and improving the surgical workflow. 6
Three robotic systems are currently in use. The use of these robots varies significantly between centers, including differences in image acquisition, trajectory planning, patient positioning, head fixation, patient registration, and the implantation method itself. 10 All these steps might influence the overall workflow, the perceived accuracy, and surgical results. 11 The differences in the surgical workflow had their origin partly in the type of previous training and in the experience and surgical subspecialty of the user, rather than an objective factor. If one was trained as a stereotactic neurosurgeon, the neurosurgeon would be more prone to use a stereotactic frame with the robot; on the other hand, those not accustomed to stereotactic procedures would prefer to use a head clamp. Similarly, if one is used to navigational systems, the neurosurgeon would likely use the robot coupled with neuronavigation instead of using stereotactic coordinates.
Proponents of robotic applications argue that the overall accuracy is better when compared to manual methods. A recent meta‐analysis, which analyzed 13 different implantation systems including frameless, frame‐based, and robotic systems, suggested that robotic systems were at least noninferior to classic frame‐based systems and superior to frameless ones 12 , 13 (Figure 1). Robotic trajectory guidance systems had an entry point error (EPE) of 1.17 mm and a target point error (TPE) of 1.71 mm, compared to an EPE of 1.43 and 2.45 and a TPE of 1.93 and 2.89 for frame‐based and frameless systems, respectively. 12 However, those comparisons were performed based on retrospective frame‐based and frameless systems series, and there are no adequate comparisons between robotic trajectory guidance systems with other techniques or between robotic trajectory guidance systems themselves. It is unlikely, though, that robotic systems would be more accurate than modern frame‐based systems. Although rarely discussed, the choice of imaging for referencing and the referencing method itself affect the overall accuracy of a method considerably. Computerized tomography (CT) frame‐based referencing was shown to be the most precise method for determining entry points, 14 with an EPE below 1 mm, followed by CT laser referencing with an EPE below 2 mm, and finally, by magnetic resonance imaging (MRI) laser referencing with an EPE of about 3 mm. 15 Accordingly, previous studies also showed that fiducial‐based referencing was more accurate than surface referencing and that CT surface referencing was more accurate than using MRI. To minimize radiation exposure, especially in the pediatric group, 3T MRI proved to be adequate for most stereotactic procedures. 16 In addition, novel three‐dimensional (3D) computerized models can now further increase the accuracy and safety by automated vessel detection. 8
Figure 1.
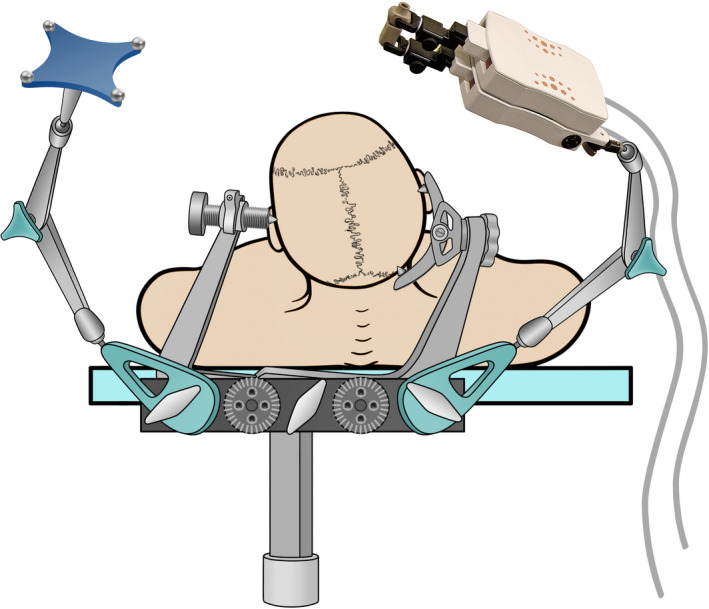
Illustration of a miniature robotic device used for implantation of depth electrodes with the help of neuronavigation. The head is fixed in a standard head clamp with the reference for the navigation and the robot attached to it via an adapter (for details please refer to reference 10)
The question remains, however, if this drive toward increased accuracy is justified and if an accuracy comparable to deep brain stimulation (DBS) procedures is at all necessary while planning for SEEG implantation. If the answer is no, then one of the main arguments to use robotic applications would be relativized. In contrast to DBS, the typical mean trajectory length for depth electrodes ranges between 4 and 5 cm, which is about half of the typical DBS trajectory. Furthermore, the common target for depth electrodes is far less defined. The accuracy needed for depth electrode implantation might depend on the type of trajectory planning. Strict orthogonal trajectories that cross sulci or the Sylvian fissure need higher accuracy compared to an implantation method planning trajectories that avoid crossing sulci. Longer trajectories for insular coverage need higher accuracy than the typical shorter ones to evaluate mesial or superficial cortex. A combination of depth and subdural electrodes would need different planning. The coverage of the frontal, parietal, and occipital mesial cortex can be accomplished both by interhemispheric subdural electrodes and longer orthogonal depth electrodes. These conceptual differences influenced the length of the trajectories, and therefore, the level of accuracy needed. The reported low complication rate with depth electrode use might reflect the fact that surgeons do well in anticipating the error margins of their applied method when planning the trajectories. 17
An argument often made in favor of robotic applications is that they might offer a decrease in global operative time and time needed per electrode. Indeed, the mean time per electrode using robotic applications was reported to be around 10 minutes, which was slightly faster than the reported 12‐15 minutes using manual neuronavigational techniques. 17 These reports, however, did not consider the pre‐procedural work‐up and the additional time in the operating theatre needed to use robotic applications. Taking this additional time into account might diminish the reported operative time saving. The actual operative time might be less dependent on technical aspects than on the team and infrastructure involved in the procedure. These factors are difficult to evaluate and rely on national, site‐specific regulations, and organizational and administrative processes as well. The abrupt change in invasive recording paradigm in many centers, favoring the use of SEEG over SDE, often disregarded the fact that when cortical mapping is needed, it is usually better achieved using SDEs. Therefore, both SEEG and SDEs should be available in each center, and patient‐based selection of the adequate technique is mandatory. In addition, robotic systems are expensive, and had been used mainly for SEEG implantation only. The system's pay‐back time is long, and although a drop in the hardware price could be expected over time, sometimes pay‐back would not be achieved. It would be reasonable to foresee that some institutions would not embark on purchasing the technology at this point, and especially so when there is a well‐established frame‐based stereotactic program.
1.2. Laser interstitial thermal therapy–expanding indications and patient selection
MRI‐guided laser interstitial thermal therapy (LITT, also called stereotactic laser ablation, or SLA) is an increasingly used method, which requires accurate trajectories. LITT is a minimally invasive surgical technique. 18 It takes advantage of three critical elements: stereotactic methods to position a laser probe precisely within a therapeutic target; time‐dependent thermal tissue ablation from a surgical laser system; and MRI thermography to monitor changes in temperature and tissue destruction in real time. 18 Two systems are available in the United States. Patients treated with LITT require a very brief hospital stay, pain is usually minimal, and recovery is often short. For these reasons, LITT is very appealing to patients.
The best evidence supporting the efficacy of LITT to treat patients with epilepsy with curative intent comes from the ablation of structurally well‐defined targets, including hypothalamic hamartoma and mesial temporal sclerosis. 19 , 20 , 21 As experience increases, LITT is also being used to treat a growing list of epileptogenic substrates, including focal cortical dysplasia (FCD), cavernous malformation, and lesions in challenging anatomic locations such as the insula and functionally eloquent cortex. 22 , 23 , 24 This increasing list of pathologies treated with LITT contrasts with the fact that there is no class I evidence comparing the efficacy and complication rates of LITT with those of standard surgical treatment. Furthermore, no specimen suitable for adequate pathological examination is obtained during LITT procedures. This would likely make it difficult to explore surgical outcome according to pathology and might make it more complicated to develop patient‐specific new pharmacogenetic or gene therapies in the future.
Hypothalamic hamartoma (HH) may be diagnosed by MRI with high accuracy without the need for histologic verification. Open and endoscopic surgical disconnection were associated with high morbidity and moderate efficacy. 19 LITT was introduced as a minimally invasive surgical option to treat HH and was rapidly adopted as the standard surgical technique for patients with HH in some centers (Figure 2). LITT for HH showed higher efficacy regarding seizure and behavior control and low complication rates. 25 Seizure freedom after laser ablation of HH ranged from 66% to 81% of the patients, with 20% of patients experiencing permanent morbidity. The largest series to date included 71 patients (age range 5 months‐20 years, 68% male) with gelastic seizures. Overall, 93% of patients were free of gelastic seizures at 12 months, although 23% of those patients required more than one LITT procedure. The rates of memory and endocrine complications observed were much lower than those published for open craniotomy and endoscopic approaches. This series encompassed the evolution of a new surgical technique through experience over time, and refinements included decreasing low‐temperature safety limit cutoffs and placing these heat constraints further away from normal structures to minimize the risk of thermal injury. 19 There is no study demonstrating that LITT would be superior to other stereotactic disconnection techniques, such as radiofrequency ablation, although results with LITT appear to be superior to those obtained using open techniques. The sharpness of the lesion's periphery obtained using LITT and its ability to use MR thermography, as well as a better understanding of the thermosensitivity of the lesion itself and of heat‐sinks in an area surrounded by cerebrospinal fluid (CSF) might prove critical for LITT superiority in the future.
Figure 2.
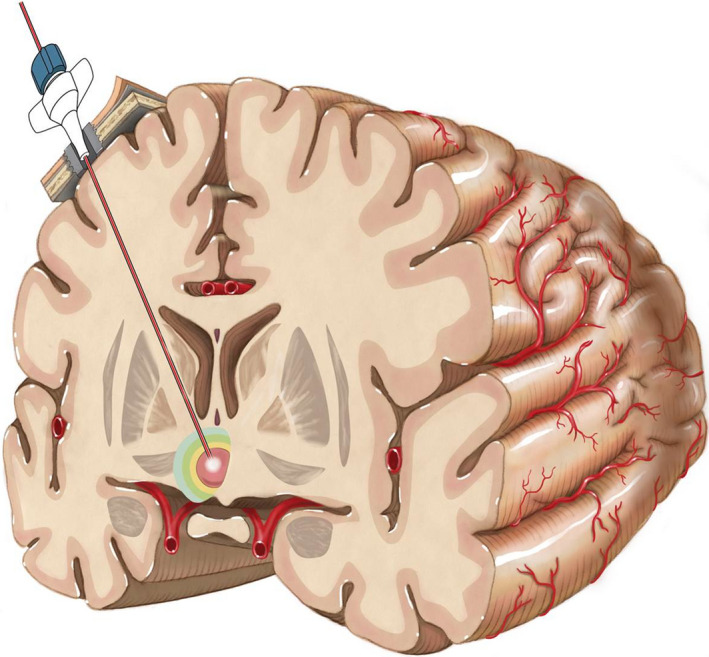
Laser interstitial thermal therapy (LITT) procedure. The laser probe is inserted into the targeted area via a fixation bolt using different stereotactic or navigational platforms. The approach for the treatment of a hypothalamic hamartoma is shown
Mesial temporal sclerosis is the second most common pathological substrate treated with LITT. The rationale for performing stereotactic hippocampectomy is not new. Radiofrequency stereotactic hippocampectomy had been performed over the last decades with variable results. 26 The anterior half of the hippocampus is usually included in the ablated volume using LITT. The issue of lack of any specimen for pathology is also often neglected in the literature but might represent a major burden in the future. Pathological findings were correlated with outcome regarding seizures 27 and memory in several studies, and pathological findings might be especially useful in patients with normal MRI. Punch biopsies are feasible during LITT procedures but would not preserve the regional 3D anatomy or that of the hippocampus 28 (Figure 3). Some reports, which included a small number of patients, suggested a better neuropsychological outcome after LITT, but adequate comparison to the other techniques is not available. 29 It is possible that these better cognitive findings would be related to the smaller amount of ablated tissue and incomplete disconnection during LITT when compared to open techniques. It is also unclear if cognitive preservation, if present, would come at the expense of a lower seizure control rate. The largest retrospective series to date on mesial temporal ablation using LITT comprised 234 patients and showed a 58.0% Engel class I outcome at both 1 and 2 years after LITT. 20 This outcome was worse than that reported after conventional open temporal lobe resections. 30 On the other hand, LITT procedures are much better tolerated; there is a low overall complication rate and a fast recovery. Long‐term results after LITT are not yet available. Long‐term Engel class I result decline over time after open temporal lobe resection. 31 It might be possible that higher recurrence rates might be seen after LITT, since LITT's ablation volume is smaller than that after open resection. 32 Complications after LITT might have been underreported in the literature, but that appears to compare favorably to open resection. The most common complication following LITT, as in open resection, are visual field cuts, occurring in about 5%‐29% of the patients. 33 , 34 , 35 Automated trajectory planning tools, including tractography data hold promise to aid in the attempt of preserving visual function. An ongoing prospective multicenter observational study (“Stereotactic laser ablation for temporal lobe epilepsy,” or SLATE) might begin to address many of these questions. 34 This study's recruitment started in December 2016 with an estimated enrollment of 150 participants by May 2022 (NCT02844465).
Figure 3.
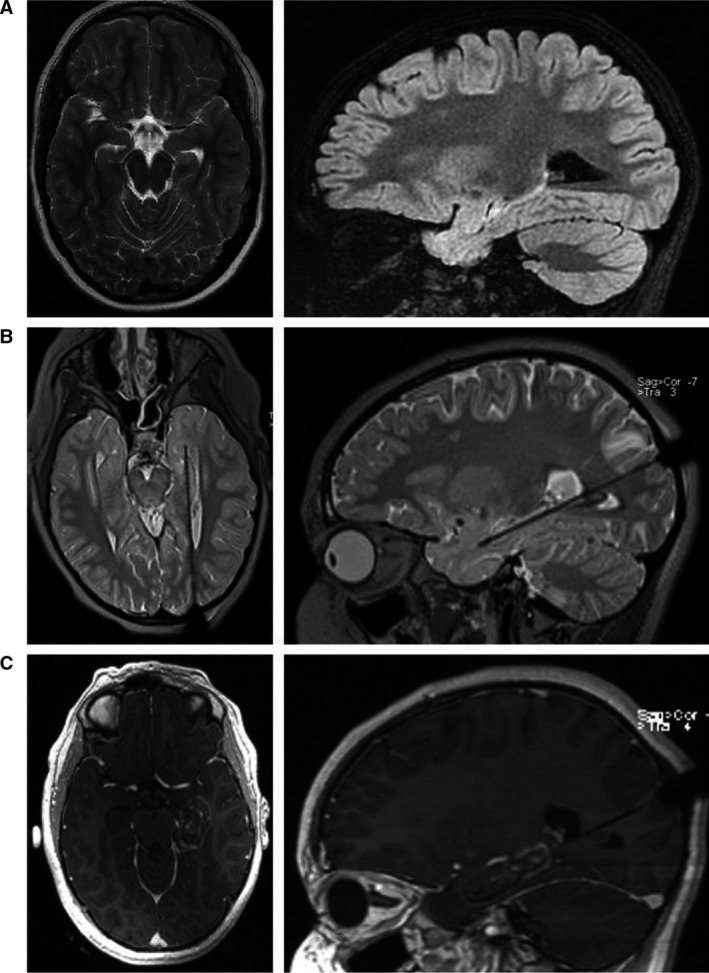
Laser interstitial thermal therapy (LITT) procedure. (A) Axial T2‐weighted and sagittal fluid‐attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) slices depicting left‐sided hippocampal sclerosis. (B) Laser probe inserted via an occipital approach along the long axis of the hippocampus. (C) Early T1 contrast‐enhanced MRI obtained after laser ablation showing the size of the lesion
Compared to other minimally invasive treatment options such as radiofrequency ablation and stereotactic radiosurgery, LITT has some advantages, such as real‐time image guidance, the potential for larger ablation volumes, and an immediate therapeutic impact without delayed risks. It is also easily coupled with other minimally invasive epilepsy surgery techniques, most notably robotic stereo‐encephalography. These advantages have led to the proliferation of LITT to treat a broad range of epileptic conditions, many of which have yet to generate sufficient evidence to determine efficacy. This includes hemispheric disconnections such as corpus callosotomy using multiple lasers, 36 lesional and nonlesional focal epilepsy in deep and functionally eloquent anatomic locations such as the insula, 37 and focal and multifocal epilepsy in patients with cavernous malformation 38 or tuberous sclerosis complex. 24
The short length of surgical recovery and low rates of morbidity associated with LITT generated significant enthusiasm in the epilepsy surgery community. It is possible that LITT could change the epilepsy surgery workflow. In such paradigm shift, a minimally invasive procedure (LITT) may seek to ameliorate seizures with a limited procedure. Recurrence would then lead to subsequent localization studies and further staged LITT procedures until seizure freedom would be obtained. Furthermore, open surgery can be easily performed after LITT. Increasing surgical experience with this technique coupled with a better understanding of patient selection, surgical targeting, and comparative therapeutic efficacy will better define the role for LITT in the surgical armamentarium for patients with drug resistant epilepsy in the future. LITT was until recently available only in the United States, and reached Europe in 2019, as shown by a recent report. 39 Consumables are expensive and the procedure is highly dependent on the presence of a manufacturer's technician, even in experienced centers. It is likely that some health systems would delay implementation of the technique until adequate cost‐effectiveness analysis is performed. Issues related to the MR interface or the probe itself are often reported. These aspects might limit the use of LITT in some centers in the short term.
1.3. Intraoperative MRI and neuronavigation
Intraoperative MRI (IMRI) has a major role in monitoring anatomical and thermographic data during LITT procedures. IMRI has also proven useful in different neurosurgical procedures. IMRI systems typically consist of the scanner itself and a navigational system linked to the scanner. 40 The scanner could either be in an adjacent room to where the patient is transferred or mounted on a rail and brought into the operating room itself when needed. A new scan might be performed, or a previous scan could be used, and images could be transferred to the navigational system. Segmentation of lesions and planning of approach trajectories may be carried out by using the planning software (Figure 4). Other scanning modalities like single‐photon emission computerized tomography (SPECT), positron emission tomography (PET), or magnetoencephalography (MEG) images could be fused to recent preoperative scans and used for defining the resection volume or targeting a lesion. 41 , 42 Intraoperative scans might determine the amount of a lesion's resection compared to the preplanned lesion contours obtained from metabolic or anatomic scans. If an incomplete resection is documented, second‐look surgery may be performed during the procedure. 43 In this case, the patient is brought back to the operating position, re‐draped, and surgery is continued after an update of the navigational system using a 3D segmentation of the residual lesion. A second IMRI scan is performed after the second look is finished. Craniotomy is closed after complete resection confirmation. Residual lesions may be approached as many times as necessary. The patient's preparation and the scanning itself takes time and might add an hour or more to the operative time.
Figure 4.
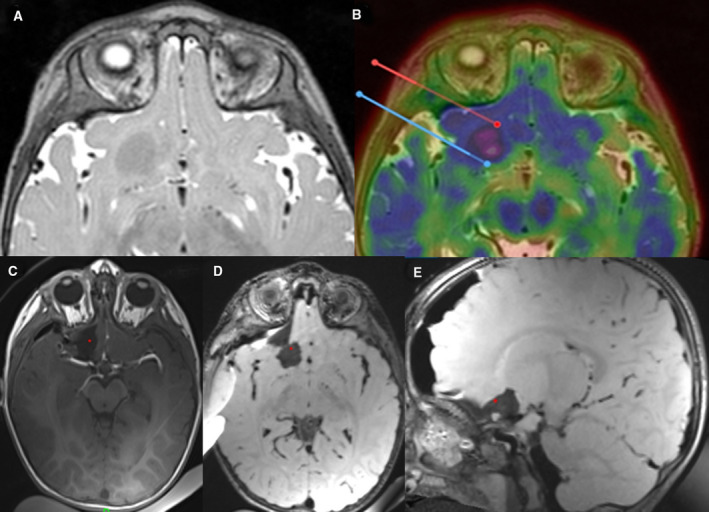
Intraoperative magnetic resonance imaging (IMRI) in a 17‐month‐old child with multiple tubers. Surface electroencephalography (EEG) suggested a right frontal seizure onset, and seizure semiology was congruent with a frontal focus. Because there were multiple tubers, including bilateral frontal tubers, α‐[11C]‐Methyl‐l‐tryptophan–PET (AMT‐PET) was used to identify the most active tuber. At surgery, the typical rubbery nature of the tuber was found and IMRI documented the complete resection. By the time of this writing, the child was seizure‐free for 8 months and showed marked improvement in neuropsychological development. A, Preoperative MRI showing a large frontobasal tuber. B, AMT‐PET suggesting this tuber was highly epileptogenic. C‐E, IMRI showing complete resection of the lesion
IMRI guidance proved particularly useful in patients with epilepsy and lesions such as FCD, long‐term epilepsy‐associated tumor (LEAT), cavernoma, or arteriovenous malformation. It might also be of help in patients whose foci are being investigated by depth‐electrode monitoring. 44 , 45 , 46 A retrospective study including patients with refractory epilepsy and FCD submitted to surgery with IMRI monitoring showed that 75% of the patients had complete resection and 89% of them had Engel class I outcome. The use of IMRI appeared to yield a higher rate of seizure‐free patients, especially when additional resection was performed after residual FCD was shown on intraoperative scanning (73% vs 38% in Engel IA, P < .05). 41 , 44 Incomplete resection of LEATs due to proximity to eloquent brain regions or misevaluation of the resected volume is a strong negative predictor for local tumor recurrence and persisting seizures. 42 , 45 , 46 The combined use of IMRI and neuronavigation yielded a 70% seizure‐free rate in patients with LEATs. 45 Patients with refractory epilepsy and cavernoma should be submitted to surgery comprising the removal of the lesion itself and its surrounding hemosiderin ring. 47 The cavernoma and the hemosiderin ring could be segmented individually, displayed at the microscope, and approached using navigational guidance. IMRI proved helpful in targeting residual cavernoma tissue or hemosiderosis yielding a high long‐term seizure‐freedom rate. 48 IMRI could be useful during depth and subdural electrodes implantation. IMRI could verify the position of depth electrodes and allows for correcting inappropriately positioned leads. 49 Comparison of preexplantation and postimplantation IMRI with or without CT fusion might be used to document lead shifting during the recording session. The surgical approach and extent of resection might need to be changed in patients with significant electrode dislocation. 50
The use of IMRI appears to provide for higher complete resection rates in patients with brain lesions, and thus better postoperative seizure control. On the other hand, no direct comparison between IMRI‐based procedures and conventional ones is available. In addition, the efficacy of using IMRI in patients with normal MRI was not adequately documented in the literature. IMRI does have drawbacks that should not be neglected. The need for technical and personnel expertise might restrict its use to centers with a high case load, including those unrelated to epilepsy. The expertise using IMRI in epilepsy surgery is so far limited when compared to its use in brain tumor surgery.
1.4. Neurostimulation–targeting epilepsy networks
Neuromodulation represents a recent development to treat refractory epilepsy, and the recognition of epilepsy as a network disease led to an increase in its use. The system includes the use of an implanted electrode array connected to a pulse generator, which can deliver electrical energy to certain neural targets. There is the obvious advantage of using a nondestructive and reversible approach to treat drug‐resistant epilepsy (Table 1). Neuromodulation was more adequately studied than many of the other epilepsy surgery techniques and major RCTs reporting on vagus nerve stimulation (VNS), 51 , 52 responsive neuromodulation (RNS), 53 and deep brain stimulation (DBS) were published. 54 , 55 The U.S. Food and Drug Administration (FDA) granted PreMarket Approval (PMA) to VNS in 1997, 56 RNS in 2013, 57 and DBS in 2018. 58 These techniques might be divided into intracranial or extracranial modalities, VNS being the main extracranial and DBS and RNS the main intracranial approaches.
Table 1.
Overview of patients and outcome for the different neuromodulation/stimulation trials
| Patient factors | Seizure type | Seizure frequency reduction in 3 mo blinded period | Seizure frequency reduction in open‐label phase | |
|---|---|---|---|---|
| RNS (64) | Age >18 y | Partial onset with 1‐2 foci | 37.9% | 44% mean (1 y) |
| Refractory to >2 AEDs | 2.1% seizure free | 53% mean (2 y) | ||
| >3 seizures/mo for >least 3 mo | 66%> 50% (8 y) | |||
| 9% seizure free | ||||
| AN‐DBS (55,56) | Age >18 y | Partial or secondarily generalized | 40.4% | 41% mean (1 y) |
| Seizures >6/mo | 12% seizure free | 56% mean (2 y) | ||
| Refractory to >3 AEDs and taking 1‐4 drugs | 16% seizure free | |||
| VNS (53,54) | Age >4 y | Partial onset seizures | 15%–28% | 22.5% >90% |
| Seizures >4/mo | No patient seizure free | 40.5% >75% | ||
| Refractory to >2 AEDs | 63.7%> 50% | |||
| 36.2% < 50% | ||||
| 15% seizure free | ||||
| HIP‐DBS (67) | Age >18 y | FIAS, FAS | 87% responders (>50% reduction) | N/A |
| Seizures >4/mo | Temporal lobe epilepsy | 50% seizure free | ||
| Refractory to >2 AEDs | ||||
| CM‐DBS (69) | Age <18 y | Secondary generalized epilepsy | N/A | 87% |
| Refractory to >2 AEDs | Atonic, tonic, myoclonic, absences | 15% seizure free | ||
| Daily seizures |
Abbreviations: AED, antiepileptic drug; AN‐DBS, deep brain stimulation of the anterior nucleus of the thalamus; CM‐DBS, deep brain stimulation of the centromedian nucleus of the thalamus; HIP‐DBS, hippocampal deep brain stimulation; mo, months; N/A, not applicable; RNS, responsive neuromodulation.
Two RCTs led to VNS approval in the United States in 1997, and more than 100,000 patients have been implanted worldwide. At least 50% of seizure frequency reduction might be expected in 50% of the patients. 59 There is an incremental response over time. 60 Some pediatric populations, like those children with Lennox‐Gastaut syndrome appear to be the best candidates for VNS. 61 The surgical technique is simple and might be performed in noncomplex hospital settings. Main complications are hoarseness, voice alterations, hardware issues, and development or worsening of sleep apnea. Quality of life is improved with the use of VNS. 62 The use of hospital facilities, especially emergency rooms, is diminished by VNS. 63 Therapy price is still high, but initial hospital costs are compensated after 5 years due to lower costs related to seizure complications. 64 More recently, a cardiac‐based closed‐loop system was introduced to the market. 65 Early studies showed noninferiority to standard VNS, but there is no adequate documentation that the closed‐loop system would be more efficacious than regular VNS. The system allows for additional stimulation cycles based on the ictal/periictal increase of heart rate. An even more versatile generator, including multiple programming capabilities, is also available, 66 but there are no data regarding its differential performance. There is no consensus on the adequate stimulation paradigm during VNS. The more commonly used parameters include cycling stimulation, but higher intensity was used to further decrease seizure frequency, and lower frequency was tried to treat side effects. 67 All seizure types and syndromes have been treated by VNS. Those patients who prove not to be candidates for cortical resection represent the main candidates for the procedure. Despite the large number of patients already submitted to the procedure, an adequate definition of the best candidates is still lacking and adequately designed cohort studies and RCTs are needed. A better understanding of its still poorly studied mechanism of action 68 would help to better use this technique.
Responsive neuromodulation has been studied in a RCT that led to its approval in the United States. 53 The RNS system is the first technology to use a closed‐loop paradigm for the treatment of epilepsy, meaning that there are sensing (detection) parameters that can be programmed that activate stimulating electrodes, which can deliver therapeutic current to the brain. The implanted hardware includes a generator that is located within the skull (which adds to the patient burden) and different types of electrodes (subdural, depth), and this technique is performed more commonly in tertiary care epilepsy centers. The sensing system utilizes line length, frequency/saturation, and trigger detections, which allow for personalization of unique seizure patterns. The pivotal RCT included patients with focal epilepsy with different localization; 37.9% were considered responders during the 3‐month blinded period. Patients commonly treated with RNS included those with bilateral mesial temporal onset, onset in eloquent and functional cortex, and suboptimal response to VNS or prior resective epilepsy surgery and in whom a discreet epileptogenic region has been identified. Long‐term seizure reduction varied from 48% to 66% between years 3 and 6. 69 This response seemed to be independent of lobe and 15% of the patients had seizure‐free periods greater than 1 year. 70 Furthermore, there did not appear to be neuropsychological decline, and there was quality of life improvement, decreased sudden unexpected death in epilepsy (SUDEP) rates, and real potential to serve as a tool for long‐term chronic monitoring. 71 , 72 , 73 RNS‐like systems could also be used to document the effectiveness of anti‐epileptic medication. 74 The use of closed‐loop paradigms is likely to increase in the future provided that the necessary hardware can be further developed and the epileptic foci dynamics can be better understood.
Deep brain stimulation has been used to treat multifocal or nonlocalizable epilepsy. Many targets were sporadically tried over the last decades, but stimulation of the anterior nucleus of the thalamus was the first one to be adequately studied in a RCT, which led to its initial approval in Europe (2010) and 8 years later in the United States. In that study, patients with focal epilepsy arising from all lobes were included. The operation is presently based on direct targeting with identification of the mammillothalamic tracts and visualization of the anterior thalamic nuclei. DBS of the anterior nucleus of the thalamus (AN‐DBS) is a stereotactic procedure using standard DBS hardware and is performed in tertiary epilepsy centers. Like other types of subcortical neuromodulation for epilepsy, AN‐DBS is mainly a palliative procedure: 40.4% of the patients were considered responders (at least 50% seizure frequency reduction), and 12.7% (14 of 110 patients) were seizure‐free for at least 6 months. 53 Long‐term 5‐year follow‐up on AN‐DBS showed 68% seizure reduction with 16% of the patients seizure‐free for at least 6 months. 75 AN‐DBS uses high‐frequency cycling stimulation, and consensus on the adequate stimulation parameters needs further exploration. There was initial worry of worsening psychiatric symptoms (especially depression) or sleep disturbances, but this did not prove to be clinically significant in larger open cohorts studied after regulatory approval. 76 Complications are uncommon and included those related to DBS devices, such as infection and hardware issues, and intracranial bleeding or thalamic (usually asymptomatic) infarcts. Although all focal epilepsies have been treated with AN‐DBS, it appears that patients who have an epileptogenic network in any way connected to the AN might represent the best candidates for this procedure. 77 More recently, an RCT reporting on hippocampal stimulation (HIP‐DBS) was published. 55 This study included only patients with refractory temporal lobe epilepsy submitted to continuous, open‐loop high‐frequency HIP‐DBS. The study design included a longer (6 months) double‐blinded phase when compared to AN‐DBS and RNS RCTs (3 months), and a longer no‐stimulation period preceding the double‐blind phase, allowing for a reduction of the impact of the insertional implantation effect. Focal aware and focal with impaired awareness seizures were significantly reduced from the first month of stimulation on. Fifty percent of the patients were rendered seizure‐free. Patients received weekly 0.4 V stimulus intensity increments, reaching 2 V at the end of that phase. These results appeared to be superior to those obtained with AN‐DBS and RNS in patients with refractory temporal lobe epilepsy. Stimulation of the centromedian nucleus of the thalamus (CM‐DBS) has also been attempted in the treatment of refractory epilepsy. Both cycling and continuous high‐frequency stimulation were tried, and there is no consensus on the adequate stimulation paradigms. CM is the only DBS target that proved efficacious in the treatment of generalized refractory epilepsy, especially in patients with Lennox‐Gastaut syndrome. 78 , 79 , 80 Seventy percent of patients might be considered responders, and 10% of them might be rendered seizure‐free by CM‐DBS. There are several open‐label studies reporting on CM‐DBS, but no adequate RCT has been carried out so far. 81 , 82
2. FUTURE DIRECTIONS: HIGH INTENSITY FOCUSED ULTRASOUND (HIFU), MULTIPURPOSE ELECTRODES AND CHRONIC WIRELESS INVASIVE RECORDINGS
The utility of high‐intensity focused ultrasound (HIFU) in the treatment of epilepsy has not yet been fully explored. Several studies have demonstrated submillimeter precision, 83 , 84 thus enabling high‐fidelity targeting in compact, highly eloquent regions. One such location could be the anterior nucleus of the thalamus, which is the current target for deep brain stimulation. 73 Other potential applications would be the acoustic ablation of amygdala, hippocampus, and piriform cortex for temporal lobe epilepsy, or ablation of lesional tissues such as focal cortical dysplasia, periventricular heterotopia, tuber, hypothalamic hamartoma, and small neoplasm. 85 , 86 , 87 Finally, disconnective procedures using HIFU such as corpus callosotomy and certain hemispheric procedures could be considered in isolation or in combination with surgical therapies, to create hybrid invasive/noninvasive approaches to the treatment of various medically refractory seizure disorders.
The primary barriers to implementing HIFU as a standard modality for the treatment of epilepsy are fourfold: (a) the adequate distribution of acoustic energy into potentially sclerotic or calcified epileptogenic tissue; (b) protection of critical neurovascular structures in the basilar cisterns near potential deep mesial foci; (c) the ability to focus energy at lateral or superficial locations where lesions or epileptogenic foci might exist; and (d) the ability to create lesions large enough to capture the entirety of the epileptogenic foci (Figure 5). Calcifications are known to cause disruption to traveling ultrasound waves. In HIFU treatment paradigms, calcifications are masked out of the stereotactic plan. Less is known about how sclerotic tissue may absorb or reflect HIFU acoustic energy. Furthermore, in a laboratory study analyzing modeled treatments for trigeminal neuralgia, there was significant spread of thermal energy to nearby cranial nerves, although the effect could be mitigated with appropriate masking. 88 A cadaveric simulation of mesial temporal lobe–focused ultrasound demonstrated thermal efficacy of target tissue, but there was concern for thermal injury to the optic, third, and fourth cranial nerves, as well as to nearby critical vasculature. 88 However, like laser ablation, creating low safety targets to abort sonication if temperatures in those locations reach above a particular low threshold could be a software solution to enable this treatment. The most efficient delivery of energy occurs for centrally located targets based on the distribution of ultrasound elements. Thus, lesions such as hypothalamic hamartoma may be more efficiently treated compared to lateral and superficial lesions. However, with further study, the ability to mask and adjust element parameters could still enable enough energy delivery to treat even these types of suboptimally located lesions. Focused ultrasound (FUS) lesions have similar appearance to radiofrequency ablations by histological analysis and by MRI. 89 The lesions tend to be relatively small, with diameters of approximately 2 mm and volumes of up to approximately 200 mm3 at 1 month. 90 Larger ablation volumes may be required to adequately treat epileptogenic networks, which may require technological modifications and advancements. 91 , 92 There are two actively enrolling clinical trials for the use of HIFU in epilepsy disorders. One is at the Ohio State University investigating HIFU thalamotomy (anterior nucleus) to prevent secondary generalization in focal onset epilepsy (ClinialTrials.gov Identifier: NCT03417297). The second is at the University of Virginia investigating HIFU for subcortical lesional epilepsy (ClinicalTrials.gov Identifier: NCT02804230). There is also one clinical trial at the University of California Los Angeles investigating low intensity FUS pulsation for temporal lobe epilepsy (ClinicalTrials.gov Identifier: NCT02151175). These clinical trials will provide critical information regarding safety, feasibility, and preliminary evidence to support the expansion of focused ultrasound to epilepsy. Although some limitations exist within the framework of current focused ultrasound systems testing is already underway to assess general safety and feasibility of this technology in various epilepsy disorders. Advancements in hardware and software platforms will continue to enhance our ability to deliver acoustic energy, which may lead to novel treatment paradigms and the potential to create a new standard for the care of patients with medically refractory epilepsy.
Figure 5.
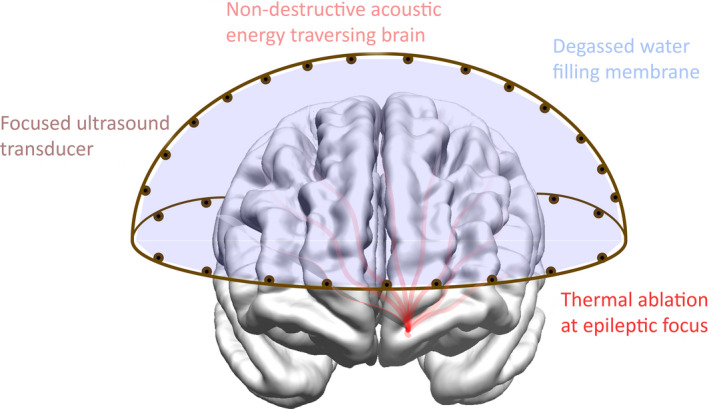
High‐intensity focused ultrasound (HIFU) schematics. This illustration shows the delivery of acoustic energy generated by the HIFU unit to a centrally located area of the brain
The growing recognition of epilepsy as a neural network disorder and the associated growth of neuromodulation for the treatment of epilepsy places even more importance on intracranial studies. There is mounting evidence from animal studies and patients with responsive neurostimulation systems implanted for epilepsy that long‐term intracranial recordings and the use of multipurpose electrodes may provide more accurate seizure‐onset and network description. 93 , 94 , 95 The use of depth electrodes with embedded microdialysis membranes to measure neurotransmitters showed that glutamate levels were elevated in the seizure‐onset region and more so in propagation nodes. 96 This finding suggested a seizure network nodal abnormality in neurotransmitters. Several epilepsy centers have reported additional insights on seizure initiation and propagation gained by microwire and single‐unit data. 97 , 98 , 99 The technology for both wireless implants and remote intracranial monitoring is now available but needs the research support for extension into humans.
Future intracranial studies will include the use of redesigned telemetered multimodal bioelectrodes capable of acquiring a wide range of data, from microwire and classical field potentials to neurotransmitters and other biometrics (temperature, blood flow, intracranial pressure, and so on). These “depth probes” will be supplemented by thin‐film dense‐array surface bioelectrodes, and the entire implant will be wirelessly telemetered. The epileptic patient might spend only a few nights in the intensive care unit/epilepsy monitoring unit (ICU/EMU) to ensure no complications, postoperative recovery, and data acquisition fidelity. Patients will then be monitored for an extended period of weeks to months in their natural environment while taking their routine antiepileptic drugs (AEDs). The limited long‐term data already available from RNS patients established the feasibility of remote data uploading and proved revolutionary in a wide array of new research endeavors. This approach and the new data streams available will exponentially increase these endeavors in the coming years. There are, however, a handful of developments that remain critical: power, multimodal bioelectrodes, electrode fidelity, and data processing and storage.
Although a variety of wireless data transmission modes are available, they presently require a good deal of power, and power often generates heat. There are understandably strict regulations on how much heat an implant can produce, usually 1°C in the human cranium. Battery size is another constraint that becomes limiting with larger power demands required to support broader bands of data. The expanding use of rechargeable technology allows for larger power loads, limited by the practical ability of battery recharge and the use of inductive charging mechanisms with intracranial implants. Institutional collaborations across expertise in neurology, neurosurgery, engineering, and computer sciences are presently working on this development. These probes would contain a combination of “off the shelf” and newly developed biosensors providing the first multimodal bioelectrode. These new bioelectrodes would be capable of monitoring parameters such as intracranial pressure, cerebral blood flow, and partial pressure of oxygen, in addition to intracranial EEG. The further development of multimodal bioprobes will enhance the value of intracranial data obtained in the study of epilepsy. 100 , 101
The expansion of the intracranial montage to microwires, small‐scale local‐field potentials, and multimodal monitoring significantly increases the mechanistic information acquired on seizure initiation and propagation. 98 The long‐term acquisition of single‐unit data is challenging and highlights the need for an intelligent approach to data acquisition as well as electrode fidelity. Although microwire low field potentials (LFP) and single‐unit data can add additional understanding of local and even cellular activity changes within a network that are associated with seizure initiation and propagation, they are subject to increased recording requirements, variable yield, and concerns regarding longer‐term stability. Several groups published on computational models related to the ability of intelligent dense arrays to accommodate the failure of a few or even several electrodes in the array. 102 New intelligent high‐density cortical electrode arrays are under development. Another consideration for high‐density electrode arrays capable of micro‐LFP and single‐unit acquisition is the potential damage caused by the insertion of the device. In this setting, neurostimulation may be useful in seizure modification and other applications such as prosthetics. Furthermore, new arrays will be designed to be utilized in parallel and provide power and wireless data transmission, being capable of both onboard closed‐loop targeted stimulation and wireless communication with outside acquisition and processing devices as well.
New technology would have a major role in the future directions of epilepsy surgery, although the superiority of new techniques and devices might need to be adequately documented. Closed‐loop neuromodulation might prove superior to open‐loop procedures in the future; new biomarkers would prove essential for further development of the techniques. On the other hand, presently there is no high‐level evidence that closed‐loop would be superior to open‐loop stimulation. Improved strategies for foci localization might improve our understanding of the epileptic network in each individual, potentially improving treatment outcome. Chronic electrocorticography and the use of multipurpose depth electrodes would likely be part of these efforts.
CONFLICT OF INTERESTS
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Dorfer C, Rydenhag B, Baltuch G, et al. How technology is driving the landscape of epilepsy surgery. Epilepsia. 2020;61:841–855. 10.1111/epi.16489
REFERENCES
- 1. Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal‐lobe epilepsy. N Engl J Med. 2001;345:311–8. [DOI] [PubMed] [Google Scholar]
- 2. Van Gompel JJ, Worrell GA, Bell ML, Patrick TA, Cascino GD, Raffel C, et al. Intracranial electroencephalography with subdural grid electrodes: techniques, complications, and outcomes. Neurosurgery. 2008;63:498–505. [DOI] [PubMed] [Google Scholar]
- 3. Lüders H, Awad I, Burgess R, Wyllie E, Van Ness P. Subdural electrodes in the presurgical evaluation for surgery of epilepsy. Epilepsy Res Suppl. 1992;5:147–56. [PubMed] [Google Scholar]
- 4. Bancaud J, Talairach J. La Stereo‐Electroencephalographie Dans L´epilepsie. Paris, France: M. CIE, 1965. [Google Scholar]
- 5. Cardinale F, Cossu M, Castana L, Casaceli G, Schiariti MP, Miserocchi A, et al. Stereoelectroencephalography: surgical methodology, safety, and stereotactic application accuracy in 500 procedures. Neurosurgery. 2013;72:353–66. [DOI] [PubMed] [Google Scholar]
- 6. Dorfer C, Minchev G, Czech T, Stefanits H, Feucht M, Pataraia E, et al. A novel miniature robotic device for frameless implantation of depth electrodes in refractory epilepsy. J Neurosurg. 2017;126:1622–8. [DOI] [PubMed] [Google Scholar]
- 7. González‐Martínez J, Bulacio J, Thompson S, Gale J, Smithason S, Najm I, et al. Technique, results, and complications related to robot‐assisted stereoelectroencephalography. Neurosurgery. 2016;78:169–79. [DOI] [PubMed] [Google Scholar]
- 8. Li K, Vakharia VN, Sparks R, Rodionov R, Vos SB, McEvoy AW, et al. Stereoelectroencephalography electrode placement: Detection of blood vessel conflicts. Epilepsia. 2019;60:1942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vakharia VN, Sparks RE, Li K, O'Keeffe AG, Pérez‐García F, França LGS, et al. Multicenter validation of automated trajectories for selective laser amygdalohippocampectomy. Epilepsia. 2019;60:1949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abel TJ, Varela Osorio R, Amorim‐Leite R, Mathieu F, Kahane P, Minotti L, et al. Frameless robot‐assisted stereoelectroencephalography in children: technical aspects and comparison with Talairach frame technique. J Neurosurg Pediatr. 2018;22:37–46. [DOI] [PubMed] [Google Scholar]
- 11. Lefranc M, Capel C, Pruvot AS, Fichten A, Desenclos C, Toussaint P, et al. The impact of the reference imaging modality, registration method and intraoperative flat‐panel computed tomography on the accuracy of the ROSA stereotactic robot. Stereot Funct Neurosurg. 2014;92:242–50. [DOI] [PubMed] [Google Scholar]
- 12. Vakharia VN, Sparks R, O'Keeffe AG, Rodionov R, Miserocchi A, McEvoy A, et al. Accuracy of intracranial electrode placement for stereoelectroencephalography: A systematic review and meta‐analysis. Epilepsia. 2017;58:921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Von Langsdorff D, Paquis P, Fontaine D. In vivo measurement of the frame‐based application accuracy of the Neuromate neurosurgical robot. J Neurosurg. 2015;122:191–4. [DOI] [PubMed] [Google Scholar]
- 14. Spyrantis A, Cattani A, Woebbecke T, Konczalla J, Strzelczyk A, Rosenow F, et al. Electrode placement accuracy in robot‐assisted epilepsy surgery: a comparison of different referencing techniques including frame‐based CT versus facial laser scan based on CT or MRI. Epilepsy Behav. 2019;91:38–47. [DOI] [PubMed] [Google Scholar]
- 15. Steinmeier R, Rachinger J, Kaus M, Ganslandt O, Huk W, Fahlbusch R. Factors influencing the application accuracy of neuronavigation systems. Stereotact Funct Neurosurg. 2000;75:188–202. [DOI] [PubMed] [Google Scholar]
- 16. Spyrantis A, Cattani A, Strzelczyk A, Rosenow F, Seifert V, Freiman TM. Robot‐guided stereoelectroencephalography without a computed tomography scan for referencing: analysis of accuracy. Int J Med Robot. 2018;14(2):e1888. [DOI] [PubMed] [Google Scholar]
- 17. Roessler K, Sommer B, Merkel A, et al. A frameless stereotactic implantation technique for depth electrodes in refractory epilepsy utilizing intraoperative MR imaging. World Neurosurg. 2016;94:206–10. [DOI] [PubMed] [Google Scholar]
- 18. Hoppe C, Witt J‐A, Helmstaedter C, Gasser T, Vatter H, Elger CE. Laser interstitial thermotherapy (LiTT) in epilepsy surgery. Seizure. 2017;48:45–52. [DOI] [PubMed] [Google Scholar]
- 19. Curry DJ, Raskin J, Ali I, Wilfong AA. MR‐guided laser ablation for the treatment of hypothalamic hamartomas. Epilepsy Res. 2018;142:131–4. [DOI] [PubMed] [Google Scholar]
- 20. Wu C, Jermakowicz WJ, Chakravorti S, Cajigas I, Sharan AD, Jagid JR, et al. Effects of surgical targeting in laser interstitial thermal therapy for mesial temporal lobe epilepsy: a multicenter study of 234 patients. Epilepsia. 2019;60:1171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palma AE, Wicks RT, Popli G, Couture DE. Corpus callosotomy via laser interstitial thermal therapy: a case series. J Neurosurg Pediatr. 2018;23:303–7. [DOI] [PubMed] [Google Scholar]
- 22. Perry MS, Donahue DJ, Malik SI, Keator CG, Hernandez A, Reddy RK, et al. Magnetic resonance imaging‐guided laser interstitial thermal therapy as treatment for intractable insular epilepsy in children. J Neurosurg Pediatr. 2017;20:575–82. [DOI] [PubMed] [Google Scholar]
- 23. McCracken DJ, Willie JT, Fernald BA, Saindane AM, Drane DL, Barrow DL, et al. Magnetic resonance thermometry‐guided stereotactic laser ablation of cavernous malformations in drug‐resistant epilepsy: imaging and clinical results. Oper Neurosurg (Hagerstown). 2016;12:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tovar‐Spinoza Z, Ziechmann R, Zyck S. Single and staged laser interstitial thermal therapy ablation for cortical tubers causing refractory epilepsy in pediatric patients. Neurosurg Focus. 2018;45:E9. [DOI] [PubMed] [Google Scholar]
- 25. Curry DJ, Gowda A, McNichols RJ, Wilfong AA. MR‐guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav. 2012;24:408–14. [DOI] [PubMed] [Google Scholar]
- 26. Parrent AG, Blume WT. Stereotactic amygdalohippocampotomy for the treatment of medial temporal lobe epilepsy. Epilepsia. 1999;40:1408–16. [DOI] [PubMed] [Google Scholar]
- 27. Deleo F, Garbelli R, Milesi G, Gozzo F, Bramerio M, Villani F, et al. Short‐ and long‐term surgical outcomes of temporal lobe epilepsy associated with hippocampal sclerosis: Relationships with neuropathology. Epilepsia. 2016;57:306–15. [DOI] [PubMed] [Google Scholar]
- 28. Baxendale S, Thompson PJ, Sander JW. Neuropsychological outcomes in epilepsy surgery patients with unilateral hippocampal sclerosis and good preoperative memory function. Epilepsia. 2013;54:e131–4. [DOI] [PubMed] [Google Scholar]
- 29. Drane DL, Loring DW, Voets NL, Price M, Ojemann JG, Willie JT, et al. Better object recognition and naming outcome with MRI‐guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 2015;56:101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Josephson CB, Dykeman J, Fiest KM, Liu X, Sadler RM, Jette N, et al. Systematic review and meta‐analysis of standard vs selective temporal lobe epilepsy surgery. Neurology. 2013;80:1669–76. [DOI] [PubMed] [Google Scholar]
- 31. Jeha LE, Najm IM, Bingaman WE, Khandwala F, Widdess‐Walsh P, Morris HH, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology. 2006;66:1938–40. [DOI] [PubMed] [Google Scholar]
- 32. Kang JY, Wu C, Tracy J, Lorenzo M, Evans J, Nei M, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 2015;57:325–34. [DOI] [PubMed] [Google Scholar]
- 33. Waseem H, Osborn KE, Schoenberg MR, Kelley V, Bozorg A, Cabello D, et al. Laser ablation therapy: an alternative treatment for medically resistant mesial temporal lobe epilepsy after age 50. Epilepsy Behav. 2015;51:152–7. [DOI] [PubMed] [Google Scholar]
- 34. Willie JT, Laxpati NG, Drane DL, Gowda A, Appin C, Hao C, et al. Real‐time magnetic resonance‐guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 2014;74:569–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jermakowicz WJ, Ivan ME, Cajigas I, Ribot R, Jusue‐Torres I, Desai MB, et al. Visual deficit from laser interstitial thermal therapy for temporal lobe epilepsy: anatomical considerations. Oper Neurosurg (Hagerstown). 2017;13:627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lehner KR, Yeagle EM, Argyelan M, Klimaj Z, Du V, Megevand P, et al. Validation of corpus callosotomy after laser interstitial thermal therapy: a multimodal approach. J Neurosurg. 2018;1:1–11. [DOI] [PubMed] [Google Scholar]
- 37. Hale AT, Sen S, Haider AS, Perkins FF, Clarke DF, Lee MR, et al. Open resection vs laser interstitial thermal therapy for the treatment of pediatric insular epilepsy. Neurosurgery. 2019;85(4):E730‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Willie JT, Malcolm JG, Stern MA, Lowder LO, Neill SG, Cabaniss BT, et al. Safety and effectiveness of stereotactic laser ablation for epileptogenic cerebral cavernous malformations. Epilepsia. 2019;60:220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schmitt FC, Büntjen L, Schütze H, Kaufmann J, Heinze H‐J, Hinrichs H, et al. Stereotactic laser thermal ablation of mesial temporal lobe epilepsy with right hippocampal sclerosis—patient decision‐making, realization and visualization of memory function. Z Epileptol. 2020;33:42–49. [Google Scholar]
- 40. Nimsky C, Kuhnt D, Ganslandt O, Buchfelder M. Multimodal navigation integrated with imaging. Acta Neurochir Suppl. 2011;109:207–14. [DOI] [PubMed] [Google Scholar]
- 41. Roessler K, Hofmann A, Sommer B, Grummich P, Coras R, Kasper BS, et al. Resective surgery for medically refractory epilepsy using intraoperative MRI and functional neuronavigation: the Erlangen experience of 415 patients. Neurosurg Focus. 2016;40:E15. [DOI] [PubMed] [Google Scholar]
- 42. Roessler K, Kasper BS, Heynold E, Coras R, Sommer B, Rampp S, et al. Intraoperative magnetic‐resonance tomography and neuronavigation during resection of focal cortical dysplasia type II in adult epilepsy surgery offers better seizure outcomes. World Neurosurg. 2018;109:e43–e49. [DOI] [PubMed] [Google Scholar]
- 43. Roessler K, Sommer B, Grummich P, Coras R, Kasper BS, Hamer HM, et al. Improved resection in lesional temporal lobe epilepsy surgery using neuronavigation and intraoperative MR imaging: favourable long term surgical and seizure outcome in 88 consecutive cases. Seizure. 2014;23:201–7. [DOI] [PubMed] [Google Scholar]
- 44. Sacino MF, Ho C‐Y, Whitehead MT, Zelleke T, Magge SN, Myseros J, et al. Resective surgery for focal cortical dysplasia in children: a comparative analysis of the utility of intraoperative magnetic resonance imaging (iMRI). Childs Nerv Syst. 2016;32:1101–7. [DOI] [PubMed] [Google Scholar]
- 45. Sacino MF, Huang SS, Keating RF, Gaillard WD, Oluigbo CO. An initial cost‐effectiveness analysis of intraoperative magnetic resonance imaging (iMRI) in pediatric epilepsy surgery. Childs Nerv Syst. 2018;34:495–502. [DOI] [PubMed] [Google Scholar]
- 46. Sommer B, Grummich P, Coras R, Kasper BS, Blumcke I, Hamer HM, et al. Integration of functional neuronavigation and intraoperative MRI in surgery for drug‐resistant extratemporal epilepsy close to eloquent brain areas. Neurosurg Focus. 2013;34:E4. [DOI] [PubMed] [Google Scholar]
- 47. Dammann P, Schaller C, Sure U. Should we resect peri‐lesional hemosiderin deposits when performing lesionectomy in patients with cavernoma‐related epilepsy (CRE)? Neurosurg Rev. 2017;40:39–43. [DOI] [PubMed] [Google Scholar]
- 48. Sommer B, Kasper BS, Coras R, Blumcke I, Hamer HM, Buchfelder M, et al. Surgical management of epilepsy due to cerebral cavernomas using neuronavigation and intraoperative MR imaging. Neurol Res. 2013;35:1076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sommer B, Rampp S, Doerfler A, Stefan H, Hamer HM, Buchfelder M, et al. Investigation of subdural electrode displacement in invasive epilepsy surgery workup using neuronavigation and intraoperative MRI. Neurol Res. 2018;40:811–21. [DOI] [PubMed] [Google Scholar]
- 50. Sommer B, Wimmer C, Coras R, Blumcke I, Lorber B, Hamer HM, et al. Resection of cerebral gangliogliomas causing drug‐resistant epilepsy: short‐ and long‐term outcomes using intraoperative MRI and neuronavigation. Neurosurg Focus. 2015;38:E5. [DOI] [PubMed] [Google Scholar]
- 51. Ben‐Menachem E, Manon‐Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35:616–26. [DOI] [PubMed] [Google Scholar]
- 52. Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial‐onset seizures. A randomized control trial. Neurology. 1998;51:48–55. [DOI] [PubMed] [Google Scholar]
- 53. Morrell MJ, King‐Stephens D, Massey AD, et al. RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–304. [DOI] [PubMed] [Google Scholar]
- 54. Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. [DOI] [PubMed] [Google Scholar]
- 55. Cukiert A, Cukiert CM, Burattini JA, Mariani PP, Bezerra DF. Seizure outcome after hippocampal deep brain stimulation in patients with refractory temporal lobe epilepsy: a prospective, controlled, randomized, double‐blind study. Epilepsia. 2017;58:1728–33. [DOI] [PubMed] [Google Scholar]
- 56. Food and Drug Administration . Premarket approval (PMA) for VNS therapy system. https://www.accessdata.fda.gov/scrIpts/cdrh/cfdocs/cfPMA/pma.cfm?id=319777. Updated S April 15, 2019. Accessed April 22, 2019.
- 57. Food and Drug Administration . Premarket approval (PMA) for neuropace RNS system. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100026. Updated April 15, 2019. Accessed April 22, 2019.
- 58. Food and Drug Administration . Premarket approval (PMA) for Medtronic DBS therapy for epilepsy. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P960009S219. Updated April 15, 2019. Accessed April 22, 2019.
- 59. Wasade VS, Schultz L, Mohanarangan K, Gaddam A, Schwalb JM, Spanaki‐Varelas M. Long‐term seizure and psychosocial outcomes of vagus nerve stimulation for intractable epilepsy. Epilepsy Behav. 2015;53:31–6. [DOI] [PubMed] [Google Scholar]
- 60. Elliott RE, Morsi A, Tanweer O, Grobelny B, Geller E, Carlson C, et al. Efficacy of vagus nerve stimulation over time: review of 65 consecutive patients with treatment‐resistant epilepsy treated with VNS > 10 years. Epilepsy Behav. 2011;20:478–83. [DOI] [PubMed] [Google Scholar]
- 61. Morris GL, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence‐based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81:1453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ryvlin P, Gilliam FG, Nguyen DK, Colicchio G, Iudice A, Tinuper P, et al. The long‐term effect of vagus nerve stimulation on quality of life in patients with pharmacoresistant focal epilepsy: the PuLsE (Open Prospective Randomized Long‐term Effectiveness) trial. Epilepsia. 2014;55:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ben‐Menachem E, Hellström K, Verstappen D. Analysis of direct hospital costs before and 18 months after treatment with vagus nerve stimulation therapy in 43 patients. Neurology. 2002;59:S44–S47. [DOI] [PubMed] [Google Scholar]
- 64. Boon P, Vonck K, Vandekerckhove T, D'have M, Nieuwenhuis L, Michielsen G, et al. Vagus nerve stimulation for medically refractory epilepsy; efficacy and cost‐benefit analysis. Acta Neurochir (Wien). 1999;141:447–52. [DOI] [PubMed] [Google Scholar]
- 65. Hamilton P, Soryal I, Dhahri P, Wimalachandra W, Leat A, Hughes D, et al. Clinical outcomes of VNS therapy with AspireSR(®) (including cardiac‐based seizure detection) at a large complex epilepsy and surgery centre. Seizure. 2018;58:120–6. [DOI] [PubMed] [Google Scholar]
- 66. Mertens A, Raedt R, Gadeyne S, Carrette E, Boon P, Vonck K. Recent advances in devices for vagus nerve stimulation. Expert Rev Med Devices. 2018;15:527–39. [DOI] [PubMed] [Google Scholar]
- 67. Cukiert A, Cukiert CM, Burattini JA, Lima AM, Forster CR, Baise C, et al. A prospective long‐term study on the outcome after vagus nerve stimulation at maximally tolerated current intensity in a cohort of children with refractory secondary generalized epilepsy. Neuromodulation. 2013;16:551–6. [DOI] [PubMed] [Google Scholar]
- 68. Raedt R, Clinckers R, Mollet L, Vonck K, El Tahry R, Wyckhuys T, et al. Increased hippocampal noradrenaline is a biomarker for efficacy of vagus nerve stimulation in a limbic seizure model. J Neurochem. 2011;117:461–9. [DOI] [PubMed] [Google Scholar]
- 69. Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King‐Stephens D, Nair D, et al. Long‐term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84:810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Geller EB, Skarpaas TL, Gross RE, Goodman RR, Barkley GL, Bazil CW, et al. Brain‐responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017;58:994–1004. [DOI] [PubMed] [Google Scholar]
- 71. Loring DW, Kapur R, Meador KJ, Morrell MJ. Differential neuropsychological outcomes following targeted responsive neurostimulation for partial‐onset epilepsy. Epilepsia. 2015;56:1836–44. [DOI] [PubMed] [Google Scholar]
- 72. Heck CN, King‐Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, et al. Two‐year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55:432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Devinsky O, Friedman D, Duckrow RB, Fountain NB, Gwinn RP, Leiphart JW, et al. Sudden unexpected death in epilepsy in patients treated with brain‐responsive neurostimulation. Epilepsia. 2018;59:555–61. [DOI] [PubMed] [Google Scholar]
- 74. Skarpaas TL, Tcheng TK, Morrell MJ. Clinical and electrocorticographic response to antiepileptic drugs in patients treated with responsive stimulation. Epilepsy Behav. 2018;83:192–200. [DOI] [PubMed] [Google Scholar]
- 75. Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, et al. Long‐term efficacy and safety of thalamic stimulation for drug‐resistant partial epilepsy. Neurology. 2015;84:1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tröster AI, Meador KJ, Irwin CP, Fisher RS, SANTE Study Group . Memory and mood outcomes after anterior thalamic stimulation for refractory partial epilepsy. Seizure. 2017;45:133–41. [DOI] [PubMed] [Google Scholar]
- 77. Sherdil A, Coizet V, Pernet‐Gallay K, David O, Chabardès S, Piallat B. Implication of anterior nucleus of the thalamus in mesial temporal lobe seizures. Neuroscience. 2019;418:279–90. [DOI] [PubMed] [Google Scholar]
- 78. Velasco AL, Velasco F, Jimenez F, Velasco M, Castro G, Carrillo‐Ruiz JD, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox‐Gastaut syndrome. Epilepsia. 2006;47:1203–12. [DOI] [PubMed] [Google Scholar]
- 79. Cukiert A, Cukiert CM, Argentoni‐Baldochi M, Baise C, Forster CR, Mello VA, et al. Intraoperative neurophysiological responses in epileptic patients submitted to hippocampal and thalamic deep brain stimulation. Seizure. 2011;20:748–53. [DOI] [PubMed] [Google Scholar]
- 80. Valentín A, García Navarrete E, Chelvarajah R, Torres C, Navas M, Vico L, et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia. 2013;54:1823–33. [DOI] [PubMed] [Google Scholar]
- 81. Cukiert A, Burattini JA, Cukiert CM, Argentoni‐Baldochi M, Baise‐Zung C, Forster CR, et al. Centro‐median stimulation yields additional seizure frequency and attention improvement in patients previously submitted to callosotomy. Seizure. 2009;18:588–92. [DOI] [PubMed] [Google Scholar]
- 82. Son BC, Shon YM, Choi JG, Kim J, Ha SW, Kim SH, et al. Clinical outcome of patients with deep brain stimulation of the centromedian thalamic nucleus for refractory epilepsy and location of the active contacts. Stereotact Funct Neurosurg. 2016;94:187–97. [DOI] [PubMed] [Google Scholar]
- 83. Jeanmonod D, Werner B, Morel A, Michels L, Zadicario E, Schiff G, et al. Transcranial magnetic resonance imaging‐guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus. 2012;32:E1. [DOI] [PubMed] [Google Scholar]
- 84. Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. A Pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2013;369:640–8. [DOI] [PubMed] [Google Scholar]
- 85. Monteith S, Sheehan J, Medel R, Wintermark M, Eames M, Snell J, et al. Potential intracranial applications of magnetic resonance–guided focused ultrasound surgery. J Neurosurg. 2012;118:215–21. [DOI] [PubMed] [Google Scholar]
- 86. Monteith S, Snell J, Eames M, Kassell NF, Kelly E, Gwinn R. Transcranial magnetic resonance–guided focused ultrasound for temporal lobe epilepsy: a laboratory feasibility study. J Neurosurg. 2016;125:1557–64. [DOI] [PubMed] [Google Scholar]
- 87. McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging‐guided focused ultrasound surgery of brain tumors: Initial findings in 3 patients. Neurosurgery. 2010;66:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Monteith SJ, Medel R, Kassell NF, Wintermark M, Eames M, Snell J, et al. Transcranial magnetic resonance–guided focused ultrasound surgery for trigeminal neuralgia: a cadaveric and laboratory feasibility study. J Neurosurg. 2012;118:319–28. [DOI] [PubMed] [Google Scholar]
- 89. Elias WJ, Khaled M, Hilliard JD, Aubry J‐F, Frysinger RC, Sheehan JP, et al. A magnetic resonance imaging, histological, and dose modeling comparison of focused ultrasound, radiofrequency, and Gamma Knife radiosurgery lesions in swine thalamus. J Neurosurg. 2013;119:307–17. [DOI] [PubMed] [Google Scholar]
- 90. Wintermark M, Druzgal J, Huss DS, Khaled MA, Monteith S, Raghavan P, et al. Imaging findings in MR imaging‐guided focused ultrasound treatment for patients with essential tremor. Am J Neuroradiol. 2014;35:891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jung HH, Kim SJ, Roh D, Chang JG, Chang WS, Kweon EJ, et al. Bilateral thermal capsulotomy with MR‐guided focused ultrasound for patients with treatment‐refractory obsessive‐compulsive disorder: a proof‐of‐concept study. Mol Psychiatry. 2015;20:1205–11. [DOI] [PubMed] [Google Scholar]
- 92. Arvanitis CD, Vykhodtseva N, Jolesz F, Livingstone M, McDannold N. Cavitation‐enhanced nonthermal ablation in deep brain targets: feasibility in a large animal model. J Neurosurg. 2016;124:1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zaatreh MM, Spencer DD, Thompson JL, Blumenfeld H, Novotny EJ, Mattson RH, et al. Frontal lobe tumoral epilepsy: clinical, neurophysiologic features and predictors of surgical outcome. Epilepsia. 2002;43:727–33. [DOI] [PubMed] [Google Scholar]
- 94. Brinkmann BH, Patterson EE, Vite C, Vasoli VM, Crepeau D, Stead M, et al. Forecasting seizures using intracranial EEG measures and SVM in naturally occurring canine epilepsy. PLoS One. 2015;10:e0133900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. King‐Stephens D, Mirro E, Weber PB, Laxer KD, Van Ness PC, Salanova V, et al. Lateralization of mesial temporal lobe epilepsy with chronic ambulatory electrocorticography. Epilepsia. 2015;56:959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Çavuş I, Romanyshyn JC, Kennard JT, Farooque P, Williamson A, Eid T, et al. Elevated basal glutamate and unchanged glutamine and GABA in refractory epilepsy: microdialysis study of 79 patients at the yale epilepsy surgery program. Ann Neurol. 2016;80:35–45. [DOI] [PubMed] [Google Scholar]
- 97. Bower MR, Stead M, Meyer FB, Marsh WR, Worrell GA. Spatiotemporal neuronal correlates of seizure generation in focal epilepsy. Epilepsia. 2012;53:807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Merricks EM, Smith EH, McKhann GM, Goodman RR, Bateman LM, Emerson RG, et al. Single unit action potentials in humans and the effect of seizure activity. Brain. 2015;138:2891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liou J‐Y, Smith EH, Bateman LM, McKhann GM, Goodman RR, Greger B, et al. Multivariate regression methods for estimating velocity of ictal discharges from human microelectrode recordings. J Neural Eng. 2017;14:044001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chen R, Canales A, Anikeeva P. Neural recording and modulation technologies. Nat Rev Mater. 2017;2:16093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tiwari S, Sharma V, Mujawar M, Mishra YK, Kaushik A, Ghosal A, et al. Biosensors for epilepsy management: state‐of‐art and future aspects. Sensors (Basel). 2019;19:1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Acharya I, Joshi B, Lanning B, Zaveri H. Reconfigurable fault‐tolerant multielectrode array for dependable monitoring of the human brain. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:652–5. [DOI] [PubMed] [Google Scholar]


