Abstract
Aim
Efficacy and safety of dapagliflozin plus saxagliptin (DAPA + SAXA) were compared with insulin glargine (INS) in patients with type 2 diabetes (T2D) in a 52‐week extension study.
Materials and methods
This international Phase 3 study randomized adults with T2D on metformin with/without sulphonylurea. They received DAPA + SAXA or INS for 24 weeks (short‐term) with a 28‐week (long‐term) extension. Week 52 exploratory endpoints included adjusted mean change from baseline in glycated haemoglobin A1c (HbA1c) and body weight, and a proportion of patients achieving optimal glycaemic response without hypoglycaemia and without requiring rescue medication.
Results
Of the 1163 patients enrolled, 643 received treatment; 600 (DAPA + SAXA, 306; INS, 294) entered the long‐term phase. At 52 weeks, HbA1c [adjusted least squares (LS) mean; 95% confidence interval (CI)] decreased more with DAPA + SAXA (−1.5% [−1.6%, −1.4%]) than with INS (−1.3% [−1.4%, −1.1%]); the LS mean difference (95% CI) was −0.25% (−0.4%, −0.1%; P = 0.009). Total body weight reduced with DAPA + SAXA [LS mean (95% CI): −1.8 kg (−2.4, −1.3)] and increased with INS [LS mean (95% CI): +2.8 kg (2.2, 3.3)]. More patients on DAPA + SAXA (17.6%) achieved HbA1c <7.0% without hypoglycaemia versus those on INS (9.1%). Rescue medication was required by 77 patients (23.8%) and 97 patients (30.4%) in the DAPA + SAXA and INS groups, respectively.
Conclusion
DAPA + SAXA treatment was non‐inferior to INS in reducing HbA1c and body weight, and in achieving optimal glycaemic control without hypoglycaemia in patients with T2D 52 weeks after initiation.
Keywords: combination therapy, dapagliflozin, insulin glargine, saxagliptin, type 2 diabetes
1. INTRODUCTION
Metformin monotherapy frequently proves inadequate for glycaemic control in patients with type 2 diabetes (T2D), necessitating additional glucose‐lowering agents.1, 2, 3, 4 A patient‐centric approach is recommended to select an appropriate add‐on therapy.5 Injectable insulin may not appeal to the patient and is associated with body weight (BW) gain and an increased risk of hypoglycaemia. The ideal oral add‐on antihyperglycaemic agent should have long‐term efficacy, attain glycated haemoglobin A1c (HbA1c) targets without hypoglycaemia through complementary mechanisms of action,5, 6 and achieve BW reduction or neutrality.
Studies have reported that the sodium‐glucose cotransporter‐2 inhibitor (SGLT‐2i) dapagliflozin (DAPA) plus the dipeptidyl peptidase‐4 inhibitor (DPP‐4i) saxagliptin (SAXA) in combination with metformin provides superior HbA1c reductions versus either agent added to metformin alone, with a lower risk of hypoglycaemia and BW gain.6, 7, 8, 9, 10, 11, 12 The risk of genital infection is lower in treatment regimens with the combination of DAPA + SAXA than DAPA monotherapy.13 However, validation is needed to determine the long‐term efficacy of DAPA + SAXA versus injectable insulin as an add‐on to metformin therapy in patients with T2D.
This Phase 3 study is part of a clinical programme supporting the development of a fixed‐dose combination (FDC) of DAPA 10 mg and SAXA 5 mg for T2D management. In an earlier 24‐week randomized trial, in patients with T2D that were inadequately controlled on metformin with or without sulphonylurea, co‐administration with DAPA + SAXA was non‐inferior to co‐administration with insulin glargine (INS) in achieving HbA1c reductions and reducing BW gain and hypoglycaemia.14 In the 28‐week extension, we compared the efficacy, tolerability and safety of DAPA + SAXA versus INS over 52 weeks with respect to HbA1c, BW and glycaemic control in patients with T2D that were inadequately controlled on metformin with or without sulphonylurea.
2. MATERIALS AND METHODS
2.1. Study design
This is a 52‐week, multicentre (112 centres across 11 countries), randomized, open‐label, two‐arm, parallel‐group, Phase 3b study (ClinicalTrials.gov identifier: NCT02551874; Figure 1).
Figure 1.

Study design. *Additional visits only for patients participating in the CGM substudy. #Increase in the daily dose of insulin (based on FPG and SMBG values) at weeks 8 and 12 was at the discretion of the investigator. The goal was to reach an acceptable and stable dose by week 12. After week 12, patients with confirmed central laboratory‐measured FPG values >200 mg/dL (11.1 mmol/L) were eligible for open‐label rescue medication (including further uptitration of daily dose of insulin). DAPA + SAXA, DAPA (10 mg/day) + SAXA (5 mg/day) + metformin ± SU; INS, INS (100 U/mL/day) + metformin ± SU. Abbreviations: CGM, continuous glucose monitoring; DAPA, dapagliflozin; FPG, fasting plasma glucose; INS, insulin glargine; SAXA, saxagliptin; SMBG, self‐monitored blood glucose; SU, sulphonylurea
The study was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice guidelines of the International Conference on Harmonisation, and approved by the relevant institutional review boards or independent ethics committees. All persons gave informed consent for both the short‐ and long‐term study periods before study inclusion.
Adult (age ≥18 years) male or female patients with T2D and inadequate glycaemic control (HbA1c ≥8% to ≤12%) receiving stable metformin therapy (≥1500 mg/day) with or without sulphonylurea (≥50% of maximal dose) for at least 8 weeks before screening were eligible. Study participants had body mass index (BMI) ≤45.0 kg/m2 at screening and baseline fasting plasma glucose (FPG) ≤270 mg/dL. Major exclusion criteria included type 1 diabetes, maturity‐onset diabetes of the young, secondary diabetes or diabetes insipidus, and a history of diabetic ketoacidosis or of renal impairment (creatinine clearance <60 mL/min and serum creatinine ≥1.5 mg/dL in men or ≥1.4 mg/dL in women). Participants were randomized 1:1 using an interactive voice response system stratified by sulphonylurea use with background metformin treatment to receive either DAPA + SAXA or INS for 24 weeks with a long‐term extension (Figure 2).
Figure 2.
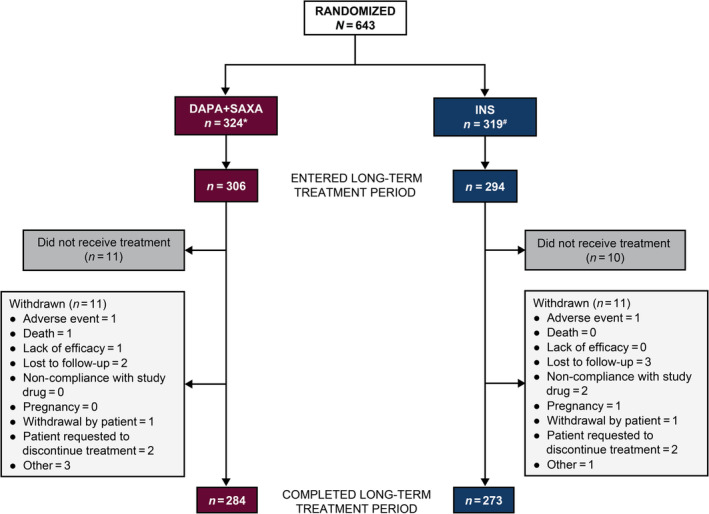
Patient disposition. *Number of patients receiving metformin + SU = 166, number of patients receiving metformin – SU = 158. #Number of patients receiving metformin + SU = 165, number of patients receiving metformin – SU = 154. DAPA + SAXA, DAPA (10 mg/day) + SAXA (5 mg/day) + metformin ± SU. INS, INS (100 U/mL/day) + metformin ± SU. Abbreviations: DAPA, dapagliflozin; INS, insulin glargine; N, total number of patients in the group; n, number of patients analysed; SAXA, saxagliptin; SU, sulphonylurea
DAPA 10 mg/day (BMS‐512148; BMS, New Brunswick, New Jersey) and SAXA 5 mg/day (BMS‐477118; BMS, Mount Vernon, Indiana) were administered orally as tablets, and 100 U/mL INS (Sanofi, Quebec, Canada) was administered as a subcutaneous injection. All patients continued to receive their previous dose regimen of metformin with or without sulphonylurea throughout the study. Insulin dose was self‐titrated by patients in 2‐U increments every 3 days, starting from 0.2 U/kg BW on day 1 until week 8, based on daily glucose monitoring and an FPG target of ≤100 mg/dL. Uptitration of the insulin dose was withheld in case of any unexplained hypoglycaemic events (FPG ≤70 mg/dL) in the preceding 3 days. The dose was increased at week 8 and/or week 12 at the investigator's discretion and was maintained stably, thereafter, to facilitate comparison of HbA1c with the main 24‐week study, unless there were safety concerns or rescue medication requirement. Patients with FPG ≥200 mg/dL after week 12 and up to and including week 24 and patients with HbA1c >8% after week 24 and up to and including week 52 were eligible for open‐label rescue medication. Insulin was recommended as the first‐line rescue therapy. Additionally, patients meeting rescue criteria received open‐label antihyperglycaemic rescue medication, initiated at the lowest starting dose and titrated according to the product label in the applicable country.
Patients completing the short‐term treatment period continued into the long‐term extension period, receiving the same open‐label study medication that they were randomized to on day 1 along with metformin with or without sulphonylurea. All patients performed a six‐point self‐monitored blood glucose (SMBG) on any three non‐consecutive days during the long‐term period and continued to record their fasting SMBG twice daily, study medication dosing, hypoglycaemic episodes and mealtimes. The investigator reviewed patients’ diary entries between visits to identify any hypoglycaemic events, including symptoms with or without SMBG ≤70 mg/dL.
2.2. Efficacy endpoints
Measurements of efficacy at week 52 included mean change in HbA1c and BW from baseline and achieving an optimal glycaemic response (HbA1c <7.0%) without hypoglycaemia. Assessments comprised central laboratory measurements of HbA1c and SMBG values during treatment to assess hypoglycaemia. BW was measured in kilograms or pounds using the same digital precision scales at each visit. Other endpoints included the proportion of patients requiring rescue medication or discontinuing due to lack of glycaemic control and change from baseline in the average postprandial glucose values measured by six‐point SMBG profiles over 52 weeks. Patient‐reported outcomes (PROs) were assessed using self‐administered PRO questionnaires (Phase V® Health Outcomes Information System Diabetes Module) comprising validated generic and diabetes‐specific modules of treatment satisfaction, quality of life and barriers to medication adherence.
2.3. Safety endpoints
Safety analyses were conducted using the treated patients’ data set (including data after rescue), defined as all patients who received at least one dose of the study medication. Safety and tolerability were evaluated based on the analyses of adverse events (AEs), vital signs, physical examinations, electrocardiograms, hypoglycaemia and clinical laboratory evaluations. AEs were classified by primary system organ class and the preferred term according to MedDRA20.0 and were sorted by decreasing frequency. All AEs (serious and non‐serious), including all hypoglycaemic events, were summarized by treatment group, where applicable. No formal comparisons were made between treatments. Two analyses‐related deaths were reported during the study in the DAPA + SAXA group, one each in the short‐ and long‐term periods. Serious AEs (SAEs), including hypoglycaemic events, were described in narratives regardless of the investigator's assessment of causality. All AEs that led to discontinuation were listed.
Separate summaries were provided for AEs of special interest (AESI) and included hypoglycaemic events, urinary tract infections, renal impairment, genital infections, hepatic injury, bone fractures, hypersensitivity reactions, volume depletion and cardiac failure. The AESI and serious AESI with an onset from day 1 of the short‐term treatment period through 4 and 30 days after the last dose date in the short‐ and long‐term treatment periods, respectively, occurred during the combined short‐ and long‐term periods.
2.4. Statistical analyses
Statistical analyses were performed using SAS® version 9.4 (SAS Institute, Cary, North Carolina) according to the statistical analysis plan. The analysis of efficacy endpoints was performed using a direct likelihood longitudinal repeated‐measures analysis, including the fixed categorical effects of treatment, week, randomization stratification factor (metformin with or without sulphonylurea background medication), and treatment‐by‐week interaction and the continuous fixed covariates of baseline measurement and baseline measurement‐by‐week interaction.15, 16 The intent‐to‐treat population comprised randomized subjects who received at least one dose of study medication (primary efficacy data set). Efficacy analyses were performed using the data collected before initiation of the rescue treatment or treatment discontinuation.
Kaplan–Meier plots of time‐to‐event variables were generated for the treatment groups. The 95% confidence interval (CI) was restricted to 0 or 1 if the estimated value of the lower bound was <0 or the upper bound was >1, respectively. An upper bound CI of <0.3% implied non‐inferiority of DAPA + SAXA versus INS group. No formal statistical testing was performed for safety endpoints; only summary statistics were provided.14
The data underlying the findings described in this study may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
3. RESULTS
3.1. Patients
In total, 1163 patients with T2D were enrolled from October 20, 2015 to October 19, 2016, of which 707 patients entered the lead‐in treatment phase. Overall, 650 patients were randomized: 324 received DAPA + SAXA treatment and 326 received INS. The mean total daily insulin dose at week 52 was 37.9 U. Seven patients from the INS group (1.1%) were excluded because they did not receive at least one dose of open‐label treatment. Of the 643 patients who received randomized treatment, 600 (93.3%) entered the long‐term treatment period. Most participants who received treatment (579; 96.2%) completed the study (Figure 2). Overall, 3.8% of patients discontinued study treatment during the 52‐week, open‐label treatment period; 3.7% in the DAPA + SAXA group and 3.9% in the INS group before week 52. Five patients (0.9%), two (0.7%) in the DAPA + SAXA group and three (1.1%) in the INS group, were lost to follow‐up. Four patients (0.7%), three (1.0%) from the DAPA + SAXA group and one (0.4%) from the INS group, withdrew from the study due to “other” reasons (personal emergency, incorrect randomization, significant cognitive impairment and inability to attend study visits). Four patients (0.7%), two (0.7%) in each treatment group, requested discontinuation of treatment and withdrew from the study. In total, 121 patients (18.8%) had at least one relevant protocol deviation. The most common deviation leading to complete exclusion was overall non‐compliance (i.e., patients who took <80% or >120% of their prescribed dose of study medication during the short‐term treatment period).
Patient demographics and baseline characteristics were well balanced between groups (Table 1). Most patients were white (80.4%); 54% were men. The mean ± standard deviation (SD) age was 55.5 ± 9.6 years and time since T2D diagnosis was 9.4 ± 6.3 years. The mean BW of patients was 89.6 ± 18.1 kg and the mean BMI was 32.2 ± 5.3 kg/m2. Most patients (92.5%) were overweight (BMI ≥25 kg/m2), while 62.4% of patients were obese (BMI ≥30 kg/m2). The mean ± SD estimated glomerular filtration rate was similar between the groups (94.6 ± 23.7 mL/min/1.73 m2 in the DAPA + SAXA group and 97.3 ± 21.8 mL/min/1.73 m2 in the INS group).
Table 1.
Patient demographics and baseline characteristics
| Characteristic | DAPA + SAXA (N = 324) | INS (N = 319) |
|---|---|---|
| Age, years, mean ± SD | 55.7 ± 9.52 | 55.3 ± 9.63 |
| Sex, n (%) | ||
| Male | 176 (54.3) | 171 (53.6) |
| Female | 148 (45.7) | 148 (46.4) |
| Race, n (%) | ||
| White | 263 (81.2) | 254 (79.6) |
| African American | 28 (8.6) | 35 (11.0) |
| Asian | 12 (3.7) | 12 (3.8) |
| American Indian | 12 (3.7) | 6 (1.9) |
| Native Hawaiian | 0 (0.0) | 1 (0.3) |
| Other | 9 (2.8) | 11 (3.4) |
| Duration of T2D, years, mean ± SD | 9.6 ± 6.5 | 9.3 ± 6.2 |
| HbA1c, %, mean ± SD | 9.0 ± 1.0 | 9.1 ± 1.1 |
| BW, kg, mean ± SD | 89.8 ± 17.7 | 89.4 ± 18.4 |
| BMI, kg/m2, mean ± SD | 32.5 ± 5.3 | 32.0 ± 5.4 |
| FPG, mg/dL, mean ± SD | 189.5 ± 55.5 | 188.6 ± 53.8 |
| eGFR, mL/min/1.73 m2, mean ± SD | 94.6 ± 23.7 | 97.3 ± 21.8 |
| Background therapiesa | ||
| Allowed concomitant medication, n (%) | 288 (88.9) | 279 (87.5) |
| HMG CoA reductase inhibitors | 139 (42.9) | 127 (39.8) |
| ACE inhibitors, plain | 111 (34.3) | 121 (37.9) |
| Platelet aggregation inhibitors, excluding heparin | 77 (23.8) | 83 (26.0) |
| β‐blockers, selective | 61 (18.8) | 65 (20.4) |
| Angiotensin II antagonists, plain | 44 (13.6) | 43 (13.5) |
| Propionic acid derivatives | 41 (12.7) | 39 (12.2) |
| Proton pump inhibitors | 39 (12.0) | 41 (12.9) |
| Anilides | 44 (13.6) | 35 (11.0) |
Abbreviations: ACE, angiotensin‐converting enzyme; BMI, body mass index; BW, body weight; DAPA, dapagliflozin; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HMG CoA, 3‐hydroxy‐3‐methylglutaryl coenzyme A; INS, insulin glargine; N, total number of patients in the group; n, number of patients analysed; SAXA, saxagliptin; SD, standard deviation; SU, sulphonylurea; T2D, type 2 diabetes.
DAPA + SAXA, DAPA (10 mg/day) + SAXA (5 mg/day) + metformin ± SU; INS, INS (100 U/mL/day) + metformin ± SU. Values are mean ± SD or n (%) unless otherwise stated.
Treated patients’ data. Percentages are based on the number of patients in the randomized data set.
Among the 643 patients in the randomized data set, 166 (51.2%) in the DAPA + SAXA group and 165 (51.7%) in the INS group received sulphonylurea at randomization, and all patients received metformin background therapy, regardless of treatment group or sulphonylurea use. The most common specific disease history terms (those reported in >10% of patients overall) were T2D (100%), hypertension (65.8%), hyperlipidaemia (41.7%), history of obesity (38.3%), dyslipidaemia (30.2%) and diabetic neuropathy (18.7%). Five patients (0.8%) had a history of amputation (one in the DAPA + SAXA group and four in the INS group). The use of allowed concomitant medications, reported for 288 patients (88.9%) in the DAPA + SAXA group and 279 patients (87.5%) in the INS group, was well balanced between the groups (Table 1 and Table S1; see Supporting Information). The most frequently used allowed concomitant medications were statins (41.4%), plain angiotensin‐converting enzyme inhibitors (36.1%), platelet aggregation inhibitors, excluding heparin (24.9%) and selective β‐blockers (19.6%). Both groups had a low incidence of disallowed concomitant medication usage, reported for nine patients (2.8%) in the DAPA + SAXA group and five patients (1.6%) in the INS group (Table S1; see Supporting Information). Treatment compliance was high and balanced between the groups.
3.2. Efficacy
All efficacy analyses of the long‐term treatment period were exploratory and reported for the randomized patient data set. Mean HbA1c decreased from baseline to week 52 in both groups (Figure 3A). The adjusted least squares (LS) mean change (95% CI) in HbA1c was −1.5% (−1.6%, −1.4%) for the DAPA + SAXA group and − 1.3% (−1.4%, −1.1%) for the INS group; the LS mean difference (95% CI) between the groups was −0.25% (−0.4%, −0.1%; P = 0.009).
Figure 3.

A, Percentage change from baseline in HbA1c over 52 weeks. Week 0 refers to the baseline value. Baseline is defined as patients in the randomized patient data set with non‐missing baseline assessment and at least one post‐baseline assessment. HbA1c assessments, collected after initiation of rescue treatment or collected more than 8 days after the last dose in the short‐term plus long‐term open‐label treatment period, were excluded from the analysis. n, number of patients. B, Change in BW from baseline over 52 weeks. Week 0 refers to the baseline value. Baseline is defined as patients in the randomized patient data set with non‐missing baseline assessment and at least one post‐baseline assessment. BW assessments, collected after initiation of rescue treatment or collected more than 8 days after the last dose in the short‐term plus long‐term open‐label treatment period, were excluded from the analysis. n, number of patients. C, Proportion of patients achieving adjusted optimal glycaemic response (HbA1c <7%) without hypoglycaemia at week 52. Percentages calculated on the randomized subject data set. *Patients with unknown status at week 52 and patients given rescue medication before week 52 were treated as non‐responders for the endpoint. #Based on the logistic regression method with adjustment for baseline HbA1c and randomization stratification factor (background medication of metformin ± SU). †Based on the logistic regression method with adjustment for baseline HbA1c (background medication of metformin ± SU and treatment by randomization stratification factor interaction). n, number of patients with an event of optimal glycaemic response at week 52. D, Proportion of patients requiring rescue or discontinuation due to lack of glycaemic control at week 52. Percentages calculated on the randomized subject data set. *Patients with unknown status at week 52 and patients given rescue medication before week 52 were treated as non‐responders for the endpoint. #Based on the logistic regression method with adjustment for baseline HbA1c and randomization stratification factor (background medication of metformin ± SU). n, number of patients needing rescue or discontinuation at week 52. DAPA + SAXA, DAPA (10 mg/day) + SAXA (5 mg/day) + metformin ± SU; INS, titrated INS (100 U/mL/day) + metformin ± SU. Abbreviations: BW, body weight; CI, confidence interval; DAPA, dapagliflozin; HbA1c, glycated haemoglobin; INS, insulin glargine; N, total number of patients in the treatment group; OR, odds ratio; SAXA, saxagliptin; SU, sulphonylurea
The mean reduction in BW from baseline to week 52 in the DAPA + SAXA group differed significantly from that in the INS group (Figure 3B). At week 52, the adjusted LS mean (95% CI) BW decreased with DAPA + SAXA by −1.8 kg (−2.4, −1.3), while increasing by +2.8 kg (2.2, 3.3) in the INS group. The LS mean difference (95% CI) between the two groups was −4.6 kg (−5.3, −3.8; P <0.001).
Overall, the proportion of patients [adjusted percentage (95% CI)] achieving an optimal glycaemic response (HbA1c <7%) at week 52 was 29.8% (24.8%, 35.3%) in the DAPA + SAXA group and 24.0% (19.5%, 29.3%) in the INS group, regardless of background sulphonylurea treatment. Optimal glycaemic response without hypoglycaemia was achieved in 57 patients (17.6%) in the DAPA + SAXA group [41 patients (25.9%) without background sulphonylurea group, 16 patients (9.6%) with background sulphonylurea] and 29 patients (9.1%) in the INS group [17 patients (11%) without background sulphonylurea group, 12 patients (7.3%) with background sulphonylurea] at week 52. The adjusted percentage (95% CI) of patients achieving an optimal glycaemic response without hypoglycaemia was 15.2% (11.6%, 19.8%) and 7.2% (4.8%, 10.6%) with DAPA + SAXA and INS, respectively [odds ratio (OR): 2.3; 95% CI: 1.4, 3.8] (Figure 3C). Confirmed hypoglycaemia was found in a smaller proportion of patients in the DAPA + SAXA group (28.7%) compared with the INS group (46.1%) (OR: 0.4; 95% CI: 0.3, 0.6), regardless of background sulphonylurea therapy.
Overall, 174 patients required rescue medication or discontinued the study due to lack of glycaemic control: 77 (23.8%) in the DAPA + SAXA group and 97 (30.4%) in the INS group at week 52. The adjusted percentage (95% CI) of patients requiring rescue medication or discontinuation at week 52 was 21.0% (16.7%, 26.1%) and 27.7% (22.8%, 33.3%) in the DAPA + SAXA and INS groups, respectively (OR: 0.7; 95% CI: 0.5, 1.0; Figure 3D).
Changes from baseline in average and postprandial glucose values were measured by a six‐point SMBG at week 52, which were similar across the groups [average SMBG level, LS mean (95% CI): DAPA + SAXA, −43.6 mg/dL (−48.2, −38.9); INS, −39.3 mg/dL (−44.2, −34.6)], with the DAPA + SAXA treatment causing greater decrease in mean postprandial SMBG level [DAPA + SAXA, −48.1 mg/dL (−53.2, −42.9)]; INS [−40.2 mg/dL (−45.7, −34.8)]. Decreases in FPG were evident at all time points beginning at week 1, with a mean change (95% CI) from baseline of −49.9 mg/dL (−56.9, −42.9) and −56.0 mg/dL (−65.0, −47.1) in the DAPA + SAXA and INS groups, respectively, at week 52 (Figure S1; see Supporting Information).
The mean overall satisfaction with treatment increased in both groups between baseline and week 52 (Table S2; see Supporting Information). The LS mean change [standard error (SE)] in the DAPA + SAXA group [9.4 (0.7)] was significantly greater (P = 0.006) than the INS group [6.0 (0.7)]. Treatment group comparisons of the regimen acceptance score, a subdomain of treatment satisfaction, also showed significantly better results with DAPA + SAXA at week 52 (LS mean difference: 4.2; P < 0.001). The short‐ and long‐term results were similar, indicating a significantly higher level of treatment satisfaction in patients receiving DAPA + SAXA than those on INS.
3.3. Safety
The mean ± SD duration exposure, regardless of rescue medication use, was similar between treatment groups (DAPA + SAXA, 338.5 ± 81.9 days; INS, 330.1 ± 93.4). Most patients in both treatment groups had a cumulative exposure of >270 days (>38 weeks). At least one AE was reported by 209 patients (64.5%) in the DAPA + SAXA group and 217 (68.0%) in the INS group (Table 2). AEs considered by the investigator to be treatment‐related were more common in the DAPA + SAXA group (11.1%) versus the INS group (4.7%). Two patients (0.6%) in the DAPA + SAXA group died during the study, one in the short‐term period (respiratory failure) and one in the long‐term period (pulmonary oedema); both deaths were not considered related to treatment. Hypoglycaemic events were fewer in the DAPA + SAXA group versus the INS group before rescue medication use. Neither group had any hypoglycaemic event leading to study drug discontinuation. The most frequently reported SAE was coronary artery disease (0.9% in the DAPA + SAXA group; 0.3% in the INS group), followed by congestive cardiac failure (0.6% in the DAPA + SAXA; 0.3% in the INS group) and acute coronary syndrome (0.3% in the DAPA + SAXA group; 0.6% in the INS group). No other SAE was reported for more than one patient. Diabetic ketoacidosis was reported by one patient in the INS group. There were no amputations in either treatment arm. No SAE was considered by the investigator to be related to treatment.
Table 2.
AEs (incident rate ≥5% for both AEs and SAEs) in any category during the 52‐week treatment period, regardless of rescue
| AE category | DAPA + SAXA (N = 324) n a (%) | INS (N = 319) n a (%) |
|---|---|---|
| At least one AE | 209 (64.5) | 217 (68.0) |
| Most common AEs | ||
| Viral upper respiratory tract infection | 25 (7.7) | 19 (6.0) |
| Upper respiratory tract infection | 17 (5.2) | 23 (7.2) |
| Urinary tract infection | 16 (4.9) | 11 (3.4) |
| Back pain | 15 (4.6) | 13 (4.1) |
| Headache | 12 (3.7) | 22 (6.9) |
| Dizziness | 8 (2.5) | 1 (0.3) |
| Diarrhoea | 7 (2.2) | 11 (3.4) |
| Nausea | 5 (1.5) | 8 (2.5) |
| Hypertension | 1 (0.3) | 15 (4.7) |
| AEs of special interest | ||
| Hypoglycaemic events | 100 (30.9) | 153 (48.0) |
| Severe hypoglycaemia | 0 (0.0) | 3 (0.9) |
| Urinary tract infections | 20 (6.2) | 16 (5.0) |
| Renal impairment/failure | 9 (2.8) | 3 (0.9) |
| Blood creatinine increased | 5 (1.5) | 1 (0.3) |
| GFR decreased | 5 (1.5) | 1 (0.3) |
| Creatinine renal clearance decreased | 1 (0.3) | 0 (0.0) |
| Genital infections | 16 (4.9) | 2 (0.6) |
| Cardiac failure | 5 (1.5) | 7 (2.2) |
| Acute congestive heart failure | 2 (0.6) | 0 (0.0) |
| Pulmonary oedema | 1 (0.3) | 0 (0.0) |
| Bone fractures | 3 (0.9) | 4 (1.3) |
| Hypersensitivity reactions | 14 (4.3) | 11 (3.4) |
| Hepatic injury | 7 (2.2) | 10 (3.1) |
| Volume depletion (hypotension, dehydration, hypovolaemia) | 6 (1.9) | 2 (0.6) |
| Hypotension | 4 (1.2) | 1 (0.3) |
| BP decreased | 1 (0.3) | 0 (0.0) |
| Syncope | 1 (0.3) | 1 (0.3) |
| Any AE leading to death | 2 (0.6) | 0 (0.0) |
| AE leading to discontinuation of IPb | 8 (2.5) | 2 (0.6) |
| Investigations | 5 (1.5) | 0 (0.0) |
| Blood creatinine increased | 2 (0.6) | 0 (0.0) |
| GFR decreased | 2 (0.6) | 0 (0.0) |
| Blood potassium abnormal | 1 (0.3) | 0 (0.0) |
| Respiratory disorders | 2 (0.6) | 0 (0.0) |
| COPD | 1 (0.3) | 0 (0.0) |
| Pulmonary oedema | 1 (0.3) | 0 (0.0) |
| Respiratory failure | 1 (0.3) | 0 (0.0) |
| Vascular disorders | 1 (0.3) | 0 (0.0) |
| Hypotension | 1 (0.3) | 0 (0.0) |
| Nervous system disorders | 0 (0.0) | 1 (0.3) |
| Ischaemic stroke | 0 (0.0) | 1 (0.3) |
| Neoplasms (benign, malignant, unspecified) | 0 (0.0) | 1 (0.3) |
| Colon cancer | 0 (0.0) | 1 (0.3) |
| Treatment‐emergent AEsc | 36 (11.1) | 15 (4.7) |
| Infections and infestations | 15 (4.6) | 2 (0.6) |
| Urinary tract infection | 5 (1.5) | 2 (0.6) |
| Vulvovaginal mycotic infection | 3 (0.9) | 0 (0.0) |
| Gastrointestinal disorders | 5 (1.5) | 3 (0.9) |
| Nervous system disorders | 3 (0.9) | 1 (0.3 |
| Dizziness | 3 (0.9) | 0 (0.0) |
| Cardiac disorders | 1 (0.3) | 0 (0.0) |
| Palpitations | 1 (0.3) | 0 (0.0) |
| Metabolism and nutrition disorders | 2 (0.6) | 1 (0.3) |
| Vascular disorders | 1 (0.3) | 0 (0.0) |
| At least one SAE | 20 (6.2) | 13 (4.1) |
| Most common SAEsd | ||
| Cardiac disorders | 7 (2.2) | 4 (1.3) |
| Coronary artery disease | 3 (0.9) | 1 (0.3) |
| Cardiac failure congestive | 2 (0.6) | 1 (0.3) |
| Acute coronary syndrome | 1 (0.3) | 2 (0.6) |
| Acute myocardial infarction | 1 (0.3) | 0 (0.0) |
| Neoplasms (benign, malignant, unspecified) | 4 (1.2) | 1 (0.3) |
| Injury, poisoning, procedural complications | 4 (1.2) | 1 (0.3) |
| Respiratory disorders | 3 (0.9) | 0 (0.0) |
| COPD | 1 (0.3) | 0 (0.0) |
| Pulmonary embolism | 1 (0.3) | 0 (0.0) |
| Renal and urinary disorders | 2 (0.6) | 1 (0.3) |
| Haematuria | 1 (0.3) | 0 (0.0) |
| Acute kidney injury | 0 (0.0) | 1 (0.3) |
| Gastrointestinal disorders | 1 (0.3) | 0 (0.0) |
| Metabolism and nutrition disorders | 0 (0.0) | 1 (0.3) |
| Diabetic ketoacidosis | 0 (0.0) | 1 (0.3) |
| SAE leading to discontinuation of IP | 2 (0.6) | 1 (0.3) |
Abbreviations: AE, adverse event; BP, blood pressure; COPD, chronic obstructive pulmonary disease; DAPA, dapagliflozin; GFR, glomerular filtration rate; INS, titrated insulin glargine; IP, investigational product (study drug); N, total number of patients in the group; n, number of patients analysed; PT, preferred term; SAE, serious adverse event; SAXA, saxagliptin; SOC, system organ class; SU, sulphonylurea.
DAPA + SAXA, DAPA (10 mg/day) + SAXA (5 mg/day) + metformin ± SU; INS, INS (100 U/mL/day) + metformin ± SU.
Patients with multiple events in the same category were counted only once in that category. Patients with events in more than one category were counted once in each of those categories. The deaths were considered non‐treatment‐related.
Number (%) of patients with an AE leading to discontinuation of IP, sorted by international order for SOC and by decreasing frequency of PT within each SOC for DAPA + SAXA. Patients with multiple AEs leading to discontinuation were counted once for each SOC/PT.
Causally related to any study medication, as assessed by the investigator.
SAEs by system organ class and preferred term during the 52‐week open‐label treatment period, regardless of rescue.
4. DISCUSSION
Guidelines recommend combination therapies with glucose‐lowering agents to manage the progressive nature of T2D when metformin monotherapy fails to achieve or sustain glycaemic control.5 To the best of our knowledge, this study is the first to evaluate the efficacy and safety of an FDC of DAPA + SAXA versus INS in patients with T2D inadequately controlled on metformin with or without sulphonylurea over a long period (52 weeks). The mean total daily insulin dose at week 52 in the current study was 37.9 U, which was lower than that reported in most treat‐to‐target studies where the daily insulin dose varied between 37 and 62 U.17, 18, 19, 20 Glycaemic endpoint in the INS arm is dependent on the aggressiveness of the insulin dose titration. However, although hypoglycaemia is relatively rare at insulin initiation, it is essential to achieve a balance between a lower glucose level and hypoglycaemic risk. In the current study, a proportion of patients without hypoglycaemia did not achieve target HbA1c levels, indicating that the insulin titrations could have been more aggressive. However, daytime glucose control was crucial, as approximately 50% of patients receiving insulin had hypoglycaemia, which would probably have become pronounced with a more aggressive titration schedule. FDCs with different classes of glucose‐lowering agents are advantageous over monotherapies, as they enhance adherence to therapy and reduce treatment burden, improve glycaemic control, increase patient satisfaction and lower healthcare costs.21, 22 An FDC therapy with DAPA + SAXA differs from the other classes of antihyperglycaemic drugs by its complementary mechanism of action, low risk for hypoglycaemia without increasing the risk of BW gain,10, 23, 24 and no requirement for dose titration, which simplifies the therapy compared with insulin. Results of our short‐term trial showed that oral combination therapy with DAPA 10 mg and SAXA 5 mg resulted in non‐inferior reductions in HbA1c, with a beneficial BW profile and a lower prevalence of hypoglycaemia versus insulin, regardless of the presence or absence of background sulphonylurea.14 The current extension study demonstrated the sustained efficacy of oral combination therapy with DAPA + SAXA as add‐on to metformin with or without sulphonylurea over 52 weeks in reducing HbA1c and BW from baseline, with a lower prevalence of hypoglycaemia versus INS. These results were consistent with those of a previous 52‐week study comparing DAPA with sulphonylureas as add‐ons to metformin therapy; hypoglycaemic events with DAPA were significantly lower versus sulphonylureas.25
The clinically relevant and sustained change in BW from baseline to week 52 in the DAPA + SAXA group, which was significantly greater compared with the INS group, supports results from previous studies showing that treatment with DAPA alone or in combination with SAXA was associated with BW reduction, whereas insulin treatment was associated with BW gain.10, 23, 26 The higher proportion of patients achieving optimal glycaemic control after 52 weeks with a lower incidence of hypoglycaemia in the DAPA + SAXA group compared with those receiving INS corroborates previous evidence.27, 28, 29 However, evidence is lacking regarding the effect of these drugs alone compared with insulin in achieving optimal glycaemic control without hypoglycaemia or BW gain. More patients required rescue medication in the INS group than the DAPA + SAXA group.
Overall, AEs and SAEs were balanced between the two groups. The safety and tolerability profile of DAPA + SAXA was consistent with that of the individual agents, supporting observations from previous studies.5, 10, 12, 30, 31 Oral administration of glucose‐lowering drugs is often preferred to injections by both patients and healthcare professionals.32 A treatment strategy including oral, insulin‐independent, glucose‐lowering drugs instead of injectable basal insulin in patients with uncontrolled T2D is a welcome alternative. The function of DPP‐4is with sulphonylurea mechanistically relies on residual β‐cell activity, while SGLT‐2is work complementarily to DPP4is, reducing hyperglycaemia through renal excretion of glucose, independent of insulin secretion, thus reducing glucotoxicity33 and β‐cell burden. The risk of genital infections with combination therapy is lower than that observed with DAPA alone, which is suggestive of a protective effect.13, 34, 35
An important strength of this study is its randomized and multinational design. Most patients who entered the long‐term extension completed the study. These results are particularly promising because patients included in the study had high baseline HbA1c levels. Hence, the oral treatment combination of DAPA + SAXA is an efficient and valid alternative to insulin for patients with high glucose levels. However, a limitation is that the study was not blinded, although both patient groups received active treatment. Furthermore, although DAPA + SAXA showed durability up to 52 weeks when added to metformin with or without sulphonylurea, durability beyond 1 year with this combination remains to be determined.
In conclusion, the present study showed that administration of an oral combination therapy of DAPA + SAXA allowed patients with T2D to achieve better glycaemic control compared with INS over a long period. DAPA + SAXA prevented BW gain and lowered the risk of hypoglycaemia. Previous studies have shown an association between lower hypoglycaemia, reduced risk of cardiovascular disease and cognitive impairment, and better quality of life.36, 37 SGLT2is are associated with a beneficial cardiovascular profile.38, 39 Therefore, compared with insulin, the combination therapy of DAPA + SAXA as an add‐on to metformin with or without sulphonylurea is a viable long‐term treatment option for patients with T2D because of easier administration, lower healthcare resource utilization and a beneficial metabolic profile.
AUTHOR CONTRIBUTIONS
E.J. and E.E. were involved in the study design and conduct, interpretation of results, and drafting and critically reviewing the manuscript. N.D. was involved in the study design, statistical analysis plan, analysis of data, interpretation of results, and drafting and critically reviewing the manuscript. M.L., T.V. and S.A.J. were involved in the study design, recruitment, analysis of data, interpretation of results, and drafting and critically reviewing the manuscript. R.G.‐S. was involved in the interpretation of results and critically reviewing the manuscript. T.V. is the guarantor of this work and, as such, takes responsibility for the integrity of the data and the accuracy of the data analysis.
CONFLICT OF INTEREST
E.J., E.E. and N.D. are employees of AstraZeneca. M.L. has received research grants from AstraZeneca, Dexcom, Novo Nordisk and Pfizer; has been a consultant or received honoraria from AstraZeneca, Dexcom, Eli Lilly, Medtronic and Novo Nordisk; and has participated in advisory boards for MSD and Novo Nordisk. S.A.J. is a consultant for AstraZeneca, Eli Lilly and Janssen. T.V. has served on scientific advisory panels and/or speakers’ bureaus or has served as a consultant to and/or received research support from Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Eli Lilly, MSD/Merck, Novo Nordisk, Sanofi and Sun Pharma. R.G.S. is a former employee of AstraZeneca and current employee of BMS.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
The authors thank the patients, their families, and all investigators involved in this study. Medical writing support was provided by Debasri Mukherjee, PhD, of Cactus Communications, and was funded by AstraZeneca.
Vilsbøll T, Ekholm E, Johnsson E, et al. Efficacy and safety of dapagliflozin plus saxagliptin versus insulin glargine over 52 weeks as add‐on to metformin with or without sulphonylurea in patients with type 2 diabetes: A randomized, parallel‐design, open‐label, Phase 3 trial. Diabetes Obes Metab. 2020;22:957–968. 10.1111/dom.13981
An abstract of this work has been presented as a poster at the 54th Annual Meeting of the European Association for the Study of Diabetes, 1–5 October 2018, Berlin, Germany.
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13981.
Funding information AstraZeneca
REFERENCES
- 1. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61(12):2461‐2498. [DOI] [PubMed] [Google Scholar]
- 2. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies MJ, D'Alessio DA, Fradkin J, et al. Correction to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2019;62(5):873. [DOI] [PubMed] [Google Scholar]
- 4. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care. 2018;41(2):356‐363. [DOI] [PubMed] [Google Scholar]
- 5. Mathieu C, Ranetti AE, Li D, et al. Randomized, double‐blind, phase 3 trial of triple therapy with dapagliflozin add‐on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38(11):2009‐2017. [DOI] [PubMed] [Google Scholar]
- 6. Forst T, Alghdban MK, Fischer A, et al. Sequential treatment escalation with dapagliflozin and saxagliptin improves beta cell function in type 2 diabetic patients on previous metformin treatment: an exploratory mechanistic study. Horm Metabol Res. 2018;50(5):403‐407. [DOI] [PubMed] [Google Scholar]
- 7. Dey J. SGLT2 inhibitor/DPP‐4 inhibitor combination therapy ‐ complementary mechanisms of action for management of type 2 diabetes mellitus. Postgrad Med. 2017;129(4):409‐420. [DOI] [PubMed] [Google Scholar]
- 8. Lingvay I. Sodium glucose cotransporter 2 and dipeptidyl peptidase‐4 inhibition: promise of a dynamic duo. Endocr Pract. 2017;23(7):831‐840. [DOI] [PubMed] [Google Scholar]
- 9. Williams DM, Stephens JW. Combination therapy with saxagliptin and dapagliflozin for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2015;16(15):2373‐2379. [DOI] [PubMed] [Google Scholar]
- 10. Rosenstock J, Hansen L, Zee P, et al. Dual add‐on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double‐blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38(3):376‐383. [DOI] [PubMed] [Google Scholar]
- 11. Muller‐Wieland D, Kellerer M, Cypryk K, et al. Efficacy and safety of dapagliflozin or dapagliflozin plus saxagliptin versus glimepiride as add‐on to metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20(11):2598‐2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mathieu C, Herrera Marmolejo M, Gonzalez Gonzalez JG, et al. Efficacy and safety of triple therapy with dapagliflozin add‐on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(11):1134‐1137. [DOI] [PubMed] [Google Scholar]
- 13. Yu H, Woo VC. Emerging use of combination therapies for the management of type 2 diabetes ‐ focus on saxagliptin and dapagliflozin. Diabetes Metab Syndr Obes. 2017;10:317‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vilsboll T, Ekholm E, Johnsson E, Dronamraju N, Jabbour S, Lind M. Dapagliflozin plus saxagliptin add‐on therapy compared with insulin in patients with type 2 diabetes poorly controlled by metformin with or without sulphonylurea therapy: a randomized clinical trial. Diabetes Care. 2019;42(8):1464‐1472. [DOI] [PubMed] [Google Scholar]
- 15. Zhang M, Tsiatis AA, Davidian M. Improving efficiency of inferences in randomized clinical trials using auxiliary covariates. Biometrics. 2008;64(3):707‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsiatis AA, Davidian M, Zhang M, Lu X. Covariate adjustment for two‐sample treatment comparisons in randomized clinical trials: a principled yet flexible approach. Stat Med. 2008;27(23):4658‐4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riddle MC, Rosenstock J, Gerich J. The treat‐to‐target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080‐3086. [DOI] [PubMed] [Google Scholar]
- 18. Yki‐Jarvinen H, Juurinen L, Alvarsson M, et al. Initiate Insulin by Aggressive Titration and Education (INITIATE): a randomized study to compare initiation of insulin combination therapy in type 2 diabetic patients individually and in groups. Diabetes Care. 2007;30(6):1364‐1369. [DOI] [PubMed] [Google Scholar]
- 19. Buse JB, Wolffenbuttel BH, Herman WH, et al. DURAbility of basal versus lispro mix 75/25 insulin efficacy (DURABLE) trial 24‐week results: safety and efficacy of insulin lispro mix 75/25 versus INS added to oral antihyperglycemic drugs in patients with type 2 diabetes. Diabetes Care. 2009;32(6):1007‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Swinnen SG, Dain MP, Aronson R, et al. A 24‐week, randomized, treat‐to‐target trial comparing initiation of INS once‐daily with insulin detemir twice‐daily in patients with type 2 diabetes inadequately controlled on oral glucose‐lowering drugs. Diabetes Care. 2010;33(6):1176‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bailey CJ, Day C. Fixed‐dose single tablet antidiabetic combinations. Diabetes Obes Metab. 2009;11(6):527‐533. [DOI] [PubMed] [Google Scholar]
- 22. Blonde L, San Juan ZT. Fixed‐dose combinations for treatment of type 2 diabetes mellitus. Adv Ther. 2012;29(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 23. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2010;375(9733):2223‐2233. [DOI] [PubMed] [Google Scholar]
- 24. DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32(9):1649‐1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add‐on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52‐week, double‐blind, active‐controlled noninferiority trial. Diabetes Care. 2011;34(9):2015‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on BW, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020‐1031. [DOI] [PubMed] [Google Scholar]
- 27. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double‐blind, placebo‐controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frederich R, McNeill R, Berglind N, Fleming D, Chen R. The efficacy and safety of the dipeptidyl peptidase‐4 inhibitor saxagliptin in treatment‐naïve patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetol Metab Syndr. 2012;4(1):36‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bailey CJ, Marx N. Cardiovascular protection in type 2 diabetes: insights from recent outcome trials. Diabetes Obes Metab. 2019;21(1):3‐14. [DOI] [PubMed] [Google Scholar]
- 30. Del Prato S, Rosenstock J, Garcia‐Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post‐hoc analysis of concomitant add‐on versus sequential add‐on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab. 2018;20(6):1542‐1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mathieu C, Catrinoiu D, Ranetti AE, et al. Characterization of the open‐label lead‐in period of two randomized controlled phase 3 trials evaluating dapagliflozin, saxagliptin, and metformin in type 2 diabetes. Diabetes Ther. 2018;9(4):1703‐1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Factors associated with psychological insulin resistance in individuals with type 2 diabetes. Diabetes Care. 2010;33(8):1747‐1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium‐glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124(2):499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matthaei S, Aggarwal N, Garcia‐Hernandez P, et al. One‐year efficacy and safety of saxagliptin add‐on in patients receiving dapagliflozin and metformin. Diabetes Obes Metab. 2016;18(11):1128‐1133. [DOI] [PubMed] [Google Scholar]
- 35. Matthaei S, Catrinoiu D, Celinski A, et al. Randomized, double‐blind trial of triple therapy with saxagliptin add‐on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care. 2015;38(11):2018‐2024. [DOI] [PubMed] [Google Scholar]
- 36. Zinman B, Marso SP, Christiansen E, Calanna S, Rasmussen S, Buse JB. Hypoglycemia, cardiovascular outcomes, and death: the LEADER experience. Diabetes Care. 2018;41(8):1783‐1791. [DOI] [PubMed] [Google Scholar]
- 37. Standl E, Stevens SR, Armstrong PW, et al. Increased risk of severe hypoglycemic events before and after cardiovascular outcomes in TECOS suggests an at‐risk type 2 diabetes frail patient phenotype. Diabetes Care. 2018;41(3):596‐603. [DOI] [PubMed] [Google Scholar]
- 38. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 39. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


