Summary
This phase 2 study evaluated the activity and safety of ibrutinib, a Bruton’s tyrosine kinase inhibitor, plus rituximab in adults with previously untreated follicular lymphoma. Patients received once‐daily ibrutinib 560 mg continuously plus once‐weekly rituximab 375 mg/m2 for 4 weeks beginning Week 1 (Arm 1, n = 60) or Week 9 (following an 8‐week ibrutinib lead‐in) to explore biomarkers (Arm 2, n = 20). The primary endpoint was the best overall response rate (ORR). The median age was 58 years; most had an Eastern Cooperative Oncology Group Performance Status of 0 (74%) and Stage III/IV disease (84%). At a median study follow‐up of 34 months in Arm 1 and 29 months in Arm 2, ORRs were 85% [95% confidence interval (CI) 73–93] and 75% (95% CI 51–91), respectively, with complete responses in 40% and 50%. The median duration of response was not reached in either arm; 30‐month progression‐free and overall survival rates were 67% and 97% (Arm 1) and 65% and 100% (Arm 2). The most common adverse events were fatigue, diarrhoea and nausea. Higher grade (Grade 3/4) haematological, haemorrhagic and cardiac events occurred infrequently. Ibrutinib plus rituximab was active and tolerable in first‐line follicular lymphoma.
Keywords: follicular lymphoma, ibrutinib, rituximab
Introduction
Follicular lymphoma (FL), a neoplasm of germinal centre B cells, is the most common subtype of indolent non‐Hodgkin lymphoma (Armitage & Weisenburger, 1998; Relander et al., 2010) with a yearly USA incidence of approximately 3·18 new cases per 100 000 persons (Morton et al., 2006). FL frequently presents at advanced stage and commonly recurs following conventional therapy (Gallagher et al., 1986; Armitage & Weisenburger, 1998; Hiddemann et al., 2005).
Rituximab, a chimeric anti‐CD20 monoclonal antibody, is a first‐line treatment for FL administered as monotherapy or with chemotherapy (Subramanian et al., 2017). Overall response rates (ORRs) to first‐line, single‐agent rituximab range from 45% to 75% in FL, with complete response (CR) rates from 7% to 37%, depending on treatment duration and assessment timing (Colombat et al., 2001; Hainsworth et al., 2002). Addition of rituximab to conventional chemotherapeutic regimens (i.e. R‐CHOP [rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone], R‐CVP [rituximab, cyclophosphamide, vincristine, and prednisone] and BR [bendamustine and rituximab]) achieves ORRs of 81–100% in previously untreated patients (Hiddemann et al., 2005; Marcus et al., 2005; van Oers et al., 2006; Marcus et al., 2008; Rummel et al., 2013; Flinn et al., 2014; Mondello et al., 2018). However, chemoimmunotherapy regimens are associated with considerable toxicity (Colombat et al., 2001; Hainsworth et al., 2002; Hiddemann et al., 2005; Marcus et al., 2005; van Oers et al., 2006; Marcus et al., 2008; Mondello et al., 2018). Combining rituximab with targeted therapies may yield disease control with manageable toxicity.
Inhibition of Bruton’s tyrosine kinase (BTK) blocks signalling downstream of the B‐cell receptor (Honigberg et al., 2010), thereby interfering in a key pathway in the pathogenesis of most B‐cell lymphomas, including FL (Kuppers, 2005; Irish et al., 2006). Ibrutinib, the only once‐daily BTK inhibitor, is indicated by the US Food and Drug Administration (FDA) for the treatment of several B‐cell malignancies and chronic graft‐versus‐host disease. In a phase 1 dose‐escalation study of ibrutinib in patients with B‐cell malignancies, those with relapsed/refractory FL demonstrated an ORR of 38% (six of 16, including three CRs) (Advani et al., 2013). Subsequent phase 2 studies of single‐agent ibrutinib in relapsed/refractory FL demonstrated ORRs of 21–38% (CR rates: 11–13%) (Gopal et al., 2016; Bartlett et al., 2018), suggesting ibrutinib may be an active, safe treatment for patients with FL. This phase 2 study evaluated the combination of ibrutinib and rituximab in first‐line FL.
Patients and methods
Study design
Study PCYC‐1125‐CA (NCT01980654) was an open‐label, non‐randomised, multicentre, phase 2 study that evaluated ibrutinib in combination with rituximab in patients with previously untreated FL. The study included two treatment arms (Arms 1 and 2) to assess the efficacy and safety of ibrutinib plus rituximab, with Arm 2 (including an 8‐week ibrutinib lead‐in) added to explore biomarkers during single‐agent ibrutinib therapy before rituximab administration. Patients were enrolled independently in each arm, with enrolment in Arm 2 starting once enrolment in Arm 1 was almost completed. Dosing regimens are described in Fig 1. At study closure, all patients who were receiving ibrutinib treatment were provided the option to continue on ibrutinib in a long‐term follow‐up study.
Fig 1.
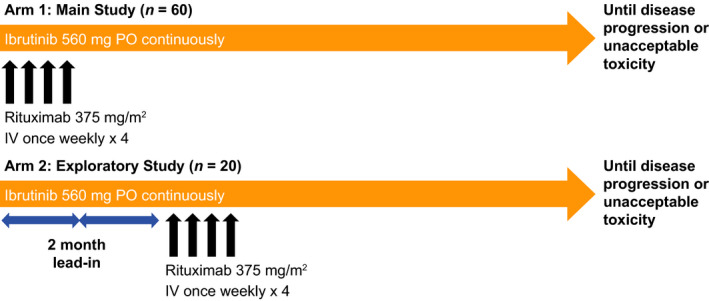
Treatment schema. IV, intravenous; PO, by mouth.
Eligibility criteria
Adults aged ≥18 years with previously untreated, histologically documented FL (Grades 1–3A) were eligible if they had Stage II–IV disease, life expectancy >3 months, one or more measurable lesions ≥2 cm in longest diameter by computed tomography (CT) scan, and the investigator’s expectation that the patient would benefit from therapy, with or without meeting Groupe d'Etude des Lymphomes Folliculaires (GELF) criteria (see Supplement for additional eligibility criteria).
Endpoints and assessments
The primary endpoint was the ORR, defined as the proportion of patients achieving either CR or partial response (PR) as a best response using Cheson criteria (Cheson et al., 2007). Secondary endpoints included duration of response (DOR; i.e. interval between patients’ first documented CR/PR and progressive disease [PD]/death), progression‐free survival (PFS) and overall survival (OS); imaging assessment details are described in the Supplement). The median time to response was defined as the time from initial study treatment to the initial response of either CR or PR.
Treatment‐emergent adverse events (TEAEs) were reported from the first dose of study drug through 30 days after the last dose and classified by severity (Common Terminology Criteria for Adverse Events, version 4·03) and relatedness to study treatment.
Bone marrow and peripheral blood (PB) samples were collected for BCL‐2 t(14;18) testing to evaluate minimal residual disease (MRD) for FL. Viably cryopreserved samples were assessed using a validated BCL‐2 real‐time quantitative polymerase chain reaction assay with a sensitivity of ≥1 × 104 cells (0·01%; Hematologics Inc., Seattle, WA, USA).
Statistical analyses
Efficacy analyses were performed in the modified intent‐to‐treat population (eligible patients who received ≥1 dose of ibrutinib). A sample size of approximately 60 eligible patients in Arm 1 was planned; the sample size of 20 patients in Arm 2 had insufficient power for a comparison with Arm 1. Patients’ ORRs were compared to the average ORR of 53% in first‐line rituximab for FL from two comparable studies (Hainsworth et al., 2002; Freedman et al., 2009); see Supplement for statistical considerations.
Study oversight
The study was implemented in accordance with local regulations, the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. The study protocol and all amendments were approved by institutional review boards and independent ethics committees. Patients provided written informed consent.
Results
Patients
A total of 80 patients were enrolled (Arm 1, n = 60; Arm 2, n = 20). The median patient age was 58 years, with 26 (33%) patients aged ≥65 years. Most patients had an Eastern Cooperative Oncology Group Performance Status of 0 (74%) and a disease Stage of III (40%) or IV (44%); 50% of patients in each arm had a high‐risk Follicular Lymphoma International Prognostic Index (FLIPI) score (i.e. 3–5) and 16% of patients had bulky disease (longest axis of target lesion ≥7 cm). Patient demographics and clinical characteristics in the treatment arms are shown in Table 1.
Table 1.
Baseline demographic and clinical characteristics.
| Variable |
Arm 1 (n = 60) |
Arm 2 (n = 20) |
|---|---|---|
| Age, years | ||
| Median (range) | 58 (32–84) | 55 (30–75) |
| ≥65, n (%) | 18 (30) | 8 (40) |
| Male sex, n (%) | 28 (47) | 12 (60) |
| Cytopenia at baseline, n (%) | ||
| ANC ≤1·5 × 109/L | 2 (3) | 0 (0) |
| Haemoglobin ≤110 g/l | 5 (8) | 0 (0) |
| Platelets ≤100 × 109/l | 2 (3) | 0 (0) |
| Disease Grade, n (%) | ||
| 1 | 19 (32) | 11 (55) |
| 2 | 35 (58) | 8 (40) |
| 3a | 6 (10) | 1 (5) |
| Disease Stage, n (%) | ||
| II | 12 (20) | 1 (5) |
| III | 24 (40) | 8 (40) |
| IV | 24 (40) | 11 (55) |
| ECOG Performance Status, n (%) | ||
| 0 | 47 (78) | 12 (60) |
| 1 | 13 (22) | 8 (40) |
| ≥2 | 0 | 0 |
| FLIPI score, n (%) | ||
| Low risk (0–1) | 7 (12) | 1 (5) |
| Intermediate risk (2) | 23 (38) | 9 (45) |
| High risk (3–5) | 30 (50) | 10 (50) |
| Time from first FL diagnosis to first study dose, months, median (range) | 3·1 (0·1–178·5) | 2·6 (0·2–57·5) |
| Target lesion SPD, cm2, median (range) | 23·7 (2·9–135·5) | 15·7 (3·0–161·0) |
| Bulky disease, n (%) | ||
| ≥5 cm in ≥1 site | 23 (38) | 3 (15) |
| ≥7 cm in ≥1 site | 12 (20) | 1 (5) |
| ≥10 cm in ≥1 site | 2 (3) | 1 (5) |
ANC, absolute neutrophil count; ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; FLIPI, Follicular Lymphoma International Prognostic Index; SPD, sum of the product of diameters.
Treatment exposure
All patients received ibrutinib and were included in the modified intent‐to‐treat population for response evaluations; all patients in Arm 1 and 17 of 20 patients in Arm 2 received rituximab. Of the three patients in Arm 2 who did not receive rituximab, two had PD during the first 8 weeks of treatment with single‐agent ibrutinib and one had Grade 3 abnormal hepatic function. The primary reasons for discontinuation of ibrutinib were study termination by the sponsor with the option to enrol in a long‐term follow‐up study, PD, patient decision and adverse events (AEs) (Table II). Two (10%) patients in Arm 2 discontinued rituximab and continued ibrutinib treatment per investigator decision (Table II). The median (range) duration of ibrutinib treatment was 25·5 (0·8–41·9) months in Arm 1 and 28·9 (1·0–35·0) months in Arm 2. At study closure, 24 patients remained on ibrutinib in a long‐term follow‐up study.
Table 2.
Patient disposition and study treatment exposure.
|
Arm 1 (n = 60) |
Arm 2 (n = 20) |
|
|---|---|---|
| Received ibrutinib, n (%) | 60 (100) | 20 (100) |
| Discontinued ibrutinib, n (%) | ||
| Study termination by the sponsor with the option to enrol in a long‐term follow‐up study | 16 (27) | 8 (40) |
| Progressive disease | 15 (25) | 6 (30) |
| Patient decision | 12 (20) | 3 (15) |
| Adverse event | 12 (20) | 2 (10) |
| Investigator decision | 3 (5) | 0 |
| Death | 2 (3) | 0 |
| Patient required a prohibited concomitant medication | 0 | 1 (5) |
| Duration of treatment with ibrutinib, months, median (range) | 25·5 (0·8–41·9) | 28·9 (1·0–35·0) |
| Relative ibrutinib dose intensity, % (range) | 97·4 (63·2–100) | 97·13 (79·7–100) |
| Received rituximab, n (%) | 60 (100) | 17 (85) |
| Discontinued rituximab, n (%) | ||
| Investigator decision | 0 | 2 (10) |
| No. of rituximab doses, median (range) | 4·0 (3–4) | 4·0 (0–4) |
Activity
The median (range) follow‐up in Arm 1 was 34 (5·8–42+) months and 29 (4–35) months in Arm 2. In Arm 1, the ORR based on the best response was 85% (95% confidence interval [CI] 73–93), including 24 (40%) patients with CR and 27 (45%) with PR (Fig 2A). Because the lower bound of the 95% CI in Arm 1 (73%) is much higher than the 53% (the null hypothesis of the study), this study suggests favourable efficacy of the combination compared with rituximab alone for previously untreated patients with low‐grade FL. In Arm 2, the ORR after rituximab initiation was 75% (95% CI 51–91), including 10 (50%) patients with CR and five (25%) with PR; 15% of patients in each arm demonstrated stable disease. The median (range) time to initial response was 2·7 (1·1–16·5) months in Arm 1 and 4·3 (1·8–18·0) months in Arm 2. Consistent with the response evaluations, all patients in Arm 1 experienced an improvement in the sum of the product of diameters (SPD) of their target lesions (Fig 3A). In Arm 2, the SPD was reduced in 17 (85%) patients at week 8 (Fig 3B) and in 18 (90%) patients overall (Fig 3C). In some patients, responses deepened (Fig 2B) and a greater reduction in SPD was seen over time (data not shown). The median DOR was not reached in either arm (Arm 1: 95% CI 30·2 months–not estimable [NE]; Arm 2: 95% CI 24·6 months–NE), with median follow‐up times of 30·5 months in Arm 1 and 22·1 months in Arm 2.
Fig 2.
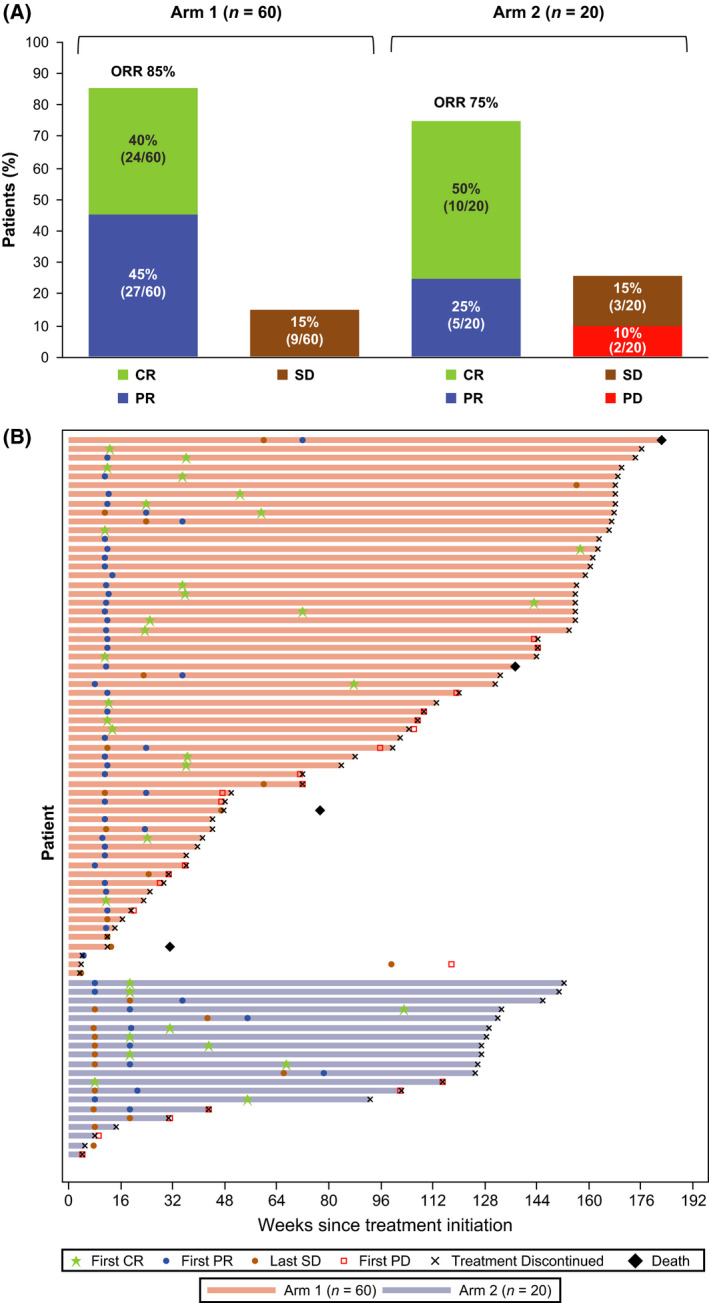
Best response (A) and treatment and response duration (B). Data are presented for all patients, as all received ≥1 dose of ibrutinib and were included in the modified intent‐to‐treat population. Response was evaluated according to the International Working Group criteria for non‐Hodgkin lymphoma. CR, complete response; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Fig 3.
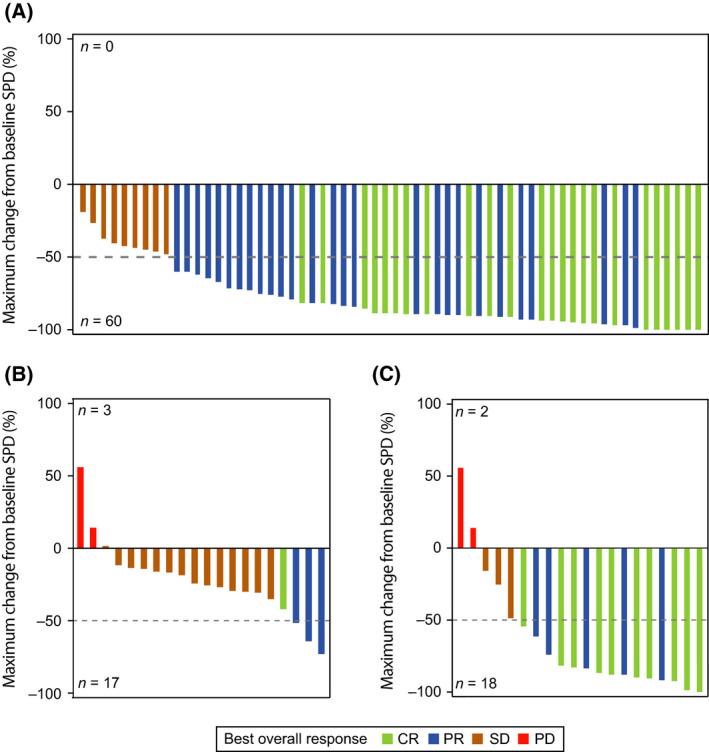
Percentage change in tumour size. Maximum percentage change from baseline in tumour size, calculated as the target lesion SPD, is shown separately for Arm 1 (A), Arm 2 at week 8 with single‐agent ibrutinib before rituximab initiation (B), and Arm 2 overall (C). In Arm 1, 51 of 60 (85%) patients had a >50% reduction in SPD, and in Arm 2, 15 of 20 (75%) patients had a >50% reduction in SPD. Colours correspond to each patient’s best response; n represents the number of patients with an increase or decrease from baseline in tumour size. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; SPD, sum of the product of diameters.
The median PFS was 41·9 months (95% CI 31·6–41·9) in Arm 1 and was not reached (95% CI 23·5 months–NE) in Arm 2 (Fig 4). PFS rates at 30 months for Arms 1 and 2 were 67% (95% CI 52–79) and 65% (95% CI 37–83), respectively. At study closure, 10 patients had a follow‐up of ≥36 months. The earliest enrolled patient who had the longest PFS of 41·9 months died, and nine patients who were still on ibrutinib were rolled over to a long‐term follow‐up study. Thus, the estimate of median PFS by the Kaplan–Meier method is not reliable for this study due to the lack of sufficient follow‐up time. Four patients in Arm 1 died; the 30‐month OS rate for Arms 1 and 2 were 97% (95% CI 87–99) and 100% (95% CI 100–100), respectively. Arm 1 patients were on study for a median (range) of 34 (5·8–42+) months and Arm 2 patients for 29 (4·4–35) months. Subsequent anticancer therapy was received by 18 (30%) patients in Arm 1 and five (25%) in Arm 2.
Fig 4.
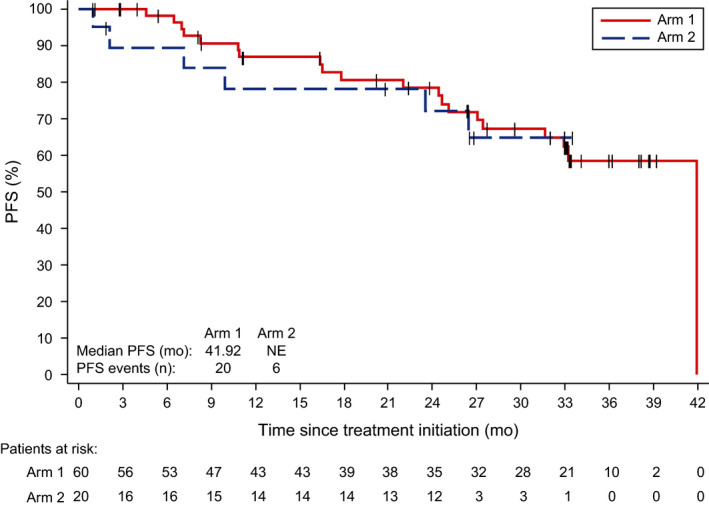
PFS. Data are shown for each study arm as of the data cut‐off for the end of the study. Some patients did not have documented progressive disease or death as of the cut‐off date. NE, not estimable; PFS, progression‐free survival.
An analysis of tumour burden based on FLIPI scores showed high response rates (78–100%) in both arms, regardless of risk scores. The median PFS and OS were not reached in either arm for patients with low‐ or intermediate‐risk FLIPI scores; of those with high‐risk FLIPI scores, the median PFS and OS were 41·9 months in Arm 1 and not reached in Arm 2. BCL‐2 translocation in PB was used to determine the MRD status of 28 patients with evaluable samples. Major breakpoint or minor cluster regions were detected in 12 patients at baseline; after CR, all 12 were MRD negative (<0·01% FL cells in PB).
Safety
Treatment‐emergent adverse event frequency was similar between treatment arms. All patients experienced AEs, and 51 (64%) experienced a Grade 3/4 TEAE (Table 3). Serious TEAEs occurred in 20 (25%) patients; the most common serious TEAEs were pyrexia (n = 5, 6%) and pneumonia (n = 4, 5%).
Table 3.
Summary of TEAEs.
| TEAE | Arm 1 (n = 60) | Arm 2 (n = 20) | Total (n = 80) | |||
|---|---|---|---|---|---|---|
| Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | |
| Any TEAE, n (%) | 60 (100) | 37 (62) | 20 (100) | 14 (70) | 80 (100) | 51 (64) |
| TEAEs in ≥ 20% of patients in any arm, n (%) | ||||||
| Fatigue | 41 (68) | 7 (12) | 15 (75) | 1 (5) | 56 (70) | 8 (10) |
| Diarrhoea | 34 (57) | 1 (2) | 12 (60) | 4 (20) | 46 (58) | 5 (6) |
| Nausea | 29 (48) | 1 (2) | 10 (50) | 0 | 39 (49) | 1 (1) |
| Myalgia | 20 (33) | 3 (5) | 9 (45) | 2 (10) | 29 (36) | 5 (6) |
| Cough | 20 (33) | 0 | 6 (30) | 1 (5) | 26 (33) | 1 (1) |
| Maculopapular rash | 18 (30) | 3 (5) | 8 (40) | 2 (10) | 26 (33) | 5 (6) |
| Headache | 19 (32) | 0 | 5 (25) | 1 (5) | 24 (30) | 1 (1) |
| Arthralgia | 20 (33) | 2 (3) | 3 (15) | 2 (10) | 23 (29) | 4 (5) |
| Constipation | 16 (27) | 1 (2) | 6 (30) | 0 | 22 (28) | 1 (1) |
| Dizziness | 13 (22) | 0 | 9 (45) | 1 (5) | 22 (28) | 1 (1) |
| Vomiting | 16 (27) | 1 (2) | 6 (30) | 0 | 22 (28) | 1 (1) |
| Muscle spasms | 16 (27) | 0 | 5 (25) | 1 (5) | 21 (26) | 1 (1) |
| Pyrexia | 16 (27) | 2 (3) | 5 (25) | 3 (15) | 21 (26) | 5 (6) |
| Stomatitis | 10 (17) | 1 (2) | 11 (55) | 0 | 21 (26) | 1 (1) |
| Upper respiratory infection | 13 (22) | 0 | 7 (35) | 0 | 20 (25) | 0 |
| Dry eye | 15 (25) | 0 | 4 (20) | 0 | 19 (24) | 0 |
| Urinary tract infection | 13 (22) | 1 (2) | 5 (25) | 2 (10) | 18 (23) | 3 (4) |
| Paraesthesia | 9 (15) | 1 (2) | 8 (40) | 1 (5) | 17 (21) | 2 (3) |
| Abdominal pain | 12 (20) | 1 (2) | 4 (20) | 0 | 16 (20) | 1 (1) |
| Dyspnoea | 12 (20) | 1 (2) | 4 (20) | 0 | 16 (20) | 1 (1) |
| Infusion‐related reaction | 14 (23) | 0 | 1 (5) | 0 | 15 (19) | 0 |
| Oropharyngeal pain | 10 (17) | 0 | 5 (25) | 0 | 15 (19) | 0 |
| Vision blurred | 8 (13) | 0 | 6 (30) | 0 | 14 (18) | 0 |
| Memory impairment | 9 (15) | 0 | 5 (25) | 0 | 14 (18) | 0 |
| Sinusitis | 7 (12) | 0 | 5 (25) | 0 | 12 (15) | 0 |
| Hypomagnesaemia | 6 (10) | 0 | 4 (20) | 0 | 10 (13) | 0 |
| Lacrimation increased | 3 (5) | 0 | 6 (30) | 0 | 9 (11) | 0 |
| Peripheral swelling | 5 (8) | 0 | 4 (20) | 0 | 9 (11) | 0 |
| Dry mouth | 4 (7) | 0 | 4 (20) | 0 | 8 (10) | 0 |
| Any serious TEAE, n (%) | 14 (23) | 11 (18) | 6 (30) | 5 (25) | 20 (25) | 16 (20) |
| Any Grade 5 TEAE, n (%) | 2 (3) | 2 (3) | 0 | 0 | 2 (3) | 2 (3) |
| Dose modifications due to a TEAE, n (%) | ||||||
| Ibrutinib dose reduction | 9 (15) | 6 (10) | 5 (25) | 4 (20) | 14 (18) | 10 (13) |
| Withholding of rituximab dose | 1 (2) | 1 (2) | 1 (5) | 1 (5) | 2 (3) | 2 (3) |
| Discontinuation due to a TEAE, n (%) | ||||||
| Discontinuation of ibrutinib | 14 (23) | 11 (18) | 2 (10) | 1 (5) | 16 (20) | 12 (15) |
| Discontinuation of rituximab | 0 | 0 | 0 | 0 | 0 | 0 |
TEAE, treatment‐emergent adverse event.
Most patients (96%) experienced TEAEs deemed related to ibrutinib, including 54% with a Grade 3/4 TEAE. The most frequently reported ibrutinib‐related TEAEs were fatigue (n = 52, 65%), diarrhoea (n = 39, 49%), nausea (n = 35, 44%), myalgia (n = 26, 33%) and maculopapular rash (n = 25, 31%). Rituximab‐related TEAEs were reported for 67 (84%) patients, including 18 (23%) with a Grade 3/4 rituximab‐related TEAE. The most common rituximab‐related TEAEs were fatigue (n = 39, 49%) and diarrhoea (n = 25, 31%).
Treatment‐emergent adverse events led to dose reductions of ibrutinib in 14 (18%) patients, mostly due to fatigue (n = 6, 8%). Rituximab was withheld in two patients due to TEAEs (increased aminotransferase levels and macular rash). TEAEs led to ibrutinib discontinuation in 16 (20%) patients; no patient discontinued rituximab due to a TEAE. Four deaths were deemed unrelated to study treatment: Hodgkin lymphoma, multisystem organ failure (exact cause unknown), and two unknown causes. The death from Hodgkin lymphoma and one of the deaths from unknown causes occurred several months after study discontinuation.
The frequency of Grade 3/4 haematological TEAEs was low, with neutropenia reported in seven (9%) patients and febrile neutropenia in two (3%). No Grade 3/4 anaemia or thrombocytopenia events were reported. Bleeding (any grade) was reported in 32 (40%) patients. Two Grade 3/4 bleeding events (rectal and ear haemorrhage) occurred. Cardiac TEAEs occurred in 11 (14%) patients: cardiac flutter (n = 2); palpitations (n = 2); and atrial flutter, irregular heart rate, bradycardia, sinus bradycardia, tachycardia, sinus tachycardia, paroxysmal tachycardia, ventricular tachycardia and syncope (n = 1 each). Six (8%) patients experienced atrial fibrillation, including two (3%) with Grade 3/4 events. Two patients experienced Grade 3/4 cardiac TEAEs (ventricular tachycardia and syncope). No opportunistic infections occurred except one case of clinically asymptomatic lung cryptococcosis.
Second malignancies [defined by a conservative Standardised Medical Dictionary for Regulatory Activities (MedDRA) Query] were reported in seven (9%) patients: non‐malignant skin cancer (n = 2) and transformation to diffuse large B‐cell lymphoma, fallopian tube cancer, gastrointestinal stromal tumour, lung adenocarcinoma and neuroendocrine tumour (n = 1 each).
Discussion
The current standard of care for first‐line FL is rituximab or obinutuzumab, with or without chemotherapy or radioimmunotherapy (Subramanian et al., 2017). Rituximab‐based combination regimens (e.g. R‐CHOP, R‐CVP, BR, rituximab‐lenalidomide) demonstrated efficacy in patients with previously untreated FL, with ORRs of 81–100% and CR rates of 20–72% (Czuczman et al., 2004; Hiddemann et al., 2005; Marcus et al., 2005; Marcus et al., 2008; Rummel et al., 2013; Flinn et al., 2014; Fowler et al., 2014; Martin et al., 2017; Mondello et al., 2018; Morschhauser et al., 2018). No single regimen is currently favoured in clinical practice (Subramanian et al., 2017). The high response rates on rituximab plus chemo‐immunotherapy can be accompanied by substantial toxicity. Obinutuzumab plus chemo‐immunotherapy in a patient population with high tumour bulk also demonstrates high response rates (89%), but is associated with significant cytopenia (Marcus et al., 2017). Due to the relatively favourable toxicity profile, single‐agent rituximab is often recommended for patients with low tumour burden or who are not expected to tolerate chemo‐immunotherapy; however, single‐agent rituximab is associated with lower remission rates (ORR 47–73%) compared to chemotherapy combinations in first‐line treatment of FL (Colombat et al., 2001; Hainsworth et al., 2002). Data sufficient for assessing tumour burden according to GELF criteria were not systematically collected, and tumour burden was assessed via an analysis of bulky disease.
The primary objective, achieving an ORR surpassing that of single‐agent rituximab in FL (53%) (Hainsworth et al., 2002; Freedman et al., 2009), was reached with ibrutinib and rituximab in Arm 1 (85%, 95% CI 73–93), suggesting favourable efficacy of the combination versus rituximab alone. Arm 2 was exploratory and was not powered for primary endpoint assessment.
The ORR of ≥75% observed is also higher than ORRs (21–38%) reported for single‐agent ibrutinib in phase 1 and 2 studies of relapsed/refractory FL (Advani et al., 2013; Gopal et al., 2016; Bartlett et al., 2018). The higher ORR may be due to the addition of rituximab and to the previously untreated versus relapsed/refractory FL patient populations. Although the study was not designed and powered to compare across treatment arms, we did not observe different response rates in regard to rituximab infusion timing. Of note, two patients in Arm 2 who did not receive rituximab at Week 9 because of PD did not stabilise during study treatment; all patients in Arm 1 achieved stable disease or better.
The efficacy of ibrutinib plus rituximab combination therapy can be compared with published studies using non‐chemotherapeutic rituximab regimens in patients with FL. In the RESORT (Rituximab Extended Schedule or Retreatment Trial) study, 70·8% of patients with Grade 1 or 2 FL receiving induction rituximab monotherapy (four doses of 375 mg/m2 weekly) achieved a PR or better, with 11·8% achieving CR (including unconfirmed CR); among responsive patients subsequently assigned to receive maintenance rituximab therapy (375 mg/m2 every 13 weeks), 78% were progression‐free at 3 years, and those assigned to receive retreatment with rituximab upon PD had a 50% 3‐year PFS rate (Kahl et al., 2014). In a separate study, untreated patients with FL who received rituximab induction therapy (four doses of 375 mg/m2 weekly) and achieved an objective response or stable disease and subsequently received maintenance rituximab therapy (375 mg/m2 weekly for 4 weeks repeated every 6 months) achieved a response rate of 76% (Hainsworth et al., 2002). Interpretation of these findings, as well as those from the RESORT study, should take into consideration that patients who progressed after rituximab induction were excluded; thus, the population was selected for patients responsive to rituximab. In a study involving patients with asymptomatic, advanced‐stage, low tumour burden FL, the overall response rate was 84% (CR/CR unconfirmed/PR; CR, 69%) with rituximab induction therapy followed by rituximab maintenance therapy (375 mg/m2 every 4 weeks followed by 12 infusions of rituximab given at 2‐monthly intervals for 2 years). At month 25, an ORR of 57% (CR 33%) with rituximab induction therapy (375 mg/m2 every 4 weeks) was reported (Ardeshna et al., 2014). These data are noteworthy given the similarity in baseline disease characteristics compared with the present study (similar proportions of patients with FL Grade 1 or 2 and Stage II, III or IV disease), although the present study had slightly more patients with a high‐risk FLIPI score. In the Swiss Group for Clinical Cancer Research (SAKK) 35/10 study, after a median follow‐up of 4 years, untreated patients with Grade I–IIIa FL had substantially improved PFS with rituximab combination therapy; the median PFS was 5 years with rituximab (375 mg/m2 on day 1 and at weeks 1–4 and 12–15) plus lenalidomide (15 mg daily for 18 weeks), whereas the median PFS was 2·3 years with single‐agent rituximab (same dose and regimen) (Zucca et al., 2019). Combination therapy with a triplet regimen of rituximab, lenalidomide and ibrutinib was evaluated in previously untreated FL and demonstrated an ORR of 94% and a CR rate of 38% (Ujjani et al., 2016). Taken together, these results and the present study’s median PFS of 41·9 months on rituximab‐ibrutinib treatment demonstrate the benefit of non‐chemotherapeutic rituximab combination regimens for FL.
Minimal residual disease analysis was performed for patients who achieved CR, but was limited due to sample availability. No evidence of MRD was found in 12 patients [with major breakpoint or minor cluster regions detected in BCL‐2 t(14;18) at baseline] who achieved a CR. Ongoing collaborations with investigators from similar studies in indolent lymphoma to identify and analyse larger sample sets, including genetic analyses and MRD, may generate additional predictive and prognostic biomarkers.
The combination of ibrutinib plus rituximab was well tolerated, with a low rate of treatment discontinuation due to TEAEs. No new safety signals were identified, but, as expected, the proportions of patients who experienced diarrhoea, nausea and maculopapular rash over the course of study treatment were higher than those reported in patients with FL on single‐agent rituximab (Hainsworth et al., 2002; Freedman et al., 2009). Few individual Grade 3/4 TEAEs were observed in more than five (6%) patients. Notably, Grade 3/4 haematological, haemorrhagic and cardiac TEAEs, as well as infections were uncommon in this study, and the rate of Grade 3/4 neutropenia was 9%. The rate of Grade 3/4 AEs overall (64%) was similar to the rates observed in the RELEVANCE study [Rituximab Lenalidomide versus Any Chemotherapy] for rituximab plus lenalidomide (65%) and rituximab plus chemotherapy (R‐CHOP, R‐CVP, or BR, 68%) (Morschhauser et al., 2018).
In general, the safety profile of ibrutinib plus rituximab was similar to that of single‐agent ibrutinib (Advani et al., 2013; Gopal et al., 2016; Bartlett et al., 2018). The present study’s low rate of haematological TEAEs contrasts with the safety profiles seen with regimens that combine rituximab and chemotherapy in first‐line FL. For example, patients with previously untreated FL who received R‐CVP had a 24% rate of Grade 3/4 neutropenia (Marcus et al., 2008) and 63% of patients with previously untreated FL given R‐CHOP had Grade 3/4 granulocytopenia (Hiddemann et al., 2005). Similarly, in the RELEVANCE study in first‐line FL, the rituximab‐chemotherapy combination (either R‐CHOP, R‐CVP, or BR) showed a 50% rate of Grade 3/4 neutropenia (Morschhauser et al., 2018). The rates of Grade 3/4 neutropenia with the combination of ibrutinib plus rituximab (9%) compared favourably with another non‐chemotherapy containing combination of rituximab plus lenalidomide also evaluated in the RELEVANCE study (32%) (Morschhauser et al., 2018). However, differences in patient populations and prescribed evaluations between the present analysis and RELEVANCE make additional investigation of ibrutinib plus rituximab treatment necessary to draw definitive conclusions about the combination’s relative efficacy and toxicity.
A limitation of the present analysis was that GELF criteria were not systematically collected for all patients. Tumour burden was assessed by an analysis of bulky disease. As there was no requirement for patients to have a high tumour burden, many patients with low tumour burden were enrolled. Only 20% of patients in Arm 1 and 5% of patients in Arm 2 had bulky disease (target lesion longest axis ≥7 cm in one or more sites). Furthermore, even smaller proportions of patients in Arm 1 (3%) and Arm 2 (5%) had bulky disease with a target lesion longest axis ≥10 cm in one or more sites. The effect of tumour burden on response to treatment is being studied in an ongoing phase 3 study.
In conclusion, the combination of ibrutinib with four weekly infusions of rituximab demonstrated clinical activity and durable responses in first‐line FL. This combination was well tolerated, with a manageable overall safety profile consistent with those of single‐agent ibrutinib or rituximab. Additional randomised studies are needed to determine whether ibrutinib plus rituximab provides additional benefit over rituximab alone in the untreated setting. Based on the results of this study, an international phase 3 study, PCYC‐1141‐CA (PERSPECTIVE; NCT02947347), is ongoing. PERSPECTIVE is evaluating ibrutinib plus rituximab versus rituximab plus placebo and includes rituximab maintenance in patients who are elderly and/or unfit, including those with low tumour burden.
Data sharing agreement
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu
Author contributions
Nathan H. Fowler, Karl Eckert, Darrin M. Beaupre and Jutta K. Neuenburg designed the study; Nathan H. Fowler, Loretta Nastoupil, Sven De Vos, Mark Knapp, Ian W. Flinn, Robert Chen, Ranjana H. Advani, Sumeet Bhatia, Peter Martin, Raul Mena, Richard Eric Davis, Sattva S. Neelapu and M. Lia Palomba contributed to the accrual and treatment of patients and data acquisition; Karl Eckert, Jerry Ping, Darrin M. Beaupre, Melannie Co and Jutta K. Neuenburg analysed the data. All authors contributed to interpretation of the data, critically reviewed or revised the manuscript, approved the final manuscript for publication and vouch for data accuracy and completeness.
Conflicts of interest
Nathan H. Fowler received research funding and consulting fees from Celgene, Roche, Janssen, AbbVie and TG Therapeutics; and travel/accommodation expenses from Janssen, Celgene, TG Therapeutics and Roche. Loretta Nastoupil received research funding from Celgene, Genentech, Janssen, Lam Therapeutics, TG Therapeutics and Karus Therapeutics; and honoraria from Bayer, Celgene, Genentech, Gilead, Janssen, Novartis, Spectrum and TG Therapeutics. Sven De Vos received consulting fees from Bayer and Verastem. Mark Knapp received research funding from Genentech, Pharmacyclics LLC, an AbbVie Company and Merck; and consulting fees from Kite Pharma and Incyte. Ian W. Flinn received research funding from AbbVie, AcertaPharma, Agios, ArQule, Astra Zeneca, BeiGene, Calithera, Celgene, Constellation, Curis, Forma, Forty Seven, Genentech, Gilead, Incyte, Infinity, Janssen, Juno, Karyopharm Therapeutics, Kite Pharma, Merck, MorphoSys, Novartis, Pfizer, Pharmacyclics LLC, an AbbVie Company, Portola, Roche, Takeda, Teva, TG Therapeutics, Trillium, Unum and Verastem; consulting fees from AbbVie, Seattle Genetics, TG Therapeutics and Verastem. Robert Chen has no conflicts of interest to disclose. Ranjana H. Advani received research funding from Seattle Genetics, Genentech, Forty Seven, Merck, Celgene, Millennium, Agensys, Kura Therapeutics, Pharmacyclics LLC, an AbbVie Company and Regeneron; and consulting fees from Seattle Genetics, Spectrum, Roche/Genentech, Pharmacyclics LLC, an AbbVie Company, NanoString, Gilead/Kite Pharma, Bristol‐Myers Squibb, AstraZeneca, Autolus, Takeda and Kyowa. Sumeet Bhatia is employed and has a leadership role with CHOP LLC; has stock ownership in Acceleron, Agios, Amgen, Blueprint, Forty Seven, Loxo Oncology, Nektar and Rial; has received consulting fees from Agendia, Amgen, Eli Lilly and Genomic Health; and has received honoraria from Agendia, Amgen, Genomic Health and Rial. Peter Martin received consulting fees from Celgene, Janssen, AstraZeneca, Sandoz and Karyopharm. Raul Mena has no conflicts of interest to disclose. Richard Eric Davis received research funding from Karus Therapeutics. Sattva S. Neelapu received research funding from Gilead/Kite Pharma, Merck, Bristol‐Myers Squibb, Cellectis, Pharmacyclics LLC, an AbbVie Company, Poseida, Karus Therapeutics, Acerta and Unum Therapeutics; consulting fees from Gilead/Kite Pharma, Merck, Celgene, Novartis, Unum Therapeutics, Pfizer, Precision Biosciences, CellMedica and Incyte; honoraria from BioAscend, Medscape and Dava Oncology; and received travel/accommodation expenses from Gilead/Kite Pharma, Merck, Celgene, Novartis, Unum Therapeutics, Pfizer and Precision Biosciences. Karl Eckert and Jerry Ping are employed by Pharmacyclics LLC, an AbbVie Company and have stock ownership in AbbVie. Melannie Co was previously employed by Pharmacyclics LLC, an AbbVie Company and has stock ownership in AbbVie. Darrin M. Beaupre was previously employed by Pharmacyclics LLC, an AbbVie Company; is employed with Pfizer; has stock ownership in, received travel/accommodation expenses from and received research funding from Pfizer and AbbVie; and has patents/royalties with AbbVie. Jutta K. Neuenburg is employed by Pharmacyclics LLC, an AbbVie Company. M. Lia Palomba received consulting fees from Merck, Pharmacyclics LLC, an AbbVie Company and Celgene; received honoraria from and has patents/royalties with Seres and Juno; and has stock ownership in Seres.
Supporting information
Data S1. Supplement.
Acknowledgments
We thank all patients who participated in the PCYC‐1125‐CA phase 2 study and their families, as well as the study sub‐investigators, research nurses and co‐ordinators at each study site. We also thank Catherine Cheung for clinical study support and data collection. Medical writing and editorial assistance was provided by Kimberly Brooks, PhD, CMPP and supported by funding from Pharmacyclics, LLC, an AbbVie Company. This study was sponsored by Pharmacyclics, LLC, an AbbVie Company.
References
- Advani, R.H. , Buggy, J.J. , Sharman, J.P. , Smith, S.M. , Boyd, T.E. , Grant, B. , Kolibaba, K.S. , Furman, R.R. , Rodriguez, S. , Chang, B.Y. , Sukbuntherng, J. , Izumi, R. , Hamdy, A. , Hedrick, E. & Fowler, N.H. (2013) Bruton tyrosine kinase inhibitor ibrutinib (PCI‐32765) has significant activity in patients with relapsed/refractory B‐cell malignancies. Journal of Clinical Oncology, 31, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshna, K.M. , Qian, W. , Smith, P. , Braganca, N. , Lowry, L. , Patrick, P. , Warden, J. , Stevens, L. , Pocock, C.F. , Miall, F. , Cunningham, D. , Davies, J. , Jack, A. , Stephens, R. , Walewski, J. , Ferhanoglu, B. , Bradstock, K. & Linch, D.C. (2014) Rituximab versus a watch‐and‐wait approach in patients with advanced‐stage, asymptomatic, non‐bulky follicular lymphoma: an open‐label randomised phase 3 trial. The Lancet Oncology, 15, 424–435. [DOI] [PubMed] [Google Scholar]
- Armitage, J.O. & Weisenburger, D.D. (1998) New approach to classifying non‐Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non‐Hodgkin's Lymphoma Classification Project. Journal of Clinical Oncology, 16, 2780–2795. [DOI] [PubMed] [Google Scholar]
- Bartlett, N.L. , Costello, B.A. , LaPlant, B.R. , Ansell, S.M. , Kuruvilla, J.G. , Reeder, C.B. , Thye, L.S. , Anderson, D.M. , Krysiak, K. , Ramirez, C. , Qi, J. , Siegel, B.A. , Griffith, M. , Griffith, O.L. , Gomez, F. & Fehniger, T.A. (2018) Single‐agent ibrutinib in relapsed or refractory follicular lymphoma: a phase 2 consortium trial. Blood, 131, 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson, B.D. , Pfistner, B. , Juweid, M.E. , Gascoyne, R.D. , Specht, L. , Horning, S.J. , Coiffier, B. , Fisher, R.I. , Hagenbeek, A. , Zucca, E. , Rosen, S.T. , Stroobants, S. , Lister, T.A. , Hoppe, R.T. , Dreyling, M. , Tobinai, K. , Vose, J.M. , Connors, J.M. , Federico, M. & Diehl, V. (2007) Revised response criteria for malignant lymphoma. Journal of Clinical Oncology, 25, 579–586. [DOI] [PubMed] [Google Scholar]
- Colombat, P. , Salles, G. , Brousse, N. , Eftekhari, P. , Soubeyran, P. , Delwail, V. , Deconinck, E. , Haioun, C. , Foussard, C. , Sebban, C. , Stamatoullas, A. , Milpied, N. , Boue, F. , Taillan, B. , Lederlin, P. , Najman, A. , Thieblemont, C. , Montestruc, F. , Mathieu‐Boue, A. , Benzohra, A. & Solal‐Celigny, P. (2001) Rituximab (anti‐CD20 monoclonal antibody) as single first‐line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood, 97, 101–106. [DOI] [PubMed] [Google Scholar]
- Czuczman, M.S. , Weaver, R. , Alkuzweny, B. , Berlfein, J. & Grillo‐Lopez, A.J. (2004) Prolonged clinical and molecular remission in patients with low‐grade or follicular non‐Hodgkin's lymphoma treated with rituximab plus CHOP chemotherapy: 9‐year follow‐up. Journal of Clinical Oncology, 22, 4659–4664. [DOI] [PubMed] [Google Scholar]
- Flinn, I.W. , van der Jagt, R. , Kahl, B.S. , Wood, P. , Hawkins, T.E. , Macdonald, D. , Hertzberg, M. , Kwan, Y.L. , Simpson, D. , Craig, M. , Kolibaba, K. , Issa, S. , Clementi, R. , Hallman, D.M. , Munteanu, M. , Chen, L. & Burke, J.M. (2014) Randomized trial of bendamustine‐rituximab or R‐CHOP/R‐CVP in first‐line treatment of indolent NHL or MCL: the BRIGHT study. Blood, 123, 2944–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, N.H. , Davis, R.E. , Rawal, S. , Nastoupil, L. , Hagemeister, F.B. , McLaughlin, P. , Kwak, L.W. , Romaguera, J.E. , Fanale, M.A. , Fayad, L.E. , Westin, J.R. , Shah, J. , Orlowski, R.Z. , Wang, M. , Turturro, F. , Oki, Y. , Claret, L.C. , Feng, L. , Baladandayuthapani, V. , Muzzafar, T. , Tsai, K.Y. , Samaniego, F. & Neelapu, S.S. (2014) Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open‐label, phase 2 trial. The lancet Oncology, 15, 1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman, A. , Neelapu, S.S. , Nichols, C. , Robertson, M.J. , Djulbegovic, B. , Winter, J.N. , Bender, J.F. , Gold, D.P. , Ghalie, R.G. , Stewart, M.E. , Esquibel, V. & Hamlin, P. (2009) Placebo‐controlled phase III trial of patient‐specific immunotherapy with mitumprotimut‐T and granulocyte‐macrophage colony‐stimulating factor after rituximab in patients with follicular lymphoma. Journal of Clinical Oncology, 27, 3036–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher, C.J. , Gregory, W.M. , Jones, A.E. , Stansfeld, A.G. , Richards, M.A. , Dhaliwal, H.S. , Malpas, J.S. & Lister, T.A. (1986) Follicular lymphoma: prognostic factors for response and survival. Journal of Clinical Oncology, 4, 1470–1480. [DOI] [PubMed] [Google Scholar]
- Gopal, A.K. , Schuster, S.J. , Fowler, N. , Trotman, J. , Hess, G. , Hou, J.Z. , Yacoub, A. , Lill, M. , Martin, P. , Vitolo, U. , Jurczak, W. , Morton, J. , Osmanov, D. , Gartenberg, G. , Vermeulen, J. , Balasubramanian, S. , Wang, S.S. , Deshpande, S. & Salles, G.A. (2016) Ibrutinib as treatment for chemoimmunotherapy‐resistant patients with follicular lymphoma: first results from the open‐label, multicenter, phase 2 DAWN study. Blood, 128, 1217. [DOI] [PubMed] [Google Scholar]
- Hainsworth, J.D. , Litchy, S. , Burris, H.A. III , Scullin, D.C. Jr , Corso, S.W. , Yardley, D.A. , Morrissey, L. & Greco, F.A. (2002) Rituximab as first‐line and maintenance therapy for patients with indolent non‐Hodgkin's lymphoma. Journal of Clinical Oncology, 20, 4261–4267. [DOI] [PubMed] [Google Scholar]
- Hiddemann, W. , Kneba, M. , Dreyling, M. , Schmitz, N. , Lengfelder, E. , Schmits, R. , Reiser, M. , Metzner, B. , Harder, H. , Hegewisch‐Becker, S. , Fischer, T. , Kropff, M. , Reis, H.E. , Freund, M. , Wormann, B. , Fuchs, R. , Planker, M. , Schimke, J. , Eimermacher, H. , Trumper, L. , Aldaoud, A. , Parwaresch, R. & Unterhalt, M. (2005) Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced‐stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low‐Grade Lymphoma Study Group. Blood, 106, 3725–3732. [DOI] [PubMed] [Google Scholar]
- Honigberg, L.A. , Smith, A.M. , Sirisawad, M. , Verner, E. , Loury, D. , Chang, B. , Li, S. , Pan, Z. , Thamm, D.H. , Miller, R.A. & Buggy, J.J. (2010) The Bruton tyrosine kinase inhibitor PCI‐32765 blocks B‐cell activation and is efficacious in models of autoimmune disease and B‐cell malignancy. PNAS, 107, 13075–13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish, J.M. , Czerwinski, D.K. , Nolan, G.P. & Levy, R. (2006) Altered B‐cell receptor signaling kinetics distinguish human follicular lymphoma B cells from tumor‐infiltrating nonmalignant B cells. Blood, 108, 3135–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl, B.S. , Hong, F. , Williams, M.E. , Gascoyne, R.D. , Wagner, L.I. , Krauss, J.C. , Habermann, T.M. , Swinnen, L.J. , Schuster, S.J. , Peterson, C.G. , Sborov, M.D. , Martin, S.E. , Weiss, M. , Ehmann, W.C. & Horning, S.J. (2014) Rituximab extended schedule or re‐treatment trial for low‐tumor burden follicular lymphoma: eastern cooperative oncology group protocol e4402. Journal of Clinical Oncology, 32, 3096–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppers, R. (2005) Mechanisms of B‐cell lymphoma pathogenesis. Nature Reviews Cancer, 5, 251–262. [DOI] [PubMed] [Google Scholar]
- Marcus, R. , Imrie, K. , Belch, A. , Cunningham, D. , Flores, E. , Catalano, J. , Solal‐Celigny, P. , Offner, F. , Walewski, J. , Raposo, J. , Jack, A. & Smith, P. (2005) CVP chemotherapy plus rituximab compared with CVP as first‐line treatment for advanced follicular lymphoma. Blood, 105, 1417–1423. [DOI] [PubMed] [Google Scholar]
- Marcus, R. , Imrie, K. , Solal‐Celigny, P. , Catalano, J.V. , Dmoszynska, A. , Raposo, J.C. , Offner, F.C. , Gomez‐Codina, J. , Belch, A. , Cunningham, D. , Wassner‐Fritsch, E. & Stein, G. (2008) Phase III study of R‐CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. Journal of Clinical Oncology, 26, 4579–4586. [DOI] [PubMed] [Google Scholar]
- Marcus, R. , Davies, A. , Ando, K. , Klapper, W. , Opat, S. , Owen, C. , Phillips, E. , Sangha, R. , Schlag, R. , Seymour, J.F. , Townsend, W. , Trneny, M. , Wenger, M. , Fingerle‐Rowson, G. , Rufibach, K. , Moore, T. , Herold, M. & Hiddemann, W. (2017) Obinutuzumab for the first‐line treatment of follicular lymphoma. New England Journal of Medicine, 377, 1331–1344. [DOI] [PubMed] [Google Scholar]
- Martin, P. , Jung, S.H. , Pitcher, B. , Bartlett, N.L. , Blum, K.A. , Shea, T. , Hsi, E.D. , Ruan, J. , Smith, S.E. , Leonard, J.P. & Cheson, B.D. (2017) A phase II trial of lenalidomide plus rituximab in previously untreated follicular non‐Hodgkin's lymphoma (NHL): CALGB 50803 (Alliance). Annals of Oncology, 28, 2806–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello, P. , Steiner, N. , Willenbacher, W. , Cerchione, C. , Nappi, D. , Mauro, E. , Ferrero, S. , Cuzzocrea, S. & Mian, M. (2018) Bendamustine plus Rituximab versus R‐CHOP as first‐line treatment for patients with Follicular Lymphoma grade 3A: evidence from a multicenter, retrospective study. The Oncologist, 23, 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morschhauser, F. , Fowler, N.H. , Feugier, P. , Bouabdallah, R. , Tilly, H. , Palomba, M.L. , Fruchart, C. , Libby, E.N. , Casasnovas, R.O. , Flinn, I.W. , Haioun, C. , Maisonneuve, H. , Ysebaert, L. , Bartlett, N.L. , Bouabdallah, K. , Brice, P. , Ribrag, V. , Daguindau, N. , Le Gouill, S. , Pica, G.M. , Martin Garcia‐Sancho, A. , Lopez‐Guillermo, A. , Larouche, J.F. , Ando, K. , Gomes da Silva, M. , Andre, M. , Zachee, P. , Sehn, L.H. , Tobinai, K. , Cartron, G. , Liu, D. , Wang, J. , Xerri, L. & Salles, G.A. (2018) Rituximab plus lenalidomide in advanced untreated Follicular Lymphoma. New England Journal of Medicine, 379, 934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton, L.M. , Wang, S.S. , Devesa, S.S. , Hartge, P. , Weisenburger, D.D. & Linet, M.S. (2006) Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood, 107, 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers, M.H. , Klasa, R. , Marcus, R.E. , Wolf, M. , Kimby, E. , Gascoyne, R.D. , Jack, A. , Van't Veer, M. , Vranovsky, A. , Holte, H. , van Glabbeke, M. , Teodorovic, I. , Rozewicz, C. & Hagenbeek, A. (2006) Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non‐Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood, 108, 3295–3301. [DOI] [PubMed] [Google Scholar]
- Relander, T. , Johnson, N.A. , Farinha, P. , Connors, J.M. , Sehn, L.H. & Gascoyne, R.D. (2010) Prognostic factors in follicular lymphoma. Journal of Clinical Oncology, 28, 2902–2913. [DOI] [PubMed] [Google Scholar]
- Rummel, M.J. , Niederle, N. , Maschmeyer, G. , Banat, G.A. , von Grünhagen, U. , Losem, C. , Kofahl‐Krause, D. , Heil, G. , Welslau, M. , Balser, C. , Kaiser, U. , Weidmann, E. , Dürk, H. , Ballo, H. , Stauch, M. , Roller, F. , Barth, J. , Hoelzer, D. , Hinke, A. & Brugger, W. (2013) Bendamustine plus rituximab versus CHOP plus rituximab as first‐line treatment for patients with indolent and mantle‐cell lymphomas: an open‐label, multicentre, randomised, phase 3 non‐inferiority trial. Lancet, 381, 1203–1210. [DOI] [PubMed] [Google Scholar]
- Subramanian, J. , Cavenagh, J. , Desai, B. & Jacobs, I. (2017) Rituximab in the treatment of follicular lymphoma: the future of biosimilars in the evolving therapeutic landscape. Cancer Manag Res, 9, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujjani, C.S. , Jung, S.H. , Pitcher, B. , Martin, P. , Park, S.I. , Blum, K.A. , Smith, S.M. , Czuczman, M. , Davids, M.S. , Levine, E. , Lewis, L.D. , Smith, S.E. , Bartlett, N.L. , Leonard, J.P. & Cheson, B.D. (2016) Phase 1 trial of rituximab, lenalidomide, and ibrutinib in previously untreated follicular lymphoma: alliance A051103. Blood, 128, 2510–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca, E. , Rondeau, S. , Vanazzi, A. , Ostenstad, B. , Mey, U.J.M. , Rauch, D. , Wahlin, B.E. , Hitz, F. , Hernberg, M. , Johansson, A.S. , de Nully Brown, P. , Hagberg, H. , Ferreri, A.J.M. , Lohri, A. , Novak, U. , Zander, T. , Bersvendsen, H. , Bargetzi, M. , Mingrone, W. , Krasniqi, F. , Dirnhofer, S. , Hayoz, S. , Hawle, H. , Vilei, S.B. , Ghielmini, M. & Kimby, E. (2019) Short regimen of rituximab plus lenalidomide in follicular lymphoma patients in need of first‐line therapy. Blood, 134, 353–362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplement.


