Summary
Background
Patient‐reported outcome (PRO) measures historically used in inflammatory bowel disease have been considered inadequate to support future drug labelling claims by regulatory agencies.
Aims
To develop PRO tools for use in Crohn's disease (CD) and ulcerative colitis (UC) following guidance issued by the US FDA and the ISPOR (International Society for Pharmacoeconomics and Outcomes Research).
Methods
Concept elicitation and cognitive interviews were conducted in adult patients (≥18 years) across the United States and Canada. Semi‐structured interview guides were used to collect data, and interview transcripts were coded and analysed. Concept elicitation results were considered alongside existing literature and clinical expert opinion to identify candidate PRO items. Cognitive interviews evaluated concept relevance, interpretability and structure, and facilitated instrument refinement. Concept elicitation participants, except those with an ostomy, underwent centrally read endoscopy to assess inflammatory status.
Results
In all, 54 participants (mean age: 46.2 years; 66.7% female) were included in the CD concept elicitation interviews. In total, 80 symptom concepts and 61 impact concepts were identified. After three waves of cognitive interviews, the 31‐item Symptoms and Impacts Questionnaire for CD (SIQ‐CD) was developed. In the UC concept elicitation phase, 53 participants were interviewed (mean age: 41.4 years; 49.1% female). In total, 79 symptoms concepts and 49 impact concepts were identified. Following two waves of cognitive interviews, the 29‐item Symptoms and Impacts Questionnaire for UC (SIQ‐UC) was developed. Both instruments include four symptom and six impact domains.
Conclusions
We developed PROs to support CD and UC drug labelling claims. Psychometric validation studies to evaluate instrument reliability and responsiveness are ongoing.
1. INTRODUCTION
The inflammatory bowel diseases, Crohn's disease and ulcerative colitis, are idiopathic disorders characterised by chronic intestinal inflammation. Treatment options have improved over the past two decades with the introduction of several new classes of therapeutic agents; nevertheless, a substantial proportion of patients do not respond or lose response to available treatments. Consequently, multiple compounds are currently in early and late phase clinical trials. 1 , 2
An important limitation to the development of novel inflammatory bowel disease drugs is that historic Crohn's disease and ulcerative colitis outcome measures 3 —including the Inflammatory Bowel Disease Questionnaire (IBDQ) 4 —were not designed as valid patient‐reported outcomes (PROs), which are considered by regulatory agencies to be the gold standard for quantifying patient experience. Guidance documents issued by the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) indicate that a co‐primary endpoint consisting of a PRO measure and an endoscopic outcome is required in future inflammatory bowel disease registration trials. 5 , 6 While the Crohn's Disease Patient‐Reported Outcomes Signs and Symptoms (CD‐PRO/SS) diary 7 and Ulcerative Colitis Patient‐Reported Outcomes Signs and Symptoms (UC‐PRO/SS) diary 8 were established according to the FDA‐endorsed pathway, endoscopic disease activity was not evaluated in the development studies. This represents an important potential shortcoming given that objective measures of inflammatory bowel disease activity do not necessarily correspond to symptom‐based assessments.
PROs, defined as “any report of the status of a patient's health condition that comes directly from the patient, without interpretation of the patient's response by a clinician or anyone else,” 9 are widely used to study chronic diseases. According to the aforementioned FDA guidance, a valid and reliable PRO instrument is capable of measuring clinically meaningful aspects of disease activity that are most relevant to patients and therefore allows for assessment of the relative benefits of new treatments in a readily interpretable manner. PRO instrument creation and validation is a rigorous, resource‐intensive process that can take several years to complete. 2 , 10 In addition to the CD‐PRO/SS and UC‐PRO/SS measures, relevant examples in gastrointestinal research include the Experience Sampling Method Patient‐Reported Outcome (ESM‐PROM) 11 for the assessment of irritable bowel syndrome and the Eosinophilic Esophagitis Activity Index Patient‐Reported Outcome (EEsAI PRO). 12
PRO instruments are composed of individual items, which take the form of a question, statement or task, that evaluate specific aspects (concepts) relevant to patients' well‐being. These concepts are often aggregated into sub‐concepts, or “domains.” 9 For example, in the ESM‐PROM, the item “I am having abdominal pain” is grouped under the “physical status” domain. 11
PRO development begins with a literature review that identifies concepts of relevance to patients, in addition to existing PRO instruments. Subsequently, semi‐structured interviews are conducted to obtain patient input and generate new concepts and item wording. It is essential that participating patients are clinically well characterised and reflect the study populations in which the PRO instrument will ultimately be used. 9 In the context of inflammatory bowel disease research, endoscopic evaluation is required because demonstration of mucosal inflammation is a critical eligibility criterion for clinical trials of anti‐inflammatory drugs. Preliminary PRO concepts and items are then evaluated and refined through iterative waves of cognitive comprehension interviews before being aggregated into a prototypic instrument for the assessment of measurement properties.
The creation of PRO instruments for use in inflammatory bowel disease trials is an urgent research priority. In response to this imperative, we developed the Symptoms and Impacts Questionnaire for Crohn's Disease and Ulcerative Colitis (SIQ‐CD and SIQ‐UC, respectively) using objective assessments of disease activity and regulatory guidelines.
2. MATERIALS AND METHODS
The best practice recommendations for the development of PRO tools as outlined by the FDA and ISPOR (International Society for Pharmacoeconomics and Outcomes Research) were followed. 13 , 14 , 15 , 16 This methodology is based upon a mixed‐methods approach that involves qualitative and quantitative assessments of disease phenotype and activity, patient interviews, content analysis and concept rating exercises (Figure 1).
FIGURE 1.
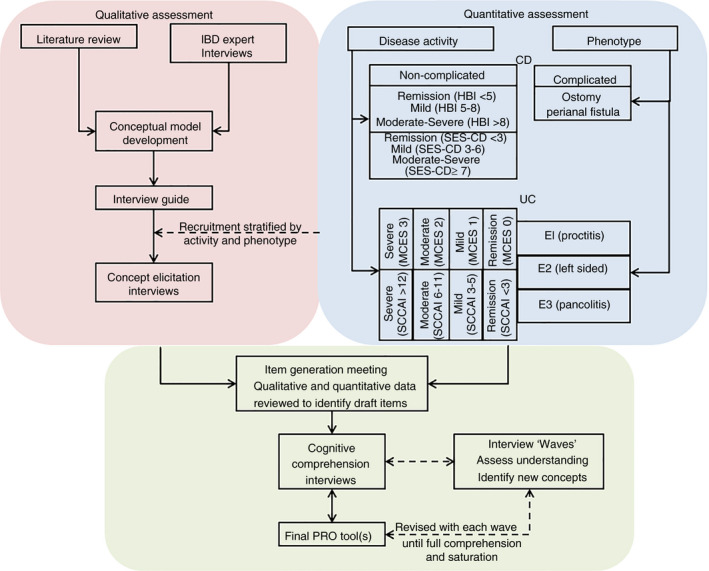
SIQ‐CD and SIQ‐UC development activities. We performed quantitative and qualitative assessments when building novel patient‐reported outcome (PRO) instruments for use in Crohn's disease (CD) and ulcerative colitis (UC). Disease activity and phenotype were evaluated during the screening process. CD patients were categorised as having “complicated” (ie an ostomy or perianal fistula) or “non‐complicated” disease. The Harvey Bradshaw Index (HBI) was used to quantify clinical disease activity. Participants without complications were required to undergo endoscopy at baseline, and a centrally read Simple Endoscopic Score for Crohn's Disease (SES‐CD) was collected to characterise the study population. All UC participants underwent endoscopy at baseline, and centrally read Mayo Clinic Endoscopic Scores (MCES) were used to assess endoscopic disease activity. Simple Clinical Colitis Activity Index (SCCAI) scores and disease extent were also collected to characterise the study population. Qualitative assessments included a literature review, interviews with key opinion leaders and concept elicitation interviews. Once the concept elicitation interview results were analysed, an item generation meeting took place to review draft PRO items. The draft CD and UC instruments were piloted in waves of cognitive comprehension interviews to assess patient understanding and feasibility. Revisions to the draft instruments were made based on the cognitive compression interview results
2.1. Concept elicitation
2.1.1. Preliminary conceptual model
A conceptual model summarises components of the patient experience with respect to having the disease and undergoing treatment(s). We constructed a preliminary conceptual model consisting of symptom and impact concepts using two sources: (a) separate reviews of Crohn's disease and ulcerative colitis literature (which were also designed to identify existing PRO instruments) and (b) input from clinical experts in the treatment of inflammatory bowel disease. 9 Crohn's disease and ulcerative colitis literature searches were conducted in PubMed on 14 May 2014 and 15 April 2016, respectively, using pre‐defined search terms and inclusion criteria (Appendix S1). Ten clinical experts across North America and Europe who specialise in inflammatory bowel disease were then asked to provide feedback on the preliminary conceptual model. Clinicians were selected based on academic expertise and community practice patterns, with practice volumes ranging from several hundred to several thousand inflammatory bowel disease patients. In accordance with the FDA guidance, draft concepts were added, reviewed, revised and prioritised during the clinical expert interviews.
Concept elicitation interview guide
Following the clinical expert interviews, qualitative researchers with expertise in PRO instrument development (KPM, MLM) built concept elicitation interview guides that reflected the preliminary conceptual model and the scientific objectives of the study. Within each guide, questions were semi‐structured to obtain both spontaneous and probed input, including the specific language used by study participants. The severity (ie the level of intensity) and bothersomeness (ie the level of annoyance or aggravation) of each symptom concept and the degree of difficulty experienced by participants while coping with each impact concept were separately rated on a scale from 0 (none) to 10 (extremely severe/bothersome/difficult). Open‐ended questions with follow‐up probing by the interviewer ensured that the full patient experience was reflected in the interviews.
Participant recruitment and quantitative assessments
Previous research indicates that approximately 99% of concepts emerge by the 25th elicitation interview in clinical outcome instrument development. 17 We aimed to conduct approximately 60 Crohn's disease and 60 ulcerative colitis concept elicitation interviews to allow for adequate concept emergence and support exploratory analyses in important sub‐populations, such as patients with an ostomy or perianal fistulising Crohn's disease. A convenience sample of adult (≥18 years) Crohn's disease and ulcerative colitis patients were prospectively and consecutively recruited from academic and community practice clinics across the United States and Canada to participate in the concept elicitation interviews.
Local site investigators performed quantitative clinical assessments of disease activity using the Harvey Bradshaw Index (HBI) 18 for Crohn's disease and the Simple Clinical Colitis Activity Index (SCCAI) 19 for ulcerative colitis. These tools were selected because they accurately evaluate clinical disease activity and are relatively easy to administer in a routine practice setting. 3 HBI thresholds were used to define clinical remission (HBI < 5), mild‐to‐moderate disease (HBI = 5‐8) and severe disease (HBI > 8). 18 For ulcerative colitis, SCCAI scores were used to define clinical remission (SCCAI < 3), mild disease (SCCAI = 3‐5), moderate disease (SCCAI = 6‐11) and severe disease (SCCAI > 11). 19
To evaluate endoscopic disease activity, Crohn's disease participants without complications (ie patients without an ostomy or a perianal fistula) underwent a colonoscopy, which was centrally read in a blinded manner, and the Simple Endoscopic Score for Crohn's Disease (SES‐CD) was calculated. 20 SES‐CD thresholds were used to define endoscopic remission (SES‐CD = 0‐2), mild disease (SES‐CD = 3‐6) and moderate‐to‐severe disease (SES‐CD > 7). Endoscopy was not required in participants with an ostomy or perianal fistula. In the ulcerative colitis cohort, all participants underwent a colonoscopy, which was centrally read in a blinded manner, and the Mayo Clinic Endoscopic Subscore (MCES) 21 and Ulcerative Colitis Endoscopic Index of Severity (UCEIS) score 22 was calculated. Participants were classified as having endoscopic remission (MCES = 0), mild disease (MCES = 1), moderate disease (MCES = 2) or severe disease (MCES = 3). Endoscopic scores were independently calculated by two central readers. If there was disagreement between the two centrally read scores, a third central reader selected the most valid score, which is consistent with methodology used in regulatory clinical trials.
Concept elicitation interviews
Trained interviewers with experience in qualitative data collection for the purposes of PRO development conducted the concept elicitation interviews by telephone using the condition‐specific guide. The interviews, which lasted between 60 and 90 minutes, were audio‐recorded and transcribed. A quality check was performed to confirm the accuracy of the transcription and redact patient identifiers.
Two trained coders independently identified symptom and impact concepts within the concept elicitation interview transcripts using a pre‐defined process. A concept is an idea or notion expressed by a participant during an interview. For example, a patient may describe their abdominal pain, making abdominal pain a reported and coded concept. Tables were prepared to present the frequency at which concepts emerged, the proportion of the patient sample that expressed each concept, and group means from the rating exercises for symptom severity, symptom bothersomeness and difficulty coping with impacts.
ATLAS.ti (version 7.1.0) software was used to tag patient quotations with concept codes and organise concepts according to similarity. Of the total transcripts, 10% were coded by two independent coders, and inter‐rater agreement (IRA) was calculated. An IRA value of 90% or greater was considered acceptable. 14
Saturation of concept (the point at which no new concept‐level data are derived) 9 was evaluated to identify whether a sufficient number of interviews had been conducted. Concept elicitation interview transcripts were ordered by date and divided into six groups of approximately nine interview transcripts per group. Results from each interview group were compared against those from the previous group to identify the emergence of new concepts.
Concept justification tables were created to summarise the level of support for each symptom and impact concept as determined by the literature review, clinical expert input and the qualitative interview results.
2.2. Draft PRO instrument development: item generation
The qualitative research experts and study investigators met in‐person to review the overall results and discuss each concept from a clinical and measurement perspective before deciding whether to include the concept in PRO measurement. Selected concepts were then cross‐referenced against commonly used PRO instruments in inflammatory bowel disease to examine whether existing instruments provided adequate content coverage and if development of novel content was warranted.
During PRO item generation, wording was informed by patient quotations coded from the concept elicitation interview transcripts. Item structure, response options and recall period were chosen by the methods experts and study investigators.
2.3. Cognitive comprehension and instrument refinement
2.3.1. Cognitive comprehension interview process
Cognitive comprehension interviews were conducted in‐person to evaluate participant understanding. After self‐administration of the draft PRO instrument, participants were asked to respond to the interviewer's questions about their comprehension of the instructions, item language, fit, and sufficiency of the response options, and the overall structure and comprehensiveness of the content covered.
Cognitive comprehension interviews were conducted in waves comprising approximately four to six participants each. After each wave, study investigators reviewed findings and implemented refinements to the draft PRO instrument. Questions were added to the interview guide in the final interview wave to assess whether there were differences in the interpretation of the paper and electronic versions of the draft instrument. All cognitive comprehension interview sessions were audio‐recorded and transcribed.
2.3.2. Recruitment and quantitative assessments
For the cognitive comprehension exercise, we planned to enrol approximately 18 Crohn's disease and 18 ulcerative colitis participants. Previous research suggests that approximately 7 to 10 interviews are sufficient to confirm participant understanding. 13 , 23 The recruitment process and eligibility criteria for the concept elicitation and cognitive comprehension interviews were identical, expect except that endoscopic assessment was not required to partake in the cognitive comprehension interviews since the goal of this component of the study was to determine participant understanding. Otherwise, the recruitment process and eligibility criteria for the concept elicitation and cognitive comprehension interviews were identical.
2.4. Ethics approval
The study protocol and interview forms were approved by Quorum Review IRB (Seattle, WA, USA). Where required, local institutional review board approval was obtained before site initiation. All participants provided written informed consent prior to participating in study activities. The study was conducted in compliance with the Declaration of Helsinki, and no changes were made to the participants’ existing care in this observational study.
2.5. Statistical analyses
Qualitative interview data from the interview transcripts were coded as described above. Quantitative data from the screening and enrolment processes and rating exercises were entered into SPSS (version 18.0) to generate tables of descriptive statistics.
3. RESULTS
3.1. Concept elicitation: Crohn's disease
3.1.1. Concept elicitation interview guide and baseline characteristics
The concept elicitation interview guide for Crohn's disease consisted of symptom and impact content derived from the 30 relevant studies identified by the literature review and expert opinion (Figure S1). A total of 54 patients with Crohn's disease were recruited from seven sites to participate in the concept elicitation process. Demographic and clinical characteristics are provided in Table 1. The mean participant age was 46.2 years (standard deviation [SD] 15.3). Females comprised 66.7% (36/54) of the study population, and the mean disease duration since diagnosis was 15.5 years (SD 12.2). Two‐thirds (66.7%, 36/54) of participants had luminal Crohn's disease without complications, 22.2% (12/54) had an ostomy and 11.1% (6/54) had an active perianal fistula.
TABLE 1.
Baseline demographic and clinical characteristics of participants included in the concept elicitation interviews
| Characteristic | CD | UC | |||
|---|---|---|---|---|---|
| Total (N = 54) | Without complications (N = 36) | With complications | Total (N = 53) | ||
| Ostomy (N = 12) | Perianal fistula (N = 6) | ||||
| Age (years) | |||||
| Mean (SD) | 46.2 (15.3) | 45.8 (15.3) | 51.3 (14.5) | 38.0 (15.7) | 41.4 (14.3) |
| Range | 19‐77 | 19‐77 | 32‐70 | 20‐67 | 19‐79 |
| Gender | |||||
| Male | 18 (33.3%) | 12 (33.3%) | 2 (16.7%) | 4 (66.7%) | 27 (50.9%) |
| Female | 36 (66.7%) | 24 (66.7%) | 10 (83.3%) | 2 (33.3%) | 26 (49.1%) |
| Marital status | |||||
| Married or living as married | 27 (50.0%) | 20 (55.6%) | 5 (41.7%) | 2 (33.3%) | 32 (60.4%) |
| Widowed | 3 (5.6%) | 2 (5.6%) | 1 (8.3%) | 0 | 1 (1.9%) |
| Separated or divorced | 11 (20.4%) | 5 (13.9%) | 4 (33.3%) | 2 (33.3%) | 3 (5.7%) |
| Never married | 13 (24.1%) | 9 (25.0%) | 2 (16.7%) | 2 (33.3%) | 17 (32/1%) |
| Highest level of education completed | |||||
| High school | 18 (33.3%) | 14 (38.9%) | 2 (16.7%) | 2 (33.3%) | 7 (13.2%) |
| Some college | 14 (25.9%) | 7 (19.4%) | 5 (41.7%) | 2 (33.3%) | 17 (32.1%) |
| Bachelor's degree | 15 (27.8%) | 13 (36.1%) | 2 (16.7%) | 0 | 14 (26.4%) |
| Graduate/professional school | 6 (11.1%) | 2 (5.6%) | 3 (25.0%) | 1 (16.7%) | 15 (28.3%) |
| Missing/refused to answer | 1 (1.9%) | 0 | 0 | 1 (16.7%) | 0 |
| Employment | |||||
| Employed full‐time for wages | 19 (35.2%) | 15 (41.7%) | 1 (8.3%) | 3 (50.0%) | 29 (54.7%) |
| Employed part‐time for wages | 0 | 0 | 0 | 0 | 6 (11.3%) |
| Self‐employed | 1 (1.9%) | 0 | 0 | 1 (16.7%) | 3 (5.7%) |
| Out of work | 2 (3.7%) | 2 (5.6%) | 0 | 0 | 2 (3.8%) |
| Homemaker | 5 (9.3%) | 3 (8.3%) | 2 (16.7%) | 0 | 1 (1.9%) |
| Student | 3 (5.6%) | 2 (5.6%) | 0 | 1 (16.7%) | 5 (9.4%) |
| Retired | 10 (18.5%) | 6 (16.7%) | 4 (33.3%) | 0 | 3 (5.7%) |
| Unable to work | 13 (24.1%) | 8 (22.2%) | 5 (41.7%) | 0 | 4 (7.5%) |
| Missing/refused to answer | 1 (1.9%) | 0 | 0 | 1 (16.7%) | 0 |
| Hispanic or Latino origin | |||||
| Non‐Hispanic or Latino | 54 (100%) | 36 (100%) | 12 (100%) | 6 (100%) | 46 (86.8%) |
| Hispanic or Latino | 0 | 0 | 0 | 0 | 7 (13.2%) |
| Race | |||||
| Black or African American | 6 (11.1%) | 6 (16.7%) | 0 | 0 | 2 (3.8%) |
| White | 46 (85.2%) | 29 (80.6%) | 11 (91.7%) | 6 (100%) | 45 (84.9%) |
| Other | 1 (1.9%) | 1 (2.8%) | 0 | 0 | 6 (11.3%) |
| Missing/refused to answer | 1 (1.9%) | 0 | 1 (8.3%) | 0 | 0 |
| Time since diagnosis (years) | |||||
| Mean (SD) | 15.5 (12.2) | 12.7 (10.9) | 23.8 (6.3) | 14.8 (12.5) | 8.7 (6.9) |
| Range | 0‐43 | 0‐35 | 2‐43 | 2‐29 | 1‐33 |
| HBI score | |||||
| Mean (SD) | 7.8 (5.6) | 6.4 (4.8) | 12.3 (5.5) | 8.0 (5.6) | — |
| Range (0‐18 a ) | 0‐23 | 0‐20 | 4‐23 | 3‐18 | — |
| HBI score category | |||||
| <5 | 16 (29.6%) | 13 (36.1%) | 1 (8.3%) | 2 (33.3%) | — |
| 5‐8 | 18 (33.3%) | 14 (38.9%) | 3 (25.0%) | 1 (16.7%) | — |
| >8 | 20 (37.0%) | 9 (25.0%) | 8 (66.7%) | 3 (50.0%) | — |
| SCCAI score | |||||
| Mean (SD) | — | — | — | — | 3.6 (3.5) |
| Range (0‐19) | — | — | — | — | 0‐14 |
| SCCAI score category | |||||
| <3 | — | — | — | — | 26 |
| 3‐5 | — | — | — | — | 10 |
| 6‐11 | — | — | — | — | 16 |
| >11 | — | — | — | — | 1 |
| SES‐CD (N = 34) b , c | |||||
| Median (IQR) | — | 3.5 (0‐7) | — | 6.0 (4‐14) | — |
| Range (0‐56) | — | 0‐19 | — | 3‐15 | — |
| SES‐CD category (N = 34) b , c | |||||
| 0‐2 | — | 12 | — | 0 | — |
| 3‐6 | — | 13 | — | 4 | — |
| >7 | — | 9 | — | 2 | — |
| MCES category | |||||
| 0 | — | — | — | — | 6 (11.3%) |
| 1 | — | — | — | — | 19 (35.8%) |
| 2 | — | — | — | — | 12 (22.6%) |
| 3 | — | — | — | — | 16 (30.2%) |
| UCEIS score | |||||
| Mean (SD) | — | — | — | — | 2.5 (1.9) |
| Range (0‐8) | — | — | — | — | 0‐8 |
| Disease extent | |||||
| E1 (proctitis) | — | — | — | — | 17 (32.1%) |
| E2 (distal to splenic flexure) | — | — | — | — | 17 (32.1%) |
| E3 (proximal to splenic flexure) | — | — | — | — | 19 (35.8%) |
| Comorbidities/extraintestinal manifestations | |||||
| Allergies | 25 (47.2%) | ||||
| Arthritis/rheumatism | 8 (15.1%) | ||||
| Bile acid diarrhoea | 4 (7.4%) | 4 (11.1%) | 0 | 0 | — |
| Back problems | — | — | — | — | 6 (11.3%) |
| Depression | — | — | — | — | 12 (22.6%) |
| Diabetes or high blood pressure | — | — | — | — | 7 (13.2%) |
| Headache or migraine | — | — | — | — | 11 (20.8%) |
| Heart trouble | — | — | — | — | 2 (3.8%) |
| High blood pressure or hypertension | — | — | — | — | 7 (13.2%) |
| Intestinal bacterial overgrowth | 1 (1.9%) | 1 (2.8%) | 0 | 0 | — |
| Liver problems | — | — | — | — | 4 (7.5%) |
| Nervousness or anxiety disorder | — | — | — | — | 14 (26.4%) |
| Primary sclerosing cholangitis | — | — | — | — | 2 (3.8%) |
| Thyroid problems | — | — | — | — | 3 (5.7%) |
| Trouble hearing (even with hearing aid) | — | — | — | — | 3 (5.7%) |
| Trouble seeing (even with glasses/contacts) | — | — | — | — | 8 (15.1%) |
| Stricture with obstructive symptoms | 3 (5.6%) | 2 (5.6%) | 1 (8.3%) | 0 | — |
| Treatment history: disease‐related procedures | |||||
| Ileocolonic resection with ileocecal valve resection and ileocolonic anastomosis | 15 (27.8%) | 7 (19.4%) | 6 (50.0%) | 2 (33.3%) | — |
| None | 53 (100.0%) d | ||||
| Previous Billroth I gastrectomy | 1 (1.9%) | 0 | 0 | 1 (16.7%) | — |
Abbreviations: CD, Crohn's Disease; HBI, Harvey‐Bradshaw Index; MCES, Mayo Clinic Endoscopic Subscore; SCCAI, Simple Clinical Colitis Activity Index; SD, Standard Deviation; SES‐CD, Simple Endoscopic Score for Crohn's Disease; UC, Ulcerative Colitis; UCEIS, Ulcerative Colitis Endoscopic Index of Severity.
HBI scores range from 0 to 18, plus 1 point for each liquid stool per day.
Two patients in the group without comorbidities were missing endoscopic videos, however, still images were available.
All six participants with fistulising CD underwent baseline endoscopy and the SES‐CD was calculated, although this was not required.
Participants with UC were asked whether they had ever undergone IPAA surgery, colostomy, ileostomy, sub‐total colectomy or proctocolectomy.
The mean HBI score was 7.8 (SD 5.6). In participants without complications, HBI‐defined clinical disease activity was relatively well distributed: 36.1% (13/36) of participants were in clinical remission, 38.9% (14/36) had mild‐to‐moderate disease and 25.0% (9/36) had severe disease. Of the participants with an ostomy, 8.3% (1/12) were in clinical remission, 25.0% (3/12) had mild‐to‐moderate disease and 66.7% (8/12) had severe disease. There was at least one participant with a perianal fistula in each HBI‐defined category.
The median SES‐CD for the Crohn's disease subgroup without complications was 3.5 (interquartile range [IQR] 0‐7). In total, 35.3% (12/34) of these participants were in endoscopic remission, and 64.7% (22/34) had active endoscopic disease activity. Missing endoscopic videos prevented two of the 36 Crohn's disease participants from being evaluated in the SES‐CD analyses.
All six participants with active perianal fistula also underwent baseline endoscopy. The median SES‐CD was 6.0 (IQR 4‐14) for this subgroup; all participants with active perianal fistula had active endoscopic disease activity.
3.1.2. Coding, inter‐rater agreement and saturation of concept
The final coding framework for the 54 Crohn's disease transcripts contained a total of 141 concept codes. Five of the 54 interview transcripts were coded by two independent coders to evaluate IRA. For each of the five transcript pairs, IRA values ranged from 91.3% to 93.6% for concept identification, and from 90.5% to 97.7% for concept assignment. Saturation of concept was observed by the fifth transcript group (ie after approximately 45 of the 54 interviews) as no novel concepts were identified in subsequent transcripts.
3.1.3. Symptoms
In total, 80 symptom concepts were identified during Crohn's disease concept elicitation interviews (Table S1). “Abdominal pain” was the most frequently expressed symptom (8.0% [171/2134] of the total symptom expressions). Other symptom concepts mentioned in over 100 expressions were “diarrhoea” (153 expressions), “joint pain” (132 expressions), “fatigue” (103 expressions) and “abdominal cramping” (101 expressions). Spontaneous and probed symptom expressions are reported by frequency in Table S1.
Symptoms reported by at least 25% of Crohn's disease study population included “abdominal pain” (74.1%, 40/54), “diarrhoea” (74.1%, 40/54), “watery/loose stools” (68.5%, 37/54), “joint pain” (64.8%, 35/54), “urgency” (64.8%, 35/54), “fatigue” (63%, 34/54), “frequent bowel movements” (57.4%, 31/54), “nausea” (51.9%, 28/54), “abdominal cramping” (50%, 27/54), “tiredness” (50%, 27/54), “weakness” (50%, 27/54), “reduced appetite” (46.3%, 25/54), “rectal bleeding” (40.7%, 22/54), “low energy” (38.9%, 21/54), “weight loss” (37.0%, 20/54), “fever” (37.0%, 20/54), “faecal incontinence” (35.2%, 19/54), “vomiting” (27.8%, 15/54), “unspecified pain” (27.8%, 15/54), “skin lesion/rash” (27.8%, 15/54), “gas” (25.9%, 14/54) and “exhaustion” (25.9%, 14/54).
The mean severity and bothersome rating for each symptom concept are reported in Table S1. Some symptoms, such as “fistula drainage” and “irritation at stoma site” were rated the maximum score of 10 for severity or bothersomeness; however, only one or two participants rated these symptoms. Symptoms rated by at least three participants with the highest mean severity ratings included “back pain” (9.3, SD 1.0), “muscle cramps” (9.0, SD 1.0) and “headache” (8.7, SD 2.3), while those rated by at least three participants with high mean bothersomeness ratings included “watery/loose stools” (8.3, SD 2.3), “low energy” (8.3, SD 2.1) and “back pain” (7.8, SD 1.7).
3.1.4. Impacts
Crohn's disease concept elicitation interviews identified 61 impact concepts (Table S2). “Dietary changes” was the most frequently recorded impact concept, closely followed by “work limitations” (6.7% [79/1176] and 6.6% [78/1176] of the total impact expressions, respectively). Other impact concepts mentioned in over 40 expressions were “limitations to leisure activities” (61 expressions), “limitations to social activities” (55 expressions), “limitations to overall functioning” (46 expressions), “sadness/depression” (45 expressions) and “eats less/avoids eating” (44 expressions). Spontaneous and probed impact expressions are reported by frequency in Table S2.
Impacts reported by at least 25% of Crohn's disease study population included “sadness/depression” (44.4%, 24/54), “limitations to work” (66.7%, 36/54), “dietary changes” (53.7%, 29/54), “limitations to overall functioning” (46.3%, 25/54), “limitations to leisure activities” (46.3%, 25/54), “decreased quality of life” (44.4%, 24/54), “need to be near restroom” (40.7%, 22/54), “limitations to social activities” (40.7%, 22/54), “eats less/avoids eating” (38.9%, 21/54), “limitations to physical functioning” (37.0%, 20/54), “embarrassment” (33.3%, 18/54), “fear of incontinence” (33.3%, 18/54), “reduced sleep quality” (29.6%, 16/54), “sexual function issues” (29.6%, 16/54), “worry or fear” (27.8%, 15/54), “anxiety” (25.9%, 14/54), “housework/chores limitations” (25.9%, 14/54) and “relationships with family” (25.9%, 14/54).
The mean difficulty rating for each impact concept is reported in Table S2. Two impact concepts, “waking up to use the restroom” and “being misunderstood by doctors,” were rated the maximum difficulty score of 10; however, only one participant rated each of these impacts. Of the impact concepts rated by at least three participants, “limitations to parenting/caregiving” (8.5, SD 2.4), “frustration” (7.9, SD 1.6) and “negative self‐image” (7.7, SD 2.4) had the highest mean difficulty ratings.
3.2. Concept elicitation: ulcerative colitis
3.2.1. Concept elicitation interview guide and baseline characteristics
The concept elicitation interview guide for ulcerative colitis consisted of symptom and impact content included in the 29 relevant studies identified by the literature review and expert opinion (Figure S2). In all, 53 patients with ulcerative colitis were recruited from three sites to participate in the concept elicitation exercise. Demographic and clinical characteristics are provided in Table 1. The mean age of participants at baseline was 41.4 years (SD 14.3), 49.1% (26/53) were female, and the mean disease duration since diagnosis was 8.7 years (SD 6.9).
The mean SCCAI score at baseline was 3.6 (SD 3.5), and the study population was generally representative of the disease activity spectrum. A broad range of endoscopic disease activity was represented in the ulcerative colitis study. The mean UCEIS score was 2.5 (SD 1.9). Approximately 10% of the ulcerative colitis participants were in endoscopic remission (11.3%, 6/53) and 35.8% (19/53), 22.6% (12/53) and 28.3% (15/53) had mild, moderate and severe endoscopic disease, respectively.
3.2.2. Interview coding and saturation of concept
The final coding framework for the 53 ulcerative colitis transcripts contained a total of 128 concept codes. Four of the 53 interview transcripts were coded by two independent coders to evaluate IRA. For each of the four transcript pairs, IRA values ranged from 89.5% to 93.9% for concept identification, and from 95.2% to 98.6% for concept assignment. Saturation of concept was observed by the fifth transcript group (ie after approximately 45 of the 53 interviews) as no new concepts were identified in subsequent transcripts.
3.2.3. Symptom concepts
A total of 79 symptom concepts were identified during the ulcerative colitis concept elicitation interviews (Table S1). “Rectal bleeding” was most often mentioned, followed by “urgency” (8.5% [296/3500] and 8.3% [291/3500] of the total symptom expressions, respectively). Other symptom concepts mentioned in over 200 expressions were “frequent bowel movements” (232 expressions) and “abdominal pain” (221 expressions). Spontaneous and probed symptom expressions are reported by frequency in Table S1.
Symptoms reported by at least 25% of the ulcerative colitis study population included “rectal bleeding” (96.2%, 51/53), “urgency” (92.5%, 49/53), “frequent bowel movements” (86.8%, 46/53), “diarrhoea” (79.2%, 42/53), “tiredness” (71.7%, 38/53), “faecal incontinence” (69.8%, 37/53), “gas” (69.8%, 37/53), “mucus in stool” (69.8%, 37/53), “inability to distinguish gas from stool” (67.9%, 36/53), “weight loss” (64.2%, 34/53), “bloating” (62.3%, 33/53), “abdominal cramping” (62.3%, 33/53), “loose stools” (58.5%, 31/53), “weakness” (54.7%, 29/53), “liquid stools” (54.7%, 29/53), “anaemia” (50.9%, 27/53), “fatigue” (50.9%, 27/53), “difficulty staying asleep” (50.9%, 27/53), “dehydration” (49.1%, 26/53) “reduced appetite” (47.2%, 25/53), “odour to gas or stool” (47.2%, 25/53), “joint pain” (45.3%, 24/53), “low energy” (43.4%, 23/53), “back pain” (41.5%, 22/53), “constipation” (37.7%, 20/53), “nausea” (34%, 18/53), “skin problems/inflammation” (32.1%, 17/53), “irregular/rapid heartbeat” (30.2%, 16/53), “light‐headedness” (28.3%, 15/53), “vomiting” (26.4%, 14/53) and “sweating” (26.4%, 14/53).
The mean severity and bothersome rating for each symptom concept are reported in Table S1. Some symptoms, such as “tenesmus” and “nail problems,” were rated the maximum score of 10 for severity or bothersomeness; however, only one or two participants rated these symptoms. Of the symptom concepts rated by at least three participants, “headache” (9.4, SD 1.2), “diarrhoea” (9.1, SD 1.3) and “urgency” (8.8, SD 1.8) had the highest mean severity ratings, while “difficulty staying asleep” (9.3, SD 1.2), “faecal incontinence” (8.8, SD 1.9) and “headache” (8.8, SD 1.0) had the highest mean bothersomeness ratings.
3.2.4. Impact concepts
In all, 49 impact concepts were described in the ulcerative colitis concept elicitation interviews (Table S2). “Limitations to work” was most frequently recorded (7.2% [141/1952] of the total impact expressions). Other impact concepts mentioned in over 100 expressions were “worry or fear” (125 expressions) and “dietary changes” (119 expressions). Spontaneous and probed impact expressions are reported by frequency in Table S2.
Impacts reported by at least 25% of the ulcerative colitis study population included “limitations to overall functioning” (73.6%, 39/53), “dietary changes” (73.6%, 39/53), “need to be near restroom” (71.7%, 38/53), “worry or fear” (71.7%, 38/53), “financial burden” (67.9%, 36/53), “limitations to social activities” (64.2%, 34/53), “fear of incontinence” (60.4%, 32/53), “frustration” (58.5%, 31/53), “eats less/avoids eating” (56.6%, 30/53), “travel impacted” (56.6%, 30/53), “decreased quality of life” (56.6%, 30/53), “embarrassment” (50.9%, 27/53), “emotional health in general” (47.2%, 25/53), “limitations to exercise/sports” (45.3%, 24/53), “difficulty concentrating” (43.4%, 23/53), “anxiety” (41.5%, 22/53), “limitations to physical functioning” (37.7%, 20/53), “treatment burden” (35.8%, 19/53), “depression” (34%, 18/53), “stress” (34%, 18/53), “limitations to leisure activities” (34%, 18/53), “daily routine impacted” (32.1%, 17/53), “relationship with partner/spouse affected” (32.1%, 17/53), “altered clothing choices” (32.1%, 17/53), “housework/chores limitations” (28.3%, 15/53), “preparation for incontinence” (28.3%, 15/53), “sexual function issues” (28.3%, 15/53), “limitations to driving/transport” (26.4%, 14/53) and “relationships with friends affected” (26.4%, 14/53).
The mean difficulty rating for each impact concept is reported in Table S2. “Lack of control” and “fertility issues” received the maximum mean difficulty score of 10; however, these impacts were each rated by only two participants. Of the impact concepts rated by at least three participants, “overall emotional health” (9.3, SD 1.2), “limitations to personal care” (8.8, SD 1.5) and “general functioning” (8.9, SD 1.2) had the highest mean difficulty ratings.
3.3. Cognitive comprehension interviews and draft instruments
3.3.1. Crohn's disease
The literature review and experts did not identify existing PRO instruments that incorporated all the concepts that were determined to be relevant for PRO measurement; thus, the need for a novel tool was established. The Crohn's disease concept generation meeting resulted in the removal of 62 symptom and 43 impact concepts (Table S3), leaving 18 symptom and 18 impact concepts for inclusion in the preliminary draft PRO instrument. Recall periods and response options incorporated in the draft questionnaire were based on input from the clinical experts and PRO methodologists. A translatability assessment was conducted prior to the cognitive comprehension interviews to identify areas that may require revision if the instrument is used in global clinical studies. No issues that would impact translation were identified.
The 36‐item draft instrument was evaluated by 17 Crohn's disease participants (Table S4) during three waves of cognitive comprehension interviews. A cognitive summary table was generated for each wave of interviews to determine whether the questionnaire was feasible and whether participants had difficulty understanding the instrument content. Several symptom concepts (“diarrhoea” and “watery/loose stools,”; “frequent bowel movements” and “using the restroom frequently”; and “fatigue” and “low energy”) and impact concepts (“limitations to work” and “limitations to school”) were combined according to feedback collected in the cognitive comprehension interviews. This resulted in the 31‐item draft SIQ‐CD, which consists of 14 symptom and 17 impact concepts (Table 2).
TABLE 2.
Symptoms and Impacts Questionnaire for Crohn's Disease (SIQ‐CD)
|
Daily Bowel Movement Report 1. When did you have this bowel movement? – Just now – Earlier today (please enter time) 2. How strong was your urge to use the restroom before this bowel movement? – Not strong at all – Mild – Moderate – Very strong – Extremely strong 3. Were you able to reach the toilet in time with this bowel movement? – Yes, I made it to the toilet on time – No, I had an accident before reaching the toilet 4. How severe was your rectal bleeding with this bowel movement? – No bleeding at all – Mild – Moderate – Severe – Very severe 5. Please select the picture and description that best resembles your stool. Daily Symptom Diary 1. How severe was your worst abdominal cramping during the last 24 hours? – No cramping at all – Mild – Moderate – Severe – Very severe 2. How severe was your worst abdominal pain during the last 24 hours? – No pain at all – Mild – Moderate – Severe – Very severe 3. How severe was your worst joint pain during the last 24 hours? – No pain at all – Mild – Moderate – Severe – Very severe 4. How would you rate your worst feelings of tiredness during the last 24 hours? – No tiredness at all – Mild – Moderate – Severe – Very severe 5. How often did you experience low energy during the last 24 hours? – Never – Rarely – Sometimes – Often – Always 6. How often did you feel weak in the last 24 hours? – Never – Rarely – Sometimes – Often – Always 7. How often did you have a poor appetite during the last 24 hours? – Never – Rarely – Sometimes – Often – Always 8. How often did you experience nausea during the last 24 hours? – Never – Rarely – Sometimes – Often – Always 9. How many times did you vomit during the last 24 hours (enter number of times)? Weekly Impact Assessment 1. Over the last 7 days, how limited were you in the types of food you could eat because of your Crohn's disease? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 2. Over the last 7 days, how limited were you in the amount of food you could comfortably eat because of your Crohn's disease? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 3. Over the last 7 days, how limited were your activities because of the need to be near a restroom? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 4. Over the past 7 days, how limited were your social activities because of your Crohn's disease? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 5. Over the past 7 days, how limited were your leisure activities because of your Crohn's disease? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 6. Over the past 7 days, how limited was your overall functioning because of your Crohn's disease? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 7. Over the past 7 days, how difficult was it for you to complete your responsibilities at work or school because of your Crohn's disease? – Not difficult at all – A little difficult – Moderately difficult – Very difficult – Extremely difficult – Not applicable: I did not work or attend school in the past 7 days because of my Crohn's disease – Not applicable: I did not work or attend school in the past 7 days for reasons not related to my Crohn's disease 8. Over the past 7 days, how difficult was it to complete housework or chores because of your Crohn's disease? – Not difficult at all – A little difficult – Moderately difficult – Very difficult – Extremely difficult 9. Over the past 7 days, how difficult was it to complete your family responsibilities because of your Crohn's disease? – Not difficult at all – A little difficult – Moderately difficult – Very difficult – Extremely difficult – Not applicable: my Crohn's disease has made me unable to have children or has influenced my choice not to have children – Not applicable: I do not have children or others who depend on me, for reasons not related to my Crohn's disease 10. Over the past 7 days, how limited were you in physical activities because of your Crohn's disease? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 11. Over the past 7 days, how difficult were relationships with friends because of your Crohn's disease? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 12. Over the past 7 days, how difficult were relationships with family because of your Crohn's disease? – Not difficult at all – A little difficult – Moderately difficult – Very difficult – Extremely difficult 13. Over the past 7 days, how limited were your sexual activities because of your Crohn's disease? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited – Not applicable: I did not attempt sexual activities in the past 7 days because of my Crohn's disease – Not applicable: I did not attempt sexual activities in the past 7 days for reasons not related to my Crohn's disease 14. Over the past 7 days, how much has Crohn's disease interfered with your quality of life? – Not at all – A little bit – Moderately – Very much – Extremely 15. Over the past 7 days, how often have you worried about having an accident related to your Crohn's disease? – Never – Rarely – Sometimes – Often – Always 16. Over the past 7 days, how often has your Crohn's disease caused you to feel embarrassed? – Never – Rarely – Sometimes – Often – Always 17. Over the past 7 days, how often has your Crohn's disease caused you to feel sad? – Never – Rarely – Sometimes – Often – Always |
During the final wave of cognitive comprehension interviews, the SIQ‐CD was administered using a smartphone‐based electronic PRO format and evaluated alongside a paper presentation. No conceptual differences between the paper and electronic versions were identified, which provides support for platform neutrality of the instrument content. It took participants approximately 6 minutes to complete the draft instrument.
3.3.2. Ulcerative colitis
The ulcerative colitis generation meeting led to a reduction from 77 to 16 symptom concepts and 47 to 13 impact concepts for inclusion in the novel ulcerative colitis PRO instrument (Table S5). When the Crohn's disease and ulcerative colitis preliminary draft measures were compared, two‐thirds of the symptom concepts and half of the impact concepts were found to be common to both instruments. The ulcerative colitis translatability assessment did not identify issues that would impact translation.
Given the sizeable overlap across the preliminary draft Crohn's disease and ulcerative colitis measures—along with the completed cognitive work for the Crohn's disease measure that demonstrated acceptable structure, instructional text, response options, recall period and electronic PRO presentation—the targeted number of ulcerative colitis cognitive comprehension interviews was reduced. Seven ulcerative colitis cognitive comprehension interviews (one wave of five participants and one wave of two participants) were conducted, with the results yielding few revisions to the preliminary draft ulcerative colitis questionnaire (Table S4).
Both the paper and electronic versions of the draft ulcerative colitis instrument were assessed in cognitive comprehension interviews to determine whether there was conceptual equivalence across modes of administration. Similar to the draft SIQ‐CD instrument, no issues with comprehension or feasibility were identified, nor were there substantial differences in the paper versus electronic presentations. Thus, the 29‐item draft SIQ‐UC remained intact (Table 3). Participants completed the draft SIQ‐UC instrument in approximately 6 minutes.
TABLE 3.
Symptoms and Impacts Questionnaire for Ulcerative Colitis (SIQ‐UC)
|
Daily Bowel Movement Report 1. When did you have this bowel movement? – Just now – Earlier today (please enter time) 2. How strong was your urge to use the restroom before this bowel movement? – Not strong at all – Mild – Moderate – Very strong – Extremely strong 3. Were you able to reach the toilet in time with this bowel movement? – Yes, I made it to the toilet on time – No, I had an accident before reaching the toilet 4. How severe was your rectal bleeding with this bowel movement? – No bleeding at all – Mild – Moderate – Severe – Very severe 5. Please select the picture and description that best resembles your stool. Daily Symptom Diary 1. How severe was your worst abdominal cramping during the last 24 hours? – No cramping at all – Mild – Moderate – Severe – Very severe 2. How severe was your worst abdominal pain during the last 24 hours? – No pain at all – Mild – Moderate – Severe – Very severe 3. How severe was your worst joint pain during the last 24 hours? – No pain at all – Mild – Moderate – Severe – Very severe 4. How would you rate your worst feelings of tiredness during the last 24 hours? – No tiredness at all – Mild – Moderate – Severe – Very severe 5. How would you rate the severity of any constipation that you have experienced in the last 24 hours? – No constipation at all – Mild – Moderate – Severe – Very severe 6. How severe was your worst abdominal bloating during the last 24 hours? – No bloating at all – Mild – Moderate – Severe – Very severe 7. How severe was your worst gas during the last 24 hours? – No gas at all – Mild – Moderate – Severe – Very severe 8. During the last 24 hours, how often did you have difficulty telling the difference between gas and a bowel movement? – Never – Rarely – Sometimes – Often – Always 9. How often did you experience low energy during the last 24 hours? – Never – Rarely – Sometimes – Often – Always 10. How often did you feel weak in the last 24 hours? – Never – Rarely – Sometimes – Often – Always 11. How often did you have a poor appetite in the last 24 hours? – Never – Rarely – Sometimes – Often – Always Weekly Impact Assessment 1. Over the last 7 days, how limited were you in the types of food you could eat because of your ulcerative colitis? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 2. Over the last 7 days, how limited were you in the amount of food you could comfortably eat because of your ulcerative colitis? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 3. Over the past 7 days, how limited were your activities because of the need to be near a restroom? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 4. Over the past 7 days, how limited were your social activities because of your UC? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 5. Over the past 7 days, how limited were your leisure activities because of your UC? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 6. Over the past 7 days, how limited was your overall functioning because of your ulcerative colitis? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 7. Over the past 7 days, how difficult was it to complete your responsibilities at work or school because of your UC? – Not difficult at all – A little difficult – Moderately difficult – Very difficult – Extremely difficult – Not applicable: I did not work or attend school in the past 7 days because of my UC – Not applicable: I did not work or attend school in the past 7 days for reasons not related to my UC 8. Over the past 7 days, because of your UC, how difficult was it for you to stay asleep after going to bed? – Not difficult at all – A little difficult – Moderately difficult – Very difficult – Extremely difficult 9. Over the past 7 days, how limited was your participation in exercise or sports because of your UC? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 10. Over the past 7 days, how limited was your ability to travel because of your UC? – Not limited at all – A little limited – Moderately limited – Very limited – Extremely limited 11. Over the past 7 days, how much has UC interfered with your quality of life? – Not at all – A little bit – Moderately – Very much – Extremely 12. Over the past 7 days, how often have you worried about having an accident related to your UC? – Never – Rarely – Sometimes – Often – Always 13. Over the past 7 days, how often has your UC caused you to feel embarrassed? – Never – Rarely – Sometimes – Often – Always |
The concepts, sub‐concepts and domains included in the final draft SIQ‐CD and SIQ‐UC are depicted in Figure 3. Questionnaire items are grouped into three modules: a daily bowel movement report, a daily symptom assessment and a weekly impact assessment. The first two modules are completed once daily over a 7‐day period, while the third module is completed once at the end of a 7‐day period. These recall periods were selected based on expert opinion. A modified version of the draft SIQ‐CD tool was also designed to accommodate Crohn's disease patients with an ostomy.
FIGURE 3.
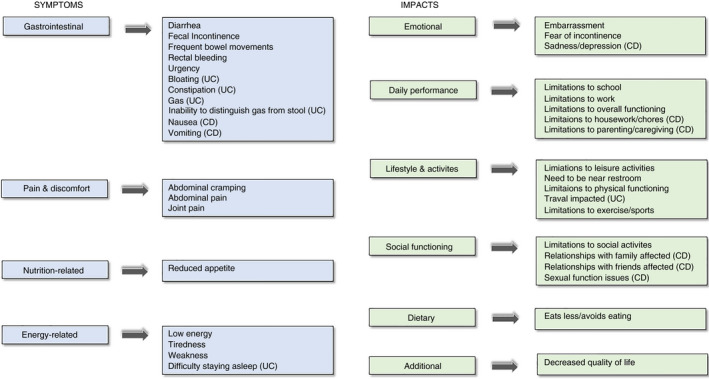
Domains and sub‐domains included in the SIQ‐CD and SIQ‐UC. The “symptoms” domain consists of four sub‐domains (gastrointestinal, pain and discomfort, nutrition‐related and energy‐related symptoms), while the “impacts” domain consists of six sub‐domains (emotional, daily performance, lifestyle and activities, social functioning, dietary and additional impacts)
4. DISCUSSION
In recent years, the FDA and EMA have recommended that clinical parameters, endoscopic findings and patient‐reported symptoms be separately quantified and reported in inflammatory bowel disease trials. 5 , 24 Conversely, historical outcome measures such as Crohn's Disease Activity Index (CDAI), 25 HBI, Mayo Clinic Score (MCS) 21 and SCCAI will no longer be accepted in PRO‐based labelling claims. To encourage rigorous PRO instrument development, the FDA has issued a guidance document, and in keeping with this roadmap, we developed two novel PRO instruments, the SIQ‐CD and the SIQ‐UC, to facilitate inflammatory bowel disease drug development programmes and labelling claims.
The FDA guidance states that item generation should incorporate patients with variations in disease severity. To date, our study is the only PRO initiative in which inflammatory bowel disease patients with a range of both clinical and endoscopic disease severity were included in the development population. Given that our goal was to build a PRO tool for use in inflammatory bowel disease registration trials, we incorporated central reading of endoscopy, which is the gold standard approach recommended by regulatory authorities for evaluating inflammatory disease.
In the ulcerative colitis concept elicitation phase of our study, endoscopic disease was assessed at baseline and recruitment was monitored to ensure that MCES‐defined categories were equally characterised. Additionally, SCCAI and disease extent scores were collected. At least one ulcerative colitis participant belonged to each SCCAI category, and disease extent was evenly distributed. Approximately one‐third of participants had proctitis, left‐sided colitis and pancolitis, respectively. In the Crohn's disease concept elicitation phase, recruitment was monitored to ensure that HBI‐defined clinical disease activity categories were equally represented. The HBI was chosen in favour of the SES‐CD because Crohn's disease participants with an ostomy or perianal fistula were not required to undergo endoscopy at baseline. However, endoscopy was required in all patients without complications, and it was confirmed that at minimum one participant from this subgroup belonged to each of the three SES‐CD categories.
While at least one other inflammatory bowel disease PRO initiative has cited the FDA guidance, endoscopy was not incorporated in the development of the CD‐PRO/SS and UC‐PRO/SS measures. 7 , 8 Participants in the CD‐PRO/SS development study were enrolled based on physician‐confirmed biopsy and clinical disease activity was assessed using the Sandler estimated Crohn's Disease Activity Index (SeCDAI), without endoscopy. 7 , 26 It is well established that Crohn's disease symptoms do not correlate with the severity of endoscopic disease, 27 , 28 , 29 and that clinical assessments of disease activity are susceptible to bias. 30 Similarly, baseline endoscopy was not performed in the development of the UC‐PRO/SS. It is therefore unclear how many Crohn's disease and ulcerative colitis patients were experiencing active disease at the time of study participation and whether a spectrum of endoscopic disease was incorporated. Furthermore, there was a lack of variation in clinical disease activity among the CD‐PRO/SS development population. The majority of participants (83%) had moderate or severe disease, defined as a SeCDAI score of 220 or greater. In our Crohn's disease study, enrolment was stratified by HBI score to ensure a range of clinical disease activity and SES‐CD values were collected.
Another important strength of the SIQ‐CD and SIQ‐UC is the incorporation of an impacts section designed to assess functioning related to disease status. These multi‐domain instruments may be able to support claims related to improvement in not only symptoms but also ability to function and emotional state. 9
It is notable there was considerable overlap in Crohn's disease and ulcerative colitis concept elicitation results. Two‐thirds (12/18) of the symptom concepts and one‐half (10/20) of the impact concepts overlap, notwithstanding that the two development processes were independent of each other. This finding holds out the possibility that with future development a robust combined instrument could be created.
Comparisons of the IBDQ, the tools developed by Higgins et al, and the novel questionnaires described in the current manuscript also reveal a sizeable proportion of shared items (Tables S6 and S7). For example, 65% (20/31) and 55% (16/29) of the items included in the SIQ‐CD and SIQ‐UC are included in the IBDQ, respectively. This is interesting given that the IBDQ was developed two decades before the FDA PRO guidance was issued and raises the question of whether strict adherence to the guidance principles is inherently beneficial, especially since heterogeneous clinical trial outcome measures impede between‐study comparisons and meta‐analyses.
Several limitations to the current study should be acknowledged. First, blinded centrally read endoscopy was not used to prospectively guide recruitment in Crohn's disease cohort. Rather, it was used to confirm that a spectrum of objectively confirmed endoscopic disease was represented in Crohn's disease study population. The study population included only one participant with an SES‐CD value greater than 15. Similarly, while a spectrum of endoscopic disease activity was incorporated in the ulcerative colitis cohort, only one participant had a SCCAI score greater than 11. Second, the SIQ‐CD and SIQ‐UC were both developed in an English‐speaking, North American population. While no issues were identified in the translatability assessments of these instruments, additional psychometric testing may be required if substantial adaptations are made to the SIQ‐CD and SIQ‐UC in the future. Finally, while we used rigorous qualitative and mixed‐methods approaches to identify patient‐reported concepts, refine the underlying conceptual frameworks, and provide evidence of content validity for the newly developed SIQ‐CD and SIQ‐UC, cross‐sectional and longitudinal measurement properties need to be evaluated in adequately powered studies before these instruments can be used to support labelling claims. Finally, prospective validation is required to confirm the recall periods, as they were selected using expert consensus, and determine instrument scaling and scoring.
In conclusion, the SIQ‐CD and SIQ‐UC are novel PRO draft measures for use in Crohn's disease and ulcerative colitis trials, respectively. They were developed in consonance with a regulatory framework, and hold promise for evaluating both inflammatory bowel disease‐related symptoms and impacts in patients with a range of clinical and endoscopic disease severity. Further validation efforts are currently underway within clinical trials programmes to assess validity, reliability and responsiveness.
AUTHORSHIP
Guarantor of the article: William J. Sandborn.
Author contributions: Study concept and design: PSD, VJ, RK, KPM, MLM, JM, BGF, WJS; data collection and analysis: JM, KPM, MLM; analysis and interpretation of data: PSD, VJ, KPM, MLM, CP, BGF, WJS; drafting of the manuscript: PSD, VJ, CEP, KPM, MLM, BGF, WJS; critical revision of the manuscript for important intellectual content: PSD, VJ, RK, CM, KPM, MLM, CEP, BGF, WJS; study supervision: VJ, BGF, WJS.
All listed authors have made substantial contributions to the conception and design, or acquisition of data, or analysis and interpretation of data; been involved in drafting the manuscript or critically revising it for important intellectual content; given final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors approved the final version of the manuscript.
5.
FIGURE 2.

Venn diagram of concepts elicited for Crohn's disease (CD) and ulcerative colitis (UC). The concept elicitation exercise identified a total of 141 concept codes (80 symptoms and 61 impacts) relevant to CD and 128 concept codes (79 symptoms and 49 impacts) relevant to UC. Based on the cognitive comprehension exercise, literature review and expert option, 31 concepts (14 symptoms and 17 impacts) were identified for inclusion in the CD instrument and 29 concepts (16 symptoms and 13 impacts) were identified for inclusion in the UC instrument
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Thank you to Leonardo Guizzetti of Robarts Clinical Trials Inc for providing statistical support.
Declaration of personal interests: PD has received consulting fees and research support from Takeda, AbbVie, Janssen, Pfizer, Polymedco and Bulhmann. VJ has received consulting fees from AbbVie, Takeda, Eli Lilly, Pfizer, Janssen, Ferring, Shire, Merck, GSK, Celltrion and Robarts Clinical Trials Inc; serves as an advisory board member for AbbVie, Takeda, Janssen, Arena, GSK, Eli Lilly and Ferring; has received speakers' bureau fees from Takeda, AbbVie, Janssen, Pfizer, Shire and Ferring. RK has received scientific advisory board fees from AbbVie, Janssen, Pfizer and Takeda; consulting fees from AbbVie, Janssen, Takeda and Robarts Clinical Trials Inc; and payments for lectures/speakers' bureau fees from AbbVie, Janssen, Shire and Takeda. CM has received scientific advisory board fees from AbbVie, Janssen, Pfizer and Takeda; consulting fees from Robarts Clinical Trials, Inc; and payment for lectures/speakers' bureau fees from AbbVie, Janssen, Pfizer and Takeda. KPM was an employee of Health Research Associates (HRA) at the time the work was conducted. HRA received contract research funding from Robarts Clinical Trials Inc to conduct the studies detailed in this manuscript. MM was an employee of Health Research Associates (HRA) at the time the work was conducted. HRA received contract research funding from Robarts Clinical Trials Inc to conduct the studies detailed in this manuscript. CEP is an employee of Robarts Clinical Trials, Inc JM is an employee of Robarts Clinical Trials, Inc BGF has received grant/research support from AbbVie Inc, Amgen Inc, AstraZeneca/MedImmune Ltd., Atlantic Pharmaceuticals Ltd., Boehringer‐Ingelheim, Celgene Corporation, Celltech, Genentech Inc/Hoffmann‐La Roche Ltd., Gilead Sciences Inc, GlaxoSmithKline (GSK), Janssen Research & Development LLC., Pfizer Inc, Receptos Inc/Celgene International, Sanofi, Santarus Inc, Takeda Development Center Americas Inc, Tillotts Pharma AG, and UCB; consulting fees from Abbott/AbbVie, Akebia Therapeutics, Allergan, Amgen, Applied Molecular Transport Inc, Aptevo Therapeutics, Astra Zeneca, Atlantic Pharma, Avir Pharma, Biogen Idec, BioMx Israel, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, Galapagos, GiCare Pharma, Gilead, Gossamer Pharma, GSK, Inception IBD Inc, JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nestle, Nextbiotix, Novonordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Progenity, Protagonist, Receptos, Salix Pharma, Shire, Sienna Biologics, Sigmoid Pharma, Sterna Biologicals, Synergy Pharma Inc, Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, Vivelix Pharma, VHsquared Ltd., and Zyngenia; speakers bureau fees from Abbott/AbbVie, JnJ/Janssen, Lilly, Takeda, Tillotts, and UCB Pharma; is a scientific advisory board member for Abbott/AbbVie, Allergan, Amgen, Astra Zeneca, Atlantic Pharma, Avaxia Biologics Inc, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Celgene, Centocor Inc, Elan/Biogen, Galapagos, Genentech/Roche, JnJ/Janssen, Merck, Nestle, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Sterna Biologicals, Takeda, Teva, TiGenix, Tillotts Pharma AG, and UCB Pharma; and is the Senior Scientific Officer of Robarts Clinical Trials Inc WJS has received research grant support from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, AbbVie, Janssen, Takeda, Lilly and Celgene/Receptos; consulting fees from AbbVie, Allergan, Amgen, Boehringer Ingelheim, Celgene, Conatus, Cosmo, Escalier Biosciences, Ferring, Genentech, Gilead, Gossamer Bio, Janssen, Lilly, Miraca Life Sciences, Nivalis Therapeutics, Novartis Nutrition Science Partners, Oppilan Pharma, Otsuka, Paul Hastings, Pfizer, Precision IBD, Progenity, Prometheus Laboratories, Ritter Pharmaceuticals, Robarts Clinical Trials Inc (owned by Health Academic Research Trust or HART), Salix, Shire, Seres Therapeutics, Sigmoid Biotechnologies, Takeda, TiGenix, Tillotts Pharma, UCB Pharma, and Vivelix; and stock options from Ritter Pharmaceuticals, Oppilan Pharma, Escalier Biosciences, Gossamer Bio, Precision IBD, and Progenity.
Dulai PS, Jairath V, Khanna R, et al. Development of the symptoms and impacts questionnaire for Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. 2020;51:1047–1066. 10.1111/apt.15726
The Handling Editor for this article was Professor Jonathan Rhodes, and it was accepted for publication after full peer‐review.
The SIQ‐CD and SIQ‐UC are available for use; please contact PRO‐engagement@robartsinc.com for inquiries about permission to use the paper and/or electronic versions.
Funding information
AbbVie Inc., Gilead Sciences, Inc, Pfizer Inc. and Robarts Clinical Trials Inc. Funders, except for Robarts Clinical Trials Inc., were not involved in the study design or conduct.
REFERENCES
- 1. Hindryckx P, Vande Casteele N, Novak G, et al. The expanding therapeutic armamentarium for inflammatory bowel disease: how to choose the right drug[s] for our patients? J Crohns Colitis. 2018;12:105‐119. [DOI] [PubMed] [Google Scholar]
- 2. Khanna R, Jairath V, Vande Casteele N, et al. Efficient early drug development for ulcerative colitis. Gastroenterology. 2016;150:1056‐1060. [DOI] [PubMed] [Google Scholar]
- 3. De Jong MJ, Huibregtse R, Masclee AAM, Jonkers D, Pierik MJ. Patient‐reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2018;16:648‐663. [DOI] [PubMed] [Google Scholar]
- 4. Guyatt G, Mitchell A, Irvine EJan, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804‐810. [PubMed] [Google Scholar]
- 5. European Medicines Agency .Guideline on the development of new medicinal products for the treatment of Crohn's disease. https://www.ema.europa.eu/documents/scientific‐guideline/guideline‐development‐new‐medicinal‐products‐treatment‐crohns‐disease‐revision‐2_en.pdf. Accessed October 10, 2019.
- 6. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research ( CDER ) . Ulcerative colitis: clinical trial endpoints guidance for industry. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/ulcerative‐colitis‐clinical‐trial‐endpoints‐guidance‐industry. Accessed October 10, 2019.
- 7. Higgins PDR, Harding G, Leidy NK, et al. Development and validation of the Crohn's disease patient‐reported outcomes signs and symptoms (CD‐PRO/SS) diary. J Patient Rep Outcomes. 2017;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higgins PDR, Harding G, Revicki DA, et al. Development and validation of the Ulcerative Colitis patient‐reported outcomes signs and symptoms (UC‐pro/SS) diary. J Patient Rep Outcomes. 2017;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research ( CDER ), Center for Biologics Evaluation and Research ( CBER ), Center for Devices and Radiological Health ( CDRH ) . Patient‐reported outcome measures use in medical product development to support labeling claims 2009. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed October 10, 2019.
- 10. Jairath V, Levesque BG, Vande Casteele N, et al. Evolving concepts in phases I and II drug development for Crohn's disease. J Crohns Colitis. 2017;11:246‐255. [DOI] [PubMed] [Google Scholar]
- 11. Vork L, Keszthelyi D, Mujagic Z, et al. Development, content validity, and cross‐cultural adaptation of a patient‐reported outcome measure for real‐time symptom assessment in irritable bowel syndrome. Neurogastroenterol Motil. 2018;30:e13244. [DOI] [PubMed] [Google Scholar]
- 12. Schoepfer AM, Straumann A, Panczak R, et al. Development and validation of a symptom‐based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1255‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity–establishing and reporting the evidence in newly developed patient‐reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2–assessing respondent understanding. Value Health. 2011;14:978‐988. [DOI] [PubMed] [Google Scholar]
- 14. Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity–establishing and reporting the evidence in newly developed patient‐reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1–eliciting concepts for a new PRO instrument. Value Health. 2011;14:967‐977. [DOI] [PubMed] [Google Scholar]
- 15. Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient‐reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8:94‐104. [DOI] [PubMed] [Google Scholar]
- 16. Williet N, Sandborn WJ, Peyrin‐Biroulet L. Patient‐reported outcomes as primary end points in clinical trials of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:1246‐1256. [DOI] [PubMed] [Google Scholar]
- 17. Turner‐Bowker DM, Lamoureux RE, Stokes J, et al. Informing a priori sample size estimation in qualitative concept elicitation interview studies for clinical outcome assessment instrument development. Value Health. 2018;21:839‐842. [DOI] [PubMed] [Google Scholar]
- 18. Harvey RF, Bradshaw JM. A simple index of Crohn's‐disease activity. Lancet. 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 19. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES‐CD. Gastrointest Endosc. 2004;60:505‐512. [DOI] [PubMed] [Google Scholar]
- 21. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625‐1629. [DOI] [PubMed] [Google Scholar]
- 22. Travis SPL, Schnell D, Krzeski P, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut. 2012;61:535‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Willis G. Cognitive Interviewing. Thousand Oaks, CA: Sage Publications, Inc; 2005. [Google Scholar]
- 24. U.S. Department of Health and Human Services, Food and Drug Administration . FDA Great III Workshop: Gastroenterology regulatory endpoints and the advancement of therapeutics 2015.
- 25. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439‐444. [PubMed] [Google Scholar]
- 26. Sandler RS, Jordan MC, Kupper LL. Development of a Crohn's index for survey research. J Clin Epidemiol. 1988;41:451‐458. [DOI] [PubMed] [Google Scholar]
- 27. Cellier C, Sahmoud T, Froguel E, et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn's disease. A prospective multicentre study of 121 cases. The Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones J, Loftus EV, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2008;6:1218‐1224. [DOI] [PubMed] [Google Scholar]
- 29. Ricanek P, Brackmann S, Perminow G, et al. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand J Gastroenterol. 2011;46:1081‐1091. [DOI] [PubMed] [Google Scholar]
- 30. Feagan BG, Sandborn WJ, D'Haens G, et al. The role of centralized reading of endoscopy in a randomized controlled trial of mesalamine for ulcerative colitis. Gastroenterology. 2013;145:149‐157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


