Abstract
Aim
To study effect of delmopinol hydrochloride (DEL) in comparison with chlorhexidine digluconate (CHX) and a placebo (PLA) in addition to non‐surgical mechanical debridement in patients with peri‐implant mucositis.
Materials and methods
Eighty‐nine patients with at least one implant diagnosed with peri‐implant mucositis were randomly assigned to one of three study groups (DEL, CHX and PLA). Professional non‐surgical mechanical debridement was performed at baseline. Mouth rinsing was carried out by the patients twice a day in addition to their regular oral hygiene practices. Assessments of efficacy were performed for the primary outcome ‐ Implant bleeding on probing (IBOP%) and secondary outcomes ‐ modified Bleeding Index (mBI) and modified Plaque Index (mPI) at 1 and 3 months.
Results
At 3 months, there was statistically significant reduction in IBOP% and mBI within the study groups compared to baseline. However, there was no statistically significant difference between the study groups at 3 months follow‐up. Moreover, there was a statistically significant difference according to mPI at 1 month between the chlorhexidine and placebo group (p = .004).
Conclusions
This study confirms that mechanical debridement combined with oral hygiene instruction is effective in treatment of peri‐implant mucositis. The clinical effects between groups were comparable.
Keywords: chlorhexidine, delmopinol, mouthrinse, non‐surgical mechanical debridement, peri‐implant mucositis
Clinical Relevance.
Scientific rationale for the study: Optimal biofilm removal around dental implants is essential for treatment of peri‐implant mucositis. Despite all efforts, complete resolution of peri‐implant mucositis remains challenging. Till today, no studies are available on the effect of delmopinol on treatment of peri‐implant mucositis.
Principal findings: Delmopinol as well as the control groups showed reduced bleeding on probing and mBI throughout the study period.
Practical implications: This study explored the possibility of combining mechanical debridement with delmopinol treating peri‐implant mucositis. There is no evidence for additional clinical effect of using mouthrinse beside mechanical debridement based on the current study.
1. INTRODUCTION
Dental implant therapy has proven to be reliable in replacing missing or lost teeth. Despite high implant survival and success rates, it has long been realized that osseointegrated implants can suffer from biological complications, collectively termed peri‐implant diseases (Klinge, Klinge, Bertl, & Stavropoulos, 2018).
Peri‐implant diseases include peri‐implant mucositis and peri‐implantitis: inflammatory conditions of the soft and hard tissues around dental implants. Peri‐implant mucositis has been defined as an inflammatory lesion of the mucosa surrounding an endosseous implant without loss of supporting peri‐implant bone. The clinical signs of peri‐implant inflammation are bleeding on probing, while additional signs may include erythema, swelling and suppuration (Heitz‐Mayfield & Salvi, 2018). According to a systematic review, the prevalence of peri‐implant mucositis ranges from 19% to 65% at patient level (Derks & Tomasi, 2015).
Biofilm formation on dental implants plays an important role in peri‐implant mucositis. There is strong evidence from both animal and human experimental studies that plaque is the aetiological factor for peri‐implant mucositis (Berglundh et al., 2018). The current recommendation for the treatment of peri‐implant mucositis is mechanical debridement with or without antiseptics in addition to reinforcement of self‐performed oral hygiene (Heitz‐Mayfield, Needleman, Salvi, & Pjetursson, 2014). Therapy of peri‐implant mucositis should be considered as a preventive measure for the onset of peri‐implantitis (Salvi & Zitzmann, 2014).
Previous studies (De Siena, Francetti, Corbella, Taschieri, & Del Fabbro, 2013; Hallström, Lindgren, & Twetman, 2017; Heitz‐Mayfield et al., 2011; Menezes, Fernandes‐Costa, Silva‐Neto, Calderon, & Gurgel, 2016; Pulcini et al., 2019) suggest that peri‐implant mucositis can be treated with non‐surgical mechanical debridement with or without the use of adjunctive antiseptics or therapeutic agents such as chlorhexidine and essential oils. However, peri‐implant mucositis was not completely resolved in all cases.
Anti‐plaque agents have shown to be helpful in disrupting and preventing the formation of biofilm in the oral environment. Antibiofilm agent delmopinol is proven to be effective against plaque formation, treatment of experimentally induced gingivitis and has been suggested as an alternative for chlorhexidine (Addy, Moran, & Newcombe, 2007; Collaert, Attström, De Bruyn, & Movert, 1992; Hase, Attström, Edwardsson, Kelty, & Kisch, 1998). It has also been used as a decontamination agent for implants in treating peri‐implantitis in dogs (Berglundh, Lindhe, Marinello, Ericsson, & Liljenberg, 1992).
Delmopinol is a surface‐active agent with low antimicrobial properties, prevents plaque formation and possess plaque dissolving properties (Klinge, Matsson, Attström, Edwardsson, & Sjödin, 1996; Simonsson, Hvid, Rundegren, & Edwardsson, 1991). In short‐term and long‐term clinical trials, delmopinol has shown to have moderate anti‐plaque and anti‐gingivitis efficacy (Collaert, Edwardsson, Attström, Hase, & Aström, 1993; Lang et al., 1998). When compared with chlorhexidine, delmopinol had lower stain and calculus scores (Baehni & Takeuchi, 2003). Moreover, the use of chlorhexidine may cause an alteration of the oral microflora due to differences in sensitivities among the bacterial species. Such alterations can result in an overgrowth of Gram‐negative bacteria or yeasts and development of resistance (Elworthy et al., 1995). It was earlier shown that rinsing with delmopinol did not induce any shift into abnormal composition of the oral microflora. Treatment with delmopinol appeared to delay the plaque consolidation. Compared to chlorhexidine, delmopinol affected the oral microflora to a much less extent (Hase, 1998b).
An in vitro study model using 0.2% delmopinol showed a significant amount of reduction in biofilm‐associated bacterial population when titanium surfaces were treated for 20 min (Ready et al., 2015). The results of a recent animal model study indicated that 0.2% delmopinol rinse might play a role in the prevention of peri‐implant disease development (Levin et al., 2019). To our knowledge, there are still no studies available to assess the effect of antibiofilm agent delmopinol as an adjunct to non‐surgical mechanical debridement in treating peri‐implant mucositis.
Aim of the present study is to determine the clinical effect of delmopinol on peri‐implant mucositis when used in addition to mechanical debridement. The null hypothesis is that there is no difference in bleeding on probing around dental implants on the treatment of peri‐implant mucositis with mechanical debridement alone, or in combination with mechanical debridement and use of mouthrinse delmopinol or chlorhexidine during a period of 1 month and follow‐up of 3 months.
2. MATERIAL AND METHODS
2.1. Study design
This study was designed as a 3‐month, double‐blinded, randomized clinical trial with three parallel groups. Before commencement, the trial was registered as Trial NL5159 (NTR5299) in the Netherlands Trial Register and was approved by an independent Medical Research Ethics Committee, Vrije Universiteit Medical Centre, Amsterdam. The trial was conducted in compliance with the provisions of the Declaration of Helsinki and reported using the CONSORT guidelines (Schulz, Altman, & Moher, for the CONSORT Group, 2010).
All participants gave their written informed consent after verbal and written information. Once the entry criteria had been confirmed the subjects were entered to the study and assigned a patient number. Assignment to delmopinol (DEL), chlorhexidine (CHX) or placebo (PLA) group was done using randomization, stratified by ASA score and a block size of 9 patients. A staff member not involved in the examination or treatment of the patients gave the patients the respective blinded mouthrinse. Composition of placebo mouthrinse was made up to resemble delmopinol and positive control chlorhexidine as much as possible. All mouthrinses were prepared in a pharmaceutical laboratory (Apotheek A 15, Netherlands) and packed in identical bottles. Both participants and examiner were blinded to group assignment. The code for the mouthrinse was broken once the study was completed and the data set was locked.
The study population included individuals who visited the department of Oral Implantology, Academic Centre for Dentistry Amsterdam (ACTA) for regular dental implant maintenance between May 2017 and October 2017. The inclusion criteria of the study were as follows: (a) individuals 18 years of age and older with at least one titanium dental implant, (b) single/three‐unit fixed implant‐supported restoration with bleeding on gentle probing (BOP) and/pus and (c) implant in function for at least 1 year, with no progressive radiographic bone loss compared to the baseline peri‐apical radiograph.
Exclusion criteria were as follows: (a) implants with >2 mm bone loss as identified by comparisons between current peri‐apical radiographs with radiographs taken at placement of the prosthetic restoration, (b) untreated or recurrent periodontitis, (c) full‐mouth plaque score >20%, (d) smoking > 20 cigarettes/day, (e) uncontrolled diabetes mellitus, (f) antibiotic and anti‐inflammatory drug used in the last one month before the start of the study, (g) usage of antidepressants or anticholinergic drugs or (h) pregnancy/ lactation.
2.2. Interventions
After baseline examination, the participants received full‐mouth supra gingival scaling and polishing according to their periodontal conditions. The dental implants were debrided using an ultrasonic device (Master Piezon EMS) with a high‐tech plastic material coated tip (PI Instrument; EMS, Nyon, Switzerland) placed in the peri‐implant pocket (Riben‐Grundstrom, Norderyd, André, & Renvert, 2015). Afterwards, subjects were randomly assigned to one of the three groups and received the corresponding mouthrinse. The mouthrinses were as follows: Decapinol Mouthrinse consisting of 2.0 mg/ml (0.2%) delmopinol hydrochloride, herb flavour, saccharin sodium, ethanol 99.5%, sodium hydroxide and purified water, Positive control Chlorhexidine mouthrinse containing 2.0 mg/ml (0.2%) chlorhexidine digluconate, peppermint oil, sorbitol, ethanol 96% and purified water and Placebo mouthrinse consisting peppermint oil, saccharin sodium, ethanol 96% and purified water. No specific toothpaste was supplied to the participants. The participants were instructed not to change any dentifrice and to continue using the same fluoride containing dentifrice which they used before the start of the trial. Each subject received individualized oral hygiene instructions and interdental brushes. Besides, a diary was provided to keep record of the mouthrinse use and the patients were asked to bring the used mouthrinse bottles with them to the 1‐month follow‐up visit. Supragingival maintenance care was provided at month 1 and 3. After each visit, the dentition was polished using a rubber cup and polishing paste.
2.3. Clinical and radiographic examination
During screening appointment, participants were asked to fill in a questionnaire including any medication and smoking habits. Stability of the suprastructure and access for self‐performed oral hygiene were ensured. BOP was considered to be present only if at least grade 2 mBI, that is blood forms a confluent red line on margin within 30 s after probing was present. An intra‐oral peri‐apical radiograph of the implant site was taken if no recent radiographs were available in order to exclude peri‐implantitis. Each visit commenced with an update on adverse effect. All the measurements were performed by a single, experienced dentist. The same examiner was also trained on specific typodont and was calibrated before the start of the clinical trial.
If the subject had more than one implant with peri‐implant mucositis, all implants were treated but one implant with the highest percentage of BOP sites was included for analysis. The clinical examination started with dental implant assessment followed by full‐mouth assessment. At baseline and during the 1 and 3‐month follow‐up visits, the following assessments were performed on six surfaces of both implants and teeth.
At implant level: (a) modified Plaque Index (mPI) (Mombelli, van Oosten, Schüch, &Lang 1987), (b) Implant Pocket Probing Depth (IPPD), (c) modified Bleeding Index (mBI) (Mombelli et al., 1987) and (d) Implant Bleeding on Probing sites (IBOP%) using a plastic probe with a standardized probing force of 0.2 N (Hawe Click‐Probe, Hawe Neos Dental, Switzerland) and expressed as percentage of sites with bleeding of the total number of available sites.
At full‐mouth level: (a) Full Mouth Plaque Index (FMPI) recorded according to the Silness and Löe plaque index (Löe, 1967), (b) Full Mouth Pocket Probing Depth (FMPPD) recorded using Hu Friedy Colorvue Oxford Probe with UNC‐15 tips, (c) Full Mouth Gingival Index (FMGI) recorded according to Löe and Silness Gingival Index (Löe, 1967) and (d) Full Mouth Bleeding on Probing sites (FMBOP%) expressed as the percentage of sites with bleeding of the total number of available sites.
2.4. Sample size calculation
The sample size calculation was based on a change in the IBOP. The standard deviation was estimated to 14%, and a difference of 12% units between placebo and delmopinol was considered worth detecting (Ramberg, Lindhe, Botticelli, & Botticelli, 2009). The calculation, which was based on a power of 80% and a significance level of p = .05, resulted in a required number of at least 25 subjects in each study group.
2.5. Statistical Analysis
The primary outcome variable was the change in IBOP. Secondary outcomes included mean changes in mPl, mBI and IPPD. Mean values and standard deviation (mean; SD) for the clinical parameters were calculated for three groups. Disease resolution was defined as the absence of BOP, and the frequency distribution of resolved sites was calculated. Castor EDC was used as software for randomization, data entry and logical checks. A software package (IBM SPSS Statistics 25.0; SPSS) was used for the statistical analysis. Comparisons over time for the investigated variables were performed using Analysis of Variance (ANOVA). Results were considered statistically significant at p < .05. Repeated measures analysis of variance (ANOVA), corrected for multiple testing (Bonferroni) was used to test for changes within the groups, Univariate analysis of Covariance (ANCOVA) was used to test changes between the groups at different follow‐up times. In the ANCOVAs, clinical parameters at each single evaluation were used as dependent variables, and the corresponding parameters at baseline were used as covariates and treatment allocation as a fixed factor.
3. RESULTS
3.1. Sample description
One hundred patients were screened of which eight did not fulfil the study requirements and three declined to participate in the investigation. Thirty‐one individuals were treated with DEL, thirty individuals with CHX and twenty‐eight individuals with PLA. One patient could not attend the 1‐month examination, but all 89 patients participated at the 3‐month examination (Figure 1). The primary analysis was intention to treat and involved all patients who were randomly assigned. The trial was ended upon achieving the estimated sample size and completing the 3‐month follow‐up.
FIGURE 1.
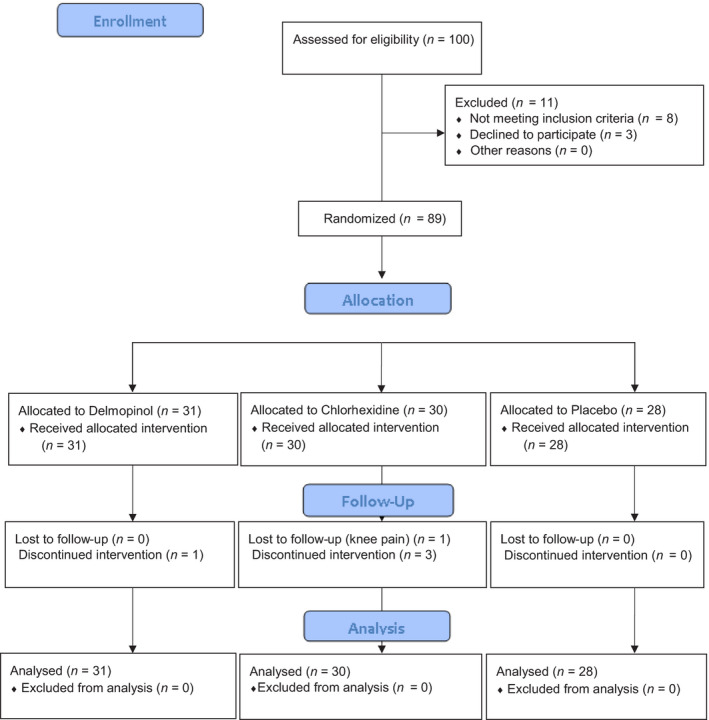
Consolidated Standards of Reporting Trials (CONSORT) Flow Diagram of the study
Table 1 depicts the clinical and demographic variables at baseline, with no statistically significant differences between groups with regard to age, sex or medical status. The mean age was 61.87 years (SD = 10.22). Nine patients were current smokers. All participants had fixed single crown/three‐unit bridge with titanium abutments.
TABLE 1.
Baseline demographic and clinical characteristics of the study groups
| Variable | Delmopinol (n = 31) | Chlorhexidine (n = 30) | Placebo (n = 28) | p‐value |
|---|---|---|---|---|
| Gender (male/female) | 16/15 | 16/14 | 16/12 | .91 |
| Mean age (years; SD) | 59 (10.6) | 62 (9.3) | 65 (10.3) | .15 |
| ASA score (1/2) | 14/17 | 15/15 | 12/16 | .66 |
| Smokers (current) | 4 | 2 | 3 | .72 |
| Mean number of implants | 3 (2.0) | 4 (1.9) | 3 (2.7) | .58 |
| Implant brand | ||||
| Straumann group implants (Straumann Dental Implant System) | 21 | 23 | 13 | .10 |
| Astra Tech group implants (Astra Tech Implant System) | 4 | 0 | 4 | .32 |
| Nobel Biocare group implants (Brånemark System) | 2 | 3 | 0 | .25 |
| Others | 4 | 4 | 11 | .86 |
| Suprastructure | ||||
| Cemented/screw retained | 11/20 | 8/22 | 11/17 | .57 |
| Single unit/multiunit | 19/12 | 22/8 | 17/11 | .59 |
| Tissue level/Bone level | 16/15 | 17/13 | 17/11 | .77 |
| Bone augmentation (yes/no) | 10/21 | 11/19 | 14/14 | .35 |
| Periodontal treatment history (yes/no) | 6/25 | 5/25 | 3/25 | .65 |
| Width of keratinized tissue (max 2 mm/more than 2 mm) | 9/22 | 17/13 | 13/15 | .20 |
| Location | ||||
| Maxilla/mandible | 19/12 | 19/11 | 20/8 | .70 |
| Anterior/posterior | 7/24 | 5/25 | 7/21 | .75 |
3.2. Clinical outcomes
Table 2 shows the mean values and standard deviation of clinical outcomes at baseline, 1 and 3 months.
TABLE 2.
Mean values for clinical outcomes at each visit
| Group | Baseline | 1 month | 3 months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | p‐value | n | Mean | SD | p‐value | n | Mean | SD | p‐value | ||
| Implant level | |||||||||||||
| mPI | DEL | 31 | 0.55 | 0.47 | .85 | 31 | 0.36 | 0.34 | .02* | 31 | 0.37 | 0.38 | .33 |
| CHX | 30 | 0.61 | 0.54 | 29 | 0.24 | 0.26 | 30 | 0.52 | 0.41 | ||||
| PLA | 28 | 0.60 | 0.50 | 28 | 0.48 | 0.27 | 28 | 0.33 | 0.25 | ||||
| mBI | DEL | 31 | 1.00 | 0.49 | .70 | 31 | 0.25 | 0.41 | .22 | 31 | 0.13 | 0.23 | .42 |
| CHX | 30 | 1.03 | 0.44 | 29 | 0.26 | 0.38 | 30 | 0.28 | 0.30 | ||||
| PLA | 28 | 1.08 | 0.52 | 28 | 0.18 | 0.27 | 28 | 0.19 | 0.32 | ||||
| IBOP % | DEL | 31 | 45.16 | 25.52 | .91 | 31 | 7.56 | 16.57 | .89 | 31 | 3.22 | 10.01 | .14 |
| CHX | 30 | 43.88 | 22.52 | 29 | 9.00 | 18.15 | 30 | 8.88 | 12.17 | ||||
| PLA | 28 | 47.02 | 24.45 | 28 | 6.35 | 13.09 | 28 | 7.73 | 13.96 | ||||
| IPPD | DEL | 31 | 3.18 | 0.69 | .23 | 31 | 2.89 | 0.64 | 0.28 | 31 | 2.65 | 0.54 | .03* |
| CHX | 30 | 3.44 | 0.60 | 29 | 2.90 | 0.43 | 30 | 2.76 | 0.47 | ||||
| PLA | 28 | 3.17 | 0.78 | 28 | 2.70 | 0.51 | 28 | 2.40 | 0.67 | ||||
| Full‐mouth level | |||||||||||||
| FMPI | DEL | 31 | 0.62 | 0.34 | .41 | 31 | 0.07 | 0.09 | .004* | 31 | 0.06 | 0.07 | .51 |
| CHX | 30 | 0.50 | 0.23 | 29 | 0.05 | 0.05 | 30 | 0.08 | 0.07 | ||||
| PLA | 28 | 0.61 | 0.27 | 28 | 0.06 | 0.07 | 28 | 0.06 | 0.08 | ||||
| FMGI | DEL | 31 | 0.38 | 0.40 | .56 | 31 | 0.25 | 0.16 | .58 | 31 | 0.32 | 0.25 | .51 |
| CHX | 30 | 0.32 | 0.33 | 29 | 0.18 | 0.13 | 30 | 0.36 | 0.23 | ||||
| PLA | 28 | 0.30 | 0.41 | 28 | 0.33 | 0.21 | 28 | 0.29 | 0.19 | ||||
| FMBOP% | DEL | 31 | 12.40 | 12.33 | .28 | 31 | 1.92 | 3.24 | .67 | 31 | 1.79 | 2.23 | .62 |
| CHX | 30 | 10.27 | 8.82 | 29 | 1.60 | 1.80 | 30 | 2.26 | 2.14 | ||||
| PLA | 28 | 8.64 | 6.89 | 28 | 2.06 | 2.45 | 28 | 1.86 | 2.98 | ||||
| FMPPD | DEL | 31 | 2.72 | 0.27 | .006* | 31 | 2.64 | 0.36 | .82 | 31 | 2.68 | 0.35 | .18 |
| CHX | 30 | 2.67 | 0.31 | 29 | 2.61 | 0.31 | 30 | 2.62 | 0.30 | ||||
| PLA | 28 | 2.46 | 0.38 | 28 | 2.59 | 0.30 | 28 | 2.50 | 0.47 | ||||
p‐values in bold indicate statistically significant differences between groups.
Abbreviation: FMBOP%, full mouth bleeding on probing; FMGI, full mouth gingival index; FMPI, Full Mouth Plaque Index; FMPPD, Full Mouth Pocket Probing Depth; IBOP%, Implant Bleeding on Probing; IPPD, Implant Pocket Probing Depth; mBI, modified Bleeding Index; mPI, modified Plaque Index; SD, Standard Deviation.
There was a statistically significant reduction in mean mBI from baseline to 1 month (p = .001) and from baseline to three months (p = .001) within all the three study groups. Difference in mean mBI between baseline and 3 months was highest for DEL [mean difference = 0.90; 95% CI (0.80; 1.00)]. However, the difference between baseline and 3 months was not statistically significant between any groups. At 3 months, the reduction in mean mBI for DEL in comparison with PLA was [mean difference = 0.06 (p = .86)]. The corresponding figures for CHX was 0.08 (p = .16). While DEL showed more reduction in IBOP% than CHX, no statistically significant difference was achieved between these two groups either at 1 or at 3 months. The mean reduction in IBOP% for DEL in comparison with PLA was −0.01 at 1month (p = 1.00) and 0.05 (p = .38) at 3 months. The corresponding figures for CHX was −0.02 (p = 1.00) at 1 month and −0.01 (p = 1.00) at 3 months, respectively. Reduction of IBOP% between baseline and 3 months was highest in DEL group [mean difference = 0.42; 95% CI (0.38; 0.46)] even though no statistically significant difference was found between any groups. There was significant change in mean IBOP% at both 1 month (p = .001) and 3 months (p = .001) within all three study groups. However, no statistically significant difference existed between any groups. Similarly, changes between baseline and 3 months for these parameters did not significantly differ between groups.
The number of diseased sites (pocket depth < 5 mm with BOP) before and after treatment is presented in Table 3. At the end of 3 months, the diseased sites were reduced to 13% in the DEL group, 40% in the CHX group and 29% in the PLA group. However, no statistically significant differences were found between the three groups.
TABLE 3.
Number of patients (percentages) who achieved complete disease resolution (no BOP sites) at 1 month and 3 months
| Timepoint | Delmopinol (n = 31) | Chlorhexidine (n = 30) | Placebo (n = 28) | p‐value |
|---|---|---|---|---|
| 1 month | 24 (77%) | 22 (73%) | 21 (75%) | .69 |
| 3 months | 27 (87%) | 18 (60%) | 20 (71%) | .29 |
There was statistically significant reduction in mean mPI scores at 1 month between CHX and PLA groups [mean difference = 0.25; 95% CI (0.06; 0.44); p = .004)].The corresponding values for DEL were [mean difference = 0.11; 95% CI (−0.07; −0.33); p = .43)]. There is a statistically significant difference according to mPI cores between baseline and 1 month within CHX group [mean difference = 0.37;(p = .009; 95% CI (0.08; 0.66)]. Between baseline and 3 months, there was statistically significant reduction in IPPD (p = .001) within all the three groups. At 3 months, the reduction in mean mPI and mean IPPD was not statistically significant between any of the groups.
Reduction in FMBOP% between baseline and 3 months was highest in DEL than any other group [mean difference = 9.24; 95% CI (8.4–10.09)]. However, no statistically significant difference could be achieved between any groups. Reduction in both FMBOP% and FMPI was significant within all three groups at 1 month (p = .0001).
No serious adverse events were reported in this study. The most common adverse event reported in the DEL group was a transient anaesthetic sensation in the oral mucosa, especially at the tip of the tongue, while in CHX group was staining of the teeth or tongue. Another common adverse event reported by the subjects rinsing with both DEL and CHX was taste alteration.
4. DISCUSSION
The aim of the present study was to investigate primarily the effect of delmopinol on BOP and secondly on mBI and mPI around dental implants with peri‐implant mucositis when administered as an adjuvant treatment to mechanical debridement. The effect of rinsing with delmopinol was evaluated in comparison with chlorhexidine and placebo during 1 month with a 3‐month follow‐up period. All three study groups showed a statistically significant reduction according to BOP around dental implants within the groups after 1‐month rinsing and at the 3‐month follow‐up. However, no statistically significant difference according to BOP, mBI and mPI was found between the study groups at end of 3‐month follow‐up period. One possible explanation for the positive outcome, besides the effect of mouthrinse, could be the effect of mechanical debridement combined with patient motivation and adherence to the customized oral hygiene instructions. However, it is interesting to note that BOP around dental implants continued to reduce only in the delmopinol group till the 3‐month follow‐up and also there was a significant increase in the implant plaque score after stopping the use of mouthrinse in CHX group.
The 0.2% delmopinol hydrochloride solution was less effective for reduction in plaque scores when compared to the positive control chlorhexidine. However, this difference was not statistically significant. This is in accordance with previous studies where delmopinol was compared with chlorhexidine (Hase, Edwardsson, Rundegren, Attström, & Kelty, 1998; Lang et al., 1998).
Although all three groups responded well to mechanical debridement, there were still sites with BOP after 3 months. The DEL group showed a maximum reduction in BOP sites at 3 months compared to the baseline even though this was not statistically significant compared to CHX and PLA groups.
A transient anaesthetic sensation especially located at the tip of tongue (42%) was the dominant adverse event reported in the DEL group while it was staining of the teeth or tongue in the CHX group (7%). While staining in DEL group was more likely to be registered on inspection by investigator than by subjects and easy removal of staining was possible, staining due to chlorhexidine was mostly reported by patients themselves and was less easily eradicated. This difference in ease of eradication of staining may be related to the proposition that delmopinol destabilizes existing plaque as well as prevents new plaque formation. These results agree with previous studies using the same rinsing solutions (Hase, Edwardsson, et al., 1998; Lang et al., 1998).
Coming to the limitations of the current trial, the ideal primary endpoint would be absence of BOP. This means that larger sample size is required to study the complete resolution of the disease. However, the present study could contribute to studying the possibility of combining mechanical debridement with chemical agents in the treatment of peri‐implant mucositis. Furthermore, many different factors such as implant surface, abutment connection, host genetic and microbial factors may have an influence on the disease resolution.
The PLA group showed also similar improvements in the clinical parameters assessed. This might be due to the combination of mechanical debridement and Hawthorne effect. The participants perhaps have been more alert about self‐performed oral hygiene measures due to participation in a clinical trial which might have masked the added benefit of the mouthrinse. Placebo mouthrinse contained peppermint oil (<0.0002%) and ethanol (1.5%). We consider the influence of these excipients in such low concentration negligible. However, assessment of the exact effect remains difficult to be determined due to a lack of comparable clinical studies.
While previous studies examined the role of delmopinol mouthrinse in controlling dental plaque, gingivitis and in improving periodontal health around natural teeth (Hase, Ainamo, Etemadzadeh, & Aström, 1995; Lang et al., 1998) this present study examined the effects of 0.2% delmopinol mouthrinse on a target implant and full mouth. Novel findings from the current study were that delmopinol mouthrinse use resulted in statistically significant reductions in bleeding on probing and gingivitis on both dental implants and full mouth.
Non‐surgical mechanical biofilm control administered by the patient as well as the oral healthcare professional is considered the standard treatment for the management of peri‐implant mucositis (Heitz‐Mayfield & Salvi 2018).Previous studies have reported the effect of self‐administered adjunctive therapies including essential oil mouthrinse (Ciancio, Lauciello, Shibly, Vitello, & Mather, 1995) chlorhexidine mouthrinse (De Siena et al., 2013; Menezes et al., 2016; Thöne‐Mühling et al., 2010) chlorhexidine gel (Heitz‐Mayfield et al., 2011) and chlorhexidine brush‐on gel (Hallström et al., 2017). The current study shows that the benefits of using mouthrinse with 0.2% DEL or 0.2% CHX as an adjunct to proper self‐care are limited. This is in accordance with a recent systematic review (Salvi & Ramseier, 2015).
This study confirms that the standard treatment for peri‐implant mucositis is professional and patient‐administered non‐surgical mechanical debridement combined with oral hygiene instructions.
Clinical trials with larger sample sizes are needed. It is crucial to follow the long‐term effects and peri‐implant stability after mechanical debridement in peri‐implant mucositis. Another point of consideration might be the duration of mouth rinsing because there is evidence that peri‐implant tissues heal slowly compared to gingival inflammation around natural dentition (Salvi et al., 2012).
5. CONCLUSION
In conclusion, this study showed that mechanical debridement and oral hygiene instructions with and without 0.2% delmopinol mouthrinse were effective in the treatment of peri‐implant mucositis. Further, no differences in clinical effects were found between delmopinol and neither chlorhexidine nor placebo mouthrinses. However, complete resolution of peri‐implant mucositis was not achieved in any of the study groups. Therefore, there is a need for further study on the treatment and ultimately the prevention of peri‐implant diseases.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest regarding the current study.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The contributions of dr. Aartman, I.H.A. for assistance in statistical analysis and Ms. Ringers, F. dental hygienist, are greatly acknowledged and appreciated.
Philip J, Laine ML, Wismeijer D. Adjunctive effect of mouthrinse on treatment of peri‐implant mucositis using mechanical debridement: A randomized clinical trial. J Clin Periodontol. 2020;47:883–891. 10.1111/jcpe.13295
Clinical Trial registration number: NL5159 (NTR5299).
Funding information
This study was self‐funded by the Department of Oral Implantology and Prosthetic Dentistry, Academic Center for Dentistry Amsterdam, The Netherlands. The authors thank Sinclair Pharma (Decapinol Brand acquired by Alliance Pharma PLC) for providing Decapinol mouthrinse for this study.
REFERENCES
- Addy, M. , Moran, J. , & Newcombe, R. G. (2007). Meta‐analyses of studies of 0.2% delmopinol mouth rinse as an adjunct to gingival health and plaque control measures. Journal of Clinical Periodontology, 34(1), 58–65. 10.1111/j.1600-051X.2006.01013.x [DOI] [PubMed] [Google Scholar]
- Baehni, P. C. , & Takeuchi, Y. (2003). Anti‐plaque agents in the prevention of biofilm‐associated oral diseases. Oral Diseases, 9(Suppl 1), 23–29. 10.1034/j.1601-0825.9.s1.5.x [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Armitage, G. , Araujo, M. G. , Avila‐Ortiz, G. , Blanco, J. , Camargo, P. M. , … Zitzmann, N. (2018). Peri‐implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri‐ Implant Diseases and Conditions. Journal of Clinical Periodontology, 45(Suppl 20), S286–S291. 10.1111/jcpe.12957 [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Lindhe, J. , Marinello, C. , Ericsson, I. , & Liljenberg, B. (1992). Soft tissue reaction to de novo plaque formation on implants and teeth. An experimental study in the dog. Clinical Oral Implants Research, 3(1), 1–8. 10.1034/j.1600-0501.1992.030101.x [DOI] [PubMed] [Google Scholar]
- Ciancio, S. G. , Lauciello, F. , Shibly, O. , Vitello, M. , & Mather, M. (1995). The effect of an antiseptic mouth rinse on implant maintenance: Plaque and peri‐implant gingival tissues. Journal of Periodontology, 66(11), 962–965. 10.1902/jop.1995.66.11.962 [DOI] [PubMed] [Google Scholar]
- Collaert, B. , Attström, R. , De Bruyn, H. , & Movert, R. (1992). The effect of delmopinol rinsing on dental plaque formation and gingivitis healing. Journal of Clinical Periodontology, 19(4), 274–280. 10.1111/j.1600-051X.1992.tb00466.x [DOI] [PubMed] [Google Scholar]
- Collaert, B. , Edwardsson, S. , Attström, R. , Hase, J. C. , & Aström, M. (1993). Microbiology of early supragingival plaque development after delmopinol treatment. Oral Microbiology and Immunology, 8(1), 36–41. 10.1111/j.1399-302X.1993.tb00540.x [DOI] [PubMed] [Google Scholar]
- De Siena, F. , Francetti, L. , Corbella, S. , Taschieri, S. , & Del Fabbro, M. (2013). Topical application of 1% chlorhexidine gel versus 0.2% mouthwash in the treatment of peri‐implant mucositis. An observational study. International Journal of Dental Hygiene, 11(1), 41–47. 10.1111/idh.12002 [DOI] [PubMed] [Google Scholar]
- Derks, J. , & Tomasi, C. (2015). Peri‐implant health and disease. A systematic review of current epidemiology. Journal of Clinical Periodontology, 42(Suppl 16), S158–S171. 10.1111/jcpe.12334 [DOI] [PubMed] [Google Scholar]
- Elworthy, A. J. , Edgar, R. , Moran, J. , Addy, M. , Movert, R. , Kelty, E. , & Wade, W. G. (1995). A 6‐month home‐usage trial of 0.1% and 0.2% delmopinol mouthwashes (II). Effects on the plaque microflora. Journal of Clinical Periodontology, 22(7), 527–532. 10.1111/j.1600-051X.1995.tb00800.x [DOI] [PubMed] [Google Scholar]
- Hallström, H. , Lindgren, S. , & Twetman, S. (2017). Effect of a chlorhexidine‐containing brush‐on gel on peri‐implant mucositis. International Journal of Dental Hygiene, 15(2), 149–153. 10.1111/idh.12184 [DOI] [PubMed] [Google Scholar]
- Hase, J. C. , Ainamo, J. , Etemadzadeh, H. , & Aström, M. (1995a). Plaque formation and gingivitis after mouth rinsing with 0.2% delmopinol hydrochloride, 0.2% chlorhexidine digluconate and placebo for 4 weeks, following an initial professional tooth cleaning. Journal of Clinical Periodontology, 22(7), 533–539. 10.1111/j.1600-051X.1995.tb00801.x [DOI] [PubMed] [Google Scholar]
- Hase, J. C. , Attström, R. , Edwardsson, S. , Kelty, E. , & Kisch, J. (1998). 6‐month use of 0.2% delmopinol hydrochloride in comparison with 0.2% chlorhexidine digluconate and placebo. (I). Effect on plaque formation and gingivitis. Journal of Clinical Periodontology, 25(9), 746–753. 10.1111/j.1600-051X.1998.tb02516.x [DOI] [PubMed] [Google Scholar]
- Hase, J. C. , Edwardsson, S. , Rundegren, J. , Attström, R. , & Kelty, E. (1998). 6‐month use of 0.2% delmopinol hydrochloride in comparison with 0.2% chlorhexidine digluconate and placebo (II). Effect on plaque and salivary microflora. Journal of Clinical Periodontology, 25(11), 841–849. 10.1111/j.1600-051X.1998.tb02380.x [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. J. A. , Needleman, I. , Salvi, G. E. , & Pjetursson, B. J. (2014). Consensus statements and clinical recommendations for prevention and management of biologic and technical implant complications. The International Journal of Oral and Maxillofacial Implants, 29, 346–350. 10.11607/jomi.2013.g5 [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. J. A. , & Salvi, G. E. (2018). Peri‐implant mucositis. Journal of Clinical Periodontology, 45(Suppl 20), S237–S245. 10.1111/jcpe.12953 [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. J. A. , Salvi, G. E. , Botticelli, D. , Mombelli, A. , Faddy, M. , & Lang, N. P. (2011). Anti‐infective treatment of peri‐implant mucositis: a randomised controlled clinical trial. Clinical Oral Implants Research, 22(3), 237–241. 10.1111/j.1600-0501.2010.02078.x [DOI] [PubMed] [Google Scholar]
- Klinge, B. , Klinge, A. , Bertl, K. , & Stavropoulos, A. (2018). Peri‐implant diseases. European Journal of Oral Sciences, 126(Suppl 1), 88–94. 10.1111/eos.12529 [DOI] [PubMed] [Google Scholar]
- Klinge, B. , Matsson, L. , Attström, R. , Edwardsson, S. , & Sjödin, T. (1996). Effect of local application of delmopinol hydrochloride on developing and early established supragingival plaque in humans. Journal of Clinical Periodontology, 23(6), 543–547. 10.1111/j.1600-051X.1996.tb01822.x [DOI] [PubMed] [Google Scholar]
- Lang, N. P. , Hase, J. C. , Grassi, M. , Hämmerle, C. H. , Weigel, C. , Kelty, E. , & Frutig, F. (1998). Plaque formation and gingivitis after supervised mouth rinsing with 0.2% delmopinol hydrochloride, 0.2% chlorhexidine digluconate and placebo for 6 months. Oral Diseases, 4(2), 105–113. 10.1111/j.1601-0825.1998.tb00266.x [DOI] [PubMed] [Google Scholar]
- Levin, L. , Barbu, H. , Kurgan, S. , Comӑneanu, R. M. , Referendaru, D. , & Lorean, A. (2019). Evaluation of 0.2% delmopinol mouth rinse for prevention of peri‐implant mucositis and peri‐implantitis: A randomized controlled canine study. Clinical Implant Dentistry and Related Research., 21, 46–51. [DOI] [PubMed] [Google Scholar]
- Löe, H. (1967). The gingival index, the plaque index and the retention index systems. The Journal of Periodontology, 38(6, suppl), 610–616. 10.1902/jop.1967.38.6.610 [DOI] [PubMed] [Google Scholar]
- Menezes, K. M. , Fernandes‐Costa, A. N. , Silva‐Neto, R. D. , Calderon, P. S. , & Gurgel, B. C. V. (2016). Efficacy of 0.12% chlorhexidine gluconate for non‐surgical treatment of peri‐Implant mucositis. Journal of Periodontology, 87(11), 1305–1313. 10.1902/jop.2016.160144 [DOI] [PubMed] [Google Scholar]
- Mombelli, A. , van Oosten, M. A. C. , Schürch, E. Jr , & Lang, N. P. (1987). The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiology and Immunology, 2(4), 145–151. 10.1111/j.1399-302X.1987.tb00298.x [DOI] [PubMed] [Google Scholar]
- Pulcini, A. , Bollaín, J. , Sanz‐Sánchez, I. , Figuero, E. , Alonso, B. , Sanz, M. , & Herrera, D. (2019). Clinical effects of the adjunctive use of a 0.03% chlorhexidine and 0.05% cetylpyridinium chloride mouth rinse in the management of peri‐implant diseases: A randomized clinical trial. Journal of Clinical Periodontology, 46(3), 342–353. 10.1111/jcpe.13088 [DOI] [PubMed] [Google Scholar]
- Ramberg, P. , Lindhe, J. , Botticelli, D. , & Botticelli, A. (2009). The effect of a triclosan dentifrice on mucositis in subjects with dental implants: A six‐month clinical study. The Journal of Clinical Dentistry, 20(3), 103–107. [PubMed] [Google Scholar]
- Ready, D. , Theodoridis, G. , Green, I. , Ciric, L. , Pratten, J. , Tay, W. , & McDonald, A. (2015). In vitro evaluation of the antibiofilm properties of chlorhexidine and delmopinol on dental implant surfaces. International Journal of Antimicrobial Agents, 45(6), 662–666. 10.1016/j.ijantimicag.2015.01.020 [DOI] [PubMed] [Google Scholar]
- Riben‐Grundstrom, C. , Norderyd, O. , André, U. , & Renvert, S. (2015). Treatment of peri‐implant mucositis using a glycine powder air‐polishing or ultrasonic device: A randomized clinical trial. Journal of Clinical Periodontology, 42(5), 462–469. 10.1111/jcpe.12395 [DOI] [PubMed] [Google Scholar]
- Salvi, G. E. , Aglietta, M. , Eick, S. , Sculean, A. , Lang, N. P. , & Ramseier, C. A. (2012). Reversibility of experimental peri‐implant mucositis compared with experimental gingivitis in humans. Clinical Oral Implants Research, 23(2), 182–190. 10.1111/j.1600-0501.2011.02220.x [DOI] [PubMed] [Google Scholar]
- Salvi, G. E. , & Ramseier, C. A. (2015). Efficacy of patient‐administered mechanical and/or chemical plaque control protocols in the management of peri‐implant mucositis. A systematic review. Journal of Clinical Periodontology, 42(Suppl 16), S187–S201. 10.1111/jcpe.12321 [DOI] [PubMed] [Google Scholar]
- Salvi, G. E. , & Zitzmann, N. U. (2014). The effects of anti‐infective preventive measures on the occurrence of biologic implant complications and implant loss: A systematic review. The International Journal of Oral & Maxillofacial Implants, 29(Suppl), 292–307. 10.11607/jomi.2014suppl.g5.1 [DOI] [PubMed] [Google Scholar]
- Schulz, K. F. , Altman, D. G. , & Moher, D. , for the CONSORT Group . (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ, 340(Mar23 1), c332 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsson, T. , Hvid, E. B. , Rundegren, J. , & Edwardsson, S. (1991). Effect of delmopinol on in vitro dental plaque formation, bacterial acid production and the number of microorganisms in human saliva. Oral Microbiology and Immunology, 6(5), 305–309. 10.1111/j.1399-302X.1991.tb00498.x [DOI] [PubMed] [Google Scholar]
- Thöne‐Mühling, M. , Swierkot, K. , Nonnenmacher, C. , Mutters, R. , Flores‐de‐Jacoby, L. , & Mengel, R. (2010). Comparison of two full‐mouth approaches in the treatment of peri‐implant mucositis: A pilot study. Clinical Oral Implants Research, 21(5), 504–512. 10.1111/j.1600-0501.2009.01861.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


