Abstract
Humoral alloimmunity mediated by anti‐human leucocyte antigen (HLA) antibodies is a major challenge in kidney transplantation and impairs the longevity of the transplanted organ. The immunological risk of an individual patient is currently mainly assessed by detection of HLA antibodies in the serum, which are produced by long‐lived bone marrow‐residing plasma cells. However, humoral alloimmunity is complex, and alloreactive memory B cells constitute an additional factor in the interplay of immune cells. These recirculating “silent” cells are responsible for the immunological recall response by differentiating into antibody‐producing cells upon antigen re‐encounter. Historically, due to the lack of appropriate and routinely applicable assays to determine the presence and HLA specificity of alloreactive memory B cells, their contribution to the humoral alloimmune response has clinically often been suspected but could not be determined. In this review, we give an overview of recent advances in techniques to detect alloreactive memory B cells and discuss their strengths and limitations. Furthermore, we summarize experiences with these techniques in alloimmunized individuals and transplant recipients, thereby emphasizing unmet needs to be addressed in future studies.
Keywords: antibody‐mediated rejection, donor‐specific antibodies, HLA antibodies, humoral alloimmune response, kidney transplantation, memory B cells, single‐antigen bead assay
1. INTRODUCTION
The extremely polymorphic human leucocyte antigen (HLA) system represents a major hurdle in solid organ transplantation, since genetic disparities between patient and donor HLA may evoke both cellular and humoral arms of the adaptive immune response. While advances in immunosuppression over the years achieved to prevent T‐cell‐mediated rejection in a substantial proportion of transplant recipients, less well‐controlled humoral alloimmune responses can lead to antibody‐mediated rejection (ABMR) and are a major cause of graft loss (Lefaucheur & Loupy, 2018). In kidney transplantation, presence of donor‐specific HLA antibodies (DSA) has been clearly shown to be associated with both acute and chronic ABMR (Lefaucheur et al., 2010; Mohan et al., 2012; Wehmeier et al., 2017). These antibodies either develop de novo after transplantation or already exist before as a consequence of foreign HLA encounter via previous transplantations, pregnancies or blood transfusions. While exposure to allogeneic HLA leads to generation of plasma cells responsible for spontaneous production of HLA antibodies, recognition of non‐self HLA also gives rise to formation of HLA‐specific memory B cells. Even though the contribution of HLA‐specific memory B cells to the humoral alloimmune response has clinically often been suspected, their detection has historically been hampered by the unavailability of suitable methods. In this regard, recent advances aiming at the assessment of the alloreactive memory B‐cell repertoire may have cleared the way to further study and better understand their role in clinical transplantation. In this review, we provide an overview of the available methods for HLA‐specific memory B‐cell detection and focus on their strengths and limitations for clinical routine application. In addition, we summarize experiences gained by application of these techniques and discuss open questions to be addressed by future studies.
2. THE HUMORAL IMMUNE RESPONSE IN KIDNEY TRANSPLANTATION
Antibody formation following naïve B‐cell activation by protein antigens such as HLA is a T‐cell‐dependent, multi‐step process. It takes place primarily in secondary lymphoid organs, even though local HLA antibody formation in tertiary lymphoid organs within the allograft has also been shown in solid organ transplantation (Thaunat et al., 2005; Wehner et al., 2010). Following complete activation of naïve B cells upon recognition of the alloantigen via the B‐cell receptor (BCR) and through subsequent interactions with cognate CD4 + T helper cells, some of the activated naïve B cells can immediately give rise to extrafollicular short‐lived plasma cells, mainly producing low‐affinity IgM antibodies. While a fraction of activated B cells develops into germinal centre‐independent memory B cells, other enter germinal centres and undergo somatic hypermutation, class switching and affinity maturation upon interaction with follicular dendritic cells and follicular helper T cells. It is yet incompletely understood what determines the B cell fate following naïve B‐cell activation. This process appears to be multifactorial, as illustrated by a recent study using a murine model of ABMR. This study showed that a relative higher number of antigen‐specific helper T cells compared to B cells is evoking a strong extrafollicular response while a relative predominance of B cells promotes germinal centre reactions (Alsughayyir et al., 2018). Germinal centre‐experienced B cells can leave germinal centres as isotype‐switched memory B cells or plasma cells producing high‐affinity HLA antibodies. Plasma cells then move to the bone marrow or mucosal tissues and continue to spontaneously produce isotype‐switched HLA antibodies as long‐lived plasma cells. These antibodies appear in the serum of an individual and can be diagnostically assessed by several HLA antibody detection methods, among which solid phase and, in particular, single‐antigen bead (SAB) assays have emerged as the most sensitive tool (Wehmeier, Hoenger, & Schaub, 2020). Determination of serum HLA antibodies is currently the mainstay of pre‐transplant immunological risk assessment (Tait et al., 2013; Tambur et al., 2018).
Unlike plasma cells, quiescent memory B cells that have left germinal centres continuously circulate between the secondary lymphoid organs and peripheral blood to patrol for their cognate antigen. Upon re‐encountering the same HLA, memory B cells respond in a rapid and enhanced way and differentiate into antibody‐producing cells, thereby contributing to the serum HLA antibody repertoire. Importantly, also bystander activation as provided by the cytokine environment of infections or trauma may result in memory B‐cell activation in a non‐specific manner (Amanna & Slifka, 2010; Bernasconi, Traggiai, & Lanzavecchia, 2002; D'Orsogna, van den Heuvel, van Kooten, Heidt, & Claas, 2017). Knowledge on the fate of B cells after germinal centre reactions gained from studies in the field of vaccination and infection suggests that memory B cells are precursors of long‐lived terminally differentiated plasma cells and replenish their pool (Amanna, Carlson, & Slifka, 2007; Crotty et al., 2003; Dogan et al., 2009). This concept has also been challenged by a recent study in rhesus macaques showing that plasma cells alone might be able to maintain antibody responses to pathogens without requiring continuous replenishment by memory B cells (Hammarlund et al., 2017). Interestingly, in the setting of tetanus toxoid vaccination, the antigen‐specific memory B‐cell repertoire has been shown to be more diverse than the serum antibody pool (Lavinder, Horton, Georgiou, & Ippolito, 2015).
While these studies pinpoint the enormous complexity of the immune system, they also indicate that serum HLA antibody analysis ignoring the contribution of HLA–specific memory B cells likely provides only an incomplete picture of the humoral immune response.
3. HLA‐SPECIFIC MEMORY B‐CELL ASSAYS: WHICH ONE TO CHOOSE?
Inspired from methods to tackle pathogen or vaccine‐specific memory B cells and with the aim to be used in a clinical setting, several HLA‐specific memory B‐cell assays have been developed (Karahan, Claas, & Heidt, 2015). These assays can be grouped according to the three main approaches used (Figure 1).
Figure 1.
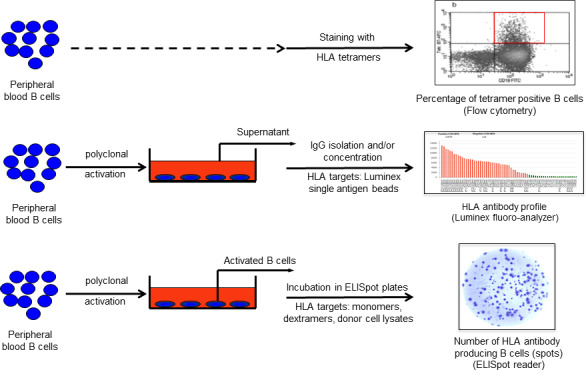
HLA‐specific memory B‐cell assays. HLA‐specific memory B cells can be detected by (i) tetramer analysis taking advantage of B‐cell receptors to be able to bind synthetic HLA molecules (upper row), (ii) culture supernatant analysis based on the ability of B cells to produce antibodies upon in vitro polyclonal activation (middle row) and (iii) ELISpot assays using either synthetic or donor‐derived HLA molecules performed by combining both of the aforementioned properties of B cells (lower row)
The first approach to detect HLA‐specific memory B cells is based on the ability of the BCR to bind to synthetic HLA molecules and can be achieved by flow cytometric analysis upon staining B cells with HLA tetramers (Mulder et al., 2003; Zachary, Kopchaliiska, Montgomery, & Leffell, 2007) (Figure 1, upper row). This technique, however, requires a second step of sorting and culturing to confirm the antibody production capacity of the tetramer‐positive cells. Since flow cytometric cell sorting inherently leads to a significant loss of cells, this route will not provide a complete picture of the memory B‐cell compartment.
In order to induce antibody production by memory B cells in vitro, polyclonal activation is required (Bernasconi et al., 2002). So far, several polyclonal activation cocktails have been successfully used. These cocktails are based on ligation of either the BCR, Toll‐like receptors and/or costimulatory molecules, and are often supplemented with a combination of B‐cell cytokines and growth factors (Heidt et al., 2008; Karahan, Eikmans, Anholts, Claas, & Heidt, 2014). Among the described cocktails, stimulation with Toll‐like receptor 7/8 agonist (Resiquimod‐R848) and interleukin 2 has been shown to preferentially stimulate memory B cells among peripheral blood mononuclear cells without causing isotype switching in the naïve B‐cell population (Karahan et al., 2017; Pinna, Corti, Jarrossay, Sallusto, & Lanzavecchia, 2009) and is, therefore, increasingly applied. The second approach of HLA‐specific memory B cells analysis is based on profiling of memory B‐cell‐derived HLA antibodies in culture supernatant produced upon such polyclonal stimulation by using luminex SAB assays (Bernasconi et al., 2002; Han, Rogers, Lavingia, & Stastny, 2009; Karahan et al., 2019) (Figure 1, middle row).
Lastly, polyclonally activated B cells can be used to perform enzyme‐linked immunospot (ELISpot) assays (Figure 1, lower row). Importantly, since ELISpot assays rely on the capacity of activated B cells to produce antibodies, viability of the stimulated cells at the end of the pre‐culture phase plays a major role for the quality and accuracy of the results. Hence, in contrast to supernatant analysis favouring longer culture periods up to 10–12 days to achieve accumulation of IgG in the culture supernatants (Karahan et al., 2019), cells to be used for ELISpot assays are generally cultured for 6–7 days in order to obtain an optimum of antibody‐producing viable cells (Heidt et al., 2012; Karahan et al., 2014, 2017). Following culturing, in most protocols activated B cells are transferred to anti‐IgG‐coated ELISpot plates and incubated overnight. In a second phase (visualization), antibody fingerprints can be visualized as a single spot representative of one HLA antibody‐producing cell using either synthetic HLA molecules (monomeric or multimeric), or donor lysate‐derived HLA molecules serving as detection matrix (Heidt et al., 2012; Karahan et al., 2017; Karahan, de Vaal, et al., 2015; Lucia et al., 2015). In order to enable detection of two different HLA specificities in the same plate well, a fluorescence‐based technique using HLA dextramers labelled by distinct fluorochromes was recently described (Luque et al., 2019). It is critical to include adequate controls in these assays. While successful polyclonal activation inducing IgG production is confirmed by performing total IgG ELISpot, HLA‐specific hybridoma cells secreting HLA antibodies of a defined specificity can assure that the HLA target used as detection matrix, regardless of its source, gives rise to a positive signal (Duquesnoy et al., 2014; Mulder et al., 2010). Since no HLA antibody production is expected to occur against the recipient's own HLA, autologous controls using self HLA for detection serve as negative controls. For reporting of the frequency of HLA‐specific memory B cells, usually expressed as a ratio of HLA‐specific memory B cells per number of IgG‐producing cells, both controls need to be taken into account (Karahan et al., 2017; Lucia et al., 2015).
All these techniques have strengths and limitations, and the specific research question will dictate which technique is most suitable (Table 1). Only ELISpot‐based assays can quantify the number of memory B cells capable of producing HLA antibodies. In contrast, tetramer analysis provides information only on the percentage of tetramer‐positive B cells and cannot be considered a fully quantitative assay when interested in B cells actually capable of HLA antibody production. While all assays utilizing synthetic HLA such as monomers, dextramers or tetramers are restricted by the availability of these molecules, this limitation can be overcome by using donor‐specific ELISpot assays, in which lysates prepared from donor cells serve as HLA targets (Karahan et al., 2017). Importantly, both tetramer staining and ELISpot assays are very labour‐intensive methods yielding unfamiliar read‐outs for the transplantation community, which render them virtually impossible to use when comparison with serum HLA antibody profiles is desired. In this regard, analysis of polyclonally activated B‐cell culture supernatants for the presence of HLA antibodies using luminex SAB assay allows for such a comparison at similar sensitivity to serum analysis, particularly when IgG is not only concentrated but also affinity‐purified from culture supernatants (Karahan et al., 2019). In addition to being a qualitative assay, supernatant analysis by luminex is also restricted by the HLA specificities included in SAB panels.
Table 1.
Overview of methods to detect alloreactive memory B cells
| Memory B‐cell assay | Assay type | Protocol based on | Read‐out | Labour intensity | Pros | Cons | References |
|---|---|---|---|---|---|---|---|
| Tetramer staining | Quantitative | Binding of BCR to synthetic HLA molecules | Percentage of tetramer‐positive B cells | +++ |
No B‐cell culture phase before tetramer staining May allow for first line screening of tetramer‐positive memory B cells |
Not all tetramer‐positive cells secrete antibodies Second step of sorting and culturing is necessary |
Mulder et al., 2003, Zachary, Kopchaliiska, Montgomery, Melancon, et al., 2007 |
| Culture supernatant analysis | Qualitative | In vitro polyclonal activation | Luminex single‐antigen bead MFI | + |
Direct comparison to serum HLA antibody profiles with similar sensitivity Familiar read‐out Easy to implement in HLA‐ labs and in clinical practice |
Does not allow for quantification of HLA‐specific memory B cells |
Han et al., 2009, Karahan et al., 2019 |
| ELISpot assay | Quantitative | In vitro polyclonal activation and IgG binding to synthetic or lysate HLA |
Number of memory B cells/IgG‐producing B cells Number of memory B cells/total B cells |
+++++ |
Only assay that can quantify the number of antibody‐secreting HLA‐specific memory B cells |
Expertise and specialized equipment required Difficult to standardize for clinical use |
Heidt et al., 2012, Karahan et al., 2017, Lucia et al., 2015 |
Abbreviations: BCR, B‐cell receptor; MFI, mean fluorescence intensity.
Inherent to all antigen‐specific B‐cell detection methods, as long as the peripheral blood is screened, there is always a possibility that HLA‐specific memory B cells may not be circulating at the time of sampling but are instead residing in secondary lymphoid organs, precluding their detection (Kamburova et al., 2013). This also emphasizes that negative results of current assays should always be interpreted with caution since absence from detection assays may not be equal to non‐existence. Investigation of tissue from secondary lymphoid organs such as lymph nodes or spleen might, in this regard, provide novel insights, even though it is more difficult to obtain, may require an invasive procedure and is therefore of limited usefulness in practice.
From our perspective, the ideal memory B‐cell assay for clinical routine should utilize easily accessible biological material such as peripheral blood and be applicable in all laboratories without a further need for additional specialized equipment and standardization. Since most HLA laboratories have become familiar with interpretation of luminex SAB results, culture supernatant of activated B cells analysis moves into focus.
4. CLINICAL VALUE OF DETECTING ALLOREACTIVE MEMORY B CELLS
Several of the above‐mentioned techniques have been applied in small clinical studies, providing insights into the potential value of detecting and tracking HLA‐specific memory B cells.
4.1. For pre‐transplant risk stratification
Ideally, assessment of the peripheral HLA‐specific memory B‐cell pool should enhance the accuracy of pre‐transplant risk assessment. By using HLA tetramers, Zachary et al were the first to describe a correlation between detection of HLA‐specific B cells before and appearance of DSA shortly after transplantation (Zachary, Kopchaliiska, Montgomery, Melancon, & Leffell, 2007). The same group later showed that the CD20 antibody rituximab, known to deplete memory B cells but not plasma cells, was able to abrogate an antibody recall among allograft recipients who were pre‐transplant HLA tetramer‐positive but serum antibody‐negative (Zachary, Lucas, Montgomery, & Leffell, 2013). This suggested that assessment of HLA‐specific memory B cells could aid in uncovering previous alloimmunization when serum antibodies are absent. Indeed, serum HLA antibody titres becoming too low for detection may result from exhaustion of the, in comparison with memory B cells, lower number of alloantigen‐specific long‐lived plasma cells (Lavinder et al., 2015), while memory B cells remain detectable. Interestingly, several recent studies revealed that detection of pre‐transplant HLA‐specific memory B cells in the absence of serum antibodies is not as frequent as expected. By screening allograft recipients for memory B‐cell‐derived DSA in culture supernatants following polyclonal activation, Han et al found 8% of antibodies (4 out of 50) to be present in supernatants only. Seventy percent of specificities were detectable in both serum and supernatants (Han et al., 2009). In another study using a similar technical approach, Snanoudj et al detected memory B‐cell‐derived antibodies in 18 patients awaiting a kidney transplant and 86% of the specificities found were also present in the serum collected on the same day (Snanoudj et al., 2015). These results are in accordance with our own experience studying HLA antibody profiles in culture supernatants. While complete overlap of HLA antibody profiles in serum and culture supernatants as well as a broader repertoire in serum were the most frequently observed patterns in a small cohort consisting of 11 kidney re‐transplant candidates and 2 multiparous women, only 10% of specificities were solely detected in culture supernatants (Karahan et al., 2019). Interestingly, Lucia et al, using an ELISpot assay, investigated a small cohort of kidney transplant recipients and detected donor‐specific memory B cells at the time of ABMR but also before transplantation, with some patients not having detectable serum DSA pre‐transplant (Lucia et al., 2015). These data suggest that, despite being overall rare, memory B‐cell detection in the absence of serum antibodies might be helpful on an individual patient level. A logical next step would be to apply current assays in patient populations where memory B‐cell existence seems likely despite absence of serum antibodies. In this regard, seemingly “non‐immunized” re‐transplant candidates or husband‐to‐wife or child‐to‐mother transplantations, historically always thought to confer a higher immunological risk due to memory B‐cell existence, are suitable patient groups to screen. While detailed information on the sensitizing HLA is required, it might be also of interest to investigate the timely correlation with alloimmunization, since especially high‐affinity and class‐switched IgG memory B cells may also be lost over the years (Chong & Ansari, 2018; Pape, Taylor, Maul, Gearhart, & Jenkins, 2011).
Given that detection of alloreactive memory B cells in the absence of serum antibodies is not the most frequently observed pattern, it might be useful to focus instead on concurrent detection of serum antibodies and HLA‐specific memory B cells of the same specificity. Pre‐transplant assessment of the memory B‐cell repertoire may thereby add in determining the pathogenicity and relevance of HLA antibody specificities found in serum. This relies on the concept that activated IgG + memory B cells differentiating into plasma cells following antigen re‐exposure may continuously replenish the serum antibody pool post‐transplant (Chong & Ansari, 2018; Dogan et al., 2009; Pape et al., 2011), which may lead to a more durable and eventually more harmful antibody response. Several studies already showed that post‐transplant risk of antibody‐mediated rejection was associated with persistence or rebound of HLA antibodies (Burns et al., 2008; Kimball, Baker, Wagner, & King, 2011; Redondo‐Pachon et al., 2018). Our group found in a recent pilot study focusing on 20 pre‐transplant DSA‐positive patients that DSA persisted more frequently post‐transplant when concurrent donor‐specific memory was detectable before transplantation. Concurrent detection of pre‐transplant DSA and donor‐specific memory was associated with a higher 1‐year incidence of ABMR as well as more severe microvascular inflammation in allograft biopsies. Furthermore, 7 out of 9 patients with persisting DSA developed ABMR in the first year post‐transplant (Wehmeier et al., 2019). These findings will need to be validated in a larger cohort and by further investigating DSA kinetics with or without pre‐transplant concurrent detectable memory B cells over the post‐transplant course as well as at time of rejection. Importantly, such studies should also consider the emerging knowledge on HLA epitopes (Duquesnoy, 2018; Kramer, Roelen, Heidt, & Claas, 2017), especially since one study already indicated that HLA antibodies produced by memory B cells may show a restricted reactivity pattern on the epitope level (Snanoudj et al., 2015).
Another important issue to address will be the question, whether donor‐specific memory B‐cell detection is of additional value as compared to serum DSA characteristics such as mean fluorescence intensity (MFI). So far, it has been described by several groups that MFI of serum antibodies positively correlates with detection of alloreactive memory B cells (Lucia et al., 2015; Luque et al., 2019; Snanoudj et al., 2015; Wehmeier et al., 2019). While reasons remain speculative, one might argue that a “strong” sensitizing event will lead to robust alloimmunization on both plasma cell and memory B‐cell level, potentially being reflected by a high MFI serum antibody. In those cases, memory B‐cell detection may not provide much information beyond serum antibody MFI. However, since also serum antibodies with lower MFI might have concurrently detectable B‐cell memory, this additional test could aid in recognizing the harmful specificities especially among those that cannot be attributed to a sensitizing event.
4.2. For post‐transplant monitoring
Potentially, memory B‐cell detection can be helpful for post‐transplant management of patients. One recent study investigated the value of post‐transplant tracking donor‐specific memory B‐cell frequencies by ELISpot. In addition to patients with acute ABMR, donor‐specific memory B cells were also detected in chronic ABMR and also in the absence of DSA at this time. Furthermore, in an additional cohort of non‐sensitized patients undergoing surveillance biopsies, donor‐specific memory B‐cell detection allowed for prediction of subclinical ABMR in some cases (Luque et al., 2019). Another group used time‐of‐flight mass cytometry and found, at the time of de novo DSA detection, enriched percentages of B cells with antibody‐secreting and memory phenotypes only in paediatric kidney graft recipients who developed ABMR later on (Fischman et al., 2019). Even though the latter study did not address HLA specificity, these results appear promising and warrant further studies on the kinetics of de novo memory B‐cell formation. If development of de novo HLA‐specific memory B‐cell responses would precede de novo DSA detection, insufficient immunosuppression could be identified at an earlier time point and consequently increased. This could, in light of currently missing effective options to treat chronic ABMR (Bohmig, Eskandary, Doberer, & Halloran, 2019; Eskandary et al., 2018; Moreso et al., 2018), display a major step forward. Furthermore, a clear impact of HLA‐specific memory B‐cell detection on occurrence of chronic ABMR would also stimulate attempts to more specifically target the memory B‐cell compartment by immunosuppressive agents.
5. CONCLUSIONS
Alloreactive memory B cells can nowadays be detected by using several techniques. As an intrinsic limitation, all of them consider only HLA‐specific memory B cells present in the peripheral blood. Regardless of the technique used, small clinical studies have so far supported the historical assumption that alloreactive memory B‐cell detection is of clinical value. While refinement of the pre‐transplant risk stratification for prevention of allograft rejection processes should clearly be the main goal, memory B‐cell detection may also aid in post‐transplant management of kidney allograft recipients. In our opinion, current techniques should now be applied prospectively to larger and well‐defined cohorts to expand our experiences and to ultimately provide successful translation into routine clinical practice.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Wehmeier C, Karahan GE, Heidt S. HLA‐specific memory B‐cell detection in kidney transplantation: Insights and future challenges. Int J Immunogenet. 2020;47:227–234. 10.1111/iji.12493
REFERENCES
- Alsughayyir, J. , Chhabra, M. , Qureshi, M. S. , Mallik, M. , Ali, J. M. , Gamper, I. , … Pettigrew, G. J. (2018). Relative frequencies of alloantigen‐specific helper CD4 T cells and B cells determine mode of antibody‐mediated allograft rejection. Frontiers in Immunology, 9, 3039 10.3389/fimmu.2018.03039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanna, I. J. , Carlson, N. E. , & Slifka, M. K. (2007). Duration of humoral immunity to common viral and vaccine antigens. New England Journal of Medicine, 357(19), 1903–1915. 10.1056/NEJMoa066092 [DOI] [PubMed] [Google Scholar]
- Amanna, I. J. , & Slifka, M. K. (2010). Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunological Reviews, 236, 125–138. 10.1111/j.1600-065X.2010.00912.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi, N. L. , Traggiai, E. , & Lanzavecchia, A. (2002). Maintenance of serological memory by polyclonal activation of human memory B cells. Science, 298(5601), 2199–2202. 10.1126/science.1076071 [DOI] [PubMed] [Google Scholar]
- Bohmig, G. A. , Eskandary, F. , Doberer, K. , & Halloran, P. F. (2019). The therapeutic challenge of late antibody‐mediated kidney allograft rejection. Transplant International, 32(8), 775–788. 10.1111/tri.13436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns, J. M. , Cornell, L. D. , Perry, D. K. , Pollinger, H. S. , Gloor, J. M. , Kremers, W. K. , … Stegall, M. D. (2008). Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. American Journal of Transplantation, 8(12), 2684–2694. 10.1111/j.1600-6143.2008.02441.x [DOI] [PubMed] [Google Scholar]
- Chong, A. S. , & Ansari, M. J. (2018). Heterogeneity of memory B cells. American Journal of Transplantation, 18(4), 779–784. 10.1111/ajt.14669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty, S. , Felgner, P. , Davies, H. , Glidewell, J. , Villarreal, L. , & Ahmed, R. (2003). Cutting edge: Long‐term B cell memory in humans after smallpox vaccination. Journal of Immunology, 171(10), 4969–4973. 10.4049/jimmunol.171.10.4969 [DOI] [PubMed] [Google Scholar]
- Dogan, I. , Bertocci, B. , Vilmont, V. , Delbos, F. , Megret, J. , Storck, S. , … Weill, J. C. (2009). Multiple layers of B cell memory with different effector functions. Nature Immunology, 10(12), 1292–1299. 10.1038/ni.1814 [DOI] [PubMed] [Google Scholar]
- D'Orsogna, L. , van den Heuvel, H. , van Kooten, C. , Heidt, S. , & Claas, F. H. J. (2017). Infectious pathogens may trigger specific allo‐HLA reactivity via multiple mechanisms. Immunogenetics, 69(8–9), 631–641. 10.1007/s00251-017-0989-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquesnoy, R. J. (2018). Epitope‐based human leukocyte antigen matching for transplantation: A personal perspective of its future. Current Opinion in Organ Transplantation, 23(4), 486–492. 10.1097/MOT.0000000000000539 [DOI] [PubMed] [Google Scholar]
- Duquesnoy, R. J. , Marrari, M. , Tambur, A. R. , Mulder, A. , Sousa, L. C. , da Silva, A. S. , & do Monte, S. J. H. (2014). First report on the antibody verification of HLA‐DR, HLA‐DQ and HLA‐DP epitopes recorded in the HLA epitope registry. Human Immunology, 75(11), 1097–1103. 10.1016/j.humimm.2014.09.012 [DOI] [PubMed] [Google Scholar]
- Eskandary, F. , Regele, H. , Baumann, L. , Bond, G. , Kozakowski, N. , Wahrmann, M. , … Bohmig, G. A. (2018). A randomized trial of bortezomib in late antibody‐mediated kidney transplant rejection. Journal of the American Society of Nephrology, 29(2), 591–605. 10.1681/ASN.2017070818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman, C. , Fribourg, M. , Fabrizio, G. , Cioni, M. , Comoli, P. , Nocera, A. , … Cravedi, P. (2019). Circulating B cells with memory and antibody‐secreting phenotypes are detectable in pediatric kidney transplant recipients before the development of antibody‐mediated rejection. Transplant Direct, 5(9), e481 10.1097/TXD.0000000000000914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund, E. , Thomas, A. , Amanna, I. J. , Holden, L. A. , Slayden, O. D. , Park, B. , … Slifka, M. K. (2017). Plasma cell survival in the absence of B cell memory. Nature Communications, 8(1), 1781 10.1038/s41467-017-01901-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, M. , Rogers, J. A. , Lavingia, B. , & Stastny, P. (2009). Peripheral blood B cells producing donor‐specific HLA antibodies in vitro. Human Immunology, 70(1), 29–34. 10.1016/j.humimm.2008.10.013 [DOI] [PubMed] [Google Scholar]
- Heidt, S. , Roelen, D. L. , de Vaal, Y. J. , Kester, M. G. , Eijsink, C. , Thomas, S. , … Mulder, A. (2012). A NOVel ELISPOT assay to quantify HLA‐specific B cells in HLA‐immunized individuals. American Journal of Transplantation, 12(6), 1469–1478. 10.1111/j.1600-6143.2011.03982.x [DOI] [PubMed] [Google Scholar]
- Heidt, S. , Roelen, D. L. , Eijsink, C. , van Kooten, C. , Claas, F. H. , & Mulder, A. (2008). Effects of immunosuppressive drugs on purified human B cells: Evidence supporting the use of MMF and rapamycin. Transplantation, 86(9), 1292–1300. 10.1097/TP.0b013e3181874a36 [DOI] [PubMed] [Google Scholar]
- Kamburova, E. G. , Koenen, H. J. , Borgman, K. J. , ten Berge, I. J. , Joosten, I. , & Hilbrands, L. B. (2013). A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function. American Journal of Transplantation, 13(6), 1503–1511. 10.1111/ajt.12220 [DOI] [PubMed] [Google Scholar]
- Karahan, G. E. , Claas, F. H. , & Heidt, S. (2015). Detecting the humoral alloimmune response: We need more than serum antibody screening. Transplantation, 99(5), 908–915. 10.1097/TP.0000000000000724 [DOI] [PubMed] [Google Scholar]
- Karahan, G. E. , de Vaal, Y. J. H. , Krop, J. , Wehmeier, C. , Roelen, D. L. , Claas, F. H. J. , & Heidt, S. (2017). A memory B cell crossmatch assay for quantification of donor‐specific memory B cells in the peripheral blood of HLA‐immunized individuals. American Journal of Transplantation, 17(10), 2617–2626. 10.1111/ajt.14293 [DOI] [PubMed] [Google Scholar]
- Karahan, G. E. , de Vaal, Y. J. , Roelen, D. L. , Buchli, R. , Claas, F. H. , & Heidt, S. (2015). Quantification of HLA class II‐specific memory B cells in HLA‐sensitized individuals. Human Immunology, 76(2–3), 129–136. 10.1016/j.humimm.2015.01.014 [DOI] [PubMed] [Google Scholar]
- Karahan, G. E. , Eikmans, M. , Anholts, J. D. , Claas, F. H. , & Heidt, S. (2014). Polyclonal B cell activation for accurate analysis of pre‐existing antigen‐specific memory B cells. Clinical and Experimental Immunology, 177(1), 333–340. 10.1111/cei.12305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahan, G. E. , Krop, J. , Wehmeier, C. , de Vaal, Y. J. H. , Langerak‐Langerak, J. , Roelen, D. L. , … Heidt, S. (2019). An Easy and sensitive method to profile the antibody specificities of HLA‐specific memory B cells. Transplantation, 103(4), 716–723. 10.1097/TP.0000000000002516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball, P. M. , Baker, M. A. , Wagner, M. B. , & King, A. (2011). Surveillance of alloantibodies after transplantation identifies the risk of chronic rejection. Kidney International, 79(10), 1131–1137. 10.1038/ki.2010.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, C. S. M. , Roelen, D. L. , Heidt, S. , & Claas, F. H. J. (2017). Defining the immunogenicity and antigenicity of HLA epitopes is crucial for optimal epitope matching in clinical renal transplantation. Hla, 90(1), 5–16. 10.1111/tan.13038 [DOI] [PubMed] [Google Scholar]
- Lavinder, J. J. , Horton, A. P. , Georgiou, G. , & Ippolito, G. C. (2015). Next‐generation sequencing and protein mass spectrometry for the comprehensive analysis of human cellular and serum antibody repertoires. Current Opinion in Chemical Biology, 24, 112–120. 10.1016/j.cbpa.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Lefaucheur, C. , & Loupy, A. (2018). Antibody‐mediated rejection of solid‐organ allografts. New England Journal of Medicine, 379(26), 2580–2582. 10.1056/NEJMc1813976 [DOI] [PubMed] [Google Scholar]
- Lefaucheur, C. , Loupy, A. , Hill, G. S. , Andrade, J. , Nochy, D. , Antoine, C. , … Suberbielle‐Boissel, C. (2010). Preexisting donor‐specific HLA antibodies predict outcome in kidney transplantation. Journal of the American Society of Nephrology, 21(8), 1398–1406. 10.1681/ASN.2009101065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucia, M. , Luque, S. , Crespo, E. , Melilli, E. , Cruzado, J. M. , Martorell, J. , … Bestard, O. (2015). Preformed circulating HLA‐specific memory B cells predict high risk of humoral rejection in kidney transplantation. Kidney International, 88(4), 874–887. 10.1038/ki.2015.205 [DOI] [PubMed] [Google Scholar]
- Luque, S. , Lucia, M. , Melilli, E. , Lefaucheur, C. , Crespo, M. , Loupy, A. , … Bestard, O. (2019). Value of monitoring circulating donor‐reactive memory B cells to characterize antibody‐mediated rejection after kidney transplantation. American Journal of Transplantation, 19(2), 368–380. 10.1111/ajt.15055 [DOI] [PubMed] [Google Scholar]
- Mohan, S. , Palanisamy, A. , Tsapepas, D. , Tanriover, B. , Crew, R. J. , Dube, G. , … Radhakrishnan, J. (2012). Donor‐specific antibodies adversely affect kidney allograft outcomes. Journal of the American Society of Nephrology, 23(12), 2061–2071. 10.1681/ASN.2012070664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreso, F. , Crespo, M. , Ruiz, J. C. , Torres, A. , Gutierrez‐Dalmau, A. , Osuna, A. , … Seron, D. (2018). Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: A multicenter, prospective, randomized, double‐blind clinical trial. American Journal of Transplantation, 18(4), 927–935. 10.1111/ajt.14520 [DOI] [PubMed] [Google Scholar]
- Mulder, A. , Eijsink, C. , Kardol, M. J. , Franke‐van Dijk, M. E. , van der Burg, S. H. , Kester, M. , … Claas, F. H. (2003). Identification, isolation, and culture of HLA‐A2‐specific B lymphocytes using MHC class I tetramers. Journal of Immunology, 171(12), 6599–6603. 10.4049/jimmunol.171.12.6599 [DOI] [PubMed] [Google Scholar]
- Mulder, A. , Kardol, M. J. , Arn, J. S. , Eijsink, C. , Franke, M. E. , Schreuder, G. M. , … Claas, F. H. (2010). Human monoclonal HLA antibodies reveal interspecies crossreactive swine MHC class I epitopes relevant for xenotransplantation. Molecular Immunology, 47(4), 809–815. 10.1016/j.molimm.2009.10.004 [DOI] [PubMed] [Google Scholar]
- Pape, K. A. , Taylor, J. J. , Maul, R. W. , Gearhart, P. J. , & Jenkins, M. K. (2011). Different B cell populations mediate early and late memory during an endogenous immune response. Science, 331(6021), 1203–1207. 10.1126/science.1201730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna, D. , Corti, D. , Jarrossay, D. , Sallusto, F. , & Lanzavecchia, A. (2009). Clonal dissection of the human memory B‐cell repertoire following infection and vaccination. European Journal of Immunology, 39(5), 1260–1270. 10.1002/eji.200839129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo‐Pachon, D. , Perez‐Saez, M. J. , Mir, M. , Gimeno, J. , Llinas, L. , Garcia, C. , … Crespo, M. (2018). Impact of persistent and cleared preformed HLA DSA on kidney transplant outcomes. Human Immunology, 79(6), 424–431. 10.1016/j.humimm.2018.02.014 [DOI] [PubMed] [Google Scholar]
- Snanoudj, R. , Claas, F. H. , Heidt, S. , Legendre, C. , Chatenoud, L. , & Candon, S. (2015). Restricted specificity of peripheral alloreactive memory B cells in HLA‐sensitized patients awaiting a kidney transplant. Kidney International, 87(6), 1230–1240. 10.1038/ki.2014.390 [DOI] [PubMed] [Google Scholar]
- Tait, B. D. , Susal, C. , Gebel, H. M. , Nickerson, P. W. , Zachary, A. A. , Claas, F. H. , … Opelz, G. (2013). Consensus guidelines on the testing and clinical management issues associated with HLA and non‐HLA antibodies in transplantation. Transplantation, 95(1), 19–47. 10.1097/TP.0b013e31827a19cc [DOI] [PubMed] [Google Scholar]
- Tambur, A. R. , Campbell, P. , Claas, F. H. , Feng, S. , Gebel, H. M. , Jackson, A. M. , … Nickerson, P. (2018). Sensitization in Transplantation: Assessment of Risk (STAR) 2017 Working Group Meeting Report. American Journal of Transplantation, 18(7), 1604–1614. 10.1111/ajt.14752 [DOI] [PubMed] [Google Scholar]
- Thaunat, O. , Field, A. C. , Dai, J. , Louedec, L. , Patey, N. , Bloch, M. F. , … Nicoletti, A. (2005). Lymphoid neogenesis in chronic rejection: Evidence for a local humoral alloimmune response. Proceedings of the National Academy of Sciences of the United States of America, 102(41), 14723–14728. 10.1073/pnas.0507223102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeier, C. , Hoenger, G. , Cun, H. , Amico, P. , Hirt‐Minkowski, P. , Georgalis, A. , … Schaub, S. (2017). Donor specificity but not broadness of sensitization is associated with antibody‐mediated rejection and graft loss in renal allograft recipients. American Journal of Transplantation, 17(8), 2092–2102. 10.1111/ajt.14247 [DOI] [PubMed] [Google Scholar]
- Wehmeier, C. , Hoenger, G. , & Schaub, S. (2020). Caveats of HLA antibody detection by solid‐phase assays. Transplant International, 33(1), 18–29. 10.1111/tri.13484 [DOI] [PubMed] [Google Scholar]
- Wehmeier, C. , Karahan, G. E. , Krop, J. , de Vaal, Y. , Langerak‐Langerak, J. , Binet, I. , … the Swiss Transplant Cohort Study . (2019). Donor‐specific B cell memory in alloimmunized kidney transplant recipients ‐ first clinical application of a novel method. Transplantation, 104(5), 1026–1032. 10.1097/TP.0000000000002909 [DOI] [PubMed] [Google Scholar]
- Wehner, J. R. , Fox‐Talbot, K. , Halushka, M. K. , Ellis, C. , Zachary, A. A. , & Baldwin, W. M. 3rd (2010). B cells and plasma cells in coronaries of chronically rejected cardiac transplants. Transplantation, 89(9), 1141–1148. 10.1097/TP.0b013e3181d3f271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary, A. A. , Kopchaliiska, D. , Montgomery, R. A. , & Leffell, M. S. (2007). HLA‐specific B cells: I. A method for their detection, quantification, and isolation using HLA tetramers. Transplantation, 83(7), 982–988. 10.1097/01.tp.0000259017.32857.99 [DOI] [PubMed] [Google Scholar]
- Zachary, A. A. , Kopchaliiska, D. , Montgomery, R. A. , Melancon, J. K. , & Leffell, M. S. (2007). HLA‐specific B cells: II. Application to transplantation. Transplantation, 83(7), 989–994. 10.1097/01.tp.0000259019.68244.d7 [DOI] [PubMed] [Google Scholar]
- Zachary, A. A. , Lucas, D. P. , Montgomery, R. A. , & Leffell, M. S. (2013). Rituximab prevents an anamnestic response in patients with cryptic sensitization to HLA. Transplantation, 95(5), 701–704. 10.1097/TP.0b013e31827be3c1 [DOI] [PubMed] [Google Scholar]


