Abstract
Background
Hypoglycaemia is the most frequent complication of treatment with insulin or insulin secretagogues in people with diabetes. Severe hypoglycaemia, i.e. an event requiring external help because of cognitive dysfunction, is associated with a higher risk of adverse cardiovascular outcomes and all‐cause mortality, but underlying mechanism(s) are poorly understood. There is also a gap in the understanding of the clinical, psychological and health economic impact of ‘non‐severe’ hypoglycaemia and the glucose level below which hypoglycaemia causes harm.
Aim
To increase understanding of hypoglycaemia by addressing the above issues over a 4‐year period.
Methods
Hypo‐RESOLVE is structured across eight work packages, each with a distinct focus. We will construct a large, sustainable database including hypoglycaemia data from >100 clinical trials to examine predictors of hypoglycaemia and establish glucose threshold(s) below which hypoglycaemia constitutes a risk for adverse biomedical and psychological outcomes, and increases healthcare costs. We will also investigate the mechanism(s) underlying the antecedents and consequences of hypoglycaemia, the significance of glucose sensor‐detected hypoglycaemia, the impact of hypoglycaemia in families, and the costs of hypoglycaemia for healthcare systems.
Results
The outcomes of Hypo‐RESOLVE will inform evidence‐based definitions regarding the classification of hypoglycaemia in diabetes for use in daily clinical practice, future clinical trials and as a benchmark for comparing glucose‐lowering interventions and strategies across trials. Stakeholders will be engaged to achieve broadly adopted agreement.
Conclusion
Hypo‐RESOLVE will advance our understanding and refine the classification of hypoglycaemia, with the ultimate aim being to alleviate the burden and consequences of hypoglycaemia in people with diabetes.
Background
Diabetes is a major non‐communicable threat to global health, affecting 10% of adults worldwide 1, and is among the most expensive chronic health conditions, imposing an increasing burden on healthcare resources 2. Despite different underlying causes and approaches to treatment, both type 1 and type 2 diabetes can have devastating effects on physical and mental health and can continue to reduce both quality of life and life expectancy. Maintaining glucose at or close to recommended target levels has been shown to decrease risks of microvascular and macrovascular complications 3, 4, 5, 6, 7, and improves overall survival in people with recent‐onset type 2 diabetes 6.
Insulin and insulin secretagogues (e.g. sulfonylureas) are effective in managing hyperglycaemia, but increase hypoglycaemia risk because their action is independent of glycaemic levels. Hypoglycaemia causes profound physiological and cellular stress, with marked stimulation of the autonomic nervous system and the hypothalamic–pituitary–adrenal axis, leading to symptoms ranging from feelings of unpleasantness to cognitive impairment, serious harm or death. In type 1 diabetes, 4–8% of premature deaths have been attributed to hypoglycaemia 8, 9, not including the so‐called ‘dead‐in‐bed’ syndrome, a mode of sudden death thought to result from hypoglycaemia‐induced fatal arrhythmia 10. Severe hypoglycaemia, defined as an event causing sufficient cognitive dysfunction to require assistance from another person for recovery 11, is predictive of two‐ to threefold higher risks of cardiovascular morbidity and mortality in people with type 2 diabetes 12. A recent analysis calculated that an unrealistically high prevalence of comorbid diseases would be needed to explain the association by frailty alone 13, yet the mechanisms underlying a causative relationship remain undiscovered.
On average, hypoglycaemia occurs at a rate of two events per week in those with type 1 and two per month in those with insulin‐treated type 2 diabetes 14. Reported rates of severe hypoglycaemia vary between 0.3 and 3 events per year in unselected cohorts of people with type 1 diabetes 15. The distribution is highly skewed to those with impaired awareness of hypoglycaemia 16, 17, a condition already recognized early following the discovery of insulin 18 and characterized by reduced ability to detect the onset of hypoglycaemia. Most estimates ignore ‘silent’ events that occur in these individuals or during sleep. Significant advances in diabetes management (e.g. insulin analogues, subcutaneous pumps, glucose sensor technology and structured education) appear to have had only a relatively modest impact on the incidence of hypoglycaemia 19.
The myriad of definitions for hypoglycaemia contribute to the variation in reported incidence rates of hypoglycaemia. The glucose cut‐offs below which counter‐regulatory responses to, and symptoms of, hypoglycaemia appear are not fixed and depend on both non‐modifiable factors (e.g. age and duration) and modifiable factors (e.g. prior exposure to hyper‐ and hypoglycaemia) 20, 21. Despite agreement about the clinical relevance of any event of low plasma glucose that carries the potential to cause harm 22, uncertainty remains about where such harm starts and in whom, making it difficult to assign a single glucose threshold value to define hypoglycaemia. The International Hypoglycaemia Study Group (IHSG) has proposed a three‐level classification: level 1: glucose ≤3.9 mmol/l (70 mg/dl), representing an alert value; level 2: glucose <3.0 mmol/l (54 mg/dl) to indicate ‘clinically important hypoglycaemia’, based on its association with cognitive dysfunction, reductions in hypoglycaemic awareness and mortality; and level 3: severe hypoglycaemia, as defined above 11. This classification has been adopted by the American Diabetes Association, the European Association for the Study of Diabetes, the International Society for Paediatric and Adolescent Diabetes 23 and the European Medicines Agency (EMA), and by an international expert panel on continuous glucose monitoring 24.
The evidence base supporting this classification remains relatively limited 25, particularly with respect to interstitial glucose measurements, which is relevant given the increasing use of glucose sensors, both clinically and in trials. Also, data on the health‐economic consequences and on patient‐reported outcomes related to ‘non‐severe’ hypoglycaemia are largely lacking, but highly relevant in clinical practice, as hypoglycaemia affects virtually everyone using insulin. Thus, there is an urgent need to increase understanding of hypoglycaemia in diabetes and to provide more robust and comprehensive evidence to strengthen and/or refine the IHSG‐proposed classification.
Project aim
The Hypoglycaemia REdefining SOLutions for better liVEs (Hypo‐RESOLVE) project's overall aim is to reduce the burden and consequences of hypoglycaemia in people with diabetes by increasing our understanding of hypoglycaemia, using a comprehensive multilevel approach that addresses unanswered questions and unmet needs 26. In the present paper, we explain how clinicians and scientists with expertise in basic, clinical, psychological/behavioural and health‐economic aspects of hypoglycaemia will work collaboratively with data science and biostatistics experts, industry partners, people with diabetes, healthcare organizations and other stakeholders to achieve this aim.
Project outline
The work of Hypo‐RESOLVE is structured across eight work packages (WPs), each of which has a distinct (scientific) focus, with strong interconnected linkages (Fig. 1). Central to the project is the further elucidation of the link between hypoglycaemia, whether measured by finger pricks or continuous glucose monitoring, and clinical (i.e. cardiovascular and mortality) consequences, its underlying mechanism(s), and patient‐reported and health‐economic outcomes. We will also map definitions of hypoglycaemia as they are currently being used in national and international guidelines and maintain a close dialogue with regulators, including the EMA, and other stakeholders.
Figure 1.
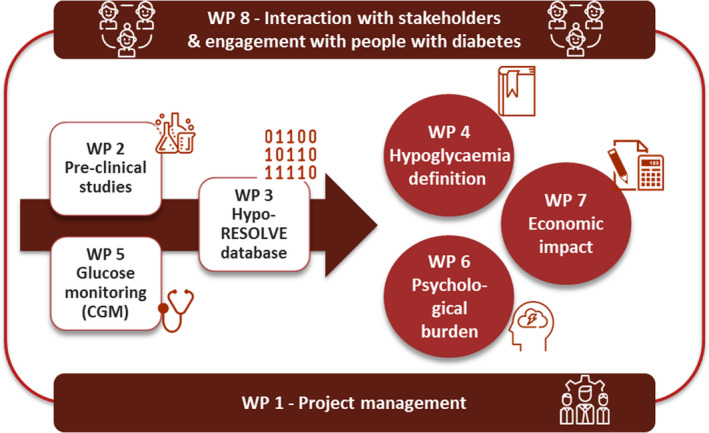
Organizational structure and interaction of the work in Hypo‐RESOLVE: project management [work package (WP)1], animal and human pre‐clinical experiments (WP2), construction of the sustainable Hypo‐RESOLVE database (WP3), analysis of the Hypo‐RESOLVE database to refine and solidify the classification of hypoglycaemia (WP4), glucose monitoring studies (WP5), psychological burden of hypoglycaemia (WP6), health‐economic impact of hypoglycaemia (WP7) and interaction with stakeholders and engagement with people affected by or living with diabetes (WP8).
Hypo‐RESOLVE database: WP3 and WP4
It is standard practice for hypoglycaemic events to be secondary or safety outcomes in clinical trials involving insulin treatment. A key objective of Hypo‐RESOLVE is to collect these events alongside a range of clinical outcomes from over 100 clinical trials on insulin treatment and/or glucose monitoring in people with type 1 or type 2 diabetes, to construct a large, sustainable database, offering the statistical power needed to establish the glucose threshold(s) below which hypoglycaemia constitutes a risk. Entry criteria for these trials are: randomized trials initiated in 2001 or later; participants with type 1 or type 2 diabetes; insulin treatment alone or in combination with other drugs; and trial duration of at least 12 weeks. After a process of de‐identification to ensure participant anonymity, original data from each trial will be extracted, reformatted and harmonized to the standard Clinical Data Interchange Standards Consortium (CDISC) Study Data Tabulation Model (SDTM) format (http://www.cdisc.org/; Fig. 2). De‐identified trial data will be uploaded to a secure server behind a firewall, inaccessible for downloads or changes, and subsequently harmonized and pooled into a single database, reachable for a variety of statistical analysis tools, implemented in the R statistical framework. After agreeing on categorization of hypoglycaemia, e.g. defined by presence (or absence) of symptoms, help from another person, certain glucose thresholds and duration, the associations with (un)known clinical and psychological consequences will be examined. A statistical analysis plan is being constructed and tested to perform analyses on the risk factors for, and consequences of, hypoglycaemia. Using data from the Scottish Diabetes Research Network Type 1 Bioresource 27, a Bayesian posterior distribution method was found to be a better fit to model the risk factors for the prediction of hypoglycaemia than the standard approach of negative binomial regression (manuscript in preparation).
Figure 2.
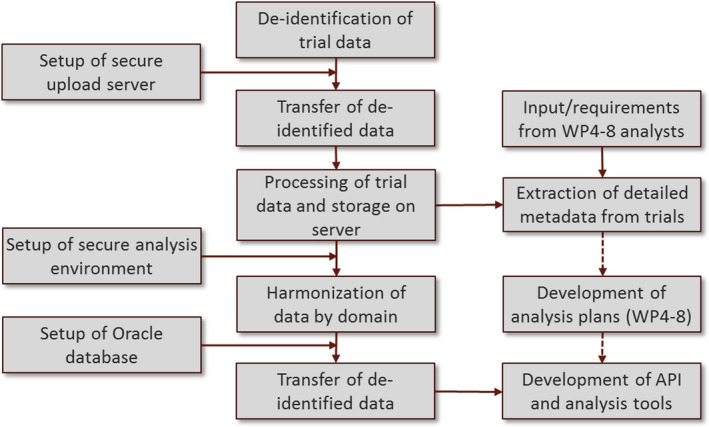
Workflow for the construction of the Hypo‐RESOLVE database. API, application programming interface; WP, work package.
Mechanistic studies: WP2
The focus of basic research will be first on further elucidation of the pathogenesis of impaired awareness of hypoglycaemia. Animal studies with state‐of‐the‐art transgenic approaches will also be used to discover novel pathways that contribute to the development of impaired awareness of hypoglycaemia as well as to identify therapeutic targets that may help to restore hypoglycaemia awareness.
A second crucial element of the project is to reveal the potential mechanism(s) underlying the association between (severe) hypoglycaemia and cardiovascular outcomes, which would complement analyses of the above‐mentioned Hypo‐RESOLVE clinical database. Recent research has shown hypoglycaemia to be a pro‐inflammatory, potentially pro‐atherogenic, stimulus 28, with the resulting response potentially modifiable by prior exposure to hypoglycaemia or the level of overall glucose control. We will characterize in detail the nature and time course of such inflammatory responses, including underlying processes, using hyperinsulinaemic hypoglycaemic glucose clamps. During and up to 1 week after the clamp, blood will be sampled and immune cells isolated for extensive phenotyping. The primary outcome is the effect of experimental hypoglycaemia on inflammatory responses; secondary outcomes include underlying molecular, metabolic and functional mechanisms, epigenetic profiles and oxidative stress, as well as cardiac function, assessed by echocardiography. The experiments will be conducted in people with: type 1 diabetes with intact awareness of hypoglycaemia; type 1 diabetes with impaired awareness of hypoglycaemia; type 1 diabetes with suboptimal glycaemia (HbA1c >64 mmol/mol [8%]); and insulin‐treated type 2 diabetes (n=16 each). One group of volunteers without diabetes (n=16), matched to those with type 2 diabetes, will undergo the same procedure. Another group (n=32), matched to participants with type 1 diabetes, will be randomized to prior exposure to either two episodes of hypoglycaemia or normoglycaemia on the day before the clamp procedure. Sample sizes are based on a power calculation to meet the primary outcome for each subgroup separately, with comparisons between groups to be conducted in subsequent analyses. The broad inclusion criteria of this study, registered at clinicaltrials.gov (NCT03976271), will enable us to assess the impact of awareness of hypoglycaemia, HbA1c, prior hypoglycaemia and duration of diabetes. Moreover, complimentary rodent studies will determine whether the effects in immune cells are replicated in specific organs (e.g. the heart) and tissues (e.g. adipose tissue), and to examine the impact of recurrent hypoglycaemia on end‐organ histopathology and function.
Using continuous glucose monitoring to understand hypoglycaemia: WP5
In the management of insulin‐treated diabetes, self‐monitoring of blood glucose, with its major limitations of (relatively) infrequent glucose measurements at (more or less) standard time points, is being superseded by (semi‐)continuous glucose monitoring techniques. In the near future, these techniques will become increasingly important for monitoring glucose levels and patterns, assessing glycaemic targets and evaluating the effects of interventions, particularly in type 1 diabetes and in countries where individuals or the healthcare system can afford it. As continuous glucose monitoring starts to become used as an outcome measure to determine risk of hypoglycaemia, we also recognize that device metrics may be unstable over short durations. Glucose sensor data collected and harmonized in the constructed database (WP3) will enable analysis of the optimum duration of glucose sensor use and the relative contribution of sampling interval to determine an individual's risk of hypoglycaemia. We will use in silico modelling techniques to glucose sensor data to identify behavioural factors, including self‐monitoring of blood glucose, carbohydrate counting and missed insulin administration, that modify the risk of hypoglycaemia.
Recent data show that, even in those with intact awareness of hypoglycaemia, up to 60% of episodes are asymptomatic and undetected by the individual 29. This causes a clinical dilemma as to whether such events need to be treated or ignored, and raises issues about how people deal, psychologically, with visibility of the potentially overwhelming amount of (semi‐)continuous data. We will therefore conduct a multinational observational study aiming to understand the impact of both symptomatic and asymptomatic episodes of low glucose sensor readings. Participants will be asked to wear blinded glucose sensors for 10 weeks, in addition to their usual means of glucose monitoring. They will also use an app to register all self‐treated episodes of hypoglycaemia and answer questions in real‐time relevant to various domains of life that may be affected by hypoglycaemia. The primary objective is to determine the optimal definition of sensor hypoglycaemia in terms of threshold and duration by elucidating the optimum value of these variables that provides the best sensitivity and specificity for patient‐reported hypoglycaemia. Secondary outcomes include the impact of symptomatic as well as asymptomatic hypoglycaemia and low interstitial glucose values on clinical, psychosocial and health‐economic variables, such as activity, sleep quality, energy levels, well‐being, quality of life and productivity. A sample size calculation based on the number of expected episodes of patient‐reported hypoglycaemia and sensor‐detected hypoglycaemia taken from a series of large observational datasets, calculated that 180 adults with type 1 and 325 with type 2 diabetes are required to meet our primary objective. Allowing for withdrawals and to be able to evaluate outcomes in those with impaired awareness, we aim to recruit 250 participants with type 1 diabetes, 50 of whom have impaired awareness, and 350 with type 2 diabetes (n=600 in total). We will investigate differences in the impact of hypoglycaemia between type 1 and type 2 diabetes, and the impact of other aspects such as sleep and baseline hypoglycaemia rate on these factors.
Psychological burden and health‐economic impact: WP6 and WP7
The potential of hypoglycaemia to cause cognitive decline, social embarrassment, loss of consciousness, accidents and physical injury is a well‐known cause of fear of hypoglycaemia 30 among individuals with diabetes, as well as partners, parents and other family members 31, 32. Substantial fear of hypoglycaemia often results in a coping strategy of maintaining glucose levels in the higher range, thus accepting increased exposure to hyperglycaemia and greater risks of associated vascular complications 33. Indisputably, hypoglycaemia (and the fear of it) remains a major barrier to people with diabetes achieving and maintaining the glucose levels necessary to avoid the long‐term complications associated with chronic hyperglycaemia 34.
To achieve better insight into the overall burden of hypoglycaemia for people with diabetes and their families, extensive systematic literature reviews will be performed to collate and critique all quantitative and qualitative studies investigating the impact of hypoglycaemia on quality of life and associated psychological outcomes among adults or children with diabetes and family members. Findings will be summarized and, where possible, subjected to meta‐analyses. Existing patient‐reported outcome instruments relevant to assessing the burden of hypoglycaemia will be identified, and their psychometric characteristics will be summarized and critiqued. This knowledge will be used to develop new or refine existing patient‐reported outcome instruments that can be used in various populations for health‐economic evaluation purposes. In addition, these instruments will be developed into a preference‐based measure, such that it can be used to generate quality‐adjusted life‐years for use in cost–utility analyses. As such, Hypo‐RESOLVE will be able to precisely map the health‐economic consequences of hypoglycaemia, including the costs of hypoglycaemia for healthcare systems in Europe and elsewhere.
In parallel, a qualitative multi‐country online survey will be conducted to explore the unmet psychological needs of people with diabetes and their families in relation to the prevention, experience and management of hypoglycaemia. We plan to include 800–1000 participants from Denmark, Germany, the Netherlands and the UK, stratified into groups of adults with type 1 or type 2 diabetes, children and adolescents with diabetes, and family members. The findings will subsequently be used to design a multinational quantitative online survey to further address knowledge gaps, including the impact of (severe and self‐treated) hypoglycaemia in adults and adolescents and on relationships, unmet care needs and barriers to talking about hypoglycaemia.
Integration of the results of Hypo‐RESOLVE: WP8
The findings from Hypo‐RESOLVE will be collated into a comprehensive summary for high‐level communication with stakeholders and development of a consensus guideline with uniformly agreed definitions and data collection methods for the standardization of clinical investigations with regard to hypoglycaemia. A dialogue has thus been established with key European and American regulators, Health Technology Assessment (HTA) body representatives, professional and diabetes organizations and other stakeholders.
Project organization: WP1
The Hypo‐RESOLVE consortium comprises 23 partner organizations from academic centres across Europe, industry and biotech partners owning the vast majority of global insulin producing capacity and the manufacturing of glucose monitoring devices, respectively, organizations representing people with diabetes, and a project management office. A multi‐layered governance model has been agreed to ensure suitable levels of cooperation, scientific exchange and project management, as well as a decision‐making process, in order to achieve the project's objectives. An international Patient Advisory Committee has been formed that interacts closely with each of the WPs to ensure that the voice of people with diabetes resonates in the direction, goals and strategy of the project. Finally, multi‐disciplinary Strategic Advisory and Ethics Boards have been appointed to provide reflection and feedback from scientific and ethical perspectives, respectively.
Expected impact
Using detailed and varied statistical analyses, Hypo‐RESOLVE will provide robust evidence about risk factors and clinical, psychological and health‐economic consequences of asymptomatic (i.e. sensor‐detected), non‐severe and severe hypoglycaemia in people with diabetes. Integration of these data offers the unique opportunity to refine and solidify an evidence‐based classification of hypoglycaemia, whether measured in (capillary) blood or the interstitium, for its use in clinical practice, trials design and research, and for the development of clinical guidelines. The results of Hypo‐RESOLVE are expected to be of considerable interest to a broad range of stakeholders, including people with diabetes, healthcare professionals, the scientific community, HTA bodies, regulators, industry and payers (Table 1). The project's progress will be reported through scientific publications, the Hypo‐RESOLVE website (hypo‐resolve.eu), social media (@HypoResolve), and stakeholder meetings, to ensure widespread dissemination to, and engagement with, all those potentially interested in the project's progress and outcomes and to endorse the ramifications of its analyses for classifying hypoglycaemia.
Table 1.
Expected impact of Hypo‐RESOLVE for stakeholders
| Stakeholder | Expected impact |
|---|---|
| People with diabetes |
|
| Healthcare professionals |
|
| Payers |
|
| Regulatory authorities |
|
| Scientific community |
|
| Industry |
|
CGM, continuous glucose monitoring; SMBG, self‐monitoring of blood glucose.
Hypo‐RESOLVE limitations
Although comprehensive, Hypo‐RESOLVE will not provide answers to all questions relating to hypoglycaemia in people with diabetes. In particular, the Hypo‐RESOLVE database may be (too) limited for directly examining harm of sensor‐detected hypoglycaemia and investigating hypoglycaemia in certain populations. Children, the elderly, pregnant women, ethnic subgroups and people at highest risk of (severe) hypoglycaemia, including those with impaired awareness of hypoglycaemia, chronic kidney disease or other chronic comorbidities are usually excluded from most clinical trials. However, the harm of sensor‐detected hypoglycaemia may be derived indirectly from the observational clinical study on continuous glucose monitoring. Also, examining the impact of hypoglycaemia on children, and in particular their carers, is a specific objective of the ‘psychology’ WP, whereas elderly populations will be included in the health‐economic work. Finally, people with impaired awareness of hypoglycaemia will be recruited to the experimental, psychological and clinical studies, and animal studies will include models of this condition.
Conclusion
Despite almost 100 years of experience with insulin as a treatment for diabetes and major advances in its management, hypoglycaemia remains a continuous threat to people with diabetes who require treatment with insulin or secretagogues. The clinical, psychological and health‐economic consequences of hypoglycaemia and, in particular, the glucose threshold identifying increased risk for harm, remain to be fully established. Hypo‐RESOLVE will apply a comprehensive multi‐level, multi‐disciplinary scientific approach to examine the links between hypoglycaemia (at various levels) and potentially harmful outcomes and its underlying mechanism(s), where applicable. Thus, Hypo‐RESOLVE will advance our understanding of hypoglycaemia, so as to alleviate its burden and improve the lives of all people affected by diabetes.
Funding sources
Hypo‐RESOLVE has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 777460. The JU receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA and T1D Exchange, JDRF, International Diabetes Federation (IDF) and The Leona M. and Harry B. Helmsley Charitable Trust. The industry partners supporting the JU include Abbott Diabetes Care, Eli Lilly, Medtronic, Novo Nordisk and Sanofi‐Aventis. The funder had no role in the design of the project or its WPs, the collection or analysis of data, the writing of the manuscript or the decision to submit for publication.
Competing interests
B.D.G. has served on scientific advisory boards for Novo Nordisk and received research grant support from AstraZeneca and Sanofi. S.R.H. has served on advisory boards for Eli Lilly, Novo Nordisk, Sanofi Aventis and Zealand Pharma and been a member of speaker panels for Novo Nordisk. R.J.M. has served on advisory boards for Eli Lilly, Novo Nordisk, Sanofi Aventis and been a member of speaker panels for Novo Nordisk. J.S. has served on the advisory boards of Janssen, Medtronic, Roche Diabetes Care and Sanofi Diabetes. Her research group (ACBRD) has received honoraria in respect of these activities, and she has also received unrestricted educational grants and in‐kind support from Abbott Diabetes Care, AstraZeneca, Medtronic, Roche Diabetes Care and Sanofi Diabetes, sponsorship to attend educational meetings from Medtronic, Roche Diabetes Care and Sanofi Diabetes, and consultancy income and/or speaker fees from Abbott Diabetes Care, AstraZeneca, Medtronic, Novo Nordisk, Roche Diabetes Care and Sanofi Diabetes. M.R. is an employee of Eli Lilly and company. C.T. has served on scientific advisory boards for Merck and Novo Nordisk and received research grant support from AstraZeneca. M.M. is employee of Novo Nordisk A/S. All other authors declare no conflicts of interest.
Diabet. Med. 37, 1066–1073(2020)
References
- 1. International Diabetes Federation . IDF Diabetes Atlas. 8th edn Brussels: IDF, 2017. [Google Scholar]
- 2. Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J et al Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 293–301. [DOI] [PubMed] [Google Scholar]
- 3. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 4. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 5. Zoungas S, Arima H, Gerstein HC, Holman RR, Woodward M, Reaven P et al Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta‐analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol 2017; 5: 431–437. [DOI] [PubMed] [Google Scholar]
- 6. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 7. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ et al Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tunbridge WM. Factors contributing to deaths of diabetics under fifty years of age. On behalf of the Medical Services Study Group and British Diabetic Association. Lancet 1981; 2: 569–572. [DOI] [PubMed] [Google Scholar]
- 9. Gagnum V, Stene LC, Jenssen TG, Berteussen LM, Sandvik L, Joner G et al Causes of death in childhood‐onset Type 1 diabetes: long‐term follow‐up. Diabet Med 2017; 34: 56–63. [DOI] [PubMed] [Google Scholar]
- 10. Tanenberg RJ, Newton CA, Drake AJ. Confirmation of hypoglycemia in the "dead‐in‐bed" syndrome, as captured by a retrospective continuous glucose monitoring system. Endocr Pract 2010; 16: 244–248. [DOI] [PubMed] [Google Scholar]
- 11. International Hypoglycaemia Study Group . Glucose Concentrations of Less Than 3.0 mmol/L (54 mg/dL) Should Be Reported in Clinical Trials: A Joint Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017; 40: 155–157. [DOI] [PubMed] [Google Scholar]
- 12. Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L et al Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363: 1410–1418. [DOI] [PubMed] [Google Scholar]
- 13. Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta‐analysis with bias analysis. BMJ 2013; 347: f4533. [DOI] [PubMed] [Google Scholar]
- 14. International Hypoglycaemia Study Group . Minimizing Hypoglycemia in Diabetes. Diabetes Care 2015; 38: 1583–1591. [DOI] [PubMed] [Google Scholar]
- 15. Pedersen‐Bjergaard U, Thorsteinsson B. Reporting Severe Hypoglycemia in Type 1 Diabetes: Facts and Pitfalls. Curr Diab Rep 2017; 17: 131. [DOI] [PubMed] [Google Scholar]
- 16. UK Hypoglycaemia Study Group . Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007; 50: 1140–1147. [DOI] [PubMed] [Google Scholar]
- 17. Pedersen‐Bjergaard U, Pramming S, Thorsteinsson B. Recall of severe hypoglycaemia and self‐estimated state of awareness in type 1 diabetes. Diabetes Metab Res Rev 2003; 19: 232–240. [DOI] [PubMed] [Google Scholar]
- 18. Lawrence RD. Insulin hypoglycaemia: changes in nervous manifestations. Lancet 1941; 2: 602–604. [Google Scholar]
- 19. Lipska KJ, Ross JS, Wang Y, Inzucchi SE, Minges K, Karter AJ et al National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med 2014; 174: 1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boyle PJ, Schwartz NS, Shah SD, Clutter WE, Cryer PE. Plasma glucose concentrations at the onset of hypoglycemic symptoms in patients with poorly controlled diabetes and in nondiabetics. N Engl J Med 1988; 318: 1487–1492. [DOI] [PubMed] [Google Scholar]
- 21. Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 1991; 40: 223–226. [DOI] [PubMed] [Google Scholar]
- 22. Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo‐Jack S, Fish L et al Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36: 1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abraham MB, Jones TW, Naranjo D, Karges B, Oduwole A, Tauschmann M et al ISPAD Clinical Practice Consensus Guidelines 2018: Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes 2018; 19 (Suppl. 27): 178–192. [DOI] [PubMed] [Google Scholar]
- 24. Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH et al International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017; 40: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heller SR, Buse JB, Ratner R, Seaquist E, Bardtrum L, Hansen CT et al Redefining Hypoglycemia in Clinical Trials: Validation of Definitions Recently Adopted by the American Diabetes Association/European Association for the Study of Diabetes. Diabetes Care 2019; pii: dc182361 10.2337/dc18-2361. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hypo‐RESOLVE Investigates Hypoglycaemia and its Impact in Diabetes. Available at https://www.hypo-resolve.eu/. Last accessed 1 December 2019.
- 27. McKeigue PM, Spiliopoulou A, McGurnaghan S, Colombo M, Blackbourn L, McDonald TJ et al Persistent C‐peptide secretion in Type 1 diabetes and its relationship to the genetic architecture of diabetes. BMC Med 2019; 17: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ratter JM, Rooijackers HM, Tack CJ, Hijmans AG, Netea MG, de Galan BE et al Proinflammatory Effects of Hypoglycemia in Humans With or Without Diabetes. Diabetes 2017; 66: 1052–1061. [DOI] [PubMed] [Google Scholar]
- 29. Henriksen MM, Andersen HU, Thorsteinsson B, Pedersen‐Bjergaard U. Hypoglycemic Exposure and Risk of Asymptomatic Hypoglycemia in Type 1 Diabetes Assessed by Continuous Glucose Monitoring. J Clin Endocrinol Metab 2018; 103: 2329–2335. [DOI] [PubMed] [Google Scholar]
- 30. Hendrieckx C, Halliday JA, Bowden JP, Colman PG, Cohen N, Jenkins A, et al. Severe hypoglycaemia and its association with psychological well‐being in Australian adults with type 1 diabetes attending specialist tertiary clinics. Diabetes Res Clin Pract 2014; 103: 430–436. [DOI] [PubMed] [Google Scholar]
- 31. Gonder‐Frederick L, Nyer M, Shepard JA, Vajda K, Clarke W. Assessing fear of hypoglycemia in children with Type 1 diabetes and their parents. Diabetes Manag (Lond) 2011; 1: 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderbro T, Gonder‐Frederick L, Bolinder J, Lins PE, Wredling R, Moberg E et al Fear of hypoglycemia: relationship to hypoglycemic risk and psychological factors. Acta Diabetol 2015; 52: 581–589. [DOI] [PubMed] [Google Scholar]
- 33. Gonder‐Frederick LA, Vajda KA, Schmidt KM, Cox DJ, Devries JH, Erol O et al Examining the Behaviour subscale of the Hypoglycaemia Fear Survey: an international study. Diabet Med 2013; 30: 603–609. [DOI] [PubMed] [Google Scholar]
- 34. Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia 2002; 45: 937–948. [DOI] [PubMed] [Google Scholar]


