Abstract
Introduction
Low‐dose aspirin (LDA) prophylaxis has been shown to reduce women’s preeclampsia risk. Evidence regarding LDA adherence rates of pregnant women is based almost exclusively on clinical trials, giving a potentially biased picture. Moreover, these studies do not report on determinants of adherence. Since 2017, obstetric healthcare professionals in a Dutch region have assessed women’s preeclampsia risk by means of a prediction tool and counseled those with an above‐population average risk on LDA as a prophylactic measure.
Material and methods
From 2017 to 2018, 865 women were recruited in multiple centers and prospectively followed using web‐based surveys (Expect Study II). Rates and determinants of LDA usage among women with an increased preeclampsia risk in daily practice were assessed. Results were compared with findings in a similar cohort from a care‐as‐usual setting lacking risk‐based counseling (Expect Study I, n = 2614). Netherlands Trial Register NTR4143.
Results
In total, 306 women had a predicted increased preeclampsia risk. LDA usage was higher for women receiving risk‐based care than care‐as‐usual (29.4% vs 1.5%, odds ratio 19.1, 95% confidence interval 11.2‐32.5). Daily LDA usage was positively correlated with both predicted risk and women’s concerns regarding preeclampsia. Most reported reasons for non‐ or incomplete use were unawareness of LDA as a preventive intervention, concerns about potential adverse effects and doubts regarding the benefits.
Conclusions
Risk‐based counseling was associated with a higher prevalence of LDA usage, but general usage rates were low. Future research regarding potential factors improving the usage of LDA during pregnancy is necessary.
Keywords: adherence, aspirin, preeclampsia, pregnancy
Abbreviations
- CI
confidence interval
- LDA
low‐dose aspirin
- PE
preeclampsia
- PTB
preterm birth
- SGA
small‐for‐gestational‐age
Key message.
Low‐dose aspirin usage strongly increased using risk‐based counseling with the aid of a prediction tool, but general usage rates were low.
1. INTRODUCTION
Preeclampsia (PE) is an important cause of serious maternal and fetal complications. Despite improved management, curative options preserving the pregnancy remain absent. Preventive measures reducing the risk of PE are therefore an essential part of strategies aimed at decreasing the burden of PE.1
Besides lifestyle interventions and adequate calcium intake, low‐dose aspirin (LDA) treatment is currently one of the key interventions for the prevention of PE.2, 3, 4 Reduction of PE risk has been shown at aspirin dosages between 80 and 150 mg/day.5 The majority of publications on LDA with respect to PE focus on its effectiveness. They mainly differ regarding dosing, gestational window or target group.2, 5 Published LDA adherence rates are fairly high (66%‐90%) but are mostly measured within clinical trials.2, 6 It is unlikely that women who would not opt for LDA during their pregnancy would be willing to participate in a trial involving LDA usage. Thus, trial‐based adherence rates may be seriously biased upwards. Relatively little is known regarding the daily LDL usage rates among pregnant women in daily practice.7
Several obstetric authorities recommend LDA for women with an increased PE risk, including the American College of Obstetricians and Gynecologists (ACOG), the US Preventive Services Task Force, and the National Institute for Health and Clinical Excellence (NICE, UK).8, 9, 10 Nevertheless, “increased risk of PE” has been defined in different ways and no consensus has yet been achieved. Assessment of PE risk can be performed using either unweighted or weighted combinations of multiple risk factors. The latter method (ie, prediction models) has been shown to outperform the use of unweighted risk factors (ie, NICE criteria) in terms of predictive ability.11, 12
Recently, healthcare professionals in the southeastern part of the Netherlands implemented an externally validated prediction tool to assess, during the first trimester of pregnancy, the risk of developing PE.11, 13, 14 In the case of an increased risk, the option of LDA prophylaxis is discussed using a shared‐decisional approach. In such an approach, healthcare professionals share the best available evidence with the women in order to make an informed decision together.15 This observational study reports on LDA usage rates by women with an increased PE risk, as well as on determinants and reasons given for use and non‐use.
2. MATERIAL AND METHODS
In 2017, members of the Limburg Obstetric Consortium (located in the southeastern part of the Netherlands) started to assess women’s PE risk during the first antenatal visits by means of a prediction tool. This tool embedded Syngelaki’s prediction model, externally validated and recalibrated by Meertens et al.11, 16 This model is based on maternal characteristics (age, body mass index, ethnicity, mode of conception, family history, medical history and obstetric history) and was made available for all healthcare professionals of the region.
A detailed description of the content of risk‐based care is reported elsewhere.13, 17 In short, women with a PE risk exceeding the population average risk (>3.0%; sensitivity 75%, specificity 64%) should be counseled regarding the option of LDA prophylaxis (80‐100 mg daily) in a shared‐decisional approach. All women ≥18 years old with a singleton pregnancy were eligible for inclusion. Women were recruited from 2017 to 2018 at their first prenatal visit (<16 weeks of pregnancy), at which time their healthcare professional used the prediction tool. Women were recruited from multiple centers, five hospitals and 26 autonomous midwifery practices, all belonging to the geographical area of the Limburg Obstetric Consortium. For the analyses in this paper, women with incomplete data regarding LDA usage or a contraindication for LDA usage were excluded. A detailed study protocol has been published previously.13 Briefly, after providing informed consent, the results of the risk assessment were automatically logged. Enrolled women received four online surveys at intervals (at enrollment and at 24 weeks of pregnancy, 34 weeks of pregnancy, and 6 weeks after the due date). In the case of preterm birth, women were automatically redirected to the postpartum questionnaire when completing the questionnaire sent at 24 or 34 weeks of pregnancy. In addition, medical records and discharge letters were retrieved.
The first survey contained questions related to the first antenatal visits. Women were asked whether they were informed regarding LDA and whether they intended to use LDA. Additionally, women were questioned how often they worried about complications related to PE, such as PE itself, small‐for‐gestational‐age (SGA) infancy and preterm birth (PTB). They could choose from the options not at all, sometimes, regularly and often. Answers were transformed to a 4‐point scale (0, not at all; 1, sometimes; 2, regularly; 3, often).
The postpartum survey included questions related to LDA usage throughout the pregnancy. Women who stated that they used LDA, received additional questions regarding the gestational window of LDA usage and whether they took it daily. Women stating that they did not use LDA, received additional questions with respect to their most decisive reason of non‐use. Women were able to choose from predefined options but were also able to provide a different reason and leave additional remarks.
2.1. Statistical analyses
Usage of LDA was analyzed with respect to women’s estimated PE risk. Any LDA usage was defined as LDA usage regardless of the numbers of pills taken, duration or frequency. Per protocol LDA usage was defined as the usage as described in the risk‐based care pathways: daily LDA usage from <16+0 weeks of gestation up to 36 weeks of gestation or, in the case of preterm birth, up to 1 week before birth. We cross‐tabulated the proportions of women who reported having discussed the option of LDA, any LDA usage and per protocol LDA usage with respect to the estimated PE risk (low risk/increased risk).
Data of the Expect Study I (n = 2614), a similar multicenter prospective cohort study conducted in the same region from 2013 to 2015, were used to represent the care‐as‐usual approach with no risk‐based recommendations.11, 18 For Expect Study I, a paper and pencil questionnaire was available on request. However, the vast majority of women completed the web‐based version of the questionnaires. The data contained information on usage of LDA but not whether LDA was used in accordance with the risk‐based care recommendations. As a result, only the proportions of any LDA usage could be compared between risk‐based care and former care‐as‐usual.
The proportions of any LDA usage by women who received care‐as‐usual and women who received risk‐based care were plotted using the estimated risk as a continuous variable. A nonparametric local weighted regression (loess regression) was applied to fit the curves.19
For analysis of determinants correlated with per protocol LDA usage, a multiple logistic regression was performed. This analysis was restricted to women with an increased risk who were informed by their healthcare professional regarding LDA, since only these women are able to make an informed decision. Factors taken into account were estimated PE risk (continuous); reported educational level (tertiary yes/no); concerns regarding developing PE (continuous); concerns regarding developing complications related to PE (SGA, continuous; PTB, continuous); type of healthcare professional responsible for LDA counseling (midwife/gynecologist). For the continuous determinants, we used frequency plots to verify that assumptions of linearity were not violated. All statistical analyses were performed using R statistical software version 3.6.0.20
2.2. Ethical approval
The Medical Ethical Committee of the Maastricht University Medical Center evaluated both Expect Study protocols I and II and declared that neither observational study fell under the Medical Research Involving Human Subjects Act (METC‐13‐4‐053 and METC‐17‐4‐057, respectively). Netherlands Trial Register NTR4143. Online informed consent was obtained from all participants.
3. RESULTS
Figure 1 displays a flowchart of study enrollment. Informed consent was provided by 865 women. Of these, 30 women were excluded from the study cohort for various reasons. Additionally, 121 women were excluded from the current analysis because of either incomplete data (n = 104) or a contraindication for LDA usage (n = 17). In total, 714 women were available for the analyses. Those excluded (n = 121) were more likely to have a primary/secondary educational level (57.7%) than those included (n = 714) in the study (38.2%). Otherwise, no differences in characteristics were observed for parity, body mass index, age, ethnicity, unassisted conception or estimated PE risk (data not presented).
Figure 1.
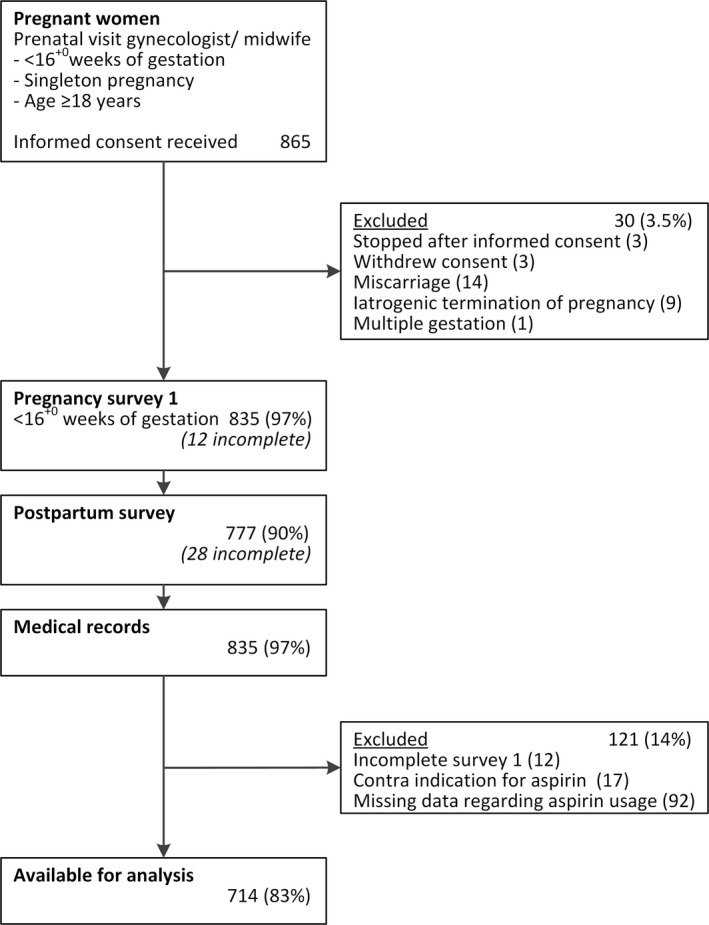
Flowchart participant enrollment of Expect Study II
An overview of baseline characteristics for women enrolled in Expect Study I or II (women received care‐as‐usual and risk‐based care, respectively) is given in Table 1. At baseline, the characteristics of women enrolled in the two studies do not substantially differ. However, for Expect Study II, relatively more women had a history of PE. As a result, the percentage of women identified with an increased PE risk was slightly higher (37.2% vs 42.9%). According to the recommendations of the regional consortium, women in risk‐based care who have been identified with an increased PE risk (risk >3.0%) should be informed regarding LDA usage for the prevention of PE. A large majority of the women (79%, n = 241) reported having discussed LDA with their healthcare provider, indicating a high, but not optimal, adherence rate to regional recommendations by healthcare professionals. Of these women, 94 (39%) intended to use LDA throughout the pregnancy, of which 52 eventually used LDA according to protocol, a per protocol usage rate of 22% (Figure 2).
Table 1.
Baseline characteristics of the Expect Study cohorts I and II
| Baseline characteristics <16 weeks of gestation | Expect Study I care‐as‐usual cohort (n = 2614) | Expect Study II risk‐based care cohort (n = 714) |
|---|---|---|
| Age, years; mean ± SD | 30.2 ± 3.9 | 30.8 ± 4.0 |
| Ethnicity | ||
| Caucasian; n (%) | 2533 (96.9) | 698 (97.8) |
| Other; n (%) | 81 (3.1) | 16 (2.2) |
| Educational level | ||
| Primary or secondary; n (%) | 1194 (45.7) | 273 (38.2) |
| Tertiary level of education; n (%) | 1420 (54.3) | 441 (61.8) |
| Body mass index, kg/m2; mean ± sd | 24.2 ± 4.3 | 24.8 ± 4.6 |
| Smoking during pregnancy | ||
| Yes | 319 (12.2) | 32 (4.5) |
| No | 2137 (81.8) | 682 (95.5) |
| Chronic hypertension | 28 (1.1) | 16 (2.2) |
| Conception | ||
| Natural; n (%) | 2440 (93.3) | 644 (90.2) |
| Ovulation induction; n (%) | 93 (3.6) | 35 (4.9) |
| In vitro fertilization; n (%) | 81 (3.1) | 35 (4.9) |
| Obstetric history | ||
| Nulliparous; n (%) | 1326 (50.7) | 360 (50.4) |
| Prior PE; n (%) | 72 (2.8) | 38 (5.3) |
| No prior PE; n (%) | 1216 (46.5) | 316 (44.3) |
| Family history of PE; n (%) | 131 (5.0) | 36 (5.0) |
| Counseling of PE risk | ||
| by midwife; n (%) | NA | 523 (73.2) |
| by obstetrician; n (%) | NA | 191 (26.8) |
| Estimated PE risk %; median (interquartile range) | 2.5 (1.0‐3.6) | 2.7 (1.1‐4.2) |
| Increased PE risk; n (%) | 974 (37.2) | 306 (42.9) |
| Estimated PE risk % for women identified with an increased risk; median (interquartile range) | 4.2 (3.4‐5.8) | 4.7 (3.6‐6.8) |
Abbreviations: NA, not available; PE, preeclampsia; SD, standard deviation.
Figure 2.
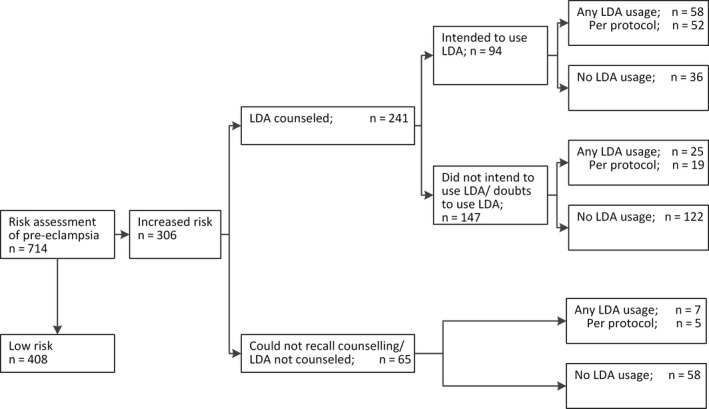
Women’s intentions regarding low‐dose aspirin (LDA) usage after risk‐based counseling and reported LDA usage
Postpartum, of all enrolled women, 113 (15.8%) reported having used LDA during their pregnancy and 87 (12.2%) used it according to protocol (Table 2). Among women with an increased PE risk (>3%), this results in an average usage rate of 29.4% and a per protocol usage rate of 24.8%. Furthermore, a small amount of women (n = 11) used LDA throughout the pregnancy despite not being identified with an increased PE risk.
Table 2.
Proportions of counseling and usage of low‐dose aspirin in relation to predicted preeclampsia risk
| All women (n = 714) | PE risk ≤3% (n = 408) | PE risk >3% (n = 306) | |
|---|---|---|---|
| Total | 714 (100) | 408 (100) | 306 (100) |
| Aspirin prophylaxis discussed | |||
| Yes | 295 (41.3) | 54 (13.2) | 241 (78.8) |
| No | 419 (58.7) | 354 (86.8) | 65 (21.2) |
| Uncertain | 19 (2.7) | 13 (3.2) | 6 (2.0) |
| Aspirin used | |||
| Yes | 113 (15.8) | 23 (5.6) | 90 (29.4) |
| According to protocol | 87 (12.2) | 11 (2.7) | 76 (24.8) |
| No | 601 (84.2) | 385 (94.4) | 216 (70.6) |
Abbreviation: PE, preeclampsia.
The majority of women who started using LDA during their pregnancy in risk‐based care, used it according to protocol. Of the 26 women who used LDA, but not according to protocol, three stopped due to complaints they attributed to LDA (diarrhea n = 1, nose bleeding n = 2). Two women reported they forgot to continue the LDA prophylaxis, and 11 women ended LDA usage at the beginning of their third trimester. Additionally, we could not assess per protocol usage for 9 women who did not recall the date they stopped using LDA.
For the care‐as‐usual approach (Expect Study I, 2013‐2015), LDA usage was nearly non‐existent, with only 23 of 2614 women reporting having used LDA (0.9%). We retrospectively calculated the PE risk of these women, resulting in 974 women being classified with an increased PE risk of which 15 (1.5%) used LDA. In risk‐based care, women with an increased PE risk estimation were more likely to use LDA (odds ratio 19.1; 95% confidence interval [CI] 11.2‐32.5). This disparity even rises for higher PE risk estimations.
Figure S1 provides an overview of the distribution of observed PE risk estimates. Figure 3 displays the proportions of any LDA usage by estimated PE risk for both risk‐based care and the care‐as‐usual approach. We limited the graph to PE risk estimates of ≤15%, which constitutes 99% of the observations. Furthermore, per protocol LDA usage rates are also shown for the risk‐based care cohort. This graph indicates a positive correlation between estimated PE risk and LDA usage in women receiving risk‐based care.
Figure 3.
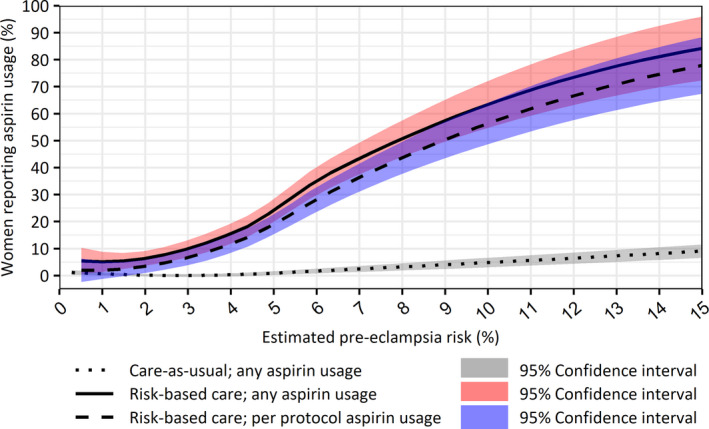
Estimated preeclampsia risks and low‐dose aspirin usage rates by women receiving care‐as‐usual or risk‐based care [Color figure can be viewed at wileyonlinelibrary.com]
The type of healthcare professional (midwife or obstetrician) informing women about LDA was significantly correlated with per protocol LDA usage (odds ratio 2.34, indicating higher usage under obstetric‐gynecological care; 95% CI 1.32‐4.18). However, this association was no longer apparent when correcting for the estimated PE risk (adjusted odds ratio 1.32; 95% CI 0.66‐2.60). In the adjusted analysis (Table 3) only the degree of women’s concerns regarding a pregnancy complicated by PE was statistically significantly associated with per protocol LDA usage when controlling for the estimated PE risk (adjusted odds ratio 1.99; 95% CI 1.35‐2.98).
Table 3.
Multiple logistic regression of potential determinants of per protocol low‐dose aspirin usage among women with an increased risk with whom aspirin usage was discussed
| No. of participants | No. with per protocol aspirin usage, n (%; 95% CI) | Unadjusted odds ratio (95% CI) | Adjusted odds ratioa (95% CI) | |
|---|---|---|---|---|
| All | 241 | 71 (29; 24‐36) | — | — |
| Estimated PE risk | 1.23 (1.14‐1.35) | 1.18 (1.09‐1.30) | ||
| Educational level | ||||
| Primary or secondary | 106 | 30 (28; 21‐38) | 1 [Reference] | 1 [Reference] |
| Tertiary | 135 | 41 (30; 23‐39) | 1.10 (0.63‐1.94) | 1.36 (0.72‐2.62) |
| Concerns regarding PE | 2.23 (1.64‐3.09) | 1.99 (1.35‐2.98) | ||
| Concerns regarding SGA | 1.20 (0.87‐1.66) | 0.98 (0.65‐1.46) | ||
| Concerns regarding PTB | 1.31 (0.97‐1.77) | 0.79 (0.52‐1.19) | ||
| Counseling of PE risk | ||||
| by midwife | 162 | 38 (23; 18‐31) | 1 [Reference] | 1 [Reference] |
| by obstetrician | 79 | 33 (42; 32‐53) | 2.34 (1.32‐4.18) | 1.32 (0.66‐2.60) |
Abbreviations: CI, confidence interval; PE, preeclampsia; PTB, preterm birth; SGA, small‐for‐gestational‐age infancy.
Odds ratios adjusted for variables listed in lefthand column.
Using a semiqualitative approach, we analyzed women’s reasons for not using LDA during the pregnancy. A list of mentioned reasons for not using LDA and their frequencies is shown in Table 4. Surprisingly, despite having an increased PE risk, 92 of 216 women (43%) reported that they believed that the LDA recommendations were not applicable to their situation. This proportion was similar in subgroups with higher PE risk estimates. This questions whether these women received and understood the information regarding LDA usage. Indeed, 39 of these 92 women reported during the first survey that they were not informed regarding LDA.
Table 4.
Reported reasons for not using low‐dose aspirin during pregnancy
| Specified reason | Preeclampsia risk >3% n (%) | Preeclampsia risk >5% n (%) |
|---|---|---|
| It was not applicable to my situation | 92 (43.2) | 27 (39.1) |
| It was not recommended by my healthcare professional | 14 (6.6) | 6 (8.7) |
| The potential benefit is too low for my situation | 64 (30) | 17 (24.6) |
| Because aspirin is a drug | 27 (12.7) | 8 (11.6) |
| No clear reason (eg, forgotten) | 8 (3.8) | 5 (7.2) |
| Miscellaneous | 5 (2.3) | 3 (4.3) |
| Unknown | 6 (2.8) | 3 (4.3) |
| Total | 216 (100) | 69 (100) |
Other frequently mentioned reasons for not using LDA were that women felt that either the potential benefit of LDA was too low (n = 64; 30%) or that they did not want to use (preventive) medication during their pregnancy (n = 27; 13%). In the remarks section of the questionnaire, concerns regarding potential adverse effects of LDA and medicalization of the pregnancy were frequently expressed as important reasons for not using LDA. Interestingly, these proportions did not differ greatly between women with high PE risk estimates and women with a history of PE.
4. DISCUSSION
Our prediction tool identified 306 women (43%) with an increased PE risk. The majority of these women (n = 241; 79%) reported that their healthcare professional discussed the option of LDA prophylaxis with them, suggesting adequate adherence of healthcare professionals to the risk‐based care recommendations. Usage rates of LDA increased as compared with care‐as‐usual (29.4% vs 1.5%, odds ratio 19.1, 95% CI 11.2‐32.5). Daily aspirin usage was positively correlated with both predicted risk and the degree of women’s concerns regarding PE. Most reported reasons for non‐ or incomplete use were unawareness of LDA as preventive intervention, concerns about potential adverse effects and doubts regarding the benefits.
This is a large observational study to investigate LDA usage rates by women with an increased PE risk, as well as determinants and reasons given for use and non‐use. Another strength is the multicenter study design. Combined with the broad inclusion criteria, this should have ensured an unselected population as possible. Nevertheless, women of Caucasian origin in our cohort are overrepresented and the majority of women are well educated. Since impaired health literacy is correlated with nonadherence,21 usage rates in our study may be somewhat overestimated.
Another potential limitation in this paper is that LDA usage was based upon self‐report. We were unable to verify reliably LDA usage with medical records or pharmacy registries because LDA is available over‐the‐counter in the Netherlands. However, there is no clear gold standard available to assess medication use in large‐scale studies.22 It could be possible that women answered in a socially acceptable manner, resulting in an overestimation of the usage rate.23 On the other hand, in risk‐dependent care, counseling of LDA took the form of a shared decisional process. Usage of medication during pregnancy is not generally perceived as “good” or “bad”, since women are aware medication may cause adverse effects but could be beneficial for their health as well.21, 24 Moreover, women were informed that survey results would be processed anonymously and would not be shared with their healthcare professional. The researchers who distributed the web‐based surveys were not involved in the care of participants. Therefore, the potential overestimation with respect to the adherence rate due to self‐report is probably limited.
Besides socially acceptable answers, self‐report of medication usage is also prone to recall biases. However, women reporting non‐usage are likely to be telling the truth.23 Furthermore, underreporting for pregnancy‐related medications as well as medication prescribed for a longer period is limited in prospective studies.25
Women’s adherence regarding medication during pregnancy has been studied for several drugs, such as anti‐diabetics, medicines for chronic airway conditions and anti‐inflammatory drugs, with varying adherence rates from 40% to 80%.21, 24 However, these drugs are prescribed because of an apparent (chronic) medical condition such as diabetes, asthma or inflammatory bowel disease. Therefore, these situations likely differ compared with LDA, which is recommended to prevent PE. Most women with an increased PE risk do not have any medical complaints warranting LDA usage, which probably leads to different risk‐benefit evaluations.
Studies of pregnant women’s adherence regarding LDA in particular are limited and mostly result from clinical trials.2, 6 These trials indicate high adherence rates (66%‐90%). However, trial‐based adherence rates may be seriously biased upwards, as women who do not want to use any drugs (ie, LDA), are unlikely to be willing to participate in such a trial. We found one observational study indicating a lower adherence rate (54%) as well, but within a small cohort (n = 42) and restricted to women with high‐risk pregnancies.7 Another observational study, conducted among high‐risk women in Iran, did not provide absolute adherence rates.26 Compared with these reports, the rate of LDA usage of 25% in our cohort is low but is probably a more realistic estimation of LDA usage in daily practice.
Most guidelines recommend LDA prophylaxis to women with an increased PE risk, but there is no consensus yet as to how to identify women with an increased PE risk.8, 9, 27 In our study, an externally validated prediction model was used to estimate women’s PE risk during the first antenatal visits. Since the risk assessment was used as starting point of the shared decisional process regarding LDA usage, a risk threshold with a relatively high detection rate was used.17 As a result, women identified with an increased PE risk in our study may have had a lower PE risk on average compared with other studies. This may have contributed to the lower usage rate. Furthermore, LDA usage was strongly correlated with the predicted PE risk, resulting in high usage rates among women with the highest risks, similar to the rates previously reported.
Despite the lower usage rates in general, LDA usage still improved strongly with an absolute increase of 27.9%. However, during enrollment of the care‐as‐usual cohort (2013‐2015) there was no uniform Dutch guideline recommending LDA prophylaxis, although many obstetric healthcare professionals were familiar with the NICE guideline for hypertensive disorders,27 especially gynecologists, LDA recommendation depended mainly on the decision of individual healthcare professionals. As a result, the increase of LDA usage may mainly reflect adequate implementation of risk‐based‐care and uptake of its recommendations by healthcare professionals.
To the best of our knowledge, no studies have yet reported on determinants of LDA usage or women’s reasons for non‐usage of LDA in particular. In the unadjusted analysis, the LDA usage rate was associated with the type of healthcare professional responsible for LDA counseling. However, in the Dutch maternity care system, low‐risk women remain primarily under the supervision of autonomous midwives. As a result, women’s risk should be taken into account. Indeed, when correcting for PE risk at baseline, this effect was no longer apparent. The degree of concern about possible complications related to PE (SGA infancy and PTB) was not significantly linked to the usage rate in the adjusted analysis. However, women may be unaware that PE may result into SGA infancy or (iatrogenic) PTB.
The adjusted analysis also indicates that both the estimated PE risk and the level of concern regarding PE are positively correlated with LDA usage. This is in line with previous research which suggests that women’s beliefs about medication and its effectiveness are a crucial factor in determining their adherence.21, 24 This also fits with our finding that the most frequent reasons of non‐use were concerns regarding potential adverse effects of LDA and doubts regarding the potential benefits resulting from LDA prophylaxis. Moreover, the finding that most women who started using LDA, used it according to protocol, suggests those women made their choice consciously.
Informing women about the low prevalence of effects of LDA, which are also mild,28, 29, 30 may be a central factor to improve adherence rates. Furthermore, a substantial proportion of women stating that LDA was not applicable to their situation, reported that LDA had not been discussed with them. With our data, it is not possible to distinguish whether LDA was not discussed by the healthcare professional or whether these women could not recall that LDA was discussed. Clear communication of PE risk and adequate counseling regarding potential benefits and harms of LDA may positively influence women’s decision regarding LDA usage during pregnancy. Future qualitative research, for example with the aid of focus groups among both healthcare professionals as well as pregnant women, may improve our insight and understanding regarding the key elements at play in the decisional process regarding preventive LDA usage.
5. CONCLUSION
Implementation of risk‐based care improved LDA usage by pregnant women with an increased PE risk, especially among high‐risk women. Nevertheless, general usage rates were relatively low. To improve LDA usage rates, more insight into this decisional process is necessary, underlining the importance of future (qualitative) research regarding preventive LDA usage by pregnant women.
CONFLICT OF INTEREST
None.
Supporting information
van Montfort P, Scheepers HCJ, van Dooren IMA, et al. Low‐dose‐aspirin usage among women with an increased preeclampsia risk: A prospective cohort study. Acta Obstet Gynecol Scand. 2020;99:875–883. 10.1111/aogs.13808
Funding information
ZonMw (The Netherlands Organization for Health Research and Development; federal funding) grant numbers 209020007 and 505200098150.
REFERENCES
- 1. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre‐eclampsia. Lancet. 2016;387:999‐1011. [DOI] [PubMed] [Google Scholar]
- 2. Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218:287‐293.e1. [DOI] [PubMed] [Google Scholar]
- 3. Hofmeyr GJ, Lawrie TA, Atallah AN, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2018;(10):CD001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allen R, Rogozinska E, Sivarajasingam P, Khan KS, Thangaratinam S. Effect of diet‐ and lifestyle‐based metabolic risk‐modifying interventions on preeclampsia: a meta‐analysis. Acta Obstet Gynecol Scand. 2014;93:973‐985. [DOI] [PubMed] [Google Scholar]
- 5. Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta‐analysis. Am J Obstet Gynecol. 2017;216:110‐120.e6. [DOI] [PubMed] [Google Scholar]
- 6. Navaratnam K, Alfirevic Z, Pirmohamed M, Alfirevic A. How important is aspirin adherence when evaluating effectiveness of low‐dose aspirin? Eur J Obstet Gynecol Reprod Biol. 2017;219:1‐9. [DOI] [PubMed] [Google Scholar]
- 7. Abheiden CN, van Reuler AV, Fuijkschot WW, de Vries JI, Thijs A, de Boer MA. Aspirin adherence during high‐risk pregnancies, a questionnaire study. Pregnancy Hypertens. 2016;6:350‐355. [DOI] [PubMed] [Google Scholar]
- 8. Hypertension in pregnancy . Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122‐1131. [DOI] [PubMed] [Google Scholar]
- 9. LeFevre ML. Low‐dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:819‐826. [DOI] [PubMed] [Google Scholar]
- 10. National Collaborating Centre for Women’s Children’ Health . National Institute for Health and Clinical Excellence: Guidance. Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. London: RCOG Press; 2010. [PubMed] [Google Scholar]
- 11. Meertens L, Scheepers H, van Kuijk S, et al. External validation and clinical usefulness of first trimester prediction models for the risk of preeclampsia: a prospective cohort study. Fetal Diagn Ther. 2019;45:381‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wright D, Syngelaki A, Akolekar R, Poon LC, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal characteristics and medical history. Am J Obstet Gynecol. 2015;213:62.e1‐62.e10. [DOI] [PubMed] [Google Scholar]
- 13. van Montfort P, Willemse JPPM, Dirksen CD, et al. Implementation and effects of risk‐dependent obstetric care in the Netherlands (Expect Study II): protocol for an impact study. JMIR Res Protoc. 2018;7:e10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200‐209. [DOI] [PubMed] [Google Scholar]
- 15. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Syngelaki A, Bredaki FE, Vaikousi E, Maiz N, Nicolaides KH. Body mass index at 11–13 weeks’ gestation and pregnancy complications. Fetal Diagn Ther. 2011;30:250‐265. [DOI] [PubMed] [Google Scholar]
- 17. van Montfort P, Smits LJM, van Dooren IMA, et al. Implementing a preeclampsia prediction model in obstetrics: cutoff determination and health care professionals’ adherence. Med Decis Making. 2019. 10.1177/0272989X19889890 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meertens LJE, Scheepers HCJ, De Vries RG, et al. External validation study of first trimester obstetric prediction models (Expect Study I): Research protocol and population characteristics. JMIR Res Protoc. 2017;6:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cleveland WS. LOWESS: a program for smoothing scatterplots by robust locally weighted regression. Am Stat. 1981;35(1):54. [Google Scholar]
- 20. R Core Team . R: A Language and Environment for Statistical Computing. 3.6.0 edn. Vienna: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 21. Lupattelli A, Spigset O, Nordeng H. Adherence to medication for chronic disorders during pregnancy: results from a multinational study. Int J Clin Pharm. 2014;36:145‐153. [DOI] [PubMed] [Google Scholar]
- 22. van Gelder MMHJ, de Jong LAA, te Winkel B, et al. Assessment of medication use during pregnancy by Web‐based questionnaires, pharmacy records and serum screening. Reprod Toxicol. 2019;84:93‐97. [DOI] [PubMed] [Google Scholar]
- 23. George J. Patient deviations from treatment recommendations: does it matter what we call it? Int J Pharm Pract. 2010;18:3‐5. [DOI] [PubMed] [Google Scholar]
- 24. Sawicki E, Stewart K, Wong S, Leung L, Paul E, George J. Medication use for chronic health conditions by pregnant women attending an Australian maternity hospital. Aust N Z J Obstet Gynaecol. 2011;51:333‐338. [DOI] [PubMed] [Google Scholar]
- 25. van Gelder M, Vorstenbosch S, Te Winkel B, van Puijenbroek EP, Roeleveld N. Using web‐based questionnaires to assess medication use during pregnancy: a validation study in 2 prospectively enrolled cohorts. Am J Epidemiol. 2018;187:326‐336. [DOI] [PubMed] [Google Scholar]
- 26. Lin C‐Y, Broström A, Nilsen P, Pakpour AH. Using extended theory of planned behavior to understand aspirin adherence in pregnant women. Pregnancy Hypertens. 2018;12:84‐89. [DOI] [PubMed] [Google Scholar]
- 27. National Institute for Health and Clinical Excellence . The Management of Hypertensive Disorders During Pregnancy. London: RCOG Press; 2010. [PubMed] [Google Scholar]
- 28. Nørgård B, Puhó E, Czeizel AE, Skriver MV, Sørensen HT. Aspirin use during early pregnancy and the risk of congenital abnormalities: a population‐based case‐control study. Am J Obstet Gynecol. 2005;192:922‐923. [DOI] [PubMed] [Google Scholar]
- 29. Roberge S, Bujold E, Nicolaides KH. Meta‐analysis on the effect of aspirin use for prevention of preeclampsia on placental abruption and antepartum hemorrhage. Am J Obstet Gynecol. 2018;218:483‐489. [DOI] [PubMed] [Google Scholar]
- 30. Ahrens KA, Silver RM, Mumford SL, et al. Complications and safety of preconception low‐dose aspirin among women with prior pregnancy losses. Obstet Gynecol. 2016;127:689‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


