Abstract
Aims
In CARMELINA®, linagliptin demonstrated cardiovascular and renal safety in patients with type 2 diabetes (T2D) with high renal and cardiovascular disease (CVD) risk. We investigated safety and efficacy of this dipeptidyl peptidase‐4 inhibitor in older participants.
Materials and methods
Subjects aged ≥18 years with T2D and established CVD with urinary albumin‐to‐creatinine ratio (UACR) >30 mg/g, and/or prevalent kidney disease, were randomized to linagliptin or placebo added to usual care. The primary endpoint (time to first occurrence of 3P‐MACE: cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke) and other outcomes were evaluated across age groups <65 (n = 2968), 65 to <75 (n = 2800) and ≥75 years (n = 1211).
Results
Mean age was 65.9 years (17.4% and 5.9% aged ≥75 and 80, respectively) and median follow‐up was 2.2 years. The hazard ratio (HR) for 3P‐MACE with linagliptin versus placebo was 1.02 [95% confidence interval (CI) 0.89, 1.17] with no significant interaction between age and treatment effect (P = 0.0937). HRs for participants aged <65, 65 to <75 and ≥75 years were 1.11 (95% CI 0.89, 1.40), 1.09 (0.89, 1.33) and 0.76 (0.57, 1.02), respectively. Linagliptin did not increase the risk of adverse kidney outcomes or hospitalization for heart failure across age groups. The incidence of adverse events, including hypoglycaemia, increased with age but was similar with linagliptin and placebo despite glycated haemoglobin A1c reduction with linagliptin.
Conclusions
Linagliptin did not increase risk for cardiovascular events or hypoglycaemia and kidney function remained stable in older people with T2D and established CVD with albuminuria and/or kidney disease.
1. INTRODUCTION
Population ageing over recent decades has dramatically shifted the epidemiology of diabetes towards older age. Currently, approximately 123 million of the estimated 425 million people with diabetes globally are ≥65 years,1 and >25% of people aged >65 years have diabetes in the United States.2 However, despite the high prevalence in the elderly, older patients have historically been under‐represented in clinical trials of glucose‐lowering drugs, in particular, individuals aged >75 years. An analysis in 2013 found that only 0.6% of interventional trials in diabetes had specifically targeted patients >65 years, while 31% had actively excluded them. Furthermore, most trials had excluded individuals >75 years.3
This paucity of data poses a challenge for evidence‐based treatment of type 2 diabetes (T2D) in the older patient population, in which there is a high prevalence of comorbidities, frailty and polypharmacy. The choice of glucose‐lowering drugs, in particular, is complicated by the common comorbidities of cardiovascular disease (CVD), heart failure and, especially, chronic kidney disease with the added need to avoid hypoglycaemia. Impaired kidney function increases the risk of hypoglycaemia and necessitates dosage adjustment or avoidance of most glucose‐lowering drugs.4, 5 Despite the limited evidence base, various individuals and organizations have developed consensus‐ or opinion‐based guidelines for diabetes care of older patients.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Their recommendations generally emphasize safety as a prime consideration for glucose‐lowering treatment of older patients. In particular, one needs to avoid hypoglycaemia, and thus glycaemic targets often are less stringent in this population.
Dipeptidyl peptidase‐4 (DPP‐4) inhibitors are a modern class of glucose‐lowering drugs that are considered well tolerated. Linagliptin is the only globally available DPP‐4 inhibitor that is not primarily excreted by the kidneys and hence does not require dosage adjustment for patients with kidney disease.20, 21 Linagliptin demonstrated glycaemic efficacy and tolerability in people aged ≥70 years in a dedicated phase III randomized clinical trial,22 as well as in a pooled analysis of participants aged ≥65 years from this study and six other phase III trials in individuals aged 18–80 years.23 However, the long‐term cardiovascular and renal safety of linagliptin in older patients has not been investigated previously.
In the recent CARMELINA® cardiovascular safety study in people with T2D who had established CVD and albuminuria, and/or kidney disease, linagliptin did not increase the risk of cardiorenal events.24, 25 CARMELINA® enrolled people aged at least 18 years with no maximum age restriction. We report here a prespecified subgroup analysis evaluating clinical outcomes and adverse events according to participant age at baseline, including those aged over 65, 75 or 80 years.
2. MATERIALS AND METHODS
2.1. Study design
The study design and main results of CARMELINA® have been described previously.24, 25, 26 In brief, CARMELINA® was a cardiovascular event‐driven, placebo‐controlled, double‐blind, randomized clinical trial conducted between August 2013 and January 2018 in 27 countries across Asia, Europe, Latin America and North America (ClinicalTrials.gov, number NCT01897532). The trial was approved by local authorities at each study site, and was conducted under the auspices of the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonisation.27, 28 All patients provided written informed consent at initial screening.
Individuals with T2D were eligible to participate if they were ≥18 years at screening, had a glycated haemoglobin A1c (HbA1c) level between 6.5% and 10.0% inclusive, and body mass index (BMI) ≤45 kg/m2. Participants also had to have established CVD (previous myocardial infarction or stroke, and/or current coronary, carotid or peripheral artery disease) together with a urinary albumin‐to‐creatinine ratio (UACR) >30 mg/g, and/or impaired kidney function [estimated glomerular filtration rate (eGFR) 15 to <45 mL/min/1.73 m2 or eGFR ≥45 to 75 mL/min/1.73 m2 with UACR >200 mg/g]. Both drug‐naïve patients and those on glucose‐lowering pharmacotherapies were eligible, unless the latter were taking DPP‐4 inhibitors, glucagon‐like peptide‐1 (GLP‐1) receptor agonists and/or sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors.
Participants were randomized to receive once‐daily oral treatment with linagliptin 5 mg (the licensed dose) or placebo until at least 611 subjects had had a primary endpoint event. Study investigators, blinded to study drug allocation, were instructed to treat their patients according to local standards of care, including optimizing glycaemic control using additional glucose‐lowering medications, if needed.
The primary endpoint was the first occurrence of cardiovascular death, non‐fatal myocardial infarction, or non‐fatal stroke: the three‐point major adverse cardiovascular events (3P‐MACE) composite outcome. The key secondary endpoint was a composite kidney outcome comprising death because of kidney disease, progression to end‐stage kidney disease, or sustained decrease in eGFR from baseline of ≥40%. Progression of albuminuria was also assessed: change from normoalbuminuria (UACR <30 mg/g) to microalbuminuria (UACR 30–300 mg/g) or macroalbuminuria (UACR >300 mg/g), or from microalbuminuria to macroalbuminuria. Other prespecified clinical endpoints included hospitalization for heart failure. Centralized independent clinical events committees blinded to treatment assignment adjudicated all reported cardiovascular and kidney events. Metabolic outcomes included change in HbA1c from baseline. Adverse events reported by investigators were categorized using the Medical Dictionary for Regulatory Activities29 version 20.1.
2.2. Subgroup analysis by baseline age of participants
This was a prespecified subgroup analysis of outcomes in CARMELINA® participants based on their age at screening. Predefined age group categories were <65, 65 to <75, and ≥75 years, with the latter group split post hoc into those aged 75 to <80 and ≥80 years for some assessments. Clinical outcomes and adverse events were evaluated for all participants randomized and treated with at least one dose of study drug. Differences between treatment groups in clinical outcomes were assessed using Cox proportional hazards models with terms for treatment group, geographic region, age category, and interaction between treatment group and age category; the model for hospitalization for heart failure also included an additional term for history of heart failure. Sensitivity analyses of clinical outcomes were conducted for events occurring only during the time patients were receiving study drug. Changes from baseline in HbA1c, body weight, blood pressure and lipids were evaluated post hoc using mixed models for repeated measures that included terms for treatment, geographical region, baseline value, week, subgroup, treatment‐by‐week interaction, treatment‐by‐subgroup interaction, week‐by‐subgroup interaction, treatment‐by‐week‐by‐subgroup interaction and baseline value‐by‐week interaction. The proportion of participants initiating new glucose‐lowering medications was analysed post hoc using a Cox regression model with terms for treatment group, region, age category, and interaction between treatment group and age category. Adverse events were summarized with descriptive statistics.
3. RESULTS
3.1. Study participants, drug exposure and duration
At the start of CARMELINA®, the 6979 randomized participants who subsequently received treatment with the study drug were aged 27–92 years (mean: 65.9 years). In total, 2968 participants (42.5%) were aged <65 years, while 2800 (40.1%) were 65 to <75 years, 1211 (17.4%) were ≥75 years and 412 (5.9%) were ≥80 years. The demographic and clinical characteristics of participants in these age groups are shown in Table 1. Overall, most participants were white (~80%), obese (mean BMI 30–32 kg/m2) and moderately hyperglycaemic (mean HbA1c 7.7%–8.1%). With increasing age, there was an increase in the duration of diabetes as well as the prevalence of females, non‐smokers, North Americans, reduced kidney function and atrial fibrillation. The number of participants taking metformin decreased with increasing age: 63.1%, 52.9% and 42.0% of those aged <65, 65 to <75 and ≥75 years, respectively; however, the use of insulin by the majority of participants was relatively constant across age groups: 57.5%, 59.1% and 56.9%, respectively. Other characteristics were generally similar across age groups. Within each age group, baseline characteristics were mostly comparable between the linagliptin and placebo groups.
Table 1.
Baseline demographic and clinical characteristics of patients by age
| Age <65 years | Age 65 to <75 years | Age ≥75 years | ||||
|---|---|---|---|---|---|---|
| Linagliptin (n = 1467) | Placebo (n = 1501) | Linagliptin (n = 1405) | Placebo (n = 1395) | Linagliptin (n = 622) | Placebo (n = 589) | |
| Age, years | 57.6 ± 5.67 | 57.3 ± 5.73 | 69.2 ± 2.8 | 69.1 ± 2.9 | 78.9 ± 3.4 | 78.8 ± 3.5 |
| Sex, n (%) | ||||||
| Male | 973 (66.3) | 1005 (67.0) | 838 (59.6) | 901 (64.6) | 337 (54.2) | 336 (57.0) |
| Female | 494 (33.7) | 496 (33.0) | 567 (40.4) | 494 (35.4) | 285 (45.8) | 253 (43.0) |
| Race, n (%) | ||||||
| White | 1145 (78.1) | 1157 (77.1) | 1159 (82.5) | 1147 (82.2) | 523 (84.1) | 465 (78.9) |
| Asian | 146 (10.0) | 149 (9.9) | 112 (8.0) | 118 (8.5) | 49 (7.9) | 66 (11.2) |
| Black or African‐American | 98 (6.7) | 104 (6.9) | 73 (5.2) | 82 (5.9) | 23 (3.7) | 31 (5.3) |
| American Indian or Alaska Native | 77 (5.2) | 87 (5.8) | 57 (4.1) | 44 (3.2) | 25 (4.0) | 25 (4.2) |
| Native Hawaiian or Other Pacific Islander | 1 (0.1) | 4 (0.3) | 4 (0.3) | 4 (0.3) | 2 (0.3) | 2 (0.3) |
| Region, n (%) | ||||||
| Europe | 592 (40.4) | 585 (39.0) | 625 (44.5) | 637 (45.7) | 256 (41.2) | 239 (40.6) |
| Latin America | 557 (38.0) | 597 (39.8) | 431 (30.7) | 404 (29.0) | 168 (27.0) | 153 (26.0) |
| North America | 187 (12.7) | 185 (12.3) | 251 (17.9) | 260 (18.6) | 155 (24.9) | 142 (24.1) |
| Asia | 131 (8.9) | 134 (8.9) | 98 (7.0) | 94 (6.7) | 43 (6.9) | 55 (9.3) |
| Smoking status, n (%) | ||||||
| Never smoker | 753 (51.3) | 775 (51.6) | 781 (55.6) | 742 (53.2) | 363 (58.4) | 339 (57.6) |
| Ex‐smoker | 483 (32.9) | 509 (33.9) | 517 (36.8) | 539 (38.6) | 231 (37.1) | 228 (38.7) |
| Current smoker | 230 (15.7) | 215 (14.3) | 104 (7.4) | 113 (8.1) | 28 (4.5) | 22 (3.7) |
| Missing | 1 (0.1) | 2 (0.1) | 3 (0.2) | 1 (0.1) | 0 | 0 |
| History of heart failure, n (%) | 387 (26.4) | 392 (26.1) | 390 (27.8) | 378 (27.1) | 175 (28.1) | 151 (25.6) |
| Ischaemic heart disease, n (%) | 823 (56.1) | 884 (58.9) | 852 (60.6) | 847 (60.7) | 354 (56.9) | 321 (54.5) |
| History of hypertension, n (%) | 1317 (89.8) | 1348 (89.8) | 1276 (90.8) | 1288 (92.3) | 578 (92.9) | 542 (92.0) |
| Atrial fibrillation, n (%) | 73 (5.0) | 81 (5.4) | 153 (10.9) | 164 (11.8) | 93 (15.0) | 109 (18.5) |
| eGFR (MDRD), mL/min/1.73 m2 | 61.8 ± 28.5 | 61.8 ± 28.0 | 51.4 ± 21.7 | 51.1 ± 21.7 | 45.3 ± 18.1 | 44.2 ± 17.2 |
| eGFR (MDRD), mL/min/1.73 m2, n (%) | ||||||
| ≥90 | 271 (18.5) | 276 (18.4) | 75 (5.3) | 80 (5.7) | 17 (2.7) | 9 (1.5) |
| ≥60 | 720 (49.1) | 772 (51.4) | 455 (32.4) | 462 (33.1) | 119 (19.1) | 103 (17.5) |
| ≥45 to <60 | 253 (17.2) | 237 (15.8) | 299 (21.3) | 297 (21.3) | 138 (22.2) | 124 (21.1) |
| ≥30 to <45 | 298 (20.3) | 273 (18.2) | 439 (31.2) | 419 (30.0) | 257 (41.3) | 252 (42.8) |
| <30 | 196 (13.4) | 219 (14.6) | 212 (15.1) | 217 (15.6) | 108 (17.4) | 110 (18.7) |
| UACR, mg/g, median (IQR) | 227 (58–1058) | 231 (62–950) | 139 (39–617) | 155 (42–719) | 104 (27–405) | 96.4 (23–349) |
| <30, n (%) | 233 (15.9) | 231 (15.4) | 299 (21.3) | 290 (20.8) | 164 (26.4) | 175 (29.7) |
| 30–300, n (%) | 581 (39.6) | 596 (39.7) | 604 (43.0) | 587 (42.1) | 278 (44.7) | 248 (42.1) |
| >300, n (%) | 652 (44.4) | 673 (44.8) | 502 (35.7) | 518 (37.1) | 179 (28.8) | 166 (28.2) |
| Missing, n (%) | 1 (0.1) | 1 (0.1) | 0 | 0 | 1 (0.2) | 0 |
| BMI,a kg/m2 | 31.6 ± 5.4 | 31.7 ± 5.5 | 31.2 ± 5.4 | 31.4 ± 5.3 | 30.4 ± 4.7 | 30.2 ± 5.0 |
| HbA1c, % | 8.0 ± 1.0 | 8.1 ± 1.0 | 7.9 ± 1.0 | 7.9 ± 1.0 | 7.8 ± 0.9 | 7.7 ± 1.0 |
| Fasting plasma glucose,b mg/dL | 154.1 ± 47.1 | 154.8 ± 47.5 | 150.5 ± 45.6 | 150.1 ± 45.2 | 145.7 ± 43.2 | 144.6 ± 43.0 |
| Diabetes duration, years | 12.8 ± 8.4 | 12.5 ± 8.2 | 15.8 ± 9.3 | 15.7 ± 9.3 | 18.3 ± 11.6 | 17.0 ± 10.5 |
| Systolic blood pressure, mmHg | 139.5 ± 17.4 | 139.7 ± 17.9 | 141.2 ± 18.4 | 141.0 ± 18.0 | 140.7 ± 16.9 | 142.0 ± 18.4 |
| Diastolic blood pressure, mmHg | 80.3 ± 10.0 | 80.4 ± 9.9 | 76.9 ± 10.3 | 76.8 ± 10.1 | 73.8 ± 10.6 | 74.1 ± 10.9 |
| Heart rate,c beats per min | 71.9 ± 12.4 | 71.5 ± 11.9 | 68.8 ± 11.9 | 68.8 ± 12.6 | 67.2 ± 11.4 | 67.7 ± 12.2 |
| Total cholesterol,d mg/dL | 179.4 ± 52.9 | 176.6 ± 51.0 | 169.2 ± 46.7 | 168.1 ± 44.8 | 164.3 ± 43.2 | 164.0 ± 42.1 |
| LDL cholesterol,e mg/dL | 96.5 ± 43.3 | 94.3 ± 42.2 | 89.7 ± 38.9 | 88.6 ± 36.5 | 84.8 ± 35.1 | 85.5 ± 33.4 |
| HDL cholesterol,f mg/dL | 43.7 ± 13.5 | 43.5 ± 12.6 | 44.8 ± 12.4 | 44.7 ± 12.7 | 46.4 ± 13.0 | 46.3 ± 13.7 |
| Triglycerides,g mg/dL | 209.3 ± 160.7 | 205.2 ± 162.1 | 178.8 ± 122.0 | 177.8 ± 100.3 | 167.4 ± 88.0 | 161.8 ± 88.8 |
| Glucose‐lowering medication, n (%) | ||||||
| Metformin | 893 (60.9) | 925 (61.6) | 732 (52.1) | 749 (53.7) | 256 (41.2) | 253 (43.0) |
| Sulphonylurea | 470 (32.0) | 496 (33.0) | 430 (30.6) | 460 (33.0) | 202 (32.5) | 184 (31.2) |
| Insulin | 869 (59.2) | 838 (55.8) | 832 (59.2) | 823 (59.0) | 355 (57.1) | 334 (56.7) |
| GLP‐1 receptor agonisth | 0 | 0 | 0 | 0 | 0 | 0 |
| SGLT2 inhibitorh | 0 | 0 | 0 | 0 | 0 | 0 |
| Antihypertensive medication, n (%) | 1392 (94.9) | 1430 (95.3) | 1345 (95.7) | 1360 (97.5) | 600 (96.5) | 564 (95.8) |
| ACE inhibitor or ARB | 1208 (82.3) | 1200 (79.9) | 1147 (81.6) | 1146 (82.2) | 505 (81.2) | 452 (76.7) |
| β‐blocker | 867 (59.1) | 856 (57.0) | 839 (59.7) | 862 (61.8) | 374 (60.1) | 355 (60.3) |
| Diuretic | 712 (48.5) | 768 (51.2) | 780 (55.5) | 806 (57.8) | 400 (64.3) | 362 (61.5) |
| Calcium antagonist | 553 (37.7) | 583 (38.8) | 613 (43.6) | 609 (43.7) | 267 (42.9) | 254 (43.1) |
| Aspirin, n (%) | 917 (62.5) | 946 (63.0) | 878 (62.5) | 891 (63.9) | 371 (59.6) | 341 (57.9) |
| Statins, n (%) | 1030 (70.2) | 1066 (71.0) | 1001 (71.2) | 1035 (74.2) | 464 (74.6) | 422 (71.6) |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide 1; HbA1c, glycated haemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MDRD, Modification of Diet in Renal Disease study equation; SGLT2, sodium‐glucose co‐transporter‐2; UACR, urinary albumin‐to‐creatinine ratio.
Note: Data are mean ± SD unless otherwise stated.
BMI data missing for one patient aged <65 years (placebo), one aged 65 to <75 (placebo) and one aged ≥75 (placebo).
Fasting plasma glucose data missing for 34 patients aged <65 years (21 linagliptin, 13 placebo), 34 aged 65 to <75 (18 linagliptin, 16 placebo) and 11 aged ≥75 (five linagliptin, six placebo).
Heart rate data missing for 208 patients aged <65 years (91 linagliptin, 117 placebo), 221 aged 65 to <75 (109 linagliptin, 112 placebo) and 87 aged ≥75 (46 linagliptin, 41 placebo).
Total cholesterol data missing for 105 patients aged <65 years (43 linagliptin, 62 placebo), 100 aged 65 to <75 (54 linagliptin, 46 placebo) and 83 aged ≥75 (47 linagliptin, 36 placebo).
LDL cholesterol data missing for 109 patients aged <65 years (47 linagliptin, 62 placebo), 101 aged 65 to <75 (55 linagliptin, 46 placebo) and 34 aged ≥75 (18 linagliptin, 16 placebo).
HDL cholesterol data missing for 106 patients aged <65 years (44 linagliptin, 62 placebo), 100 aged 65 to <75 (54 linagliptin, 46 placebo) and 34 aged ≥75 (18 linagliptin, 16 placebo).
Triglyceride data missing for 105 patients aged <65 years (43 linagliptin, 62 placebo), 100 aged 65 to <75 (54 linagliptin, 46 placebo) and 34 aged ≥75 (18 linagliptin, 16 placebo).
Patients already taking a GLP‐1 receptor agonist or SGLT2 inhibitor were excluded from the study by design.
As previously reported,24 the median duration of treatment was 1.9 years in both the linagliptin and placebo groups overall, while the median observation time (including treatment) was 2.2 years in both groups. Within each age group (<65, 65 to <75, ≥75 years), the median treatment and observation times were very similar to these overall values (Table S1; see Supporting Information).
3.2. Cardiovascular and kidney outcomes
In the overall population, the primary endpoint (3P‐MACE) occurred in 434 participants (12.4%) in the linagliptin group and 420 (12.1%) in the placebo group; the hazard ratio (HR) for a first event with linagliptin compared with placebo was 1.02 [95% confidence interval (CI) 0.89, 1.17].24 There was no significant interaction between age and treatment effect (P = 0.0937 for interaction between treatment group and age category). In the age groups evaluated here, the HR for 3P‐MACE for linagliptin compared with placebo was 1.11 (95% CI 0.89, 1.40) for participants aged <65 years, 1.09 (95% CI 0.89, 1.33) for those aged 65 to <75 years, and 0.76 (95% CI 0.57, 1.02) for those aged ≥75 years (Figure 1).
Figure 1.
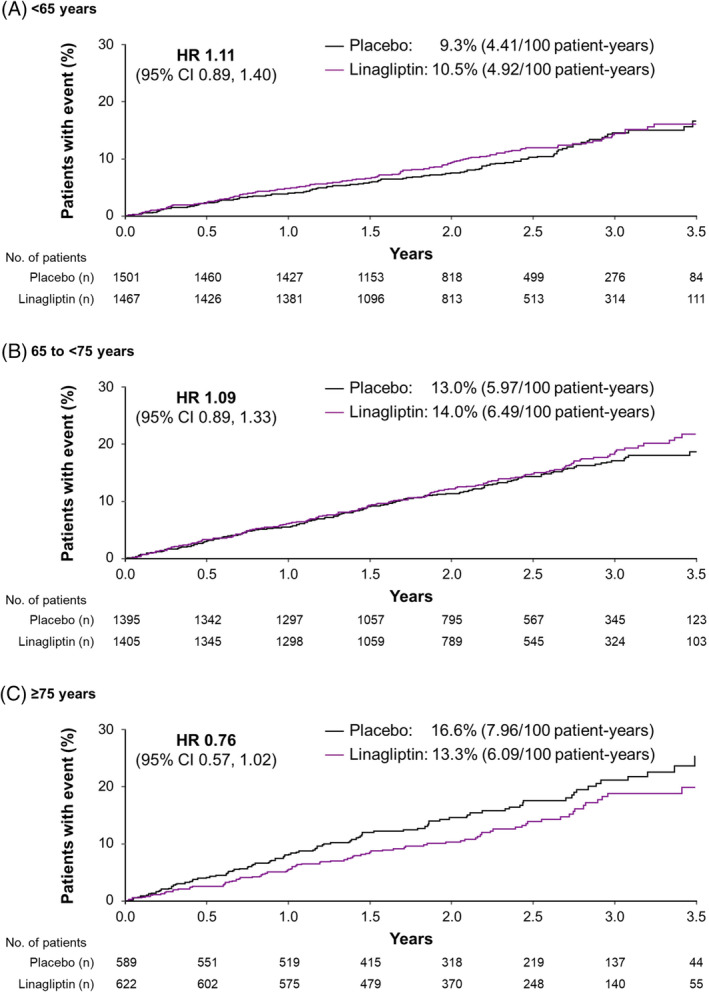
Time to first occurrence of 3P‐MACE by age. CI, confidence interval; HR, hazard ratio; 3P‐MACE, three‐point major adverse cardiovascular event (cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke)
Overall, hospitalization for heart failure occurred in 209 of 3494 linagliptin‐treated participants (6.0%) and 226 of 3485 (6.5%) placebo‐treated participants (HR 0.90; 95% CI 0.74, 1.08).24 There was no significant interaction between age and treatment effect (P = 0.9788), with HRs for linagliptin versus placebo 0.87 (95% CI 0.63, 1.21), 0.89 (95% CI 0.67, 1.18) and 0.92 (95% CI 0.63, 1.35) in participants aged <65, 65 to <75 and ≥75 years, respectively (Figure 2).
Figure 2.
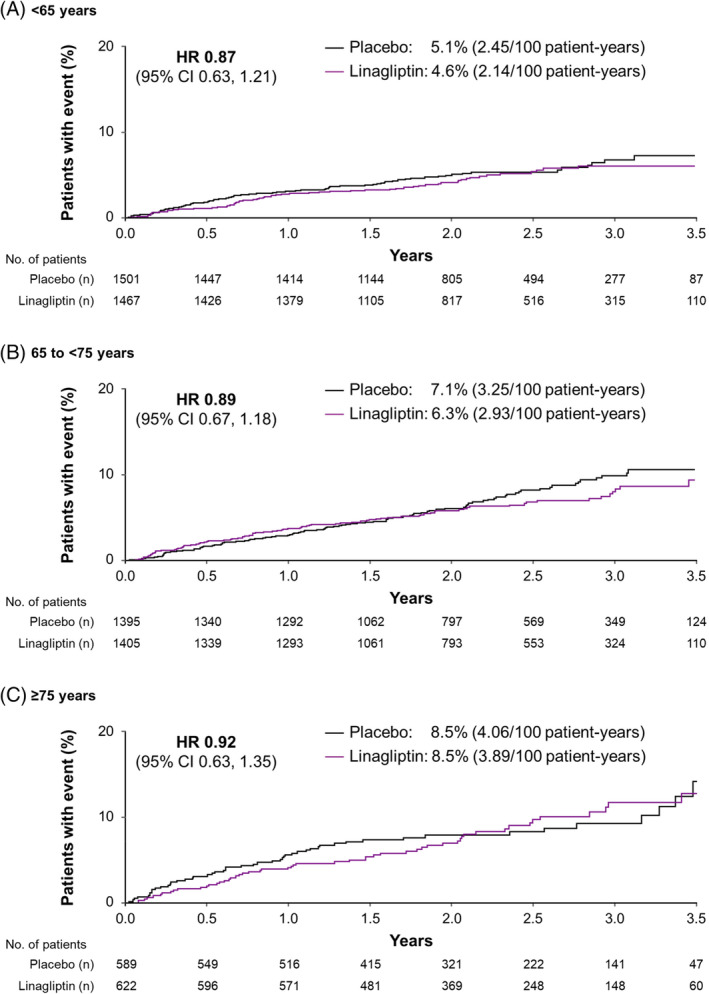
Time to first occurrence of hospitalization for heart failure by age. CI, confidence interval; HR, hazard ratio
The time to first occurrence of 3P‐MACE, its component endpoints and hospitalization for heart failure across these age groups are summarized in Figure S1 (see Supporting Information).
The key secondary endpoint (death because of kidney disease, progression to end‐stage kidney disease, or a sustained decrease in eGFR from baseline of ≥40%) occurred in 9.4% and 8.8% of linagliptin‐ and placebo‐treated participants, respectively (HR 1.04; 95% CI 0.89, 1.22).24 As for 3P‐MACE and hospitalization for heart failure, the treatment effect was consistent across different age groups for the composite kidney outcome (P = 0.9968 for interaction between treatment group and age category). HRs for this kidney endpoint for linagliptin versus placebo were 1.05 (95% CI 0.85, 1.29), 1.06 (95% CI 0.81, 1.38) and 1.06 (95% CI 0.64, 1.75) in participants aged <65, 65 to <75, and ≥75 years, respectively (Figure 3).
Figure 3.
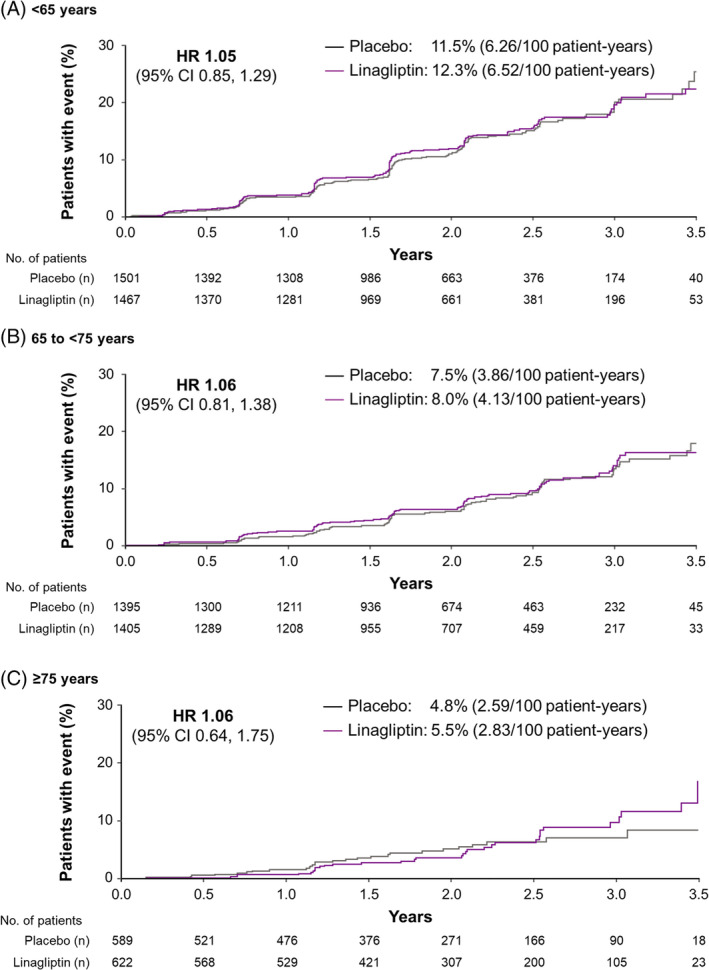
Time to first occurrence of the key secondary kidney composite endpoint† by age. CI, confidence interval; HR, hazard ratio. †Death because of kidney disease, progression to end‐stage kidney disease, or a sustained decrease in estimated glomerular filtration rate from baseline of ≥40%
The sensitivity analyses of cardiovascular and kidney events occurring only until permanent treatment discontinuation had very similar findings to the main analyses, with no significant interaction between age and the treatment effect of linagliptin, which was consistently neutral (Figure S2; see Supporting Information).
Evaluation of outcomes by use, or not, of metformin at baseline found no significant interaction, in general, between patient age and the treatment effect of linagliptin on 3P‐MACE or the composite kidney outcome. However, there was a nominally significant interaction with age for 3P‐MACE in patients not taking metformin (Figure S3; see Supporting Information).
Progression of albuminuria occurred in significantly fewer patients in the linagliptin group (35.3%) than in the placebo group (38.5%) (HR 0.86; 95% CI 0.78, 0.95).24 Once again, the treatment effect was consistent across different age groups for this outcome (P = 0.4318 for interaction between treatment group and age category). HRs for albuminuria progression for linagliptin versus placebo were 0.93 (95% CI 0.79, 1.09), 0.84 (95% CI 0.72, 0.98) and 0.78 (95% CI 0.63, 0.97) in participants aged <65, 65 to <75 and ≥75 years, respectively.
The HRs for the key secondary endpoint, death because of kidney disease or end‐stage kidney disease, and progression of albuminuria across age groups are summarized in Figure S4 (see Supporting Information).
Furthermore, in participants aged ≥75 years, there was also no increased risk for 3P‐MACE, hospitalization for heart failure, or the composite kidney outcome with linagliptin compared with placebo in individuals aged 75 to <80 or ≥80 years (Figure S5; see Supporting Information).
3.3. Glycaemia and cardiovascular risk factors
There was a significant mean reduction in HbA1c with linagliptin compared with placebo after 12 weeks of treatment: adjusted differences of −0.50% (95% CI –0.57, −0.44), −0.53% (95% CI –0.60, −0.46) and − 0.46% (95% CI –0.57, −0.36) in participants aged <65, 65 to <75 and ≥75 years, respectively (P < 0.0001 for all). This was at a time when the protocol indicated that diabetes management was to remain unchanged and only the study drugs were to be added. The weighted average difference over the full study duration showed a significant treatment effect in all age groups (−0.30% to −0.40%; P < 0.0001 for all) (Figure S6; see Supporting Information).
In each age category, fewer participants in the linagliptin group than in the placebo group required additional glucose‐lowering medications, including insulin; HRs were 0.71 (95% CI 0.62, 0.83), 0.78 (95% CI 0.67, 0.90) and 0.86 (95% CI 0.68, 1.07) for ages <65, 65 to <75 and ≥75 years, respectively (Figure S7; see Supporting Information).
There were no meaningful changes from baseline in body weight, blood pressure or lipids in any treatment group or age category during the study (data not shown), and the weighted average mean difference across the study between the linagliptin and placebo groups was not significant for any of these parameters in any age group (Table S2; see Supporting Information).
3.4. Adverse events
More older participants reported adverse events than those aged <65 years; however, within each age category, the percentage of the linagliptin group reporting adverse events was generally comparable with the placebo group (Table 2). Slightly fewer participants in the linagliptin group reported adverse events leading to discontinuation of study drug or serious adverse events, compared with the placebo group (Table 2).
Table 2.
Adverse events by age
| Age <65 years | Age 65 to <75 years | Age 75 to <80 years | Age ≥80 years | |||||
|---|---|---|---|---|---|---|---|---|
| n (%) of patients | Linagliptin (n = 1467) | Placebo (n = 1501) | Linagliptin (n = 1405) | Placebo (n = 1395) | Linagliptin (n = 402) | Placebo(n = 397) | Linagliptin(n = 220) | Placebo(n = 192) |
| Overall summary | ||||||||
| Any AE | 1087 (74.1) | 1142 (76.1) | 1093 (77.8) | 1084 (77.7) | 340 (84.6) | 333 (83.9) | 177 (80.5) | 164 (85.4) |
| Serious AE | 466 (31.8) | 518 (34.5) | 545 (38.8) | 548 (39.3) | 176 (43.8) | 178 (44.8) | 106 (48.2) | 99 (51.6) |
| AE leading to discontinuation | 108 (7.4) | 146 (9.7) | 164 (11.7) | 168 (12.0) | 52 (12.9) | 57 (14.4) | 35 (15.9) | 31 (16.1) |
| Selected AEs | ||||||||
| Hypoglycaemiaa | 403 (27.5) | 425 (28.3) | 429 (30.5) | 423 (30.3) | 141 (35.1) | 117 (29.5) | 63 (28.6) | 59 (30.7) |
| Plasma glucose <54 mg/dL or severeb | 210 (14.3) | 242 (16.1) | 233 (16.6) | 218 (15.6) | 76 (18.9) | 73 (18.4) | 38 (17.3) | 39 (20.3) |
| Severeb | 39 (2.7) | 37 (2.5) | 40 (2.8) | 45 (3.2) | 15 (3.7) | 14 (3.5) | 12 (5.5) | 12 (6.3) |
| Hypersensitivity reactionsc | 38 (2.6) | 39 (2.6) | 44 (3.1) | 42 (3.0) | 22 (5.5) | 20 (5.0) | 10 (4.5) | 8 (4.2) |
| With concomitant ACE inhibitor/ARB use at baseline | 28 (2.3d) | 32 (2.7d) | 33 (2.9d) | 35 (3.1d) | 17 (5.4d) | 13 (4.2d) | 9 (4.8d) | 5 (3.5d) |
| Cancere , f | 21 (1.4) | 37 (2.5) | 65 (4.6) | 59 (4.2) | 16 (4.0) | 23 (5.8) | 14 (6.4) | 15 (7.8) |
| Colon cancerg | 3 (0.2) | 2 (0.1) | 2 (0.1) | 3 (0.2) | 0 | 2 (0.5) | 1 (0.5) | 1 (0.5) |
| Pancreatic cancer (adjudication‐confirmed)h | 2 (0.1) | 2 (0.1) | 7 (0.5) | 0 | 2 (0.5) | 1 (0.3) | 0 | 1 (0.5) |
| Gastric cancerg | 0 | 2 (0.1) | 0 | 1 (0.1) | 0 | 0 | 0 | 0 |
| Pemphigoidg | 0 | 0 | 2 (0.1) | 0 | 3 (0.7) | 0 | 2 (0.9) | 0 |
| Skin lesionsi | 1 (0.1) | 1 (0.1) | 0 | 0 | 2 (0.5) | 0 | 2 (0.9) | 0 |
| Acute pancreatitis (adjudication‐confirmed)f , h | 3 (0.2) | 3 (0.2) | 3 (0.2) | 2 (0.1) | 2 (0.5) | 0 | 1 (0.5) | 0 |
| Chronic pancreatitis (adjudication‐confirmed)f , h | 0 | 1 (0.1) | 0 | 1 (0.1) | 2 (0.5) | 1 (0.3) | 0 | 0 |
Note: MedDRA Version 20.1 was used to classify AEs.
Abbreviations: ACE, angiotensin‐converting enzyme; AE, adverse event; ARB, angiotensin‐receptor blocker; MedDRA, Medical Dictionary for Regulatory Activities; SMQ, Standardized MedDRA Query.
Any investigator‐reported hypoglycaemia AE.
Any hypoglycaemia AE requiring the assistance of another person to administer actively carbohydrate, glucagon or other resuscitative actions.
Narrow SMQ: “hypersensitivity”.
Percentage of the number of patients on ACE inhibitor/ARB at baseline in the respective treatment and age group, not the total number.
SMQ “Malignant Tumours” and SMQ “Tumours of unspecified malignancy”.
All events from first intake of study drug onwards were included for cancer and pancreatitis (unlike other AEs for which events up to 7 days after permanent treatment discontinuation were included).
MedDRA preferred term.
Adjudicated by an independent clinical events committee blinded to treatment assignment.
Narrow SMQ: “severe cutaneous adverse reactions”.
Hypoglycaemia occurred in less than one‐third of participants in all age groups at a similar incidence in the linagliptin and placebo arms, despite better glucose control in the former (Table 2). Severe hypoglycaemia occurred in <4% of participants aged <80 years at a similar incidence with linagliptin or placebo. In those aged ≥80 years, severe hypoglycaemia was more common than in the younger age groups, occurring in 5.5% of the linagliptin group and 6.3% of the placebo group (Table 2).
Hypersensitivity reactions and cancer occurred in only a low number of participants, and their incidence was generally balanced between linagliptin and placebo across all age groups (Table 2). A small number of pemphigoid cases occurred (n = 7), all of which were in linagliptin‐treated participants aged ≥65 years (zero, two, three and two cases in those aged <65, 65 to <75, 75 to <80 and ≥80 years, respectively).
4. DISCUSSION
The older participants in CARMELINA® comprise one of the highest‐risk cohorts studied in cardiovascular outcomes trials of glucose‐lowering drugs conducted following the US Food and Drug Administration (FDA) requirement for such drugs to demonstrate cardiovascular safety.30 The overall rate of 3P‐MACE in the placebo group (5.6 participants per 100 patient‐years24) was higher than in most other cardiovascular outcomes trials, while the rate in participants aged 65 to <75 or ≥75 years was even higher: 6.0 and 8.0, respectively, compared with 4.4 in those aged <65 years. Thus, age exacerbated the already high cardiovascular risk conferred by the comorbidities of established CVD with albuminuria and/or kidney disease required for inclusion in this trial. Our analysis found that even in such high‐risk older patients, including those aged >75 years, linagliptin did not increase the risk of atherosclerotic cardiovascular events, heart failure or adverse kidney outcomes. Furthermore, linagliptin improved glycaemic control compared with placebo in all age groups without increasing the risk of hypoglycaemia or most other adverse events.
Chronic kidney disease is highly prevalent among older people with T2D. CARMELINA® studied an enriched study population for kidney disease and was thus able to evaluate kidney outcomes robustly. With no increase in adverse kidney events, linagliptin exhibited renal safety in all age groups, including older participants who had reduced kidney function (mean eGFR of approximately 45–50 mL/min/1.73 m2), hence advancing the evidence base in this older population.
Safety is of paramount concern for the management of elderly people with T2D, who are a fast‐growing proportion of the patient population with T2D yet have been largely understudied to date. Because of the paucity of studies evaluating glucose‐lowering drugs in older people, there was little specific guidance in this area before the European Diabetes Working Party for Older People published guidelines in 2011.6 This was followed by other national and international guidelines and position statements either dedicated specifically to older age groups or including a consideration of patient age, such as those from the American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD),9, 19 International Diabetes Federation,11 Japan Diabetes Society/Japan Geriatrics Society,13 American Geriatrics Society,10 Endocrine Society17 and others.8, 12, 14, 15, 16, 18 In general, these guidelines emphasize that the safety of glucose‐lowering treatment should be the primary consideration in older people, particularly the frail elderly with comorbidities who have a high risk of adverse events and may have a limited life expectancy. Indeed, the incidence of adverse events in CARMELINA® was higher in older than younger age groups, but was generally similar within each age group in the linagliptin and placebo arms. Importantly, there was no increase in hypoglycaemia with linagliptin in older participants, either overall episodes or severe hypoglycaemia in particular despite this DPP‐4 inhibitor reducing overall HbA1c by at least 0.3% over the study period.
It is noteworthy that the improved glycaemic control with linagliptin occurred despite the CARMELINA® trial being designed to achieve similar glucose lowering in both treatment arms (glycaemic equipoise) by encouraging study investigators to optimize glycaemic control for all participants. Most other cardiovascular outcomes trials in T2D have also found glycaemic differences between the active treatment and placebo arms, despite being similarly designed to achieve glycaemic equipoise. This phenomenon may reflect the clinical inertia often seen among physicians treating people with T2D in clinical practice.31, 32
The guidelines on older people with T2D also generally suggest that their glycaemic goals may be relaxed compared with younger patients. Their HbA1c target is usually recommended to be 7.5%–8.0%, in contrast to the target of <7.0% normally set for younger diabetic individuals. However, this recommendation is partly suggested for safety reasons, mainly to avoid hypoglycaemia. Indeed, several guidelines recommend a lower HbA1c target for non‐frail older diabetic individuals using modern medications that do not cause hypoglycaemia, such as DPP‐4 inhibitors.13, 15, 16, 17 In fact, the guidelines also emphasize that glycaemic control should not be abandoned in older people, as chronic hyperglycaemia is associated with complications such as dehydration, urinary infections, dizziness, falls and acute hyperglycaemic crises (diabetic ketoacidosis, hyperglycaemic hyperosmolar syndrome and hyperosmolar ketoacidosis). With this in mind, it is notable that the glycaemic reductions with linagliptin in older participants in the CARMELINA® trial achieved mean HbA1c levels in the 7.0%–8.0% range, as recommended by most guidelines.
Apart from being informative for management of the older individual with T2D, our subgroup analysis is also relevant in the context of the current consensus report from the ADA and EASD.33, 34 This report recommends that people with T2D and indicators of high cardiovascular risk, established CVD, heart failure or chronic kidney disease, including older individuals, who have insufficient glycaemic control from metformin alone should receive second‐line treatment with a glucose‐lowering drug with proven cardiovascular, heart failure or chronic kidney disease benefit, such as an SGLT2 inhibitor or GLP‐1 receptor agonist.33, 34 For people unable to tolerate these drugs, or needing third‐line therapy, the report recommends other glucose‐lowering drugs with proven cardiovascular safety.33, 34 Our analysis of CARMELINA® data by participant age suggests that linagliptin has cardiovascular safety in older people at very high cardiovascular risk, even those aged >75 years, as well as in younger individuals. The data on heart failure are particularly reassuring given the concern about this arising from the cardiovascular safety studies of saxagliptin35 and alogliptin.36
Our analysis has some specific strengths and limitations. The parent study was a rigorously designed and well‐conducted randomized clinical trial that fulfilled the criteria for demonstrating cardiovascular safety mandated by the FDA30 and the European Medicines Agency.37 With no upper age limit, its inclusion of a large number of older individuals, notably >1200 participants aged >75 years and >400 aged >80 years, facilitated our prespecified analysis of outcomes by participant age. Furthermore, unlike most other cardiovascular outcomes trials of glucose‐lowering drugs, CARMELINA® was designed to include people with chronic kidney disease as well as to evaluate adverse renal outcomes, both of which are prevalent among the elderly population in clinical practice. Among the limitations, the nature of an event‐driven trial in a high‐risk population means that although its duration (median 2.2 years) was sufficient to establish cardiovascular and kidney safety, it might have been too short to uncover potential cardiorenal benefits of linagliptin manifesting over the longer term. Finally, as CARMELINA® was not a dedicated study in the elderly, frailty and functional status were not assessed. However, CARMELINA® did include a unique preplanned cognition substudy, with cognitive function tests being conducted at baseline and the end of treatment. This substudy found a neutral effect on cognitive function over time with linagliptin, including in the subgroups aged <70 and ≥70 years.38
In conclusion, our prespecified analysis has demonstrated that linagliptin treatment in older people with T2D, established CVD with albuminuria and/or chronic kidney disease did not increase their cardiorenal risk compared with placebo, but did improve their glycaemic control without increasing the risk of hypoglycaemia. These findings should be informative for clinicians managing older individuals with T2D.
CONFLICT OF INTEREST
M.E.C. has received fees for advisory services and honoraria from Boehringer Ingelheim, Eli Lilly, Sanofi, Servier, Bayer, Astra Zeneca, Novartis, Reata, MundiPharma and MSD, and a grant from NovoNordisk. J.R. has served on scientific advisory boards and received honoraria or consulting fees from Eli Lilly, Sanofi, Novo Nordisk, Janssen, Applied Therapeutics, Boehringer Ingelheim, and Intarcia; he has also received grants/research support from Merck, Pfizer, Sanofi, Novo Nordisk, Bristol‐Myers Squibb, Eli Lilly, GlaxoSmithKline, Genentech, Janssen, Lexicon, Boehringer Ingelheim, Oramed, Applied Therapeutics and Intarcia. T.K. reports consulting/lecture fees from Abbott, Asahi Mutual Life Insurance, Astellas Pharma Inc., AstraZeneca K.K., Bayer, Boehringer Ingelheim, Cosmic, Daiichi Sankyo Company, Limited, Eli Lilly and Company, Fujifilm, FUJIREBIO, Johnson & Johnson Co., Ltd, Kissei Pharmaceutical Co., Ltd, Kowa Co., Ltd, Kyowa Hakko Kirin Co., Ltd, Medical Review, Medscape Education, Medtronic Sofamor Danek, Mitsubishi Tanabe Pharma Corporation, MSD, Musashino Foods, Nipro, Novartis International AG, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Sanofi S.A., Sanwa Kagaku Kenkyusho Co., Ltd, Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd, Takeda Pharmaceutical Company Limited, and Terumo; grants from Astellas Pharma Inc., Daiichi Sankyo Company, Limited, Eli Lilly and Company, Kissei Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Sanofi S.A., Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd and Takeda Pharmaceutical Company Limited; contracted research from AstraZeneca K.K. and Takeda Pharmaceutical Company Limited; joint research from Daiichi Sankyo Company, Limited; endowed chair from Asahi Mutual Life Insurance, Boehringer Ingelheim, Kowa Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd and Takeda Pharmaceutical Company Limited. Y.S. has received lecture fees from MSD, K.K., Kao, Taisho, Boehringer Ingelheim, Taisho Toyama, Takeda, Becton Dickinson and Novo Nordisk; and research support from Terumo, Bayer, Boehringer Ingelheim, Ono, Sumitomo Dainippon, Taisho Toyama and Novo Nordisk. C.W. has received fees for advisory services to Boehringer Ingelheim and MSD as well as honoraria for lecturing from AstraZeneca, Eli Lilly and Sanofi. S.S., D.C. and O.E.J. are employees of Boehringer Ingelheim.
AUTHOR CONTRIBUTIONS
M.E.C., J.R., C.W., S.S. and O.E.J. participated in design of the study, conduct and data collection, and interpretation of data. M.E.C and O.E.J drafted the first version, and all authors subsequently revised the manuscript. S.S. also performed the statistical analysis. T.K, Y.S. and D.C. participated in interpretation of data and revision of the manuscript. All authors have approved the final version of the manuscript.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
The CARMELINA® trial was funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Giles Brooke, PhD, CMPP, of Elevate Scientific Solutions during the preparation of this manuscript. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development and have approved the final version.
Cooper ME, Rosenstock J, Kadowaki T, et al. Cardiovascular and kidney outcomes of linagliptin treatment in older people with type 2 diabetes and established cardiovascular disease and/or kidney disease: A prespecified subgroup analysis of the randomized, placebo‐controlled CARMELINA® trial. Diabetes Obes Metab. 2020;22:1062–1073. 10.1111/dom.13995
CARDIORENAL SAFETY OF LINAGLIPTIN IN OLDER PEOPLE WITH T2D
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13995.
Funding information Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017. [Google Scholar]
- 2. Centers for Disease Control and Prevention National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 3. Lakey WC, Barnard K, Batch BC, Chiswell K, Tasneem A, Green JB. Are current clinical trials in diabetes addressing important issues in diabetes care? Diabetologia. 2013;56:1226‐1235. [DOI] [PubMed] [Google Scholar]
- 4. Davies M, Chatterjee S, Khunti K. The treatment of type 2 diabetes in the presence of renal impairment: what we should know about newer therapies. Clin Pharmacol. 2016;8:61‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tong L, Adler S. Glycemic control of type 2 diabetes mellitus across stages of renal impairment: information for primary care providers. Postgrad Med. 2018;130:381‐393. [DOI] [PubMed] [Google Scholar]
- 6. Sinclair AJ, Paolisso G, Castro M, et al. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab. 2011;37(Suppl 3):S27‐S38. [DOI] [PubMed] [Google Scholar]
- 7. Sinclair A, Morley JE, Rodriguez‐Manas L, et al. Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc. 2012;13:497‐502. [DOI] [PubMed] [Google Scholar]
- 8. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60:2342‐2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus , Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc. 2013;61:2020‐2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunning T, Sinclair A, Colagiuri S. New IDF Guideline for managing type 2 diabetes in older people. Diabetes Res Clin Pract. 2014;103:538‐540. [DOI] [PubMed] [Google Scholar]
- 12. Sinclair A, Dunning T, Rodriguez‐Manas L. Diabetes in older people: new insights and remaining challenges. Lancet Diabetes Endocrinol. 2015;3:275‐285. [DOI] [PubMed] [Google Scholar]
- 13. Japan Diabetes Society /Japan Geriatrics Society Joint Committee on Improving Care for Elderly Patients with Diabetes , Haneda M, Ito H. Glycemic targets for elderly patients with diabetes. Diabetol Int. 2016;7:331‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sinclair AJ, Abdelhafiz A, Dunning T, et al. An international position statement on the management of frailty in diabetes mellitus: Summary of recommendations 2017. J Frailty Aging. 2018;7:10‐20. [DOI] [PubMed] [Google Scholar]
- 15. Strain WD, Hope SV, Green A, Kar P, Valabhji J, Sinclair AJ. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty. A national collaborative stakeholder initiative. Diabet Med. 2018;35:838‐845. [DOI] [PubMed] [Google Scholar]
- 16. Diabetes Canada Clinical Practice Guidelines Expert Committee , Meneilly GS, Knip A, et al. Diabetes in older people. Can J Diabetes. 2018;42(Suppl 1):S283‐S295. [DOI] [PubMed] [Google Scholar]
- 17. LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of diabetes in older adults: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1520‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sinclair AJ, Abdelhafiz AH, Forbes A, Munshi M. Evidence‐based diabetes care for older people with type 2 diabetes: a critical review. Diabet Med. 2019;36:399‐413. [DOI] [PubMed] [Google Scholar]
- 19. American Diabetes Association . 12. Older adults: standards of medical care in diabetes‐2019. Diabetes Care. 2019;42:S139‐S147. [DOI] [PubMed] [Google Scholar]
- 20. Graefe‐Mody U, Retlich S, Friedrich C. Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clin Pharmacokinet. 2012;51:411‐427. [DOI] [PubMed] [Google Scholar]
- 21. Friedrich C, Emser A, Woerle HJ, Graefe‐Mody U. Renal impairment has no clinically relevant effect on the long‐term exposure of linagliptin in patients with type 2 diabetes. Am J Ther. 2013;20:618‐621. [DOI] [PubMed] [Google Scholar]
- 22. Barnett AH, Huisman H, Jones R, von Eynatten M, Patel S, Woerle HJ. Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2013;382:1413‐1423. [DOI] [PubMed] [Google Scholar]
- 23. Schernthaner G, Barnett AH, Patel S, Hehnke U, von Eynatten M, Woerle HJ. Safety and efficacy of the dipeptidyl peptidase‐4 inhibitor linagliptin in elderly patients with type 2 diabetes: a comprehensive analysis of data from 1331 individuals aged >/= 65 years. Diabetes Obes Metab. 2014;16:1078‐1086. [DOI] [PubMed] [Google Scholar]
- 24. Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: The CARMELINA randomized clinical trial. JAMA. 2019;321:69‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGuire DK, Alexander JH, Johansen OE, et al. Linagliptin effects on heart failure and related outcomes in individuals with type 2 diabetes mellitus at high cardiovascular and renal risk in CARMELINA. Circulation. 2019;139:351‐361. [DOI] [PubMed] [Google Scholar]
- 26. Rosenstock J, Perkovic V, Alexander JH, et al. Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA[R]): a randomized, double‐blind, placebo‐controlled clinical trial in patients with type 2 diabetes and high cardio‐renal risk. Cardiovasc Diabetol. 2018;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonized tripartite guideline: Guideline for Good Clinical Practice. J Postgrad Med. 2001;47:45–50. [PubMed]
- 28. ICH Expert Working Group . ICH harmonised tripartite guideline: guideline for good clinical practice E6 (R1). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. June 10, 1996.
- 29. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20:109‐117. [DOI] [PubMed] [Google Scholar]
- 30. Center for Drug Evaluation and Research. Guidance for industry. Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes [Internet], December 2008. Silver Spring, MD, U.S. Food and Drug Administration: Available from http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf. Accessed July 31, 2019. [Google Scholar]
- 31. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411‐3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fu AZ, Sheehan JJ. Treatment intensification for patients with type 2 diabetes and poor glycaemic control. Diabetes Obes Metab. 2016;18:892‐898. [DOI] [PubMed] [Google Scholar]
- 33. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63:221‐228. [DOI] [PubMed] [Google Scholar]
- 35. Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR‐TIMI 53 randomized trial. Circulation. 2014;130:1579‐1588. [DOI] [PubMed] [Google Scholar]
- 36. Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double‐blind trial. Lancet. 2015;385:2067‐2076. [DOI] [PubMed] [Google Scholar]
- 37.Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus [Internet], May 14, 2012. London, UK. European Medicines Agency. Available from http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf. Accessed July 31, 2019.
- 38. Biessels GJ, Verhagen C, Janssen J, et al. Effect of linagliptin on cognitive performance in patients with type 2 diabetes and cardiorenal comorbidities: The CARMELINA randomized trial. Diabetes Care. 2019;42:1930‐1938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


