Summary
Patients with atopic dermatitis (AD) have an increased risk of bacterial skin infections, which cause significant morbidity and, if untreated, may become systemic. Staphylococcus aureus colonizes the skin of most patients with AD and is the most common organism to cause infections. Overt bacterial infection is easily recognized by the appearance of weeping lesions, honey‐coloured crusts and pustules. However, the wide variability in clinical presentation of bacterial infection in AD and the inherent features of AD – cutaneous erythema and warmth, oozing associated with oedema, and regional lymphadenopathy – overlap with those of infection, making clinical diagnosis challenging. Furthermore, some features may be masked because of anatomical site‐ and skin‐type‐specific features, and the high frequency of S. aureus colonization in AD makes positive skin swab culture of suspected infection unreliable as a diagnostic tool. The host mechanisms and microbial virulence factors that underlie S. aureus colonization and infection in AD are incompletely understood. The aim of this article is to present the latest evidence from animal and human studies, including recent microbiome research, to define the clinical features of bacterial infections in AD, and to summarize our current understanding of the host and bacterial factors that influence microbial colonization and virulence.
Patients with atopic dermatitis (AD; also known as ‘atopic eczema’) have an increased risk of recurrent skin infections.1, 2, 3, 4 Staphylococcus aureus is the most common infectious organism, although beta‐haemolytic streptococci may also be involved.5, 6, 7, 8
The mechanisms underlying bacterial infection in AD are multifactorial and include both host and bacterial factors. The reduced skin barrier, cutaneous innate and adaptive immune abnormalities and trauma from scratching all contribute to the increased risk of skin infection.9, 10, 11, 12, 13 The host skin microbiota may play a role in protecting against S. aureus colonization and infection in patients with AD.14, 15, 16, 17 Bacterial virulence factors, such as the superantigens, proteases and cytolytic phenol‐soluble modulins (PSMs) secreted by S. aureus, cause skin inflammation and may also contribute to bacterial persistence and/or epithelial penetration and infection.12, 18, 19
The complex interaction between bacteria and host results in wide variability in the clinical presentation of infection in AD and can make the diagnosis challenging. Cutaneous infection may be associated with concomitant AD flares, and the classic signs of infection (erythema, oozing and crusting and increased cutaneous warmth) are masked by similar clinical features of AD itself. Increases in erythema in individuals with darker skin types are more difficult to appreciate, making diagnosis yet more challenging. Pustules are an uncommon sign of bacterial infection in AD, but if present they can allow the diagnosis to be made with greater certainty. Diagnosis and management decisions are further complicated by the fact that the main causative organism, S. aureus, commonly colonizes even nonlesional, clinically unaffected AD skin, thus limiting the usefulness of bacterial cultures in identifying the causative organism.
Untreated bacterial skin infection in AD may become systemic and lead to life‐threatening complications including sepsis, endocarditis and bone and joint infections.20, 21, 22 Despite the significant morbidity caused by bacterial skin infection in AD, there is a lack of consensus on how to define and treat associated bacterial colonization and infection. Although there are many diagnostic criteria for AD itself, there are no validated diagnostic criteria for infected AD.23
The International Eczema Council, a group of approximately 100 experts in AD worldwide, has recently initiated a taskforce to define the role of bacterial skin infections and their management in AD through consensus statements in an effort to provide level D evidence. It is hoped that input from clinical experts will contribute to better defining the wide‐ranging clinical presentations of S. aureus infection in AD and, more importantly, to identify better those who may benefit from existing or novel antimicrobial treatments. Based on a systematic search of the literature, including terms for AD and ‘infection’, ‘bacteria’, ‘staphylococcus aureus’ and ‘microbiome’ (detailed search strategy available on request), this narrative review defines the clinical features of bacterial infection in AD and our current understanding of the host and bacterial factors that influence microbial colonization and virulence.
Clinical features of bacterial skin infection in atopic dermatitis
The typical clinical signs of overt bacterial skin infection in AD are well recognized. More specific signs of S. aureus infection in AD lesions include weeping, honey‐coloured crusts, and pustules, both interfollicular and follicular based (folliculitis) (Fig. 1a, b).6, 24 Pustules are an uncommon feature of infection in AD, but may be associated with significant pruritus and even pain (Fig. 1c).25 By contrast, beta‐haemolytic streptococcal infection may present with well‐defined, bright red erythema, thick‐walled pustules and heavy crusting (Fig. 1d).7, 26 In severe cases, cutaneous bacterial infection may cause abscesses – especially with methicillin‐resistant S. aureus (MRSA) infection – fever and lymphadenopathy. A complication in diagnosing infection in AD is the common association with a disease flare. Features of flared AD (increased erythema, oedema, papulation, oozing and excoriation) can mask and/or resemble signs of infection.
Figure 1.
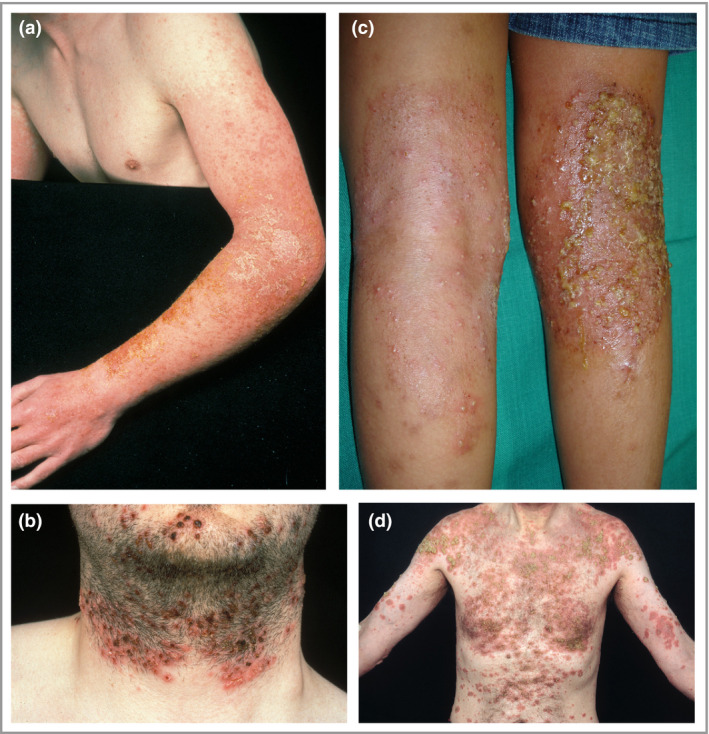
Clinical features of bacterial skin infection in atopic dermatitis. Clinical features of S. aureus infection in atopic dermatitis lesions include (a) weeping, honey‐coloured crusts; (b) folliculitis; and (c) pustulation. (d) Beta‐haemolytic streptococcal infection may present with well‐defined bright red erythema.
Concomitant viral infection
Several nonbacterial infections can occur concomitantly with bacterial skin infection and can resemble bacterial infections, requiring consideration in the differential diagnosis. For instance, eczema herpeticum (EH) is caused by the local spread of herpes simplex virus, which favours AD lesional skin and is commonly observed in the context of an AD flare.27 Early in the course of EH the characteristic skin lesions are superficial clusters of dome‐shaped vesicles and/or small, round, punched‐out erosions (Fig. 2a, b).27 As the disease progresses, lesions may become superficially infected with S. aureus and may develop an impetiginized scale (Fig. 2c, d).12 EH typically arises in involved AD skin, most frequently on the face, neck, upper trunk and antecubital/popliteal areas with AD, and is often accompanied by fever, malaise and lymphadenopathy.28, 29 Moderate‐to‐severe AD, filaggrin loss‐of‐function mutation, a history of S. aureus skin infection, greater allergen sensitization and type 2 immunity are important risk factors for EH.30, 31, 32 Staphylococcal α‐toxin and reductions in the tight junction protein claudin‐1 result in greater epidermal spread of herpes simplex virus in vitro.33, 34 This infection can spread rapidly and, in severe cases, may lead to keratoconjunctivitis and encephalitis.
Figure 2.
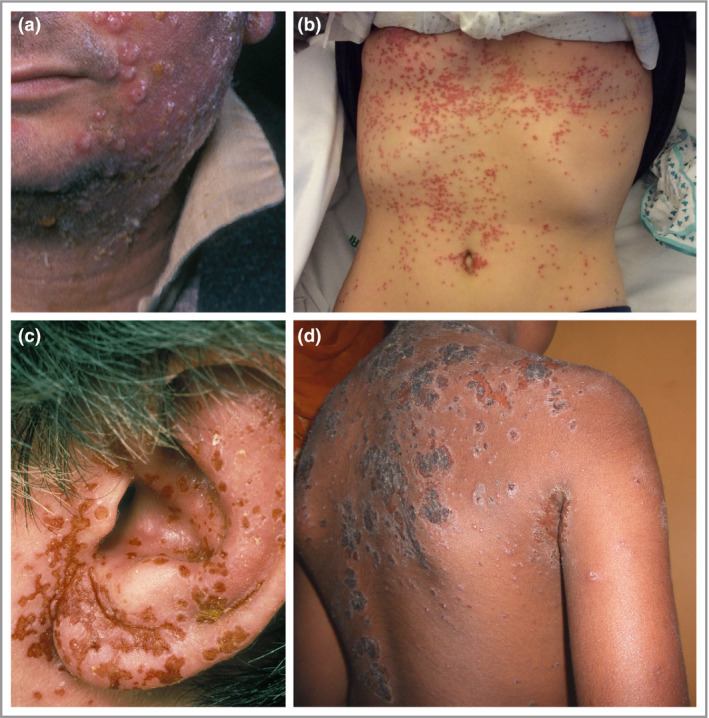
Clinical features of eczema herpeticum. (a, b) Early eczema herpeticum lesions are superficial clusters of dome‐shaped vesicles and/or small, round, punched‐out erosions. (c, d) As the disease progresses, the lesions commonly become superficially infected with Staphylococcus aureus and may have the characteristic impetiginized scale.
Concomitant fungal colonization
Fungal colonization can also complicate the clinical picture of AD. For instance, Malassezia colonization is thought to drive inflammation in AD in a subset of patients who typically have dermatitis in areas with a high density of sebaceous glands (e.g. head, neck, and upper chest and back) (Fig. 3). This seborrhoeic distribution overlaps with, but is distinct from, the distribution of allergic contact dermatitis or airborne allergy, which typically involve the upper face, eyelids and periorbital regions, anterior neck, postauricular area and exposed areas on the arms. Malassezia is a commensal yeast. Although it is not more abundant on AD skin,35 patients with AD are more frequently sensitized to Malassezia.36, 37, 38 In some patients, sensitization to yeast antigens induces autoreactivity to human proteins via molecular mimicry, leading to sustained skin inflammation.39, 40 Cross‐reactivity between Malassezia‐specific IgE and Candida albicans has also been shown.41 A systematic review of the eight published randomized controlled trials evaluating the benefit of antifungal therapy found that five trials demonstrated a benefit from antifungal drugs and three trials found no benefit compared with placebo or standard therapy.38
Figure 3.
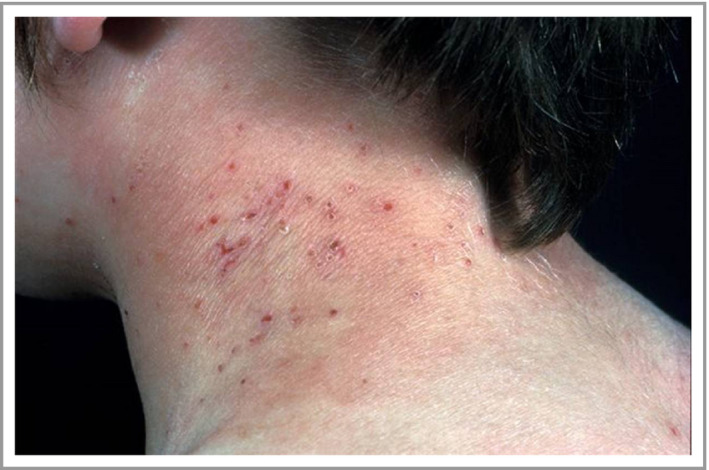
Malassezia colonization in atopic dermatitis, which may drive inflammation in patients who have head and neck dermatitis.
Bacterial skin infection in different ethnic skin types
There is wide variation in the clinical manifestation of AD in different ethnic groups. This may be a result of underlying genetic variation, which influences AD susceptibility and clinical presentation, inadequate early intervention because of masking of erythema in dark skin, and differences in both treatment response and environmental exposures.42 In dark‐skinned individuals, perifollicular accentuation is often present and erythema appears violaceous and often muted (Fig. 4).43, 44, 45 This can lead to poor recognition of inflammation, underestimation of disease severity and inadequate intervention. Patients with AD of African descent often have extensor disease rather than the characteristic flexural lesions.45 Importantly, S. aureus strain differences, including variability in the presence of superantigen genes, has been shown between European American, African American and Mexican American patients with AD.46
Figure 4.
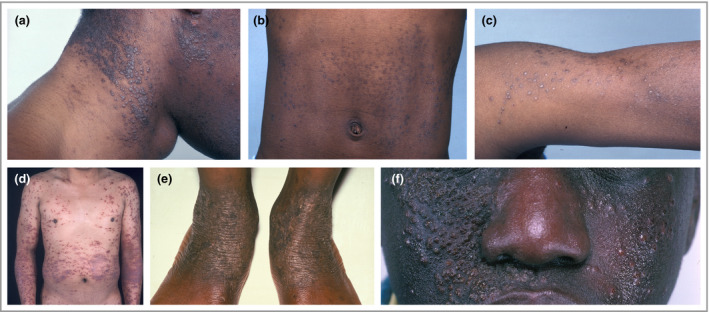
Atopic dermatitis in different ethnic skin types. In dark‐skinned individuals perifollicular accentuation is often present in atopic dermatitis, and erythema appears violaceous.
Methicillin‐resistant Staphylococcus aureus
As in healthy people who are colonized by MRSA, patients with AD often have recurrent infections and disease flares that are resistant to standard treatment regimens (Fig. 5). The prevalence of MRSA skin colonization varies significantly with geographical location and study setting in both healthy and diseased populations. It is therefore difficult to compare accurately the prevalence of MRSA colonization between AD and healthy cohorts. For example, in the U.S.A. there is significant state‐wide variation, with the rate of MRSA colonization varying between 0·3% and 13% in people with AD.3, 47, 48, 49 In another study, 4–19% of children with AD from the U.K. and Ireland were found to be colonized with MRSA.50, 51 The reported prevalence of MRSA colonization in patients with AD in Sri Lanka is 8%, and in Korea 3–14%.52, 53, 54, 55 A meta‐analysis of MRSA colonization in the general population reported a prevalence of 0·2–7% worldwide.56 The authors describe significant study heterogeneity. In a subgroup analysis that excluded people with prior healthcare contact, the prevalence of MRSA colonization was found to be very low (0·2%).
Figure 5.
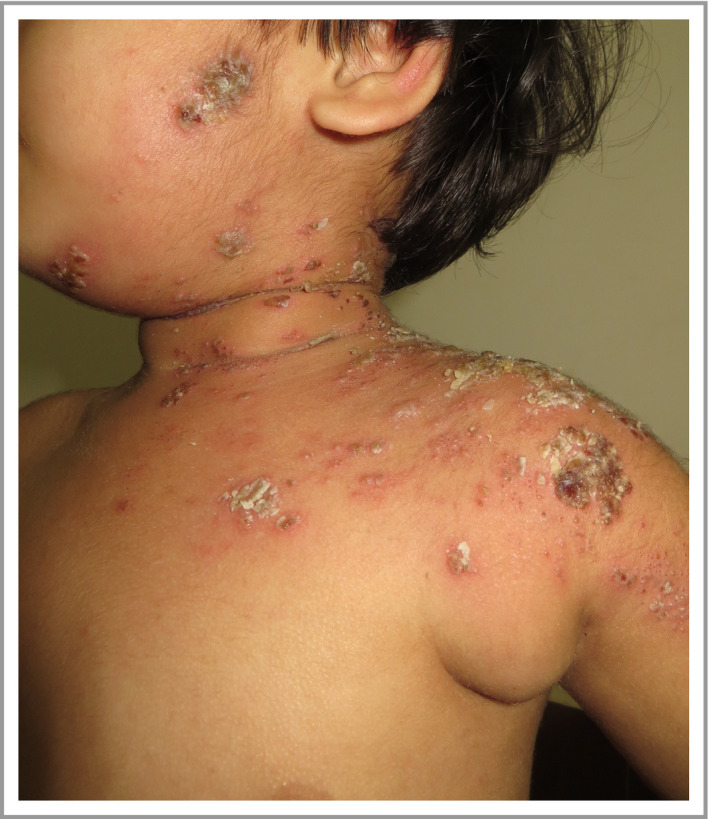
Methicillin‐resistant Staphylococcus aureus infection in atopic dermatitis may cause recurrent flares that are resistant to standard treatment regimens.
Although some studies suggest that MRSA colonization rates are higher in people with AD than in the general population, other studies have found much lower rates. For instance, a cross‐sectional study of 200 patients with AD in Canada found MRSA in only one individual.57 Similarly, children with AD from San Diego were found to have a lower rate of community‐acquired MRSA colonization than the general outpatient paediatric population.58 Further research is needed to understand the significance of MRSA in AD.
Staphylococcus aureus colonization in atopic dermatitis
Most patients with AD are colonized by S. aureus. A recent meta‐analysis found that the pooled prevalence of S. aureus colonization of lesional AD skin is 70%, of nonlesional AD skin 39% and of the nares 62%.59 However, the prevalence varies greatly across studies, from 22% to 99% in lesional skin and 3% to 79% in nonlesional skin.59, 60, 61, 62, 63 Most patients colonized by S. aureus do not exhibit overt signs of infection, and 10% of healthy individuals carry S. aureus.62, 64
Staphylococcus aureus colonization can be associated with three main clinical scenarios in AD: (i) stable or baseline AD without clinical evidence of overt infection; (ii) AD flare without clinical evidence of overt infection; and (iii) overtly infected AD with the classical symptoms as described above. Although antimicrobial therapy is clearly essential for patients with overtly infected AD, the clinical significance, recognition and management of S. aureus colonization without clinical evidence of infectious disease are not fully understood. Some studies show that patients with AD improve with topical and systemic antibiotic treatments, even without overt signs of secondary infection.65, 66, 67, 68, 69, 70 However, other studies have found no clinical benefit of antibiotic treatment over corticosteroid therapy alone.63, 71 A 2010 Cochrane review found no support for routine topical or systemic antistaphylococcal interventions in AD that is not clinically infected, although the studies were generally short term and of poor quality.72
It is likely that the density of S. aureus is more relevant than simply the presence of the bacteria. The density of S. aureus colonization correlates with the severity of AD.73, 74, 75, 76 Williamson and Kligman used an early method of quantitative bacteriology to compare the effects of topical and systemic antibiotics on S. aureus in AD.77 The detergent scrub technique was used on AD lesions to obtain bacterial samples, which were incubated before the S. aureus density was measured. They found that appreciable clinical improvement with antibiotic therapy occurred only in patients whose AD lesions were infected by S. aureus at a density of greater than 106 colony‐forming units per cm2.61, 68 Similarly, microbiome studies of paediatric patients with AD show that the relative abundance of S. aureus is associated with disease flares and correlates with severity.78, 79, 80, 81
In addition to bacterial abundance, there are several additional factors that determine whether S. aureus successfully colonizes the skin in AD and whether this results in clinically relevant infection. Casadevall and Pirofski described the ‘damage–response framework’ approach to microbial pathogenesis.82, 83 The basic tenets of this concept are that host and microbe interact to create a spectrum of possible states, ranging from commensalism and colonization to disease. Disease results from damage to the host, which can come from the host response, the microbe or both. The damage–response framework defines infection as the acquisition of a microbe, but it does not necessarily mean the microbe is causing disease. Infection results in disease when the host–microbe interaction produces sufficient damage to become clinically apparent.84
This approach is a framework that advances thinking beyond the classic microbe‐centric Koch's postulates that dominated microbiological thought for more than a century. It may be a useful approach for understanding the S. aureus–host interaction in AD and the range of clinical scenarios that can arise (Fig. 6). We have some understanding of the various bacterial and host factors that contribute during S. aureus infection in AD. However, the key questions to be answered are (i) which of these factors lead to worsening inflammation in AD? and (ii) can a threshold of host damage resulting from the S. aureus–host interaction be defined, beyond which antibiotics prove beneficial? If the key host and microbial factors that determine these outcomes are identified, then targeting of these specific factors with novel immunotherapies or selective antimicrobial therapies may become a reality.14, 85
Figure 6.
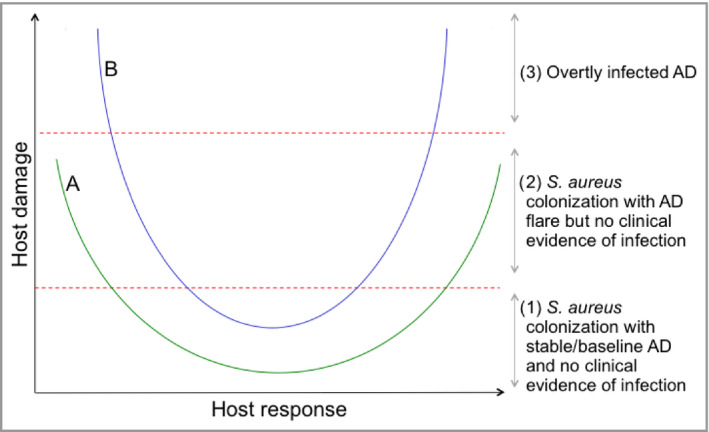
Hypothetical damage–response framework for Staphylococcus aureus in atopic dermatitis (AD).82 Different host–S. aureus interactions result in different damage–response relationships. Curves A and B represent the damage–response relationships of S. aureus with two different hosts or those of a single host with two different S. aureus strains. The outcome for the host depends on the strength of the host response to S. aureus or the virulence of S. aureus. During intermediate host responses neither interaction (A or B) causes clinical evidence of infection, as the amount of damage incurred by the host is insufficient (1). However, in the setting of weak or strong responses both interactions cause an AD flare (2) and interaction B causes overtly infected AD (3). The position of the curve is determined by multiple host and S. aureus factors.
Host factors associated with Staphylococcus aureus colonization
Adults with AD who are colonized with S. aureus have more severe disease, and greater T helper type 2 (Th2) immune deviation, allergen sensitization and barrier dysfunction than noncolonized patients with AD.86 Some studies have found that filaggrin mutations are associated with S. aureus colonization in AD, but others have not.86, 87, 88 The increased susceptibility to S. aureus colonization and infection in AD is multifactorial and driven by both skin barrier abnormalities and innate and adaptive immune responses (Fig. 7).
Figure 7.
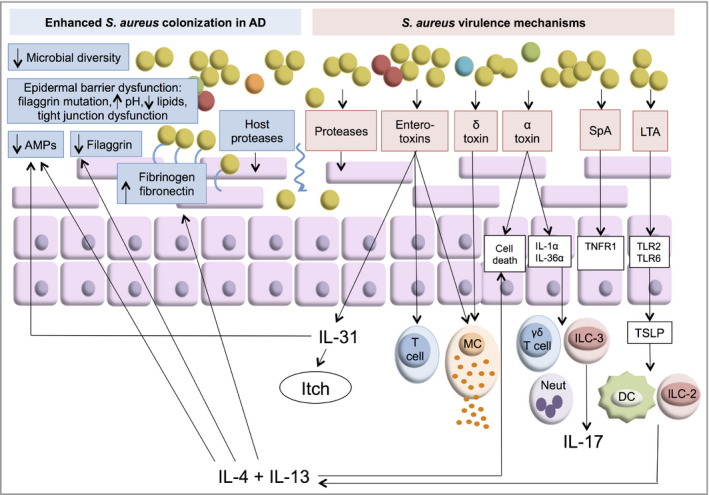
Possible mechanisms of Staphylococcus aureus colonization and virulence in atopic dermatitis (AD). Staphylococcus aureus colonization is increased in AD skin. This may be due to epidermal barrier dysfunction, reduced levels of antimicrobial peptides (AMPs), reduced microbial diversity or increased fibrinogen and fibronectin. Proteases produced by the host and S. aureus allow the bacteria to penetrate into the deeper layers of the skin. Staphylococcal enterotoxins (SEs) stimulate polyclonal T‐cell responses, SE‐specific IgE responses and interleukin (IL)‐31 expression. α‐Toxin can cause keratinocyte death and can activate keratinocyte IL‐1α and IL‐36α production to stimulate γδT cells, innate lymphoid cell (ILC)‐3‐mediated IL‐17 release and neutrophil (Neut) recruitment. δ‐Toxin causes mast cell (MC) degranulation. Staphylococcal protein A (SpA) activates proinflammatory pathways via tumour necrosis factor receptor 1 (TNFR1) on keratinocytes. Staphylococcus aureus lipoteichoic acid (LTA) and lipoproteins activate Toll‐like receptor (TLR)2 and TLR6 to produce thymic stromal lymphopoietin (TSLP), which activates dendritic cells (DC) and ILC‐2, leading to production of T helper cell (Th)2 cytokines.
The impaired skin barrier
The impaired skin barrier in AD is characterized by reduced very‐long‐chain epidermal lipids, defective tight junctions, differentiation in protein deficiency (including from filaggrin loss‐of‐function mutations), enhanced protease activity and increased skin‐surface pH. This impaired barrier provides a favourable environment for S. aureus colonization.89, 90, 91, 92 The deposition of stratum corneum (SC) fibronectin, to which S. aureus adheres, is increased in AD.26, 93, 94 Staphylococcus aureus clumping factor B binds to loricrin and cytokeratin 10 and promotes adhesion of S. aureus to the stratum corneum in AD.95 Antimicrobial peptides (AMPs) such as β‐defensins and cathelicidins are also reduced in AD lesions.96
Type 2 inflammation
Type 2 inflammatory pathways, in which the cytokines interleukin (IL)‐4 and IL‐13 play a major role, drive inflammation in AD. Th2 cytokines reduce expression of important skin barrier proteins: filaggrin, loricrin and involucrin.97, 98 The expression of fibronectin is increased by IL‐4 and may facilitate S. aureus adherence in AD.99 The failure to mount an appropriate AMP response in AD may also be due to the suppressive effects of IL‐4 and IL‐13, and may enhance S. aureus colonization further.12, 13, 100
A recent pooled analysis of seven randomized, placebo‐controlled dupilumab trials in adults with moderate‐to‐severe AD found that bacterial skin infections were significantly less common in the dupilumab groups than in the placebo group.101 Similarly, a meta‐analysis of data from eight dupilumab trials found that patients treated with dupilumab had a lower risk of skin infection than those treated with placebo.102 The reduced rate of skin infection with dupilumab supports the role of a Th2‐driven host skin barrier defect in infection in AD, which after treatment may become a less favourable environment for bacteria. This shift may be mediated by inhibition of type 2 inflammatory cytokines, reduced scratching, or microbiome changes induced by dupilumab. Dupilumab treatment results in increased microbial diversity and decreased S. aureus abundance in AD.103
The skin microbiome
Microbial diversity is reduced in AD and inversely correlates with disease severity.78, 79, 81 Skin commensal microbes, including coagulase‐negative staphylococci (CoNS), may aid skin homeostasis and provide protection against S. aureus. Thus, the diminution of commensal skin microbiota with flares may promote S. aureus colonization and infection in AD. During flares of paediatric AD, both Staphylococcus epidermidis and S. aureus are increased, suggesting a compensatory role for S. epidermidis.78 This skin commensal promotes AMP expression by cultured keratinocytes via Toll‐like receptor 2 signalling.104 Furthermore, S. epidermidis produces PSMγ and PSMδ, which enhance AMP effects and inhibit growth of S. aureus and group A Streptococcus in vitro.105 Cutaneous application of antimicrobial CoNS strains to adults with AD decreased colonization by S. aureus within 24 h of a single application.14
In addition to inhibiting S. aureus colonization, CoNS also reduce S. aureus‐driven skin inflammation. CoNS from healthy skin produce autoinducing peptides that inhibit the S. aureus accessory gene regulatory quorum sensing system, leading to reduced expression of the S. aureus virulence factor PSMα in vitro and reduced S. aureus‐induced skin barrier damage in mice.16 Cutibacterium acnes supresses growth of MRSA in mouse skin through glycerol fermentation, leading to short‐chain fatty acid production and reduced bacterial intracellular pH.15 Treatment with the Gram‐negative Roseomonas mucosa, collected from healthy human skin, inhibits the growth of S. aureus in vitro and results in reduced inner‐ear thickness in a mouse model of AD.106 In human studies, spraying R. mucosa onto lesional AD skin of the antecubital area improved AD severity and reduced the need for topical corticosteroids.17 MRSA colonization is associated with reduced microbial diversity compared with methicillin‐sensitive S. aureus colonization of AD lesional skin and greater decreases in the relative abundance of skin commensal bacteria, including Cutibacterium, Streptococcus and Corynebacterium.47 Further research is needed to understand the interactions between S. aureus and commensal organisms, and how these organisms relate to host immune responses.
Staphylococcus aureus factors promoting colonization and virulence
Staphylococcus aureus exacerbates AD by secreting virulence factors that affect the epidermis (leading to inflammation and skin barrier disruption) and factors that hamper innate and adaptive immune responses (Fig. 7). Staphylococcal superantigens activate polyclonal T‐cell responses without prior antigen processing and by activating epithelial cells via CD40.107, 108, 109 Several of the staphylococcal enterotoxins can also act as allergens to stimulate staphylococcal exotoxin‐specific IgE production.110 Staphylococcal enterotoxin B increases the expression of IL‐31, which is well known to cause pruritus in AD.111 IL‐31 also suppresses filaggrin and AMP expression, resulting in increased S. aureus colonization.112, 113 Superantigen‐producing strains are found in over 80% of S. aureus isolates from patients with AD.114 MRSA produces higher levels of superantigen enterotoxins than methicillin‐sensitive S. aureus.115
Additional toxins such as the staphylococcal PSMs, including δ‐toxin and α‐toxin, may additionally enhance the virulence of S. aureus in AD. The δ‐toxin is a potent inducer of mast cell degranulation in vitro and in mouse models of AD.116 α‐Toxin treatment of AD skin causes keratinocyte death, which is enhanced by IL‐4 and IL‐13.117 Recent studies have shown that α‐toxin activates keratinocyte IL‐1α and IL‐36α production, which stimulates γδT cells, innate lymphoid cell (ILC)‐3‐mediated IL‐17 release and neutrophil recruitment.118, 119 Filaggrin protects keratinocytes by mediating the secretion of sphingomyelinase, an enzyme that reduces the number of α‐toxin binding sites on the keratinocyte surface.120 Staphylococcus aureus growth and virulence factor production are reduced in the presence of filaggrin breakdown products.121 These studies suggest that S. aureus‐produced mediators potentiate the effects of S. aureus in AD, and filaggrin‐deficient epidermis may be particularly susceptible to S. aureus.
Staphylococcal protein A activates proinflammatory pathways via tumour necrosis factor receptor 1 on keratinocytes.122 Staphylococcus aureus lipoteichoic acid and lipoproteins activate Toll‐like receptors 2 and 6 to exacerbate AD and stimulate release of thymic stromal lymphopoietin (TSLP) from keratinocytes. TSLP activates dendritic cells and ILC‐2, leading to further production of type 2 cytokines.12, 123 Staphylococcus aureus proteases are required for penetration of the bacteria into the deeper layers of the skin and the induction of Th2 cytokine production.124 Staphylococcus aureus also stimulates keratinocytes to increase their endogenous protease activity.125 Whole‐genome sequencing of S. aureus has recently revealed higher levels of antimicrobial resistance genes in S. aureus isolates from children with AD than in those from healthy control children, suggesting additional potential S. aureus virulence mechanisms in AD.52, 126
Conclusions
Bacterial infection in AD is common and causes significant morbidity. Overt bacterial infection is easily recognized. However, less overt manifestation of infection may be more difficult to diagnose, especially given the greater risk of infection with flares (themselves associated with increased erythema and oozing), as well as the limited value of culture, given the high rates of colonization. Although we have some understanding of how S. aureus colonizes the skin and causes inflammation in AD, many questions related to this complex relationship remain unanswered. Further research is needed for better definition of features that distinguish infection from colonization. Future work of the International Eczema Council, through expert consensus statements, aims to provide guidance regarding the practical use of antimicrobial therapy in atopic dermatitis. Improving our understanding of S. aureus virulence mechanisms and downstream host immune mediators of S. aureus‐driven inflammatory pathways may help to identify novel therapeutic targets for infection in AD.
Conflicts of interest: A.S.P. is an investigator for AbbVie, AnaptysBio, Castle Creek, Eli Lilly, Galderma, Incyte, Janssen, LEO, Novartis and Regeneron; and a consultant for AbbVie, Amgen, Asana, Castle Creek, Dermavant, Dermira, Galderma, Eli Lilly, Forte, LEO, Matrisys, Menlo, Morphosys/Galapagos, Novartis, Patagonia, Pfizer, Pierre Fabre, Regeneron, Sanofi Genzyme and UCB. L.A.B. is an investigator for AbbVie, Eli Lilly, LEO Pharma, Pfizer and Regeneron; and a consultant for AbbVie, Allakos, Arena Pharma, AstraZeneca, Connect Biopharma, Incyte, LEO Pharma, Lilly, Novan, Novartis, Pfizer, Regeneron, Sanofi and UCB; and has stock in Pfizer and Medtronics. S.D. is a key opinion leader and member of the scientific advisory boards of Galderma India, Sun Pharma, ALKEM Pharma, Curatio Health Care, BIOCON Pharma and Sanofi India. G.G. has been principal investigator in clinical trials sponsored by and/or and has received personal fees from AbbVie, Abiogen, Almirall, Amgen, Biogen, Celgene, Eli Lilly, Genzyme, LEO Pharma, Menlo Therapeutics, Novartis, Pfizer, Regeneron, Samsung, Sandoz and Sanofi. A.D.I. is a coinvestigator of the U.K. National Institute for Health Research‐funded TREAT trial (ISRCTN15837754) and the U.K.–Irish Atopic Eczema Systemic Therapy Register (A‐STAR; ISRCTN11210918). He is a principal investigator in the European Union Horizon 2020‐funded BIOMAP Consortium (http://www.biomap-imi.eu). He has received consulting fees from AbbVie, Genentech, Janssen, LEO Pharma, Novartis, Regeneron, Sanofi Genzyme and Pfizer. P.S. has consulted in the past for Sanofi (11 October 2017) and AbbVie (4 December 2017) (unpaid) and is involved in performing clinical trials with many pharmaceutical companies that manufacture drugs used for the treatment of conditions including psoriasis and atopic dermatitis, for which financial compensation is paid to the department. J.S. has been an investigator, consultant or advisor for AbbVie, Amgen, Eli Lilly, Janssen/JNJ, Meda, Novartis, Pfizer, Pierre Fabre and Sanofi. J.P.T. is an advisor for Sanofi‐Genzyme, Pfizer, AbbVie, LEO Pharma and Eli Lilly & Co; and an investigator for AbbVie, LEO Pharma, Eli Lilly & Co and Sanofi‐Genzyme, presently for Sanofi‐Genzyme, LEO Pharma and Regeneron. C.V. is an investigator and lecturer for LEO Pharma, Sanofi‐Genzyme, AbbVie, Galapagos, Novartis and Pfizer. T.W., as principal investigator of the German TREAT registry on atopic dermatitis, has received honoraria for invited talks or scientific advice and research grants from AbbVie, Almirall, ALK Scherax, Astellas, Janssen/JNJ, LEO, Lilly, Meda, Novartis, Pfizer, Regeneron/Sanofi, Roche, Stallergen, Takeda and Ziarco. A.W. has been an investigator, advisor or lecturer for AbbVie, Almirall, Chugai, Eli Lilly, Galapagos, Galderma, LEO Pharma, MedImmune, Novartis, Pfizer, Pierre Fabre, Regeneron and Sanofi‐Aventis. M.D. is a consultant, investigator, scientific advisory board member and/or lecturer for AbbVie, Eli Lilly, Galapagos, LEO Pharma, Novartis, Pfizer, La Roche‐Posay, Regeneron Pharmaceuticals, Sanofi‐Genzyme, Almirall and Pierre Fabre. C.F. is chief investigator of the U.K. NIHR‐funded TREAT and SOFTER trials, and A‐STAR. He is also a principal investigator in the European Union Horizon 2020‐funded BIOMAP Consortium. His department has also received funding from Sanofi‐Genzyme for skin microbiome work.
Funding sources C.F. holds a National Institute for Health Research (NIHR) Career Development Fellowship (CDF‐2014‐07‐037). He is also supported by the NIHR Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King's College London. Corporate sponsorship was provided to the International Eczema Council by AbbVie, Amgen, Asana, Celgene, Chugai, Dermavant, Dermira, Eli Lilly, Galderma, Incyte, LEO Pharma, Kyowa Kirin, Novartis, Pierre Fabre, Pfizer, Sanofi Genzyme, Regeneron Pharmaceuticals, Sienna and Valeant. The sponsors had no influence on the content and viewpoints in this article. The cost of publication was covered by the International Eczema Council.
Conflicts of interest Conflicts of interest statements can be found in the Appendix.
References
- 1. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl 1980; 92:44–7. [Google Scholar]
- 2. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population‐based study. J Allergy Clin Immunol 2014; 133:1041–7. [DOI] [PubMed] [Google Scholar]
- 3. Narla S, Silverberg JI. Association between atopic dermatitis and serious cutaneous, multiorgan and systemic infections in US adults. Ann Allergy Asthma Immunol 2018; 120:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Langan SM, Abuabara K, Henrickson SE et al Increased risk of cutaneous and systemic infections in atopic dermatitis – a cohort study. J Invest Dermatol 2017; 137:1375–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brook I, Frazier EH, Yeager JK. Microbiology of infected atopic dermatitis. Int J Dermatol 1996; 35:791–3. [DOI] [PubMed] [Google Scholar]
- 6. David T, Cambridge G. Bacterial infection and atopic eczema. Arch Dis Child 1986; 61:20–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sugarman JL, Hersh AL, Okamura T et al A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol 2011; 28:230–4. [DOI] [PubMed] [Google Scholar]
- 8. Altunbulakli C, Reiger M, Neumann AU et al Relations between epidermal barrier dysregulation and Staphylococcus species‐dominated microbiome dysbiosis in patients with atopic dermatitis. J Allergy Clin Immunol 2018; 142:1643–7. [DOI] [PubMed] [Google Scholar]
- 9. Cork MJ, Danby SG, Vasilopoulos Y et al Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol 2009; 129:1892–908. [DOI] [PubMed] [Google Scholar]
- 10. Hata TR, Gallo RL. Antimicrobial peptides, skin infections, and atopic dermatitis. Semin Cutan Med Surg 2008; 27:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuo I‐H, Yoshida T, De Benedetto A, Beck LA. The cutaneous innate immune response in patients with atopic dermatitis. J Allergy Clin Immunol 2013; 131:266–78. [DOI] [PubMed] [Google Scholar]
- 12. Ong PY, Leung DYM. Bacterial and viral infections in atopic dermatitis: a comprehensive review. Clin Rev Allergy Immunol 2016; 51:329–37. [DOI] [PubMed] [Google Scholar]
- 13. Eyerich K, Pennino D, Scarponi C et al IL‐17 in atopic eczema: linking allergen‐specific adaptive and microbial‐triggered innate immune response. J Allergy Clin Immunol 2009; 123:59–66. [DOI] [PubMed] [Google Scholar]
- 14. Nakatsuji T, Chen TH, Narala S et al Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 2017; 9:eaah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shu M, Wang Y, Yu J et al Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin‐resistant Staphylococcus aureus . PLOS ONE 2013; 8:e55380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams MR, Costa SK, Zaramela LS et al Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci Transl Med 2019; 11:eaat8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Myles IA, Earland NJ, Anderson ED et al First‐in‐human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 2018; 3:120608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leung DYM. The role of Staphylococcus aureus in atopic eczema. Acta Derm Venereol 2008; Suppl. 216:21–7. [Google Scholar]
- 19. Geoghegan JA, Irvine AD, Foster TJ. Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship. Trends Microbiol 2018; 26:484–97. [DOI] [PubMed] [Google Scholar]
- 20. Benenson S, Zimhony O, Dahan D et al Atopic dermatitis – a risk factor for invasive Staphylococcus aureus infections: two cases and review. Am J Med 2005; 118:1048–51. [DOI] [PubMed] [Google Scholar]
- 21. Patel D, Jahnke MN. Serious complications from Staphylococcal aureus in atopic dermatitis. Pediatr Dermatol 2015; 32:792–6. [DOI] [PubMed] [Google Scholar]
- 22. Serrano L, Patel KR, Silverberg JI. Association between atopic dermatitis and extracutaneous bacterial and mycobacterial infections: a systematic review and meta‐analysis. J Am Acad Dermatol 2019; 80:904–12. [DOI] [PubMed] [Google Scholar]
- 23. Brenninkmeijer EEA, Schram ME, Leeflang MMG et al Diagnostic criteria for atopic dermatitis: a systematic review. Br J Dermatol 2008; 158:754–65. [DOI] [PubMed] [Google Scholar]
- 24. Lyons JJ, Milner JD, Stone KD. Atopic dermatitis in children: clinical features, pathophysiology, and treatment. Immunol Allergy Clin North Am 2015; 35:161–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hanifin JM, Rogge JL. Staphylococcal infections in patients with atopic dermatitis. Arch Dermatol 1977; 113:1383–6. [PubMed] [Google Scholar]
- 26. Lubbe J. Secondary infections in patients with atopic dermatitis. Am J Clin Dermatol 2003; 4:641–54. [DOI] [PubMed] [Google Scholar]
- 27. Leyden JJ, Baker DA. Localized herpes simplex infections in atopic dermatitis. Arch Dermatol 1979; 115:311–12. [PubMed] [Google Scholar]
- 28. Wollenberg A, Zoch C, Wetzel S et al Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol 2003; 49:198–205. [DOI] [PubMed] [Google Scholar]
- 29. Peng WM, Jenneck C, Bussmann C et al Risk factors of atopic dermatitis patients for eczema herpeticum. J Invest Dermatol 2007; 127:1261–3. [DOI] [PubMed] [Google Scholar]
- 30. Beck LA, Boguniewicz M, Hata T et al Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J Allergy Clin Immunol 2009; 124:260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Traidl S, Kienlin P, Begemann G et al Patients with atopic dermatitis and history of eczema herpeticum elicit herpes simplex virus‐specific type 2 immune responses. J Allergy Clin Immunol 2018; 141:1144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao P‐S, Rafaels NM, Hand T et al Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol 2009; 124:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bin L, Kim BE, Brauweiler A et al Staphylococcus aureus α‐toxin modulates skin host response to viral infection. J Allergy Clin Immunol 2012; 130:683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Benedetto A, Slifka MK, Rafaels NM et al Reductions in claudin‐1 may enhance susceptibility to herpes simplex virus 1 infections in atopic dermatitis. J Allergy Clin Immunol 2011; 128:242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Darabi K, Hostetler SG, Bechtel MA, Zirwas M. The role of Malassezia in atopic dermatitis affecting the head and neck of adults. J Am Acad Dermatol 2009; 60:125–36. [DOI] [PubMed] [Google Scholar]
- 36. Johansson C, Sandstrom MH, Bartosik J et al Atopy patch test reactions to Malassezia allergens differentiate subgroups of atopic dermatitis patients. Br J Dermatol 2003; 148:479–88. [DOI] [PubMed] [Google Scholar]
- 37. Glatz M, Bosshard P, Schmid‐Grendelmeier P. The role of fungi in atopic dermatitis. Immunol Allergy Clin North Am 2017; 37:63–74. [DOI] [PubMed] [Google Scholar]
- 38. Tsakok T, Schulenburg H, Smith C et al The role of yeast in atopic dermatitis revisited: a critical appraisal. Curr Dermatol Rep 2015; 4:228–40. [Google Scholar]
- 39. Schmid‐Grendelmeier P, Fluckiger S, Disch R et al IgE‐mediated and T cell‐mediated autoimmunity against manganese superoxide dismutase in atopic dermatitis. J Allergy Clin Immunol 2005; 115:1068–75. [DOI] [PubMed] [Google Scholar]
- 40. Balaji H, Heratizadeh A, Wichmann K et al Malassezia sympodialis thioredoxin‐specific T cells are highly cross‐reactive to human thioredoxin in atopic dermatitis. J Allergy Clin Immunol 2011; 128:92–9. [DOI] [PubMed] [Google Scholar]
- 41. Morita E, Hide M, Yoneya Y et al An assessment of the role of Candida albicans antigen in atopic dermatitis. J Dermatol 1999; 26:282–7. [DOI] [PubMed] [Google Scholar]
- 42. Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups – variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol 2018; 27:340–57. [DOI] [PubMed] [Google Scholar]
- 43. Silverberg NB. Typical and atypical clinical appearance of atopic dermatitis. Clin Dermatol 2017; 35:354–9. [DOI] [PubMed] [Google Scholar]
- 44. Ben‐Gashir MA, Seed PT, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol 2002; 147:920–5. [DOI] [PubMed] [Google Scholar]
- 45. Vachiramon V, Tey HL, Thompson AE, Yosipovitch G. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol 2012; 29:395–402. [DOI] [PubMed] [Google Scholar]
- 46. Merriman JA, Mueller EA, Cahill MP et al Temporal and racial differences associated with atopic dermatitis Staphylococcus aureus and encoded virulence factors. mSphere 2016; 1:e00295–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shi B, Leung DYM, Taylor PA, Li H. Methicillin‐resistant Staphylococcus aureus colonization is associated with decreased skin commensal bacteria in atopic dermatitis. J Invest Dermatol 2018; 138:1668–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suh L, Coffin S, Leckerman KH et al Methicillin‐resistant Staphylococcus aureus colonization in children with atopic dermaitis. Pediatr Dermatol 2008; 25:528–34. [DOI] [PubMed] [Google Scholar]
- 49. Warner JA, McGirt LY, Beck LA. Biomarkers of Th2 polarity are predictive of staphylococcal colonization in subjects with atopic dermatitis. Br J Dermatol 2009; 160:183–5. [DOI] [PubMed] [Google Scholar]
- 50. Arkwright PD, Daniel TO, Sanyal D et al Age‐related prevalence and antibiotic resistance of pathogenic staphylococci and streptococci in children with infected atopic dermatitis at a single‐specialty center. Arch Dermatol 2002; 138:939–41. [DOI] [PubMed] [Google Scholar]
- 51. Harkins CP, McAleer MA, Bennett D et al The widespread use of topical antimicrobials enriches for resistance in Staphylococcus aureus isolated from patients with atopic dermatitis. Br J Dermatol 2018; 179:951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Park JM, Jo JH, Jin H et al Change in antimicrobial susceptibility of skin‐colonizing Staphylococcus aureus in Korean patients with atopic dermatitis during ten‐year period. Ann Dermatol 2016; 28:470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jung MY, Chung JY, Lee HY et al Antibiotic susceptibility of Staphylococcus aureus in atopic dermatitis: current prevalence of methicillin‐resistant Staphylococcus aureus in Korea and treatment strategies. Ann Dermatol 2015; 27:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chung HJ, Jeon HS, Sung H et al Epidemiological characteristics of methicillin‐resistant Staphylococcus aureus isolates from children with eczematous atopic dermatitis lesions. J Clin Microbiol 2008; 46:991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gomes PLR, Malavige GN, Fernando N et al Characteristics of Staphylococcus aureus colonization in patients with atopic dermatitis in Sri Lanka. Clin Exp Dermatol 2011; 36:195–200. [DOI] [PubMed] [Google Scholar]
- 56. Salgado CD, Farr BM, Calfee DP. Community‐acquired methicillin‐resistant Staphylococcus aureus: a meta‐analysis of prevalence and risk factors. Clin Infect Dis 2003; 36:131–9. [DOI] [PubMed] [Google Scholar]
- 57. Balma‐Mena A, Lara‐Corrales I, Zeller J et al Colonization with community‐acquired methicillin‐resistant Staphylococcus aureus in children with atopic dermatitis: a cross‐sectional study. Int J Dermatol 2011; 50:682–8. [DOI] [PubMed] [Google Scholar]
- 58. Matiz C, Tom WL, Eichenfield LF et al Children with atopic dermatitis appear less likely to be infected with community acquired methicillin‐resistant Staphylococcus aureus: the San Diego experience. Pediatr Dermatol 2011; 28:6–11. [DOI] [PubMed] [Google Scholar]
- 59. Totte JEE, van der Feltz WT, Hennekam M et al Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta‐analysis. Br J Dermatol 2016; 175:687–95. [DOI] [PubMed] [Google Scholar]
- 60. Aly R, Maibach HI, Shinefield HR. Microbial flora of atopic dermatitis. Arch Dermatol 1977; 113:780–2. [PubMed] [Google Scholar]
- 61. Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol 1974; 90:525–30. [DOI] [PubMed] [Google Scholar]
- 62. Goodyear HM, Watson PJ, Egan SA et al Skin microflora of atopic eczema in first time hospital attenders. Clin Exp Dermatol 1993; 18:300–4. [DOI] [PubMed] [Google Scholar]
- 63. Ewing CI, Ashcroft C, Gibbs AC et al Flucloxacillin in the treatment of atopic dermatitis. Br J Dermatol 1998; 138:1022–9. [DOI] [PubMed] [Google Scholar]
- 64. Matsui K, Nishikawa A, Suto H et al Comparative study of Staphylococcus aureus isolated from lesional and non‐lesional skin of atopic dermatitis patients. Microbiol Immunol 2000; 44:945–7. [DOI] [PubMed] [Google Scholar]
- 65. Polano M, De Vries H. Analysis of the results obtained in the treatment of atopic dermatitis with corticosteroid and neomycin containing ointments. Dermatologica 1960; 120:191–9. [DOI] [PubMed] [Google Scholar]
- 66. Lever R, Hadley K, Downey D, Mackie R. Staphylococcal colonization in atopic dermatitis and the effect of topical mupirocin therapy. Br J Dermatol 1988; 119:189–98. [DOI] [PubMed] [Google Scholar]
- 67. Ramsay CA, Savoie JM, Gilbert M et al The treatment of atopic dermatitis with topical fusidic acid and hydrocortisone acetate. J Eur Acad Dermatology Venereol 1996; 7 (Suppl. 1):S15–22. [Google Scholar]
- 68. Leyden JJ, Kligman AM. The case for steroid–antibiotic combinations. Br J Dermatol 1977; 96:179–87. [DOI] [PubMed] [Google Scholar]
- 69. Dhar S, Kanwar AJ, Kaur S et al Role of bacterial flora in the pathogenesis and management of atopic dermatitis. Indian J Med Res 1992; 95:234–8. [PubMed] [Google Scholar]
- 70. Breuer K, Haussler S, Kapp A, Werfel T. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol 2002; 147:55–61. [DOI] [PubMed] [Google Scholar]
- 71. Hjorth N, Schmidt K, Thomsen K. Fusidic acid plus betamethasone in infected or potentially infected eczema. Pharmatherapeutica 1985; 4:126–31. [PubMed] [Google Scholar]
- 72. Bath‐Hextall FJ, Birnie AJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema: an updated Cochrane review. Br J Dermatol 2010; 163:12–26. [DOI] [PubMed] [Google Scholar]
- 73. Nilsson EJ, Henning CG, Magnusson J. Topical corticosteroids and Staphylococcus aureus in atopic dermatitis. J Am Acad Dermatol 1992; 27:29–34. [DOI] [PubMed] [Google Scholar]
- 74. Nilsson E, Henning C, Hjörleifsson ML. Density of the microflora in hand eczema before and after topical treatment with a potent corticosteroid. J Am Acad Dermatol 1986; 15:192–7. [DOI] [PubMed] [Google Scholar]
- 75. Travers JB, Kozman A, Yao Y et al Treatment outcomes of secondarily impetiginized pediatric atopic dermatitis lesions and the role of oral antibiotics. Pediatr Dermatol 2012; 29:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tauber M, Balica S, Hsu C‐Y et al Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol 2016; 137:1272–4. [DOI] [PubMed] [Google Scholar]
- 77. Williamson P, Kligman AM. A new method for the quantitative investigation of cutaneous bacteria. J Invest Dermatol 1965; 45:498–503. [DOI] [PubMed] [Google Scholar]
- 78. Kong HH, Oh J, Deming C et al Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 2012; 22:850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Byrd AL, Deming C, Cassidy SKB et al Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med 2017; 9:eaal4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Totte JEE, Pardo LM, Fieten KB et al Nasal and skin microbiomes are associated with disease severity in paediatric atopic dermatitis. Br J Dermatol 2019; 181:796–804. [DOI] [PubMed] [Google Scholar]
- 81. Li W, Xu X, Wen H et al Inverse association between the skin and oral microbiota in atopic dermatitis. J Invest Dermatol 2019; 139:1779–87. [DOI] [PubMed] [Google Scholar]
- 82. Casadevall A, Pirofski L. The damage–response framework of microbial pathogenesis. Nat Rev Microbiol 2003; 1:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Casadevall A, Pirofski L. What is a host? Attributes of individual susceptibility. Infect Immun 2018; 86:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pirofski L, Casadevall A. The meaning of microbial exposure, infection, colonisation, and disease in clinical practice. Lancet Infect Dis 2002; 2:628–35. [DOI] [PubMed] [Google Scholar]
- 85. Clowry J, Irvine AD, McLoughlin RM. Next‐generation anti‐Staphylococcus aureus vaccines: a potential new therapeutic option for atopic dermatitis? J Allergy Clin Immunol 2019; 143:78–81. [DOI] [PubMed] [Google Scholar]
- 86. Simpson EL, Villarreal M, Jepson B et al Atopic dermatitis subjects colonized with Staphylococcus aureus have a distinct phenotype and endotype. J Invest Dermatol 2018; 138:2224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Clausen M‐L, Edslev SM, Andersen PS et al Staphylococcus aureus colonization in atopic eczema and its association with filaggrin gene mutations. Br J Dermatol 2017; 177:1394–400. [DOI] [PubMed] [Google Scholar]
- 88. Berents TL, Carlsen KCL, Mowinckel P et al Skin barrier function and Staphylococcus aureus colonization in vestibulum nasi and fauces in healthy infants and infants with eczema: a population‐based cohort study. PLOS ONE 2015; 10:e0130145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Palmer CNA, Irvine AD, Terron‐Kwiatkowski A et al Common loss‐of‐function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006; 38:441–6. [DOI] [PubMed] [Google Scholar]
- 90. Fluhr JW, Elias PM. Stratum corneum pH: formation and function of the ‘acid mantle’. Exog Dermatol 2002; 1:163–75. [Google Scholar]
- 91. Miller SJ, Aly R, Shinefeld HR, Elias PM. In vitro and in vivo antistaphylococcal activity of human stratum corneum lipids. Arch Dermatol 1988; 124:209–15. [PubMed] [Google Scholar]
- 92. Georas SN, Cheadle C, Berger AE. Tight junction defects in atopic dermatitis. J Allergy Clin Immunol 2012; 127:773–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cho SH, Strickland I, Boguniewicz M, Leung DY. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. J Allergy Clin Immunol 2001; 108:269–74. [DOI] [PubMed] [Google Scholar]
- 94. Foster TJ, Geoghegan JA, Ganesh VK, Hook M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus . Nat Rev Microbiol 2014; 12:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fleury OM, McAleer MA, Feuillie C et al Clumping factor B promotes adherence of Staphylococcus aureus to corneocytes in atopic dermatitis. Infect Immun 2017; 85:e00994–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ong PY, Ohtake T, Brandt C et al Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 2002; 347:1151–60. [DOI] [PubMed] [Google Scholar]
- 97. Howell MD, Gallo RL, Boguniewicz M et al Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity 2006; 24:341–8. [DOI] [PubMed] [Google Scholar]
- 98. Kim BE, Leung DYM, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down‐regulated by Th2 cytokines through STAT‐6. Clin Immunol 2008; 126:332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Postlethwaite AE, Holness MA, Katai H, Raghow R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Invest 1992; 90:1479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nomura I, Goleva E, Howell MD et al Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol 2003; 171:3262–9. [DOI] [PubMed] [Google Scholar]
- 101. Eichenfield LF, Bieber T, Beck LA et al Infections in dupilumab clinical trials in atopic dermatitis: a comprehensive pooled analysis. Am J Clin Dermatol 2019; 20:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ou Z, Chen C, Chen A et al Adverse events of dupilumab in adults with moderate‐to‐severe atopic dermatitis: a meta‐analysis. Int Immunopharmacol 2018; 54:303–10. [DOI] [PubMed] [Google Scholar]
- 103. Callewaert C, Nakatsuji T, Knight R et al IL‐4Rα blockade by dupilumab decreases Staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J Invest Dermatol 2019; doi: 10.1016/j.jid.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lai Y, Cogen AL, Radek KA et al Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol 2010; 130:2211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cogen AL, Yamasaki K, Sanchez KM et al Selective antimicrobial action is provided by phenol‐soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol 2010; 130:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Myles IA, Williams KW, Reckhow JD et al Transplantation of human skin microbiota in models of atopic dermatitis. JCI Insight 2016; 1:86955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Saloga J, Gelfand E, Knop J. Superantigens. Exp Dermatol 1996; 5:65–72. [DOI] [PubMed] [Google Scholar]
- 108. Schlievert PM, Cahill MP, Hostager BS et al Staphylococcal superantigens stimulate epithelial cells through CD40 to produce chemokines. MBio 2019; 10:e00214–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Stach CS, Herrera A, Schlievert PM. Staphylococcal superantigens interact with multiple host receptors to cause serious diseases. Immunol Res 2014; 59:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Leung DY, Harbeck R, Bina P et al Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest 1993; 92:1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sonkoly E, Muller A, Lauerma AI et al IL‐31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol 2006; 117:411–17. [DOI] [PubMed] [Google Scholar]
- 112. Cornelissen C, Marquardt Y, Czaja K et al IL‐31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol 2012; 129:426–33. [DOI] [PubMed] [Google Scholar]
- 113. van Drongelen V, Haisma EM, Out‐Luiting JJ et al Reduced filaggrin expression is accompanied by increased Staphylococcus aureus colonization of epidermal skin models. Clin Exp Allergy 2014; 44:1515–24. [DOI] [PubMed] [Google Scholar]
- 114. Leung DYM, Hanifin JM, Pariser DM et al Effects of pimecrolimus cream 1% in the treatment of patients with atopic dermatitis who demonstrate a clinical insensitivity to topical corticosteroids: a randomized, multicentre vehicle‐controlled trial. Br J Dermatol 2009; 161:435–43. [DOI] [PubMed] [Google Scholar]
- 115. Schlievert PM, Strandberg KL, Lin YC et al Secreted virulence factor comparison between methicillin‐resistant and methicillin‐sensitive Staphylococcus aureus, and its relevance to atopic dermatitis. J Allergy Clin Immunol 2010; 125:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Nakamura Y, Oscherwitz J, Cease KB et al Staphylococcus delta‐toxin induces allergic skin disease by activating mast cells. Nature 2013; 503:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Brauweiler AM, Goleva E, Leung DYM. Th2 cytokines increase Staphylococcus aureus alpha toxin‐induced keratinocyte death through the signal transducer and activator of transcription 6 (STAT6). J Invest Dermatol 2014; 134:2114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nakagawa S, Matsumoto M, Katayama Y et al Staphylococcus aureus virulent PSMα peptides induce keratinocyte alarmin release to orchestrate IL‐17‐dependent skin inflammation. Cell Host Microbe 2017; 22:667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Liu H, Archer NK, Dillen CA et al Staphylococcus aureus epicutaneous exposure drives skin inflammation via IL‐36‐mediated T‐cell responses. Cell Host Microbe 2017; 22:653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Brauweiler AM, Bin L, Kim BE et al Filaggrin‐dependent secretion of sphingomyelinase protects against staphylococcal alpha‐toxin‐induced keratinocyte death. J Allergy Clin Immunol 2013; 131:421–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus . J Allergy Clin Immunol 2010; 126:1184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Classen A, Kalali BN, Schnopp C et al TNF receptor I on human keratinocytes is a binding partner for staphylococcal protein A resulting in the activation of NF kappa B, AP‐1, and downstream gene transcription. Exp Dermatol 2011; 20:48–52. [DOI] [PubMed] [Google Scholar]
- 123. Vu AT, Baba T, Chen X et al Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll‐like receptor 2–Toll‐like receptor 6 pathway. J Allergy Clin Immunol 2010; 126:985–93. [DOI] [PubMed] [Google Scholar]
- 124. Nakatsuji T, Chen TH, Two AM et al Staphylococcus aureus exploits epidermal barrier defects in atopic dermatitis to trigger cytokine expression. J Invest Dermatol 2016; 136:2192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Williams MR, Nakatsuji T, Sanford JA et al Staphylococcus aureus induces increased serine protease activity in keratinocytes. J Invest Dermatol 2017; 137:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Harkins CP, Holden MTG, Irvine AD. Antimicrobial resistance in atopic dermatitis. Ann Allergy Asthma Immunol 2018; 122:236–40. [DOI] [PubMed] [Google Scholar]


